Abstract
Objectives. We examined associations between the socioeconomic characteristics of census tracts and racial/ethnic disparities in the incidence of bacteremic community-acquired pneumonia among US adults.
Methods. We analyzed data on 4870 adults aged 18 years or older with community-acquired bacteremic pneumonia identified through active, population-based surveillance in 9 states and geocoded to census tract of residence. We used data from the 2000 US Census to calculate incidence by age, race/ethnicity, and census tract characteristics and Poisson regression to estimate rate ratios (RRs) and 95% confidence intervals (CIs).
Results. During 2003 to 2004, the average annual incidence of bacteremic pneumonia was 24.2 episodes per 100 000 Black adults versus 10.1 per 100 000 White adults (RR = 2.40; 95% CI = 2.24, 2.57). Incidence among Black residents of census tracts with 20% or more of persons in poverty (most impoverished) was 4.4 times the incidence among White residents of census tracts with less than 5% of persons in poverty (least impoverished). Racial disparities in incidence were reduced but remained significant in models that controlled for age, census tract poverty level, and state.
Conclusions. Adults living in impoverished census tracts are at increased risk of bacteremic pneumonia and should be targeted for prevention efforts.
Bacterial pneumonia is an important cause of morbidity and mortality in adults1 and is a potentially serious complication of influenza infection.2,3 Racial/ethnic disparities in the incidence of bacterial pneumonia are a public health problem. Among US adults, rates of bacteremic pneumonia caused by Streptococcus pneumoniae (pneumococcus), the leading cause of community-acquired pneumonia, are higher among Blacks than among Whites.4 Vaccination against pneumococcal disease is recommended for persons aged 65 years and older, for cigarette smokers, and for persons with certain chronic conditions.5 Vaccination coverage with 23-valent pneumococcal polysaccharide vaccine is lower, however, among Hispanic and non-Hispanic Black adults with indications for vaccination than among non-Hispanic White adults.6,7 The introduction of pneumococcal conjugate vaccination for children in 2000 led to lower pneumococcal disease incidence in all age groups8,9 and narrowed the difference between incidence in Black and White children, although disparities persist among adults.10,11 Less is known about the factors influencing racial/ethnic disparities in the incidence of bacteremic pneumonia caused by other common pathogens. A better understanding of the underlying factors that sustain disparities in bacterial disease would help to direct prevention efforts.
Several previous studies examined the contributions of socioeconomic inequalities to racial/ethnic disparities in invasive pneumococcal disease.12–15 Between 1960 and 1970, a series of population-based studies in US communities identified socioeconomic measures, including average income and education, as strongly associated with both race/ethnicity and bacterial meningitis incidence.16–18 However, socioeconomic data have not routinely been collected in US surveillance systems for invasive bacterial disease. With geocoding software, individual cases can be mapped to census tract of residence.15 Census tract–level socioeconomic measures can then be included in analyses of health outcomes to better understand racial/ethnic disparities in disease incidence.19 We used a large population-based surveillance system for invasive bacterial infections to examine associations between the socioeconomic characteristics of census tracts and racial/ethnic disparities in the incidence of bacteremic community-acquired pneumonia among US adults. We sought to identify a single, publicly available census tract characteristic that could be used to target interventions to geographic areas likely to have high incidences of bacteremic pneumonia and to monitor inequalities in disease burden.
METHODS
Active, population-based surveillance for invasive bacterial disease caused by selected pathogens was conducted through the US Centers for Disease Control and Prevention's Active Bacterial Core surveillance (ABCs)/Emerging Infections Program network. Invasive bacterial disease is defined as the isolation of pathogenic bacteria from normally sterile sites, including blood, cerebrospinal fluid, or pleural fluid. ABCs personnel regularly contact all clinical laboratories serving the residents of the surveillance population to identify cases, and periodic audits of laboratory records are conducted to ensure complete case ascertainment. Clinical and demographic data, including residence address, are collected from medical records for all episodes of invasive bacterial disease caused by specific pathogens among residents of the surveillance area.
Data for this analysis were limited to episodes of physician-diagnosed pneumonia among adults 18 years or older for which S. pneumoniae, Haemophilus influenzae, group A streptococcus, or group B streptococcus was isolated from blood or pleural fluid. We included only the first episode of bacterial pneumonia caused by each pathogen in an individual patient during the surveillance period. Bacterial pneumonia cases with a date of culture more than 2 days after hospital admission (approximately 3% of all cases) were considered health care–associated and were excluded from the analysis. Analyses included episodes with onset during 2003 or 2004 in 9 surveillance sites: Connecticut (entire state), Minnesota (entire state), California (1 county), Colorado (5 counties), Georgia (20 counties), Maryland (6 counties), New York (15 counties), Oregon (3 counties), and Tennessee (11 counties). According to the 2000 US Census20, the 9 surveillance areas included 18.1 million adults aged 18 years or older (8.7% of the US adult population), including approximately 13.7 million non-Hispanic White adults, 2.4 million non-Hispanic Black adults, 1.0 million Hispanic adults, 670 000 Asian adults, and 75 000 American Indian or Alaska Native adults. Approximately 227 000 adults (1.3% of adult surveillance area residents) were of other racial/ethnic groups.20
Race or ethnicity was identified in medical records for 91% of the patients according to 2000 US Census categories, ranging from 62% in Oregon to more than 99% in Connecticut. Patients with missing ethnicity (53%) were assumed to be non-Hispanic. Patients were identified as HIV-infected if HIV or AIDS was recorded in the medical charts. For analyses on the subset of non–HIV infected patients, we excluded all patients from New York State, which does not report HIV status.
Geocoding
According to the US Census Bureau, census tracts were designed to be relatively permanent statistical subdivisions whose boundaries are nested within counties and are delineated by using visible features expected to be stable over many decades. At the time they are established, census tracts are designed to be homogeneous with regard to socioeconomic indicators and generally include between 1000 and 8000 persons (with an optimum size of 4000 persons).21 For the 2000 US Census, surveillance areas included in this analysis were divided into 5553 census tracts (Minnesota [1303 census tracts], Connecticut [819], Georgia [660], Tennessee [616], Maryland [615], New York [537], Colorado [514], Oregon [313], and California [176]). Census tract of residence was determined for 4524 (93%) of 4870 adult surveillance area residents who experienced an episode of invasive bacterial pneumonia in 2003 or 2004. Census tract of residence was determined by using home addresses from medical records and using geocoding software including ArcView/ArcGIS (ESRI, Redlands, CA), MapMarker (Pitney Bowes MapInfo Inc, Troy, NY), and Address Object/GeoCoder Object (Melissa DATA Corp, Rancho Santa Margarita, CA). Geocoding was performed at Emerging Infections Program sites. Geocoding success ranged from 86% for patients in the California surveillance area to 98% in Tennessee surveillance areas. Overall, geocoding success ranged from 93% to 94% for Whites, Blacks, and Hispanics and was 89% for patients of unknown race/ethnicity; geocoding success ranged from 92% to 94% across age groups.
Area-Based Socioeconomic Measures
For each census tract in the surveillance area, 11 socioeconomic variables were created with 2000 US Census data. Socioeconomic variables were defined in accordance with the Public Health Disparities Geocoding Project.22 Percentage of census tract poverty was defined as the percentage of the census tract's population living below the federal poverty level and was entered as a continuous variable or was stratified into 4 levels: 0% to 4.9%, 5.0% to 9.9%, 10.0% to 19.9%, and 20% or more of the population (the federally defined threshold for “poverty area” designation). Percentage of census tract crowding (households with at least 1 person per room) and percentage of expensive homes (houses worth at least 400% of the US median value of owner-occupied homes) were stratified by using the same 4 levels (0% to 4.9%, 5.0% to 9.9%, 10.0% to 19.9%, and 20% or more). Percentage of the census tract population with low education (persons aged 25 years or older with less than a 12th-grade education) and with high education (persons aged 25 years or older with at least 4 years of college education) were each grouped into 4 levels (0% to 14.9%, 15.0% to 24.9%, 25.0% to 39.9%, and 40% or more). The percentage of the census tract population employed in working-class occupations was categorized into 4 levels (0% to 49.9%, 50.0% to 64.9%, 65.0% to 74.9%, and 75% or more). Census tracts were stratified into quintiles for median household income, percentage of low-income households (income less than 50% of the US median household income), and percentage of high-income households (income at least 400% of the US median household income). Census tract quintiles were also created for the modified Townsend index (a composite measure of deprivation, standardized to the US population, that includes percentage crowding, percentage unemployment, percentage no car ownership, and percentage renters). The percentage of the census tract population living in rural areas was dichotomized as 0% to 49.9% or 50% or more.
Statistical Analysis
The annual incidence rate of invasive bacterial pneumonia in each census tract was estimated by dividing the average number of cases in 2003 and 2004 by the number of adult census tract residents, according to age group and racial/ethnic category from 2000 US Census data. Rate ratios (RRs) and 95% confidence intervals (CIs) were calculated by using Poisson regression. For Poisson regression models, the dependent variable was the number of cases in each census tract for each age group and racial/ethnic category. Independent variables included age group, racial/ethnic category, surveillance site, and a census tract–level socioeconomic measure. In models containing a socioeconomic indicator variable, we examined all 2-way interactions between age group, racial/ethnic category, and the census tract–level socioeconomic measure. Population denominator offsets were calculated for each combination of age category, racial/ethnic category, and census tract. To permit examination of the independent effects of age group, racial/ethnic category, and area-based socioeconomic measure on the incidence of bacteremic pneumonia, rates were not age-standardized, as was done by the Public Health Disparities Geocoding Project.19 To identify the single area-based socioeconomic measure that best explained observed racial disparities in the incidence of bacteremic pneumonia (with control for age group and ABCs site), we compared RRs for incidence among Black versus White adults in a base model (including age group, state, and race/ethnicity) with 11 independent models each containing 1 of the socioeconomic indicator variables in addition to the variables included in the base model. We used the Akaike Information Criterion (AIC) to compare the goodness of fit of independent Poisson models including different census tract–level socioeconomic measures.23 Results were not substantially different when using negative binomial regression to account for clustering of cases within census tracts or when using multilevel Poisson and negative binomial models with census tracts treated as random effects.24 We present the results from the Poisson models. Statistical analyses were conducted with SAS Version 9.1 (SAS Institute Inc., Cary, NC). Significance was defined as a 2-tailed P < .05.
RESULTS
We identified 4870 cases of invasive community-acquired bacterial pneumonia among adults aged 18 years or older in 9 US surveillance areas in 2003 and 2004. Sixty-one percent of the patients identified were non-Hispanic White, 25% were non-Hispanic Black, 3% were Hispanic, 2% were other racial/ethnic categories, and 9% were of unknown race/ethnicity. Males accounted for 51% of the patients. S. pneumoniae was isolated in 4006 (82%) episodes, H. influenzae in 337 (7%), group B streptococcus in 281 (6%), and group A streptococcus in 246 (5%). Because restricting the analyses to cases of pneumococcal pneumonia yielded similar results (data not presented), we present only the combined results of all 4 causes of bacteremic pneumonia. Twelve percent of the patients had documented HIV infection (excluding patients from New York State).
The average annual incidence of bacteremic pneumonia during the 2-year period was 12.6 episodes per 100 000 adults. Incidence varied significantly among surveillance areas, ranging from 8.4 episodes per 100 000 in Minnesota to 19.8 episodes per 100 000 in Maryland.
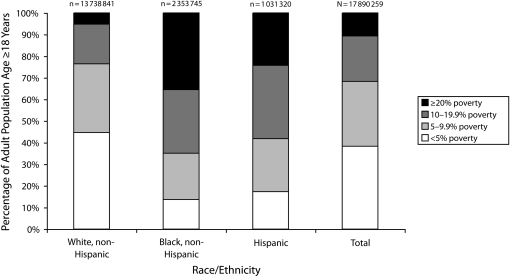
We identified cases of bacteremic pneumonia among residents of 2727 (49%) of 5553 census tracts (range of cases per census tract, 1–15) in all surveillance areas. The distribution of adults in ABCs surveillance areas by race/ethnicity and census tract–level percentage of persons living in poverty, according to the 2000 US Census, is shown in Figure 1. Forty-five percent of the White population versus less than 20% of Blacks and Hispanics resided in census tracts with less than 5% of persons living in poverty; 36% of Blacks versus 24% of Hispanics and 5% of Whites lived in the most impoverished census tracts. White adults in census tracts with less than 5% of persons living in poverty accounted for 34% of the surveillance population but for only 23% of cases; Blacks and Hispanics in census tracts with 20% or more of persons living in poverty together accounted for only 6% of the population but for 15% of cases.
FIGURE 1.
Distribution of the adult population according to percentage of census tract poverty, by race/ethnicity: Active Bacterial Core surveillance areas, United States, 2000.
Note. Percentage of census tract poverty was defined according to the percentage of persons living below the federal poverty threshold according to the 2000 US Census.20 The total adult population included all racial/ethnic groups except multiracial categories.
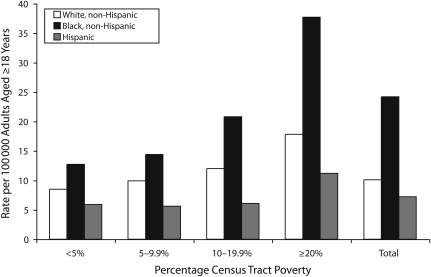
The incidence of bacteremic pneumonia by census tract poverty level, age category, and racial/ethnic category is shown in Table 1. Incidence generally increased with increasing age. Among adults aged 18 to 79 years in all racial/ethnic groups, the incidence of bacteremic pneumonia tended to be highest among persons living in census tracts in which 20% or more of the population lived below the poverty level. At all levels of census tract poverty, Blacks had a higher overall incidence of bacteremic pneumonia than did Whites and Hispanics (Figure 2). The RR comparing the incidence between Black adults and White adults was 2.40 (95% CI = 2.24, 2.57). Overall incidence rates among Hispanic adults generally were similar to rates among non-Hispanic Whites. When the analyses were restricted to bacteremic pneumonia caused by H. influenzae, group A streptococcus, and group B streptococcus, the same pattern was observed of a higher incidence in Blacks than in Whites and in census tracts with 20% or more of persons living in poverty than in those with less than 5% of persons living in poverty (data not presented).
TABLE 1.
Incidence of Bacteremic Pneumonia in Adults According to Race/Ethnicity, Age, and Proportion of Residents Living in Poverty: ABCs Areas, United States, 2003–2004
| Race/Ethnicity by Age |
Population Count |
No. of Casesa |
Incidence of Bacteremic Pneumonia per 100 000 Peopleb | ||||
| < 5% Poverty |
5%–9.9% Poverty |
10%–19.9% Poverty |
≥ 20% Poverty |
Overall |
|||
| Total adult populationc | 17 890 259 | 4 524 | 9.4 | 11.1 | 14.1 | 26.2 | 12.6 |
| White, non-Hispanic | 13 738 841 | 2 767 | 8.5 | 9.9 | 12.0 | 17.8 | 10.1 |
| Black, non-Hispanic | 2 353 745 | 1 140 | 12.7 | 14.4 | 20.8 | 37.7 | 24.2 |
| Hispanic | 1 031 320 | 148 | 5.9 | 5.6 | 6.1 | 11.2 | 7.2 |
| Adults aged 18–34 y | 5 795 245 | 341 | 1.9 | 2.5 | 3.0 | 5.9 | 2.9 |
| White, non-Hispanic | 3 970 653 | 141 | 1.6 | 1.9 | 1.9 | 2.2 | 1.8 |
| Black, non-Hispanic | 929 317 | 142 | 2.6 | 5.0 | 7.5 | 11.4 | 7.6 |
| Hispanic | 572 531 | 31 | 4.3 | 2.6 | 1.4 | 3.8 | 2.7 |
| Adults aged 35–49 y | 5 858 541 | 1 061 | 3.9 | 6.6 | 10.6 | 39.6 | 9.1 |
| White, non-Hispanic | 4 523 687 | 439 | 3.1 | 5.3 | 6.4 | 18.0 | 4.9 |
| Black, non-Hispanic | 804 471 | 487 | 13.4 | 14.8 | 21.2 | 58.1 | 30.3 |
| Hispanic | 291 768 | 45 | 0.8 | 3.5 | 9.9 | 16.2 | 7.7 |
| Adults aged 50–64 y | 3 535 963 | 1 152 | 10.6 | 13.4 | 20.3 | 47.3 | 16.3 |
| White, non-Hispanic | 2 902 523 | 684 | 9.2 | 11.2 | 14.6 | 37.5 | 11.8 |
| Black, non-Hispanic | 390 922 | 315 | 22.2 | 24.5 | 36.8 | 58.7 | 40.3 |
| Hispanic | 114 049 | 31 | 10.3 | 12.4 | 11.8 | 19.9 | 13.6 |
| Adults aged 65–79 y | 1 960 136 | 1 027 | 24.5 | 23.0 | 30.2 | 34.7 | 26.2 |
| White, non-Hispanic | 1 678 195 | 759 | 21.9 | 19.3 | 26.8 | 34.8 | 22.6 |
| Black, non-Hispanic | 177 412 | 114 | 24.8 | 27.9 | 36.3 | 32.6 | 32.1 |
| Hispanic | 42 494 | 29 | 19.1 | 35.5 | 27.8 | 50.8 | 34.1 |
| Adults aged ≥ 80 y | 740 374 | 943 | 69.2 | 61.1 | 61.4 | 59.8 | 63.7 |
| White, non-Hispanic | 663 783 | 744 | 62.7 | 53.5 | 50.3 | 55.2 | 56.0 |
| Black, non-Hispanic | 51 623 | 82 | 105.7 | 84.1 | 114.9 | 55.8 | 79.4 |
| Hispanic | 10 478 | 12 | 141.3 | 16.7 | 62.2 | 40.1 | 57.3 |
Note. ABCs = Active Bacterial Core surveillance.
Number of successfully geocoded cases.
Incidence is the average annual rate by census tract poverty level. Census tract poverty level was defined according to the percentage of persons living below the federal poverty threshold per the 2000 US Census.20 Incidence rates are not age-adjusted.
Total adult population included all racial/ethnic groups.
FIGURE 2.
Average annual incidence of invasive bacteremic pneumonia in adults, by race/ethnicity and percentage of census tract poverty: Active Bacterial Core surveillance areas, United States, 2003–2004.
Note. Percentage of census tract poverty was defined according to the percentage of persons living below the federal poverty threshold according to the 2000 US Census.20
Incidence among Blacks residing in the most impoverished census tracts was 4.44 times that among Whites in the least impoverished census tracts, 2.12 times that among Whites in the most impoverished census tracts, and 2.97 times that among Blacks in the least impoverished census tracts. In each of the 9 surveillance areas, Blacks had a significantly higher incidence of bacteremic pneumonia than did Whites in models including age groups. In the multivariate analysis of factors associated with the incidence of bacteremic pneumonia, the RR for Blacks compared with Whites was 2.61 (95% CI = 2.42, 2.81) in the model that included age category, racial/ethnic category, and surveillance area. When percentage of census tract poverty was added to the model as a categorical variable (Table 2), the RR for Blacks compared with Whites declined 50% to 1.84 (95% CI = 1.69, 2.00). When percentage of census tract poverty was alternatively added as a continuous variable, the RR for Blacks compared with Whites also declined 50% to 1.83 (95% CI = 1.68, 2.00). In the full multivariable model (Table 2), significant independent, positive associations were also observed between the incidence of bacteremic pneumonia and increasing age and increasing percentage of census tract poverty, respectively. The incidence of bacteremic pneumonia did not differ significantly for Whites and Hispanics when the analysis controlled for other factors. Restricting the analysis to patients without HIV infection resulted in lower RRs for Blacks versus Whites before (1.84; 95% CI = 1.68, 2.01) and after (1.45; 95% CI = 1.31, 1.60) control for percentage of census tract poverty.
TABLE 2.
Results of Multivariable Poisson Regression Analysis of Invasive Bacterial Pneumonia Incidence in Adults, by Age, Race/Ethnicity, and Census Tract–Level Socioeconomic Measures: ABCs Areas, United States, 2003–2004
| Variable |
Model 1, ARR (95% CI) |
Model 2, ARR (95% CI) |
Model 3, ARR (95% CI) |
Model 4, ARR (95% CI) |
| Age, y | ||||
| 18–34 (Ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| 35–49 | 3.15 (2.78, 3.58) | 3.30 (2.91, 3.75) | 3.34 (2.95, 3.79) | 3.34 (2.94, 3.80) |
| 50–64 | 5.74 (5.05, 6.52) | 5.92 (5.21, 6.72) | 5.98 (5.28, 6.77) | 6.04 (5.32, 6.86) |
| 65–79 | 9.34 (8.21, 10.63) | 9.27 (8.15, 10.56) | 9.21 (8.11, 10.46) | 9.48 (8.33, 10.79) |
| ≥ 80 | 23.73 (20.81, 27.07) | 23.02 (20.18, 26.25) | 22.40 (19.69, 25.50) | 23.15 (20.30, 26.40) |
| Race/ethnicity | ||||
| White, non-Hispanic (Ref) | 1.00 | 1.00 | 1.00 | 1.00 |
| Black, non-Hispanic | 2.61 (2.42, 2.81) | 1.84 (1.69, 2.00) | 1.93 (1.77, 2.09) | 1.94 (1.78, 2.11) |
| Hispanic | 1.12 (0.94, 1.33) | 0.87 (0.73, 1.04) | 0.91 (0.76, 1.08) | 0.89 (0.75, 1.06) |
| Percentage of census tract povertya | ||||
| < 5 (Ref) | 1.00 | |||
| 5–9.9 | 1.17 (1.07, 1.27) | |||
| 10–19.9 | 1.45 (1.32, 1.58) | |||
| ≥ 20 | 2.39 (2.16, 2.64) | |||
| Median incomeb | ||||
| Lowest quintile | 2.45 (2.19, 2.74) | |||
| Second quintile | 1.67 (1.49, 1.86) | |||
| Third quintile | 1.50 (1.34, 1.67) | |||
| Fourth quintile | 1.27 (1.14, 1.41) | |||
| Highest quintile (Ref) | 1.00 | |||
| Townsend indexb | ||||
| Lowest quintile (Ref) | 1.00 | |||
| Second quintile | 1.15 (1.03, 1.29) | |||
| Third quintile | 1.25 (1.12, 1.40) | |||
| Fourth quintile | 1.50 (1.34, 1.67) | |||
| Highest quintile | 2.17 (1.94, 2.43) |
Note. ABCs = Active Bacterial Core surveillance; ARR = adjusted rate ratio; CI = confidence interval. All models included age category, racial/ethnic category, and surveillance site.
Percentage of census tract poverty was defined according to the percentage of persons living below the federal poverty threshold set by the 2000 US Census.20
Defined at the census tract level.
We also considered the effect on the RR comparing the incidence of bacteremic pneumonia between Blacks and Whites of including different area-based socioeconomic measures in separate models (supplemental Figure A, available as a supplement to the online version of this article at http://www.ajph.org). Rate ratios comparing the incidence among Black adults with the incidence among White adults were significantly lower when an area-based socioeconomic measure was included in models, with the exception of models that included percentage expensive homes or percentage rural. The reduction in the Black to White RR was greatest in the model including percentage of census tract poverty, followed by the model with median income category (adjusted RR = 1.93; 95% CI = 1.77, 2.09) and the model including the modified Townsend index (adjusted RR = 1.94; 95% CI = 1.78, 2.11; Table 2). According to the goodness-of-fit measure (lowest AIC score), the model including census tract poverty level explained more of the variance in disease rates than did the base model or any of the other 10 models that included a census tract–level socioeconomic measure. In each of the 9 surveillance populations, Black to White RRs were lower in models including census tract poverty level; the RR was significantly different for the Maryland surveillance area (Black to White RR in the model without percentage of census tract poverty = 3.18; 95% CI = 2.75, 3.68; Black to White RR in the model including percentage of census tract poverty = 1.89; 95% CI = 1.58, 2.27).
The percentage of the incidence of bacteremic pneumonia among Blacks that would be eliminated if they had the same risk as Whites was 58.3%. Similarly, 64.1% of the incidence of bacteremic pneumonia among adults in the most impoverished census tracts would be eliminated if these adults had the same risk as those in the least impoverished census tracts.
In the full multivariable model including age category, racial/ethnic category, census tract poverty level, and surveillance area, there were significant 2-way interactions between race/ethnicity and age group and between census tract poverty level and age group. Differences in the incidence of bacteremic pneumonia between Blacks and Whites and across census tract poverty levels were lower among adults aged 65 years or older than in adults aged 18 to 64 years (Table 3). Similar 2-way interactions were observed in models that included other census tract–level socioeconomic indicator variables, such as median income or the Townsend index (data not shown). There were no significant 2-way interactions between race/ethnicity and census tract–level socioeconomic measures.
TABLE 3.
Results of Multivariable Poisson Regression Analysis of Invasive Bacterial Pneumonia Incidence in Adults, by Age, Race/Ethnicity, and Percentage of Census Tract Poverty: ABCs Areas, United States, 2003–2004
| Variable by Age |
ARR (95% CI) |
| Race/Ethnicity | |
| Adults aged 18–34 y | |
| White, non-Hispanic (Ref) | 1.00 |
| Black, non-Hispanic | 2.93 (2.26, 3.79) |
| Hispanic | 1.20 (0.80, 1.79) |
| Adults aged 35–49 y | |
| White, non-Hispanic (Ref) | 1.00 |
| Black, non-Hispanic | 2.66 (2.28, 3.11) |
| Hispanic | 0.87 (0.63, 1.19) |
| Adults aged 50–64 y | |
| White, non-Hispanic (Ref) | 1.00 |
| Black, non-Hispanic | 1.70 (1.44, 1.99) |
| Hispanic | 0.70 (0.48, 1.02) |
| Adults aged 65–79 y | |
| White, non-Hispanic (Ref) | 1.00 |
| Black, non-Hispanic | 0.93 (0.74, 1.16) |
| Hispanic | 1.15 (0.79, 1.68) |
| Adults aged ≥ 80 y | |
| White, non-Hispanic (Ref) | 1.00 |
| Black, non-Hispanic | 1.21 (0.93, 1.56) |
| Hispanic | 0.66 (0.34, 1.27) |
| Percentage of census tract povertya | |
| Adults aged 18–34 y | |
| < 5% (Ref) | 1.00 |
| 5%–9.9% | 1.16 (0.82, 1.63) |
| 10%–19.9% | 1.29 (0.91, 1.81) |
| ≥ 20% | 2.06 (1.45, 2.91) |
| Adults aged 35–49 y | |
| < 5% (Ref) | 1.00 |
| 5%–9.9% | 1.61 (1.31, 1.97) |
| 10%–19.9% | 2.13 (1.73, 2.62) |
| ≥ 20% | 5.69 (4.64, 6.99) |
| Adults aged 50–64 y | |
| < 5% (Ref) | 1.00 |
| 5%–9.9% | 1.28 (1.08, 1.52) |
| 10%–19.9% | 1.74 (1.46, 2.09) |
| ≥ 20% | 3.37 (2.77, 4.10) |
| Adults aged 65–79 y | |
| < 5% (Ref) | 1.00 |
| 5%–9.9% | 0.98 (0.83, 1.16) |
| 10%–19.9% | 1.35 (1.14, 1.61) |
| ≥ 20% | 1.51 (1.19, 1.92) |
| Adults aged ≥ 80 y | |
| < 5% (Ref) | 1.00 |
| 5%–9.9% | 0.92 (0.78, 1.08) |
| 10%–19.9% | 0.91 (0.76, 1.09) |
| ≥ 20% | 0.77 (0.58, 1.02) |
Note. ABCs = Active Bacterial Core surveillance; ARR = adjusted rate ratio; CI = confidence interval. The model included the main effects of age category, racial/ethnic category, percentage of census tract poverty, and surveillance site, and 2-way interactions between age and race/ethnicity and between age and percentage of census tract poverty.
Percentage of census tract poverty was defined according to the percentage of persons living below the federal poverty threshold per the 2000 US Census.20
DISCUSSION
The results of this analysis showed a strong association between the socioeconomic characteristics of census tracts and the incidence of bacteremic community-acquired pneumonia among adults. For all racial/ethnic groups examined, the incidence of bacteremic pneumonia was dramatically higher in the most impoverished census tracts than in the least impoverished census tracts. Incidence rates and trends among Hispanic adults were similar to those among non-Hispanic Whites. The racial differences in incidence were diminished but not eliminated when we included an area-based socioeconomic measure in the models. Rather, area-based socioeconomic level and race/ethnicity were independently associated with disease risk. The incidence of bacteremic pneumonia among Black adults living in the most impoverished census tracts was twice that of Whites in the most impoverished areas and more than 4 times that of Whites in the least impoverished census tracts. Collection and analysis of data according to socioeconomic measures are important for assessing public health programs aimed at eliminating disparities in health outcomes.
This was the first multisite analysis of data from the ABCs/Emerging Infections Program network on invasive bacterial disease in the US population that included census tract–level socioeconomic measures. Studies in individual surveillance areas have shown that the incidence of invasive pneumococcal disease is associated with county and census tract characteristics, including median household income and county AIDS prevalence.13,15 The findings of this multisite analysis are relevant for public health practice because data from this surveillance system have been used to set and track progress toward national health goals, including Healthy People 2010.10,25 We found that analysis according to census tract poverty level, categorized a priori into 4 strata,19 was both feasible and informative for examination of racial/ethnic differences in the incidence of bacteremic pneumonia among US adults. Overall, census tracts were identified for 90% of patients on the basis of information obtained from medical charts. Census tract characteristics were available from the 2000 US Census. Notably, the composite Townsend index performed no better in Poisson models than did census tract poverty level. Although area-based socioeconomic measures were shown to be poor surrogates for self-reported income in 1 study of pneumococcal pneumonia,14 self-reported income data are not routinely collected in ABCs. As a single measure, census tract poverty level could be used by public health programs to measure and track area-based socioeconomic inequalities in the incidence of bacteremic pneumonia and should be included in future analyses examining racial/ethnic disparities.
Associations between area-based socioeconomic measures and health outcomes have been described for a variety of communicable and noncommunicable diseases.19 Such measures likely reflect a mixture of individual and environmental risk factors for bacterial pneumonia in adults. Differences in the prevalence of cigarette smoking, HIV/AIDS, asthma, and diabetes mellitus likely contribute to higher incidence in more impoverished census tracts and among Black adults than among White adults.13,26,27 Restricting the analyses to patients without HIV infection markedly reduced the Black to White RR, although it remained significant before and after we adjusted for census tract poverty level. Pneumococcal vaccination is generally lower among Black and Hispanic adults, although trends by income level are not pronounced.28 Survival of healthier individuals from all racial/ethnic and socioeconomic groups might explain the reduced racial/ethnic disparity and socioeconomic inequalities observed in older age groups. In addition to individual-level risk factors, poorer communities may have greater exposure to indoor and outdoor air pollutants that increase the risk of respiratory diseases.29–32 Physiologic responses to chronic stress associated with racism, poverty, and other forms of social deprivation have been shown to decrease immune function and may also contribute to disease incidence.33–36 Targeting public health interventions to poorer communities with high rates of disease, in addition to targeting specific risk groups, could help to improve effectiveness where prevention efforts have stalled.37
Limitations
This analysis had several limitations. Race/Ethnicity-specific incidence was underestimated as the result of missing data on race/ethnicity in medical records. Data on race/ethnicity were missing for similar proportions of patients according to age category and census-tract socioeconomic stratum. Findings regarding patients of Hispanic ethnicity should be interpreted with caution; ethnicity was not recorded in almost half of the medical charts, and patients with missing ethnicity were assumed to be non-Hispanic. The incidence in Hispanic adults could have been higher than that for White adults if Hispanic ethnicity was underreported. Also, self-described race/ethnicity in census data may differ from how race/ethnicity is recorded in medical records. Medical records generally do not record multiple-race categories; therefore, patients were classified according to single-race categories found in the 2000 US Census.20 Furthermore, geocoding of patients to census tract of residence was incomplete and may have resulted in differential underestimation of incidence in rural and more impoverished census tracts.
Conclusions
Our study highlights the importance of collecting and analyzing socioeconomic data to inform interventions and measure progress toward eliminating racial/ethnic differences in health status that may result from socioeconomic inequalities. Coverage with pneumococcal polysaccharide vaccine in adults has stalled well below the Healthy People 2010 target of 90% in all racial/ethnic groups.7,25 New strategies are needed to increase vaccination coverage at the community level. Introduction of pneumococcal conjugate vaccines for adults is expected to reduce pneumococcal disease incidence and may have an impact on socioeconomic and racial/ethnic disparities. Prevention and treatment of chronic diseases and HIV/AIDS and improved access to smoking cessation programs in the poorest communities could also lessen disparities in the incidence of pneumonia. The results of our study also suggest that economically disadvantaged communities are likely to be hotspots for influenza–bacterial coinfection and should receive particular attention in pandemic influenza research and planning. Analysis of socioeconomic data underscores the need to address differential exposures to poverty and other social determinants of health as a means of reducing overall disease burden and promoting health equity.38
Acknowledgments
The study was funded by the Centers for Disease Control and Prevention's Emerging Infections Program.
The authors thank the California Emerging Infections Program, including Susan Brooks and Pam Daily Kirley; the Connecticut Emerging Infections Program, including M. Zachariah Fraser, James L. Hadler, and Katherine Purviance; the Colorado Emerging Infections Program, including Steve Burnite, Nicole Comstock, and Tessa Crume; the Georgia Emerging Infections Program, including Wendy Baughman and Paul Malpiedi; the Maryland Emerging Infections Program, including Rosemary A. Hollick, Kim Holmes, and Elisabeth Vaeth; the Minnesota Emerging Infections Program, including Brenda Jewell, Lindsey Lesher, Catherine Lexau, Craig Morin, Jean Rainbow, and Lori Triden; the New York Emerging Infections Program, including Jillian Karr, Glenda Smith, and Nancy Spina; the Oregon Emerging Infections Program, including Karen Stefonek; the Tennessee Emerging Infections Program, including Brenda Barnes and Terri McMinn; and the US Centers for Disease Control and Prevention, including Tamar Pilishvili, Tami Hilger Skoff, Carolyn Wright, and Elizabeth Zell.
Note. N. M. Bennett and L. H. Harrison have received consulting fees from Wyeth and Merck.
Human Participant Protection
CDC's institutional review board has determined that the protocol for Active Bacterial Core surveillance is exempt from human subjects review as routine public health surveillance.
References
- 1.Marston BJ, Plouffe JF, File TM, Jr, et al. Incidence of community-acquired pneumonia requiring hospitalization: results of a population-based active surveillance study in Ohio. Arch Intern Med. 1997;157(15):1709–1718 [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)–United States, May-August 2009. MMWR Morb Mortal Wkly Rep. 2009;58(38):1071–1074 [PubMed] [Google Scholar]
- 3.Klugman KP, Chien YW, Madhi SA. Pneumococcal pneumonia and influenza: a deadly combination. Vaccine. 2009;27(suppl 3):C9–C14 [DOI] [PubMed] [Google Scholar]
- 4.Robinson KA, Baughman W, Rothrock G, et al. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995-1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285(13):1729–1735 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Recommended adult immunization schedule–United States, 2009. MMWR Morb Mortal Wkly Rep. 2008;57(53):Q1–Q4 [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Racial/ethnic disparities in influenza and pneumococcal vaccination levels among persons aged > or =65 years–United States, 1989-2001. MMWR Morb Mortal Wkly Rep. 2003;52(40):958–962 [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention QuickStats from the National Center for Health Statistics. Percentage of adults aged >65 years who ever received a pneumococcal vaccination, by sex, age group, and race/ethnicity–National Health Interview Survey, United States, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(26):723 [Google Scholar]
- 8.Centers for Disease Control and Prevention Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998-2003. MMWR Morb Mortal Wkly Rep. 2005;54(36):893–897 [PubMed] [Google Scholar]
- 9.Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–1746 [DOI] [PubMed] [Google Scholar]
- 10.Flannery B, Schrag S, Bennett NM, et al. Impact of childhood vaccination on racial disparities in invasive Streptococcus pneumoniae infections. JAMA. 2004;291(18):2197–2203 [DOI] [PubMed] [Google Scholar]
- 11.Talbot TR, Poehling KA, Hartert TV, et al. Elimination of racial differences in invasive pneumococcal disease in young children after introduction of the conjugate pneumococcal vaccine. Pediatr Infect Dis J. 2004;23(8):726–731 [DOI] [PubMed] [Google Scholar]
- 12.Breiman RF, Spika JS, Navarro VJ, et al. Pneumococcal bacteremia in Charleston County, South Carolina. A decade later. Arch Intern Med. 1990;150(7):1401–1405 [PubMed] [Google Scholar]
- 13.Harrison LH, Dwyer DM, Billmann L, et al. Invasive pneumococcal infection in Baltimore, Md: implications for immunization policy. Arch Intern Med. 2000;160(1):89–94 [DOI] [PubMed] [Google Scholar]
- 14.Flory JH, Joffe M, Fishman NO, et al. Socioeconomic risk factors for bacteraemic pneumococcal pneumonia in adults. Epidemiol Infect. 2009;137(5):717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen FM, Breiman RF, Farley M, et al. Geocoding and linking data from population-based surveillance and the US Census to evaluate the impact of median household income on the epidemiology of invasive Streptococcus pneumoniae infections. Am J Epidemiol. 1998;148(12):1212–1218 [DOI] [PubMed] [Google Scholar]
- 16.Fraser DW, Darby CP, Koehler RE, et al. Risk factors in bacterial meningitis: Charleston County, South Carolina. J Infect Dis. 1973;127(3):271–277 [DOI] [PubMed] [Google Scholar]
- 17.Fraser DW, Geil CC, Feldman RA. Bacterial meningitis in Bernalillo County, New Mexico: a comparison with three other American populations. Am J Epidemiol. 1974;100(1):29–34 [DOI] [PubMed] [Google Scholar]
- 18.Fraser DW, Henke CE, Feldman RA. Changing patterns of bacterial meningitis in Olmsted County, Minnesota, 1935-1970. J Infect Dis. 1973;128(3):300–307 [DOI] [PubMed] [Google Scholar]
- 19.Krieger N, Chen JT, Waterman PD, et al. Painting a truer picture of US socioeconomic and racial/ethnic health inequalities: the Public Health Disparities Geocoding Project. Am J Public Health. 2005;95(2):312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bureau of the Census, US Department of Commerce US Census 2000. [Google Scholar]
- 21.Bureau of the Census, US Department of Commerce Decennial Management Division Glossary. Washington, DC: Bureau of the Census; Available at: http://www.census.gov/dmd/www/glossary.html#C. Accessed July 29, 2009 [Google Scholar]
- 22.Krieger N, Chen JT, Waterman PD, et al. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–482 [DOI] [PubMed] [Google Scholar]
- 23.Stapleton JH. Linear Statistical Models. New York, NY: Wiley; 1995 [Google Scholar]
- 24.McCullagh P, Nelder JA. Generalized Linear Models. New York, NY: Chapman and Hall; 1989 [Google Scholar]
- 25.US Department of Health and Human Services Healthy People 2010. Washington, DC: US Department of Health and Human Services; 2000 [Google Scholar]
- 26.Kyaw MH, Rose CE, Jr, Fry AM, et al. The influence of chronic illnesses on the incidence of invasive pneumococcal disease in adults. J Infect Dis. 2005;192(3):377–386 [DOI] [PubMed] [Google Scholar]
- 27.Nuorti JP, Butler JC, Farley MM, et al. Cigarette smoking and invasive pneumococcal disease. N Engl J Med. 2000;342(10):681–689 [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System survey data. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2008. Available at: http://www.cdc.gov/BRFSS. Accessed July 29, 2009 [Google Scholar]
- 29.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schikowski T, Sugiri D, Reimann V, et al. Contribution of smoking and air pollution exposure in urban areas to social differences in respiratory health. BMC Public Health. 2008;8:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villeneuve PJ, Burnett RT, Shi Y, et al. A time-series study of air pollution, socioeconomic status, and mortality in Vancouver, Canada. J Expo Anal Environ Epidemiol. 2003;13(6):427–435 [DOI] [PubMed] [Google Scholar]
- 32.Zeger SL, Dominici F, McDermott A, et al. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000-2005). Environ Health Perspect. 2008;116(12):1614–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aiello AE, Simanek AM, Galea S. Population levels of psychological stress, herpesvirus reactivation and HIV. AIDS Behav. 2010;14(2):308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brunner E. Stress and the biology of inequality. BMJ. 1997;314(7092):1472–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Culhane JF, Rauh VA, Goldenberg RL. Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep. 2006;8(6):459–464 [DOI] [PubMed] [Google Scholar]
- 36.Littrell J. The mind-body connection: not just a theory anymore. Soc Work Health Care. 2008;46(4):17–37 [DOI] [PubMed] [Google Scholar]
- 37.Whitehead SJ, Cui KX, De AK, et al. Identifying risk factors for underimmunization by using geocoding matched to census tracts: a statewide assessment of children in Hawaii. Pediatrics. 2007;120(3):e535–e542 [DOI] [PubMed] [Google Scholar]
- 38.Marmot M, Friel S, Bell R, et al. Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–1669 [DOI] [PubMed] [Google Scholar]