Abstract
Objectives. We sought to determine smoking-related hazard ratios (HRs) and population-attributable risk percentage (PAR%) for serious clinical events and death among HIV-positive persons, whose smoking prevalence is higher than in the general population.
Methods. For 5472 HIV-infected persons enrolled from 33 countries in the Strategies for Management of Antiretroviral Therapy clinical trial, we evaluated the relationship between baseline smoking status and development of AIDS-related or serious non-AIDS events and overall mortality.
Results. Among all participants, 40.5% were current smokers and 24.8% were former smokers. Adjusted HRs were higher for current than for never smokers for overall mortality (2.4; P < .001), major cardiovascular disease (2.0; P = .002), non-AIDS cancer (1.8; P = .008), and bacterial pneumonia (2.3; P < .001). Adjusted HRs also were significantly higher for these outcomes among current than among former smokers. The PAR% for current versus former and never smokers combined was 24.3% for overall mortality, 25.3% for major cardiovascular disease, 30.6% for non-AIDS cancer, and 25.4% for bacterial pneumonia.
Conclusions. Smoking contributes to substantial morbidity and mortality in this HIV-infected population. Providers should routinely integrate smoking cessation programs into HIV health care.
Highly active antiretroviral therapy (HAART) for HIV has led to a decrease in AIDS-related events and deaths.1–3 However, HIV-infected persons are also at risk for a variety of serious non–AIDS-defining diseases, including cardiovascular, renal, and hepatic disease, as well as certain cancers and infections not included in the AIDS case definition.1,3–5 Studies conducted in the general population demonstrate that cigarette smoking increases the likelihood of many of these serious clinical conditions, including cardiovascular, pulmonary, and neoplastic diseases.6,7 For example, smoking is a major risk factor for peripheral vascular and coronary artery disease, increasing the risk for cardiovascular disease (CVD) complications, including myocardial infarction and stroke.6–8 Respiratory complications of smoking include chronic obstructive pulmonary disease and respiratory infections such as bacterial pneumonia or pulmonary tuberculosis.6,7,9 Smoking increases the risk for many types of cancer, including cancers of the oral cavity, pharynx, esophagus, stomach, pancreas, larynx, lung, cervix, urinary bladder, and kidney.6,7,10
Studying smoking-related morbidity and mortality among persons with HIV is especially important because their smoking prevalence is higher than that of the general population. Twenty-one percent of US adults are current cigarette smokers,11 but many recent studies have reported rates 2 or 3 times as high (46%–76%) among HIV-positive persons.12–18 HIV-infected current smokers are reported to smoke an average of 6 to 23 cigarettes daily and to have smoked for an average of 23 to 24 years.15,16,18,19
Many smoking-related illnesses significantly affect HIV-infected persons. Lung cancer and other malignancies are important causes of death among persons with HIV.20,21 HIV infection or use of antiretroviral drugs may contribute to CVD risk,22,23 and use of effective HAART has resulted in increasing numbers of aging HIV-infected patients, who may develop metabolic syndrome, obesity, and other CVD risk factors.24,25 HIV-infected patients may develop a variety of pulmonary diseases, including bacterial pneumonia26,27; recurrent pneumonia is considered an AIDS-defining condition.28
Because persons with HIV are at risk for these serious and life-threatening clinical syndromes, critical prevention questions are whether and to what extent smoking further increases the risk of developing these diseases, especially in the era of HAART. Because smoking is a modifiable risk factor, it is important to define the magnitude of smoking's effect on overall mortality among HIV-infected patients and its effect on development of specific adverse clinical conditions, including those that are not AIDS defining. It is also important to identify the proportion of disease among an HIV-infected population that is attributable to smoking. This information can be used to help estimate the effect of smoking cessation on reducing disease and improving survival for HIV-infected persons and to counsel individual patients about ways to optimize their health.
We evaluated data from a large multisite international study of 2 HAART treatment strategies. We determined the relative risks associated with smoking for development of different serious clinical events and on all-cause mortality, with adjustment for a variety of important potential confounders. We also calculated the population-attributable risk percentage (PAR%) associated with smoking for these clinical syndromes and all-cause mortality.
METHODS
The Strategies for Management of Antiretroviral Therapy (SMART) clinical trial compared continuous HAART use to episodic use on the basis of CD4+ lymphocyte count criteria among 5472 HIV-infected persons enrolled during 2002 to 2006 from 318 sites in 33 countries.29
Health Conditions and Variables
In our analysis of data from this trial, we defined AIDS-related events as clinical diseases meeting the revised Centers for Disease Control and Prevention AIDS case definition,28 plus other conditions considered to be attributable to HIV-related immunodeficiency; a full list of these conditions is available elsewhere.29 Serious non-AIDS events included major CVD events, any CVD event (according to an expanded definition), non-AIDS cancer, major hepatic disease, major renal disease, and initial episodes of bacterial pneumonia. Major CVD events included nonfatal myocardial infarction requiring hospitalization or diagnosed by serial Q-wave change on an electrocardiogram, nonfatal stroke, coronary artery disease requiring surgery or invasive procedures, or death from CVD. The expanded CVD definition consisted of major CVD events, congestive heart failure, coronary artery disease requiring drug treatment, or peripheral vascular disease. Major renal disease was defined as nonfatal or fatal kidney failure; major hepatic disease was nonfatal or fatal cirrhosis.
We considered only the first event of a given type in an individual if more than 1 was reported. Specific criteria and standard definitions were developed for all AIDS-related and serious non-AIDS clinical events in SMART. An independent endpoint review committee blinded to treatment status reviewed and adjudicated all clinical events and reviewed all death reports to assign underlying cause of death.20
The baseline data collection form identified whether participants were current, former, or never smokers. Current smoking status was reascertained at each annual follow-up visit. Although our analysis focused on baseline smoking status as the primary exposure of interest, we also evaluated data on annual smoking status to determine whether the proportion of current smokers significantly changed over the course of follow-up. Other baseline variables in our analysis included history of injection drug use (IDU), history of alcohol abuse, diabetes, and use of lipid- or blood pressure–lowering drugs. Body mass index was calculated from height and weight. Baseline laboratory measurements included CD4+ count; high- and low-density lipoprotein cholesterol, triglyceride, and HIV RNA levels; and evidence of hepatitis B (HBV) and C (HCV) virus. HCV infection was defined as presence of HCV antibody, and HBV infection as persistence of HBV surface antigen for 6 months or longer.
Analyses
We compared characteristics across baseline smoking categories (never, former, current) with the Kruskal-Wallis nonparametric test for continuous variables and the χ2 test for categorical variables. Baseline smoking status was included as a fixed covariate. We constructed Kaplan–Meier curves by baseline smoking categories and used the log-rank test to compare cumulative event proportions. We estimated hazard ratios (HRs) for comparison of smoking categories for clinical events from Cox proportional hazards models. We then calculated adjusted HRs (AHRs) with additional variables entered into the final model.
All adjusted models contained the following baseline covariates: age, gender, race/ethnicity (Black, Hispanic, other), SMART treatment arm, baseline HIV RNA, baseline CD4+ count, and nadir CD4+ count. For different clinical events, we added variables to the final adjusted model that we considered relevant to that endpoint. For all-cause mortality and AIDS-related events, these additional variables were history of alcohol abuse or IDU and previous AIDS events. For CVD events, additional variables were body mass index, diabetes, use of lipid-lowering and blood pressure–lowering drugs, low-density lipoprotein cholesterol level, and triglyceride level. For cancer events and liver events, additional variables were history of alcohol abuse or IDU and HBV or HCV infection.
We calculated the PAR% of a clinical endpoint attributable to smoking as Pe (HR − 1) / [Pe (HR − 1) + 1], where Pe was the proportion of the population with the exposure (e.g., proportion who reported current smoking at baseline), and HR represented the relative risk estimate.30,31 In PAR% calculations that compared only 2 smoking subgroups (e.g., current versus former smokers), we adjusted the population at risk to incorporate only participants belonging to those 2 subgroups. We used AHRs in the final calculations of PAR%. We calculated 95% confidence intervals (CIs) for rates, HRs, and PAR%.
RESULTS
Baseline characteristics for the 5472 participants are shown in Table 1. Mean follow-up time was 33 months; the total follow-up time was 15 095 person-years. At baseline, 2215 HIV-infected persons (40.5%) were current smokers, 1358 (24.8%) were former smokers, and 1899 (34.7%) reported never smoking. Comparison of the 3 smoking groups by demographic and other baseline characteristics revealed significant differences for many variables, although baseline smoking status was similar between SMART treatment arms.
TABLE 1.
Baseline Participant Characteristics by Baseline Smoking Status: Strategies for Management of Antiretroviral Therapy Clinical Trial, 2002–2006
| Never Smokers (n = 1899) | Former Smokers (n = 1358) | Current Smokers (n = 2215) | P | |
| Treatment arm, episodic ART, % | 48.9 | 49.3 | 50.7 | .47 |
| Age, y, median | 42 | 46 | 43 | <.001 |
| Female, % | 36.1 | 21.4 | 23.0 | <.001 |
| Race/ethnicity, % | <.001 | |||
| Latino | 26.1 | 24.0 | 19.5 | |
| Black | 26.7 | 25.9 | 28.7 | |
| White | 36.1 | 46.3 | 48.3 | |
| Other | 11.1 | 3.8 | 3.5 | |
| Region, % | <.001 | |||
| North America | 48.6 | 63.0 | 59.3 | |
| South America | 13.2 | 10.0 | 7.3 | |
| Europe | 25.7 | 21.3 | 28.2 | |
| Australia/New Zealand | 3.0 | 3.5 | 3.2 | |
| Asia/Africa | 9.5 | 2.2 | 2.0 | |
| History of injection drug use, % | 2.5 | 9.7 | 15.9 | <.001 |
| History of alcohol abuse, % | 3.2 | 12.6 | 17.0 | <.001 |
| Body mass index, kg/m2, median | 25.4 | 25.5 | 24.2 | <.001 |
| HIV RNA ≤ 400, % | 76.9 | 73.3 | 66.2 | <.001 |
| CD4+ cells/mm3, median | 589 | 594 | 606 | .005 |
| CD4+ nadir cells/mm3, median | 246 | 245 | 260 | .002 |
| Previous AIDS disease, % | 21.9 | 25.3 | 25.5 | .02 |
| Diabetes, % | 6.9 | 9.1 | 5.9 | .001 |
| Lipid-lowering drugs, % | 14.1 | 22.2 | 13.4 | <.001 |
| Blood pressure–lowering drugs, % | 16.6 | 24.2 | 17.1 | <.001 |
| Hepatitis B, % | 2.1 | 2.1 | 2.9 | .17 |
| Hepatitis C, % | 6.1 | 15.1 | 22.2 | <.001 |
| LDL cholesterol, mg/dL, median | 112 | 113 | 110 | .02 |
| HDL cholesterol, mg/dL, median | 42 | 40 | 40 | .004 |
| Triglycerides, mg/dL, median | 155 | 179 | 159 | <.001 |
Note. ART = antiretroviral therapy; HDL = high-density lipoprotein; LDL = low-density lipoprotein.
Smoking status did not change greatly during the follow-up period. The proportion of current smokers was 38% at 12 months, 39% at 36 months, and 36% at 60 months. Among baseline current smokers, 89% reported current smoking on at least 1 follow-up visit; 89% of baseline former or never smokers reported not currently smoking at each follow-up visit.
Baseline Smoking Status and Risk
All-cause mortality.
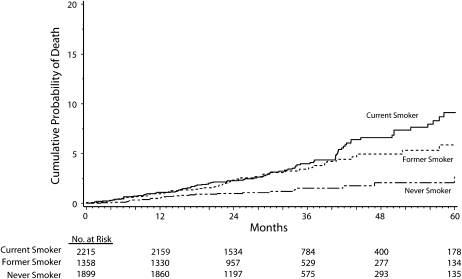
Three percent (167) of participants died from any cause. We calculated death rates by baseline smoking status, with the highest rate (1.5 per 100 person-years) for current smokers (Table 2). The estimated percentage of participants who died by 24 and 48 months was, respectively, 1.0% and 2.1% among never smokers, 2.3% and 4.9% among former smokers, and 2.3% and 6.6% among current smokers (P < .001; Figure 1). The AHR for all-cause mortality was significantly higher for current than for never smokers (2.4; P < .001; Table 3). The AHR for all-cause mortality was also significantly higher for current smokers than for former ones (1.5; P = .04).
TABLE 2.
All-Cause Mortality and Specific Clinical Events by Smoking Status at Baseline: Strategies for Management of Antiretroviral Therapy Clinical Trial, 2002–2006
| Never Smokers |
Former Smokers |
Current Smokers |
||||
| Clinical Event | No. | Ratea (95% CI) | No. | Ratea (95% CI) | No. | Ratea (95% CI) |
| All-cause mortality | 25 | 0.5 (0.3, 0.7) | 47 | 1.2 (0.9, 1.5) | 95 | 1.5 (1.2, 1.8) |
| AIDS-related disease | 43 | 0.9 (0.6, 1.1) | 34 | 0.9 (0.6, 1.2) | 86 | 1.4 (1.1, 1.7) |
| Major CVD | 33 | 0.7 (0.4, 0.9) | 39 | 1.0 (0.7, 1.3) | 74 | 1.2 (0.9, 1.5) |
| Expanded CVDb | 53 | 1.1 (0.8, 1.4) | 65 | 1.7 (1.3, 2.1) | 120 | 2.0 (1.7, 2.4) |
| Non-AIDS cancer | 35 | 0.7 (0.5, 0.9) | 29 | 0.8 (0.5, 1.0) | 72 | 1.2 (0.9, 1.5) |
| Major Renal disease | 1 | 0.0 (0.0, 0.1) | 9 | 0.2 (0.1, 0.4) | 8 | 0.1 (0.0, 0.2) |
| Major Hepatic disease | 5 | 0.1 (0.0, 0.2) | 8 | 0.2 (0.1, 0.3) | 12 | 0.2 (0.1, 0.3) |
| Bacterial pneumoniac | 38 | 0.8 (0.5, 1.0) | 51 | 1.3 (1.0, 1.7) | 115 | 1.9 (1.6, 2.3) |
Note. CI = confidence interval; CVD = cardiovascular disease.
Per 100 person-years.
Major CVD events, as well as congestive heart failure, coronary artery disease requiring drug treatment, or peripheral vascular disease.
Initial occurrence.
FIGURE 1.
Cumulative probability of death (all-cause mortality) by months of follow-up, among current, former, and never smokers: Strategies for Management of Antiretroviral Therapy clinical trial, 2002–2006.
TABLE 3.
Unadjusted and Adjusted Hazard Ratios for All-Cause Mortality and Specific Clinical Events by Baseline Smoking Status: Strategies for Management of Antiretroviral Therapy Clinical Trial, 2002–2006
| Current Versus Never Smokers |
Current Versus Former Smokers |
|||
| Clinical Event | HR (95% CI) | P | HR (95% CI) | P |
| All-cause mortality | ||||
| Unadjusted | 3.0 (1.9, 4.7) | <.001 | 1.3 (0.9, 1.8) | .15 |
| Adjusted | 2.4 (1.5, 3.8) | <.001 | 1.5 (1.0, 2.1) | .04 |
| AIDS-related disease | ||||
| Unadjusted | 1.6 (1.1, 2.3) | .01 | 1.6 (1.1, 2.4) | .02 |
| Adjusted | 1.3 (0.9, 1.9) | .18 | 1.6 (1.0, 2.3) | .03 |
| Major CVD | ||||
| Unadjusted | 1.8 (1.2, 2.8) | .004 | 1.2 (0.8, 1.8) | .31 |
| Adjusted | 2.0 (1.3, 3.1) | .002 | 1.6 (1.1, 2.4) | .02 |
| Expanded CVDa | ||||
| Unadjusted | 1.9 (1.3, 2.6) | <.001 | 1.2 (0.9, 1.6) | .25 |
| Adjusted | 1.9 (1.4, 2.7) | <.001 | 1.5 (1.1, 2.1) | .009 |
| Non-AIDS cancer | ||||
| Unadjusted | 1.7 (1.1, 2.5) | .01 | 1.6 (1.0, 2.5) | .03 |
| Adjusted | 1.8 (1.2, 2.8) | .008 | 2.3 (1.5, 3.6) | <.001 |
| Major Renal disease | ||||
| Unadjusted | 6.6 (0.8, 52.4) | .08 | 0.6 (0.2, 1.5) | .24 |
| Adjusted | 6.6 (0.8, 53.9) | .08 | 0.7 (0.2, 1.7) | .4 |
| Major Hepatic disease | ||||
| Unadjusted | 1.9 (0.7, 5.5) | .22 | 0.9 (0.4, 2.3) | .91 |
| Adjusted | 0.6 (0.2, 1.8) | .32 | 0.8 (0.3, 2.1) | .69 |
| Bacterial pneumoniab | ||||
| Unadjusted | 2.5 (1.7, 3.6) | <.001 | 1.5 (1.0, 2.0) | .03 |
| Adjusted | 2.3 (1.6, 3.3) | <.001 | 1.5 (1.1, 2.1) | .01 |
Note. CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio. All models adjusted for treatment group, age, gender, race/ethnicity (Black, Hispanic, other), baseline HIV RNA, and baseline and nadir CD4+ counts. Death and AIDS endpoints additionally adjusted for alcohol abuse, injection drug use, and previous AIDS disease. CVD endpoints additionally adjusted for body mass index, diabetes, blood pressure–lowering drugs, lipid-lowering drugs, low-density lipoprotein cholesterol, and triglycerides. Cancer and liver endpoints additionally adjusted for alcohol abuse, injection drug use, and hepatitis B or C.
Major CVD events, as well as congestive heart failure, coronary artery disease requiring drug treatment, or peripheral vascular disease.
Initial occurrence.
AIDS-related events.
Three percent (163) of participants experienced an AIDS-related event. The AIDS-related event rate was highest among current smokers (1.4 cases per 100 person-years; Table 2). The AHR for all AIDS-related events for current versus never smokers was 1.3 (P = .18) and for current versus former smokers was 1.6 (P = .03; Table 3). We conducted a subanalysis for esophageal candidiasis, the most common AIDS-related disease, with 36 (68%) of 53 cases among current smokers. The AHR for current versus never smokers was 2.5 (95% CI = 1.2, 5.4; P = .02); the AHR for current versus former smokers was 2.6 (95% CI = 1.2, 5.7; P = .02).
Cardiovascular disease.
Three percent (146) of participants experienced a major CVD event, and 4% (238) had any (expanded definition) CVD event. The event rate for both major and expanded CVD events was highest for current smokers (1.2 and 2.0 per 100 person-years, respectively; Table 2). The AHR for major CVD events for current versus never smokers was 2.0 (P = .002) and for current versus former smokers was 1.6 (P = .02; Table 3). The estimated percentage with at least 1 expanded definition CVD event by 24 and 48 months was, respectively, 2.2% and 4.1% among never smokers, 2.7% and 8.0% among former smokers, and 3.8% and 8.0% among current smokers (P < .001). The AHR for expanded CVD events for current versus never smokers was 1.9 (P < .001), and for current versus former smokers was 1.5 (P = .009; Table 3).
Non-AIDS cancer.
Two percent (136) of participants developed a non-AIDS cancer. Current smokers had the highest event rate (1.2 per 100 person-years; Table 2). The cumulative estimated percentage with a non-AIDS cancer at 24 and 48 months was, respectively, 1.2% and 3.2% among never smokers, 1.4% and 2.7% among former smokers, and 2.0% and 4.6% among current smokers (P = .02). The AHR for non-AIDS cancer for current versus never smokers was 1.8 (P = .008) and for current versus former smokers was 2.3 (P < .001; Table 3).
In our subanalysis for the 2 most common cancers, 15 (41%) of 37 nonmelanoma skin cancers and 13 (72%) of 18 lung cancers occurred in current smokers. The AHR for nonmelanoma skin cancer for current versus never smokers was 0.9 (95% CI = 0.4, 1.9; P = .82); the AHR for current versus former smokers was 2.5 (95% CI = 0.9, 6.7; P = .06). The AHR for lung cancer for current versus never smokers was 9.4 (95% CI = 1.1, 76.3; P = .04); the AHR for current versus former smokers was 3.0 (95% CI = 0.9, 9.6; P = .07).
Bacterial pneumonia.
Four percent (204) of participants developed bacterial pneumonia at least once. The highest rate for pneumonia (1.9 per 100 person-years) was among current smokers (Table 2). The cumulative estimated percentage at 24 and 48 months with bacterial pneumonia was, respectively, 1.5% and 3.2% among never smokers, 2.6% and 5.8% among former smokers, and 3.2% and 8.1% among current smokers (P < .001). The AHR for bacterial pneumonia for current versus never smokers was 2.3 (P < .001) and for current versus former smokers was 1.5 (P = .01; Table 3).
Renal or hepatic disease.
Eighteen participants experienced a major renal event, and 25 had a major hepatic event. The overall numbers and rates for renal and hepatic disease were relatively small, with limited power to detect differences (Table 2); none of the AHRs were statistically significant (Table 3).
Additional analyses.
We recalculated AHRs with CD4+ count and HIV RNA level as time-dependent covariates. We also recalculated AHRs by confining our analysis to the continuous HAART group. In both cases, although some numerical estimates changed (usually slightly), we detected little change in overall significant results or the magnitude of association. For example, in the continuous HAART group, the AHR for current versus never smokers was 2.2 (95% CI = 1.1, 4.3) for all-cause mortality. An additional sensitivity analyses was performed adding as a covariate years of education (≤ 12 years vs > 12 years) as one reflection of socioeconomic status; AHRs remained almost exactly the same.
Population-Attributable Risk Percentage
The PAR% was calculated for overall mortality and selected clinical endpoints. We first compared current smokers to nonsmokers (i.e., both former and never smokers) to help estimate what percentage of disease was attributable to current smoking. We used the AHRs and a current smoking prevalence of 40.5% to calculate a PAR% estimate of 24.3% (95% CI = 10.6%, 37.6%) for all cause-mortality, 25.3% (95% CI = 10.9%, 39.1%) for major CVD events, 22.9% (95% CI = 11.6%, 33.9%) for any CVD event, 30.6% (95% CI = 15.7%, 44.6%) for non-AIDS cancer, and 25.4% (95% CI = 13.6%, 36.8%) for bacterial pneumonia.
We limited the second analysis to current and former smokers, to help estimate the reduction in incidence that would be observed among current smokers if they all became former smokers. Within this subpopulation, the PAR% estimate was 15.6% (95% CI = 0.5%, 30.8%) for all-cause mortality, 20.1% (95% CI = 3.3%, 36.6%) for major CVD events, 17.4% (95% CI = 4.3%, 30.5%) for any CVD event, 34.3% (95% CI = 15.8%, 51.1%) for non-AIDS cancer, and 17.8% (95% CI = 3.8%, 31.7%) for bacterial pneumonia.
DISCUSSION
A significant proportion (40.5%) of HIV-infected participants in this large, multinational clinical trial reported current smoking at enrollment; more than one third also reported smoking at 1-, 3-, and 5-year follow-up visits. Our results show that smoking contributed to substantial morbidity and mortality in this population. Current smokers had significantly higher rates of non-AIDS cancers, CVD, bacterial pneumonia, and all-cause mortality than did nonsmokers. These findings remained robust: significantly higher risk persisted after adjustment for multiple potential confounders, CD4+ count, and HIV RNA level. The association between current smoking and adverse clinical outcomes also persisted after we controlled for SMART treatment arm and in analyses restricted to the continuous HAART group.
Smoking in HIV-infected persons is of great concern for many reasons. Smoking prevalence is higher among persons with HIV than among the general population.12–18 Many diseases that occur more often among smokers than among nonsmokers are more common in HIV-infected persons, and smoking may even further increase risk among this population. For example, HIV-infected persons are at increased risk for lung cancer, independent of smoking status32–34; however, smoking even further raises lung cancer risk. An analysis that considered both HIV infection and smoking as lung cancer risk factors found a multivariate HR of 1.8 for each additional pack of cigarettes smoked per day.33
Smoking may negate many positive benefits of HIV treatment, including HAART. Recent HIV treatment guidelines recommend earlier HAART initiation in part because data (from SMART and other studies) show that untreated HIV infection may be associated with development of certain non–AIDS-defining diseases, such as CVD and malignancy.35,36 In the SMART data, episodic HAART was also associated with higher rates of bacterial pneumonia.37 If smoking promotes development of these serious non-AIDS events, it may counteract many of the benefits of earlier HAART.
Although the AHR in our analysis for current versus never smokers was not statistically significant for the combined category of AIDS-related events, smoking may still increase the risk of specific AIDS-defining clinical diseases among HIV-infected persons, again weakening HIV treatment benefits. Studies of HIV-infected patients have identified smoking as a risk factor for AIDS-defining pulmonary infections, including Pneumocystis jirovecii pneumonia and tuberculosis.38,39 We also identified a significant association between current smoking and esophageal candidiasis, consistent with reports of an association between smoking and oral candidiasis.40–42 Among the possible pathogenic mechanisms for this association are mucosal injury and epithelial alterations from smoke that facilitate candida colonization, factors in cigarette smoke that directly promote candida growth, and suppression of local or systemic immunologic defenses against candida.42 Smokers' use of inhaled or systemic steroids for smoking-related diseases may further promote candidiasis.
The association of smoking and many adverse effects, supported by our findings, strongly suggests that smoking may further increase morbidity and mortality associated with HIV infection. One study of US veterans reported mortality rates per 100 person-years of 1.76 for HIV-negative never smokers, 2.35 for HIV-negative current smokers, 2.45 for HIV-positive never smokers, and 5.48 for HIV-positive current smokers.17
Our analysis had several strengths. Participants in SMART were enrolled from 33 countries and represented a variety of demographic populations and HIV risk groups.29 Our findings are therefore applicable to a diverse population of HIV-infected persons. All clinical endpoints in SMART were reviewed according to standardized criteria by an independent endpoint review committee blinded to treatment status. SMART collected data on, and our analysis adjusted for, multiple risk factors and confounders, including CD4+ count, HIV RNA level, alcohol abuse and IDU, HBV and HCV infection, body mass index, diabetes, lipid levels, and hypertension treatment.
We analyzed and reported the relationship between smoking and adverse clinical events in several ways. HRs reflected the magnitude of association, expressing the hazard of developing death or disease among exposed versus unexposed groups. Attributable risk estimates reflected excess risk of disease among exposed compared with unexposed groups. PAR% expressed the proportion of disease among a population that was attributable to an exposure; this proportion helped to estimate the proportion of disease that would be reduced if the exposure were eliminated.30 Our findings that 24% of deaths and 23% to 31% of certain serious clinical events were attributable to current smoking highlight the serious health risk that smoking represents among this HIV-infected population.
Our findings strongly support recommendations that for HIV-infected persons who smoke, smoking cessation should be a central health promotion strategy and should be routinely integrated with other HIV health care services.18,43 Proven benefits of smoking cessation include reducing the risk of clinical diseases such as lung cancer, CVD, and stroke.44–47 Clinical practice guidelines for treating tobacco use and dependence recommend that providers systematically and regularly assess their patients for tobacco use and strongly urge tobacco users to quit.48 In practice, however, many HIV providers are unaware that their patients are currently smoking or do not assess the interest of their smoking patients in quitting.14,49
For patients who indicate that they are willing to make a quit attempt, multiple strategies are available and have been used in HIV-infected patients.18,43,50–52 Behavioral interventions include telephone quit lines, motivational interviewing, cognitive behavioral interventions, and other individual or group counseling approaches.18,43,48,50–53 Nicotine replacement therapy is available in multiple forms, including gum, lozenges, transdermal patches, inhalers, and sprays.18,43,48,51–54 Other pharmacological agents used for smoking cessation include bupropion and varenicline18,43,48,54; because of the potential for drug–drug interactions, including with HAART medications,18,55,56 providers considering these drugs should first consult knowledgeable pharmacists and other current and authoritative sources of information.35 Because it is common for smokers to make multiple quit attempts before successful cessation, continued support is important.15,16,18,47 Approaches must be individualized; multiple strategies (such as behavior interventions and nicotine replacement therapy) have been employed simultaneously for some persons with HIV.18,51–53 Other underlying comorbidities, such as depression or substance abuse, may also need to be addressed.16,18
Limitations
We had no specific information on date of smoking cessation for former smokers in our study. However, consistent with other studies,17,45,46 former smokers in our analysis had risks intermediate between current smokers and never smokers for both overall mortality and several specific clinical events. The risk difference between current and former smokers would be expected to increase with increasing duration of cessation.44–47
We also had no information on amount and duration of smoking (e.g., pack-years) for current or former smokers. Although heavy or prolonged smoking has greater adverse health consequences, even light smoking (1–4 cigarettes/day) has been associated with an increased risk (compared with that of nonsmokers) of dying from ischemic heart disease and of all-cause mortality.57 Current smoking at any level therefore represents a health risk among HIV-infected populations and highlights the importance of smoking cessation.
Many clinical outcomes in our analysis, including cancer and CVD, have multiple risk factors, and we cannot rule out additional potential confounders, such as other health-related behaviors (e.g., those related to diet or exercise) or other health conditions. However, our results concerning the relationship of smoking with all-cause mortality and development of serious adverse clinical events were robust in multiple analyses and were consistent with many other studies in varied populations.
Conclusions
Among HIV-infected participants in this clinical trial of 2 HAART treatment strategies, current smoking represented a significant risk factor for both all-cause mortality and several serious clinical disease outcomes, such as CVD, non-AIDS cancers, and bacterial pneumonia. Significant reductions in morbidity and mortality among HIV-infected patients achieved by advances in HIV therapy may be undercut by increases in adverse clinical outcomes attributable to smoking. The high prevalence of smoking among this population and other HIV-infected populations should cause encouraging smoking cessation to become a high priority for clinicians and other HIV service providers to promote health and reduce morbidity and mortality in their patients.
Acknowledgments
SMART was supported by the National Institutes of Health (grants U01-AI068641, U01-AI46362, and U01-AI042170). Jose Ramon Arribas received support from the Program for Intensification of Research Activities in the National Health System.
We thank the INSIGHT executive committee and the SMART study participants.
The SMART Protocol Team cochairs were Wafaa El-Sadr and James Neaton. A full listing of study investigators and other contributors to SMART is available in the appendix to the SMART Study Group article in the New England Journal of Medicine.29
Human Participant Protection
Each of the participating sites in the SMART study was required to obtain the approval of the local institutional review board or ethics committee.
References
- 1.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34 [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–29 [DOI] [PubMed] [Google Scholar]
- 3.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44(2):179–187 [DOI] [PubMed] [Google Scholar]
- 4.Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacheco AG, Tuboi SH, May SB, et al. Temporal changes in causes of death among HIV-infected patients in the HAART era in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2009;51(5):624–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Office on Smoking and Health The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 2004 [Google Scholar]
- 7.Centers for Disease Control and Prevention Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228 [PubMed] [Google Scholar]
- 8.Pearson TA, Blair SN, Daniels SR, et al. AHA Guidelines for Primary Prevention of Cardiovascular Disease and Stroke: 2002 Update: Consensus Panel Guide to Comprehensive Risk Reduction for Adult Patients Without Coronary or Other Atherosclerotic Vascular Diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106(3):388–391 [DOI] [PubMed] [Google Scholar]
- 9.Bates MN, Khalakdina A, Pai M, Chang L, Lessa F, Smith KR. Risk of tuberculosis from exposure to tobacco smoke: a systematic review and meta-analysis. Arch Intern Med. 2007;167(4):335–342 [DOI] [PubMed] [Google Scholar]
- 10.Vineis P, Alavanja M, Buffler P, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96(2):99–106 [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(44):1227–1232 [PubMed] [Google Scholar]
- 12.Feldman JG, Minkoff H, Schneider MF, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: a report from the Women's Interagency HIV Study. Am J Public Health. 2006;96(6):1060–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohli R, Lo Y, Homel P, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006;43(1):90–98 [DOI] [PubMed] [Google Scholar]
- 14.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV-positive New Yorkers: prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14(4):824–835 [DOI] [PubMed] [Google Scholar]
- 15.Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, Rapkin BD. Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res. 2005;7(4):511–522 [DOI] [PubMed] [Google Scholar]
- 16.Benard A, Bonnet F, Tessier JF, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDS. 2007;21(2):458–468 [DOI] [PubMed] [Google Scholar]
- 17.Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21(3 Suppl):40–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahvi S, Cooperman NA. Review: the need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009;21(3 Suppl):14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. HIV-positive smokers considering quitting: differences by race/ethnicity. Am J Health Behav. 2008;32(1):3–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lifson AR, INSIGHT Cause of Death Writing Group, Belloso WH, et al. Determination of the underlying cause of death in three multicenter international HIV clinical trials. HIV Clin Trials. 2008;9(3):177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet F, Burty C, Lewden C, et al. Changes in cancer mortality among HIV-infected patients: the Mortalité 2005 Survey. Clin Infect Dis. 2009;48(5):633–639 [DOI] [PubMed] [Google Scholar]
- 22.Currier JS. Update on cardiovascular complications in HIV infection. Top HIV Med. 2009;17(3):98–103 [PubMed] [Google Scholar]
- 23.Aberg JA. Cardiovascular complications in HIV management: past, present, and future. J Acquir Immune Defic Syndr. 2009;50(1):54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39(5):557–561 [PubMed] [Google Scholar]
- 25.Wand H, Calmy A, Carey DL, et al. Metabolic syndrome, cardiovascular disease and type 2 diabetes mellitus after initiation of antiretroviral therapy in HIV infection. AIDS. 2007;21(18):2445–2453 [DOI] [PubMed] [Google Scholar]
- 26.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis. 2004;4(7):445–455 [DOI] [PubMed] [Google Scholar]
- 27.Madeddu G, Porqueddu EM, Cambosu F, et al. Bacterial community acquired pneumonia in HIV-infected inpatients in the highly active antiretroviral therapy era. Infection. 2008;36(3):231–236 [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19 [PubMed] [Google Scholar]
- 29.Strategies for Management of Antiretroviral Therapy (SMART) Study Group, El-Sadr WM, Lundgren JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355(22):2283–2296 [DOI] [PubMed] [Google Scholar]
- 30.Hennekens CH, Buring JE. Epidemiology in Medicine. Boston, MA: Little, Brown and Co; 1987:87–96 [Google Scholar]
- 31.Kelsey JL, Thompson WD, Evans AS. Methods in Observational Epidemiology. New York, NY: Oxford University Press; 1986:39–41 [Google Scholar]
- 32.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;24(9):1383–1388 [DOI] [PubMed] [Google Scholar]
- 33.Kirk GD, Merlo C, O' Driscoll P, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis. 2007;45(1):103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS. 2007;21(2):207–213 [DOI] [PubMed] [Google Scholar]
- 35.Panel on Antiretroviral Guidelines for Adults and Adolescents Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Dept of Health and Human Services. December 1, 2009. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed February 14, 2010
- 36.Strategies for Management of Antiretroviral Therapy (SMART) Study Group, Emery S, Neuhaus JA, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197(8):1133–1144 [DOI] [PubMed] [Google Scholar]
- 37.Gordin FM, Roediger MP, Girard PM, et al. Pneumonia in HIV-infected persons: increased risk with cigarette smoking and treatment interruption. Am J Respir Crit Care Med. 2008;178(6):630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miguez-Burbano MJ, Ashkin D, Rodriguez A, et al. Increased risk of Pneumocystis carinii and community-acquired pneumonia with tobacco use in HIV disease. Int J Infect Dis. 2005;9(4):208–217 [DOI] [PubMed] [Google Scholar]
- 39.Miguez-Burbano MJ, Burbano X, Ashkin D, et al. Impact of tobacco use on the development of opportunistic respiratory infections in HIV seropositive patients on antiretroviral therapy. Addict Biol. 2003;8(1):39–43 [DOI] [PubMed] [Google Scholar]
- 40.Conley LJ, Bush TJ, Buchbinder SP, Penley KA, Judson FN, Holmberg SD. The association between cigarette smoking and selected HIV-related medical conditions. AIDS. 1996;10(10):1121–1126 [PubMed] [Google Scholar]
- 41.Burns DN, Hillman D, Neaton JD, et al. Cigarette smoking, bacterial pneumonia, and other clinical outcomes in HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(4):374–383 [DOI] [PubMed] [Google Scholar]
- 42.Soysa NS, Ellepola ANB. The impact of cigarette/tobacco smoking on oral candidosis: an overview. Oral Dis. 2005;11(5):268–273 [DOI] [PubMed] [Google Scholar]
- 43.Niaura R, Shadel WG, Morrow K, Tashima K, Flanigan T, Abrams DB. Human immunodeficiency virus infection, AIDS, and smoking cessation: the time is now. Clin Infect Dis. 2000;31(3):808–812 [DOI] [PubMed] [Google Scholar]
- 44.National Cancer Institute Changes in Cigarette-Related Disease Risks and Their Implications for Prevention and Control. Smoking and Tobacco Control Monograph No. 8 Bethesda, MD: National Institutes of Health; 1997. NIH publication 97-4213 [Google Scholar]
- 45.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ. 2000;321(7257):323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawachi I, Colditz GA, Stampfer MJ, et al. Smoking cessation and time course of decreased risks of coronary heart disease in middle-aged women. Arch Intern Med. 1994;154(2):169–175 [PubMed] [Google Scholar]
- 47.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke. The Framingham Study. JAMA. 1988;259(7):1025–1029 [PubMed] [Google Scholar]
- 48.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med. 2008;35(2):158–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Decreased awareness of current smoking among health care providers of HIV-positive compared to HIV-negative veterans. J Gen Intern Med. 2007;22(6):749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidrine DJ, Arduino RC, Lazev AB, Gritz ER. A randomized trial of a proactive cellular telephone intervention for smokers living with HIV/AIDS. AIDS. 2006;20(2):253–260 [DOI] [PubMed] [Google Scholar]
- 51.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behav. 2009;13(3):545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Elzi L, Spoerl D, Voggensperger J, et al. A smoking cessation programme in HIV-infected individuals: a pilot study. Antivir Ther. 2006;11(6):787–795 [PubMed] [Google Scholar]
- 53.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. Motivation and patch treatment for HIV+ smokers: a randomized controlled trial. Addiction. 2009;104(11):1891–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills EJ, Wu P, Spurden D, Ebbert JO, Wilson K. Efficacy of pharmacotherapies for short-term smoking abstinance: a systematic review and meta-analysis. Harm Reduct J. 2009;6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robertson SM, Maldarelli F, Natarajan V, Formentini E, Alfaro RM, Penzak SR. Efavirenz induces CYP2B6-mediated hydroxylation of bupropion in healthy subjects. J Acquir Immune Defic Syndr. 2008;49(5):513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hogeland GW, Swindells S, McNabb JC, Kashuba ADM, Yee GC, Lindley CM. Lopinavir/ritonavir reduces bupropion plasma concentrations in healthy subjects. Clin Pharmacol Ther. 2007;81(1):69–75 [DOI] [PubMed] [Google Scholar]
- 57.Bjartveit K, Tverdal A. Health consequences of smoking 1–4 cigarettes per day. Tob Control. 2005;14(5):315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]