Abstract
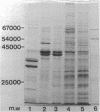
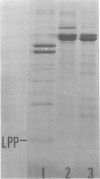
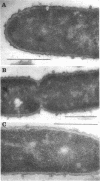
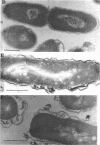
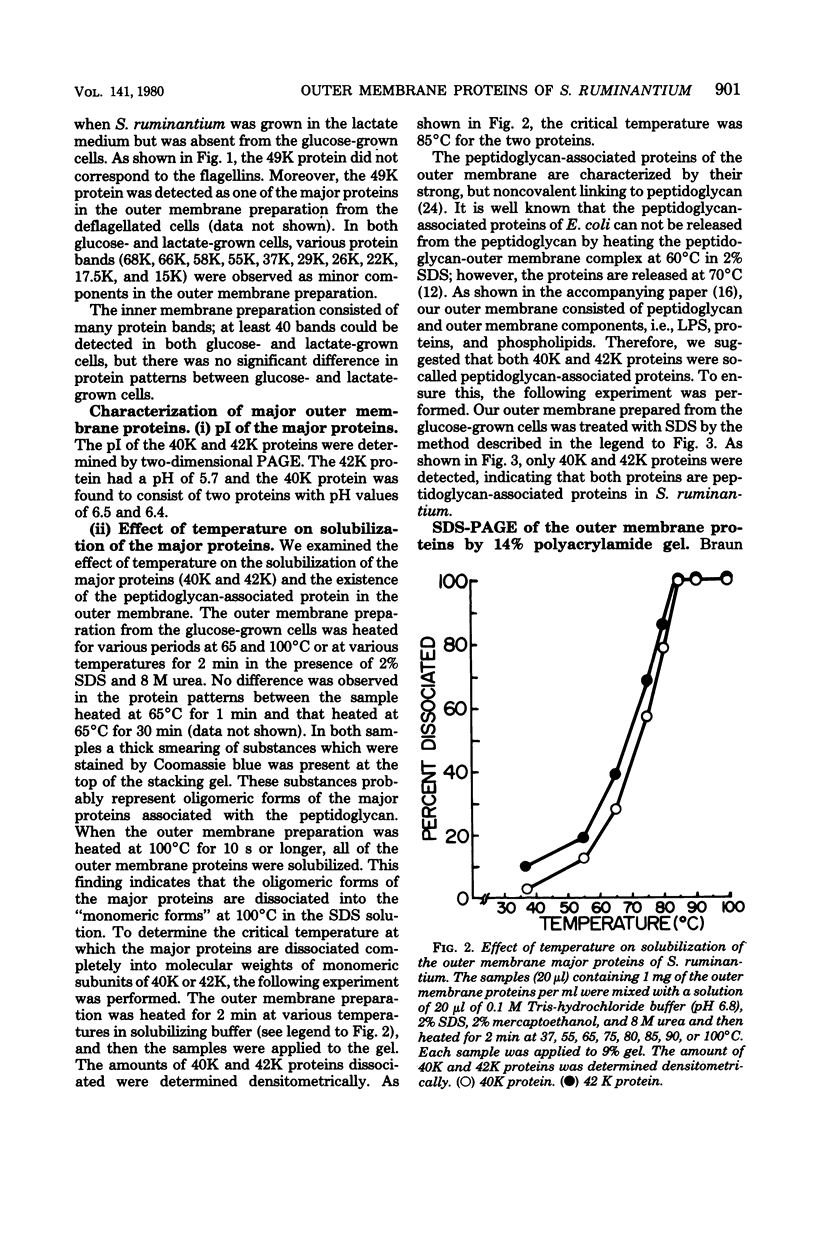
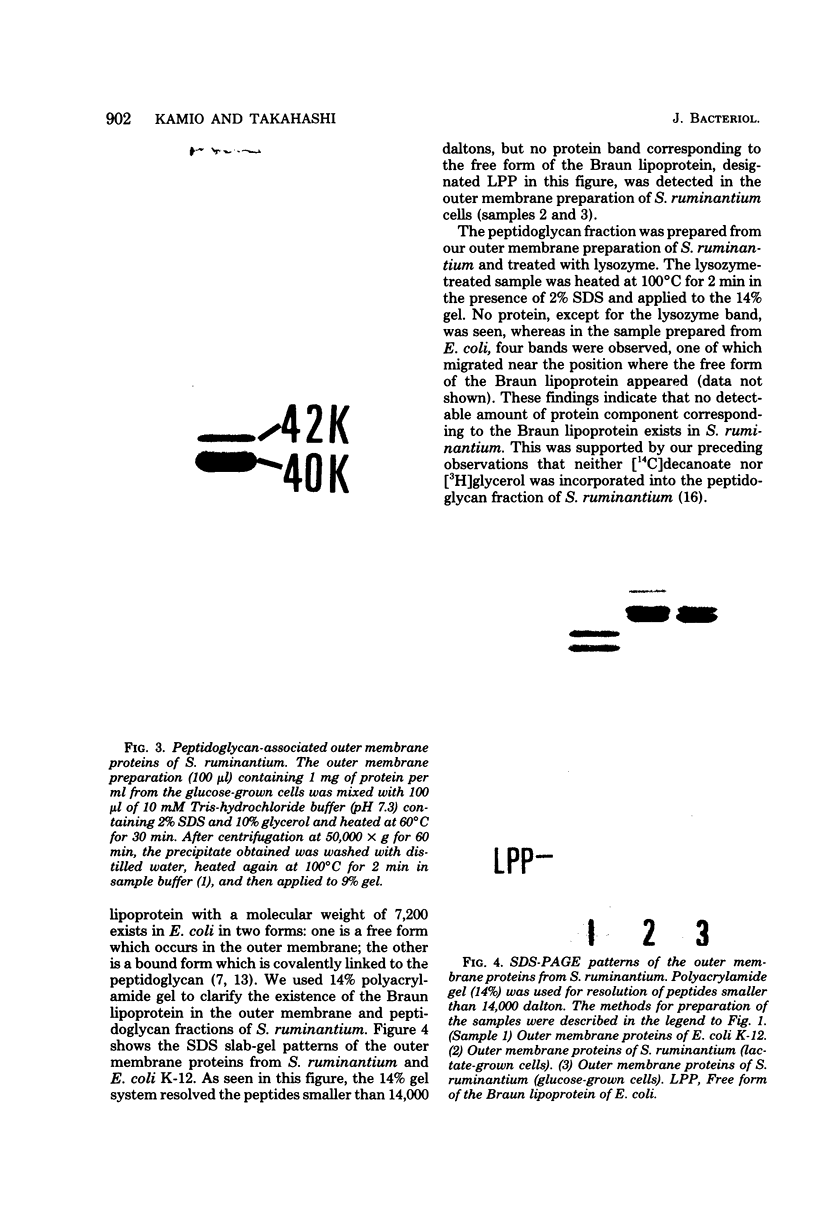
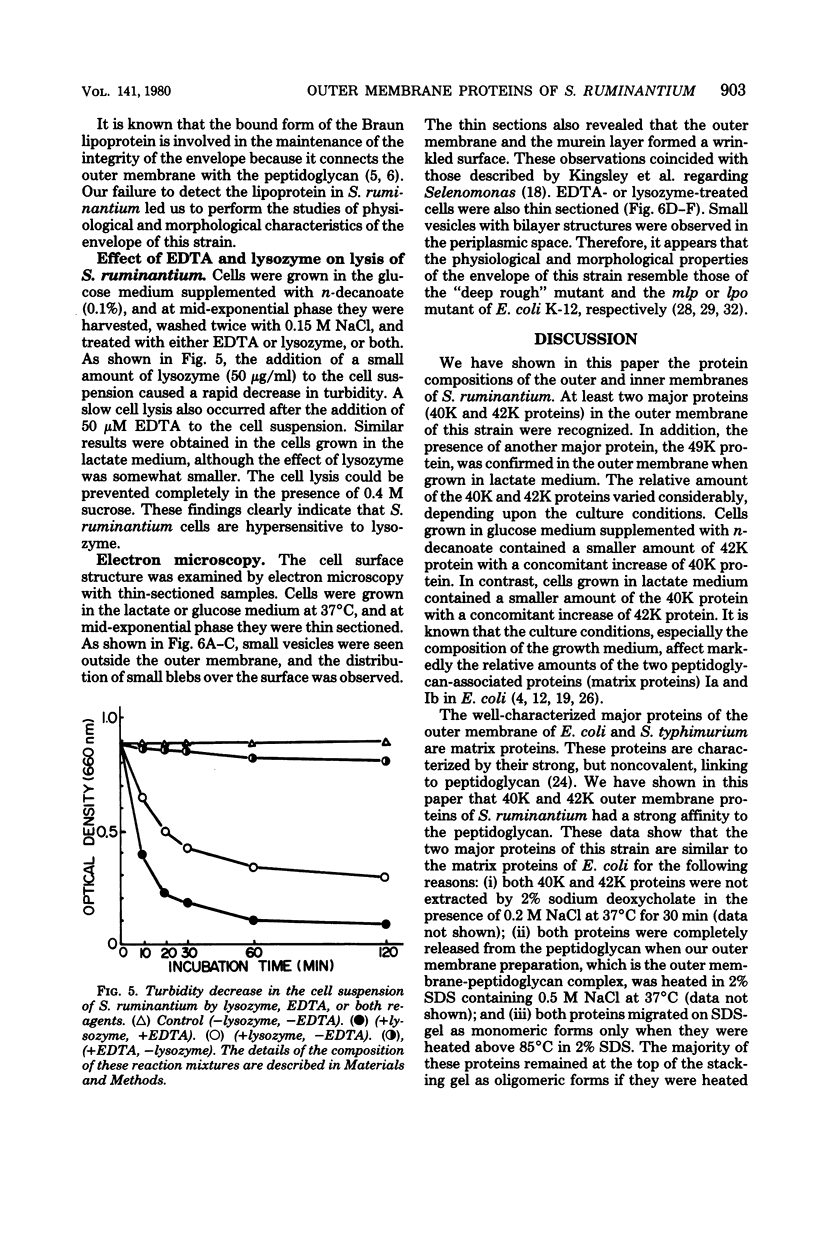
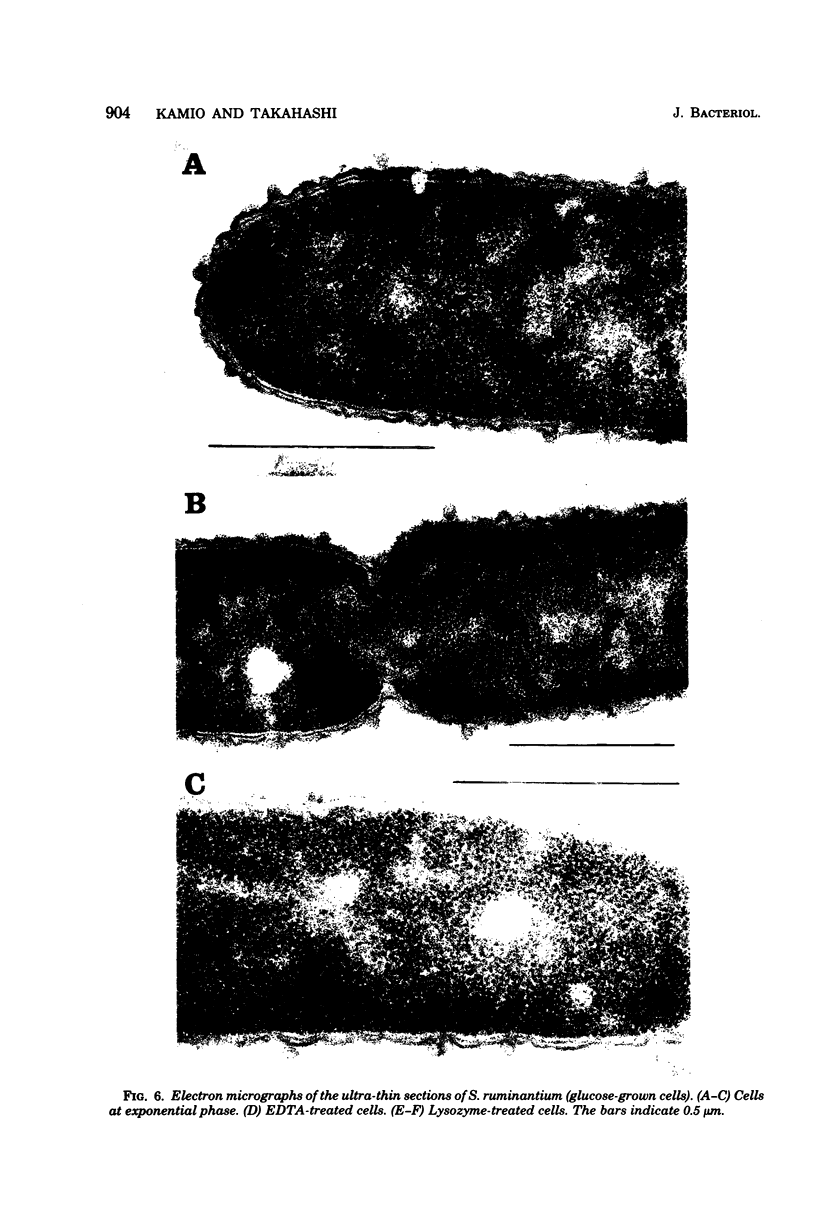
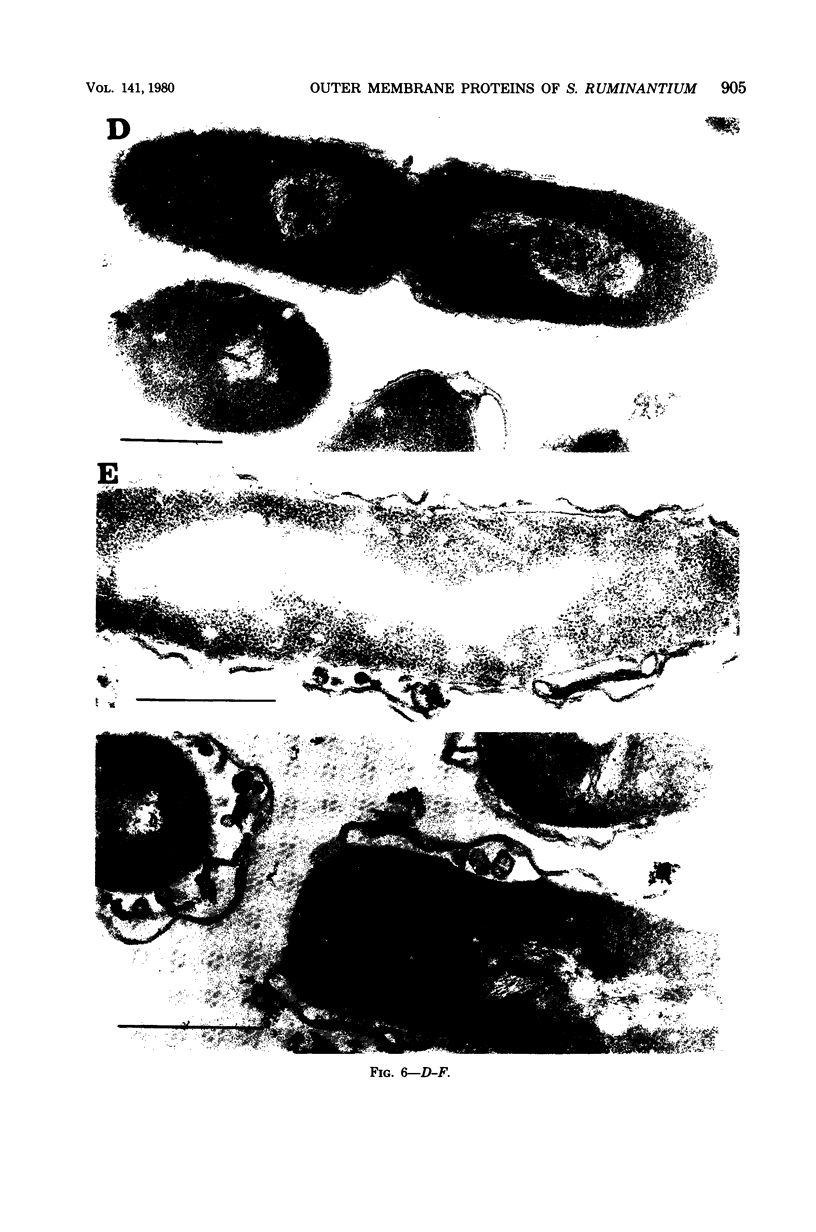
The protein compositions of the membrane preparations from Selenomonas ruminantium grown in glucose or lactate medium were determined by sodium dodecyl sulfate- and two-dimensional (first, isoelectric focusing; second, sodium dodecyl sulfate) polyacrylamide slab gel electrophoresis. The outer membrane from both glucose- and lactate-grown cells contained two major proteins with apparent molecular weights of 42,000 and 40,000. These proteins existed as peptidoglycan-associated proteins in the outer membrane. The critical temperature at which they were dissociated completely into the monomeric subunits of 42,000 and 40,000 daltons was found to be 85 degrees C. The amount of each protein varied considerably depending upon the cultural conditions. The absence of the lipoprotein of Braun in S. ruminantium was suggested in our preceding paper (Y. Kamio, and H. Takahashi, J. Bacteriol. 141:888--898, 1980), and the possible absence of the protein components corresponding to the Braun lipoprotein in this strain was confirmed by electrophoretic analysis of the outer membrane and the lysozyme-treated peptidoglycan fractions. Examination of the cell surface of S. ruminantium by electron microscopy showed that the outer membrane formed a wrinkled surface with irregular blebs, some of which pinched off forming vesicles of various sizes. Rapid cell lysis occurred with the addition of a low level of lysozyme to the cell suspension. These findings led us to conclude that the physiological and morphological properties of this strain were similar to those of "deep rough" and mlp or lpo mutants of Escherichia coli K-12, respectively.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Resolution of bacterial proteins by polyacrylamide gel electrophoresis on slabs. Membrane, soluble, and periplasmic fractions. J Biol Chem. 1974 Jan 25;249(2):634–644. [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassford P. J., Jr, Diedrich D. L., Schnaitman C. L., Reeves P. Outer membrane proteins of Escherichia coli. VI. Protein alteration in bacteriophage-resistant mutants. J Bacteriol. 1977 Aug;131(2):608–622. doi: 10.1128/jb.131.2.608-622.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Bosch V. Repetitive sequences in the murein-lipoprotein of the cell wall of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Apr;69(4):970–974. doi: 10.1073/pnas.69.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Hantke K. Biochemistry of bacterial cell envelopes. Annu Rev Biochem. 1974;43(0):89–121. doi: 10.1146/annurev.bi.43.070174.000513. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay P. K., Lai J. S., Wu H. C. Incorporation of phosphatidylglycerol into murein lipoprotein in intact cells of Salmonella typhimurium by phospholipid vesicle fusion. J Bacteriol. 1979 Jan;137(1):309–312. doi: 10.1128/jb.137.1.309-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay P. K., Wu H. C. Biosynthesis of the covalently linked diglyceride in murein lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5318–5322. doi: 10.1073/pnas.74.12.5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Inouye M., Shaw J., Shen C. The assembly of a structural lipoprotein in the envelope of Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):8154–8159. [PubMed] [Google Scholar]
- Kamio Y., Ishihara M., Terawaki Y. Alteration of flagella by a temperature sensitive R plasmid Rtsl in Escherichia coli K-12. Biochem Biophys Res Commun. 1978 Nov 14;85(1):301–308. doi: 10.1016/s0006-291x(78)80043-6. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium. Identification of proteins exposed on cell surface. Biochim Biophys Acta. 1977 Feb 4;464(3):589–601. doi: 10.1016/0005-2736(77)90033-5. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Nikaido H. Outer membrane of Salmonella typhimurium: accessibility of phospholipid head groups to phospholipase c and cyanogen bromide activated dextran in the external medium. Biochemistry. 1976 Jun 15;15(12):2561–2570. doi: 10.1021/bi00657a012. [DOI] [PubMed] [Google Scholar]
- Kamio Y., Takahashi H. Isolation and characterization of outer and inner membranes of Selenomonas ruminantium: lipid compositions. J Bacteriol. 1980 Feb;141(2):888–898. doi: 10.1128/jb.141.2.888-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley V. V., Hoeniger J. F. Growth, structure, and classification of Selenomonas. Bacteriol Rev. 1973 Dec;37(4):479–521. doi: 10.1128/br.37.4.479-521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T., Nikaido H. Outer membrane as a diffusion barrier in Salmonella typhimurium. Penetration of oligo- and polysaccharides into isolated outer membrane vesicles and cells with degraded peptidoglycan layer. J Biol Chem. 1975 Sep 25;250(18):7359–7365. [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. IV. Differences in outer membrane proteins due to strain and cultural differences. J Bacteriol. 1974 May;118(2):454–464. doi: 10.1128/jb.118.2.454-464.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Yasuda S., Nishimura A., Yamada M., Hirota Y. Murein-lipoprotein of Escherichia coli: a protein involved in the stabilization of bacterial cell envelope. Mol Gen Genet. 1978 Nov 16;167(1):1–9. doi: 10.1007/BF00270315. [DOI] [PubMed] [Google Scholar]
- Tamaki S., Matsuhashi M. Increase in sensitivity to antibiotics and lysozyme on deletion of lipopolysaccharides in Escherichia coli strains. J Bacteriol. 1973 Apr;114(1):453–454. doi: 10.1128/jb.114.1.453-454.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Hou C., Lin J. J., Yem D. W. Biochemical characterization of a mutant lipoprotein of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1388–1392. doi: 10.1073/pnas.74.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizushima S. Lipoprotein-bearing peptidoglycan sacculus as a preferred site for the in vitro assembly of membrane from dissociated components of outer membrane of Escherichia coli K-12. J Biochem. 1977 Jun;81(6):1889–1899. doi: 10.1093/oxfordjournals.jbchem.a131651. [DOI] [PubMed] [Google Scholar]
- Yem D. W., Wu H. C. Physiological characterization of an Escherichia coli mutant altered in the structure of murein lipoprotein. J Bacteriol. 1978 Mar;133(3):1419–1426. doi: 10.1128/jb.133.3.1419-1426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Stimulation by lipopolysaccharide of the binding of outer membrane proteins O-8 and O-9 to the peptidoglycan layer of Escherichia coli K--12. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1397–1402. doi: 10.1016/0006-291x(77)90597-6. [DOI] [PubMed] [Google Scholar]