Abstract
Bright light was an effective aversive stimulus for Wistar rats in punishment, escape, and avoidance paradigms. Contingent punishment of lever-pressing maintained by concurrent schedules of food delivery shifted presses to an alternate lever, and depressed overall response rates. Periodic non-contingent presentation of the light prompted escape responding (head entry into a hole). Unsignaled avoidance contingencies were not effective, but pre-pulse signaling of light supported avoidance behavior. These results demonstrate a possible alternative to foot-shock, one with greater ecological validity, and one that might avoid some of the physiological effects that accompany electric shock.
Keywords: Avoidance, Escape, Punishment, Burrow, Light Aversion, Rat
Electric footshock has served as the prototypical aversive stimulus in experimental preparations using rats [34,30]. Footshock is popular for its reliability, utility at a wide range of current, and the feasibility of titrating shock levels for individual subjects. But electric shock has its downsides: It often induces secondary effects such as long term sleep disruption [41], altered social behavior [27], reduction in locomotion, rearing, and grooming behaviors, as well as an increase in immobility and defecation [45]. Footshock has also received criticism when used to model conditions such as depression or anxiety disorder that are produced by stressors that lack a comparable component of pain [28]. Finally, the nature of footshock stimulation precludes its full inclusion in some modern experimental techniques, such as electrophysiology.
As neural, behavioral, and genetic research strives to create better animal models of human disorders, the availability of options other than footshock as aversive stimuli becomes increasingly important. Reed and Yoshino [39] recently noted the utility of a broader range of aversive stimuli, including some that might replace shock and avoid some of the drawbacks that accompany its use. For instance, they demonstrated that response suppression could be effected by a loud, short tone presented with long inter-trial intervals.
The present study aims to systematically test whether bright light is an effective aversive stimulus for rats. Early experiments demonstrated that rats will press a lever to terminate a light stimulus, and that rate of lever-pressing increases with light intensity [19]. The rate of escape lever-presses is also sensitive to the schedule of negative reinforcement [16, 17, 18]. When allowed to control their own daily exposure to light by lever pressing, albino rats decrease their exposure to light intensities greater than 1.25 lux over days, and maintain their exposure to light below this threshold [22, 23, 24, 25]. Further examination led Campbell and Messing [6] to suggest that light is likely aversive even at the lowest levels of illumination in rats. However, a considerable number of studies have proposed that light—at some levels—may not be aversive [36], and might even act as a reinforcer [2, 14, 40].
The following experiments provide a systematic test of light's efficacy as an aversive stimulus. Experiment 1 examined the aversiveness of bright light in a choice situation by adding a light contingency onto one of two otherwise identical alternatives. Experiments 2 and 3 examined escape from and avoidance of light, respectively. It has long been suggested that defensive responses are more efficiently learned when they are consistent with the natural repertoire of the animal [4]. Burrowing is a commonly observed defensive behavior in Rattus norvegicus [3, 20, 33, 35]. It was thus expected that entering a short tunnel would be an effective escape/avoidance response to light.
Experiment 1: Punishment
It has been shown that rats prefer dark or dim areas over those that are brightly lit, in both Pavlovian (e.g., [42]) and operant paradigms (e.g. [22, 23, 24, 25]). However, lights have also been used as effective reinforcers during operant conditioning [2, 27, 14]. Experiment 1 aimed to determine whether a brief bright light serves as a punisher. If it does, making the light contingent on pressing one of two levers for food should reduce preference for that lever.
Methods
Subjects
Six experimentally naive male Wistar rats were housed individually in a room with a 12 h: 12 h light: dark cycle, with dusk at 0600 h; experiments were conducted only during the dark cycle. Water was always available in home cages. Supplementary chow was provided to maintain rats at 85% of their free feeding weight, as estimated from the growth curves from the provider. Upon starting the experiment the animals were approximately 46 days old and had been food restricted for 5 days. All conditions were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines and regulations at Arizona State University.
Apparatus
Experimental sessions were conducted in a MED Associates (St. Albans, VT) modular test chamber (305 mm long, 241 mm wide, and 292 mm high), enclosed in a sound- and light-attenuating box equipped with a ventilating fan. The front and rear walls and the ceiling of the experimental chambers were made of clear plastic, and the front wall was hinged and functioned as a door to the chamber. In the center of the transparent ceiling a 64-mm diameter hole allowed direct entry of 1650 lumens of light (8180 lux at the chamber floor), generated by a 150-watt indoor/outdoor Ace brand Par 38 floodlight lamp (model # 3019759). Light measurements (light meter model # 401027, Extech Instruments, Watham, MA) were taken from just above the chamber floor, which consisted of thin metal bars above a catch pan. One of the two aluminum side panels served as a test panel. A square opening (51 mm sides) located 15 mm above the floor and centered on the test panel provided access to the hopper (MED Associates, ENV-200-R2M) for 45 mg food pellets (Noyes Precision pellets, Improved Formula A/I, Research Diets, Inc., New Brunswick, NJ). A single pellet was provided with each activation of a dispenser (ENV-203). Two retractable levers (ENV-112CM) flanked the food hopper. The center of each lever was 80 mm from the center of the food hopper, and 21 mm from the floor. Lever presses were recorded when a force of approximately 0.2 N was applied to the end of the lever. The ventilation fan mounted on the rear wall of the sound-attenuating chamber provided masking noise of approximately 60 dB. There was no ambient illumination of the test chambers during experimental sessions. Experimental events were arranged via a Med-PC® interface connected to a PC controlled by Med-PC IV® software.
Procedure
Hopper and lever training
Subjects were first hopper-trained for 2 days. During this time, food was presented non-contingently for 1 hour each day on a random schedule. Subsequently, rats were trained to press on the right lever for pellets by progressively increasing the response requirements.
Baseline
The rats were then trained on two independent concurrent variable-interval 60 s (VI 60 s VI 60 s) schedules of food reinforcement. On these schedules, after a random interval elapsed, the next response on the appropriate lever delivered a pellet. Intervals were selected from 2 independent 12-item Fleshler-Hoffman distributions [10], each with a mean duration of 60 s. This condition was effective for approximately 10 sessions, 5 days per week. The preferred lever was defined for each rat as the one with more responses during the last 5 sessions. Baseline preference was computed as the proportion of lever presses made on the preferred lever. Sessions lasted for either 1 hour or 120 trials, whichever happened first.
Choice with light on VI 60 s
Five-second activations of the floodlight contingent on lever pressing were superimposed on baseline experimental conditions. For each rat, the preferred lever was selected to activate the floodlight. Light activations on the preferred lever were delivered according to a VI 60 s schedule, independent of food reward. This condition was in effect for 15 sessions.
Choice with light on VI 30 s
The rate of floodlight activation was increased by reducing the mean interval between activations to 30 s (VI 30 s). The schedule of food reinforcement remained unchanged. This condition was in effect for 12 sessions.
Reversal of light-activating assignment
The position of the lever activating the floodlight was reversed, with the schedule of light-activation remaining VI 30 s. The lever that previously delivered light activations now delivered only food. Nine sessions were conducted under these conditions.
Data Analysis
The data collected for each of the following experiments created a hierarchical, or nested, dataset with multiple observations collected from each subject [38, 43]. The nesting of data violates the assumption of independence for inferential testing in ANOVA/regression models, which in turn can lead to an excessively high Type I error rate [43]. In order to take into account the nested structure of the dataset, a mixed ANOVA model with both fixed and random effects was used [15]. The variable that is responsible for the nesting in a dataset, (i.e., subjects), was incorporated into the ANOVA model as a random effect in which each individual nest was specified as a level of the random effect [15]. The standard errors and variance estimators used for significance testing were then adjusted by the mixed ANOVA model for the presence of a nesting variable [38, 15]. The experimental condition was specified as a fixed effect. Data from the last three sessions of each of the four conditions was analyzed in order to compare stable data between each subsequent condition, and omitting the learning curve associated with changes between conditions. Because the dependent variable was not normally distributed, robust standard errors were used, which are a distribution-free estimator, and therefore less sensitive to departures from normality [8, 38]. Tukey-Kramer post-hoc tests were conducted to examine all possible comparisons between conditions; all reported p values are Tukey-Kramer adjusted. Data analysis was conducted using SAS PROC MIXED (SAS Institute, Inc. Version 9.2)
Results and Discussion
The mixed ANOVA revealed a significant effect of experimental condition, [F(3,63)= 45.06, p <.001]. During the baseline condition, 3 rats pressed the left lever more often, and 3 rats pressed the right lever more often. After extensive training, an average 56 ± 1% of lever presses were made on the preferred lever. Figure 1 shows the average choice of the preferred lever over baseline and experimental conditions.
Figure 1.
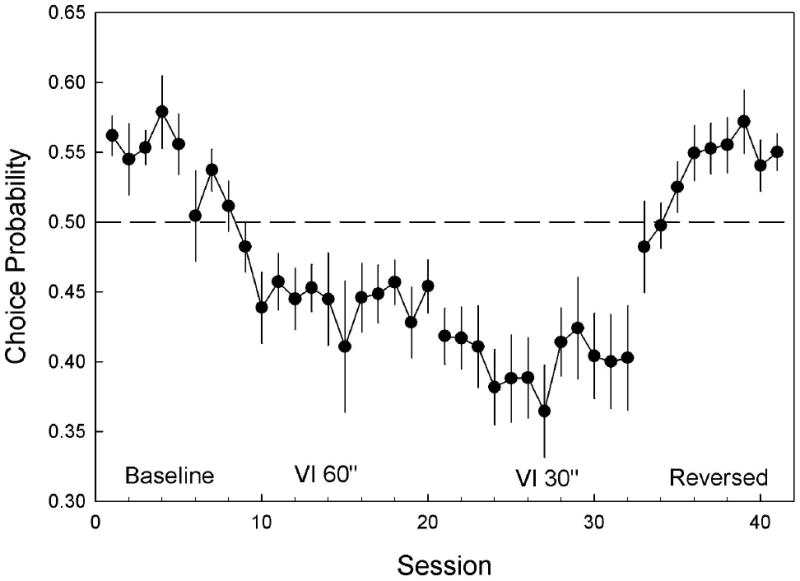
Mean (±SEM) proportion of presses on the lever that was preferred during baseline. The dashed line indicates indifference between levers. Baseline shows the initial proportion of presses on the preferred lever. When bright light was added on a VI 60 schedule, the proportion of responding shifted towards the originally non-preferred lever. When the rate of bright light presentation was increased to a VI 30 schedule a slight further decrease in preference was observed. When the bright light was Reversed onto the originally non-preferred lever, the proportion of responses on the preferred lever again increased. These data show that lever presses were less frequent on the lever that produced light. Results from the Mixed ANOVA are presented in the Experiment 1 results section.
Tukey-Kramer post hoc tests revealed that relative rates of responding on the preferred lever decreased following the introduction of the light [t(63)=3.63, p < .01], as seen in Figure 1, to an average of 44.6 ± 1.2% over the last 3 days of the VI-60 s light condition. Changing the punishment schedule to a shorter interval (VI 30 s) increased the rate of contingent light activations and caused a further reduction of responding to 40 ± 2% [t(63)=2.07, p = .17, N.S.]. Switching the light to the originally un-preferred lever quickly drove the relative rates of responding back in favor of the originally preferred lever (54.8 ± 1.3%). This increase was significantly different from the preceding condition [t(63) = -7.55, p < .001] but not significantly different from baseline [t(63)=.60, p = .93, N.S.].
The introduction of the response-activated floodlight produced an overall decrease in responding. Prior to the experimental condition, responses averaged 1248 ± 60 per session. Introduction of the light stimulus reduced this average to 800 ± 36 responses/session within 5 experimental sessions. That average was maintained for the duration of the experiment. This depression in overall responding and the differential depression of the contingent response are consistent with the hypothesis that light punished lever pressing, and that most response suppression occurred on the punished lever.
Experiment 2: Escape/Avoidance
Experiment 2 examined escape responses of albino rats using the operant burrow apparatus. The rates of head entries into the burrow were examined when a bright light was presented at various rates and durations, with entry terminating the light; and also when burrowing did not terminate the light. Given that each successful escape response produced an extended ITI, the present experiment also has a Sidman avoidance component.
Methods
Subjects
Five male Wistar rats were housed individually in a room with a 12 h: 12 h light: dark cycle, with dusk at 0600 h; experiments were conducted only during the dark cycle. The rats received food and water ad libitum. Upon starting the experiment the animals were approximately 270 days old. All five rats had prior experience in two pilot experiments with light stimuli including stroboscopic light and 40W bulbs prior to their use in the present experiment. All conditions were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines and regulations at Arizona State University.
Apparatus
A round opening 50 mm in diameter was made in one of the panels of the box used in Experiment 1, which allowed the animal to enter an aluminum cylinder 60 mm deep, closed at the distal end (the burrow, Figure 2). The center of the opening was located 85mm from the medial line of the center panel, toward the chamber door. An infrared beam (Med Associates, ENV-254) located 21mm into the cylinder recorded head entries. The same flood lamp described in Experiment 1 was used; it caused a temperature increase of no more than 4.5° C, over the course of the session. Chamber temperatures varied below this level depending on escape proficiency.
Figure 2.
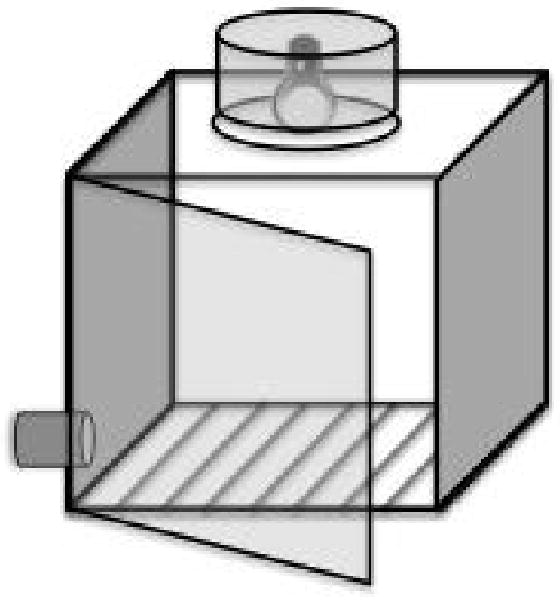
A diagram of the experimental chamber used in experiments 2 and 3. The operant burrow apparatus, located in the front lower left quadrant of the chamber measures 60 mm deep and 50 mm in diameter, and contains an infrared photo beam at 21 mm for measuring head-entries.
Procedure
Terms and basic conditions
The Light-Light (LL) interval was the interval between the response-independent termination of the light and its subsequent presentation. The Response-Light (RL) interval was the interval between the response-dependent termination of the light and its subsequent presentation. The light duration (D) represents the duration of light stimulus presentation in the absence of a successful escape response. The RL, LL, and D intervals for each of the experimental conditions is indicated below as “(RL:LL:D)”. Escapes were defined as head entries into the burrow during the light that caused a break in the infrared beam. A head removal of 2 s (during light or darkness) was required to accept subsequent escape responses. This contingency prevented subjects from avoiding the light by remaining lodged in the burrow.
Experimental conditions
Baseline 1 (20:5:10)
Subjects were exposed to D = 10 s of light following each LL interval. Head entries during the light terminated it for 20 s (the RL interval). This condition lasted for an average of 22 sessions. If no head entry occurred within the 10-s light period, the light terminated and the LL interval began.
No Light (0:0:0)
The light was discontinued. Responses during the extinction period were measured in the same way as in the previous condition, with trials defined by the 10 s interval when the light would have been presented. Subjects remained in the no-light condition for an average of 6 sessions.
Baseline 2 (20:5:5)
It was determined that all successful escape responses in baseline 1 occurred within 5 s of the stimulus presentation. Therefore, the light duration (D) was reduced to 5 s. This condition was in effect for 13 sessions.
Inescapable Light (0:5:5)
The light was made inescapable. Measurement of head-entry responses continued, but entries had no effect in changing the 5 s LL interval or 5 s light duration. Ten sessions were conducted.
Baseline 3
Subjects were returned to baseline 2 parameters (RL = 20 s, LL = 5 s, D = 5 s) for ten sessions.
Removal of Sidman Avoidance Contingency (5:5:5)
The RL interval was reduced to 5 s. Head entries to the burrow terminated the light. Ten sessions were conducted.
Data Analysis
The data for experiment two were analyzed as described above for Experiment 1. Subjects were incorporated into the ANOVA model as a random effect and the experimental condition was specified as a fixed effect. Again, only data from the last three sessions of each of the conditions were used for the analysis.
Results and Discussion
The mixed ANOVA revealed a significant effect of condition on escape probability [F(4,20)= 261.66, p<.001]. Figure 3 shows the average proportion of trials in which escape responses were emitted across experimental conditions. Subjects showed an immediate sensitivity to the light, escaping 64% ± 5.0% of the first 5 sessions and increasing to 84% ± 3.1% during the last 3 sessions of baseline 1. The mean escape latency was 2.1 s, with all successful escape responses occurring within 5 s of the stimulus presentation. Given the short latency of escapes in the first condition, the light duration for subsequent baseline conditions (baseline 2, 3) was reduced to 5 s. This may have caused a slight but non-significant decrease in the frequency of escape responses in subsequent recoveries of baseline (baseline 1 vs. 2, t(20) = 2.75, p = .11 N.S.; baseline 1 vs. 3, t(20) =2.72, p = .12, N.S.). During the no-light condition, responding dropped significantly [t(20) =15.64, p < .001], with an average escape rate of 1% ± .4%. The rats did not use the burrow unless driven there by the light.
Figure 3.
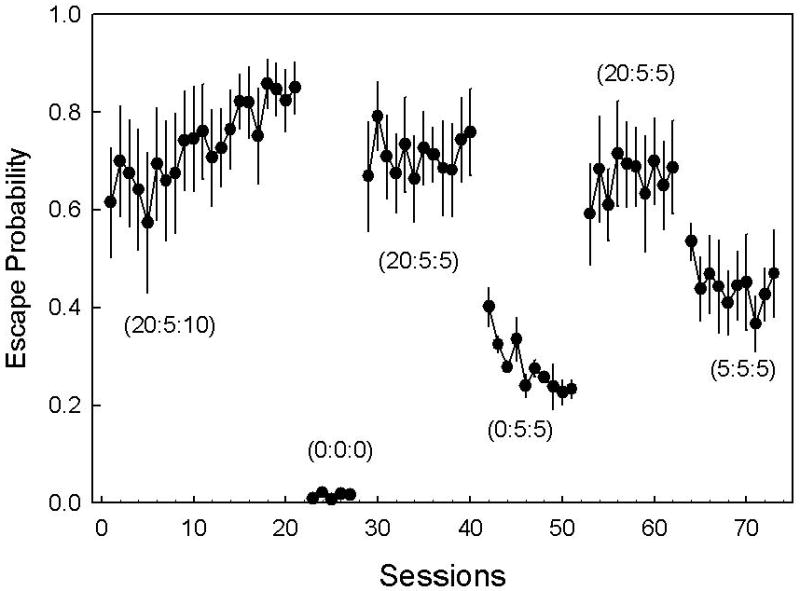
Mean (±SEM) proportion of trials in which escape responses were emitted. Escape contingencies are described in parentheses using the format RL:LL:D, where RL is the Response-Light interval, LL is the Light-Light interval, and D is the duration of the light. The three baseline [(20:5:10), (20:5:5) and (20:5:5)] conditions were intermittently followed by conditions in which no light (0:0:0) was presented, bright light was made inescapable (0:5:5) and in which the Sidman avoidance contingency was debased (5:5:5). Results from the Mixed ANOVA are presented in the Experiment 2 results section.
During 10 sessions of inescapable light, animals attempted to escape on 23% ± 1.8% of the light presentations, significantly less than baseline [t(20) = 12.03, p < .001]. Escape responses during this condition were not fully extinguished, perhaps because the escape cylinder provided some shade. Following this condition, baseline escape responding was again re-established. The last condition decreased the response contingent dark-time to 5 s, which decreased the probability of escape to 42% ± 3.8%, a significant decrement from baseline [t(20) =6.20, p < .001].
Overall, the sensitivity of escape responses to contingency manipulations confirms that light was aversive to Wistar rats. When given the opportunity, rats escaped most presentations of the light. The burrow was used substantially less when no light was presented and when the burrow was ineffective. Furthermore, escape responses were sensitive to the duration of the darkness period they produced: shorter periods supported a lower probability of escaping.
Experiment 3: Escape and Avoidance
The escape paradigm used in Experiment 2 was replicated, with additional measures taken to eliminate temperature as a factor. The ability of bright light to support an avoidance response was then tested using the burrow apparatus.
Subjects
Six male Wistar rats were housed individually in a room with a 12 h: 12 h light: dark cycle, dusk at 0600 h; experiments were conducted during the dark cycle. The rats received food and water ad libitum. Upon starting the experiment proper, the rats were approximately 155 days old. All 6 rats were tested in Experiment 1 prior to their use in the present experiment and therefore had experience with that apparatus: The bright light was identical and the dimensions and floors of the chamber were similar. All conditions were maintained in accordance with the Institutional Animal Care and Use Committee (IACUC) guidelines and regulations at Arizona State University.
Apparatus
The apparatus was similar to that used in Experiment 2. To maintain a consistent temperature, two ventilation fans were added, increasing the masking noise to 72 dB. A tone generator (MED Associates ENV-223), which could produce a tone of 3.5 kHz at 77 dB through a speaker (ENV 224AM) was also added.
Procedure
Escape only
Bright light was presented on a random basis every 10 s, but with a 5 s minimum interval and a maximum duration of 8 s. The interval between light presentations constituted the inter-trial interval (ITI). Responses within 8 s of light onset were followed by a 15 s dark period. If no response occurred within the 8 s period, the light was terminated. Either outcome was followed by an ITI. As in the previous experiment, successful escape responses required a preceding 2 s head removal from the operant burrow. This condition was in effect for 10 sessions.
Un-signaled avoidance and escape
The light presentation contingencies from the previous condition remained. Head entry responses during the ITI constituted avoidance responses and were followed by 20 s of darkness. Escape responses during the light were followed by a shorter, 5 s dark period. This condition was in effect for 10 sessions.
Signaled avoidance and escape
In addition to the preceding escape/avoidance contingencies, a tone signaled the 5-s period preceding the light onset. This condition lasted for 16 sessions.
Pre-pulse avoidance and escape
A brief (200 ms) flash of light was added at the beginning of the 5-s warning-tone period for 14 sessions. We call this light the cue-light, to distinguish it from the 8-s light it cued.
Avoidance only
The subsequent 20 sessions allowed subjects to avoid the light stimulus only through the completion of valid responses in the dark; when the light was on, it was inescapable. The warning tone from the previous condition was removed, though the cue-light remained present.
Brief light
The duration of the light was reduced from 8 s to 500 ms. 14 sessions were recorded. The cue light remained active during this condition.
Un-signaled brief light
10 sessions were conducted where the cue-light was removed, leaving only the brief flash of light, which was presented every 5 s if no avoidance responses were made, and was delayed by 20 s when an avoidance response was made.
Data Analysis
The data for experiment 2 were analyzed as described above for Experiment 1, however, responses in the light (escape) and those in the dark (avoidance) were analyzed using two separate mixed models. Subjects were incorporated into each of the ANOVAs as a random effect and the experimental condition was specified as a fixed effect. As in the previous experiments, only data from the last three sessions of each of the conditions was used for the analysis.
Results and Discussion
The mixed ANOVA revealed a significant omnibus effect of experimental condition on responses both in the light [F(5,114)= 570.75, p<.001] and in the dark [F(4,114)=88.05, P<.001]. Figures 4 and 5 show rates of head entry in the light (escape) and in the dark (avoidance), respectively. Escape behavior is not shown for the last two experimental conditions, because the brief light used in those conditions was inescapable. Subjects finished the escape-only condition with an average of 1.1 ± 0.1 escape responses/min, which cancelled the light on 47% ± 5.1 % of trials. These were lower than baseline rates demonstrated in Experiment 2. High rates of responding in the dark, which had no programmed consequences, occurred during the first session of the escape-only condition (0.69 ± 0.1 resp/min), declining to an asymptote of 0.25 ± .1 responses/min during the last 3 sessions.
Figure 4.
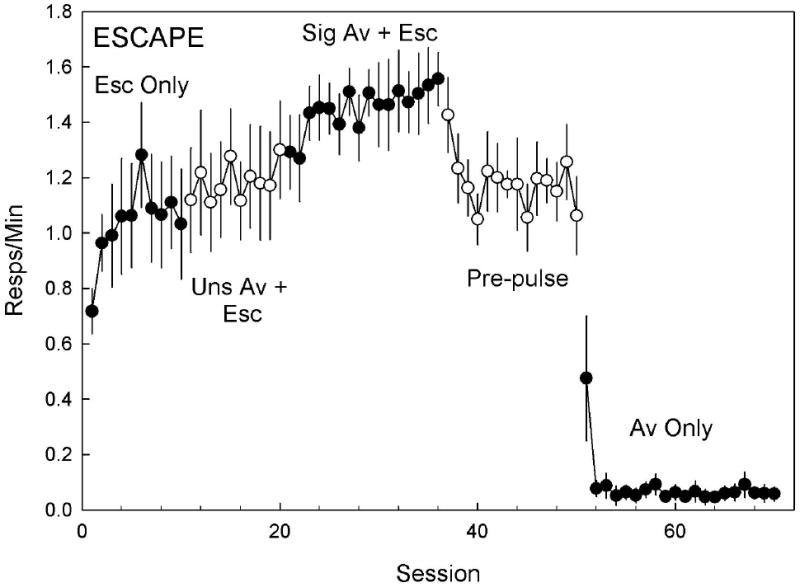
Mean (±SEM) escape rate (head entries during the light) across 5 experimental conditions. Filled and empty symbols help identify experimental conditions. In the Escape Only condition, responses during the light terminated the stimulus and produced a 15 s dark period. The addition of an avoidance contingency during the Unsignaled Avoidance + Escape condition allowed subjects to delay the light onset for 20 s by producing a head entry prior to light onset. A tone was added 5 s prior to the light onset during the Signaled Avoidance + Escape condition. A regular increase in response probability was observed across the Escape Only, and Unsignaled and Signaled Avoidance + Escape conditions. Using a pulse of bright light to signal the avoidance period during the Pre-pulse condition reduced escape probability. Removal of the escape contingency during the Avoidance Only condition quickly reduced head entries during the light to near zero levels. Results from the Mixed ANOVA are presented in the Experiment 3 results section.
Figure 5.
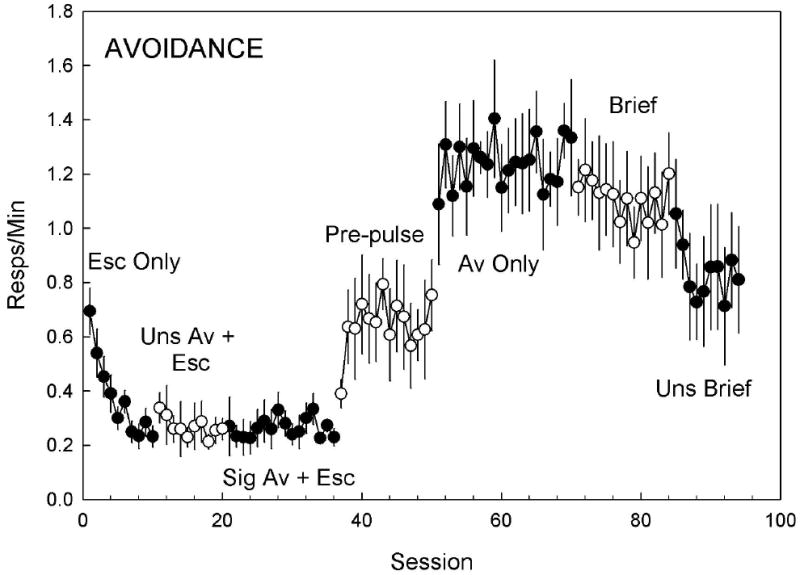
Mean (±SEM) avoidance response rate (head entries in the dark) across 7 experimental conditions. Filled and empty symbols are used to facilitate identification of experimental conditions. During the Escape Only condition responses in the dark were measured, but had no programmed effect. The addition of either an Unsignaled or Signaled Avoidance + Escape contingency produced no effect on avoidance responses. Using a pulse of bright light (Pre-pulse) to signal a 5 s avoidance period before light onset increased rates of avoidance responding. The removal of the escape contingency (Avoidance Only) further increased rates of avoidance. Reducing the duration of the aversive stimulus to match that of the pre-pulse cue (Brief) caused a slight decrease in avoidance responses. Removal of the pre-pulse cue while maintaining the shortened duration of the aversive stimulus further reduced responding. Results from the Mixed ANOVA are presented in the Experiment 3 results section.
Permitting avoidance in the second condition (Fig. 5, “Uns Av +Esc”) did not increase avoidance responding [0.24 ± 0.02 resp/min; t(114) = -2.00, p=.42 N.S.]. The addition of a warning tone in the signaled avoidance condition (Fig. 5, “Sig Av + Esc”) also caused no increase in avoidance responding (0.24 ± 0.02 resp/min), but continued the unbroken increase in escape proficiency (Fig. 4, “Sig Av + Esc”) to levels greater than those observed in the baseline condition [1.53 ± 0.07 responses/min; t(114) = -5.30, p< .0001]. The addition of a brief cue-light (Fig 4 & 5, “Pre-pulse”) to the warning tone significantly increased avoidance responding [0.66 ± 0.08 responses/min; t(114) =-3.51 p < .05], and significantly decreased escape responding [1.16 ± 0.07 responses/min; t(114) = 4.57, p< .001] from the previous condition, suggesting that the form of the warning stimulus is important for initiating the avoidance response. Maintaining the cue-light while removing the escape contingency during the avoidance-only (Fig 4 & 5, “Av Only”) condition yielded a dramatic increase in avoidance responding compared to the previous condition [1.29 ± 0.09 responses/min; t(114) = -6.60, p < .001]. Responses in the light (escape) were observed during the first avoidance-only session, but quickly reduced to near zero levels where they stabilized for the remainder of the experiment. Reducing the duration of the light to 500 ms (Fig 5, “Brief”) caused a significant decrease in avoidance responding [1.12 ± 0.09 responses/min; t(114)= 4.78, p < .001]. Finally, removing the cue-light (Fig. 5, “Uns Brief”), leaving only the 500 ms light, further reduced avoidance responding [0.80 ± 0.11 responses/min; t(114) = 3.86, p < .01].
Our data analysis show that Wistar rats both escaped and avoided bright light, and continued to avoid it even when the light was very brief (500 ms). Rats displayed an initial predisposition to produce avoidance responses (enter the burrow during the dark), even when this behavior had no consequences on light presentation. This suggests that avoidance might have been facilitated early in training, had it been reinforced. Still, this predisposition declined as escape contingencies were learned. A warning tone increased rates of escape, but was unable to facilitate avoidance. Avoidance was only acquired when a 200-ms light cued the aversive stimulus. Avoidance rates were further increased when escape contingencies were eliminated. Nearly all escape responses were eliminated within one session. It is of note that the rates of escape (number of responses in the light/total time spent in the light) and avoidance (number of responses in the dark/total time spent in the dark) were mutually exclusive measures. Reducing the duration of the avoidable light and eliminating the cue-light further decreased avoidance behavior, but not to pre-acquisition levels. These results suggest that light avoidance is strengthened if escaping is precluded, that signaled avoidance is much more effect than un-signaled, and that the avoidable light is best cued by visual, not auditory, stimuli.
General Discussion
Our results demonstrate that light may bring behavior under aversive control within three paradigms: punishment, escape, and signaled avoidance. When rats had a choice between food and food plus light (Experiment 1), they displayed an unambiguous preference for food alone. When light was presented non-contingently (Experiments 2 and 3), rats escaped to an artificial burrow; this response was rarely emitted in the dark. Changes in the frequency (Experiment 1) and duration (Experiments 2 and 3) of the light stimulus and of the efficacy of operant responses to escape and avoid it (Experiments 2 and 3) caused changes in behavior that are consistent with the hypothesis that bright light is aversive. Moreover, exposure to the light stimulus over dozens of sessions did not result in detectable levels of habituation of escape or avoidance behavior. Finally, it has been shown here and by others [1, 19] that bright light has a depressive effect upon responding, similar to that observed with shock, tail pinch, and other validated forms of punishment.
It is important to note that when the aversive light was signaled (Experiment 3), rats avoided it if the signal was visual, but not if it was auditory. These results suggest greater efficacy for warning cues that are similar to the sensory properties of the aversive stimulus [11]. While it was of interest in the present study to examine within-subject changes in behavior in order to examine possible habituation effects, it remains evident that the order-effects produced by such a design might have impeded subjects from initially learning the avoidance response in Experiment 3. Still, once produced, the avoidance response in the latter conditions was quite reliable.
It should also be noted that the pairing of the light and burrow apparatus in the present experiments provide a method by which a species-specific avoidance response can be elicited and effectively measured. Still, a number of other factors may influence the reinforcing or aversive properties of light. For instance, the angle of light presentation may be important: Overhead stimuli may be more closely associated with environmental or predatory cues [26], whereas local cues (e.g. those near a response manipulandum) may not. The reinforcing or punishing properties of luminous stimuli might also vary with the experimental apparatus (e.g. closed operant chamber versus open elevated maze) and the ambient level of stimulation in such an environment.
The present study is also limited in that only one level of illumination was tested throughout the experiments. Although bright light seems to follow the typical psychophysical curves of other aversive stimuli [6], these results are subject to interspecies differences and have also yet to be tested in concert with burrowing behavior. Future experiments will be necessary to determine the role of these and other factors in the elicitation of light aversion or approach.
Like other aversive stimuli, bright light appears to affect molecular processes involved in responses to stressors. McQuade and colleagues demonstrated that exposure to unconditioned light [28] or the conditioned cues that predict it [29] are both able to produce an increased noradrenergic response as measured by in vivo microdialysis. Activation of the locus coeruleus—the main source of noradrenaline in the brain—has also been shown to coincide with the administration of footshock [7]. The un-cued presentation of light has also been shown to increase dopamine [32], and 5-HT [31] in the occipital cortex, although only the increase in 5-HT seems to affect upstream processing in the medial prefrontal cortex [37]. Interestingly, these increases in 5-HT mirror those observed in the prefrontal cortex following fear conditioning [47].
Knutson and associates [21] showed that bright light reduces the incidence of positive-affective ultrasonic vocalizations produced in the anticipation of play with concomitants. These results are in line with decrements in positive-affective vocalizations observed when a cue predicting food reward was altered to predict footshock [5]. Unlike results seen for shock [9], however, anxiety responses to bright light as measured by open arm entries, time spent in the open arm, and the total number of arm entries in an elevated plus maze did not seem to be responsive to anxiolytic drugs [46].
It has been demonstrated previously that behavioral responses such as freezing observed in the presence of bright light differ greatly from those observed for footshock and other aversive stimuli [12, 13]. This indicates that bright light as a stressor/aversive stimulus is activating different neural and behavioral responses than those produced by footshock. Further research may elucidate the cause of these differences. Certainly, clinical disorders related to fear, stress, and anxiety are not indifferent to the nature of the stressor.
The present study provide systematic evidence that bright light can be used effectively as an aversive stimulus, producing reliable behavioral results over the course of more than 100 daily exposures. Furthermore, bright light and the burrow apparatus provide a method that is easily implemented within most behavioral paradigms, and that could be incorporated into a number of unique behavioral arrangements with little effort. These advantages, along with cost efficiency when compared to grid floors, scramblers, and so forth make bright light a practical, reliable alternative to footshock.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ayres JJB, Axelrod H, Mercker E, Muchnik F, Vigorito M. Concurrent observations of barpress suppression and freezing: effects of CS modality and on-line vs. off-line training upon posttrial behavior. Animal Learning & Behavior. 1985;13:44–50. [Google Scholar]
- 2.Barry H, Symmes D. Reinforcing effects of illumination change in different phases of the rat's diurnal cycle. Journal of Comparative and Physiological Psychology. 1963;56:117–119. [Google Scholar]
- 3.Blanchard RJ, Blanchard CD. Antipredator defensive behaviors in a visible burrow system. Journal of Comparative Psychology. 1989;103:70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- 4.Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77:32–48. [Google Scholar]
- 5.Burgdorf J, Knutson B, Panksepp J. Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats. Behav Neurosci. 2000;114:320–327. [PubMed] [Google Scholar]
- 6.Campbell BA, Messing RB. Aversion thresholds and aversion difference limens for white light in albino and hooded rats. Journal of Experimental Psychology. 1969;82:353–359. [Google Scholar]
- 7.Chen FJ, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neuroscience. 2007;144:472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. [Google Scholar]
- 9.Evenden J, Ross L, Jonak G, Zhou J. A novel operant conflict procedure using incrementing shock intensities to assess the anxiolytics and anxiogenic effects of drugs. Behavioral Pharmacology. 2009;20:226–236. doi: 10.1097/fbp.0b013e32832a8110. [DOI] [PubMed] [Google Scholar]
- 10.Fleshler M, Hoffman HS. A Progression for generating variable interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia J, Koelling RA. Relation of cue to consequence in avoidance learning. Psychonomic Science. 1966;4:123–124. [Google Scholar]
- 12.Godsil BP, Fanselow MS. Light stimulus change evokes an activity response in the rat. Learning and Behavior. 2004;32:299–310. doi: 10.3758/bf03196029. [DOI] [PubMed] [Google Scholar]
- 13.Godsil BP, Blackmore MA, Fanselow MS. Modulation of an activity response with associative and nonassociative fear in the rat: A lighting differential influences the form of defensive behavior evoked after fear conditioning. Learning & Behavior. 2005;33:454–463. doi: 10.3758/bf03193184. [DOI] [PubMed] [Google Scholar]
- 14.Goodrick C. Light- and dark-contingent bar pressing in the rat as a function of age and motivation. Journal of Comparative and Physiological Psychology. 1970;73:100–104. [Google Scholar]
- 15.Hicks CR, Turner KV. Fundamental concepts in the design of experiments. 5th. New York: Oxford University Press; 1999. [Google Scholar]
- 16.Kaplan M. The effects of noxious stimulus intensity and duration during intermittent reinforcement of escape behavior. Journal of comparative and physiological psychology. 1952;45:538–549. doi: 10.1037/h0055989. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan M. Maintenance of escape behavior under fixed-ratio reinforcement. Journal of Comparative and Physiological Psychology. 1956;49:153–157. doi: 10.1037/h0048735. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan M, Bruce J, Sparer R. Escape behavior under continuous reinforcement as a function of light intensity. The Journal of the Experimental Analysis of Behavior. 1965;8:321–323. doi: 10.1901/jeab.1965.8-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keller FS. Light-aversion in the white rat. The Psychological Record. 1941;4:233–249. [Google Scholar]
- 20.Kitaoka A. Defensive aspects of burrowing behavior in rats (Rattus Norvegicus): A descriptive and correlational study. Behavioral Processes. 1994;31:13–28. doi: 10.1016/0376-6357(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 21.Knutson B, Burgdorf J, Panksepp J. Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats. Journal of Comparative Psychology. 1998;112:65–73. doi: 10.1037/0735-7036.112.1.65. [DOI] [PubMed] [Google Scholar]
- 22.Lockard RB. Self-regulated light exposure of albino rats. Journal of Comparative and Physiological Psychology. 1962a;55:641–645. doi: 10.1037/h0042827. [DOI] [PubMed] [Google Scholar]
- 23.Lockard RB. Some effects of maintenance luminance and strain differences upon self-exposure to light by rats. Journal of Comparative and Physiological Psychology. 1962b;55:1118–1123. doi: 10.1037/h0048879. [DOI] [PubMed] [Google Scholar]
- 24.Lockard RB. Some effects of light on the behavior of rodents. Psychological Bulletin. 1963;60:509–529. doi: 10.1037/h0046113. [DOI] [PubMed] [Google Scholar]
- 25.Lockard RB. Self regulated exposure to light by pigmented and albino littermates. Journal of Comparative and Physiological Psychology. 1964;57:231–236. doi: 10.1037/h0046601. [DOI] [PubMed] [Google Scholar]
- 26.Longland WS, Price MV. Direct observations of owls and heteromyid rodents: Can predation risk explain microhabitat use? Ecology. 1991;72:2261–2273. [Google Scholar]
- 27.Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudery M. Long-term behavioural alterations in female rats after a single intense foot shock followed by situational reminders. Psychoneuroendocrinology. 2005;30:316–324. doi: 10.1016/j.psyneuen.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.McQuade R, Creton D, Stanford SC. Effects of novel environmental stimuli on rat behavior and central noradrenaline function as measured by in vivo microdialysis. Psychopharmacology. 1999;145:393–400. doi: 10.1007/s002130051073. [DOI] [PubMed] [Google Scholar]
- 29.McQuade R, Stanford SC. A microdialysis study of the noradrenergic response in rat frontal cortex and hypothalamus to a conditioned cue for aversive, naturalistic environmental stimuli. Psychopharmacology. 2000;148:201–208. doi: 10.1007/s002130050043. [DOI] [PubMed] [Google Scholar]
- 30.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biological Psychology. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 31.Muller CP, De Souza Silva MA, Huston JP. Double dissociating effects of sensory stimulation and cocaine on serotonin activity in the occipital and temporal cortices. Neuropharmacology. 52:854–862. doi: 10.1016/j.neuropharm.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Muller CP, Huston JP. Dopamine activity in the occipital and temporal cortices of rats: dissociating effects of sensory but not pharmacological stimulation. Synapse. 61:254–258. doi: 10.1002/syn.20366. [DOI] [PubMed] [Google Scholar]
- 33.Nieder L, Cagnin M, Parisi V. Burrowing and feeding behaviour in the rat. Animal Behavior. 1982;30:837–844. [Google Scholar]
- 34.Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Pinel JP, Mumby DG, Dastur FN, Pinel JG. Rat (Rattus norvegicus) defensive behavior in total darkness: risk-assessment function of defensive burying. Journal of Comparative Psychology. 1994;108:140–147. doi: 10.1037/0735-7036.108.2.140. [DOI] [PubMed] [Google Scholar]
- 36.Pum ME, Huston JP, Muller CP, De Souza Silva MA. Light induced activity in the activity-box is not aversively motivated and does not show between-trial habituation. Physiology and Behavior. 2009;96:434–439. doi: 10.1016/j.physbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Pum ME, Huston JP, De Souza Silva MA, Muller CP. Visual Sensory-motor gating by serotonin activation in the medial prefrontal and occipital, but not in the rhinal, cortices in rats. Neuroscience. 153:361–372. doi: 10.1016/j.neuroscience.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 38.Raudenbush SW, Bryk A. Hierarchical linear models: Applications and data analysis models. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 39.Reed P, Yoshino T. Effect of contingent auditory stimuli on concurrent schedule performance: An alternative punisher to electric shock. Behavioral Processes. 2008;78:421–428. doi: 10.1016/j.beproc.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Roberts CL, Marx MH, Collier G. Light onset and light offset as reinforcers for the albino rat. Journal of Comparative and Physiological Psychology. 1958;51:575–579. doi: 10.1037/h0042974. [DOI] [PubMed] [Google Scholar]
- 41.Sanford LD, Silvestri AJ, Ross RJ, Morrison AR. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapideye movement sleep. Arch Ital Biol. 2001;139:169–84. [PubMed] [Google Scholar]
- 42.Slawecki CJ. Comparison of anxiety-like behavior in adolescent and adult Sprague-Dawley rats. Behavioral neuroscience. 2005;119:1477–1483. doi: 10.1037/0735-7044.119.6.1477. [DOI] [PubMed] [Google Scholar]
- 43.Stevens J. Applied multivariate statistics for the social sciences. 4th. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- 44.Ulrich RE, Holz WC, Azrin NH. Stimulus control of avoidance behavior. Journal of the Experimental Analysis of Behavior. 1964;7:129–133. doi: 10.1901/jeab.1964.7-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Dijken HH, Ver der Heyden JAM, Mos J, Tilders FJH. Inescapable footshocks induce progressive and long-lasting behavioural changes in male rats. Physiology and Behavior. 1991;51:787–794. doi: 10.1016/0031-9384(92)90117-k. [DOI] [PubMed] [Google Scholar]
- 46.Violle N, Balandras F, Le Roux Y, Desor D, Schroeder H. Variations in illumination, closed wall transparency and/or extramaze space influence both baseline anxiety and response to diazepam in the rat elevated plus-maze. Behavioral Brain Research. 2009;203:35–42. doi: 10.1016/j.bbr.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 47.Yoshioka M, Matsumoto M, Togashi H, Saito H. Effects of conditioned fear stress on 5-HT release in the rat prefrontal cortex. Pharmacol Biochem Behav. 1995;51:515–519. doi: 10.1016/0091-3057(95)00045-x. [DOI] [PubMed] [Google Scholar]


