Abstract
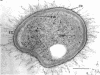
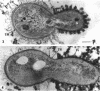
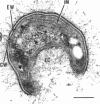
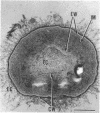
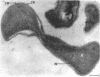
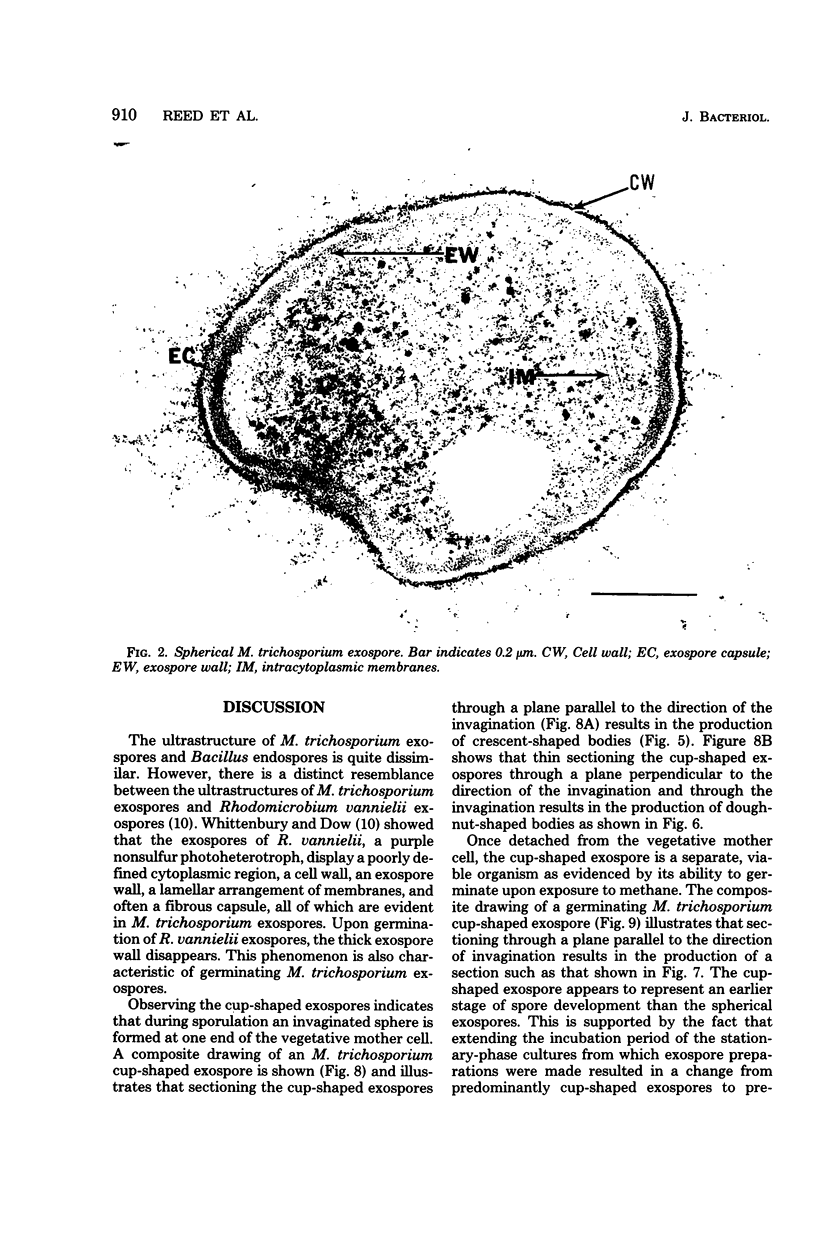
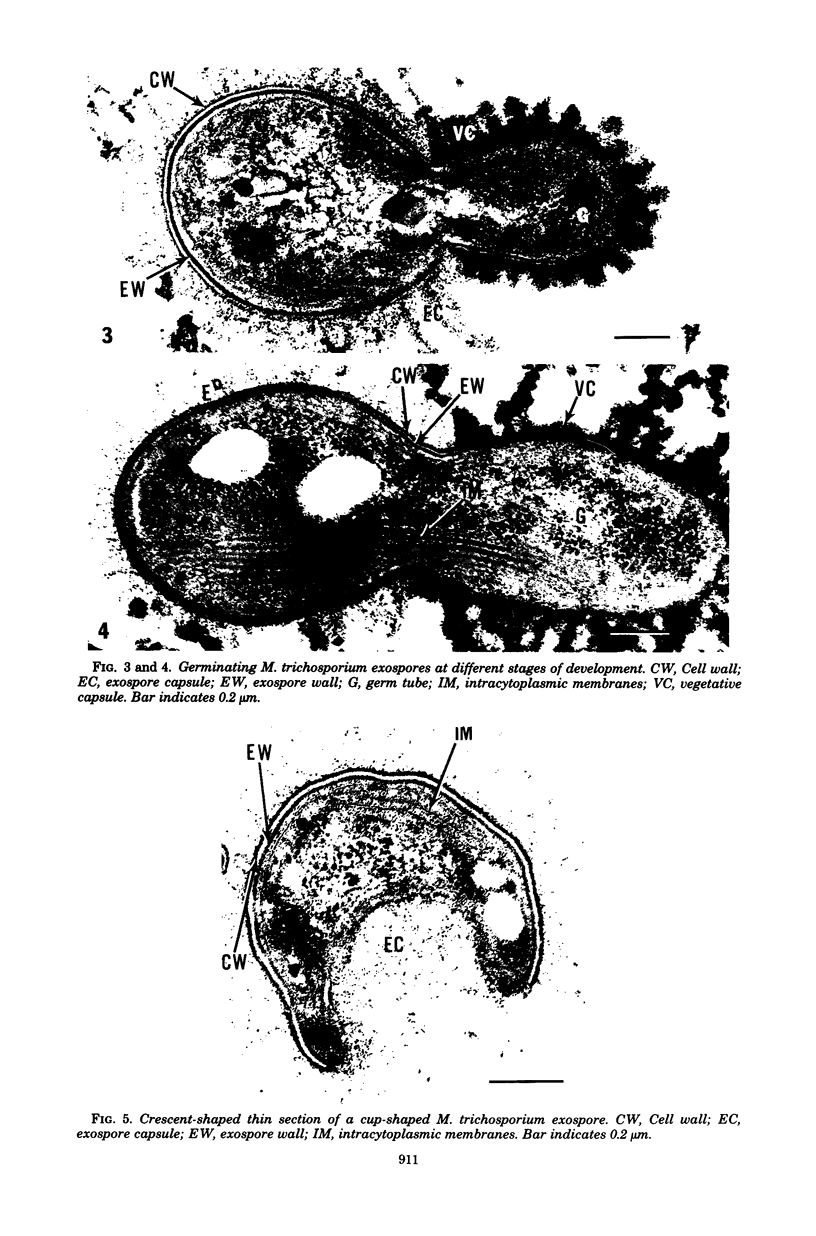
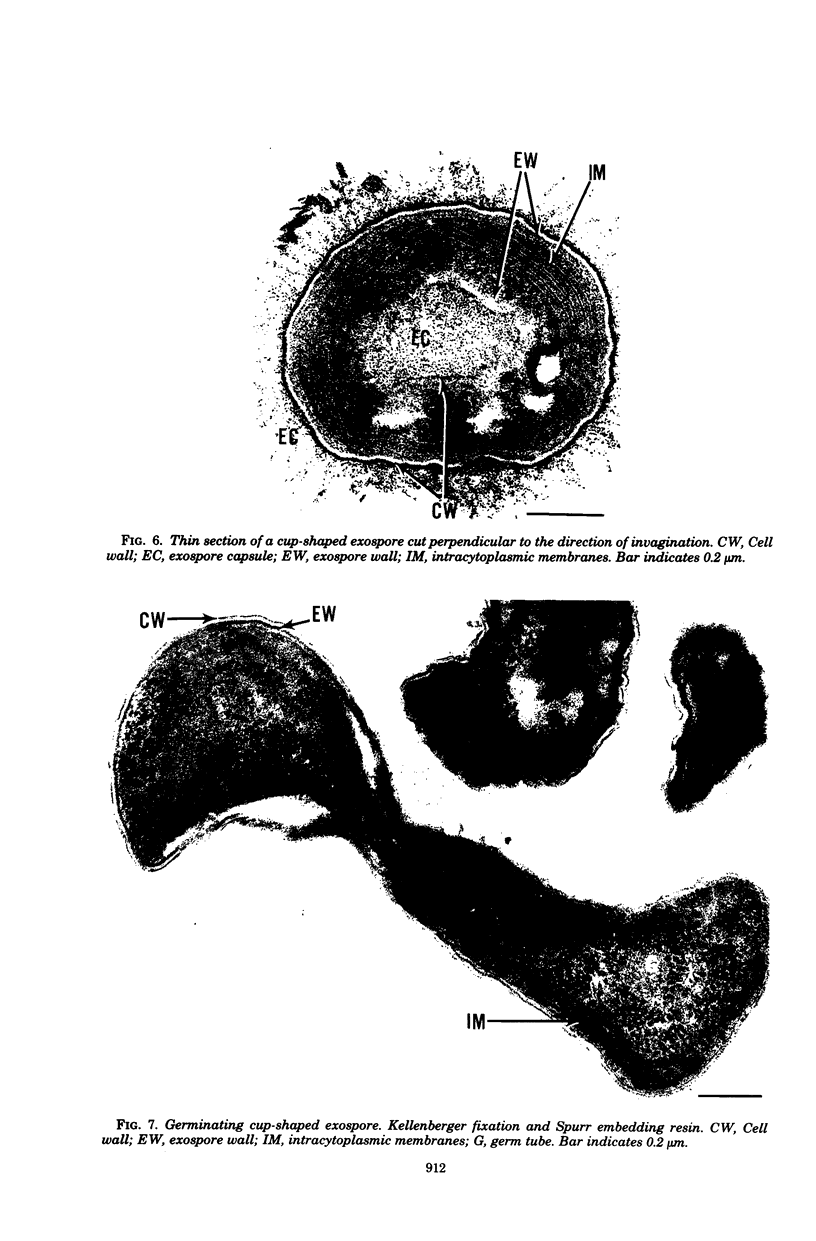
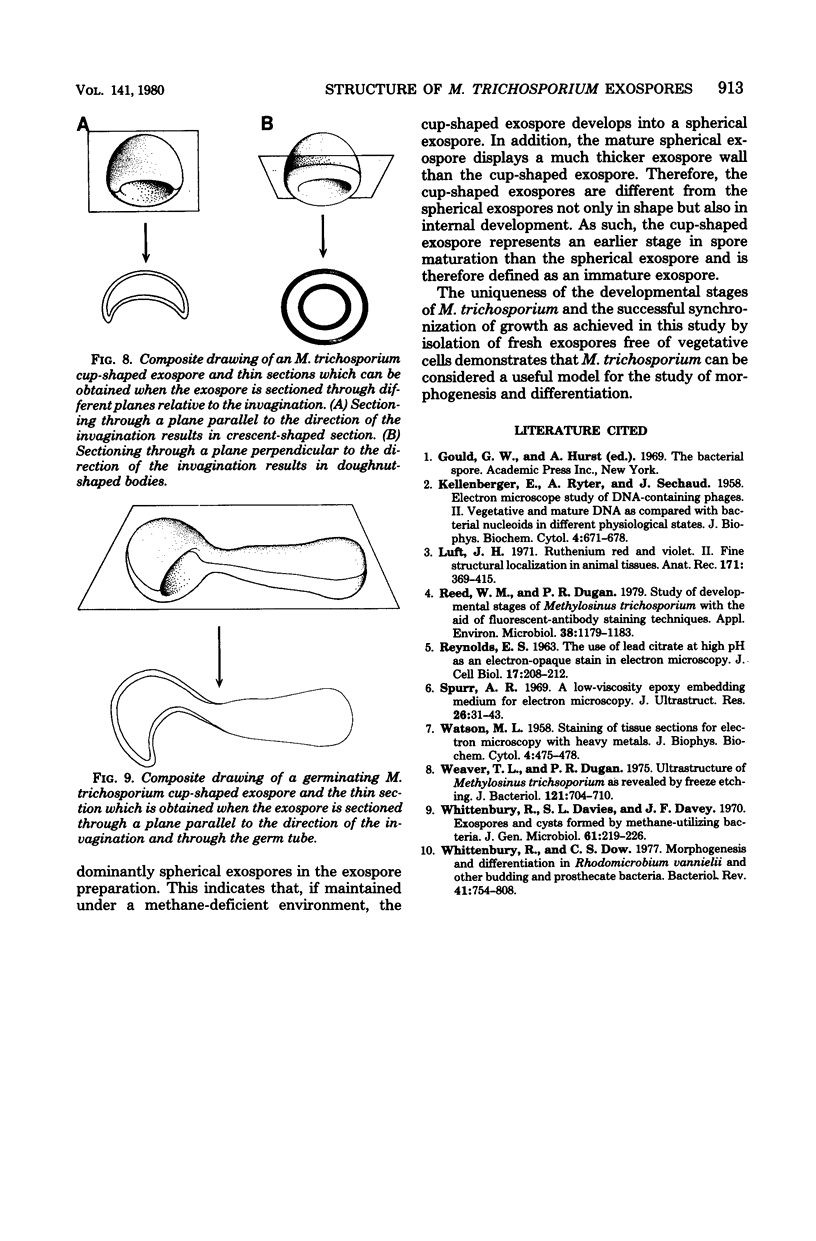
Methylosinus trichosporium exospores did not display a well-defined cortex or an exosporium. A thick, electron-dense exospore wall was characteristic of the exospores. Located on the exterior of the exospore wall was a cell wall to which a well-defined capsule was attached. An extensive lamellar intracytoplasmic membrane system characteristic of the kind in vegetative cells of this bacterium was present along the interior periphery of the exospore wall. Upon germination of M. trichosporium exospores, the thick exospore wall gradually disappeared and a germ tube formed. The intracytoplasmic membranes of the exospores extended into the germ tube which did not possess the extensive fibrillar capsule observed on the dormant exospore. Cup-shaped exospores which have an ultrastructure similar to that of mature exospores except that they are invaginated also germinated upon exposure to methane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed W. M., Dugan P. R. Study of Developmental Stages of Methylosinus trichosporium with the Aid of Fluorescent-Antibody Staining Techniques. Appl Environ Microbiol. 1979 Dec;38(6):1179–1183. doi: 10.1128/aem.38.6.1179-1183.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver T. L., Dugan P. R. Ultrastruct of Methylosinus trichosporium as revealed by freeze etching. J Bacteriol. 1975 Feb;121(2):704–710. doi: 10.1128/jb.121.2.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittenbury R., Davies S. L., Davey J. F. Exospores and cysts formed by methane-utilizing bacteria. J Gen Microbiol. 1970 May;61(2):219–226. doi: 10.1099/00221287-61-2-219. [DOI] [PubMed] [Google Scholar]
- Whittenbury R., Dow C. S. Morphogenesis and differentiation in Rhodomicrobium vannielii and other budding and prosthecate bacteria. Bacteriol Rev. 1977 Sep;41(3):754–808. doi: 10.1128/br.41.3.754-808.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]