Abstract
Recent vocal fold vibration studies have used models defined using idealized geometry. Although these models exhibit important similarities with human vocal fold vibration, some aspects of their motion are less than realistic. In this report it is demonstrated that more realistic motion may be obtained when using geometry derived from magnetic resonance imaging (MRI) data. The dynamic response of both idealized and MRI-based synthetic vocal fold models are presented. MRI-based model improvements include evidence of mucosal wave-like motion and less vertical movement. Limitations of the MRI-based model are discussed and suggestions for further synthetic model development are offered.
Introduction
Synthetic and computational vocal fold models described in the literature have typically used simplified geometries. One example is the “M5” model of Scherer et al.1 Self-oscillating models based on this geometry exhibit some life-like characteristics and have thus been used to simulate human vocal folds in research, for example: to study acoustically and aerodynamically-driven vibration modes,2 characterize supraglottic vortices,3 develop in vivo measurement devices,4 study acoustical effects of non-human mammalian air sacs on voice quality,5 estimate the sensitivity of vocal fold response to lateral material stiffness asymmetry,6 and investigate fluid-structure and acoustic-structure interactions.7
M5-based studies such as these allow for parametric physical modeling and detailed experimentation without using excised larynges, and they present several advantages and disadvantages. Advantages include low cost, low maintenance, durability, control over geometric and material property parameters, and relatively good agreement with human vocal fold characteristics, such as true self-oscillation capability and similar phonation threshold pressures. Disadvantages include (a) idealized geometry, including a uniform anterior-posterior cross section profile, and (b) some dissimilarity with true vocal fold motion, including a generally more divergent glottal profile during the entire vibratory cycle, the lack of mucosal wave-like motion, and large vertical (inferior-superior) motion.
Considering recent studies8, 9, 10 that have highlighted the role of geometry in vocal fold response, it is likely that the differences between the responses of the M5-based models and the true vocal folds are due to geometric as well as material property differences. The purpose of this letter is to show that more life-like motion can indeed be achieved when using more realistic geometry. This is done by comparing the flow-induced responses of two synthetic, self-oscillating models of matching material properties but differing geometries. One model was based on the idealized M5 geometry. The other was based on geometry derived from magnetic resonance imaging (MRI) data.11 It is hoped that these findings will stimulate and provide di-rection for research efforts aimed toward developing improved synthetic vocal fold models for human voice production research.
Methods
Geometry definition
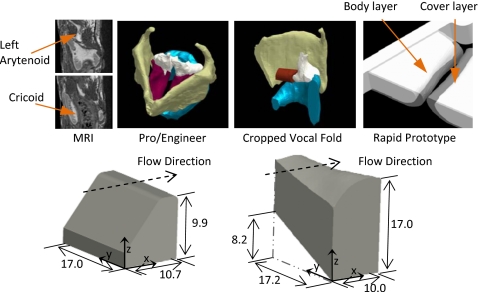
The M5 model geometry was based on the definition given by Scherer et al.1 with a 2 mm cover layer.6 The shape of the MRI-based model was obtained as follows (see Fig. 1): (1) Vocal fold tissue was outlined using MRI images via Velocity 2™ segmentation software; (2) Segmented regions were exported as three-dimensional stereolithography (STL) files; (3) STL files were imported into commercial computer-aided design (CAD) software (Pro∕ENGINEER), scaled to approximately match typical laryngeal size,12, 13 and cropped; (4) Cropped STL files (one each for cover and body layers) were exported to rapid prototyping hardware and solid models were created; and (5) Negative molds were fabricated from solid models, from which final models were fabricated as described in the next section.
Figure 1.
Top row: MRI geometry extraction sequence showing, from left to right, representative sagittal images, CAD models, cropped CAD model, and models of body and cover layers for rapid prototyping. Bottom row: Isometric views of M5 (left) and MRI (right) models. The x-, y-, and z-coordinates are aligned with the vertical (inferior-superior), anterior-posterior, and medial-lateral directions, respectively. All dimensions are in mm.
Model fabrication
Similar to previous studies5, 6 all models were made using two-part Smooth-On, Inc. Ecoflex® 0030 addition-cure silicone combined with Silicone Thinner. Increased thinner quantity yielded lower cured material Young’s modulus. Different thinner ratios were used for the body and cover layers. Models with a mixing ratio of 1:1:2 (one part each of Ecoflex® parts A and B to 2 parts thinner) for the body and 1:1:4 for the cover were fabricated, as well as models with 1:1:3 and 1:1:5 mixing ratios for the body and cover layers, respectively. Four different models were tested, here referred to by their geometry and body-cover mixing ratios: M524, M535, MRI24, and MRI35. To ensure material consistency, models with identical modulus values were created simultaneously (i.e., the M524 and MRI24 body layers were made at the same time using the same material batch, as were the cover layers, and so on). The models were mounted to 38×70×6.35 mm acrylic plates using silicone adhesive (Sil-Poxy©, Smooth-On, Inc.) applied to the lateral, anterior, and posterior surfaces. The plates were then mounted on an air flow supply tube that was connected upstream to a compressed air source.
To measure the Young’s modulus, a cylindrical test specimen of each material was fabricated concurrently with model fabrication and tested using an Instron 3342 tensile testing apparatus. The modulus values (1.5 kPa for 1:1:5, 2.94 kPa for 1:1:4, 5.2 kPa for 1:1:3, and 8.5 kPa for 1:1:2 mixing ratios) were similar to those reported in previous synthetic model studies and are generally agreeable with approximate values for human vocal fold tissue.14
Experimental setup and methods
The experimental setup was the same as that described by Pickup and Thomson,6 with additional high-speed camera positions for different views of the models. The only aspects of the setup that changed during the data acquisition process (described below) were the exchange of acrylic plates containing the different models and the high-speed camera positioning. The acrylic plates used for the MRI and M5 models were machined such that the medial surfaces of opposing models were approximately in contact when there was no flow.
Data acquired for all models during flow-induced vibration included onset and offset pressures, glottal area, model motion, and vertical (inferior-superior) displacement. Onset pressure was measured using a pressure sensor (Omega PX138–001D5V) mounted approximately 3 cm upstream of the model. The sensor fed information into a custom National Instruments LabVIEW software program that displayed pressure fluctuations during vibration. Onset pressure was identified by slowly increasing the upstream pressure until self-oscillation was induced (i.e., a steady sinusoidal wave was visible on the LabVIEW screen). Offset pressure was measured by decreasing the pressure until vibration ceased (i.e., the sinusoidal wave stopped). This process was repeated five times; averages are here reported.
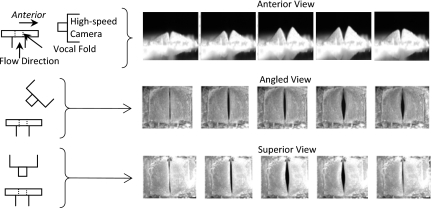
A high-speed camera (Photron APX-RS) was used to image glottal area and model motion from anterior, angled (approximately 45 degrees in the sagittal plane), and superior perspectives (Fig. 2). Images were recorded at 9000 frames per second at resolutions ranging from 400×800 to 1024×1024 pixels. Glottal area and vertical displacement measurements were obtained for each model at two pressures corresponding to 110% and 133.3% of each model’s onset pressure. Custom MATLAB subroutines were used to quantify glottal area and vertical displacement and to create videokymographic (VKG) images from the coronal midplane of the model; the latter were used to support the determination of whether mucosal wave-like motion was present.
Figure 2.
High-speed camera positioning (left) and corresponding images (right) of the M524 model at 133% Ponset.
Results and discussion
Threshold pressures
Onset and offset pressure data are listed in Table 1. The M5 models exhibited lower onset pressures than the MRI models, and were closer to human vocal fold onset phonation threshold pressures (around 0.30 kPa).15, 16 MRI model onset pressures were closer to values measured for canine vocal folds (around 1.0 kPa).17 Both models operate within ranges typical of human phonation.18 The MRI model onset pressure may be able to be reduced using other modeling methods, such as lowering the modulus cover layer materials and∕or implementing further geometric changes.
Table 1.
| Threshold pressure and maximum glottal area data for M5 and MRI models. | |||||
|---|---|---|---|---|---|
| M524 | M535 | MRI24 | MRI35 | ||
| Threshold pressure (kPa) | Onset | 0.65 | 0.91 | 2.25 | 1.86 |
| Offset | 0.52 | 0.33 | 2.06 | 1.68 | |
| Maximum glottal area (mm2) | 110% | 7.06 | 33.27 | 5.83 | 6.29 |
| 133% | 15.38 | 47.23 | 11.97 | 11.17 | |
Glottal area
Also shown in Table 1 are maximum glottal areas from the high-speed images taken from the superior perspective. Area was calculated using a custom MATLAB subroutine to identify the glottis based on pixel intensity; the uncertainty was estimated to be ±1.53 mm2. The maximum glottal areas increased with increased pressure for all models at all pressures. The M5 models had significantly larger areas than the MRI models, despite the MRI models operating at significantly higher pressures. This was attributed to reduced body layer deformation and vertical displacement (discussed below) of the MRI models.
Vertical displacement
A significant drawback of the M5 models has been their large, unnatural vertical displacement during vibration. The MRI-based models did not exhibit this behavior. This can be seen in Mm. 1 and Mm. 2, which show high-speed images of the M5 and MRI models during vibration. By comparing these two images, it is evident that the M5 model vibrated with excessive vertical and vertical-lateral displacement of the superior and medial surfaces, whereas that of the MRI model was much more realistic.
Mm. 1.
High-speed video of M5 model vibration, viewed from anterior perspective. This is a file of type “mov” (592 KB).
Mm. 2.
High-speed video of MRI-based model vibration, viewed from anterior perspective. This is a file of type “mov” (373 KB).
As a quantitative measure illustrating this behavior, Table 2 lists the vertical displacement data for each case. The data correspond to the height, H, of the highest point on the vocal fold model as seen in the images acquired from the anterior view (Fig. 2). Hmax and Hmin denote the maximum and minimum model height, respectively, over the oscillation cycle. Especially noteworthy is the net displacement, Hnet=Hmax−Hmin. For the M5 models, Hnet varied from 0.57 to 2.31 mm, indicating significant vertical displacement during vibration. For the MRI models, Hnet varied from 0.12 to 0.54 mm, which is much smaller than for the M5 models.
Table 2.
| Vertical displacement (height) data for M5 and MRI models. | ||||||||
|---|---|---|---|---|---|---|---|---|
| M524 | M535 | MRI24 | MRI35 | |||||
| 110% | 133% | 110% | 133% | 110% | 133% | 110% | 133% | |
| Hmax (mm) | 3.28 | 3.96 | 5.13 | 6.25 | 4.59 | 5.25 | 4.34 | 4.23 |
| Hmin (mm) | 2.71 | 2.83 | 3.33 | 3.94 | 4.47 | 4.89 | 3.80 | 3.68 |
| Hnet (mm) | 0.57 | 1.13 | 1.79 | 2.31 | 0.12 | 0.36 | 0.54 | 0.54 |
Model motion
Other evidence of improved MRI model motion is in the glottal profile (see Mm. 1 and Mm. 2). While not entirely visible, the M5 model glottis profile was divergent over a significant portion of the cycle (particularly apparent at low pressures). By contrast, the MRI model appeared to feature an alternating convergent-divergent pattern more typical of the human vocal folds. Other imaging methods (e.g., a hemilarynx arrangement) would be necessary to quantify such aspects of the models’ motion. An M5 model with a convergent prephonatory profile may yield more convergent-divergent motion, and this prospect should be explored.
The difference in deformation between the models can be seen in Mm. 3 and Mm. 4. Again, the M5 model has much more vertical and vertical-lateral displacement. Also, evidence of anterior-posterior mucosal wave-like motion, as well as anterior-posterior asymmetries in vibration, are evident in the MRI model videos, but not the M5 model videos.
Mm. 3.
High-speed video of M5 model vibration, viewed from angled perspective. This is a file of type “mov” (1.2 MB).
Mm. 4.
High-speed video of MRI-based model vibration, viewed from angled perspective. Evidence of mucosal wave-like motion can be seen. This is a file of type “mov” (928 KB).
Further evidence of mucosal wave-like motion can be seen in the VKG images in Fig. 3. With the M5 model, the deformation curves above and below the glottis are in-phase; however, with the MRI model, these curves are slightly out-of-phase, providing evidence of a traveling “mucosal” wave-like feature. This improvement is significant given the important role that the mucosal wave plays in human vocal fold function and clinical observation.
Figure 3.
VKG images showing mucosal wave-like motion produced by the MRI models (right), but not by the M5 models (left). Red arrows indicate direction of wave motion.
Medial surface equation
An equation approximating the medial surface of the MRI model, calculated using a 5th-order surface fit (R2=0.989) to the STL model in MATLAB, is given below (all units in μm, and a coordinate system is provided in Fig. 1). The domain spans 0<x<10 mm, 0<y<17.22 mm. z(x,y)=−3.33×10−1x5+8.92×10−2x4y+6.18x4+2.56×10−1x3y2−6.89y−25.31x3−1.88×10−2x2y3−2.53x2y2+69.29x2y−149.75x2−3.54×10−2xy4+1.39xy3−8.97xy2−169.74xy+1572.76x+2.39×10−2y5−9.94×10−1y4+14.56y3−88.12y2+434.90y+13.129×103
Conclusions and future work
The flow-induced responses of the MRI-based synthetic models showed remarkable differences compared with those of the M5 models. Improvements include (1) less vertical motion, (2) apparently more typical alternating convergent-divergent glottal profile pattern, and (3) evidence of mucosal wave-like motion. The primary disadvantage of the MRI models was the higher onset pressure, and techniques to lower the onset pressure are currently being researched. It is interesting to note that the MRI model exhibited asymmetric anterior-posterior vibrations and some irregularities in glottal area waveforms at higher pressures; these observations are not necessarily perceived as being advantageous or disadvantageous.
Since fabrication methods for both the M5 and MRI models were similar, the differences between the two models’ motions are primarily attributable to differences in geometry. It is to be expected that different geometries will yield different vibration patterns. What is significant, however, is that the MRI model showed important improvements in yielding more life-like motion than the M5 model while using the same materials.
It is emphasized that further developments are still needed to create truly life-like synthetic self-oscillating models, and research into understanding synthetic vocal fold model response is needed. For example, this research does not address the issue of which geometric features governed the various aspects of the vibratory responses of the M5 and MRI models. It is possible that the M5 model may be geometrically altered to improve its response. Systematic, parametric investigations of geometric features of the M5 and other vocal fold models, similar to the recent work by Zhang7 and Cook et al.,19 should be performed. The role of material properties in governing vocal fold model response must also continue to be explored; synthetic model materials reported in the literature (including the present ones) have typically been isotropic and linearly elastic, in contrast with the anisotropic, nonlinear material properties of vocal fold tissue. The development of more realistic synthetic materials will be an important step toward creating more life-like vocal fold models.
Acknowledgments
This work was supported by Grant R01 DC005788 from the National Institute on Deafness and Other Communication Disorders (Dr. Luc Mongeau, PI; Subcontract to Brigham Young University through McGill University). The authors thank Dr. Scott Selbie for the MRI data.
References and Links
- Scherer R. C., Shinwari D., De Witt K. J., Zhang C., Kucinschi B. R., and Afjeh A. A., “Intraglottal pressure profiles for a symmetric and oblique glottis with a divergence angle of 10 degrees,” J. Acoust. Soc. Am. 109, 1616–1630 (2001). 10.1121/1.1333420 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Neubauer J., and Berry D. A., “Aerodynamically and acoustically driven modes of vibration in a physical model of the vocal folds,” J. Acoust. Soc. Am. 120, 2841–2849 (2006). 10.1121/1.2354025 [DOI] [PubMed] [Google Scholar]
- Neubauer J., Zhang Z., Miraghaie R., and Berry D. A., “Coherent structures of the near field flow in a self-oscillating physical model of the vocal folds,” J. Acoust. Soc. Am. 121, 1102–1118 (2007). 10.1121/1.2409488 [DOI] [PubMed] [Google Scholar]
- Popolo P. S. and Titze I. R., “Qualification of a quantitative laryngeal imaging system using videostroboscopy and videokymography,” Ann. Otol. Rhinol. Laryngol. 117, 404–412 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T., Tokuda I. T., Munger J. B., and Thomson S. L., “Mammalian laryngeal air sacs add variability to the vocal tract impedance: Physical and computational modeling,” J. Acoust. Soc. Am. 124, 634–647 (2008). 10.1121/1.2924125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickup B. A. and Thomson S. L., “Influence of asymmetric stiffness on the structural and aerodynamic response of synthetic vocal fold models,” J. Biomech. 42, 2219–2225 (2009). 10.1016/j.jbiomech.2009.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., “Characteristics of phonation onset in a two-layer vocal fold model,” J. Acoust. Soc. Am. 125, 1091–1102 (2009). 10.1121/1.3050285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Scherer R. C., Wan M., Wang S., and Wu H., “Numerical study of the effects of inferior and superior vocal fold surface angles on vocal fold pressure distributions,” J. Acoust. Soc. Am. 119, 3003–3010 (2006). 10.1121/1.2186548 [DOI] [PubMed] [Google Scholar]
- Sidlof P., Svec J. G., Horaced J., Vesely J., Klepacek I., and Havlik R., “Geometry of human vocal folds and glottal channel for mathematical and biomechanical modeling of voice production,” J. Biomech. 41, 985–995 (2008). 10.1016/j.jbiomech.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Zhang Z., “Influence of flow separation location on phonation onset,” J. Acoust. Soc. Am. 124, 1689–1694 (2008). 10.1121/1.2957938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbie W. S., Zhang L., Levine W. S., and Ludlow C. L., “Using joint geometry to determine the motion of the cricoarytenoid joint,” J. Acoust. Soc. Am. 103, 1115–1127 (1998). 10.1121/1.421223 [DOI] [PubMed] [Google Scholar]
- Hunter E. J. and Titze I. R., “Individual subject laryngeal dimensions of multiple mammalian species for biomechanical models,” Ann. Otol. Rhinol. Laryngol. 114, 809–818 (2005). [DOI] [PubMed] [Google Scholar]
- Hunter E. J., Hunter L. M., and Titze I. R., “Individual subject laryngeal dimensions of multiple mammalian species for biomechanical models: A supplement,” The National Center for Voice and Speech Online Technical Memo No. 9, 2005. [DOI] [PubMed]
- Chan R. W., Fu M., Young L., and Tirunagari N., “Relative contributions of collagen and elastin to elasticity of the vocal fold under tension,” Ann. Biomed. Eng. 35, 1471–1483 (2007). 10.1007/s10439-007-9314-x [DOI] [PubMed] [Google Scholar]
- Verdolini-Marston K., Titze I. R., and Druker D. G., “Changes in phonation threshold pressure with induced conditions of hydration,” J. Voice 4, 142–151 (1990). 10.1016/S0892-1997(05)80139-0 [DOI] [Google Scholar]
- Verdolini-Marston K., Titze I. R., and Sandage M., “Effect of hydration treatments of laryngeal nodules and polyps and related measures,” J. Voice 8, 30–47 (1994). 10.1016/S0892-1997(05)80317-0 [DOI] [PubMed] [Google Scholar]
- Jiang J., Ng J., and Hanson D., “The effects of rehydration on phonation in excised canine larynges,” J. Voice 13, 51–59 (1999). 10.1016/S0892-1997(99)80061-7 [DOI] [PubMed] [Google Scholar]
- Hsiao T. Y., Liu C. M., Luschei E. S., and Titze I. R., “The effect of cricothyroid muscle action on the relation between subglottal pressure and fundamental frequency in an in vivo canine model,” J. Voice 15, 187–193 (2001). 10.1016/S0892-1997(01)00020-0 [DOI] [PubMed] [Google Scholar]
- Cook D. D., Nauman E., and Mongeau L., “Ranking vocal fold model parameters by their influence on modal frequencies,” J. Acoust. Soc. Am. 126, 2002–2010 (2009). 10.1121/1.3183592 [DOI] [PMC free article] [PubMed] [Google Scholar]