Abstract
Homeless men who have sex with men (MSM) are a particularly vulnerable population with high rates of substance dependence, psychiatric disorders, and HIV prevalence. Most need strong incentives to engage with community-based prevention and treatment programs. Contingency Management (CM) was implemented in a community HIV prevention setting and targeted reduced substance use and increased health-promoting behaviors over a 24-week intervention period. Participants in the CM condition achieved greater reductions in stimulant and alcohol use (χ2 = 27.36, p<.01) and, in particular, methamphetamine use (χ2 = 21.78, p<.01), and greater increases in health-promoting behaviors (χ2 = 37.83, p<.01) during the intervention period than those in the control group. Reductions in substance use were maintained to 9- and 12-month follow-up evaluations. Findings indicate the utility of CM for this high-risk population and the feasibility of implementing the intervention in a community-based HIV prevention program.
Keywords: contingency management, out-of-treatment, methamphetamine, homeless, men who have sex with men (MSM)
1. Introduction
California has the largest homeless population in the United States, and Los Angeles County has the largest concentration of homeless individuals in the state (Homeless Research Institute at the National Alliance to End Homelessness, 2009). Compared to 27 other U.S. cities, in Los Angeles the rate of homelessness due to substance abuse ranks as fourth highest (57%) while the rate due to mental illness is ranked 21st (14%; Lowe, 2001). The lack of affordable housing and the difficulty accessing publicly funded substance abuse treatment and mental health services without stable housing represent significant contributing factors to the ongoing health disparities experienced by this group. The precipitous economic decline in recent years has exacerbated the problem by adding to the ranks of homeless individuals. The homeless population in Los Angeles is primarily adult (mean age = 40 years); male (66%); persons of color (50% are African American and 33% are Hispanic/Latino); and many suffer from substance abuse disorders (33%–66%) and/or mental illness (25%) (Institute for the Study of Homelessness and Poverty, 2004).
Homeless individuals are difficult to engage in treatment programs due in part to the transient nature of the population and their constant struggle to survive. Among homeless individuals, men who have sex with men (MSM) are a particularly vulnerable subgroup, exhibiting high rates of substance dependence, psychiatric disorders, HIV seroprevalence and the exchange of sex for money or drugs (Reback et al., 2007). This group represents individuals at greatest risk for transmitting and contracting HIV and other sexually transmitted infections (STIs).
Contingency management (CM) utilizes positive reinforcement in the form of vouchers, money or goods/services to reduce dysfunctional behaviors and increase healthy and health-promoting behaviors. Over the past two decades, CM has been utilized successfully with HIV injection risk behaviors (Ghitza et al., 2008) and unemployment (Silverman et al., 2007), and populations including pregnant women (Jones et al., 2000, 2001; Svikis et al., 1997), substance abusers (Prendergast et al., 2006; Stitzer & Petry 2006), homeless individuals (Raczynski et al., 1993; Milby et al., 1996; Tracy et al., 2007), psychiatric patients (Bellack et al., 2006; Bennett et al., 2001; Tracy et al., 2007) and substance-dependent MSM (Shoptaw et al., 2005). CM has demonstrated efficacy for decreasing the use of marijuana (Budney et al., 2000), opioids (Silverman et al., 1996), cocaine (Higgins et al., 2000), methamphetamine (Shoptaw et al., 2005; Lee & Rawson, 2008), alcohol (Higgins & Petry, 1999), nicotine (Ledgerwood, 2008), and with polysubstance-abusing individuals (Downey et al., 2000; Pierce et al., 2006).
A significant challenge to developing effective interventions for drug-dependent individuals is the powerful operant conditioning paradigm by which drug use is maintained; positive reinforcement is provided by euphoria and other pleasurable experiences while using, and negative reinforcement is provided by the attenuation of withdrawal symptoms following additional drug use (Bigelow et al., 1981). Effective CM interventions, therefore, must provide an alternate reinforcement schedule powerful enough to compete with the established drug reinforcement paradigm (Prendergast et al., 2006). Most applications of CM with treatment-seeking substance-dependent individuals have targeted abstinence from drug use, verified by onsite urinalysis, with either an escalating reinforcement schedule or prizes for urine samples that do not indicate recent metabolites for the targeted drug. Efforts utilizing CM to target both abstinence from substances and treatment-related, health-promoting behaviors have yielded mixed results to date (see Stitzer & Petry, 2006 for review).
To date, CM has not been evaluated for reducing drug use and increasing health-promoting behaviors among out-of-treatment, substance-dependent, homeless MSM. CM may be a particularly well-suited intervention for this disenfranchised, high-risk population. Although these individuals are not seeking treatment for their substance dependence, many do access services provided by local community-based organizations. Integrating CM into a community HIV prevention program has the potential to impact public policy decisions regarding local substance abuse funding. The purpose of this study was to assess the efficacy of CM for increasing health-promoting behaviors and reducing substance use among homeless, substance-dependent MSM participating in a community-based, low-intensity, HIV prevention program. We predicted that those randomized into the CM condition would achieve more health-promoting behaviors and greater reductions in substance use than those in the control condition.
2. Materials and methods
2.1. Participants
Participants were recruited from a community-based, low-intensity, health education/risk reduction HIV prevention program serving homeless, substance-using MSM in the Hollywood/West Hollywood area of Los Angeles County. The study was conducted at Friends Community Center, the Hollywood, CA community-based site of Friends Research Institute. The study was approved by the Institutional Review Board of Friends Research Institute, Inc.
Potential participants were deemed eligible for the study if they were active participants in the service program, as defined by verified attendance in a minimum of three groups or counseling sessions; at least 18 years of age; substance-dependent (SCID-verified); non-treatment seeking; homeless; and self-reported sex with a man in the previous 12 months. Individuals were excluded if they did not meet all criteria, were unable to understand the consent forms (unable to pass a consent quiz), or were determined to have a more serious psychiatric condition.
2.2. Procedures
Participants were recruited from April 2005 through February 2008 via flyers posted at the community site and word of mouth. Following consent, intake interviews were conducted to determine study eligibility and collect baseline data including demographics, recent substance use, and psychiatric history. All potential participants, regardless of eligibility, received a $50 gift certificate to a local retail or grocery store for completing the intake interview. Randomized participants also received a $50 gift certificate for completing each 7-, 9-, and 12-month follow-up evaluation.
2.3. Intervention
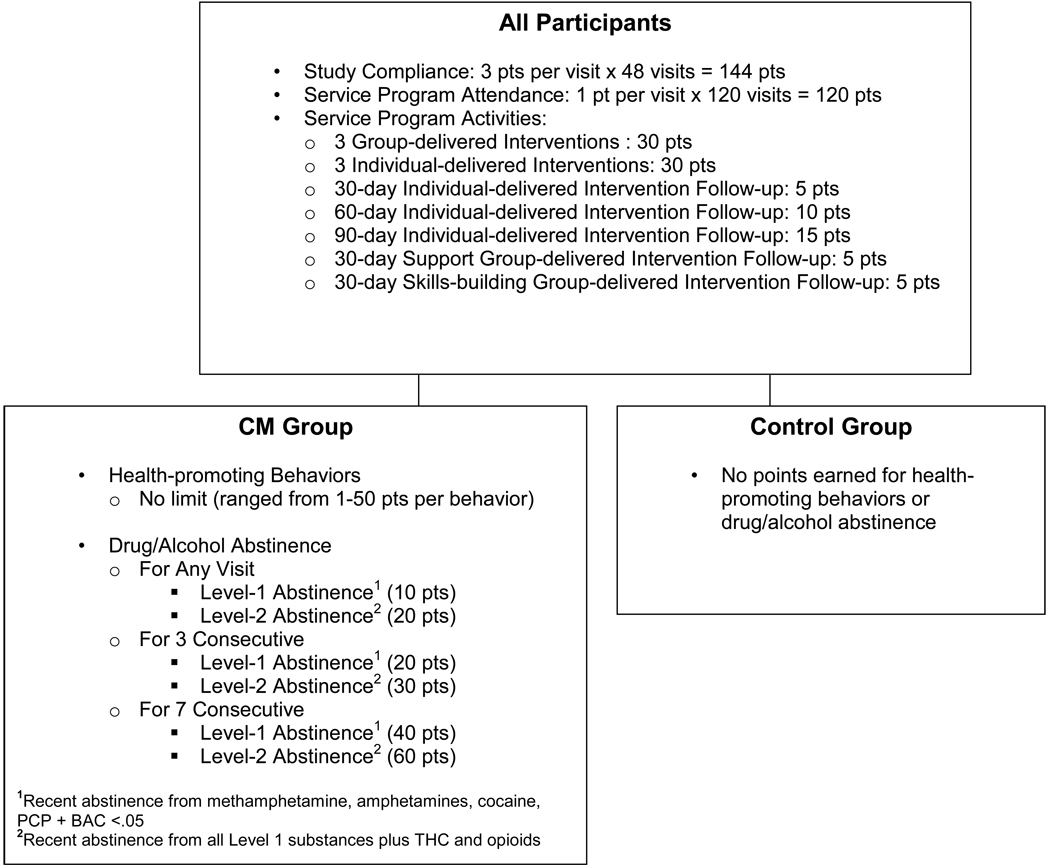
Participants were randomized into either the CM or control condition. Figure 1 illustrates the point schedule for both the CM and control condition. Participants in both conditions earned points for attending scheduled study visits and participating in the HIV prevention program activities. Participants could earn a maximum of 364 points if they completed all study and service program attendance activities.
Figure 1.
Contingency Management Schedule
Participants in the CM condition earned points for completing the targeted health-promoting behaviors and for drug/alcohol abstinence. Complex behaviors were broken down into smaller component behaviors to give participants more opportunities to earn points. Targeted health-promoting behaviors ranged in scale from low impact such as scheduling an appointment with a healthcare provider or social services agency (valued at 4 points), to medium impact such as enrolling in a GED program (valued at 20 points), to high impact such as getting a job (valued at 50 points) and keeping a job for a month (valued at an additional 50 points). Participants reported their health-promoting behaviors to study staff and, once verified, CM points were added to the participant’s existing balance. Health-promoting behaviors that could not be verified, such as condom use, were not targeted. CM points for targeted health-promoting behaviors were not limited. Points for abstaining from substance use were awarded based on a Level 1 (recent abstinence for amphetamine, methamphetamine, PCP and cocaine metabolites as well as blood alcohol <0.05) or Level 2 (recent abstinence for all Level 1 substances as well as opioid and THC metabolites) urine sample. This tier system reflects consistent findings that demonstrate that alcohol and stimulants are frequently abused substances among MSM (Reback, Shoptaw & Grella, 2008) and that stimulant use is strongly associated with HIV high-risk sexual behaviors (Stall and Purcell, 2000). In contrast, opiates are not frequently used by this population and marijuana use, while prevalent, does not have the same strong association with high-risk sexual behaviors.
Points for both conditions were earned during the 24-week intervention and were redeemable at an onsite store that participants could access during any study visit. Each point was equivalent to $1 in purchasing power. To maximize the reinforcing potential of the intervention the store was stocked with participants' preferred products (as determined by biannual focus groups) and priced with items for all earning levels (valued from 1–200 points). Points expired one week following the final 12-month follow-up evaluation.
2.4. Measures
2.4.1. Substance dependence eligibility
The Structured Clinical Interview for DSM-IV (SCID; Spitzer et al., 1995) and the SCID-II (First et al., 1996) were administered at baseline to determine substance dependence eligibility and to assess co-occurring psychiatric disorders. Individuals meeting recent (previous 12 months) dependence criteria were deemed eligible.
2.4.2. Drug and alcohol testing
At all visits a urine drug screen using a 6-panel FDA-approved urine test cup (Accutest - JANT Pharmacal, Inc.) was administered. Metabolites for amphetamines, methamphetamine, cocaine, PCP, THC and opioids were screened (the amphetamine test screens for prescription amphetamines, as opposed to methamphetamine). An alcohol breathalyzer (Alco-Sensor III, Intoximeters Inc.) was also utilized. Drug and alcohol testing was administered twice weekly, on two nonconsecutive days. Samples were immediately analyzed and the results were provided to participants during the same visit. Points earned were added to participants' existing balances and could be redeemed at the onsite store at any study visit.
2.4.3. Addiction Severity Index (ASI)
The ASI (McLellan et al., 1985) was administered to determine addiction-related problem severity profiles in one or more of the six domains assessed (substance use, medical, psychiatric, legal, family/social and employment/support). The ASI was administered at baseline, monthly during the intervention period, and at all follow-up evaluations.
2.5. Data analysis
This study utilized a two-group randomized and controlled experimental design with repeated measures. Data were collected at baseline, biweekly during the intervention period (24-weeks), and at 7-, 9- and 12-months post-randomization follow-up evaluations. All analyses were conducted using an “intent-to-treat” model (i.e. all participants were included in the analyses, regardless of whether they completed the full intervention period).
2.5.1. Health-promoting behaviors outcomes
Attendance and health-promoting behaviors data were available only as a total score calculated at the end of the intervention period. A comparison of behaviors by treatment condition and an examination of key predictors (age, race/ethnicity, and HIV status) was conducted utilizing Wilcoxon two-sample tests that corrected (Tukey correction) for multiple comparisons. Treatment by predictor interactions were assessed with two-way analysis of variance (ANOVA) tests.
2.5.2. Substance use outcomes
The urine drug analysis and breathalyzer data were evaluated for differences between the CM and control conditions on each individual substance as well as on the aggregate Level 1 score (i.e. stimulants and alcohol). Level 1 scores were transformed into Treatment Effectiveness Scores (TES; Ling et al., 1997) for repeated measures analysis. The TES is the total number of substance metabolite-free urine samples provided by each participant during the intervention period, compared to the total number of scheduled urine samples (48 in the present study).
Substance use results during the intervention period were evaluated using TES scores; substance use results at baseline and the 7-, 9-, and 12-month follow-up evaluations were analyzed using the Joint Probability Index (JPI; Ling et al., 1997). This index calculates the probability of providing a drug-free urine sample at each time point. Differences between the CM and control conditions at each evaluation were assessed using a Z test for two proportions. Percent of negative urine samples for each treatment condition accounting for non-missing responses were also calculated; differences between conditions were assessed with Z test for two proportions. Generalized Estimating Equation (GEE) modeling (Zeger & Liang, 1986) was utilized to test for differences between results of urine drug screens by condition while accounting for key predictors (age, race/ethnicity, and HIV status). Separate GEE equations were conducted to evaluate the significance between conditions for each drug as well as for the aggregate Level 1 scores.
2.5.3. Missing data
Missing data were not imputed. In order to evaluate potential biases on study findings, a GEE model tested for randomness of missing data in the urine drug screen data. This procedure allowed for evaluation of whether missingness was “completely at random,” “missing at random” (either of which would support use of GEE models to test for condition effects), or “non-ignorable missing data” (which would preclude use of GEE models to test for condition effects). This procedure was constrained to the urine drug screen data as this variable was the one with the highest number of repeated assessments and was the most vulnerable to potential biases from missing data. The analysis of missingness first classified data as missing or observed. The GEE model solutions indicated no statistically significant differences in patterns and rates of missing data between the CM and control groups, providing evidence that missing data were at least missing at random, which supports use of the described GEE models for testing intervention effects. All analyses were conducted using SAS version 9.1 for Windows (SAS Institute Inc, Cary, NC).
3. Results
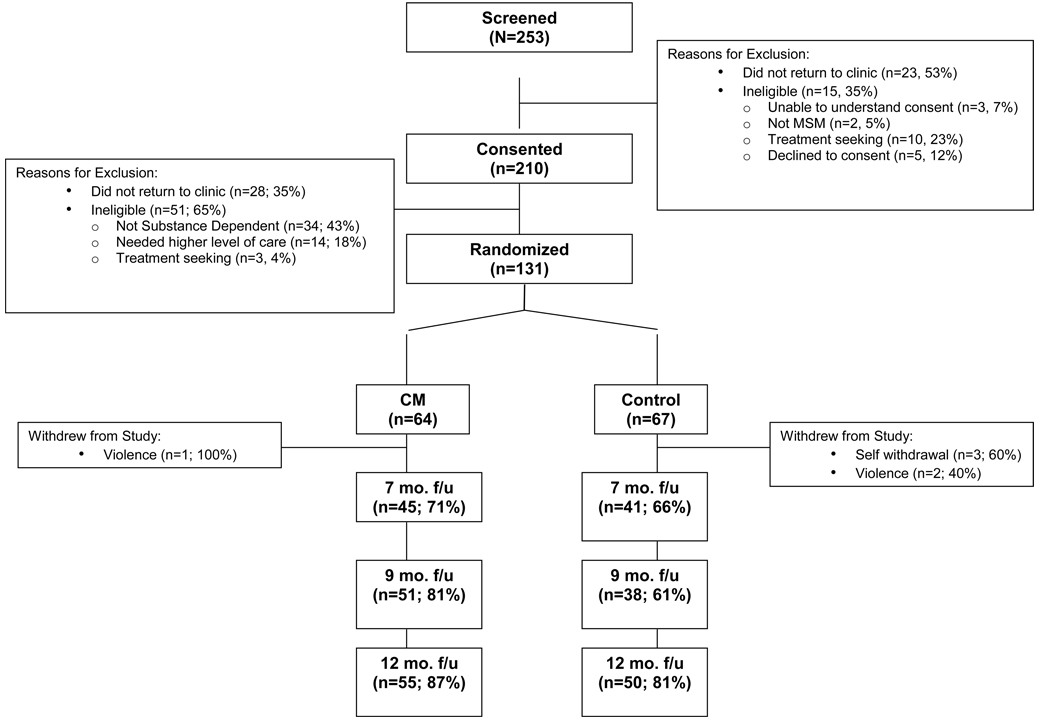
3.1. Study progression and retention
Figure 2 presents study progression and retention from initial screening through 12-month follow-up evaluations. Although the incentives for completing follow-up evaluations were equivalent for CM and control participants, completion rates were significantly higher among CM participants at the 9-month follow-up evaluation (81% vs. 61%; Z=2.33, p<.05) and trended higher at the 7-month (71% vs. 66%, ns) and 12-month (87% vs. 81%, ns) evaluations.
Figure 2.
Study Progression and Retention
3.2. Participant characteristics
Participant demographic characteristics, HIV status, and baseline substance dependence are shown in Table 1. There were no statistically significant differences between the CM and control conditions on demographic characteristics or baseline substance use or dependence. At study intake, nearly two-thirds of participants met criteria for current methamphetamine dependence, and approximately one-third each for marijuana and alcohol dependence. Cocaine and crack cocaine dependence were less prevalent, with three times as many participants meeting criteria for crack dependence as for powder cocaine. More than a quarter of the sample self-reported an HIV-positive serostatus at baseline. The HIV-seropositive participants across both conditions reported more days of methamphetamine use in the 30 days prior to intake than the HIV-seronegative participants (MHIV-positive = 9.5, MHIV-negative = 5.2; p=.006).
Table 1.
Participant Demographics and Baseline Drug Dependence
| CM (n=64) |
Control (n=67) |
Entire Cohort (N=131) |
|
|---|---|---|---|
| % (N) or mean (SD) | |||
| Race / Ethnicity | |||
| Caucasian | 54.7% (35) | 52.2% (35) | 53.4% (70) |
| African American | 21.9% (14) | 23.9% (16) | 22.9% (30) |
| Latino | 17.2% (11) | 16.4% (11) | 16.8% (22) |
| Other | 6.2% (4) | 7.5% (5) | 6.9% (9) |
| Age Range | |||
| mean (SD) | 36.3 (8.7) | 36.5 (8.7) | 36.4 (8.7) |
| Educational Attainment | |||
| mean (SD) | 12.8 (2.3) | 12.0 (3.2) | 12.4 (2.8) |
| HIV Status | |||
| negative | 71.9% (46) | 68.6% (46) | 70.2% (92) |
| positive | 26.5% (17) | 29.9% (20) | 28.3% (37) |
| unknown | 1.6% (1) | 1.5% (1) | 1.5% (2) |
| Current Methamphetamine Dependence | |||
| dependence | 64.0% (41) | 62.7% (42) | 63.4% (83) |
| Current Cannabis Dependence | |||
| dependence | 32.8% (21) | 35.8% (24) | 34.4% (45) |
| Current Alcohol Dependence | |||
| dependence | 28.1% (18) | 37.3% (25) | 32.8% (43) |
| Current Cocaine Dependence | |||
| dependence | 3.1% (2) | 9.0% (6) | 6.1% (8) |
| Current Crack Dependence | |||
| dependence | 23.4% (15) | 14.9% (10) | 19.1% (25) |
3.3. Sample attrition
Across the sample, participants provided 48.5% of all scheduled urine drug samples during the intervention period. CM participants provided a higher proportion of scheduled samples (53.3%) than control participants (43.8%; p=0.04), except for Latino participants, who provided similar proportions of scheduled samples in the CM (35.1%) and control (37.2%) conditions.
3.4. CM reinforcers
The most frequently completed health-promoting behaviors were attending a scheduled meeting with a program/service (48.8%), requesting a referral to programs/services (19.5%), and scheduling an appointment with a program/service (18.7%). The total amount spent in the CM store for the entire study period was $54,800. Participants spent an average of $418 (SD=$481; range: $0–$2,337). CM points were most frequently redeemed for grocery store gift cards (22.6%), restaurant gift cards (17.1%), and department store (most commonly Target) gift cards (14.9%).
3.5. Outcome analyses
3.5.1. Attendance and health-promoting behaviors
Table 2 illustrates the results of two-sample t-tests for attendance and health-promoting behaviors during the intervention phase. There were no statistically significant differences between the CM and control condition on rates of attendance at study visits and service program activities. Participants in the CM condition accomplished significantly more health-promoting behaviors than those in the control condition (MCM = 15.9, Mcontrol = 1.7; p<.01). In the CM condition, HIV-seropositive participants accomplished more health-promoting behaviors than HIV-seronegative participants (F = 7.40, p<.01); however, in the control group no differences based on HIV status were observed. There were no between-group differences based on ethnicity or age; however, within the CM condition Caucasian participants accomplished significantly more health-promoting behaviors than African American and Latino participants.
Table 2.
Attendance and Health-promoting Behaviors
| All Attendance Behaviors a |
Health-promoting Behaviors b |
|||
|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
|||
| CM | Control | CM | Control | |
| Condition | 25.73 | 24.50 | 15.94* | 1.67 |
| (0.07) | (0.11) | (0.001) | (0.54) | |
| HIV Status | ||||
| HIV+ | 25.47 | 30.03 | 31.05** | 2.25 |
| (19.88) | (24.33) | (46.46) | (3.22) | |
| HIV− | 25.83 | 22.15 | 10.46 | 1.45 |
| (21.70) | (19.87) | (14.23) | (2.34) | |
| Race/Ethnicity | ||||
| Caucasian | 28.80 | 26.34 | 22.80** | 2.08 |
| (21.85) | (23.02) | (34.65) | (2.88) | |
| African | 27.79 | 21.63 | 11.35 | 1.69 |
| American | (20.58) | (18.71) | (17.70) | (3.05) |
| Latino | 13.91 | 14.73 | 4.81** | 0.45 |
| (0.81) | (12.27) | (4.67) | (0.82) | |
| Other | 24.25 | 42.40 | 2.50 | 1.40 |
| (16.32) | (26.43) | (2.04) | (1.52) | |
Voucher points were awarded for attendance behaviors to all participants.
Voucher points were awarded for health-promoting behaviors only to CM participants.
p<0.01 between CM and control group.
p<0.05 between CM and control group with Tukey multiple comparisons correction.
3.5.2. Substance Use
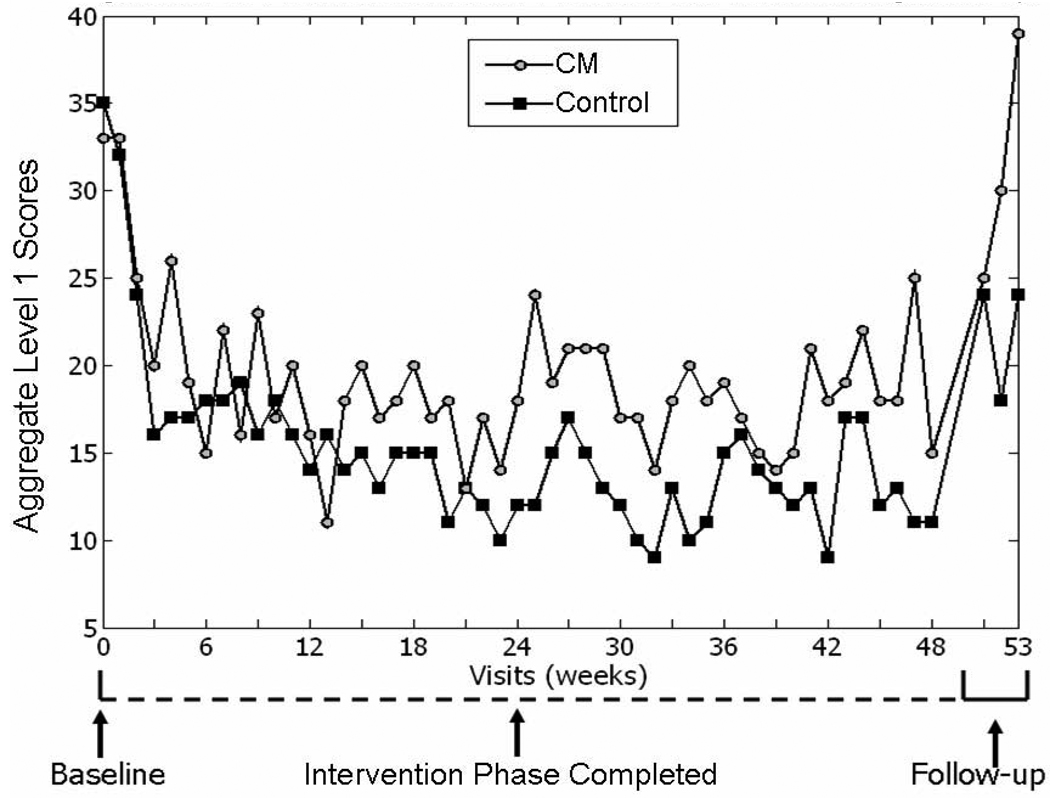
GEE modeling demonstrated that the CM condition produced significantly more drug metabolite-free urine samples for each individual substance, as well as for the Level 1 composite score (all stimulants and alcohol), than the control condition during the 24-week intervention period. The likelihood of providing a Level 1 metabolite-free urine sample was almost twice as high in the CM condition as in the control condition [β CM =0.35 (SD=0.17); Z=2.11, p<.05]. Figure 3 presents mean Level 1 scores for the entire study period. Analysis of the Level 2 composite scores (Level 1 substances plus opioids and marijuana) was not possible due to very low frequency of opioid use. While marijuana use was common, these results did not differ between the CM (43.4% of submitted urine samples) and control groups (45.0% of submitted urine samples).
Figure 3.
Mean Level 1 TES (Treatment Effectiveness Scores) by Study Visit
HIV-seronegative participants across both treatment conditions provided significantly more amphetamine [MCM = 25.2 (SD=6.2), MControl = 20.3 (SD=7.0); χ2 =24.7, p<.01], methamphetamine [MCM = 24.6 (SD=6.1), Mcontrol = 19.7 (SD=6.4); χ2 =24.8, p<.01] and Level 1 composite [MCM = 19.7 (SD=5.3), Mcontrol = 15.3 (SD=5.0); χ2 =27.36, p<.01] metabolite-free urine samples than HIV-seropositive participants. Caucasian participants achieved more Level 1 metabolite-free results than all other ethnic groups [MCaucasian = 10.2 (SD=3.1), MAfrican American = 3.3 (SD=1.7); MLatino = 2.5 (SD=1.5); MOther = 1.0 (SD=0.9); F=408.8, p<.01]. In both conditions, the probability of providing a Level 1 metabolite-free urine sample increased as participants progressed through the 24-week intervention period [β Time =0.01 (SD=0.003); Z=3.70, p<.01].
3.5.3. Follow-up evaluations
Table 3 presents the Joint Probability Index analysis of urine drug screen results indicating abstinence for individual stimulants, alcohol, and the aggregate of all stimulants plus alcohol, i.e. Level 1 TES scores, at baseline and 7-, 9-, and 12-month follow-up evaluations. CM participants were nearly twice as likely as control participants to be abstinent from alcohol and stimulants at the 9- and 12-month evaluations.
Table 3.
Stimulant and Alcohol Use at Baseline and Follow-Up Evaluations
| Variable | Cocaine | Amphetamine | Methamphetamine | Alcohol | Level 1 TESa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (N=67) |
CM (N=64) |
Control (N=67) |
CM (N=64) |
Control (N=67) |
CM (N=64) |
Control (N=67) |
CM (N=64) |
Control (N=67) |
CM (N=64) |
|
| Joint Probability Indexb | ||||||||||
| Baseline | 0.81 | 0.78 | 0.73 | 0.75 | 0.67 | 0.73 | 0.94 | 0.94 | 0.52 | 0.52 |
| 7-Month Follow-up |
0.51 | 0.55 | 0.46 | 0.52 | 0.49 | 0.52 | 0.96 | 0.98 | 0.36 | 0.39 |
| 9-Month Follow-up |
0.48 | 0.70* | 0.37 | 0.55 | 0.39 | 0.56 | 0.97 | 0.95 | 0.27 | 0.47* |
| 12-Month Follow-up |
0.58 | 0.75 | 0.46 | 0.70* | 0.48 | 0.69* | 0.96 | 0.98 | 0.36 | 0.61* |
Level 1 TES Score: Cocaine, Amphetamines, Methamphetamines, PCP & Alcohol Metabolite-Free
Joint probability index: proportion of participants that provided drug-free urine samples at a specified time point divided by number of participants randomized to that condition.
p<0.05 between CM and control groups
4. Discussion
This randomized, controlled trial of a CM study layered onto a community-based HIV prevention service program demonstrated efficacy for increasing health-promoting behaviors and reducing substance use among homeless, out-of-treatment, substance-dependent MSM. As expected, there were no differences between the CM and control condition participants on attendance at study visits and at the service program activities, for which all participants earned points. On the targeted behaviors, however, the CM condition participants accomplished significantly more health-promoting behaviors and achieved significant reductions in substance use over time compared to control condition participants. These reductions in substance use were maintained to 9- and 12-month post-randomization follow-up evaluations. CM also increased length of retention in the study, itself a key outcome.
Among participants receiving the CM intervention, Caucasian men accomplished more of the targeted behaviors and achieved more stimulant abstinence than African-American or Latino men. As well, among Latino participants, unlike all other ethnicities, there were no differences in outcomes between the CM and control conditions. It appears that this CM intervention was more effective for Caucasian men than for Latino men in particular; possible explanations include language difficulties, although at all times at least one study staff member was Spanish-speaking, or fear/distrust related to immigration status.
Regardless of treatment condition, HIV-seropositive men completed more of the targeted health-promoting behaviors than HIV-seronegative men. It is possible that the HIV-positive men were more likely to connect with health and social service agencies due to unmet health-related needs, concerns that are not shared by HIV-negative men. However, the HIV-seronegative men achieved higher rates of abstinence from methamphetamine during the study than the HIV-seropositive men. This is perhaps not surprising given that the HIV seropositive men reported nearly twice as much methamphetamine use in the 30 days prior to study intake as the HIV seronegative men. As well, previous work has demonstrated that HIV seroprevalence increases as methamphetamine use becomes more severe (Shoptaw & Reback, 2006). It is possible that the CM schedule evaluated in this work provided enough positive reinforcement to initiate new health-related behaviors but was not powerful enough to override the established drug reinforcement paradigm. Future work might evaluate CM schedules of differing magnitudes targeting methamphetamine use in HIV-seropositive MSM; a schedule providing a greater magnitude of reinforcement, particularly early in the intervention period, might demonstrate more efficacy for reducing methamphetamine use.
This is preliminary work establishing that CM can increase health-promoting behaviors and reduce substance use even in a severely impaired population. For MSM who are homeless, unemployed, and substance-dependent, the opportunity to earn points redeemable toward basic needs such as food and shelter proved to be a potent intervention. Previous work by members of this research group established the efficacy of CM for reducing methamphetamine use and sexual risk behaviors (Shoptaw et al., 2005) in a sample of higher functioning, treatment-seeking MSM, most of whom had stable housing and jobs. This study extends these findings to a much lower functioning population. The importance of increasing health-promoting behaviors and reducing substance use, particularly stimulant use, given the association between such use and HIV high-risk sexual behaviors (Shoptaw & Reback, 2006) in this population was underscored by the high HIV seroprevalence rate (28%) in this sample. These promising findings have implications for community-based substance abuse and HIV prevention programs – just as with the higher-functioning population – CM can be utilized to move participants who display disorganized lives toward higher levels of functioning. Public policy officials should consider integrating CM interventions into community substance abuse and HIV prevention programs as an evidence-based strategy for motivating out-of-treatment substance users to increase health-promoting behaviors.
These outcomes are encouraging and warrant replication efforts among other out-of-treatment populations. Participants in this study tended to be in their mid-30s and Caucasian; therefore, it is unknown whether the intervention would yield similar results among younger or older men, or among women and adolescents, or among those of varying ethnicities, although these findings indicate that CM may not be as efficacious with Latino participants as it is for Caucasian men. It remains, however, that these findings indicate the utility of CM for this high-risk population and the feasibility of implementing the intervention in a community-based HIV prevention program. The finding that substance use reductions, in the CM condition, were sustained to 9- and 12-month follow-up evaluations is very encouraging and merits further developmental work in this area.
Acknowledgements
Funding for this study was provided by NIDA Grant RO1 DA015990. Funding for the HIV prevention program was provided by Los Angeles County Department of Public Health, Office of AIDS Programs and Policy Contract H-700861.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y. A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry. 2006;63:426–432. doi: 10.1001/archpsyc.63.4.426. [DOI] [PubMed] [Google Scholar]
- Bennett ME, Bellack AS, Gearon JS. Treating substance abuse in schizophrenia. An initial report. J Subst Abuse Treat. 2001;20:163–175. doi: 10.1016/s0740-5472(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Bigelow GE, Stitzer ML, Griffiths RR, Liebson IA. Contingency management approaches to drug self-administration and drug abuse: efficacy and limitations. Addict Behav. 1981;6:241–252. doi: 10.1016/0306-4603(81)90022-8. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Radonovich KJ, Novy PL. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J Consult Clin Psychol. 2000;68:1051–1061. doi: 10.1037//0022-006x.68.6.1051. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8:176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0, 4/97 revision) Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- Ghitza UE, Epstein DH, Preston KL. Contingency management reduces injection-related HIV risk behaviors in heroin and cocaine using outpatients. Addict Behav. 2008;33:593–604. doi: 10.1016/j.addbeh.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, Budney AJ. Initial abstinence and success in achieving longer term cocaine abstinence. Exp Clin Psychopharmacol. 2000;8:377–386. doi: 10.1037//1064-1297.8.3.377. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Petry NM. Contingency management. Incentives for sobriety. Alcohol Res Health. 1999;23:122–127. [PMC free article] [PubMed] [Google Scholar]
- Homeless Research Institute at the National Alliance to End Homelessness. Washington, DC: Homelessness Counts: Changes in Homelessness from 2005–2007. 2009 Retrieved from: http://www.endhomelessness.org/files/2158_file_counts_2_final.pdf.
- Institute for the Study of Homelessness and Poverty at the Weingart Center. Los Angeles, CA: Just the Facts: Homelessness in Los Angeles. 2004 Retrieved from: http://www.thegivingspirit.org/data/resources/File/news/JusttheFactsHomelessnessLA.pdf.
- Ledgerwood DM. Contingency management for smoking cessation: where do we go from here? Curr Drug Abuse Rev. 2008;1:340–349. doi: 10.2174/1874473710801030340. [DOI] [PubMed] [Google Scholar]
- Lee NK, Rawson RA. A systematic review of cognitive and behavioural therapies for methamphetamine dependence. Drug Alcohol Rev. 2008;27:309–317. doi: 10.1080/09595230801919494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling W, Shoptaw S, Wesson D, Rawson RA, Compton M, Klett CJ. Treatment effectiveness score as an outcome measure in clinical trials. NIDA Res Monogr. 1997;175:208–220. [PubMed] [Google Scholar]
- Lowe ET. A Status Report on Hunger and Homelessness in America's Cities. The United States Conference of Mayors; 2001. 2001. [Google Scholar]
- Jones HE, Haug N, Silverman K, Stitzer M, Svikis D. The effectiveness of incentives in enhancing treatment attendance and drug abstinence in methadone-maintained pregnant women. Drug Alcohol Depend. 2001;61:297–306. doi: 10.1016/s0376-8716(00)00152-6. [DOI] [PubMed] [Google Scholar]
- Jones HE, Haug NA, Stitzer ML, Svikis DS. Improving treatment outcomes for pregnant drug-dependent women using low-magnitude voucher incentives. Addictive Behaviors. 2000;25:263–267. doi: 10.1016/s0306-4603(98)00119-1. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O’Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Milby JB, Schumacher JE, Raczynski JM, Caswell E, Engle M, Michael M, Carr J. Sufficient conditions for effective treatment of substance abusing homeless persons. Drug and Alcohol Dependence. 1996;43:39–47. doi: 10.1016/s0376-8716(96)01286-0. [DOI] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino ML, Kolodner K, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a National Drug Abuse Treatment Clinical Trials Network study. Arch Gen Psychiatry. 2006;63:201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Reback CJ, Shoptaw S, Grella CE. Methamphetamine use trends among street-recruited gay and bisexual males, from 1999 to 2007. J Urban Health. 2008;85:874–879. doi: 10.1007/s11524-008-9326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczynski JM, Schumacher J, Milby JB, et al. Comparative substance abuse treatments for the homeless: The Birmingham project. Alcohol Treatment Q. 1993;10:217–233. [Google Scholar]
- Shoptaw S, Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J Urban Health. 2006;83:1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, Veniegas RC, Freese TE, Hucks-Ortiz C. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78:125–134. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Higgins ST, Brooner RK, Montoya ID, Contoreggi C, Umbricht-Schneiter A, Schuster CR, Preston KL. Increasing opiate abstinence through voucher-based reinforcement therapy. Drug Alcohol Depend. 1996;41:157–165. doi: 10.1016/0376-8716(96)01246-x. [DOI] [PubMed] [Google Scholar]
- Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, Fingerhood M, Nuzzo P, Kolodner K. A randomizes trial of employment-based reinforcement of cocaine abstinence in injection drug users. Journal of Applied Behavioral Analysis. 2007;40:387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clincal Interview for DSM-IV. Washington D.C: American Psychiatric Press; 1995. [Google Scholar]
- Stall R, Purcell DW. Intertwining epidemics: A review of research on substance use among men who have sex with men and its connection to the AIDS epidemic. AIDS and Behavior. 2000;4:181–192. [Google Scholar]
- Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- Svikis DS, Lee J, Haug N, Stitzer M. Attendance incentives for outpatient treatment: Effects in methadone- and non-methadone-maintained pregnant drug dependent women. Drug Alcohol Depend. 1997;48:33–41. doi: 10.1016/s0376-8716(97)00101-4. [DOI] [PubMed] [Google Scholar]
- Tracy K, Babuscio T, Nich C, Kiluk B, Carroll KM, Petry NM, Rounsaville BJ. Contingency management to reduce substance use in individuals who are homeless with co-occurring psychiatric disorders. The American Journal of Drug and Alcohol Abuse. 2007;33:253–258. doi: 10.1080/00952990601174931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]