Abstract
Anterior Gradient 2 (AGR2) is a protein disulfide isomerase that plays important roles in diverse processes in multiple cell lineages as a developmental regulator, survival factor and susceptibility gene for inflammatory bowel disease. Here, we show using germline and inducible Agr2−/− mice that Agr2 plays important roles in intestinal homeostasis. Agr2−/− intestine has decreased goblet cell Mucin 2, dramatic expansion of the Paneth cell compartment, abnormal Paneth cell localization, elevated endoplasmic reticulum (ER) stress, severe terminal ileitis and colitis. Cell culture experiments show that Agr2 expression is induced by ER stress, and that siRNA knockdown of Agr2 increases ER stress response. These studies implicate Agr2 in intestinal homeostasis and ER stress and suggest a role in the etiology of inflammatory bowel disease.
Keywords: AGR2, anterior gradient 2; intestine; Goblet cell; Paneth cell; ER stress; PDI, protein disulfide isomerase
Introduction
Anterior Gradient 2 (AGR2) is a developmental regulator and survival factor with multiple functions that, although highly conserved in evolution, remain poorly characterized. Originally characterized in Xenopus laevis, heterologous AGR2 expression induces ectopic cement gland differentiation and alters neuronal cell fate (Aberger et al., 1998). In multiple mammalian cells, AGR2 stimulates cell proliferation, cell adhesion, motility and inhibits apoptosis (Fritzsche et al., 2006; Innes et al., 2006; Wang et al., 2008; Zhang et al., 2007). AGR2 mRNA and protein occur at high levels in several metastatic adenocarcinomas, e.g., colorectal, esophagus, prostate, and breast, where it stimulates proliferation and inhibits apoptosis (Fritzsche et al., 2007; Fritzsche et al., 2006; Innes et al., 2006; Smirnov et al., 2005; Valladares-Ayerbes et al., 2008; Wang et al., 2008; Zhang et al., 2007). In contrast, human AGR2 genetic variants that decrease its expression are associated with increased risk of both Crohn’s disease and ulcerative colitis (Zheng et al., 2006).
Structurally, AGR2 is a member of the endoplasmic reticulum (ER) protein disulfide isomerase (PDI) gene family (Park et al., 2009; Persson et al., 2005). PDIs facilitate the isomerization of specific client proteins into their bioactive conformations as they traffic through the ER for secretion or membrane association. When PDI substrates fail to isomerize, misfolded membrane-associated and secreted proteins accumulate in the ER, initiating ER stress, cell cycle arrest and apoptosis. For example, AGR2 binds directly to Mucin 2 (Muc2), a major component of intestinal mucus enabling the large number of Muc2 Cys residues to pair correctly. Germline deletion of mouse Agr2 disrupts Muc2 protein stability and decreases intestinal mucus production. However, Agr2 deficiency is reported only to cause modest ER stress in the intestine and the impact of Agr2 deficiency on the biology and functional state of additional intestinal cell lineages is poorly characterized (Park et al., 2009).
The lower gastrointestinal tract consists of the small intestine and colon. The lining epithelium of both organs is organized into crypts. Undifferentiated stem and progenitor cells populate the base of small intestine crypts and give rise to four major cell types: absorptive enterocytes, goblet cells, Paneth cells and enteroendocrine cells (Cheng and Leblond, 1974). Enterocytes absorb nutrients, secrete hydrolytic enzymes into the intestinal lumen and are the most common intestinal cell type. Goblet cells produce mucous to protect the intestinal barrier from infiltration by luminal contents. Paneth cells protect against microbial infection and are located at the crypt bottom. Enteroendocrine cells secrete a variety of hormones and are relatively rare. Colonic crypts contain three major cell types, including a higher proportion of goblet cells, somewhat fewer absorptive cells, and scattered enteroendocrine cells.
In normal intestine, AGR2 is expressed in several cell types. These include mature goblet, Paneth and enteroendocrine cells, as well as Musashi-1 (MSI1)-positive intestinal stem/early progenitor cells and proliferating secretory progenitors (Wang et al., 2008).
Intestinal epithelial cells are sensitive to ER stress. Targeted deletion in the mouse of the ER stress response transcription factor XBP1s, the intestine-specific ER stress response sensor IRE1β, or mice carrying missense mutations that impair Muc2 oligomerisation and protein stability of Muc2, cause intestinal cell ER stress, cell cycle arrest, apoptosis and chronic inflammation (Bertolotti et al., 2001; Heazlewood et al., 2008; Kaser et al., 2008). Therefore, based on its structure and expression pattern, it was anticipated that AGR2 might play a role in the intestinal ER stress response and suppression of inflammation.
Here, we show using germline and inducible Agr2 knockout mice and siRNA knock down in cell culture that Agr2 plays important roles in intestinal goblet and Paneth cell homeostasis, Paneth cell positioning and intestinal ER stress response. These data expand the known roles played by Agr2 in homeostasis of the intestine.
Materials and methods
Generation of Agr2−/− mice
The gene-targeting strategy is described in supplementary methods and summarized in Fig. 1. Mice were housed in a temperature (~21°C) and humid ity (~55%)-controlled room in an AALAC accredited vivarium with a 12 h light :12 h dark cycle. Entry into the vivarium was restricted to appropriate personnel, but no SPF precautions were available. Food pellets (Research Diets, New Jersey) and water were available ad libitum.
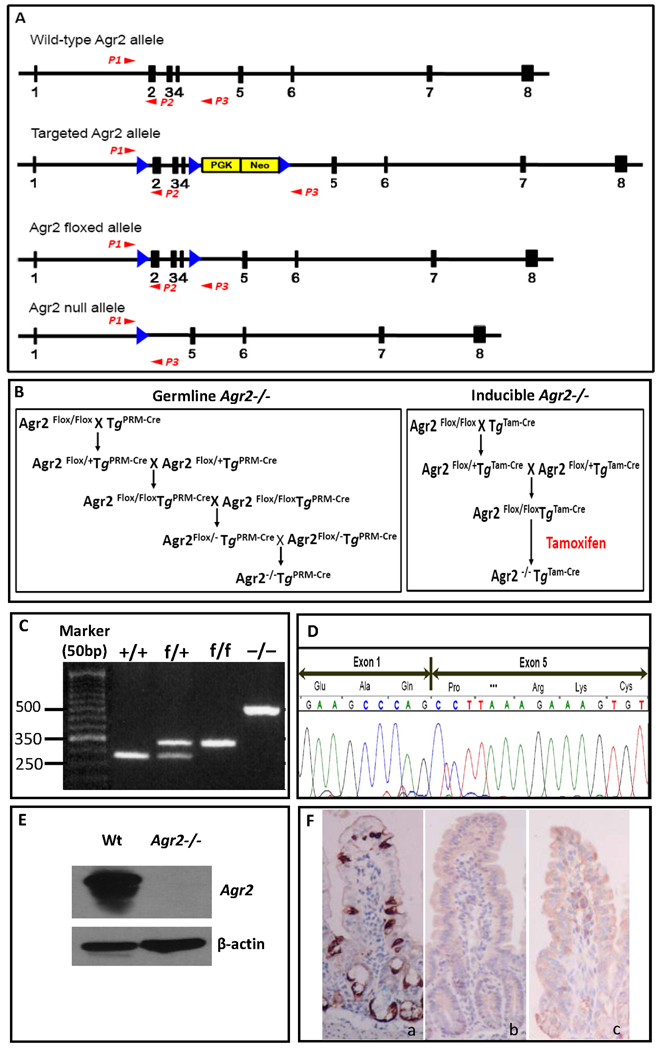
Figure 1.
Generation of Agr2−/− mice. (A) Gene-targeting strategy. The four alleles used in the gene targeting of Agr2 are shown. Black boxes with numbers denote Agr2 exons. PGK-Neo, neomycin resistance cassette, is shown as a yellow box. Blue arrows show LoxP sites. Red arrows P1–P3 depict PCR primer locations. Deletion of exon 2, 3 and 4 produces a frame shift in exon 5. (B) Breeding strategies for germline and inducible Agr2 knockout mice. Flox, flanking LoxP site. PRM-Cre, protamine promoter driven Cre recombinase. Tam-Cre, tamoxifen inducible Cre recombinase. (C) PCR products from different Agr2 genotypes. Mouse tail DNA was PCR amplified using three primers whose locations are shown in Panel A. The wild-type allele produced a band of 280bp, the floxed allele a band of 335bp and the null allele a band of 519bp. (D) DNA sequencing of cDNA showing the junction of exons 1 and 5 in the Agr2−/− intestine epithelium. (E) Western analysis shows a 19.9-kDa band specific for Agr2 absent in Agr2−/− mice. β-actin is used as a protein loading control. (F) Immunohistochemistry for Agr2 in small intestine. Agr2 protein is stained dark brown. (a) wild type; (b) germline Agr2−/−; (c) inducible Agr2−/−.
Histology, immunohistochemistry and immunofluorescence
Mouse intestines were removed and washed in cold phosphate-buffered saline (PBS), fixed by immersion in 4% paraformaldehyde, embedded in paraffin, and 5 µm sections were applied to Probe-on Plus™ slides (Fisher). For histology review, sections were stained with Hematoxylin and Eosin or Alcian blue and nuclear fast red as detailed in supplementary methods For immunohistochemistry, primary antibodies and according biotinylated secondary antibodies were using. Staining was detected with Vectastain ABC reagent (PK-6100, Vector) and development with DAB chromogen (DakoCytomation). For immunofluorescence, primary antibodies and according Texas red or fluorescein labeled secondary antibody were applied, then slides were covered with Vectashield mounting medium with DAPI (H-1200, Vector). For details and sources of antibodies, please refer to supplementary methods.
BrdU labeling proliferation assay
To test proliferation, mice were injected 2 hours before sacrifice with BrdU labeling reagent. Tissues were processed and the BrdU labeling cell proliferation assay were performed according to the manufacturer’s protocol (BrdU labeling and Detection Kit I, Roche).
Epithelial RNA isolation and quantification
Sheets of small intestinal epithelium were separated from underlying lamina propria using 3 mM EDTA, 0.5 mM DTT in PBS (Whitehead et al., 1993). Briefly, mouse intestine was removed, opened and washed in PBS 3 changes. The linearized intestine was decontaminated by soaking in 0.04% (vol/vol) sodium hypochlorite in PBS for 20 minutes at room temperature, then incubated in 3mM EDTA plus 0.5mM DTT in PBS at 22°C for 90 minutes with occasional gentle stirring. The digestion mixture of the intestine was resuspended in PBS and epithelium detached by vigorous shaking in a sealed container. Intestinal epithelium was harvested by centrifuge at 1000rpm for 5minutes. Total RNA isolated using Qiagen RNAeasy columns was reverse transcribed to cDNA by using cDNA Kit (Applied Biosystems). qRT-PCR was performed using an ABI 7900HT-SequenceDetection System with SYBR Green Master Mix. For quantitative analysis, all samples were normalized to Gapdh expression using the ΔΔCT value method. Gene-specific primers refer to supplementary methods.
Western blot
Samples were separated by SDS/PAGE and transferred to Immobilon-P PVDF (Millipore). Membranes were blocked with 5% nonfat dry milk and incubated overnight at 4 °C with primary antibodies. Band was detected by chemiluminesence using HRP-conjugated secondary antibody and ECL Western blotting reagents (GE Healthcare).
Purification and analysis of Cryptdin peptides
Cryptdin peptides were isolated using modified procedures described previously (Mastroianni and Ouellette, 2009; Selsted et al., 1992). Ilea were excised from seven inducible Agr2−/− mice (after 4 days of tamoxifen administration) and seven wild type mice (also received tamoxifen administration for 4 days). Protein extracts were prepared from “complete” ileum, consisting of tissue plus luminal contents. Details refer to supplementary methods.
XBP1 splicing assay
XBP1 splicing was measured by specific primers (Kaser et al., 2008) flanking the splicing site yielding PCR product sizes of 164 and 138 bp for human XBP1u and XBP1s, and 171 and 145 bp for mouse Xbp1. Products were resolved on 2% agarose gels and band intensity was determined densitometrically.
Cell line and small interfering RNA (siRNA) transient transfections
Pancreatic cancer cell line, Su.86.86 (American Type Culture Collection) and ON-TARGETplus SMARTpool Human siRNA-AGR2 and Non-targeting Pool control scrambled siRNA (siRNA-Scr) (Dharmacon, Inc.) were used. Details are described in supplementary methods.
SuperArray Screening
Epithelial RNA was converted to cDNA using RT2 first strand kit (SABioscience, C-03). Real-time PCR was done using SuperArray RT2 SYBR Green qPCR Master Mix (SABioscience, PA-012-12) and SuperArray RT2 Profiler™ PCR Array (PAMM−077). Thermal cycling parameters were 95°C for 10 min, followed by 40 cycles of amplifications at 95°C for 15s, 60°C for 60s. Data was analyzed by PCR Array Data Analysis Web Portal using the default set format.
In situ hybridization
Mouse ileum was fixed in 4% paraformaldehyde at 4°C overnight, then transferred to 30% sucrose solution and incubated at 4°C overnight, finally fr ozen in OCT compound and stored at −80°C. In situ hybridization was performed with 10-µm cryosections as described (Abzhanov et al., 2003). Details refer to supplementary methods.
Results
Generation of germline and inducible Agr2 −/− Mice
To understand the mechanistic roles of Agr2 we created a Cre-LoxP inducible Agr2 knockout mouse model (Fig. 1A). To generate a germline Agr2−/− mice we crossed Protamine-Cre transgenic mice (O'Gorman et al., 1997) (Tg PRM-Cre) that deletes Agr2 in the male germline. For detailed breeding strategies, see Fig. 1B. We then intercrossed Agr2 flox/− mice to make Agr2−/− mice. To generate inducible Agr2−/− mice, we used the Rosa26-CreER strain that has tightly controlled and inducible systemic Cre recombinase activation with tamoxifen (Badea et al., 2003). We crossed the Rosa26-CreER (Tg Tam-Cre) with Agr2 flox/flox to create Agr2flox/flox;TgTam-Cre mice to create inducible Agr2−/− mice. Agr2flox/flox;TgTam-Cre mice express Agr2 until mice are injected with tamoxifen (Garcia and Mills, 2002). For both germline and inducible Agr2−/− mice, deletions of exons 2,3 and 4 were confirmed by sequencing of cDNA converting from RNA isolated from intestine epithelia (Fig. 1D). Agr2 protein was undetectable as assayed by western blotting and IHC (Fig. 1E,F). Our models are therefore a null allele.
Dramatically decreased Mucin 2 in goblet cells from germline and inducible Agr2−/− mice
To analyze the phenotype in GI tract of Agr2−/− mice, we performed H+E staining which showed that germline and inducible Agr2−/− mice lack morphologically normal goblet cells (Supplementary Fig. 1A,B,C). Next, we performed Alcian Blue staining. Alcian Blue detects acid mucopolysaccharides and glycosaminoglycans, which are normally restricted to pre-goblet and goblet cells. Staining of wild type small intestine revealed numerous Alcian Blue-positive cells (Supplementary Fig. 1D). By contrast, virtually no Alcian Blue-positive cells were observed in either germline or inducible Agr2−/− mice(Supplementary Fig. 1E,F). Immunohistochemistry revealed that MUC2 protein was dramatically reduced, but detectable (Supplementary Fig. 1G,H,I). In contrast, the goblet cell marker intestinal trefoil factor (ITF) was not reduced in germline Agr2−/− mice (Supplementary Fig.1J,K). No significant differences in intestinal enteroendocrine cell chromogranin A were seen (Supplementary Fig. 1S,T,U). In summary, the goblet cell lineage continues to exist in both germline and inducible Agr2−/− small intestine but these cells lose their normal morphology and cannot be identified by H&E or Alcian blue staining.
Multiple Paneth cell abnormalities in Agr2−/− mice
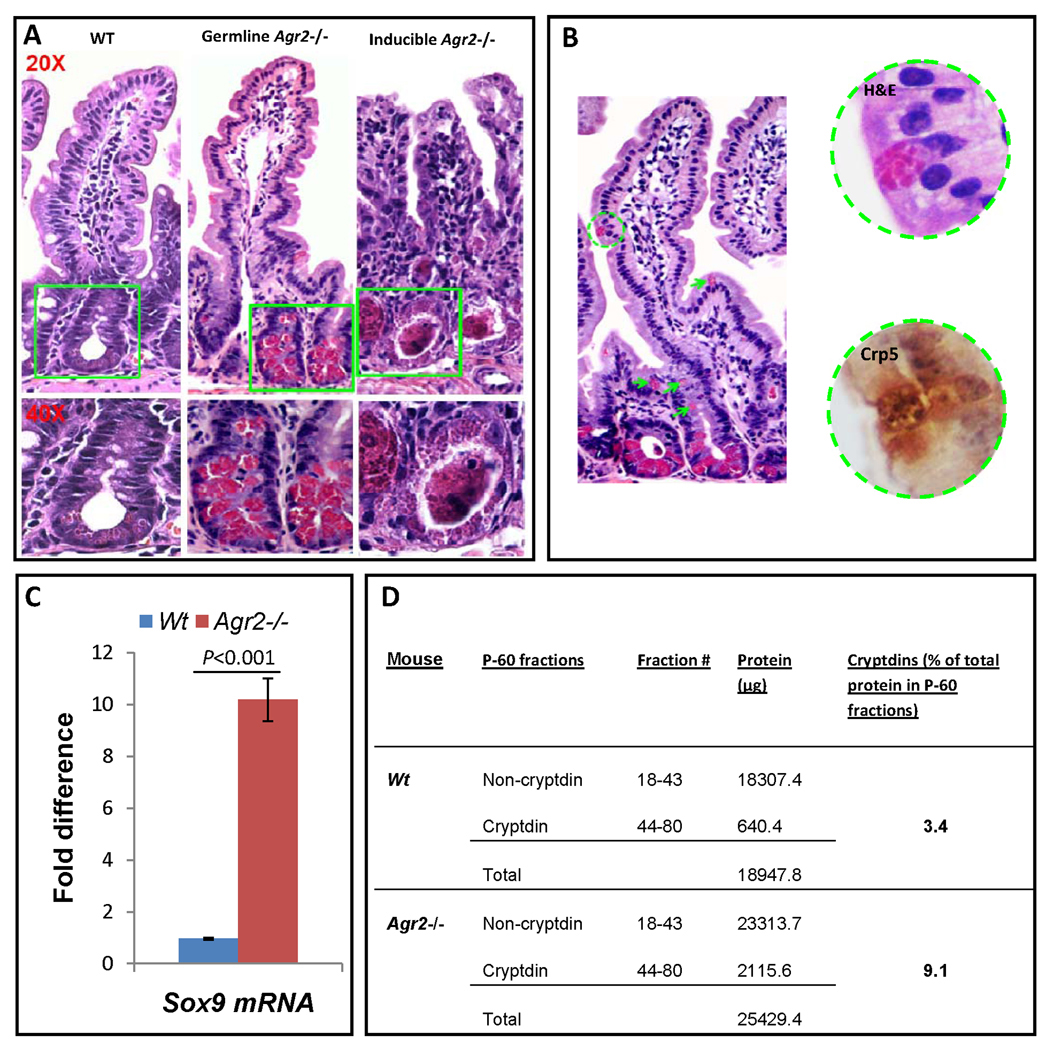
Paneth cells are specialized epithelial cells that mediate innate mucosal immunity against enteric microbial infections. Localized at the base of the crypts of Lieberkühn in the small intestine, Paneth cells release large, apically-oriented dense-core secretory granules containing cryptdins and other host defense molecules (Porter et al., 2002). Dramatic expansion of Paneth cell compartment was consistently seen in all regions of the small intestine both in the germline and inducible Agr2−/− mice (Fig. 2A) (Supplementary Fig. 2). These cells containing large, dense eosinophilic granules were confirmed to be Paneth cells with immunostudies of markers cryptdin-5, MMP7, lysozyme and Sox9 (Supplementary Figs. 1,3). Cells immunopositive for these Paneth cell markers were detected in wild type small intestine and limited to the bottom of crypts. These cells comprised a visibly greater proportion of the crypt base in both germline (Supplementary Fig. 1N,Q) and inducible (Supplementary Fig. 1O,R) Agr2−/− intestine. To confirm these studies, we examined Sox9 mRNA expression, which is elevated more than five- fold (Fig. 2C) and mature cryptdin peptides, which are elevated more than three-fold vs wild type mice (Fig. 2D) in inducible Agr2−/− intestine. Additionally, cells containing large, dense eosinophilic granules are abnormally positioned in the villi of germline Agr2−/− small intestine. Cells with dense large granules are also immunopositive for Paneth cell markers cryptdin-5, MMP7, lysozyme and Sox9 (Fig. 2B and Supplementary Fig. 3).
Figure 2.
Paneth cell abnormalities in germline and inducible Agr2 −/− mice. (A) H+E stained sections of small intestine from wild type, germline and inducible Agr2−/− mice (day5). Paneth cells containing brightly eosinophilic granules are dramatically expanded in both germline and inducible Agr2−/− mice. Intralumenal Paneth cell granule casts are easily visible in the inducible Agr2−/− small intestine. Representative crypt bases are boxed in green and shown at 20× and 40× magnifications. A marked increase in lamina propria mononuclear infiltrates can also be seen in the inducible Agr2−/− small intestine. (B) Abnormally positioned Paneth cells in the upper crypt and villus epithelium of germline Agr2−/− mice. Multiple Paneth cells are ectopically located in the upper crypt (green arrows). A Paneth cell located on the villus is indicated by a green circle. A 40× closeup image reveals characteristic Paneth cell granules that are cryptdin 5-positive by immunohistochemistry. (C) Increased Paneth cell marker Sox9 mRNA in inducible Agr2−/− small intestine (day6). TaqMan analysis of Sox9 mRNA in small intestine of inducible Agr2−/− and wild type mice. Wild-type Sox9 mRNA expression is normalized to 1.0. Mean bars of standard error are shown. The indicated P-values were determined by Student t-test. (D) Increased relative amount of cryptdins in the total protein extracted from distal small intestine of inducible Agr2−/− mice (day4). Bradford assays were performed to quantitate the amount of total protein in individual P-60 fractions from separations of inducible Agr2−/− and Wt P-60 fractions. Cryptdin P-60 fractions were those that appeared to contain mainly cryptdins by AU-PAGE. The total amount of protein was determined by adding the protein in non-cryptdin containing fractions to the protein in cryptdin containing fractions. Relative differences in the protein quantities between inducible Agr2−/− and Wt mice are listed.
Terminal ileitis and colitis in Agr2−/− mice
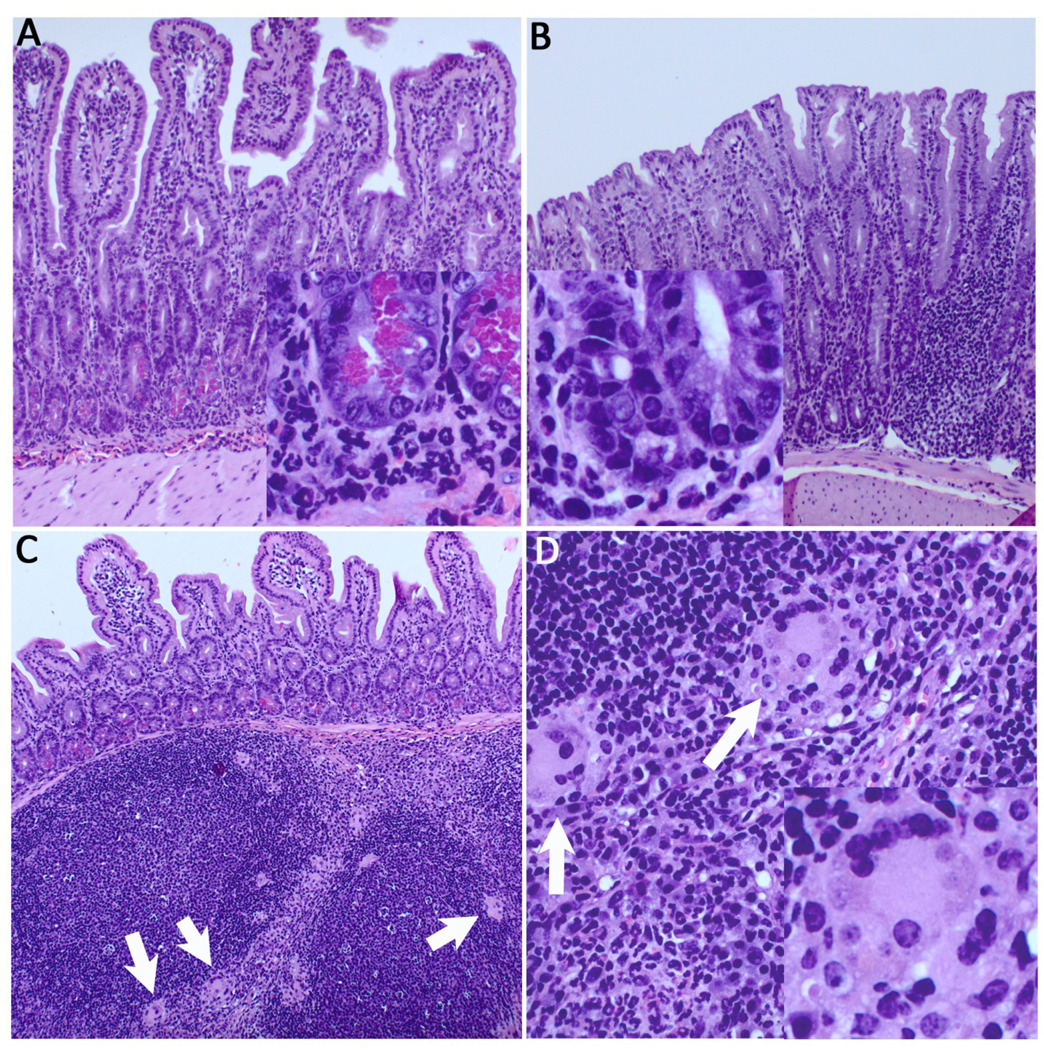
To define in more detail the normal Agr2 expression pattern in wild-type mice, we analyzed the entire lower GI tract by immunohistochemistry and Western blot analysis. Agr2 is expressed in all regions of small intestine and colon. Highest expression is in the ileum and colon (Supplementary Fig. 4). In both germline and inducible Agr2−/− mice, in addition to Paneth cell compartment expansion and decreased Mucin 2 expression in goblet cells we see profound inflammatory infiltrates in the lamina propria and submucosa. The inflammation is most severe in the terminal ileum, and to a lesser extent in the colon. In the germline Agr2−/− mice, the terminal ileitis is characterized by a dense neutrophilic infiltrate surrounding both crypt bases and submucosal perivascular spaces (Fig. 3A, inset). There is marked follicular hyperplasia of the Peyer’s patches, which also have numerous multinucleated giant cells in the interfollicular areas, suggestive of granulomatous inflammation (Fig. 3C,D, arrows). Germline deletion of Agr2 in the colon causes colitis, characterized by a mixed acute and chronic inflammatory infiltrate expanding the isolated lymphoid follicles and the lamina propria. There is significant neutrophil infiltration and marked crypt hyperplasia (Fig. 3B). No inflammation or other abnormalities were seen in Tam-Cre mice without the Agr2 flox/flox alleles (data not shown). In summary, both germline and inducible Agr2−/− mice have dramatic inflammation of the intestine, most notably the ileum, and colon.
Figure 3.
Severe ileitis and colitis in germline Agr2−/− mice. (A) H&E staining reveals severe acute ileitis, with a dense, primarily neutrophilic infiltrate in the lamina propria and submucosa. Inset reveals neutrophils surrounding hypertrophied Paneth cells. (B) Acute colitis in the proximal colon, with loss of goblet cells, crypt elongation, and infiltrating neutrophils (inset). (C) Florid lymphoid hyperplasia of ileal Peyer’s patches, with multinucleated giant cells (white arrows). (D) Multinucleated giant cells suggestive of granulomatous inflammation in interfollicular zones are seen at higher power (inset, lower right).
Paneth cell abnormalities precede those of goblet cells and enterocytes
Because germline Agr2−/− mice have a dramatic increase in both Paneth cells and inflammation, it could not be defined whether Paneth cell expansion was a primary response to acute Agr2 deficiency or a response to inflammation and bacterial invasion from a compromised intestinal epithelial barrier. To resolve this issue, we firstly analyzed the inducible Agr2−/− model at multiple time points after induced deletion. After inducing Agr2 knockout, the first observable defect is Paneth cell expansion 24 hours after tamoxifen injection (day 1) (Fig. 4A). Importantly, this Paneth cell abnormality precedes any histologic signs of inflammation. Subsequently, normal goblet cells are lost and acute inflammatory infiltrates invade the lamina propria, along with a further increase in the Paneth cell compartment (day 3). Next, enterocyte homeostasis is disrupted as the remaining absorptive enterocytes become dysplastic and the villi are blunted (day 5). To test further whether 24 hours after Agr2 deletion there is an early inflammatory response that could explain the observed Paneth cell expansion, we analyzed expression of MUC2, acid mucins (with Alcian blue staining) and 82 key genes involved in mouse inflammatory responses using SuperArray profiling. Compared to wild type mice, MUC2 immunofluorescence and Alcian blue staining at this time point are not changed (Supplementary Fig. 5), and SuperArray profiling reveals only one significantly dysregulated gene (Nos2) 24 hours after Agr2 deletion in small intestine epithelium (Supplementary Fig. 6). However, on day 5 after Agr2 deletion, when there is notable inflammation, MUC2 immunofluorescence, alcian blue staining of acid mucins are dramatically decreased (Supplementary Fig. 1I,F) and 47 out of 82 SuperArray profiled genes are dysregulated (data not shown). To test whether the impact of Agr2 deletion on enterocytes is caused by decreased proliferation or increased apoptosis, we performed BrdU and caspase 3 staining of wild type and induced Agr2−/− small intestine and colon. By day 6, the induced Agr2 deletion caused significantly less proliferation and increased apoptosis in all regions of the small intestine and colon (Fig. 4B,C). In summary, the first observable defect after Agr2 induced knockout is Paneth cell hypertrophy and expansion followed by loss of visible goblet cells, and subsequent decreased enterocyte proliferation and increased apoptosis.
Figure 4.
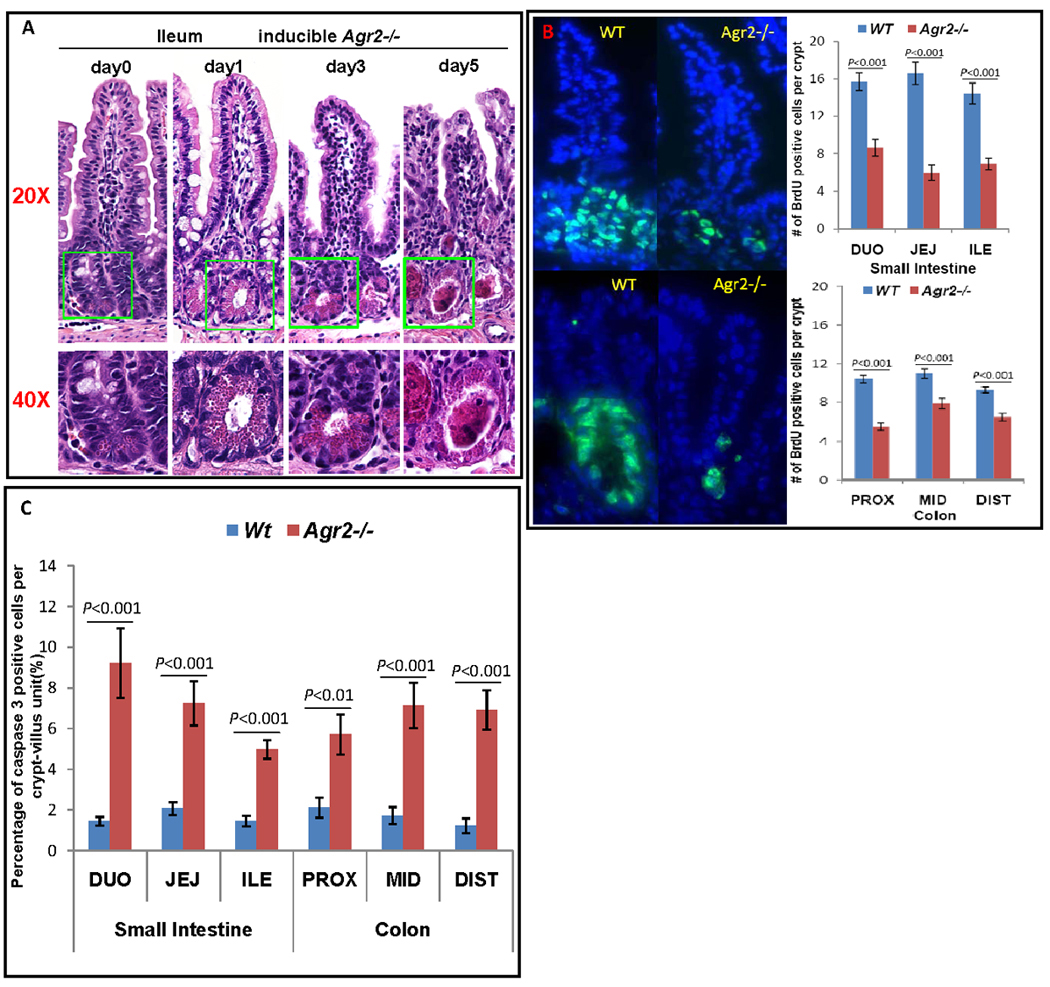
Sequential abnormalities in morphology in the intestine of inducible Agr2−/− mice. (A) H&E staining shows sequential abnormalities of Paneth cells, goblet cells and enterocytes in inducible Agr2−/− small intestine. Representative crypt bases are boxed in green and shown at 20× and 40× magnifications. Agr2 Tam-Cre flox/flox mice were injected with tamoxifen and mice analyzed over 1, 3 and 5 days. Day 0 (same day as tamoxifen injection), no visible abnormality. Day 1, crypt Paneth cell compartment expansion is seen, while goblet cells and enterocytes are unremarkable. Day 3, decrease in morphologically normal goblet cells, enterocyte dysplasia, further Paneth cell expansion and lamina propria inflammation is seen. Day 5, complete loss of morphologically normal goblet cells, severe loss of enterocytes, appearance of Paneth cell casts and increased lamina propria inflammation. (B) BrdU staining shows decreased proliferation in inducible Agr2−/− small intestine and colon (day6). Mice were injected with BrdU and sacrificed 2 hours after injection. Small intestine (top) and colon (bottom) were visualized with fluorescence microscopy. (Left) Immunoflorescence of BrdU (green) labeled crypts from Wt and induced Agr2−/− mice. (Right) Number of BrdU positive cells per crypt in small intestine (top) and colon (bottom) of Wt mice and inducible Agr2−/− mice. (C) Caspase 3 assay shows increased apoptosis in inducible Agr2−/− small intestine and colon (day6). Mean bars of standard error are shown. The indicated P-values were determined by Student t-test.
Increased ER stress in Agr2 knockout intestine and siRNA knockdown cells
Based on its structure and expression pattern, it was originally anticipated that AGR2 might play a role in the intestinal ER stress response. However, in a previous study of Agr2−/− mice, only modestly increased ER stress markers at the mRNA level were found (Park et al., 2009). The ER chaperone BiP and the spliced form of Xbp1 (Xbp1s) are well characterized biomarkers of ER stress (Ron and Walter, 2007). Therefore, we analyzed their expression in Agr2−/− intestinal epithelial preps. BiP protein levels were dramatically increased in germline Agr2−/− small intestine (Fig. 5A). To explore the expression pattern of BiP in small intestine, we first performed in situ hybridization by using a digoxigenin-labeled antisense RNA probe and a sense control probe specific for BiP. In wild type mice, the hybridization signal was detected in epithelial cells of the middle and upper crypts and at crypt-villus junctions (Supplementary Fig. 7A). In germline Agr2−/− mice, increased signal intensity was evident in the same regions as wild type mice but also extending into the lower regions of crypts and villi (Supplementary Fig. 7B). Additionally, certain epithelial cells with increased BiP hybridization signals were seen in the upper villus regions (Supplementary Fig. 7B, black arrows) as well as in cells adjacent to Paneth cells near and at the base of crypts (Supplementary Fig. 7F). No detectable signals were detected at the upper villi or crypt base of wild type mice (Supplementary Fig.7E). As Agr2 is normally expressed in multiple cell types including all three mature secretory cell lineages and MSI1-positive intestinal stem cells/early progenitor cells (Wang et al., 2008), we performed co-immunofluorescence studies to understand which cell types experience ER stress. BiP protein is increased is MSI1-positive cells at the crypt base (Supplementary Fig. 8A, white arrow) and lower crypt proliferative zone (Supplementary Fig. 8B, white arrows). Additionally, both ITF-positive goblet cells and abnormally positioned MMP7-positive Paneth cells on villi have increased BiP protein (Supplementary Fig. 8C,D). In summary, protein levels of the ER stress marker BiP are increased in MSI1-positive intestinal stem/early progenitor cells as well as in cells on villi that are immunopositive for goblet cell and Paneth cell lineage markers.
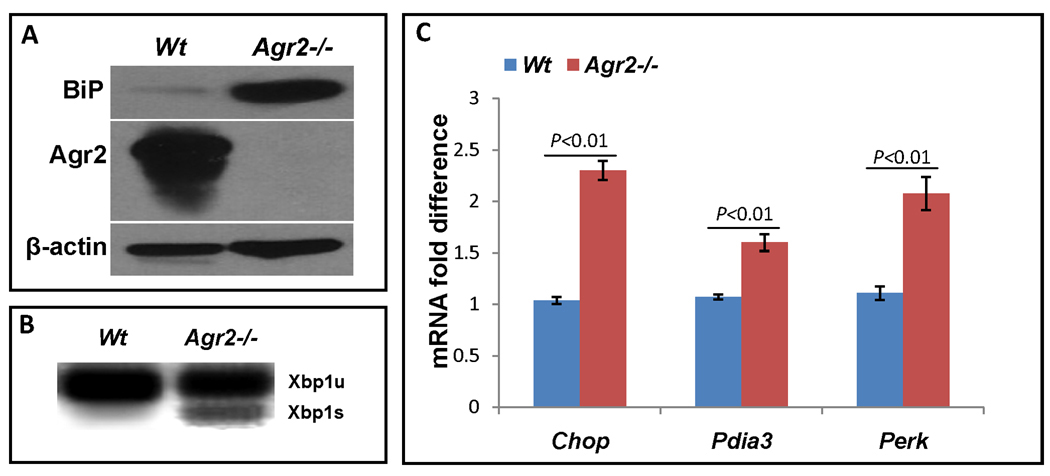
Figure 5.
Agr2 knockout increases ER stress in the intestine. (A) Germline Agr2−/− small intestine has increased levels of the ER stress response marker BiP. Agr2 protein and actin loading control are shown below. (B) Inducible Agr2−/− small intestine (day6) has increased ER stress response marker Xbp1s. Agr2 deletion was induced with tamoxifen and small intestine was assayed for Xbp1s mRNA on day6. Xbp1u, unspliced, full length form of Xbp1. Xbp1s, spliced form that is induced by ER stress. (C) Increased ER stress marker mRNA expression in induced Agr2−/− mice (day 6). qPCR of ER stress biomarker expression levels of Chop, Pdia3 and Perk. mRNA expressions of wild type are normalized to 1.0. Mean bars of standard error are shown. The indicated P-values were determined by Student t-test.
To examine ER stress in more detail, we analyzed other markers. In wild type mice, Xbp1u mRNA was detectable but Xbp1s mRNA level was not. In contrast, Xbp1s mRNA was expressed in Agr2−/− mice (Fig. 5B). mRNA levels for additional ER stress response markers Chop, Pdia3 and Perk also were elevated (Fig. 5C).
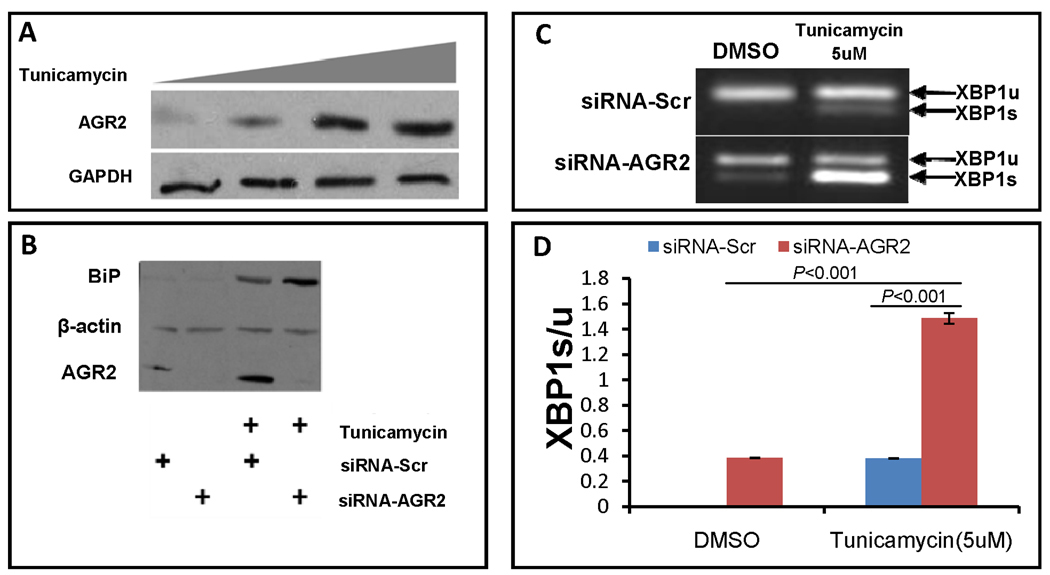
Because the intestine has a complex physiology and is populated with many different cell types, we could not determine whether the impact of Agr2 deletion directly or indirectly increases intestinal ER stress. To confirm the observations in Agr2−/− intestine and test whether Agr2 plays a direct role in this process, we used a different experimental approach, siRNA knockdown studies in cell culture. First, we tested whether AGR2 is an ER stress response gene. In SU86.86 cells, baseline AGR2 protein levels are low. Tunicamycin is a well characterized inhibitor of ER protein glycosylation and inducer of ER stress. Treatment with tunicamycin significantly increases AGR2 protein levels (Fig. 6A). Next, we looked at protein levels of the ER chaperone and ER stress response gene BiP. In untreated cells transfected with control scrambled siRNA, protein levels of the ER stress biomarker BiP are low (Fig. 6B). siRNA knockdown of AGR2 in cells effectively decreases AGR2 protein levels, but does not affect basal BiP levels. As expected, treatment with the ER stress inducer tunicamycin increases BiP levels. siRNA knockdown of AGR2 in tunicamycin treated cells further increases BiP protein levels. Consistent with Fig. 6A, AGR2 protein levels are also induced by tunicamycin, while control β-actin levels are not. Next, we tested for XBP1s. As expected, tunicamycin increases the ratio of spliced (active in ER stress) to unspliced XBP1 (XBP1s/u). siRNA knockdown in untreated cells only slightly increased the XBP1s/u ratio. However, in the presence of tunicamycin, siRNA knockdown of Agr2 significantly increased the XBP1s/u ratio (Fig. 6C,D).
Figure 6.
AGR2 siRNA knock down increases ER stress. (A) AGR2 is an ER stress response gene. Western blot analysis of SU86.86 cells in culture treated with increasing amounts of the ER stress inducer tunicamycin (0, 2.5, 5 and 7.5uM) and probed with anti-AGR2 (Top) and anti-GAPDH as a loading control (bottom). (B) AGR2 siRNA knockdown increases the ER stress biomarker BiP in tunicamycin treated cells. Western blot analysis of SU86.86 cells treated with 2.5uM tunicamycin and transfected with anti-AGR2 siRNA. BiP, ER stress biomarker BiP (Top). Beta-actin is included as a loading control (Middle). AGR2, Anterior gradient 2 (bottom). BiP, beta-actin and AGR2 all have different electrophoretic mobilities and were probed on the same transferred gel. SiRNA-Scr, negative control scrambled siRNA. SiRNA-AGR2, AGR2 specific siRNA (Open biosystems). (C,D) RT-PCR of XBP1 unspliced and spliced isoforms in SU 86.86 cells. AGR2 siRNA knockdown induces XBP1s levels in SU 86.86 cells. Cells were treated with tunicamycin at 5uM, siRNA -AGR2 increases XBP1s levels to much higher levels than siRNA-Scr, P<0.001. Standard error of the mean is shown. The indicated P-values were determined by Student t-test.
Discussion
AGR2 is an evolutionarily highly conserved protein disulfide isomerase whose roles include stimulation of cell proliferation, adhesion, motility, anti-apoptosis, Muc2 protein folding and developmental cell fate (Aberger et al., 1998; Fritzsche et al., 2007; Fritzsche et al., 2006; Innes et al., 2006; Smirnov et al., 2005; Valladares-Ayerbes et al., 2008; Wang et al., 2008; Zhang et al., 2007). In the intestine, AGR2 is strongly expressed in several distinct cell types, including the three differentiated intestinal secretory cell lineages, goblet cell progenitors and MSI1-positive intestinal stem cells/early progenitors (Wang et al., 2008). Because of this expression pattern and the fact that each protein disulfide isomerase is thought to have multiple client substrates, it was originally anticipated that AGR2 would play diverse roles in multiple intestinal cell types. Our experiments indicate that the absence of Agr2 can lead to abnormalities in multiple intestinal cell types and increased ER stress.
A recent study using germline Agr2−/− mice showed that this gene plays an essential role in the production of intestinal mucus by goblet cells(Park et al., 2009). However, in the absence of exogenous agents, there were no other abnormalities in intestinal physiology, homeostasis, or inflammation of the small intestine or colon noted. In our study, we generated germline and inducible Agr2−/− mouse models. Consistent with this study (Park et al., 2009), our germline and inducible Agr2−/− mice have virtually no morphologically normal goblet cells and decreased intestinal mucus. Also consistent with this study, the goblet cell lineage still exists and there is no apparent enteroendocrine cell phenotype.
However, in contrast to the previous study, we observe several additional abnormalities. These include dramatic intestinal Paneth cell abnormalities and severe intestinal inflammation in the absence of treatment with any exogenous agents. The Paneth cell expansion occurs along the whole small intestine and is most dramatic in the ileum. Extracellular Paneth cell granule aggregations and even casts commonly occur. Consistent with expansion of the Paneth cell compartment, expression of differentiated Paneth cell markers Sox9 and cryptdin-5 are dramatically elevated.
Another abnormality in the germline Agr2−/− mice is mislocalized Paneth cells away from the crypt base into the upper crypt and villi. This abnormal Paneth cell location phenotype is also seen in mouse knockouts of the WNT signaling components Frizzled 5, EphB3 and Apc that help specify proper cell location along the crypt-villus axis (Andreu et al., 2008; Batlle et al., 2002; van Es et al., 2005). Because Sox9 is a feedback inhibitor of WNT signaling, it is possible that the increased Sox9 expression in Agr2−/− intestine inhibits Frizzled 5, EphB3 or Apc expression and/or activity to cause inappropriate Paneth cell migration. Future studies with inducible Sox9 transgenic mice may help to answer this question.
Because Paneth cells can respond to inflammation, we performed a time course with inducible Agr2−/− mice to determine if inflammation preceded the Paneth cell increase or the reverse sequence was occurring. Expansion of the Paneth cell compartment is the first morphological abnormality caused by induced Agr2 deficiency and precedes any signs of intestinal inflammation or decreased intestinal mucus production. In summary, while we cannot exclude a low level inflammatory response 24 hours after Agr2 induced deletion that we did not detect is responsible for the expansion of the Paneth cell compartment, our data are most consistent with Paneth cell abnormalities being the first observable change from induced Agr2 deletion, followed by loss of normal goblet cells and mucin secretion. Future studies will be required to address whether expansion of the Paneth cell compartment truly is a primary effect of induced Agr2 deletion, or if inflammation below the detection level of our studies is causing this expansion. Because a recent study observed the morphological loss of goblet cells without any Paneth cell abnormalities, the mechanism of Agr2 action to inhibit goblet cell mucus production and impair normal morphology is likely to be distinct from that causing Paneth cell expansion. This interpretation is also consistent with previous observations that transgenic ablation of goblet cells does not cause spontaneous inflammation or Paneth cell expansion (Itoh et al., 1999), both of which are seen in Agr2−/− mice. Finally, in the inducible Agr2−/− model, subsequent to Paneth and goblet cell abnormalities and inflammation, enterocyte homeostasis is disrupted with decreased proliferation, increased apoptosis and blunting of villi. Because Sox9 knockout in intestinal epithelium causes increased enterocyte proliferation, hyperplasia and Paneth cell loss (the opposite of the Agr2−/− phenotype), it is possible these effects are mediated through increased levels of Sox9 in inducible Agr2−/− mice. However, given the delay in enterocyte abnormalities, these effects may well be secondary to problems occurring in other cell types rather than a primary effect of Agr2 deficiency. Future studies with intestinal cell type specific Agr2 deletion will be helpful to resolve this issue.
We do not see abnormally positioned Paneth cell in the inducible Agr2−/− mice, nor do not see decreased proliferation or increased apoptosis of enterocytes in the germline Agr2−/− mice. The reasons for these differences are not clear at present. With regard to the Paneth cell location, the acute significant disruption of crypt-villus architecture in the inducible Agr2−/− mice may preclude abnormal Paneth cell migration or differentiation in the upper crypt and villus, if it were to occur. With regard to decreased proliferation and increased apoptosis of enterocytes and overall disruption of crypt-villus architecture in the inducible but not germline Agr2−/− mice, we speculate that this difference may reflect a gradual upregulation of other protein disulfide isomerases during development in the germline knockout mice that compensate for the loss of Agr2. However, with inducible Agr2 knockout, intestinal cells cannot compensate for the acute loss. The lack of acute compensation causes increased cellular apoptosis and decreased proliferation. Future studies to identify other protein disulfide isomerases that can compensate for Agr2 loss may help to resolve this issue.
Our Agr2−/− mice develop severe acute terminal ileitis with multinucleated giant cells reminiscent of granulomatous inflammation. Overall, our findings in the germline knockouts are similar to that seen in human Crohn’s disease. In molecular epidemiology studies, AGR2 genetic variants that decrease its mRNA expression are associated with increased risk of both Crohn’s disease and ulcerative colitis(Zheng et al., 2006). While there are multiple mouse models used for the study of ulcerative colitis(Byrne and Viney, 2006; Jurjus et al., 2004) to our knowledge only TNFΔARE and SAMP1/Yit mice are currently used as models of Crohn’s disease (Bamias et al., 2007). Therefore, further studies of Agr2−/− mice may be particularly helpful to improve our mechanistic understanding of Crohn’s disease etiology.
ER stress causes cells to arrest proliferation and initiate apoptosis. Targeted deletion of the mouse ER stress response transcription factor Xbp1, the GI epithelial specific ER stress response gene Ire1β or mice carrying missense mutations in Muc2 and Mtbtps1 that increase GI epithelial cell ER stress cause decreased intestinal enterocyte proliferation, increased apoptosis, and inflammation (Bertolotti et al., 2001; Brandl et al., 2009; Heazlewood et al., 2008; Kaser et al., 2008). Protein disulfide isomerases relieve ER stress and Agr2 was previously speculated to play a role in the ER stress response (Persson et al., 2005). In our Agr2−/− mice, we find elevated intestinal levels of ER stress. ER stress is high in proliferating MSI1-positive intestinal stem cell/early progenitor cells, ITF-positive goblet lineage cells and MMP7-positive abnormally positioned Paneth cells in the villus in Agr2−/− small intestine. Surprisingly, there is no increased ER stress in the mature Paneth cells at the crypt base. Future experiments with cell lineage specific Agr2 knockout mice will be necessary to understand more precisely the role of Agr2 in the different intestinal cell types, and whether these are the same cells experiencing ER stress in Xbp1, Ire1β, Muc2 and Mtbtps1 mouse models of inflammatory bowel disease. Additionally, these experiments will help address the role of cell autonomous and non-cell autonomous increased ER stress in the intestine.
To confirm whether Agr2 deficiency increases ER stress, and test whether this effect is a direct rather than indirect consequence of Agr2 deficiency, we performed siRNA knockdown of Agr2 in cell culture. We found Agr2 protein levels increase in response to ER stress and that Agr2 knockdown causes increased ER stress levels in cells treated with the ER stress inducer tunicamycin.
In the previous study of Agr2−/− mice, the authors concluded that ER stress was not significantly elevated in this model. However, in their model the intestinal inflammation was not seen in the small intestine and extremely mild in the colon in the absence of treatment with the colitis inducer DSS(Park et al., 2009). It is not clear at present why in our Agr2−/− mouse lines intestinal inflammation is more severe, and several intestinal cell types are affected, compared to only goblet cell Muc2 production and increased DSS sensitivity in the line developed previously. In our model, we engineered deletion of Agr2 exons 2–4, while the other group deleted exons 2–3. We also cannot exclude differences in genetic background as a potential cause. For example, the C57B/L6 strain is largely resistant to intestinal inflammation by DSS and in Muc2−/− mice the colitis phenotype arises only on a permissive genetic background (Velcich et al., 2002). However, perhaps a more likely explanation is that the mice generated previously were housed in a germ free barrier facility while our mice were not. Therefore, it is tempting to speculate that commensal flora in our Agr2−/− colony contribute to the broader expressivity and increased severity of the pathology, most notably in the development of terminal ileitis. Commensal microflora have been suggested to worsen the severity of terminal ileitis in the SAMP1/YitFc model of Crohn’s disease(Bamias et al., 2007). This speculation would be consistent with our cell culture studies as well, where Agr2 knockdown in isolation did not much affect basal ER stress levels but had a significant effect when cells were treated exogenously with an ER stress inducing agent. Future studies that examine Agr2−/− intestinal inflammation in germ free conditions with exposure to individual, well characterized strains of commensal bacteria will be helpful to address this question. Since human AGR2 genetic variants that decrease its expression are associated with increased risk of Crohn’s disease and ulcerative colitis(Zheng et al., 2006), further experiments studying gene/environment interactions in Agr2−/− mice and cells may well provide important insights into the etiology of Crohn’s disease and ulcerative colitis.
Supplementary Material
Acknowledgments
Grant Support: NIH Grants DK044632, AI059346 (A.J.O.); Institutional funds (S.M. L.); NIH Grants AR 44882 (B.A.).
Abbreviations
- AGR2
Anterior Gradient 2
- ER
endoplasmic reticulum
- PDI
protein disulfide isomerase
- BrdU
bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Fang Zhao, Steven Lipkin, Bogi Andersen and Andre Ouellette participated in the study concept and design and data analysis. Fang Zhao, Diana Dizon, Jennifer Mastroianni and Mikhail Geyfman participated in data acquisition. Robert Edwards reviewed pathology.
There are no conflicts of interest to disclose for any author.
References
- Aberger F, Weidinger G, Grunz H, Richter K. Anterior specification of embryonic ectoderm: the role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–130. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Tzahor E, Lassar AB, Tabin CJ. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development (Cambridge, England) 2003;130:4567–4579. doi: 10.1242/dev.00673. [DOI] [PubMed] [Google Scholar]
- Andreu P, Peignon G, Slomianny C, Taketo MM, Colnot S, Robine S, Lamarque D, Laurent-Puig P, Perret C, Romagnolo B. A genetic study of the role of the Wnt/beta-catenin signalling in Paneth cell differentiation. Developmental biology. 2008;324:288–296. doi: 10.1016/j.ydbio.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Badea TC, Wang Y, Nathans J. A noninvasive genetic/pharmacologic strategy for visualizing cell morphology and clonal relationships in the mouse. J Neurosci. 2003;23:2314–2322. doi: 10.1523/JNEUROSCI.23-06-02314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias G, Okazawa A, Rivera-Nieves J, Arseneau KO, De La Rue SA, Pizarro TT, Cominelli F. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol. 2007;178:1809–1818. doi: 10.4049/jimmunol.178.3.1809. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide P, Darido C, Pannequin J, Kist R, Robine S, Marty-Double C, Bibeau F, Scherer G, Joubert D, Hollande F, Blache P, Jay P. Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol. 2007;178:635–648. doi: 10.1083/jcb.200704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, Clevers H. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell. 2002;111:251–263. doi: 10.1016/s0092-8674(02)01015-2. [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandl K, Rutschmann S, Li X, Du X, Xiao N, Schnabl B, Brenner DA, Beutler B. Enhanced sensitivity to DSS colitis caused by a hypomorphic Mbtps1 mutation disrupting the ATF6-driven unfolded protein response. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3300–3305. doi: 10.1073/pnas.0813036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne FR, Viney JL. Mouse models of inflammatory bowel disease. Current Opinion in Drug Discovery & Development. 2006;9:207–217. [PubMed] [Google Scholar]
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. American Journal of Anatomy. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I, Dietel M, Kristiansen G. Expression of AGR2 in non small cell lung cancer. Histol Histopathol. 2007;22:703–708. doi: 10.14670/HH-22.703. [DOI] [PubMed] [Google Scholar]
- Fritzsche FR, Dahl E, Pahl S, Burkhardt M, Luo J, Mayordomo E, Gansukh T, Dankof A, Knuechel R, Denkert C, Winzer KJ, Dietel M, Kristiansen G. Prognostic relevance of AGR2 expression in breast cancer. Clin Cancer Res. 2006;12:1728–1734. doi: 10.1158/1078-0432.CCR-05-2057. [DOI] [PubMed] [Google Scholar]
- Garcia EL, Mills AA. Getting around lethality with inducible Cre-mediated excision. Semin Cell Dev Biol. 2002;13:151–158. doi: 10.1016/s1084-9521(02)00019-8. [DOI] [PubMed] [Google Scholar]
- Heazlewood CK, Cook MC, Eri R, Price GR, Tauro SB, Taupin D, Thornton DJ, Png CW, Crockford TL, Cornall RJ, Adams R, Kato M, Nelms KA, Hong NA, Florin TH, Goodnow CC, McGuckin MA. Aberrant mucin assembly in mice causes endoplasmic reticulum stress and spontaneous inflammation resembling ulcerative colitis. PLoS Med. 2008;5:e54. doi: 10.1371/journal.pmed.0050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes HE, Liu D, Barraclough R, Davies MP, O'Neill PA, Platt-Higgins A, de Silva Rudland S, Sibson DR, Rudland PS. Significance of the metastasis-inducing protein AGR2 for outcome in hormonally treated breast cancer patients. Br J Cancer. 2006;94:1057–1065. doi: 10.1038/sj.bjc.6603065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, Beck PL, Inoue N, Xavier R, Podolsky DK. A paradoxical reduction in susceptibility to colonic injury upon targeted transgenic ablation of goblet cells. J Clin Invest. 1999;104:1539–1547. doi: 10.1172/JCI6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurjus AR, Khoury NN, Reimund JM. Animal models of inflammatory bowel disease. Journal of Pharmacological & Toxicological Methods. 2004;50:81–92. doi: 10.1016/j.vascn.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroianni JR, Ouellette AJ. Alpha-defensins in enteric innate immunity: functional Paneth cell alpha-defensins in mouse colonic lumen. The Journal of biological chemistry. 2009;284:27848–27856. doi: 10.1074/jbc.M109.050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Akiyama Y, van den Born M, van Es JH, Hamilton SR, Adams HP, Zhang J, Clevers H, de Crombrugghe B. SOX9 is required for the differentiation of paneth cells in the intestinal epithelium. Gastroenterology. 2007;133:539–546. doi: 10.1053/j.gastro.2007.05.020. [DOI] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, Killeen N, Erle DJ. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci U S A. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson S, Rosenquist M, Knoblach B, Khosravi-Far R, Sommarin M, Michalak M. Diversity of the protein disulfide isomerase family: identification of breast tumor induced Hag2 and Hag3 as novel members of the protein family. Mol Phylogenet Evol. 2005;36:734–740. doi: 10.1016/j.ympev.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Porter EM, Bevins CL, Ghosh D, Ganz T. The multifaceted Paneth cell. Cell Mol Life Sci. 2002;59:156–170. doi: 10.1007/s00018-002-8412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- Selsted ME, Miller SI, Henschen AH, Ouellette AJ. Enteric defensins: antibiotic peptide components of intestinal host defense. Journal of Cell Biology. 1992;118:929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG, Connelly MC, Terstappen LW, O'Hara SM. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993–4997. doi: 10.1158/0008-5472.CAN-04-4330. [DOI] [PubMed] [Google Scholar]
- Valladares-Ayerbes M, Diaz-Prado S, Reboredo M, Medina V, Iglesias-Diaz P, Lorenzo-Patino MJ, Campelo RG, Haz M, Santamarina I, Anton-Aparicio LM. Bioinformatics approach to mRNA markers discovery for detection of circulating tumor cells in patients with gastrointestinal cancer. Cancer Detect Prev. 2008;32:236–250. doi: 10.1016/j.cdp.2008.08.002. [DOI] [PubMed] [Google Scholar]
- van Es JH, Jay P, Gregorieff A, van Gijn ME, Jonkheer S, Hatzis P, Thiele A, van den Born M, Begthel H, Brabletz T, Taketo MM, Clevers H. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hao Y, Lowe AW. The adenocarcinoma-associated antigen, AGR2, promotes tumor growth, cell migration, and cellular transformation. Cancer Res. 2008;68:492–497. doi: 10.1158/0008-5472.CAN-07-2930. [DOI] [PubMed] [Google Scholar]
- Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Forootan SS, Liu D, Barraclough R, Foster CS, Rudland PS, Ke Y. Increased expression of anterior gradient-2 is significantly associated with poor survival of prostate cancer patients. Prostate Cancer Prostatic Dis. 2007;10:293–300. doi: 10.1038/sj.pcan.4500960. [DOI] [PubMed] [Google Scholar]
- Zheng W, Rosenstiel P, Huse K, Sina C, Valentonyte R, Mah N, Zeitlmann L, Grosse J, Ruf N, Nurnberg P, Costello CM, Onnie C, Mathew C, Platzer M, Schreiber S, Hampe J. Evaluation of AGR2 and AGR3 as candidate genes for inflammatory bowel disease. Genes Immun. 2006;7:11–18. doi: 10.1038/sj.gene.6364263. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.