Abstract
It is well established that cells must interact with their microenvironment and that such interaction is crucial for coordinated function and homeostasis. However, how cells receive and integrate external signals leading to gene regulation is far from understood. It is now appreciated that two classes of cooperative signals are implicated: a soluble class including hormones and growth factors and a class of insoluble signals emanating from the extracellular matrix (ECM) directly through contact with the cell surface. Using 3-dimensional culture systems and transgenic mice, we have been able to identify some of the elements of this ECM-signaling pathway responsible for gene regulation in rodent mammary gland differentiation and involution. Our major observations are 1) the requirement for a laminin-rich basement membrane; 2) the existence of a cooperative signaling pathway between basement membrane and the lactogenic hormone prolactin (PRL); 3) the importance of β1-integrins and bHLH transcription factor(s) and the presence of DNA response elements (exemplified by BCE-1, located on a milk protein gene, β-casein); and 4) the induction of mammary epithelial cell programmed cell death following degradation of basement membrane. We hypothesize that this cooperative signaling between ECM and PRL may be achieved through integrin- and laminin-directed restructuring of the cytoskeleton leading to profound changes in nuclear architecture and transcription factor localization. We postulate that the latter changes allow the prolactin signal to activate transcription of the β-casein gene. To further understand the molecular mechanisms underlying ECM and hormonal cooperative signaling, we are currently investigating ECM regulation of a “solid-state” signaling pathway including ECM fiber proteins, plasma membrane receptors, cytoskeleton, nuclear matrix and chromatin. We further postulate that disruption of such a pathway may be implicated in cell disorders including transformation and carcinogenesis.
I. Introduction
During embryogenesis some eukaryotic cells exit mitosis and undergo differentiation, while other cells die by programmed cell death. Some cells may also fail to properly maintain stable differentiation, eventually giving rise to tumors. These tumor cells re-express several embryonic characteristics, including proliferation and motility. An understanding of the mechanisms underlying the induction and maintenance of the differentiated state should therefore help to elucidate how an aberration in the maintenance of stability can lead to tumor development, rather than programmed cell death (Bissell and Hall, 1987). It is now well established that intact cell-cell and cell-extracellular matrix (ECM) interactions are vital for both embryonic morphogenesis (Sanes, 1989; Juliano and Haskill, 1993; Martins-Green and Bissell, 1995) and gene regulation in adult organisms (Bissell and Hall, 1987; Adams and Watt, 1989; Reid, 1990) in cooperation with soluble factors such as hormones. Our laboratory has been using the mammary gland model to elucidate how cell-cell and cell-ECM interactions regulate gene expression in mammary epithelial cells (MEC) during differentiation and programmed cell death. The mammary gland offers a unique model of post-natal differentiation and involution permitting the study of the progression between normal regulation and abnormal behavior leading to cancer. We have established relevant culture systems of MECs which mimic in vivo morphogenesis and have found that mouse midpregnant cells cultured in contact with a laminin-rich basement membrane, in the presence of lactogenic hormones, display a phenotypic (alveoli structure) and functional (milk proteins secretion) status similar to a lactating gland. Here we summarize how the ECM modulates MEC gene expression leading to either differentiation or involution and describe what the various signaling elements are from the ECM to the regulatory DNA sequences. Elucidation of the physical and soluble components of this ECM signaling pathway should help elaborate the basis for tumorigenesis.
II. Extracellular Matrix Influences the Development and Function of the Mammary Epithelium
Mammary gland development continues after birth, permitting its use for morphological and functional studies during pregnancy and lactation (Bissell and Hall, 1987). We are currently using the CID-9 cell culture system (Schmidhauser et al., 1990) derived from COMMA-ID cells (Danielson et al., 1984) which in turn were established from disassociated primary mouse MECs from 15-day midpregnant animals. These cells can be directed to achieve a functional differentiated lactating phenotype. This differentiated phenotype includes secretion of milk-specific proteins such as β-casein, which can be induced in the presence of hormones (prolactin), and an appropriate basement membrane (EHS: basement membrane matrix reconstituted from an Engelbreth-Holm-Swarm tumor) (Fig. 1). In our system, β-casein and WAP (whey acidic protein) are used as differentiation markers. β-casein represents one of the “early” classes of rodent milk proteins (its expression is measurable in the 6-day pregnant mouse mammary gland), whereas WAP represents one of the later classes of milk proteins (measurable after day 14 of pregnancy).
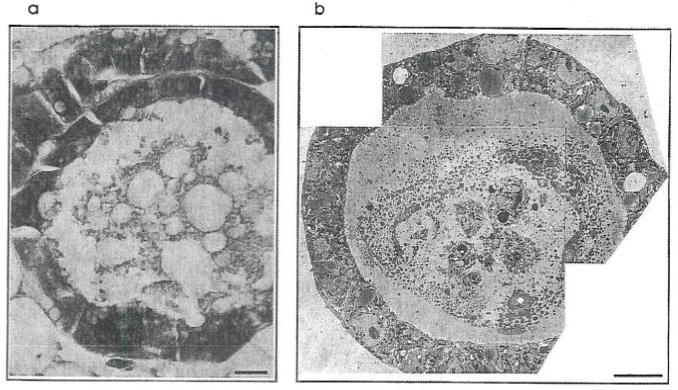
FIG. 1.
Light micrograph of a cross-section of an alveolus in a lactating mouse mammary gland, stained with hematoxylin and eosin (a). On a similar scale is an electron micrograph through a cultured spheroical structure, showing that the EHS-derived basement membrane matrix induces cultured mammary epithelial cells to form normal alveolar architecture (b). [Reproduced with permission from Streuli and Bissell, 1991.]
A complementary role for ECM and the lactogenic hormone PRL (prolactin) in differentiation was established by culturing CID-9 cells either on plastic, on attached type I collagen gels, in floating collagen gels, or on EHS matrix in the presence of lactogenic hormones (Chen and Bissell, 1989). Attached cultures were analogous to plastic and failed to express β-casein. Floating collagen gels expressed the early class of milk proteins. Expression of both classes of milk proteins required a laminin-rich basement membrane. Using this substratum induced the formation of hollow spherical structures of cells reminiscent of alveolar structures in the mammary gland in vivo, including apical β-casein and WAP secretion (Chen and Bissell, 1989; Lin and Bissell, 1993).
A single-cell assay system was used to demonstrate that cell-cell interactions were not necessary for β-casein gene expression (Sreuli et al., 1991). Addition of exogenous basement membrane (EHS) appeared to be sufficient for β-casein expression for single suspended cells; however, cell-cell interactions were necessary in collagen-1 gels to permit deposition of an endogenous basement membrane and hence β-casein expression. The regulation of both expression and deposition of ECM components was extensively studied by culturing mammary cells on different substrata (Streuli and Bissell, 1990). On plastic, the cells became squamous and nonpolarized; on attached collagen I gels, cells remained flat; while on floating collagen I, the cells were cuboidal-columnar with multicellular rearrangements (Emerman and Pitelka, 1977). Immunofluorescence analysis revealed that cells cultured on plastic deposited very little laminin or type IV collagen, although these cells had high levels of ECM mRNAs. In contrast, a basement membrane containing laminin and type IV collagen accumulated from cells cultured on floating gels but was absent in cells cultured on attached gels. This indicated that changes in shape and cell density were also required for the polarized deposition of an endogenous basement membrane. Coincident with basement membrane deposition ECM mRNA levels decreased and substantial β-casein production was observed. These results demonstrated that components of both stroma (provided here by the collagen gel matrix) and epithelial cells were required for basement membrane deposition, which in turn is required for the establishment and maintenance of the differentiated phenotype.
Observations using these culture systems were confirmed by in vivo studies using transgenic mice that inappropriately expressed an auto-activating rat stromelysin-1 gene targeted to the mammary gland, under the control of the WAP promoter (Sympson et al., 1994). The metalloproteinase stromelysin-1 has a wide range of ECM substrates, including fibronectin, laminin, type IV collagen, and proteoglycans (Chin et al., 1985). Moreover, this enzyme is implicated in the physiological control of mammary gland function during involution (Talhouk et al., 1992). In these engineered mice, expression of the transgene increased in late pregnancy and lactation and decreased during mammary gland involution. Surprisingly, low level expression of stromeylsin-1 was also observed in the adult female virgin mammary gland (Sympson et al., 1994), while expression of the stromelysin-1 transgene increased branching morphogenesis and stimulated differentiation (measured by β-casein expression) in the adult virgin female. Studies of pregnant females showed that expression of the stromelysin-1 transgene was associated with reduction of β-casein and WAP mRNAs. In lactating mice, transgenic mammary glands had reduced amounts of laminin and type IV collagen and the tissue was similar to that of an early involuting gland (Talhouk et al., 1992) both morphologicaly and functionally. Thus, by targeting basement membrane in a tissue-specific context, it was possible to confirm that basement membrane remodeling plays a fundamental role in the regulation of MEC growth and function during postnatal mammary development.
III. Involution and Programmed Cell Death Are Induced by Loss of Extracellular Matrix in Mammary Epithelial Cells
Mammary gland involution, following conversion from a lactating to a non-lactating phenotype, is a rapid process which is associated with profound tissue remodeling, including dissolution of the alveolar basement membrane (Martinez-Hernandez et al., 1976). Moreover, loss of mammary gland function during involution correlates with increased expression of ECM-degrading proteinases (Talhouk et al., 1991). Based on the fact that basement membrane integrity is required for lactation, we hypothesized that proteolysis of the basement membrane was responsible for the loss of mammary gland function during involution. Using three ways to induce involution of the mammary gland, with alterations of ECM-degrading proteinases and inhibitors, we were able to show (Talhouk et al., 1992) that a high ECM-degrading proteinase-to-inhibitor ratio correlated with loss of tissue-specific function, as indicated by both reduced β-casein expression and compromised mammary gland morphology. The metalloproteinase-to-proteinase inhibitor ratio was shown to be critical for the induction of involution. By introducing exogenous TIMP and thereby elevating the protease inhibitor-to-metalloproteinase ratio, mammary gland involution was reversed. Indeed, the higher the TIMP-to-metalloproteinase ratio, the more intact the alveoli and the longer the lactational phenotype was maintained. It is envisioned that ECM-degrading proteinases secreted by the epithelial cells, or more likely by the stromal cells, alter alveolar cell-ECM interactions, eventually leading to detachment of cells from their basement membrane and the induction of programmed cell death (Walker et al., 1989; Strange et al., 1992; Boudreau et al., 1995).
The role of ECM in mammary epithelial cell programmed cell death (apoptosis) was examined by comparing the response of CID-9 cells plated directly on tissue culture plastic in the absence of serum to those plated on an exogenous basement membrane (EHS) (Boudreau et al., 1995). After 4–5 days on plastic, CID-9 cells began to display DNA laddering, nuclear condensation, and increased expression of the SGP-2 gene, all characteristics of apoptotic cells. In contrast, cells plated on ECM failed to demonstrate similar apoptotic markers, even after 10 days of culture. Disruption of cell-ECM interactions by the addition of a β1-integrin blocking antibody induced characteristic apoptotic changes in these cells. In another set of experiments, cells plated on either fibronectin or type I collagen displayed similar degrees of apoptosis as those plated directly on plastic. These data imply that intact basement membrane or specific cell-ECM interactions were required for the prevention of programmed cell death.
We next demonstrated that proteolytic destruction of the basement membrane resulted in programmed cell death (Boudreau et al., 1995). CID-9 cells were co-transfected with an inducible expression vector encoding a stromelysin-1 auto-activating mutant, under control of the Rous sarcoma promoter containing the lac repressor-binding domain 72 hr after the induction of stromelysin-1 expression by 5 mM isopropyl-beta-D-thiogalactopyranoside, substantial apoptosis was observed in comparison to the uninduced control cells (Fig. 2). This elevated apoptosis rate was inhibited using the metalloproteinase (stromelysin-1) inhibitor. Degradation of ECM by stromelysin-1 also resulted in programmed cell death in vivo, as observed in transgenic mice expressing the stromelysin-1 gene under control of the whey acidic milk protein promoter (Sympson et al., 1994) which is activated from mid- to late pregnancy.
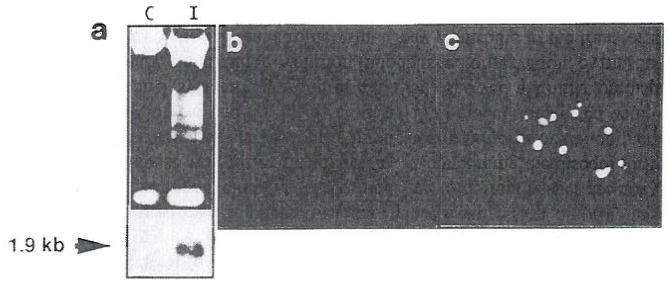
FIG. 2.
Apoptosis in cells overexpressing stromeylsin-1. Electrophoretic analysis of total DNA (20 μg) from control (C) and IPTG-induced cells (I) after 72 hours (a). Corresponding RNA blot (20 μg per lane) hybridization with a probe dial detects a 1.9-kb stromylsin-1 mRNA. In situ analysis of DNA from mammary gland of normal mice in midpregnancy (b) and in transgenics expressing stromeylsin-1 (c). Note the increase in the number of epithelial cells undergoing apoptosis and the collapsed alveoli in the transgenics compared to normal mice. Scale bar, 33 μm. [Reproduced with permission from Boudreau et al., 1995.]
Like growth and differentiation, programmed cell death requires the active and coordinated regulation of specific genes such as bcl-2 (a potent suppressor of cell death) and ICE (a potent mediator of cell death) (Yuan et al., 1993). CID-9 cells transfected with a vector encoding crmA, (a viral gene product that specifically inhibits the enzymatic activity of ICE) showed an 80% reduction in apoptosis-associated-DNA-laddering compared to control cells. Moreover, nontransfected CID-9 cells cultured on plastic contained large amounts of ICE mRNA and the ICE protein, whereas cells plated on ECM contained almost no ICE mRNA or protein (Boudreau et al., 1995). However, it is not yet clear whether it is ICE or one of its homologues which is me critical inducer of cell death in this system.
Taken in unison, these results strongly suggest that proteolytic degradation of ECM leads to the loss of the differentiated state, induction of ICE expression and activity or one of its homologues, and finally programmed cell death both in culture and in vivo.
The signal pathways implicated in the induced expression of proteinases involved in regulating development and remodeling of normal tissues are not yet understood. Likewise very little information exists concerning the disequilibrium between ECM-proteinases and inhibitors implicated in tumor growth and metastastic behavior (Golberg and Eisen, 1991). Our CID-9 model transfected with the WAP-stromelysin-1 system (Sympson et al., 1994) showed a similar predisposition to mammary gland tumor evolution (unpublished observation; Sympson et al., 1994). We hypothesize that stromelysin-1 may contribute to the transformation process by causing abnormal patterns of cell growth and death, exemplified by refractoriness to apoptosis induction by basement membrane degradation observed in pre-malignant and cancer cells. Crucial to understanding this process will be an elucidation of the molecular mechanisms implicated in ECM-signaling responsible for gene regulation.
IV. Mechanisms Underlying ECM-Genome Signaling
Experiments presented in the first two sections of this review showed that direct interactions between the basement membrane and MECs is necessary for extracellular positioning information which in turn alters the basal surface of cells, leading to the transcriptional regulation of tissue-specific genes and ultimately cell differentiation. We next discuss in detail these cell-ECM signaling components.
A. LAMININ MEDIATES TISSUE-SPECIFIC GENE EXPRESSION IN MAMMARY EPITHELIUM
The basement membrane constitutes a supramolecular network of laminin, collagen IV, glycoproteins such as entactin, and proteoglycans (Ashkenas et al., 1994). Most of these components interact directly with the cell surface through cell surface receptors.
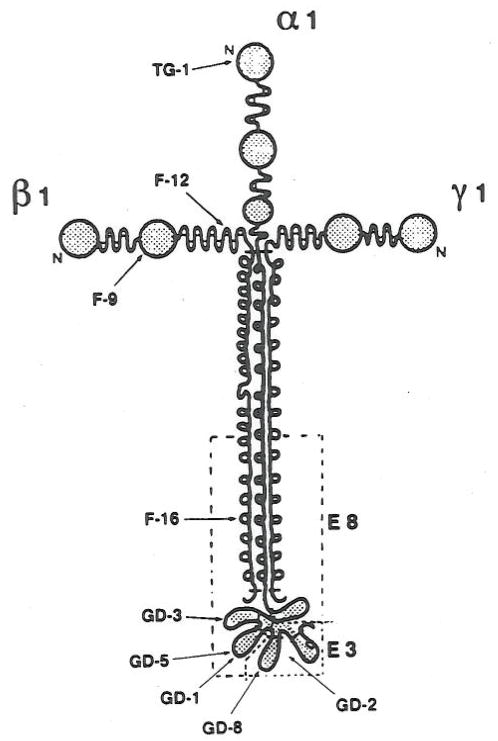
The first step in the ECM-signaling pathway includes one or several of these ECM components. To determine which ECM component was important for competent ECM signaling, we dissected the basement membrane into its component parts and examined specific ECM proteins for their differentiation-inducing potential using β-casein synthesis as the differentiation marker. Laminin, which represents 85–90% of the EHS basement membrane protein (Kleinman et al., 1986), was found to be the component capable of driving the morphological and functional differentiation of these MECs. In all of these experiments, lactogenic hormones were present and prolactin was essential. However, if ECM was not present, prolactin was not able to induce βcasein expression. Additional experiments were then performed using an ECM overlay assay, thereby minimizing independent differentiation signals emanating from secretion of ECM-protein by cultured cells. These studies involved seeding mammary cells on plastic and treating them with purified basement membrane components (Streuli et al., 1995). Purified laminin but not fibronectin, collagen I or collagen IV was able to induce substantial β-casein expression. Laminin is a complex three-chain molecule which interacts with cells through different domains (Tryggvason, 1993). A specific domain (E3) may be required for the signaling and is located within the globular region of the long arm (α-1 chain) of laminin (Fig. 3). Antibodies raised against the E3 region of laminin reduced production of β-casein, while complete E3 fragments inhibited laminin-induced β-casein expression. However, neither the E3 fragment nor a mixture of elastase-generated fragments from the complete basement membrane matrix were sufficient to induce β-casein expression. This observation implies that differentiation requires signaling from the intact laminin molecule and must include functional three-dimensional structures not produced when smaller fragments are used (Streuli et al., 1995).
FIG. 3.
Schematic diagram of laminin, indicating the location of peptides used for functional studies, and the E3 and E8 regions. [Reproduced with permission from Streuli et al., 1995.]
B. BASEMENT MEMBRANE-DEPENDENT β-CASEIN EXPRESSION AS WELL AS CELL SURVIVAL REQUIRE FUNCTIONAL β1-HNTEGRINS AND ARE DEPENDENT ON CELLULAR ARCHITECTURE
Integrins represent a superfamily of adhesion molecule receptors. These heterodimers are composed of alpha and beta subunits. Experiments using a broad spectrum β1-integrin blocking antibody prevented β-casein expression in CID-9 cells, whereas antibodies against either alpha-6 integrin or E-cadherin failed (Streuli et al., 1991). The results were obtained using a single cell culture assay system and demonstrated cell-cell interactions were not necessary for β-casein gene expression. This was the first indication that the β1-integrin was involved in ECM signal transduction induced differentiation of MECs. Among the several β1-integrins known to be laminin receptors, α3-β1 (which may interact with the E3 fragment of laminin in a human melanoma cell line) (Gehlsen et al., 1992) is a good candidate for these differentiation signals, although concomitant participation of other types of receptors (i.e. proteoglycans) cannot be ruled out.
It has been shown that clustering of β1-integrin receptors precedes biochemical signaling in other systems (Juliano and Haskill, 1993). Using a subclone of CID-9 cells “scp2” which are incapable of synthesizing a basement membrane and therefore absolutely require the addition of exogenous ECM for β-casein expression (Desprez et al., 1993), we showed that the ECM induced clustering of β1-integrin receptors (Roskelley et al., 1994, and in preparation). In addition, further studies using varying levels of polyhema demonstrated the importance of cell shape changes for this induction (Roskelley et al., in preparation). Moreover, after 2 hrs of ECM treatment, tyrosine phosphorylation was increased in at least 8 protein species and perturbation of tyrosine kinase(s) by genistein, a tyrosine kinase inhibitor, appeared to specifically inhibit β-casein synthesis. On the other hand, whereas TPA (a stimulator of protein kinase C activity) prevented β-casein expression when laminin was added to the cells, it had no effect if cellular architecture was altered by plating the cells on high-density polyhema. It appears that a biochemical response to ECM signaling involves an increase in tyrosine phosphorylation that is associated with integrin clustering, while β-casein gene expression also requires a change in the cytoskeleton. Finally, β1-integrin blocking antibodies were shown to induce apoptosis long after cell attachment had occurred, indicating that cell-ECM interactions are necessary for tissue homeostasis.
C. bHLH TRANSCRIPTION FACTOR(s) MAY COORDINATE THE GROWTH AND DIFFERENTIATION OF MAMMARY EPITHELIAL CELLS
bHLHs are basic helix-loop-helix transcription factors that regulate both growth and differentiation (Kingston, 1989). Transcriptional regulation of these factors is modulated by dimerization with Id proteins, which are ubiquitous regulators of bHLH function (Desprez et al., 1995, and associated references). Using Id-1 as a tool, we obtained the first evidence that one or more bHLH factors may coordinate the growth and differentiation of MECs. Using a scp2 cell subclone, we demonstrated that 1) ECM-induced differentiation of these cells entailed a marked decrease in Id-1 expression and arrest of their growth; 2) scp2 cells stably transfected with an Id-1 expression vector did not express β-casein in response to ECM addition, despite transitory growth arrest and formation of three-dimensional structures similar to those of control cells; and 3) scp2 cells expressing an Id-1 antisense stayed fully differentiated and stably growth arrested (Fig. 4).
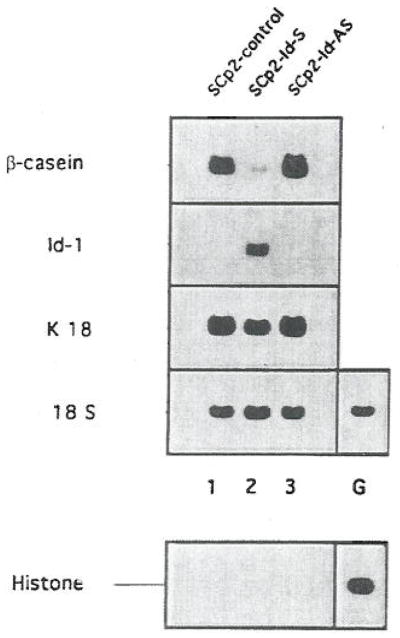
FIG. 4.
Expression of β-casein, Id-1, keratin 18 (K 18), and histone 3 mRNA in differentiated SCp2 cells transfected with control, Id-1 sense, and Id-1 anti-sense vectors. Control, SCp2-Id-S, and SCp2-Id-AS cells were plated on top of a layer of EHS-ECM in the presence of lactogenic hormones; 5 days later, RNA was isolated and analyzed for the abundance, of β-casein, Id-1, keratin 18, and histone 3 mRNA. Hybridization with an 18S rRNA probe served to control for RNA integrity, loading, and transfer. Lane G, RNA from proliferating SCp2 cells. RNA was analyzed for histone 3 mRNA as a positive control for hybridization of histone probe. [Reproduced with permission from Desprez et al., 1995.]
Id-1 may act to maintain growth potential rather than directly stimulate growth-stimulatory genes or repress growth-inhibitory genes. We hypothesize that bHLH protein(s) may allow functional differentiation in MECs by maintaining growth arrest (Desprez et al., 1995). It is known that perturbation of this balance is of particular importance in the early stages of tumorigenesis (Fingert et al., 1993); therefore, identification of bHLH transcription factors responding to ECM signaling should help to understand ECM regulation of gene expression at the genomic level.
D. IDENTIFICATION OF BCE-1 (BOVINE-CASEIN ELEMENT 1), A TRANSCRIPTIONAL ENHANCER INVOLVED IN THE PROLACTIN- AND ECM-DEPENDENT REGULATION OF β-CASEIN GENE EXPRESSION
We identified another component of ECM-signaling at the nuclear level by constructing a series of bovine β-casein-CAT fusion genes with various lengths of 5′ flanking DNA sequences at nucleotide +42 of the first noncoding bovine β-casein exon CAT (Schmidhauser et al., 1990). Co-transfection of these constructs with pSV2Neo (conferring antibiotic resistance) showed that both matrix and hormonal controls of transcription occurred at the level of a regulatory element located more than 0.8 kb upstream of the transcription site of the bovine β-casein gene (Schmidhauser et al., 1990). Within this regulatory element, we have identified a novel enhancer BCE-1 (Schmidhauser et al., 1992) that does not share any significant sequence homology with any known well-characterized enhancer elements. BCE-1 may be mammary specific, since transfected Chinese hamster ovary (CHO) cells and Mabin-Darby Canine kidney (MDCK) cells were unable to express activity in response to ECM and PRL.
Furthermore, when BCE-1 was linked to different inactive forms of a truncated β-casein promoter linked to a CAT reporter gene (Schmidhauser et al., 1992), this was sufficient to reconstitute transcriptional activity at a level which was even higher than the wild-type promoter (100–150 fold induction). It appears that prolactin and ECM-induction are functionally and kinetically separable, since addition of prolactin after ECM pre-treatment (5 days) to scp2 mammary cells induced maximal synthesis of β-casein and the other milk proteins after 1 day. In addition, a construct that contained BCE-1 and a minimal MMTV promoter could now be induced substantially by ECM when hydrocortisone was present (Schmidhauser et al., 1994). This suggests that these events are mediated by distinct sequence elements within BCE-1 (Schmidhauser et al., 1992). By using the same constructs, we showed that purified laminin could also activate transcription from the β-casein promoter (Streuli et al., 1995). These results provide evidence for a molecular signaling pathway that links specific ECM proteins with the cell’s transcriptional machinery. Thus BCE-1 will be an important tool for the analysis of the additional steps involved in ECM and prolactin signal transduction.
V. Conclusions and Perspectives: Extracellular Matrix Signaling from “Membrane Skeleton” to “Nuclear Skeleton”
In summary, our results show that MECs require a laminin-rich extracellular matrix and lactogenic hormones (prolactin) to achieve a functionally differentiated phenotype, which in turn is reflected by arrangement of the cells into alveoli-like structures and secretion of milk-specific proteins such as β-casein. There is strong evidence that ECM-induced expression of β-casein involves an “ECM-response element” (BCE-1) in the promoter of the β-casein gene that is activated by integrin-mediated signaling. Moreover, one or several bHLH transcription factors appear to be involved in the response to ECM signaling. ECM is implicated in gene regulation important for differentiation and for the control of programmed cell death associated with mammary gland involution.
The lactogenic hormone prolactin is required for β-casein synthesis, indicating that in the mammary system, ECM and soluble factors cooperate to trigger a physiological response towards differentiation. Such dual control of cell behavior also occurs in other systems (Damsky and Werb, 1992; Fuortes et al., 1993; Schwartz, 1992). Our results suggest that cooperative signaling through integrins and the prolactin receptor is necessary for the activation of a differentiated phenotype. It is possible that laminin, essential in ECM-signaling, may poise the cells to respond to additional lactogenic signals rather than induce de novo competence for expression. It has been shown that lactogenic hormonal regulation of β-casein expression is mainly at the transcriptional level (Doppler et al., 1989; Goodman and Rosen, 1990). Our results strongly suggest mat the BCE-1 enhancer of the β-casein gene contains regulatory elements for both prolactin and ECM. Thus, BCE-1 will be important for elucidating nuclear mechanisms of prolactin’s actions.
Although recent studies have begun to unravel the prolactin signal transduction pathway (Burdon et al., 1994; Wakao et al., 1994), the complexity of signals generated by cell-ECM interactions in the mammary system will need further investigation. Moreover, the cooperative signaling between ECM and prolactin requires examination. We believe this can be achieved by studying integrin and prolactin receptors and their mechanisms of action. Our working hypothesis is that laminin may be involved in the cooperative signaling effect by restructuring the cell’s internal architecture.
A model of “dynamic reciprocity” between the ECM, its transmembrane receptors, the cytoskeleton, and the nuclear matrix (“nuclear skeleton”) was postulated more than a decade ago (Bissell et al., 1982). This model predicted that there should be a direct, connected signaling pathway capable of remodeling internal cell structure, not only at the level of integrins and thus the “membrane skeleton” (which involves external interactions with ECM fibers and internal interactions with the cytoskeleton), but also at the level of the “nuclear skeleton” (nuclear matrix). The nuclear matrix is more than a physical network of proteins responsible for nuclear shape. According to the structural definition of nuclear matrix given by experimental procedures used to obtain it, this structure involves filamentous proteins such as actin (Dacheng et al., 1990; Lelievre et al., 1995), associated high molecular weight RNA (Dacheng et al., 1990), and globular DNA attachment proteins. These globular proteins are postulated to be crucial for DNA attachment either at fixed sites or by more labile interactions with specific regions of genes and are implicated in gene replication and transcription (Razin and Gromova, 1995, and associated references). Moreover, intermediate filaments irradiate from this nuclear matrix towards the cytoplasmic space (Dacheng et al., 1990). We postulate that there exists a direct physical link commencing with ECM protein fibers through plasma membrane receptors to the cytoplasmic cytoskeleton towards the nuclear matrix, ultimately terminating with the genome.
In our mammary model, it appears that both integrin clustering and the dramatic changes in the cytoplasmic cytoskeleton that are associated with cell rounding are necessary to initiate the functional signal transduction that leads to β-casein expression (Roskelley et al., 1994, 1995). In the distal portion of this physical signaling pathway called membrane skeleton, it is possible that connections among the skeleton components (e.g. actin) change in response to mechanical stress and metabolic conditions. This membrane skeleton seems partly responsible for cell shape and mechanical stability (Juna and Hitt, 1992). Membrane skeleton participates in adhesion to other cells and to the ECM through four major families of receptors, among them integrins constitute the family involved in substratum attachments. They direct focal adhesions where they bind to cytoskeletal proteins (for example, talin and alpha-actinin), apparently through the β1-integrin cytoplasmic domain (Hynes, 1992). In turn, these proteins bind to other proteins to finally create interactions with actin (Juna and Hitt, 1992). Moreover, changes in protein phosphorylation (like tyrosine phosphorylation) apparently regulate the structure and function of focal adhesions in vivo (Guan and Shalloway, 1992). Therefore, the increased tyrosine phosphorylation in scp2 cells that we observed in the presence of ECM (Roskelley et al., 1994) could be of fundamental importance for the re-arrangement of the cytoskeleton of these cells. Integrins also participate in hemidesmosome formation which mediate cell attachment to the external basement membrane and are connected to the internal intermediate filaments (Juna and Hitt, 1992). It is easy to imagine a link between membrane skeleton and nuclear skeleton through actin and even more with intermediate filaments which are known to be part of the nuclear matrix. The same argument could be also used for cell-cell interactions mediated by E-cadherins, thereby illustrating the complexity of this transduction pathway.
There are strong indications that ECM-mediated changes in cell shape lead to changes in both the cytoskeleton and the nuclear matrix which can have profound effects on gene expression (Pienta and Coffey, 1992; Bidwell et al., 1993). Demonstration of me existence of an ECM-response element in our cell system suggests that ECM signaling through the “physical pathway” could directly affect conformation or protein-binding of such elements by altering their interaction with the nuclear matrix (Boudreau et al., 1995). BCE-1 represents at this level a major candidate for further investigation. Experiments are currently under way in our laboratory to explore the consequences of ECM-signaling on both nuclear matrix composition and DNA/nuclear matrix interactions.
In conclusion, we can imagine the dual and cooperative regulation of gene expression responsible for mammary gland differentiation. The first type of regulation would be a dynamic response through a physical pathway from ECM-cell membrane-specific interactions to DNA-nuclear matrix interactions, while the second type of regulation would be mediated by lactogenic hormones which would now allow transcription factors to bind to the same DNA enhancer element.
This model predicts that deregulation of the physical signaling pathway may be at least partly responsible for tumorigenesis. Furthermore, modification of the structure of this internal physical signaling pathway would lead to re-arrangements and altered nuclear matrix interactions resulting in refractoriness to both ECM and hormonal signals necessary for competent differentiation. Thus, on the complex pathway of signal transduction, ECM regulation of gene expression appears to open some very exciting, albeit unorthodox, doors!
DISCUSSION
Jennie Mathers: Do single cells when rounded turn on milk protein gene function?
Mina Bissell: Yes, a few years ago we did a series of experiments where we allowed the cells to lose function by culturing on plastic. We then made single cells and embedded these either in basement membrane (BM) or collagen type I. Single cells in BM expressed β-casein; single cells in collagen did not. However, cell clusters in collagen could function because when cells come together, they now can make their own basement membrane and this now can signal. This was published in Journal of Cell Biology (Streuli el al., 1991).
Jennie Mathers: How specific is stromelysin in its proteolytic function?
Mina Bissell: Stromelysin is a metalloprotease that degrades basement membrane proteins. But it degrades laminin as well as other BM proteins. It is much more specific than trypsin or general proteases, however. Whether or not metalloproteases such as collagenase IV could also induce the effect I described needs to be determined. We chose stromelysin because it is not present during late pregnancy and lactation, but is increased during involution.
Terry Opgenorth: In the stromelysin transgenic mice that produce tumors, is the tumorigenesis hormone dependent? Does this occur in virgin animals?
Mina Bissell: We do get tumors even in virgin animals, but not as frequently. However, it should be remembered that the stromelysin gene is under the control of the whey acidic promoter which is really expressed at high levels only during late pregnancy and lactation. Thus the dependence on pregnancy “hormones” may be more apparent than real (i.e., the animal has to get pregnant in order to deliver the transgene which then is responsible for destroying the basement membrane and giving rise to tumors).
Nelson Horseman: Could you describe further how you conceive the ECM effect via the BCE1 site, given your observation that DNA binding activity does not change in response to ECM treatment?
Mina Bissell: We postulated that it is specific or ubiquitous factors that are present in the cell and the nucleus, but they are not at the right place or are not modified correctly unless the cells are placed on the appropriate substratum or change structure by other means. This change would reorganize the chromatin and bring the right factors together to allow the promoter to fire.
Nelson Horseman: Wouldn’t you see evidence of such factors in the deletion studies?
Mina Bissell: There appears to be a third possible site which we have not analyzed well enough. However, the factor (or factors) need not directly bind DNA. Indeed, in most probability, it would be needed to form correct complexes by protein-protein interactions. We are currently testing these ideas by in vivo footprinting and exonuclease mapping in collaboration with Gordon Hager (NIH).
Acknowledgments
This work was supported by the U.S. Department of Energy, Office of Health and Environmental Research (contracts DE-AC03–76-SF01012 and DE-AC03–76-SF0098), and by the National Institutes of Health (NCI CA 57621) to M.J. Bissell. S. Lelièvre is supported by a research fellowship from the International Agency for Research on Cancer, The World Health Organization. V.M. Weaver is supported by a research fellowship from the Canadian Medical Research Council.
References
- Adams JC, Watt FM. Nature (Lond) 1989;340:307–309. doi: 10.1038/340307a0. [DOI] [PubMed] [Google Scholar]
- Ashkenas J, Damsky CH, Bissell MJ, Werb Z. The Integrins. Academic Press; New York: 1994. Integrins, signaling, and the remodeling of the extracellular matrix; pp. 79–109. [Google Scholar]
- Bidwell JP, Van Wijnen AJ, Fey EG, Dworetzky S, Penman S, Lian JB, Stein JL, Stein GS. Proc Natl Acad Sci USA. 1993;90:3165–3166. doi: 10.1073/pnas.90.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell MJ, Hall G. Forms and function in the mammary gland : the role of extracellular matrix. In: Neville M, Daniels C, editors. The Mammary Gland. Plenum Press Publishing Corp; New York: 1987. pp. 97–146. [Google Scholar]
- Bissell MJ, Hall HG, Parry G. J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Myers C, Bissell MJ. Trends in Cell Biol. 1995a;5:1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Science. 1995b;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon TG, Demmer J, Clark DJ, Watson CJ. FEBS Lett. 1994;350:177–182. doi: 10.1016/0014-5793(94)00757-8. [DOI] [PubMed] [Google Scholar]
- Chen LH, Bissell MJ. Cell Regul. 1989;1:45–54. doi: 10.1091/mbc.1.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin JR, Murphy G, Werb Z. J Biol Chem. 1985;260:12367–12376. [PubMed] [Google Scholar]
- Dacheng H, Nickerson JA, Penman S. J Cell Biol. 1990;100:569–580. doi: 10.1083/jcb.110.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Werb Z. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Oborn CJ, Durban EM, Beutel JS, Medina D. Proc Natl Acad Sci USA. 1984;81:3756–3760. doi: 10.1073/pnas.81.12.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez PY, Roskelley CD, Campisi J, Bissell MJ. Mol Cell Differentiation. 1993;1:99–110. [Google Scholar]
- Desprez PY, Hara E, Bissell MJ, Campisi J. Mol Cell Biol. 1995;15:3398–3404. doi: 10.1128/mcb.15.6.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doppler W, Groner B, Ball RK. Proc Natl Acad Sci USA. 1989;86:104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman JT, Pitelka DR. In Vitro. 1977;13:316–328. doi: 10.1007/BF02616178. [DOI] [PubMed] [Google Scholar]
- Fingert HJ, Campisi J, Pardee AB. Cell proliferation and differentiation. In: Holland JF, Frei E, Kufe DW, Morton DL, Weichselbaum RR, editors. Cancer Medicine. Lea & Febiger; Philadelphia: 1993. pp. 1–14. [Google Scholar]
- Fuortes M, Jin WW, Nathan C. J Cell Biol. 1993;120:777–784. doi: 10.1083/jcb.120.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlsen KR, Sriramarao P, Furcht LT, Skubitz APN. J Cell Biol. 1992;117:449–459. doi: 10.1083/jcb.117.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg GI, Eisen AZ. Extracellular matrix metalloproteinases in tumor invasion and metastasis. In: Lippman ME, Dickson RB, editors. Regulatory Mechanisms in Breast Cancer. Advances in cellular and molecular biology of breast. Kluwer Academic Publishers; Boston: 1991. pp. 421–440. [DOI] [PubMed] [Google Scholar]
- Goodman HS, Rosen JM. Mol Endocrinol. 1990;11:1661–1670. doi: 10.1210/mend-4-11-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JL, Shalloway D. Nature. 1992;358:690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juna EJ, Hitt AL. Science. 1992;258:955–964. doi: 10.1126/science.1439807. [DOI] [PubMed] [Google Scholar]
- Kingston RE. Curr Opin Cell Biol. 1989;1:1081–1087. doi: 10.1016/s0955-0674(89)80054-7. [DOI] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- Lelièvre S, Soria J, Larsen AK. Bull Cancer. 1995;82:486. [Google Scholar]
- Lin CQ, Bissell MJ. FASEB J. 1993;7:737–743. doi: 10.1096/fasebj.7.9.8330681. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Fink LM, Pierce GB. Lab Invest. 1976;34:455–462. [PubMed] [Google Scholar]
- Martins-Green M, Bissell MJ. Sem Devel Biol. 1995;6:149–159. [Google Scholar]
- Pienta K, Coffey DS. J Cell Biochem. 1992;49:357–365. doi: 10.1002/jcb.240490406. [DOI] [PubMed] [Google Scholar]
- Razin SV, Gromova I. Bioassays. 1995;17:443447. doi: 10.1002/bies.950170512. [DOI] [PubMed] [Google Scholar]
- Reid LM. Curr Opin Cell Biol. 1990;2:121–130. doi: 10.1016/s0955-0674(05)80042-0. [DOI] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR. Annu Rev Neurosci. 1989;19:491–516. doi: 10.1146/annurev.ne.12.030189.002423. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Bissell MJ. Mol Carcinogenesis. 1994;10:66–71. doi: 10.1002/mc.2940100203. [DOI] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. Mol Biol Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Bissell MJ, Myers CA, Casperson GF. Proc Natl Acad Sci USA. 1990;87:9118–9122. doi: 10.1073/pnas.87.23.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. Trends Cell Biol. 1992;2:304–308. doi: 10.1016/0962-8924(92)90120-c. [DOI] [PubMed] [Google Scholar]
- Strange R, Li F, Saurer S, Burkhardt A, Fuis RR. Development. 1992;115:49–58. doi: 10.1242/dev.115.1.49. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Mammary epithelial cells, extracellular matrix, and gene expression. In: Lippman M, Dickson R, editors. Regulatory Mechanisms in Breast Cancer. Kluwer Academic Publishers; Boston: 1991. pp. 365–381. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz APN, Roskelley C, Bissell MJ. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Chin JR, Unemori EN, Werb Z, Bissell MJ. Development (Camb) 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryggvason K. Curr Opin Cell Biol. 1993;188:1271–1282. doi: 10.1016/0955-0674(93)90038-r. [DOI] [PubMed] [Google Scholar]
- Wakao H, Gouilleux H, Groner B. EMBO J. 1994;13:2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker NI, Bennett RE, Kerr JF. Am J Anat. 1989;185:19–26. doi: 10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]