Abstract
As antiretroviral treatment of HIV infection has become increasingly accessible, attention has focused on whether these drugs can used for prevention because of increased tolerability of newer medications, decreased cost, and the limitations of other approaches. We review the status of antiretroviral HIV prevention, including chemoprophylaxis, as well as the effects of treatment of infected individuals on prevention. It is possible that the life-saving agents that have transformed the natural history of AIDS can be a critical component of HIV prevention efforts, but their ultimate role in affecting HIV transmission dynamics remains to be defined.
HIV continues to spread rapidly, with more than 2.5 million new infections each year.1 Efficacious behavioral interventions, when scaled up to achieve sufficient coverage in many populations, have not resulted in durable declines in HIV incidence, and it will take years to demonstrate the efficacy of highly effective HIV-preventive vaccines.2–4 For more than a decade, increasingly well-tolerated highly active antiretroviral therapy (HAART, which incorporates 3 or more antiretroviral therapy [ART] medications) has dramatically changed HIV-associated morbidity and mortality and has improved the quality of life of HIV-infected individuals.5,6 Increasing attention has therefore focused on whether available antiretroviral drugs could be used to slow the epidemic. Recent global initiatives have concentrated on expanding access to HIV treatment in resource-limited settings7; by the end of 2010, more than 5 million people were receiving HAART.8
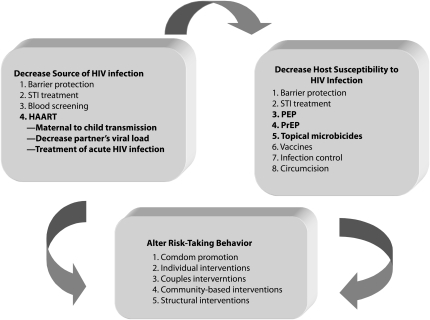
A growing group of researchers and public officials have suggested that 1 or more ART drugs may be useful not only in clinical benefits to individuals, but also in decreasing HIV transmission globally.9,10 ART has already dramatically decreased mother-to-child HIV transmission11 and could conceivably be used to prevent the sexual transmission of HIV via reductions in genital tract HIV concentrations in individuals who are already infected,12,13 or as pre- or postexposure prophylaxis for uninfected people exposed to HIV (Figure 1).14
FIGURE 1.
Potential approaches to prevent HIV transmission.
Note. HAART = highly active antiretroviral therapy; PEP = postexposure prophylaxis; PrEP = preexposure prophylaxis; STI = sexually transmitted infection. Bolded items are those that use antiretroviral agents.
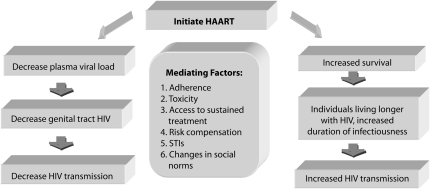
Despite increasing drug availability, however, the effectiveness of ART for prevention may be limited by concurrent sexually transmitted infections (STIs) that increase infectiousness and susceptibility, nonadherence to therapy, drug-related toxicities, viral resistance, treatment costs, and risk compensation. The effects of HAART initiation can manifest differently in diverse social settings because sexual behavior involves concerns over pleasure and procreation. Although early data from the developed world suggested that HAART could be associated with increased sexual risk,15–18 more recent data from sub-Saharan Africa has suggested that wider access to HAART is not associated with increased sexual risk-taking behaviors.19–21 The potential uses of ART for HIV prevention are shown in Figure 2.
FIGURE 2.
Potential effects of highly active antiretroviral therapy on HIV transmission.
Note. HAART = highly active antiretroviral therapy; STI = sexually transmitted infection.
SLOWING THE SPREAD OF HIV
HIV transmission remains a low-probability but high-consequence event occurring in fewer than 1 in 100 contacts on average,22–24 but the global pandemic is driven by the ubiquity of sexual intercourse and by factors that amplify infectiousness and susceptibility in specific settings. The per-contact calculation is based on composite data, and transmission probabilities vary considerably during the course of the disease, with higher transmission probability in the acute and late phases of HIV infection, when plasma and genital HIV concentrations are higher.25–29
The first empirical data about how viral suppression could lead to decreased HIV sexual transmission comes from the Rakai study of Ugandan serodiscordant couples,30 although this study was completed before generic ART had become widely accessible in Uganda. HIV was not transmitted in discordant couples when the infected partner had a plasma HIV RNA level of less than 400 copies per milliliter.30 This association between viral load and the risk of HIV transmission among serodiscordant couples was confirmed in subsequent studies in Zambia and Thailand in the pre-HAART era,31,32 and more recent data showed that HIV-infected partners in discordant relationships were substantially less likely to transmit HIV to their partners if they were on HAART.33 Another recent African study of serodiscordant couples found that HIV-infected partners who were on HAART were 92% less likely to transmit HIV to their uninfected partners.33a
However, other variables besides systemic HIV burden may affect genital tract HIV. Mucosal HIV transmission is complex: animal models suggest that either cell-free or cell-infected virus can replicate in a variety of host cells.34–36 The minimum inoculum of HIV that can cause human infection remains unclear.37 Although some studies have documented HIV-preferential binding to cervical and foreskin tissues through dendritic cells,38,39 women who have undergone hysterectomy and circumcised men can also become infected with HIV,12,40 suggesting that other urogenital cells can support HIV replication. The temporal window of opportunity for halting transmission through host defenses is very limited, because submucosal viral replication occurs within hours of exposure.41
In the first study examining the relationship between treatment and the detection of genital tract HIV, the virus was more readily cultured from seminal plasma and leukocytes in participants with leukocytospermia or advanced disease stage and less likely to be detected in semen among men taking zidovudine.42 Subsequent semen studies found that genital white blood cells were a significant source of viral burden.43,44 These data suggest that factors that increase genital tract inflammation may promote infectiousness and that ART could decrease infectiousness, presumably by decreasing viral burden.
Several studies have demonstrated that HAART suppresses viral replication not only in the blood and lymphoid tissues but also in the male and female genital tract.12,13,45,46 Although treatment generally suppresses cell-free virus in the semen, many treated individuals can still harbor proviral CD4 cells in their semen.44,47 A further complication is that various HAART drug combinations may not be equally effective in suppressing HIV in genital tissues and systemically.12,48 Differential penetration of ART drugs in genital tissues may be a function of protein binding and other pharmacodynamic properties.49 Highly protein-bound drugs, such as protease inhibitors, achieve lower concentrations in genital tract secretions than in blood plasma.14,50 Nucleoside analogs and nonnucleoside reverse transcriptase inhibitors achieve higher concentrations in genital tract secretions than in blood plasma,51–53 which might make them particularly effective in decreasing sexual transmission of HIV.
These findings have led to questions about the optimal timing for HAART initiation if a primary goal is to decrease infectiousness among those who could transmit HIV to others. One key period for treatment could be the acute phase of HIV infection, when patients have elevated viremia for about 3 weeks, until host defenses suppress replication, creating a viral set point.29,54,55 When viral replication is unimpeded during acute HIV infection, individuals may have more than 1 million copies of virus per milliliter of blood,56 with the potential to infect many individuals.54,57 Studies from North America and Africa found that between 40% and 50% of new transmissions came from recently infected patients.54,57 In Malawi, discordant HIV rapid test results and RNA pooling detected acute HIV infection in almost 2% of STI clinic patients.58
Promptly identifying microepidemics, or hot spots, of newly infected persons may present an opportunity for early ART and behavioral interventions to slow the spread of HIV in high-risk settings.59,60 Studies are under way to determine whether early identification and treatment of acutely infected individuals can have a public health impact on local epidemics, but other questions will take more time to address, such as whether treatment can be discontinued without detriment to the patient if it is initiated in the setting of acute HIV infection. Some public health challenges to identifying patients with acute infection are the very short duration of this infection period, lack of clinical detection because patients and providers often do not recognize the symptoms of acute HIV infection, and the expense of performing RNA pooling in an era of constrained health resources.
Acute HIV infection is followed by a longer period of chronic viral homeostasis in which an infected person may be asymptomatic, with good systemic virological control. This period can be interrupted by STI infections, which may override the suppressive effects of ART in the genital tract by causing inflammation, facilitating local HIV replication.27 The acute- and late-stage infection periods are relatively short, so the prolonged asymptomatic period, which can last more than a decade, may be when many transmission events occur.61–63 An analysis from sub-Saharan Africa suggests that acutely infected persons play a major role in HIV transmission during early, highly concentrated epidemics and that the contribution of chronically infected persons becomes more prominent during advanced and stabilized epidemics.61 A recent projection suggested that annual HIV testing followed by immediate initiation of ART for all HIV-infected patients regardless of CD4 cell count could have a major impact by reducing the number of new HIV infections.64
Can HAART effectively suppress genital tract HIV over a sufficiently long period to stop HIV sexual transmission?65 Studies from Taiwan and British Columbia have documented a greater than 50% reduction in anticipated number of incident HIV cases following the free provision of ART in 1997.9,66 A study among 393 couples in the pre- and post-HAART eras in Spain observed an 80% reduction in HIV transmission following the introduction of HAART.67 A recent observational study of sero- discordant African couples showed substantially lower rates of HIV transmission when the partner with HIV was receiving HAART, but these individuals were more likely to practice safer sex than were persons who remained untreated.33
To better understand the preventive effects of therapy in serodiscordant couples, the HIV Prevention Trials Network of the National Institutes of Health is conducting a randomized controlled trial to assess the impact of HAART on transmission among 1750 serodiscordant couples in 8 countries.68 HIV-infected partners in serodiscordant, monogamous relationships who have CD4 counts that would not warrant immediate initiation of HAART according to current guidelines (i.e., > 350 cells/μL) are being randomized to initiate HAART right away or to be clinically monitored until their immunological or clinical status warrants treatment. This study is fully enrolled and should provide important information in the near future.
As more individuals live longer with HIV because of HAART, the pool of persons who could transmit the infection will grow if HAART is not fully suppressive and if infected individuals increase risk-taking behavior. Studies on the effect of ART on sexual behavior among HIV-infected individuals have been inconsistent, and it is not clear whether the provision of ART may be associated with high-risk sexual behavior in some subpopulations.69,70 Although data from injection drug users and men who have sex with men (MSM) in the developed world suggest that high-risk behavior can increase with ART,17,71 studies from resource-limited settings to date have not shown that the provision of ART increases HIV risk-taking behaviors.72
Interest in evaluating test-and-treat strategies is growing, but other genital tract factors could mitigate the public health impact of early initiation of HAART. For example, concurrent STIs can raise the probability of HIV transmission73 by attracting CD4 T lymphocytes and releasing cytokines (e.g., TNF-α, IL-1) that enhance HIV transmission.37 Bacterial STIs can increase genital tract HIV concentrations,74 and interventions that provide treatment of these infections can decrease viral shedding.27,75–77 In the Rakai study, HSV-2-antibody–negative participants with viral loads greater than 38 500 copies per milliliter and HSV-2-antibody–positive participants with viral loads lower than 1700 copies per milliliter had a similar probability of transmitting HIV to their uninfected partner.78 Unfortunately, in many settings HSV-2 seroprevalence in young adult populations exceeds 50%,79 and 2 studies have documented that thymidine–kinase inhibitor chemoprophylaxis does not decrease HIV transmission to uninfected partners.80,81 It is possible that the removal of a pathogen that initiated a genitourinary inflammatory response is insufficient to restore the local cellular milieu.
Another concern about the wider use of ART for prevention is the potential for the development and transmission of antiretroviral resistance if healthy individuals are expected to be fully adherent over many years. Multiple reports have documented that resistant HIV has been sexually transmitted.82 Although ART resistance has been demonstrated with increased frequency in newly diagnosed and treatment-naive patients over time,83,84 recent findings suggest that ART-resistant HIV strains may have lower viral transmisability.85 Thus, public health systems around the world will need to track some of the potential unintended consequences of earlier initiation of antiretroviral therapy for prevention, such as risk compensation, trends in STI coepidemics, and the prevalence and incidence of transmitted ART-resistant HIV, to fully understand the costs, as well as the benefits, of treatment as prevention.
POSTEXPOSURE PROPHYLAXIS
The evidence that suggests that ART can prevent HIV acquisition comes from the success of efforts to prevent mother-to-child HIV transmission,86 animal studies,87,88 and a case–control study of postexposure prophylaxis (PEP) following needle stick injury in health care settings.89 The Centers for Disease Control and Prevention registry documented that individuals who took zidovudine monotherapy following occupational exposure were one fifth as likely to be HIV infected as were those who did not take medication.89,90 Data from the rhesus macaque model have suggested that 28 days of ART is needed for effective postexposure prophylaxis.88
Concerns have been raised that the use of PEP in nonoccupational settings might result in increased risk taking in some populations, such as among MSM. In a Brazilian MSM study, high-risk participants in a behavioral risk reduction study were educated about PEP while being counseled about safer sex, and were given 4-day starter packs of zidovudine and lamivudine. They were told that if they engaged in risky behavior and began to take the medication, they needed to come back to the study site as soon as possible, so they could get the rest of the 28-day course that they would be expected to take. About one third of the 200 high-risk men who were followed for 24 months (68 men) used PEP, a total of 109 times,91 another one third engaged in risky behavior but did not use PEP, and the remainder heeded the counseling messages and did not engage in risky behavior. The overall HIV incidence in the cohort was 2.9 per 100 person-years; 10 infections occurred among men who did not use PEP and only 1 occurred among the men who used PEP. Although HIV risk-taking behavior may not be constant over time,92 most studies of PEP after sexual exposure have not demonstrated increases in risk-taking behavior after PEP, and some have included effective counseling so that PEP could provide an entry for intensified risk-reduction interventions.91,93,94
A major impediment to the wider use of PEP in the past was the relative intolerability of some of the first-line recommended drugs, such as Azidothymidine GlaxoSmithKline (Brentford, UK) and protease inhibitors.95 However, the use of newer drugs, such as tenofovir, seems to be associated with increased tolerability and completion rates, although randomized controlled trials have not compared PEP regimens head-to-head because of logistical issues, such as the huge sample size needed to compare 2 effective regimens. Tenofovir has many features that are desirable in a chemoprophylactic agent, such as long intracellular half-life, activity in macrophages, and high concentrations in genital tissues.96 A case–control study found that men who took dual therapy with tenofovir and emtricitabine were more likely to complete a 28-day PEP regimen than were historical control participants taking 2 drug regimens containing zidovudine.97
It is still not known whether it is preferable to use 2 drugs or 3 for PEP; the argument for 2 is increased tolerability and completion rates,98 whereas the argument for 3 is that for a person already exposed to HIV, more drugs provide extra protection against drug-selected or spontaneous mutant strains. Other, newer drugs may offer opportunities for novel PEP strategies, because they are well tolerated (e.g., Atazanavir [Bristol-Myers Squibb; New York, NY] or Raltegravir [Merck; Whitehouse Station, NJ])99 or achieve high genital tract concentrations (e.g., maraviroc [Pfizer; New York, NY]).100 Although several lines of data suggest that PEP may decrease the likelihood of HIV transmission, in many settings it may be underused because of clinician concerns about risk compensation and cost and because many at-risk individuals are unfamiliar with its potential or with how to access chemoprophylactic treatment.
PREEXPOSURE PROPHYLAXIS
In situations when the likelihood of exposure to HIV can be foreseen, ART preexposure prophylaxis (PrEP), delivered either as oral therapy or as topical microbicide, could be a logical method of primary prevention. In the field of infectious disease preventive care, patients are routinely provided with prophylaxis prior to exposure when the risk of infection is imminent—examples include antituberculosis therapy and antimalarials. Data from simian studies suggests that tenofovir-containing regimens protect against infection, with rapid drug absorption and high drug levels remaining in intracellular genital tissues101 and with the possibility of intermittent dosing.102 Over the past decade, animal studies have provided the basis for clinical PrEP research,103 although concerns about access to optimal preventive services and medical treatment of vulnerable populations has impeded initial PrEP research.104 Some concerns about PrEP are possible behavioral disinhibition, ART cost, acquisition of resistant viral strains, treatment adherence, and chronic medication toxicities.
A phase II, randomized, double-blinded, placebo-controlled trial, completed 3 years ago in Cameroon, Nigeria, and Ghana, demonstrated the safety of daily oral tenofovir for HIV prevention among high-risk women also receiving HIV testing, counseling, and condoms.105 After enrollment, 8 seroconversions occurred, in 2 women who took tenofovir and 6 who received the placebo. Although this difference was not statistically significant, the trend was in the expected direction, and both groups of women reduced their behavioral risk during the course of the study. The number of sexual partners went down, and the proportion of participants reporting condom use increased over time (52% at baseline to 95% at 12 months).105,106 Despite concerns that PrEP could lead to behavioral disinhibition, an important finding in this study was that risk behavior decreased over time after initiating PrEP.
These reassuring data have encouraged public health researchers to study PrEP in efficacy trials in several high-risk populations around the world. Over the next few years, data will become available about whether oral tenofovir by itself, oral tenofovir coformulated with emtricitabine, or topical tenofovir gel is more effective than are placebos among MSM in the Americas, Thailand, and South Africa; among at-risk women in sub-Saharan Africa; among HIV-discordant couples in Africa; and among Thai injection drug users. At the recent International AIDS Society (IAS) meetings in Vienna, data were presented that found that oral tenofovir for PrEP was safe, well-tolerated, and was not associated with increases in sexual risk behavior among 400 US men who have sex with men.107
Previous studies demonstrated that topical tenofovir gel is safe and well tolerated; however in more than half of the women in a pharmacokinetic substudy, systemic levels of tenofovir were detected when the drug was administered topically to low-risk women.107a For some, this is good news, because systemic absorption means that significant genital tissue levels were achieved, but low concentrations might be less likely to be associated with clinical toxicities. Others have expressed concern because the levels were very low compared with the systemic levels obtained by a 300-mg oral dose, resulting in subinhibitory concentrations. The most significant chemoprophylaxis results were presented recently at the IAS Meetings in Vienna. The CAPRISA 004 study demonstrated the efficacy of topical 1% tenofovir gel in decreasing HIV incidence by 39% in high risk South African women who used the gel before and after sexual intercourse. The protective effect exceeded 50% among women who used the product at least 80% of the time.107b
Post-CAPRISA, major questions are how best to deliver chemoprophylaxis and which drugs to use. The ability of topical 1% tenofovir gel to deliver high drug levels to the genital tissues but with lower systematic drug levels108 led to the VOICE (Vaginal and Oral Interventions to Control the Epidemic) trial, the first study to compare daily use of 1% tenofovir gel versus oral tenofovir and emtricitabine.109,110 Studies are underway to assess other topical antiretroviral agents. Assessment of the relative merits and limitations of oral versus topical PrEP agents will require, in addition to clinical trials, careful anthropological work to elucidate whether one approach is associated with more sexual pleasure, fewer systemic side effects, and perceptions of efficacy among people in different cultures.
One broad area of concern is the development of drug resistance through the continued use of PrEP after becoming infected because of a resistant virus, suboptimal adherence, or failure of the prophylactic regimen. In an early-phase study of therapeutic uses of tenofovir, when HIV-infected patients initiated therapy with tenofovir alone, no resistance was detected after 28 days.111 In a nonhuman primate study, after exposure to drug-resistant viral challenge, drug-resistant minor variants were detected in monkeys, although tenofovir PEP was still partially effective in protecting monkeys from becoming infected.112 One study observed no tenofovir resistance after tenofovir or tenofovir–emtricitabine failure,113 and another detected intermittent emtricitabine resistance.114 When the virus develops tenofovir and emtricitabine resistance, it is hypersusceptible to zidovudine, but the activity of other nucleoside reverse transcriptase inhibitors is diminished; no effect on other classes of drugs has been found.
Two potential concerns about tenofovir prophylaxis have been mentioned in light of current treatment regimens. First, in resource-limited settings, stavudine has been often used as part of first-line drug treatment because it is inexpensive. Of concern is that the HIV subtype C virus that is most prevalent in these settings may preferentially select for a genetic mutation known as K65R. This resistance mutation may occur after exposure to stavudine-containing HAART, which could create tenofovir resistance.115,116 Thus, wider stavudine use may be selecting for a larger pool of circulating strains with K65R, which could result in failure of tenofovir prophylaxis.
Another worry is that individuals on tenofovir PrEP will develop tenofovir resistance after becoming infected. Mathematical modeling of HIV prophylaxis for primary prevention suggests that fewer than 1% of the predicted seroconversions would acquire or develop a tenofovir-resistant strain,117 but in a world where tenofovir is increasingly used for first-line antiretroviral therapy, continued monitoring to assess the potential ecological effects of PrEP is warranted. In clinical trials, participants receive frequent HIV antibody testing; patients in clinical settings will also require ongoing HIV testing to avoid substandard therapy should they become HIV infected. In either of these situations, individuals with tenofovir-resistant virus could compromise their own available treatment choices and spread resistant virus to their partners.
Clinical trials of PrEP are under way at multiple sites that will enroll more than 20 000 HIV-uninfected men and women in Asia, South and North America, and Africa and will address the role of continuous versus intermittent PrEP, topical versus oral PrEP, selection of specific drugs, and the influence of PrEP on risk practices.118 Some of the first PrEP efficacy data may be available within the next 2 years, so public health officials and clinicians will need to consider how to train providers to make the medications available to at-risk populations and provide careful monitoring and how to use PrEP to create educable moments that facilitate HIV prevention if PrEP works. Partial efficacy and potential toxicity management issues (e.g., what if tenofovir PrEP decreases the likelihood of HIV acquisition by 50%, but a few participants develop renal failure?) are examples of challenges that will arise in the development of clear and succinct messages as clinical trial data matures. One community-based organization, the AIDS Vaccine Advocacy Coalition (http://www.avac.org) has developed a PrEPWatch feature that provides continuously updated information about the status of the clinical trials.
Many clinical questions will arise regardless of the results of the first-generation PrEP studies; an overview of such studies is shown in Table 1. Other antiviral drugs are being considered for chemoprophylaxis, ranging from the oral CCR5 antagonist maraviroc, which achieves high genital tract levels, to UC-781 and TMC-120, which are poorly absorbable nonnucleoside reverse transcriptase agents and thus are being developed as topical microbicides. Injectable agents and compounds that can be delivered through a slow release ring may alleviate the adherence issues associated with stringent daily regimens. Some agents, such as lamivudine and emtricitabine, may be considered to be optimal parts of PrEP regimens because the virus that is resistant to these drugs has decreased viral fitness.119,120 Thus, individuals who become infected with this less virulent viral stain would be less likely to transmit to their partners. Because of the long intracellular half-life of drugs such as tenofovir, intermittent dosing strategies may make sense.
TABLE 1.
Current Studies of Preexposure Prophylaxis Against HIV
| Trial name | Population | Location | Drug | Means of Administration | Sample Size | Expected Results |
| Daily PrEP | ||||||
| US Extended Safety Trial (CDC 4323) | Gay men; MSM | United States | TDF | Daily oral | 400 | Study completed 2009; TDF PrEP was safe and well-tolerated. |
| iPrEX | Gay men; MSM | Brazil; Ecuador; Peru; South Africa; Thailand; United States | TDF/FTC | Daily oral | 2500 | Fully enrolled 2009; results probable early 2011 or late 2010 |
| Bangkok Tenofovir Study (CDC 4370) | Injection drug users | Thailand | TDF | Daily oral | 2400 | Completed enrollment 2010; possible results 2010 or early 2011 |
| Caprisa 004 | Heterosexual women | South Africa | TFV | Coitally dependent topical vaginal gel | 1000 | 1% TFV decreased HIV incidence by 39% |
| TDF2 (CDC 4940) | Heterosexual men and women | Botswana | TDF/FTC | Daily oral | 1200 | Enrollment stopped 2009; safety data probable 2010 |
| Partners PrEP | Serodiscordant heterosexual couples | Kenya; Uganda | TDF and TDF/FTC | Daily oral | 3900 | Enrolling; data expected 2012 |
| FEM-PrEP | Heterosexual women | Kenya; Malawi; South Africa; Tanzania; Zambia | TDF/FTC | Daily oral | 3900 | Enrolling; data expected 2013 |
| VOICE (MTN 003) | Heterosexual women | South Africa; Uganda; Zambia; Zimbabwe; additional sites to be determined | TDF; TDF/FTC | Daily oral (TDF; TDF/FTC); daily topical vaginal gel (TDF) | 5000 | Enrolling; data expected 2013 |
| Intermittent PrEP | ||||||
| IAVI E001 and E002 | Serodiscordant couples; at-risk men and women | Kenya; Uganda | TDF/FTC | Daily oral; intermittent oral (twice weekly + coital dosing) | 150 | Full enrollment expected 2010 |
| HPTN 066 | Low-risk men and women | United States | TDF/FTC | Different dosing strategies planned | 48 | Full enrollment expected 2010 |
| HPTN 067 | High-risk women and MSM | Thailand; South Africa | TDF/FTC | Fixed interval versus coitally dependent | 360 | In planning stages |
| ATN 082 | High-risk young MSM | United States | TDF/FTC | Daily; with or without a behaviorial intervention | 100 | 2011 or 2012 |
Note. ATN = Adolescent Trials Network; CDC = Centers for Disease Control and Prevention; FEM-PrEP = Study to Assess the Role of Truvada in Preventing HIV Acquisition in Women; FTC = emtricitabine; HPTN = HIV Prevention Trials Network; IAVI = International AIDS Vaccine Initiative; MSM = men who have sex with men; MTN = Microbicide Trials Network; PrEP = preexposure prophylaxis; TDF = tenofovir difurmate; TFV = tenofovir 1% gel; VOICE = Vaginal and Oral Interventions to Control the Epidemic.
A challenge for the use of ART for prevention is the possibility that at-risk persons might obtain off-label drugs even in advance of any efficacy data becoming available. One study suggested that ART was being sold at clubs and self-administered prior to high-risk sexual activity,121 but other reports from San Francisco and Boston found minimal use among MSM in the past 2 years.122,123 Clearly, the potential for widespread and unregulated use is great, and the environment could change quickly once data showing a beneficial effect from antiretroviral PrEP are released. Early work suggests that context matters: interest in using PrEP was substantially affected by perceived efficacy, side effects, and cost.122 Public health authorities, led by the Centers for Disease Control and Prevention in the United States and by the World Health Organization globally, have begun to meet regularly to anticipate community responses and will be mobilized to work with clinicians, national governments, and representatives of high-risk communities, once efficacy data become available.124
OPERATIONAL ISSUES
After nearly 2 decades of experience with HAART, clinicians and public health officials must consider how to optimally use this resource to reduce the number of new infections. Operational research into long-term safety, adherence, benefits of different modes of drug delivery and dosing, and selection of resistant virus is required. Some of these issues will be addressed in the context of phase IV expanded safety studies; others will require the strengthening of public health monitoring systems. Efforts are under way to monitor the emergence of acquired antiretroviral drug resistance in resource-limited settings via the World Health Organization's global network HIVResNet (resistance network), which provides standardized tools, training, technical assistance, laboratory quality assurance, analysis of results, and recommendations for guidelines and public health action.125
The recent expansion of World Health Organization treatment guidelines to initiate treatment at a CD4 cell count of 350 cells per microliter—rather than at 200 cells per microliter—will mean that a larger pool of HIV-infected patients will be in need of treatment.126 Recent curtailment in the US President's Emergency Plan for AIDS Relief for HIV treatment could raise further ethical concerns if providing life-saving medicine to persons already infected with HIV has to compete with efforts to prevent future HIV infections.127 Moreover, many resource-limited nations may have limited budgets for HIV treatment, and thus will have to carefully decide how to make the best use of limited resources to also decrease the number of new infections.
FUTURE OF ANTIRETROVIRALS FOR HIV PREVENTION
The degree of public health benefits reaped through the use of ART for prevention will depend on the number of HIV-infected individuals treated, the ability to effectively engage individuals most likely to transmit HIV, the relative stage of a given epidemic, the efficacy of specific ART regimens to reduce viral load in the genital tract, the development of drug-resistant viral strains, and changes in risk-taking behaviors that could compromise the protective effects of ART. Although targeting ART preventive therapy to infectious individuals or individuals at greatest risk of acquiring HIV can be a major challenge, no consensus has yet emerged about the preventive benefits of widespread administration of ART for the general population. Mathematical models have suggested that widespread provision of ART could substantially reduce HIV incidence, but this benefit could be undermined by behavioral disinhibition.128,129
The use of ART to reduce HIV transmission has moved to the forefront of public health approaches to HIV prevention because of the increased tolerability of the medications, decreased cost, expanded formulary, and limitations of other approaches. Clinical data indicate that earlier initiation of ART for infected individuals is warranted; optimizing the benefits will require attention to adherence, sustained access, behavioral risk reduction, and STI diagnosis and treatment. Although the use of ART for uninfected individuals holds great promise, public health authorities will need to assess the potential of local decreases in HIV infection relative to financial costs and ecological impact, if efficacy trial data show benefit. The field is at an early stage, with major questions remaining, such as what is the least amount of medication that can be effective with pre- and postcoital dosing and what are the optimal routes of drug delivery (e.g., topical, oral, and injectable).
It is conceivable that in the future the ART formulary will consist of drugs targeted to specific preventive and therapeutic interventions, such as topical agents for stopping viral entry. An effective HIV vaccine is still years away,3 and the utility of antiretroviral medicines for prevention will be tempered by the fact that these agents are not likely to be 100% effective in the real world. Further studies in pharmacology, virology, and behavioral science will be needed to best understand the intended, and unintended, clinical consequences of widespread ART.
ART may prove to be a critical weapon in HIV prevention, but it will need to be part of a larger arsenal aimed at reducing the number of new infections globally, including circumcision, prevention of mother-to-child HIV transmission, behavioral change, and treatment of STIs. It is possible that the life-saving agents that have transformed the natural history of HIV and the quality of life of infected patients may become part of HAARP (highly active antiretroviral prevention),130 but their ultimate potential in preventing HIV transmission remains to be fully defined.
Acknowledgments
This study was supported by Fenway Health HIV Prevention Trials Network (grant 5U01AI06948) and the Lifespan Center for Aids Research (grant P30 AI42853).
The authors would also like to recognize the assistance of Lola Wright in the preparation of the article.
Human Participant Protection
No protocol approval was required because only secondary data were used.
References
- 1.UNAIDS 2008 update on the global AIDS epidemic. Available at: http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008j. Accessed June 1, 2009
- 2.Markel H. The search for effective HIV vaccines. N Engl J Med. 2005;353(8):753–757 [DOI] [PubMed] [Google Scholar]
- 3.Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med. 2007;356(20):2073–2081 [DOI] [PubMed] [Google Scholar]
- 4.Klasse PJ, Shattock R, Moore JP. Antiretroviral drug-based microbicides to prevent HIV-1 sexual transmission. Annu Rev Med. 2008;59:455–471 [DOI] [PubMed] [Google Scholar]
- 5.Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362(9377):22–29 [DOI] [PubMed] [Google Scholar]
- 6.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization Scaling up antiretroviral therapy in resource-limited settings: treatment guidelines for a public health approach—2006 revision. Available at: http://www.who.int/hiv/pub/2009progressreport/en. Accessed June 1, 2009
- 8.World Health Organization Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Available at: http://www.who.int/hiv/mediacentre/2008progressreport/en/index.html. Published 2008. Accessed June 1, 2009
- 9.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–536 [DOI] [PubMed] [Google Scholar]
- 10.Salomon JA, Hogan DR, Stover J, et al. Integrating HIV prevention and treatment: from slogans to impact. PLoS Med. 2005;2(1):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283(9):1175–1182 [DOI] [PubMed] [Google Scholar]
- 12.Cu-Uvin S, Caliendo AM, Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14(4):415–421 [DOI] [PubMed] [Google Scholar]
- 13.Vernazza PL, Gilliam BL, Flepp M, et al. Effect of antiviral treatment on the shedding of HIV-1 in semen. AIDS. 1997;11(10):1249–1254 [DOI] [PubMed] [Google Scholar]
- 14.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146(8):591–601 [DOI] [PubMed] [Google Scholar]
- 15.Kelly JA, Otto-Salaj LL, Sikkema KJ, Pinkerton SD, Bloom FR. Implications of HIV treatment advances for behavioral research on AIDS: protease inhibitors and new challenges in HIV secondary prevention. Health Psychol. 1998;17(4):310–319 [DOI] [PubMed] [Google Scholar]
- 16.Dukers NH, Goudsmit J, de Wit JB, Prins M, Weverling GJ, Coutinho RA. Sexual risk behaviour relates to the virological and immunological improvements during highly active antiretroviral therapy in HIV-1 infection. AIDS. 2001;15(3):369–378 [DOI] [PubMed] [Google Scholar]
- 17.Ostrow DE, Fox KJ, Chmiel JS, et al. Attitudes towards highly active antiretroviral therapy are associated with sexual risk taking among HIV-infected and uninfected homosexual men. AIDS. 2002;16(5):775–780 [DOI] [PubMed] [Google Scholar]
- 18.Katz MH, Schwarcz SK, Kellogg TA, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;92(3):388–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moatti JP, Prudhomme J, Traore DC, et al. Access to antiretroviral treatment and sexual behaviours of HIV-infected patients aware of their serostatus in Côte d'Ivoire. AIDS. 2003;17(suppl 3):S69–S77 [DOI] [PubMed] [Google Scholar]
- 20.Bunnell R, Ekwaru JP, Solberg P, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20(1):85–92 [DOI] [PubMed] [Google Scholar]
- 21.Bateganya M, Colfax G, Shafer LA, et al. Antiretroviral therapy and sexual behavior: a comparative study between antiretroviral-naive and -experienced patients at an urban HIV/AIDS care and research center in Kampala, Uganda. AIDS Patient Care STDS. 2005;19(11):760–768 [DOI] [PubMed] [Google Scholar]
- 22.Chakraborty H, Sen PK, Helms RW, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001;15(5):621–627 [DOI] [PubMed] [Google Scholar]
- 23.Royce RA, Seña A, Cates W, Jr, Cohen MS. Sexual transmission of HIV. N Engl J Med. 1997;336(15):1072–1078 [DOI] [PubMed] [Google Scholar]
- 24.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17(4):455–480 [DOI] [PubMed] [Google Scholar]
- 26.Lampinen TM, Critchlow CW, Kuypers JM, et al. Association of antiretroviral therapy with detection of HIV-1 RNA and DNA in the anorectal mucosa of homosexual men. AIDS. 2000;14(5):F69–F75 [DOI] [PubMed] [Google Scholar]
- 27.Cohen MS, Hoffman IF, Royce RA, et al. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet. 1997;349(9069):1868–1873 [DOI] [PubMed] [Google Scholar]
- 28.Powers KA, Poole C, Pettifor AE, Cohen MS. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(9):553–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilcher C, Tien HC, Eron JJ, Jr, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–1792 [DOI] [PubMed] [Google Scholar]
- 30.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–929 [DOI] [PubMed] [Google Scholar]
- 31.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS Res Hum Retroviruses. 2001;17(10):901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tovanabutra S, Robison V, Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002;29(3):275–283 [DOI] [PubMed] [Google Scholar]
- 33.Sullivan P, Kayitenkore K, Chomba E, et al. Reduction of HIV transmission risk and high risk sex while prescribed ART: results from discordant couples in Rwanda and Zambia. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC: Available at: http://retroconference.org/2009. Accessed June 7, 2010 [Google Scholar]
- 33a.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. The Lancet. 2010;375(9731):2092–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stebbing J, Gazzard B, Douek DC. Where does HIV live? N Engl J Med. 2004;350(18):1872–1880 [DOI] [PubMed] [Google Scholar]
- 35.Gupta P, Collins KB, Ratner D, et al. Memory CD4(+) T cells are the earliest detectable human immunodeficiency virus type 1 (HIV-1)-infected cells in the female genital mucosal tissue during HIV-1 transmission in an organ culture system. J Virol. 2002;76(19):9868–9876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller CJ. Localization of simian immunodeficiency virus-infected cells in the genital tract of male and female Rhesus macaques. J Reprod Immunol. 1998;41(1–2):331–339 [DOI] [PubMed] [Google Scholar]
- 37.Vernazza PL, Kashuba ADM, Cohen MS. Biological correlates of sexual transmission of HIV: practical consequences and potential targets for public health. Rev Med Microbiol. 2001;12(3):131–142 [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74(13):6087–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller CJ, Vogel P, Alexander NJ, Dandekar S, Hendrickx AG, Marx PA. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab Invest. 1994;70(2):255–262 [PubMed] [Google Scholar]
- 40.Millett GA, Flores SA, Marks G, Reed JB, Herbst JH. Circumcision status and risk of HIV and sexually transmitted infections among men who have sex with men: a meta-analysis. JAMA. 2008;300(14):1674–1684 [DOI] [PubMed] [Google Scholar]
- 41.Garg S, Mandl J, Ibegbu C, et al. Low-dose simian HIVSF162P vaginal challenges reveal localized virus replication and inoculum size effects. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC: Available at: http://retroconference.org/2009. Accessed June 7, 2010 [Google Scholar]
- 42.Anderson DJ, O'Brien TR, Politch JA, et al. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA. 1992;267(20):2769–2774 [PubMed] [Google Scholar]
- 43.Quayle AJ, Xu C, Mayer KH, Anderson DJ. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176(4):960–968 [DOI] [PubMed] [Google Scholar]
- 44.Vernazza PL, Troiani L, Flepp MJ, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000;14(2):117–121 [DOI] [PubMed] [Google Scholar]
- 45.Barroso PF, Schechter M, Gupta P, et al. Effect of antiretroviral therapy on HIV shedding in semen. Ann Intern Med. 2000;133(4):280–284 [DOI] [PubMed] [Google Scholar]
- 46.Cu Uvin S, Caliendo AM, Reinert SE, Mayer KH, Flanigan TP, Carpenter CC. HIV-1 in the female genital tract and the effect of antiretroviral therapy. AIDS. 1998;12(7):826–827 [PubMed] [Google Scholar]
- 47.Zhang H, Dornadula G, Beumont M, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339(25):1803–1809 [DOI] [PubMed] [Google Scholar]
- 48.Eron JJ, Vernazza PL, Johnston DM, et al. Resistance of HIV-1 to antiretroviral agents in blood and seminal plasma: implications for transmission. AIDS. 1998;12(15):F181–F191 [DOI] [PubMed] [Google Scholar]
- 49.Taylor S, Pereira AS. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex Transm Infect. 2001;77(1):4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosn J, Chaix ML, Peytavin G, et al. Penetration of enfuvirtide, tenofovir, efavirenz, and protease inhibitors in the genital tract of HIV-1-infected men. AIDS. 2004;18(14):1958–1961 [DOI] [PubMed] [Google Scholar]
- 51.Pereira AS, Kashuba AD, Fiscus SA, et al. Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis. 1999;180(6):2039–2043 [DOI] [PubMed] [Google Scholar]
- 52.Min SS, Corbett AH, Rezk N, et al. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J Acquir Immune Defic Syndr. 2004;37(5):1577–1580 [DOI] [PubMed] [Google Scholar]
- 53.Taylor S, van Heeswijk RP, Hoetelmans RM, et al. Concentrations of nevirapine, lamivudine and stavudine in semen of HIV-1-infected men. AIDS. 2000;14(13):1979–1984 [DOI] [PubMed] [Google Scholar]
- 54.Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195(7):951–959 [DOI] [PubMed] [Google Scholar]
- 55.Cohen MS, Pilcher CD. Amplified HIV transmission and new approaches to HIV prevention. J Infect Dis. 2005;191(9):1391–1393 [DOI] [PubMed] [Google Scholar]
- 56.Pilcher CD, Price MA, Hoffman IF, et al. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004;18(3):517–524 [DOI] [PubMed] [Google Scholar]
- 57.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–1409 [DOI] [PubMed] [Google Scholar]
- 58.Pilcher CD, Fiscus SA, Nguyen TQ, et al. Detection of acute infections during HIV testing in North Carolina. N Engl J Med. 2005;352(18):1873–1883 [DOI] [PubMed] [Google Scholar]
- 59.Colfax GN, Buchbinder SP, Cornelisse PG, Vittinghoff E, Mayer K, Celum C. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS. 2002;16(11):1529–1535 [DOI] [PubMed] [Google Scholar]
- 60.Koopman JS, Jacquez JA, Welch GW, et al. The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr. 1997;14(3):249–258 [DOI] [PubMed] [Google Scholar]
- 61.Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22(9):1055–1061 [DOI] [PubMed] [Google Scholar]
- 62.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–693 [DOI] [PubMed] [Google Scholar]
- 63.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21(13):1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57 [DOI] [PubMed] [Google Scholar]
- 65.Hosseinipour M, Cohen MS, Vernazza PL, Kashuba AD. Can antiretroviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1? Clin Infect Dis. 2002;34(10):1391–1395 [DOI] [PubMed] [Google Scholar]
- 66.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190(5):879–885 [DOI] [PubMed] [Google Scholar]
- 67.Castilla J, Del Romero J, Hernando V, Marincovich B, García S, Rodríguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005;40(1):96–101 [DOI] [PubMed] [Google Scholar]
- 68.National Institutes of Health Health Prevention Trials Network. 2007. Available at: http://www.hptn.org/research_studies/hptn052.asp. Accessed September 1, 2009
- 69.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004;292(2):224–236 [DOI] [PubMed] [Google Scholar]
- 70.Hogben M, Liddon N. Disinhibition and risk compensation: scope, definitions, and perspective. Sex Transm Dis. 2008;35(12):1009–1010 [DOI] [PubMed] [Google Scholar]
- 71.Tun W, Gange SJ, Vlahov D, Strathdee SA, Celentano DD. Increase in sexual risk behavior associated with immunologic response to highly active antiretroviral therapy among HIV-infected injection drug users. Clin Infect Dis. 2004;38(8):1167–1174 [DOI] [PubMed] [Google Scholar]
- 72.Kennedy C, O'Reilly K, Medley A, Sweat M. The impact of HIV treatment on risk behaviour in developing countries: a systematic review. AIDS Care. 2007;19(6):707–720 [DOI] [PubMed] [Google Scholar]
- 73.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cu-Uvin S, Hogan JW, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract.1. Clin Infect Dis. 2001;33(6):894–896 [DOI] [PubMed] [Google Scholar]
- 75.Mcclelland RS, Wang CC, Mandaliya K, et al. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15(1):105–110 [DOI] [PubMed] [Google Scholar]
- 76.Wang CC, McClelland RS, Reilly M, et al. The effect of treatment of vaginal infections on shedding of human immunodeficiency virus type 1. J Infect Dis. 2001;183(7):1017–1022 [DOI] [PubMed] [Google Scholar]
- 77.Dyer JR, Eron JJ, Hoffman IF, et al. Association of CD4 cell depletion and elevated blood and seminal plasma human immunodeficiency virus type 1 (HIV-1) RNA concentrations with genital ulcer disease in HIV-1-infected men in Malawi. J Infect Dis. 1998;177(1):224–227 [DOI] [PubMed] [Google Scholar]
- 78.Wawer MJ, Sewankambo NK, Serwadda D, et al. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Rakai Project Study Group. Lancet. 1999;353(9152):525–535 [DOI] [PubMed] [Google Scholar]
- 79.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185(1):45–52 [DOI] [PubMed] [Google Scholar]
- 80.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358(15):1560–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9630):2109–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor S, Cane P, Workman J, Drake S, Shamanesh M, Pillay D. Identification of a Transmission Chain of HIV-1 Drug Resistant Virus. AIDS Res Hum Retroviruses. 2003;19(5):353–361 [DOI] [PubMed] [Google Scholar]
- 83.Little S, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347(6):385–394 [DOI] [PubMed] [Google Scholar]
- 84.Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002;288(2):181–188 [DOI] [PubMed] [Google Scholar]
- 85.Turner D, Brenner B, Routy JP, et al. Diminished representation of HIV-1 variants containing select drug resistance-conferring mutations in primary HIV-1 infection. J Acquir Immune Defic Syndr. 2004;37(5):1627–1631 [DOI] [PubMed] [Google Scholar]
- 86.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180 [DOI] [PubMed] [Google Scholar]
- 87.Tsai CC, Follis KE, Sabo A, et al. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270(5239):1197–1199 [DOI] [PubMed] [Google Scholar]
- 88.Tsai CC, Emau P, Follis KE, et al. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl) adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72(5):4265–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337(21):1485–1490 [DOI] [PubMed] [Google Scholar]
- 90.US Public Health Service Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1–52 [PubMed] [Google Scholar]
- 91.Schechter M, do Lago RF, Mendelsohn AB, et al. Behavioral impact, acceptability, and HIV incidence among homosexual men with access to postexposure chemoprophylaxis for HIV. J Acquir Immune Defic Syndr. 2004;35(5):519–525 [DOI] [PubMed] [Google Scholar]
- 92.Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr. 2005;39(1):82–89 [DOI] [PubMed] [Google Scholar]
- 93.Roland ME, Neilands TB, Krone MR, et al. Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clin Infect Dis. 2005;41(10):1507–1513 [DOI] [PubMed] [Google Scholar]
- 94.Guest G, Shattuck D, Johnson L, et al. Changes in sexual risk behavior among participants in a PrEP HIV prevention trial. Sex Transm Dis. 2008;35(12):1002–1008 [PubMed] [Google Scholar]
- 95.Smith DK, Grohskopf LA, Black RJ, et al. Antiretroviral postexposure prophylaxis after sexual, injection-drug use, or other nonoccupational exposure to HIV in the United States: recommendations from the US Department of Health and Human Services. MMWR Recomm Rep. 2005;54(RR-2):1–20 [PubMed] [Google Scholar]
- 96.Vourvahis M, Tappouni HL, Patterson KB, et al. The pharmacokinetics and viral activity of tenofovir in the male genital tract. J Acquir Immune Defic Syndr. 2008;47(3):329–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mayer KH, Mimiaga MJ, Cohen D, et al. Tenofovir DF plus lamivudine or emtricitabine for nonoccupational postexposure prophylaxis (NPEP) in a Boston Community Health Center. J Acquir Immune Defic Syndr. 2008;47(4):494–499 [DOI] [PubMed] [Google Scholar]
- 98.Bassett IV, Freedberg KA, Walensky RP. Two drugs or three? Balancing efficacy, toxicity, and resistance in postexposure prophylaxis for occupational exposure to HIV. Clin Infect Dis. 2004;39(3):395–401 [DOI] [PubMed] [Google Scholar]
- 99.Mayer K, Mimiaga M, Gelman M, Trufant J, Maynard S, McMorrow P. Tenofovir DF/emtricitabine/raltegravir (TDF/FTD/RAL) appears safe and well-tolerated for non-occupational post-exposure prophylaxis (NPEP). : Proceedings of the 5th IAS Conference on HIV Pathogenesis, Treatment, and Prevention, Cape Town, South Africa, July 19–22, 2009. Abstract WEAC104 [Google Scholar]
- 100.Dumond J, Patterson K, Pecha A, et al. Maraviroc (MRV) genital tract (GT) fluid and tissue pharmacokinetics (PK) in healthy female volunteers: implications for pre- or post-exposure prophylaxis (PrEP or PEP). Paper presented at: 15th Conference on Retroviruses and Opportunistic Infections (CROI); February 3–6, 2008; Boston, MA: Available at: http://retroconference.org/2008. Accessed June 6, 2009 [Google Scholar]
- 101.Dobard C, Parikh U, Sharma S, et al. Complete protection against repeated vaginal simian HIV exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC. Available at: http://retroconference.org/2009. Accessed June 7, 2010
- 102.Garcia-Lerma G, Cong ME, Mitchell J, et al. Prevention of rectal simian HIV transmission in macaques by intermittent pre-exposure prophylaxis with oral Truvada. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC. Available at: http://retroconference.org/2009. Accessed June 7, 2010
- 103.Cohen MS, Kashuba AD. Antiretroviral therapy for prevention of HIV infection: new clues from an animal model. PLoS Med. 2008;5(2):e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Grant RM, Buchbinder S, Cates W, Jr, et al. AIDS. Promote HIV chemoprophylaxis research, don't prevent it. Science. 2005;309(5744):2170–2171 [DOI] [PubMed] [Google Scholar]
- 105.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS Clin Trials. 2007;2(5):e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peterson L, Taylor D, Clarke EEK. Findings from a double-blind, randomized, placebo-controlled trial of tenofovir disoproxil fumarate (TDF) for prevention of HIV infection in women. Paper presented at: XVI International AIDS Conference; August 13–18, 2006; Toronto, ON. Available at: http://www.aids2006.org. Accessed June 6, 2009 [DOI] [PMC free article] [PubMed]
- 107.Grohskopf L, Gvetadze R, Pathak S, et al. Preliminary Analysis of Biomedical Data From the Phase II Clinical Safety Trial of Tenofovir Disoproxil Fumarate (TDF) for HIV-1 Pre-Exposure Prophylaxis (PrEP) Among US Men Who Have Sex With Men (MSM). Paper presented at: XVIII International AIDS Conference; July 18–23, 2010; Vienna, Austria [Google Scholar]
- 107a.Mayer KH, Maslankowski LA, Gai F, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20(4):543–551 [DOI] [PubMed] [Google Scholar]
- 107b.Abdool Karim Q, Abdool Karim SS. CAPRISA 004 Effectiveness and safety of vaginal microbicide 1% tenofovir gel for prevention of HIV infection in women. Paper presented at: XVIII International AIDS Conference; July 18–23, 2010; Vienna, Austria [Google Scholar]
- 108.Ayudhya U, Hopkins N, Cost M, Billitto N, Rooney J, Dezzutti C. Microbicide, tenofovir 1% gel, efficacy determined for pre- and post-coital. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC. Available at: http://retroconference.org/2009. Accessed June 7, 2010
- 109.Hillier S. Pre-exposure prophylaxis: could it work? Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC. Available at: http://retroconference.org/2009. Accessed June 7, 2010
- 110.Abdool Karim S, Coletti A, Richardson B, et al. Safety and effectiveness of vaginal microbicides buffer gel and 0.5% PRO 2000/5 gel for the prevention of HIV infection in women: results of the HPTN 035 trial. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC. Available at: http://retroconference.org/2009. Accessed June 7, 2010
- 111.Barditch-Crovo P, Deeks SG, Collier A, et al. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob Agents Chemother 2001;45(10):2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Rompay KK, Johnson JA, Blackwood EJ, et al. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology. 2007;4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194(7):904–911 [DOI] [PubMed] [Google Scholar]
- 114.Garcia-Lerma J. Prevention of rectal SHIV tranmission in macaques by tenofovir/FTC combination. Paper presented at: 13th Annual Conference on Retroviruses and Opportunistic Infections; February 5–8, 2006; Denver, CO. Available at: http://retroconference.org/2006. Accessed June 6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupta R, Hill A, Sawyer AW, Pillay D. Emergence of drug resistance in HIV type 1-infected patients after receipt of first-line highly active antiretroviral therapy: a systematic review of clinical trials. Clin Infect Dis. 2008;47(5):712–722 [DOI] [PubMed] [Google Scholar]
- 116.Brenner BG, Oliveira M, Doualla-Bell F, et al. HIV-1 subtype C viruses rapidly develop K65R resistance to tenofovir in cell culture. AIDS. 2006;20(9):F9–F13 [DOI] [PubMed] [Google Scholar]
- 117.Smith D, Kebaabetwse P, Disasi K, Fleming D, Paxton L, David M. Antiretroviral resistance is not an important risk of the oral tenofovir prophylaxis trial in Botswana: a simple mathematical modelling approach. Paper presented at: XVI International AIDS Conference; August 13–18, 2006; Toronto, Canada. Available at: http://www.aids2006.org. Accessed June 6, 2009 [Google Scholar]
- 118.Buchbinder S. HIV prevention. Paper presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8–11, 2009; Montreal, QC. Available at: http://retroconference.org/2009. Accessed June 7, 2010
- 119.Gallant JE. Drug resistance after failure of initial antiretroviral therapy in resource-limited countries. Clin Infect Dis. 2007;44(3):453–455 [DOI] [PubMed] [Google Scholar]
- 120.Van Rompay KK, Matthews TB, Higgins J, et al. Virulence and reduced fitness of simian immunodeficiency virus with the M184V mutation in reverse transcriptase. J Virol. 2002;76(12):6083–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kellerman SE, Hutchinson AB, Begley EB, Boyett BC, Clark HA, Sullivan P. Knowledge and use of HIV pre-exposure prophylaxis among attendees of minority gay pride events, 2004. J Acquir Immune Defic Syndr. 2006;43(3):376–377 [DOI] [PubMed] [Google Scholar]
- 122.Mimiaga MJ, Case P, Johnson CV, Safren SA, Mayer KH. Preexposure antiretroviral prophylaxis attitudes in high-risk Boston area men who report having sex with men: limited knowledge and experience but potential for increased utilization after education. J Acquir Immune Defic Syndr. 2009;50(1):77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu AY, Kittredge PV, Vittinghoff E, et al. Limited knowledge and use of HIV post- and pre-exposure prophylaxis among gay and bisexual men. J Acquir Immune Defic Syndr. 2008;47(2):241–247 [PubMed] [Google Scholar]
- 124.Global Advocacy for HIV Prevention AVAC Report 2009. Part of the solution: setting expectations for WHO and UNAIDS. Available at: http://www.avac.org/ht/d/sp/a/GetDocumentAction/i/2275. Accessed December 26, 2009
- 125.Bennett DE, Bertagnolio S, Sutherland D, Gilks CF. The World Health Organization's global strategy for prevention and assessment of HIV drug resistance. Antivir Ther. 2008;13(suppl 2):1–13 [PubMed] [Google Scholar]
- 126.World Health Organization Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents. Available at: http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. Published 2009. Accessed February 1, 2010 [PubMed]
- 127.Walensky RP, Kuritzkes DR. The impact of the President's Emergency Plan for AIDS Relief (PEPfAR) beyond HIV and why it remains essential. Clin Infect Dis. 2010;50(2):272–275 [DOI] [PubMed] [Google Scholar]
- 128.Law MG, Prestage G, Grulich A, Van de Ven P, Kippax S. Modelling the effect of combination antiretroviral treatments on HIV incidence. AIDS. 2001;15(10):1287–1294 [DOI] [PubMed] [Google Scholar]
- 129.Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral chemoprophylaxis on HIV-1 transmission in resource-limited settings. PLoS One. 2007;2(9):e875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372(9639):669–684 [DOI] [PMC free article] [PubMed] [Google Scholar]