Abstract
Fenvalerate (Fen), widely used for its high insecticidal potency and low mammalian toxicity, is classified as an endocrine-disrupting chemical. Recently, Fen has received great attention for its adverse effects on human reproductive health. In this study, we found that Fen (10 µM) had a stimulatory effect on the growth of both cell lines at 24 h compared with controls by MTS (p<0.01) and BrdU (p<0.01) assays by MTS colorimetric and bromodeoxyuridine (BrdU) uptake assays in hormonally responsive uterine leiomyoma (UtLM) cells and normal uterine smooth muscle cells (UtSMC). Flow cytometry reuslts showed that Fen enhanced the escape of cells from the G0-G1 checkpoint and promoted progression of both cell types into the S phase. An Annexin V assay showed that Fen had an anti-apoptotic effect on both cell types. By Real-time PCR, we found that collagen I mRNA expression increased (p<0.05) in Fen-treated cells compared to controls, although it was greater in UtLM tumor cells. Accordingly, Fen increased (p<0.05) collagen I protein level in both cell lysate and supernatant when compared to controls. To further test the mechanism of Fen’s effects, transactivation and competitive binding assays were done. The results showed Fen did not significantly stimulate the luciferase activity at concentrations of 0.1, 1.0 or 10.0 µM in either of the cell types. Competitive binding assays revealed that the affinity of Fen binding to estrogen receptors (ERs) is non-detectable compared to E2. Our data show that Fen can stimulate the growth of both UtLM cells and UtSMC, which involves a combination of enhanced cell-cycle progression and inhibition of apoptosis. Also this compound can increase collagen I expression, at both mRNA and protein levels. Interestingly, ER is less likely involved in either the hyperplasia or extracellular matrix (ECM) overproduction induced by Fen. Our results indicate that Fen exposure could be considered a novel risk factor for uterine fibroids through molecular mechanisms that do not directly involve the ERs.
Keywords: Leiomyoma, myometrium, fenvalerate, cell growth, ECM, collagen type I
1. Introduction
Uterine leiomyomas (fibroids; myomas) are one of the most common tumors clinically affecting reproductive-age women (Stewart, 2001; Walker and Stewart, 2005). Symptoms that are caused by fibroids include dysmenorrhea, recurrent miscarriage, pelvic pain and pressure, and other obstetric complications (Walker and Stewart, 2005). Nearly 50% to 80% of women will develop fibroids during their lives, and fibroids are responsible for more than 200,000 hysterectomies annually (Stewart, 2001). To date, identification and reduction of exposures to potential risk factors for fibroids is still a first line strategy in the prevention of this disease.
Many risk factors for the development of uterine leiomyomas have been examined (Flake et al., 2003; Walker and Stewart, 2005). Most of these risk factors have not been linked definitively, but causally to fibroid development and growth, and many reflect the importance of the hormonal milieu in the pathogenesis of these tumors (Flake et al., 2003; Schwartz et al., 2000). Furthermore, many studies have detailed the effects of steroid hormones on leiomyoma and myometrial growth, and the induction of hormone responsive genes (Swartz et al., 2005; Walker and Stewart, 2005). It has become widely accepted that estrogen is one of the main regulators of leiomyoma growth and certainly a major risk factor for development of this benign tumor.
As reported, leiomyomas consist of smooth muscle tumor cells and often have an abundant extracellular matrix (ECM) component, which can be up to 50% or greater than the corresponding smooth muscle component (Arslan et al., 2005; Ding et al., 2004; Sozen and Arici, 2002). Aberrant ECM metabolism has been thought to contribute to the pathogenesis of uterine leiomyomas (Ohara, 2009). Acting to up-regulate collagen synthesis, estrogen modulates collagen metabolism and induces the ECM-remodeling enzymes and collagen synthesis in uterine leiomyoma tissue and cells (Zbucka et al., 2007). The ECM of fibroids consists mainly of collagen types I and III, fibronectin, and proteoglycans. The excessive and dysregulated ECM probably plays an important role in the pathogenesis of uterine leiomyomas because it can not only enlarge tumors, but also influence the fate of resident tumor cells and serve as a reservoir for many cytokines and growth factors. Meanwhile, smooth muscle tumor cell proliferation has been validated in fibroids by many different groups including ours (Ding et al., 2004; Dixon et al., 2002; Houston et al., 2001; Moore et al., 2007; Wolanska et al., 1998).
Nearly a decade ago, hormone replacement therapy (HRT), usually in the form of estrogen, was commonly prescribed for women experiencing perimenopausal or menopausal symptoms (Ellerington et al., 1992). This kind of exogenous estrogen was considered and known to be an important risk for the development of fibroids (Sener et al., 1996; Whitcroft and Stevenson, 1992). As a result of the efforts of the FDA to strictly regulate “Hormone Therapy Drugs” due to their side effects, the high-risk of exposure of women to exogenous hormones has been reduced to a large extent. However, the incidence of fibroids still remains high, as a consequence, the search for possible hidden risks or sources of environmental exposures becomes more and more important.
Fenvalerate (Fen), one of the most common pesticides, currently widely used in agriculture and indoor. It has been considered as one of the endocrine-disrupting chemicals (EDCs) and estrogen “analogue” by the WHO (1991). Fen has been documented to have adverse effects on the nervous system, reproduction, and other human health endpoints (Chen et al., 2005; Moniz et al., 1999; Xia et al., 2004; Xiao et al., 2006). The questions asked are whether Fen plays a role in the pathogenesis or progression of uterine fibroids similar to estrogen, and if so, whether the effects are mediated by the estrogen receptor alpha (ERα) and or beta (ERβ).
2. Materials and Methods
2.1 Tissue Culture
Human UtLM cells (GM10964; Coriell Institute for Medical Research, Camden, NJ, USA) and UtSMCs (Clonetics Corporation, San Diego, CA, USA) were kept in a standard tissue culture incubator at 37°C with 5% carbon dioxide as previously reported (Swartz et al., 2005). The UtLM cells were routinely cultured in GM medium: Eagle’s minimum essential medium (Invitrogen (Gibco), Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Sigma St Louis, MO, USA) and supplemented with vitamins (Invitrogen (Gibco)), essential and non-essential amino acids (Invitrogen (Gibco)) and L-glutamine (Invitrogen (Gibco)). The UtSMCs were cultured in Smooth Muscle Cell Growth Media System (SmGM®-2 BulletKit; Clonetics). Cells were allowed to grow to 70% confluency before being exposed to concentrations of Fen (CAS# 51630-58-1; α-Cyano-3-phenoxybenzyl α-(4-chlorophenyl)-iso-valerate; #45495, Sigma-Aldrich (Fluka), St Louis, MO, USA) ranging from 0.01 to 100 µM. 24 h prior to treatment, the media were changed to Dulbecco’s Modified Eagle’s Medium Nutrient Mixture F-12 Ham (DMEM/F-12) (Hyclone Laboratories, Logan, UT, USA) phenol red free with charcoal dextran treated (stripped) FBS (Hyclone) for both cell types.
2.2 MTS Cell proliferation assay
UtLM cells and UtSMCs were seeded at 5 × 103 cells/well and 4 × 103 cells/well, respectively, in 96-well culturing plates (Corning, NY, USA). After the media were changed to DMEM/F-12 phenol red free with stripped FBS for 24 h, the cells were treated with Fen or β-Estradiol (E2; E2257, Sigma, St. Louis, MO, USA; positive control). Fen was reconstituted using 0.1% DMSO (Sigma) before it was diluted in the media. The cells were exposed to 0 µM (DMSO; control), 0.01, 0.1, 1, 10, 100µM Fen or 0.1 µM E2 for 24 h. The number of viable cells was observed using an MTS-based CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA), according to the manufacturer’s instructions. In brief, the CellTiter 96® AQueous One Solution was added to the plate and allowed to incubate for 2 h at 37°C, with 5% CO2, prior to reading the absorbance values at 490 nm using a plate reader (Molecular Devices Corporation, Sunnyvale, CA, USA). The samples were analyzed using SOFTmax Pro software (Molecular Devices Corporation, Downingtown, PA, USA). Dye intensity was proportional to the number of viable cells.
2.3 BrdU Incorporation Assay
The UtLM cells and UtSMC were plated in DMEM with 10% FBS in 96-well culture plates at 5 × 103 cells/well and 4 × 103 cells/well, respectively. 24 h after seeding, the media were changed to DMEM/F-12 supplemented with 10% stripped FBS. To analyze the effects of Fen on replication, the cells were cultured in the presence of Fen (0, 0.01, 0.1, 1, 10, 100 µM) or E2 (0.1 µM) for 24 h. Next, the BrdU incorporation into DNA was detected by immunoassay using a Bromodeoxyuridine (BrdU) Cell Proliferation Assay according to the manufacturer’s instructions (Cat. # QIA58, Calbiochem, Gibbstown, NJ, USA). In brief, the cells were incubated with BrdU label (1:2000) for 12 h. After removal of the culture medium, the cells were fixed with fixative/denaturing solution and incubated with Anti-BrdU antibody (1:100) for 1 h. Then the cells were incubated with Peroxidase Goat Anti-Mouse IgG Horse Radish Peroxidase (HRP) conjugate in conjugate diluent for 30 min. The cells were then incubated with a tetramethyl-benzidine substrate until color development was sufficient for photometric detection. The reaction product was quantified by measuring the absorbance with an ELISA reader (Molecular Devices Corporation, Sunnyvale, CA, USA) at 450 nm. The samples were analyzed with SOFTmax Pro software (Molecular Devices Corporation).
2.4 Cell Cycle Analysis by Flow Cytometry
Flow cytometry was used to assess cell growth and to determine the percentage of cells in various phases of the cell cycle. UtLM cells and UtSMCs were treated with 0 µM (DMSO; control), 10 µM Fen or 0.1 µM E2, and then incubated in culture for 24 h. Both cell types were resuspended in 1 ml of Propidium Iodide (PI) staining solution (20 ug/ml PI/ 10 Units/ml Rnase One-Promega in 1X PBS) at a density of 10,000 cells/ml for 20 min in the dark at room temperature. Then the samples were examined using a Becton Dickson Fluorescence-Activated Cell Sorting (FACS) Flow Cytometer (Franklin Lakes, NY, USA) with CellQuest software by initially gating on an area versus width PI dot plot. A minimum of 10,000 cells were collected in list mode files. PI histograms were used to analyze the cell cycle.
2.5 Annexin V-FITC Assay of Apoptosis
Induction of apoptosis and cell death was assessed using Annexin V-FITC Kit (Trevigen, Gaithersburg, MD) together with PI according to the manufacturer’s instructions. The assay was performed in UtLM and UtSMC cultures 24 h after treatment with control, 10 µM Fen or 0.1 µM E2. Each sample was added with 100 µl of Annexin V incubation reagent and incubated in the dark for 15 minutes at room temperature. Then 400 µl 1X binding buffer was added to each sample before examination. The analysis was performed on 10,000 cells using a Becton Dickinson FACS flow cytometer (Franklin Lakes, NY, USA), equipped with CellQuest software.
2.6 Western Blot
Protein was isolated using radioimmunoprecipitation assay (RIPA) buffer as previously described (Yu et al., 2008). Aliquots of the protein extracted from cultured cells treated with Fen for 24 h underwent electrophoreses on a sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and were electro-transferred onto PVDF membranes (Invitrogen, Carlsbad, CA). For the detection of protein, blots were incubated with a mouse monoclonal antibody against collagen I (CP17L; dilution 1:200, Calbiochem, USA). An HRP-conjugated secondary antibody (NA931V, ECL ™; GE Healthcare UK Limited) was used for detection. As an internal standard between the samples, HRP-labeled anti-human HPRT (sc-20975; Santa Cruz Biotechnology) was used. Films were exposed to Hyperfilm ECL for 5 min, and band intensities were quantitated using a densitometer (AlphaView, FluorChem SP, Alpha Innotech Corporation, San Leandro, CA, USA).
2.7 Real-Time Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analyses
Total RNA was extracted from cells with TRIZOL® Reagent (Invitrogen, Carlsbad, CA) and an RNeasy® Mini Kit (QIAGEN, Valencia, CA). RT was carried out using 2 µg RNA, and cDNA was generated using the SuperScript™ First-Strand Synthesis System for RT-PCR according to the manufacturer’s instructions (Invitrogen). RT-PCR was performed using the ABI Prism 7700 HT Sequence Detection System with SYBR® Green PCR Master Mix reagents (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. GAPDH was used to normalize target gene expression. The sequences for each primer set were as follows: Collagen I, 5’-GTGCTAAAGGTGCCAATGGT-3’ (forward) and 5’-ACCAGGTTCACCGCTGTTAC-3’ (reverse), and GAPDH, 5’- GAGTCAACGGATTTGGTCGT-3’ (forward) and 5’-TTGATTTTGGAGGGATATCG-3’ (reverse). Data were obtained as Ct values (cycle number at which PCR plots cross a calculated threshold line) and used to determine ΔCt values (Ct of target gene - Ct of housekeeping gene, GAPDH). These values were then used to generate mean ΔCt values ± SD for each treatment, which were employed in statistical comparisons. Visual representation of data was carried out by converting ΔCt values to fold change data relative to ΔCt values for untreated cells using the equation 2-ΔΔCt.
2.8 Transient transfection and luciferase assay in UtLM cells and UtSMCs
UtLM cells and UtSMCs were plated in 12-well plates at a density of 1 ×105 cells/well in DMEM containing 10% FBS and incubated overnight. Cells were transfected for 6 h with 3×-Vit-ERE-TATA-Luc, pRL-CMV (constitutively active Renilla reporter plasmid; Promega), pcDNA3-hERα or pRST7-ERβ by using FuGENE®6 transfection reagent (Roche Applied Science, Indianapolis, IN, USA). Afterward, we treated cells for 24 h with 10 nM E2 or various concentrations of Fen in the presence or absence of 1 µM ICI 182,780 (Sigma). We then assayed cells for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega) and an LMAXII luminometer (Molecular Devices, Sunnyvale, CA, USA).
2.9 Estrogen receptor α and β competitive binding assay
Binding affinity of E2 and Fen to ERα and ERβ was evaluated by fluorescence polarization, following the instructions provided by Estrogen Receptor Competitor Assay Red Kit (P3029 and P3032, Invitrogen). This assay, which contains insect cell-expressed, full-length, untagged, human ERα and ERβ, and a novel, tight-binding fluorescent estrogen ligand (Fluormone™ EL Red), provides a sensitive and efficient method for a high-throughput screening of potential ERα and ERβ ligands in a homogenous mix-and-read assay format. According to the protocol, in a black 96-well plate, E2 or Fen at increasing concentrations were added to the equal volume of 2× ER/Fluormone™ EL Red Complex containing a final concentration of 30 nM ERα or 60 nM ERβ, and 2 nM Fluormone™ EL Red in ER Red Assay Buffer. The plates were gently shaken to mix well and covered to protect the reagents from light, and incubate at room temperature for 4 hours. Finally, fluorescence polarization was measured for each well using a Wallac VICTOR3 V instrument (Perkin-Elmer, USA).
2.10 Statistical Analysis
MTS assay, cell cycle analysis, apoptosis assay, real-time PCR, western blot analysis, transient transfection, and receptor competitor assay were repeated at least three independent experiments with comparable results. BrdU assay were done in duplicate using 8 replicates per plate, while luciferase assay and competitive binding assays were done in triplicate using 3 replicates per plate. Cell cycle analysis data were statistically analyzed by a two-way ANOVA with Bonferroni post-tests after arcsine transformation. The data were expressed as mean ± SEM. The two-tailed Student's t-test was used to compare two different groups. The statistical significance was defined as p < 0.05. The data were analyzed by using Prism 5 (GraphPad Software, Inc).
3. Results
3.1 Cell morphology in UtLM cells and UtSMCs after treatment with Fen for 24 h
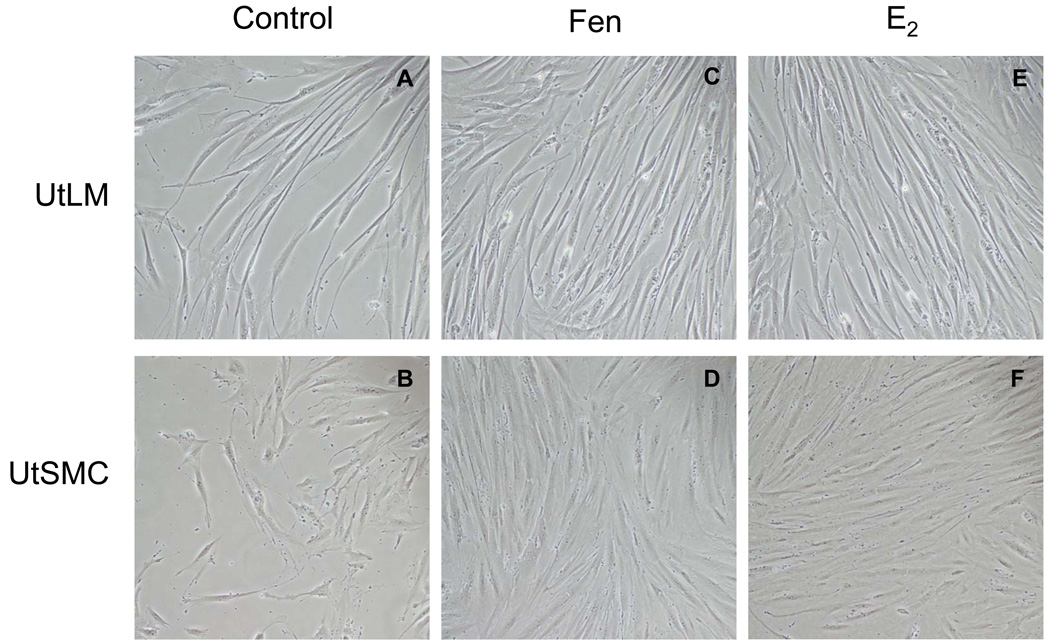
Control UtLM cells and UtSMCs appeared robust and were spindle shaped with intact oval to elongated nuclei (Figure 1 A, B). After treatment with 10 µM Fen for 24 h, both cells types had a similar histomorphology to the controls, but showed cellular crowding, suggestive of proliferation (Figure 1 C, D) and similar to UtLM cells and UtSMC treated with 0.1 µM E2 (Figure 1 E, F).
Figure 1. Cell morphology.
Morphology of UtLM cells and UtSMCs after treatment with 0, 10 µM Fen and 0.1 µM E2 at 24 h. (A) UtLM cells and (B) UtSMCs at 0 µM; (C) UtLM cells and (D) UtSMCs at 10 µM of Fen; (E) UtLM cells and (F) UtSMCs at 0.1 µM of E2. Original Magnification ×200.
3.2 Cell proliferation of Fen in UtLM cells and UtSMCs with MTS assay
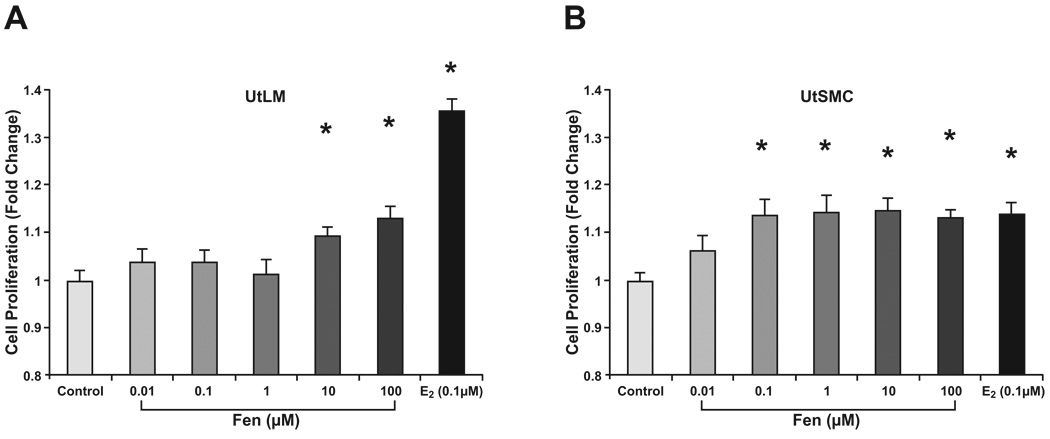
To assess the influence of Fen exposure on human UtLM cells and UtSMCs, we conducted proliferation studies with Fen at concentrations of 0.01 µM to 100 µM. At 24 h, both UtLM and UtSMC cells showed significantly (p < 0.01) increased proliferation with Fen treatment, as measured by an MTS-based assay (Fig. 2). Compared to vehicle controls, UtLM cell proliferation was increased at Fen concentrations of 10 to 100 µM range (Fig. 2A), while UtSMC cell proliferation was increased in the 0.1 to 100 µM range (Fig. 2B). E2 at a concentration of 0.1 µM served as a positive control.
Figure 2. Cell proliferation assay with MTS.
UtLM cells (A) and UtSMCs (B) were exposed to Fen for 24 h at concentrations ranging from 0.01 µM to 100 µM. E2 at a concentration of 0.1 µM was used as a positive control. Compared with proliferation in vehicle controls, UtLM cell proliferation was increased at 10 µM to 100 µM Fen; while UtSMC cell proliferation was increased at 0.1 to 100 µM Fen. Error bars represent means ± SEM. *p<0.01, Student’s t test with vehicle control.
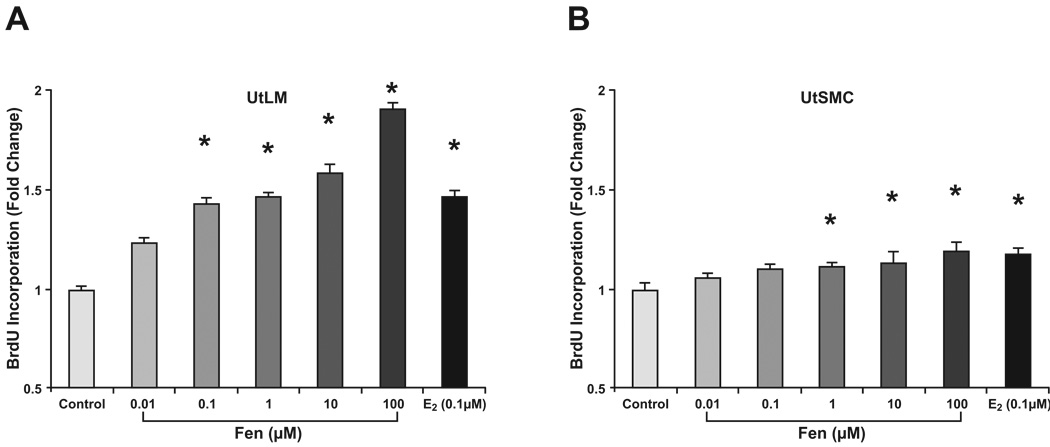
3.3 Cell proliferation of Fen in UtLM cells and UtSMCs with BrdU assay
To further examine the effects of Fen on UtLM and UtSMC cell growth, DNA synthesis and BrdU uptake were determined by BrdU labeling. We found significantly increased BrdU labeling in Fen-treated UtLM cells and UtSMC compared to untreated controls (Fig. 3). UtLM BrdU labeling was increased at 0.1 to 100µM concentrations of Fen at 24 h (Fig. 3A), whereas labeling of UtSMC cells was increased at 1 to 100 µM concentrations (Fig. 3B). In vitro study showed Fen at 10 µM archived maximal estrogenicity activity (Garey and Wolff, 1998). Based on these and acceptable daily intake (ADI) of 0–0.02 mg/Kg b.w. established for Fen by JMPR (Joint FAO/WHO Meeting on Pesticide Residues) in 1986, we chose 10 µM Fen as the concentration to conduct our further experiments.
Figure 3. Cell proliferation assay with BrdU.
UtLM cells (A) and UtSMCs (B) exposed to Fen for 24 h at concentrations ranging from 0.01 µM to 100 µM. E2 at concentration of 0.1 µM was used as a positive control. Compared with proliferation in untreated controls, BrdU labeling in UtLM cells was increased at Fen concentrations in the 0.1 µM to 100 µM range. BrdU labeling in UtSMC cells was increased in the 1 µM to 100 µM range. Representative data were shown from two separate experiments. *p<0.01 compared with vehicle control cells.
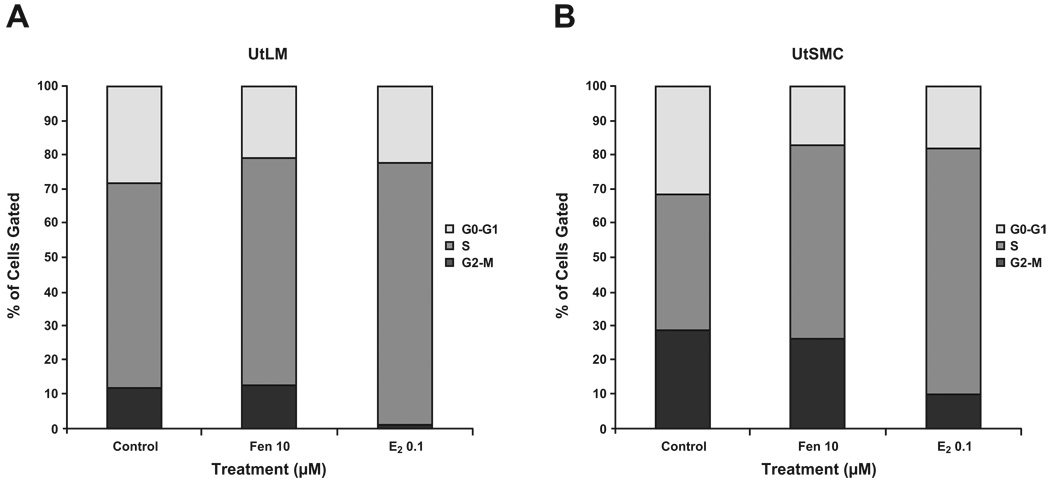
3.4 Cell cycle analysis in UtLM cells and UtSMCs after Fen treatment
We next investigated the mechanism by which Fen increased proliferation in UtLM and UtSMC cells. Using propidium iodide staining and flow cytometry analysis, we assessed the effects of Fen on cell cycle distribution in both cell lines. UtLM and UtSMC were treated with Fen at 10 µM for 24 h; E2 at concentration of 0.1 µM was used as a positive control. As depicted in Fig. 4, treatment of UtLM cells and UtSMCs with Fen significantly increased the percentage of cells in S phase, but decreased the percentage of cells in G0-G1 phase, while the percentage of cells in G2-M phase did not change significantly. However, treatment of both cell lines with E2 significantly increased the percentage of cells in S phase, but decreased the percentage of cells in both G0-G1 phase and G2-M phase. These results suggest that Fen induces UtLM and UtSMC cell cycle progression into the S phase as E2 does; however, the effects are different in that Fen decreases the percentage of cells in the G0-G1 phase, but E2 decreases cell percentages in both G0-G1 phase and G2-M phases.
Figure 4. Cell cycle analysis.
UtLM cells (A) and UtSMCs (B) were treated with Fen at 10 µM for 24 h. E2 at a concentration of 0.1 µM was used as a positive control. The values represent the number of cells in different phases of the cell cycle as a percentage (%) of the total cells observed. The data shown represent the average of three independent experiments. Representative data were shown from three separate experiments. Data were statistically analyzed by a two-way ANOVA with Bonferroni post-tests after arcsine transformation.
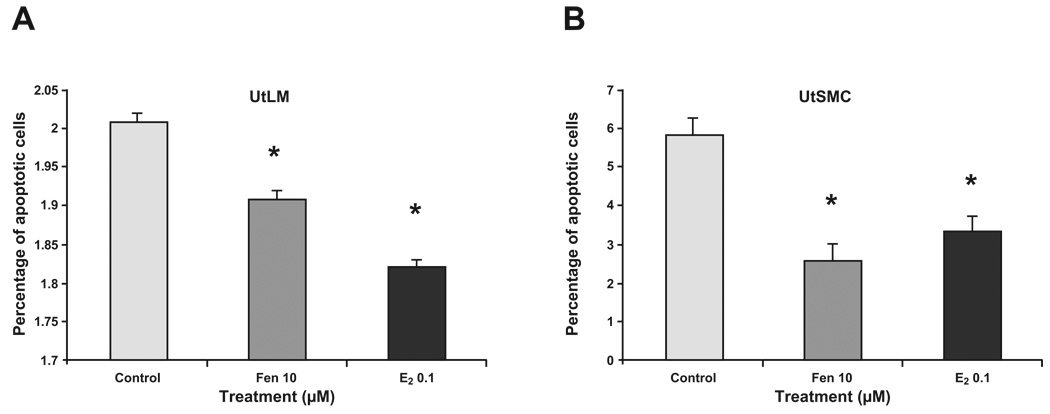
3.5 Fen inhibited cell apoptosis in UtLM cells and UtSMCs
To examine whether growth could also be attributed to an anti-apoptotic mechanism, Annexin V assays were done. UtLM cells and UtSMCs treated with 10 µM of Fen or 0.1 µM E2 for 24 h showed significantly decreased percentages of apoptotic cells (Fig. 5), and indicated that the effects of Fen on cell growth in UtLM cells and UtSMCs may be due, in part, to inhibition of apoptosis. Similar results were found in E2 treatment.
Figure 5. Analysis of apoptosis.
UtLM cells (A) and UtSMCs (B) treated with Fen at 10 µM for 24 h. E2 at a concentration of 0.1 µM was used as a positive control. After treatment, UtLM cells and UtSMCs were harvested, and stained with the apoptotic indicator Annexin V dye and were assayed by FACS flow cytometer. The values represent the percentage of apoptotic cells induced by Fen and E2 to total gated cells. Error bars represent means ± SEM. *p<0.05, Student’s t test (n = 3).
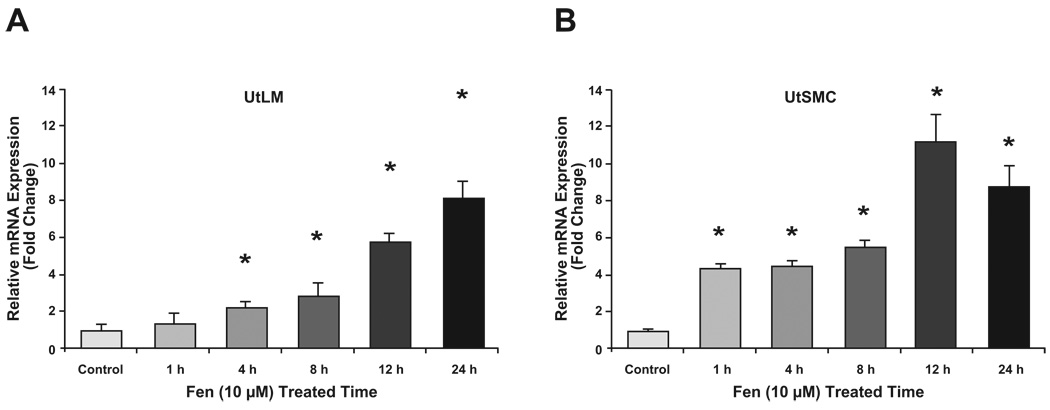
3.6 Fen induced mRNA expression of collagen type I in UtLM cells and UtSMCs
Leiomyomas are characterized by excessive ECM production. Collagen I is an ECM component that is highly expressed in leiomyomas compared with myometrium. To further characterize the effects of Fen on UtLM and UtSMC cells, we evaluated the impact of Fen on collagen type I expression in both cell types using real-time RT-PCR assays. As shown in Fig. 6, we found that the levels of collagen type I mRNA was significantly upregulated by treatment with Fen (10 µM) in a time-dependent manner in the UtLM cells. Treatment with Fen at 10 µM for 24 h induced more than an 8-fold increase in collagen type I mRNA in UtLM cells and UtSMCs.
Figure 6. Real-time PCR analysis of Collagen I in UtLM (A) and UtSMC (B) cells.
After treatment with Fen (10 µM) for 0, 4, 8, 12 and 24 h, total RNA was isolated from treated and vehicle control cells and subjected to real-time RT-PCR. Bar graphs show the mean ± SEM of three independent cell cultures. *p<0.05 compared with vehicle controls.
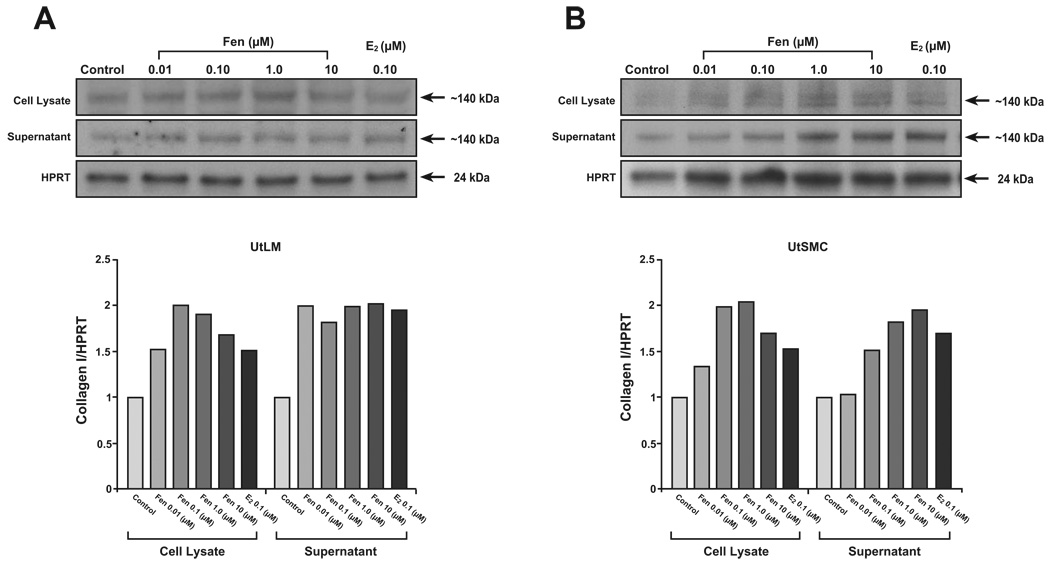
3.7 Fen upregulates collagen type I protein levels in UtLM cells and UtSMCs
To confirm the effects of Fen on collagen type I mRNA levels, western blot analyses were done to examine protein expression. As expected, Fen showed a dose-dependent increase in collagen type I protein levels in cell lysates in both UtLM cells and UtSMCs with concentrations of 0.01, 0.10, 1.0 and 10.0 µM for 24 hours. On the other hand, Fen increased collegen I in supernatant of UtLM from 0.01 to 10 µM for 24 hours, while in UtSMC from 0.1 to 10 µM. E2, as positive control, also increased the expression of collagen type I in both cell lines.
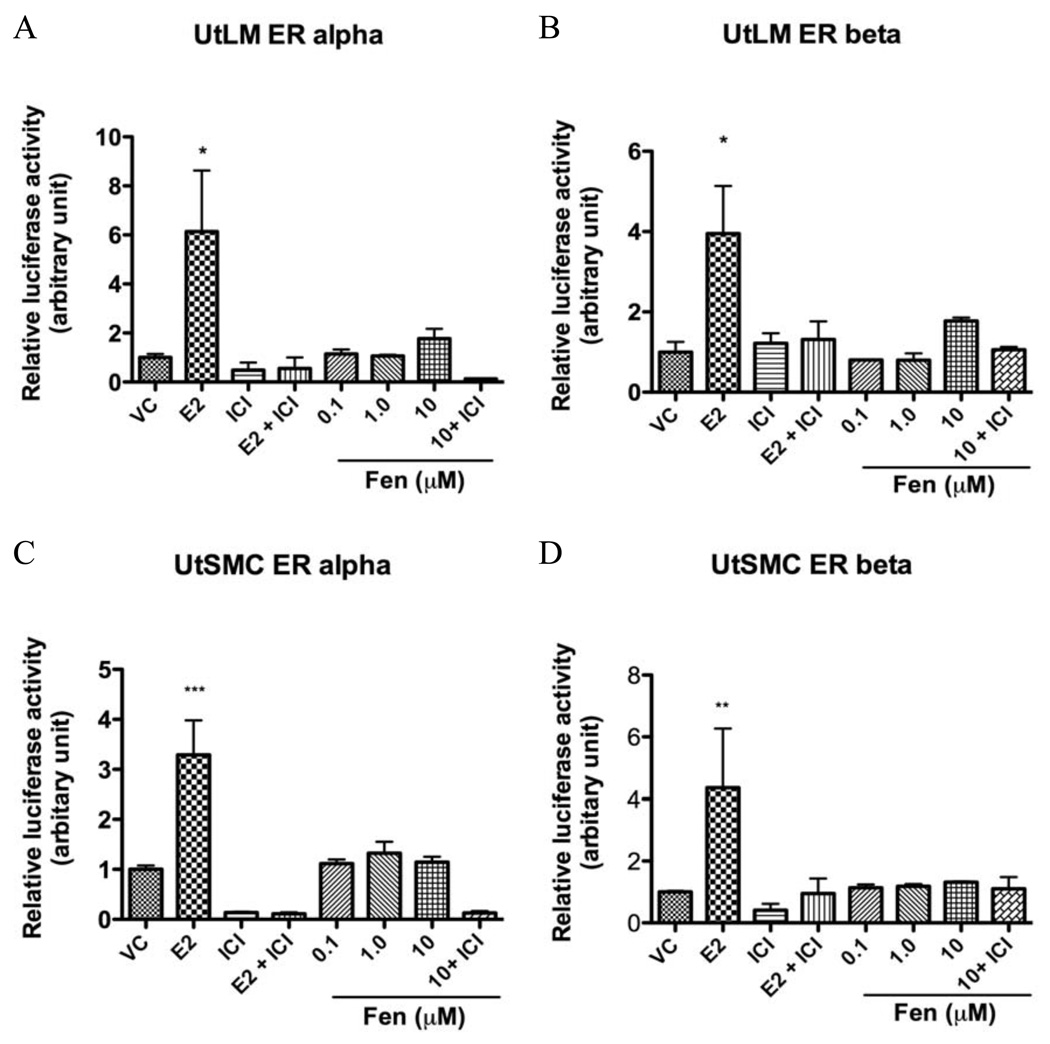
3.8 Fen may not require the region of ER for transactivation function
In order to learn whether ERs are involved in the Fen’s effects, we examined the molecular mechanism of ER-dependent gene regulation by Fen. UtLM cells and UtSMCs were transiently transfected with plasmids containing either hERα or hERβ and an estrogen-responsive element (ERE)-fused luciferase reporter plasmid (3×-Vit-ERE-TATA-Luc). Figure 8 showed that in the presence of hERα or hERβ, E2 stimulated ERE-mediated luciferase activity in 10 nM. Coincubation with 1.0 µM ICI 182,780 inhibited the transactivation of 3×-Vit-ERE-TATA-luc plasmid by Fen and E2. However, Fen did not significantly stimulate the luciferase activity in 0.1, 1.0 or 10.0 µM neither in UtLM cells nor UtSMCs. This suggested that Fen’s estrogenic actions may not be mediated through ERα or ERβ activation.
Figure 8. Transient transfection and luciferase assay in UtLM cells and UtSMCs.
Relative luciferase activity in UtLM cells and UtSMCs transfected with hERα (A, C), hERβ (B, D), and 3×-Vit-ERE-TATA-Luc plasmids that were treated with DMSO (vehicle control, VC), 10 nM E2, or 0.1, 1.0, 10 µM Fen in the presence or absence of 1.0 µM ICI 182,780. Each value was obtained from three independent experiments performed in triplicate. *p< 0.05, **p<0.01, and ***p< 0.001, compared with VC.
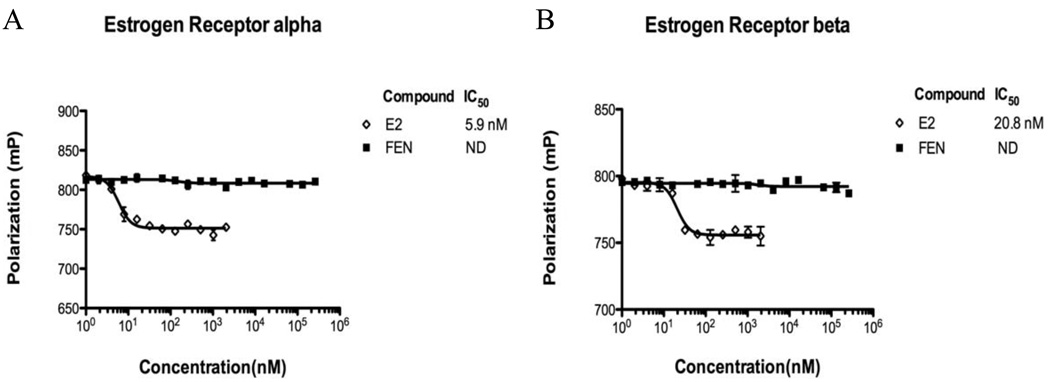
3.9 Fen does not bind to ERα or ERβ
As the estrogenic effects of E2 require the binding to a functional ER (Bolger et al., 1998), we evaluated the ability of Fen to bind to ERα and ERβ to further confirm whether the effects of Fen were mediated by the ER. The results shown in Fig. 9 A and B indicate that the affinity of Fen to ERα or ERβ was non-detectable at the concentrations ranging from 1nM to 262.144 µM; while E2 binds to ERα and ERβ with high affinity (calculated IC50 of about 5.9 nM and 20.8 nM, respectively).
Figure 9. Estrogen receptor α and β competitive binding assays.
Competitive binding curve between ER/Fluormone ™ and increasing concentrations of E2 and Fen to ERα (A) and ERβ (B), after 4 h incubation period. A 100% polarization value corresponds to no competition, due to the absence of the competitor. Results represent the means ± SEM. of three separate experiments. ND, non-detectable.
4. Discussion
Fen, a type II pyrethroid pesticide and EDC, is a class of neurotoxic pesticides registered for agriculture and residential use in United States. Consumption of pyrethroid pesticides including Fen, has continuously increased during the last two decades (Wolansky and Harrill, 2008). The current study shows that Fen, at a specific dose range can promote cell proliferation and stimulate expression of collagen I in both human uterine leiomyoma cells and myometrial cells. This part of our findings is similar to the effects of organochlorine pesticides reported by Hodges et al (Hodges et al., 2000). However, our data also show that Fen can contribute matrix expansion via over-expression of collagen I, one major type component of matrix in fibroids. To the best of our knowledge, this is the first report on effect of pesticides on the matrix turnover in leiomyoma. Fen at concentration of 10 µM achieved maximal estrogenicity activities comparable to that of 10 nM 17alpha-ethynylestradiol in Ishikawa Var-I cells (Garey and Wolff, 1998). Since the estimated maximal acceptable daily intake (ADI) for Fen is 0.02 mg/kg body weight (1991; 1996), the accumulation of this compound in human plasma could reach levels comparable to those used in this study. These data suggest that Fen should be considered as an environmental risk factor for fibroids. Based on these facts and our findings, it is prudent to be cognizant of the possible health effects of Fen exposures on women’s health and hormonally regulated diseases.
Studies in both mice and rats have shown that exposure to EDCs can increase the incidence of uterine fibroids (Howe et al., 1995; Hunter et al., 2000; Romagnolo et al., 1996). However, the potential mechanisms of EDCs in the pathogenesis of fibroids are still not fully understood. Pyrethroid insecticides are EDCs that have been considered environmental analogues of estrogen. Fen, a widely used pyrethroid insecticide, has been documented to imitate many of the actions of estrogen, such as proliferation and cancer development. Fen has been shown to mimic estrogenic activity in MCF-7 human breast carcinoma cells by inducing pS2 expression (Kasat et al., 2002). Go and coworkers (Go et al., 1999) illustrated that nanomolar concentrations of Fen were sufficient to induce cell proliferation of MCF-7 cells and induce pS2 expression levels. Garey et al found that Fen at concentration of 10 µM achieved maximal estrogenicity activity in Ishikawa Var-I cells (Garey and Wolff, 1998). In contrast, a few researchers also pointed out that Fen showed no significant estrogenic activity by using different assays. Saito et al. reported that Fen, as well as two other pyrethroids, has no significant estrogenic or antiestrogenic effects and showed their impact on the ERα mediated pathway was negligible (Saito et al., 2000). Two years later, Kunimatsu et al from the same group, confirmed their findings by using specific male and female Crj:CD(SD)IGS rats (Kunimatsu et al., 2002).
Estrogen has traditionally been identified as a major influence on the pathogenesis of fibroids, and it is known that these tumors are hyper-responsive to estrogen and exhibit elevated levels of estrogen receptors. However, the mechanisms whereby estrogen exerts its effects on fibroids are still not fully understood (Al-Hendy et al., 2004; Brahma et al., 2006; Hermon et al., 2008; Walker and Stewart, 2005). With the realization of the presence of many ubiquitous xenoestrogens in our environment and the high incidence of uterine leiomyomas in women, the potential influence of EDCs in the pathogenesis of these tumors has been called into question (Walker, 2002). Since Fen is considered to be an estrogen analogue, in this study we investigated whether Fen had effects similar to E2 on uterine leiomyoma cells and whether these effects are mediated by classical ERα and, or even possibly ERβ. ICI 182,780, a high affinity estrogen receptor antagonist that can bind to both ER subtypes (Oliveira et al., 2003; Tremblay et al., 1998; Wakeling, 2000), was used. Our data consistently showed that Fen did not bind either ERα or ERβ, as well as no significant transcriptional activity was detected after exposure to Fen in the presence or absence of ICI 182,780. These negative, but important findings suggest that the effects of Fen on UtLM cells and UtSMCs most likely occur in an ER-independent manner. Interestingly, Go et al reported that Fen exerts a proliferative effect on breast cancer cells similar to estrogen but via an estrogen receptor independent manner (Go et al., 1999). Together with our findings, it is important to further understand the differences in the molecular mechanisms of action of Fen and E2 despite their similar phenotypic impacts.
In this study, we first found increased proliferation of both UtLM cells and UtSMCs following Fen treatment by measuring two different parameters: cell proliferation and synthesis of DNA. Since cell proliferation is the endpoint of many converging biological pathways, it is important for us to clarify a specific mechanism. As a basic concept, cell proliferation is a net result of mitosis and cell death. To this end, an increase of cells in S phase is crucial to cell mitosis and can result in proliferation. On the other hand, a decrease in cell apoptosis leading to reduction in cell death is also important in cell proliferation. Our results showed that Fen could induce progression of both cell types into the S phase of the cell cycle and decrease apoptotic cells.
The understanding of these changes induced by Fen is complex and is probably multi-factorial. As human uterine muscle and leiomyoma cells differ in their rapid 17β-estradiol signaling (Nierth-Simpson et al., 2009), Fen may also activate different signal transduction pathways in these two cell types. IGF-1 could be a potential mediator of the increased progression of UtLM cells into the S-phase of the cell cycle. In previous studies, we and others have found that E2 can up-regulate the expression of IGF-1 in leiomyoma cells, and that IGF-1 could exert its mitogenic action by accelerating the progression of cells from G1 to S phase, and by inhibiting apoptosis (Leiser et al., 2006; Swartz et al., 2005). Progression through the mammalian cell cycle is regulated by cyclins, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CKIs). The function of these proteins in the irreversible growth arrest associated with terminally differentiated cells is largely unknown. A CKI, p27Kip1(p27), whose specific late G1 destruction allows progression of the cell across the G1/S boundary, is well known to be down-regulated in human fibroid tumors (Lee et al., 2004; Leiser et al., 2006). Recently, it has been reported that exogenously up-regulated cyclin-dependent kinase inhibitor, P27Kip1, could result in a reduction in S-phase in uterine leiomyoma cells (Ramachandran et al., 2008). Combining these data with our current findings, we could suggest an axis of Fen induced cell proliferation, involving Fen-IGF1-P27kip1-Cell cycle for UtLM cells. In the future, this axis could be a potential intervention target strategy against Fen or other EDCs which might be associated with fibroid pathogenesis.
Besides increased cell proliferation in uterine fibroids, excessive deposition of ECM is another important feature of these tumors. ECM is a fibrillar protein meshwork that provides a structure scaffold for tissue support and serves as a reservoir for growth factors (Arslan et al., 2005; Ding et al., 2004; Wolanska et al., 1998). Type I collagen is a major ECM component in the normal uterus, and is increased in human uterine fibroids. Again, our data indicates that Fen can increase both the expression of mRNA and protein levels of collagen type I similar to E2. The net deposition of ECM is a result of balance of synthesis and degradation. A limitation of the current study is a lack of data on the degradation of ECM. However, the collagen level in the supernatant could be considered as the final production of collagen after dynamic metabolism and this was found increased for both cell types in our study.
In summary, we believe that the data presented here will shed some light on a possible mechanism of action of EDCs in fibroids, and suggest that Fen should be considered as a possible risk factor for fibroids in women. In conclusion, we report for the first time Fen-induced cell proliferation and increased collagen type I production in UtLM cells and UtSMCs. Our studies contribute to the understanding of the effects of EDCs on women’s health and point out possible risk factors for the development and growth of uterine fibroids.
Figure 7. Effects of Fen on Collagen Type I protein levels in UtLM cells and UtSMCs.
Fen upregulates collagen type I protein levels in UtLM cells and UtSMCs. UtLM cells (A) and UtSMCs (B) were treated with Fen (0.01–10 µM) for 24 h. After treatment, cell lysate and supernatant samples were collected and analyzed by western blotting. E2 (0.10 µM) was used as a positive control. Representative immunoblots and a graph showing protein levels of collagen I relative to HPRT are shown. Data represent mean ± SEM of three independent experiments.
Acknowledgements
The authors kindly thank Dr. Gordon Flake and Ms. Retha Newbold for their critical review of this manuscript. We also thank Dr. Grace E. Kissling in the Biostatistics Branch, NIEHS for statistical data analysis, and Kathleen A. Wallace in the Environmental Carcinogenesis Division of the USEPA (Research Triangle Park, NC) for her expert technical support on fluorescence polarization. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared.
References
- Fenvalerate. IARC Monogr Eval Carcinog Risks Hum. 1991;53:309–328. [PMC free article] [PubMed] [Google Scholar]
- Fenvalerate. WHO/FAO DATA Sheets on Pesticides. 1996 No. 90.
- Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–1631. doi: 10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Arslan AA, Gold LI, Mittal K, Suen TC, Belitskaya-Levy I, Tang MS, Toniolo P. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;20:852–863. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- Bolger R, Wiese TE, Ervin K, Nestich S, Checovich W. Rapid screening of environmental chemicals for estrogen receptor binding capacity. Environ Health Perspect. 1998;106:551–557. doi: 10.1289/ehp.98106551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma PK, Martel KM, Christman GM. Future directions in myoma research. Obstet Gynecol Clin North Am. 2006;33:199–224. doi: 10.1016/j.ogc.2005.12.011. xiii. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen H, Liu R, He J, Song L, Bian Q, Xu L, Zhou J, Xiao H, Dai G, Chang HC, Wang X. Effects of fenvalerate on progesterone production in cultured rat granulosa cells. Reprod Toxicol. 2005;20:195–202. doi: 10.1016/j.reprotox.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Ding L, Xu J, Luo X, Chegini N. Gonadotropin releasing hormone and transforming growth factor beta activate mitogen-activated protein kinase/extracellularly regulated kinase and differentially regulate fibronectin, type I collagen, and plasminogen activator inhibitor-1 expression in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 2004;89:5549–5557. doi: 10.1210/jc.2004-0161. [DOI] [PubMed] [Google Scholar]
- Dixon D, Flake GP, Moore AB, He H, Haseman JK, Risinger JI, Lancaster JM, Berchuck A, Barrett JC, Robboy SJ. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Arch. 2002;441:53–62. doi: 10.1007/s00428-001-0568-7. [DOI] [PubMed] [Google Scholar]
- Ellerington MC, Whitcroft SIJ, Whitehead MI. HRT: Developments in therapy. Br Med Bull. 1992;48:401–425. doi: 10.1093/oxfordjournals.bmb.a072553. [DOI] [PubMed] [Google Scholar]
- Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111:1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey J, Wolff MS. Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochem Biophys Res Commun. 1998;251:855–859. doi: 10.1006/bbrc.1998.9569. [DOI] [PubMed] [Google Scholar]
- Go V, Garey J, Wolff MS, Pogo BG. Estrogenic potential of certain pyrethroid compounds in the MCF-7 human breast carcinoma cell line. Environ Health Perspect. 1999;107:173–177. doi: 10.1289/ehp.99107173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008;453:557–569. doi: 10.1007/s00428-008-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine leiomyoma cells in vitro. Toxicol Sci. 2000;54:355–364. doi: 10.1093/toxsci/54.2.355. [DOI] [PubMed] [Google Scholar]
- Houston KD, Hunter DS, Hodges LC, Walker CL. Uterine leiomyomas: mechanisms of tumorigenesis. Toxicol Pathol. 2001;29:100–104. doi: 10.1080/019262301301418900. [DOI] [PubMed] [Google Scholar]
- Howe SR, Gottardis MM, Everitt JI, Walker C. Estrogen stimulation and tamoxifen inhibition of leiomyoma cell growth in vitro and in vivo. Endocrinology. 1995;136:4996–5003. doi: 10.1210/endo.136.11.7588234. [DOI] [PubMed] [Google Scholar]
- Hunter DS, Hodges LC, Eagon PK, Vonier PM, Fuchs-Young R, Bergerson JS, Walker CL. Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environ Health Perspect. 2000;108 Suppl 5:829–834. doi: 10.1289/ehp.00108s5829. [DOI] [PubMed] [Google Scholar]
- Kasat K, Go V, Pogo BG. Effects of pyrethroid insecticides and estrogen on WNT10B proto-oncogene expression. Environ Int. 2002;28:429–432. doi: 10.1016/s0160-4120(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Kunimatsu T, Yamada T, Ose K, Sunami O, Kamita Y, Okuno Y, Seki T, Nakatsuka I. Lack of (anti-) androgenic or estrogenic effects of three pyrethroids (esfenvalerate, fenvalerate, and permethrin) in the Hershberger and uterotrophic assays. Regul Toxicol Pharmacol. 2002;35:227–237. doi: 10.1006/rtph.2001.1527. [DOI] [PubMed] [Google Scholar]
- Lee TK, Lee DK, Kim DI, Lee YC, Chang YC, Kim CH. Inhibitory effects of Scutellaria barbata D. Don on human uterine leiomyomal smooth muscle cell proliferation through cell cycle analysis. Int Immunopharmacol. 2004;4:447–454. doi: 10.1016/j.intimp.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Leiser AL, Anderson SE, Nonaka D, Chuai S, Olshen AB, Chi DS, Soslow RA. Apoptotic and cell cycle regulatory markers in uterine leiomyosarcoma. Gynecol Oncol. 2006;101:86–91. doi: 10.1016/j.ygyno.2005.09.055. [DOI] [PubMed] [Google Scholar]
- Moniz AC, Cruz-Casallas PE, Oliveira CA, Lucisano A, Florio JC, Nicolau AA, Spinosa HS, Bernardi MM. Perinatal fenvalerate exposure: behavioral and endocrinology changes in male rats. Neurotoxicol Teratol. 1999;21:611–618. doi: 10.1016/s0892-0362(99)00004-5. [DOI] [PubMed] [Google Scholar]
- Moore AB, Castro L, Yu L, Zheng X, Di X, Sifre MI, Kissling GE, Newbold RR, Bortner CD, Dixon D. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–2631. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nierth-Simpson EN, Martin MM, Chiang TC, Melnik LI, Rhodes LV, Muir SE, Burow ME, McLachlan JA. Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: implications for proliferation. Endocrinology. 2009;150:2436–2445. doi: 10.1210/en.2008-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara N. Sex steroidal modulation of collagen metabolism in uterine leiomyomas. Clin Exp Obstet Gynecol. 2009;36:10–11. [PubMed] [Google Scholar]
- Oliveira CA, Nie R, Carnes K, Franca LR, Prins GS, Saunders PT, Hess RA. The antiestrogen ICI 182,780 decreases the expression of estrogen receptor-alpha but has no effect on estrogen receptor-beta and androgen receptor in rat efferent ductules. Reprod Biol Endocrinol. 2003;1:75. doi: 10.1186/1477-7827-1-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Kwon KY, Shin SJ, Kwon SH, Cha SD, Bae I, Cho CH. Cyclin-dependent kinase inhibitor p27Kip1 controls growth and cell cycle progression in human uterine leiomyoma. J Korean Med Sci. 2008;23:667–673. doi: 10.3346/jkms.2008.23.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnolo B, Molina T, Leroy G, Blin C, Porteux A, Thomasset M, Vandewalle A, Kahn A, Perret C. Estradiol-dependent uterine leiomyomas in transgenic mice. J Clin Invest. 1996;98:777–784. doi: 10.1172/JCI118850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Tomigahara Y, Ohe N, Isobe N, Nakatsuka I, Kaneko H. Lack of significant estrogenic or antiestrogenic activity of pyrethroid insecticides in three in vitro assays based on classic estrogen receptor alpha-mediated mechanisms. Toxicol Sci. 2000;57:54–60. doi: 10.1093/toxsci/57.1.54. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, Marshall LM, Baird DD. Epidemiologic contributions to understanding the etiology of uterine leiomyomata. Environ Health Perspect. 2000;108 Suppl 5:821–827. doi: 10.1289/ehp.00108s5821. [DOI] [PubMed] [Google Scholar]
- Sener AB, Seckin NC, Ozmen S, Gokmen O, Dogu N, Ekici E. The effects of hormone replacement therapy on uterine fibroids in postmenopausal women. Fertil Steril. 1996;65:354–357. doi: 10.1016/s0015-0282(16)58098-4. [DOI] [PubMed] [Google Scholar]
- Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78:1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11:441–450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- Tremblay GB, Tremblay A, Labrie F, Giguere V. Ligand-independent activation of the estrogen receptors alpha and beta by mutations of a conserved tyrosine can be abolished by antiestrogens. Cancer Res. 1998;58:877–881. [PubMed] [Google Scholar]
- Wakeling AE. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer. 2000;7:17–28. doi: 10.1677/erc.0.0070017. [DOI] [PubMed] [Google Scholar]
- Walker CL. Role of hormonal and reproductive factors in the etiology and treatment of uterine leiomyoma. Recent Prog Horm Res. 2002;57:277–294. doi: 10.1210/rp.57.1.277. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Whitcroft SI, Stevenson JC. Hormone replacement therapy: risks and benefits. Clin Endocrinol (Oxf) 1992;36:15–20. doi: 10.1111/j.1365-2265.1992.tb02896.x. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Sobolewski K, Drozdzewicz M, Bankowski E. Extracellular matrix components in uterine leiomyoma and their alteration during the tumour growth. Mol Cell Biochem. 1998;189:145–152. doi: 10.1023/a:1006914301565. [DOI] [PubMed] [Google Scholar]
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Xia Y, Bian Q, Xu L, Cheng S, Song L, Liu J, Wu W, Wang S, Wang X. Genotoxic effects on human spermatozoa among pesticide factory workers exposed to fenvalerate. Toxicology. 2004;203:49–60. doi: 10.1016/j.tox.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Xiao H, Zhang XC, Zhang L, Dai XQ, Gong W, Cheng J, Gao R, Wang X. Fenvalerate modifies T-type Ca2+ channels in mouse spermatogenic cells. Reprod Toxicol. 2006;21:48–53. doi: 10.1016/j.reprotox.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Yu L, Saile K, Swartz CD, He H, Zheng X, Kissling GE, Di X, Lucas S, Robboy SJ, Dixon D. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med. 2008;14:264–275. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbucka M, Miltyk W, Bielawski T, Surazynski A, Palka J, Wolczynski S. Mechanism of collagen biosynthesis up-regulation in cultured leiomyoma cells. Folia Histochem Cytobiol. 2007;45 Suppl 1:S181–S185. [PubMed] [Google Scholar]