Abstract
Mechanisms of articular cartilage growth and maturation have been elucidated by studying composition-function dynamics during in vivo development and in vitro culture with stimuli such as insulin-like growth factor-1 (IGF-1) and transforming growth factor-beta 1 (TGF-β1). This study tested the hypothesis that IGF-1 and TGF-β1 regulate immature cartilage compressive moduli and Poisson’s ratios in a manner consistent with known effects on tensile properties. Bovine calf articular cartilage from superficial-articular (S) and middle-growth (M) regions were analyzed fresh or following culture in medium with IGF-1 or TGF-β1. Mechanical properties in confined (CC) and unconfined (UCC) compression, cartilage matrix composition, and explant size were assessed. Culture with IGF-1 resulted in softening in CC and UCC, increased Poisson’s ratios, substantially increased tissue volume, and accumulation of glycosaminoglycan (GAG) and collagen (COL). Culture with TGF-β1 promoted maturational changes in the S layer, including stiffening in CC and UCC and increased concentrations of GAG, COL, and pyridinoline crosslinks (PYR), but little growth. Culture of M layer explants with TGF-β1 was nearly homeostatic. Across treatment groups, compressive moduli in CC and UCC were positively related to GAG, COL, and PYR concentrations, while Poisson’s ratios were negatively related to concentrations of these matrix components. Thus, IGF-1 and TGF-β1 differentially regulate the compressive mechanical properties and size of immature articular cartilage in vitro. Prescribing tissue growth, maturation, or homeostasis by controlling the in vitro biochemical environment with such growth factors may have applications in cartilage repair and tissue engineering.
Keywords: articular cartilage, IGF-1, TGF-beta1, confined compression, unconfined compression, Poisson’s ratio, growth, remodeling
Introduction
As articular cartilage grows and matures, the developing geometry (e.g. thickness and surface area) and properties of the tissue contribute to its function as a low-friction, wear-resistant bearing material within a joint. Articular cartilage material properties measured at the tissue-scale, such as equilibrium tensile and compressive moduli, increase from fetus to adult (Charlebois, et al., 2004, Williamson, et al., 2003, Williamson, et al., 2001). Poisson’s ratios of cartilage may also be maturation-dependent though trends vary with animal model and testing methodology (Chahine, et al., 2004, Ficklin, et al., 2007, Jurvelin, et al., 1997, Roemhildt, et al., 2006, Wong, et al., 2000)
Changes in articular cartilage mechanical properties with growth and maturation are mirrored by and often correlate with altered matrix composition and structure. Increasing collagen (COL) and pyridinoline crosslink (PYR) concentrations stabilize the COL network during maturation (Eyre, et al., 1981, Williamson, et al., 2003). Concomitantly, sulfated glycosaminoglycans (GAG), the polyanionic polysaccharide component of the proteoglycan (PG) aggrecan providing resistance to compression, are generally maintained in concentration (Maroudas, et al., 1986, Mow, et al., 2005, Williamson, et al., 2001). The formation of distinct collagen network architecture and zonal tissue structure also contribute to the development of mechanical property anisotropy and inhomogeneity (Hunziker, et al., 2007, Mow, et al., 2005).
The dynamics of matrix composition, particularly GAG, COL, and PYR, contribute pivotally to the changing mechanical function of cartilage not only during in vivo development but also during in vitro culture with potent regulators of cartilage metabolism such as insulin-like growth factor–1 (IGF-1) and transforming growth factor–beta 1 (TGF-β1). IGF-1 stimulates PG and COL synthesis in a dose-dependent manner in calf and adult bovine cartilage explants, and it also inhibits the loss of PG from adult tissue (Sah, et al., 1996, Schalkwijk, et al., 1989). Likewise, TGF-β1 stimulates PG synthesis by calf explants and reduces the rate of PG loss (Morales and Hascall, 1991, Morales and Roberts, 1988).
Culture of calf cartilage explants in medium with IGF-1 and TGF-β1 lead to distinct in vitro tissue fates. Culture with IGF-1 is distinguished by significant tissue expansion at the expense of reduced tensile stiffness and strength (Asanbaeva, et al., 2008, Sah, et al., 1994). In contrast, culture with TGF-β1 maintains size and tensile properties (Asanbaeva, et al., 2008, Morales and Roberts, 1988). Prior experiments and theoretical modeling have suggested that collagen network properties, such as content and tensile modulus, are strong determinants of compressive Poisson’s ratios of cartilage through an inverse relationship (Ficklin, et al., 2007, Jurvelin, et al., 1997, Kiviranta, et al., 2006). However, the effects of IGF-1 and TGF-β1 on the compressive mechanical properties of immature articular cartilage have not been studied previously.
Thus, this study examined the hypothesis that culture with IGF-1 and TGF-β1 differentially affect cartilage compressive moduli and Poisson’s ratios in a manner consistent with their effects on tensile integrity. The objectives were to 1) assess changes in compressive mechanical properties, including equilibrium confined and unconfined moduli and Poisson’s ratios, biochemical composition, and size of bovine calf articular cartilage cultured with exogenous IGF-1 and TGF-β1 and 2) correlate biochemical and mechanical properties to help elucidate mechanisms by which IGF-1 and TGF-β1 alter cartilage function. The results of this study have implications for guiding tissue formation to achieve desired biomechanical maturity and size for cartilage repair and replacement (Williams, et al., 2010).
Materials and Methods
Sample Preparation and Culture
Articular cartilage blocks were harvested from the patellofemoral grooves of ten newborn (1–3 weeks) bovine calves. Day 0 (d0) control blocks were soaked for ~1 hour at 4°C in phosphate buffered saline (PBS) with protease inhibitors (+PIs) and stored at −70°C, while others were immediately prepared for culture. A superficial-articular (S) slice and adjacent middle-growth (M) slice were prepared using a vibrating microtome, targeting a thickness of 0.6mm. Actual thicknesses differed slightly (~0.8mm for S and ~0.6mm for M) according to the cutting technique to ensure sufficient material was obtained for testing. An orthogonal coordinate system was established where 1-, 2-, and 3-directions corresponded to medial-lateral, proximal-distal, and articular surface normal directions, respectively. Samples were trimmed to 6 × 6 mm2 and notched to track orientation through culture. Initial thicknesses were measured with a non-contacting laser micrometer (average of 3 points), and initial wet weights (WWi) were obtained.
The explants were cultured according to the methods of Asanbaeva et al. (2008) for 12 days (d12) in non-tissue culture treated plates with medium (DMEM with additives) and either 50 ng/ml rhIGF-1 or 10 ng/ml rhTGF-β1 (PeproTech, Rocky Hills, NJ). Cultures were conducted at 37°C in humidified 5% CO2 incubators. Medium (1.4 ml/explant) was changed every other day, and plates were changed weekly to limit cell outgrowth. Final thicknesses and wet weights (WWf) were measured upon termination. Samples were then soaked in PBS+PIs at 4°C for 1 hr and stored at −70°C.
Compression Testing
Samples were sequentially tested in confined compression (CC) followed by unconfined compression (UCC) according to established protocols (Chen, et al., 2001, Ficklin, et al., 2007, Williamson, et al., 2001). For CC testing, Ø4.8 mm disks were punched from explants and placed in a confining ring between permeable platens with PBS+PIs at room temperature in a materials testing machine (Dynastat, Northern Industrial, Albany, NY). The test sequence consisted of consecutive 400 s ramps to 15%, 30%, and 45% compression (3-direction), with stress relaxation to equilibrium determined as a change in stress of <0.003 MPa over 180 s. At each static offset, oscillatory displacements with amplitudes decreasing from 1% to 0.3% and frequencies of 0.01 to 0.5 Hz were applied. An equilibrium CC modulus (HA) was determined at each strain offset as equilibrium stress divided by strain. In addition, an equilibrium CC modulus and permeability at free-swelling thickness (HA0 and kp0) and a permeability strain-dependence parameter (M) were also estimated, as done previously (Chen, et al., 2001, Williamson, et al., 2001).
Following CC testing, disks recovered in PBS+PIs at 4°C overnight, and thicknesses were then re-measured (ratio of pre-CC to pre-UCC thickness = 1.00±0.12). For UCC testing, Ø3.2 mm disks were punched from the larger disks, to reduce relaxation times and ensure well-defined edges for photography. Samples were re-mounted in the testing machine between smooth, impermeable platens in an unconfined manner. The test sequence consisted of consecutive 400 s ramps to 15%, 30%, and 45% compression, with stress relaxation to equilibrium defined by the same criterion as above. At equilibrium, lateral-view images in the 1- and 2-directions were acquired using an optical system and camera (Ficklin, et al., 2007). An equilibrium UCC modulus (E) was determined at each strain offset as equilibrium stress divided by strain. Images were processed in MATLAB (Mathworks, Natick, MA) using a custom-written algorithm to determine lateral expansion from which Poisson’s ratios (ν31 and ν32) were calculated at each offset relative to zero strain state (resolution of ν<0.01). The observation of slip at the cartilage-platen interface during UCC testing was consistent with the assumption of a low friction condition used for analysis of material properties. Prior CC testing did not appear to affect subsequently measured UCC properties as determined by comparing UCC properties from this study to those of samples only tested in UCC in a pilot study (ratio of mean E=0.94±0.14; ratio of mean ν=1.30±0.15).
Biochemical Analyses
Tested and residual tissue portions were lyophilized and weighed to obtain dry weight. Water content was calculated as the difference between final wet and dry weights as a percentage of final wet weight. Tissue was solubilized with a solution of 0.5 mg/ml proteinase K (Roche Diagnostics, Indianapolis, IN) and assessed for DNA (Mcgowan, et al., 2002), GAG (Farndale, et al., 1986), hydroxyproline (Woessner, 1961), and PYR (Uebelhart, et al., 1993). Cell and COL contents were calculated using ratios of 7.7 pg DNA/cell (Kim, et al., 1988) and 7.25 g COL/g hydroxyproline (Herbage, et al., 1977, Pal, et al., 1981). Data were normalized to WWi and to WWf to indicate constituent content and concentration, respectively. For d0 samples, WWi = WWf. Biochemical data represented what remained in the tissue following mechanical testing, and pilot studies indicated that only a small portion of GAG (<5% of total) was lost to solutions during mechanical testing.
Statistical Analyses
Data are presented as mean ± SE. For each layer (S and M), analysis of variance (ANOVA) was used to determine the effect of culture (IGF-1 vs. TGF-β1) on the change in thickness and WW; in addition, planned comparisons were made to zero (i.e. no change) to determine if absolute changes were significant. For each layer and strain level (where applicable), the effects of culture condition on biomechanical and biochemical properties were determined by ANOVA, with Tukey post hoc testing. Hydraulic permeability data were log transformed as done previously to assure homoscedasticity (Chen et al., 2001). The significance level, α, was 0.05 for all tests.
As a first approximation of cartilage composition-function relationships, univariate and multivariate linear regression were used to analyze HA, E, ν31, and ν32 at 30% compression versus water, GAG, COL, PYR, and cells (%WWf) across treatment groups for each layer. Multivariate regression was performed via the forward selection procedure (Sokal and Rohlf, 1995). Coefficients of determination (r2) and multiple determination (R2) are reported for significant relationships (p<0.05).
Results
Volumetric Changes
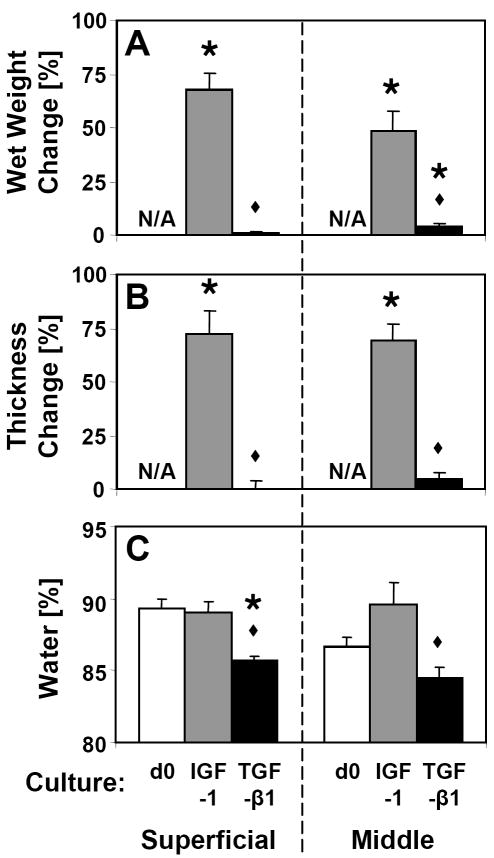
Calf articular cartilage incubated for 12 days with IGF-1 or TGF-β1 exhibited stark differences in volumetric expansion. Culture with IGF-1 resulted in large increases (~50% to 70%) in wet weight of both S and M slices, which were significantly greater than those observed in the TGF-β1 groups (p<0.001, Fig. 1A). Magnitudes of change in thickness were similar to those of wet weight (Fig. 1B). However, dimensional changes in the 1- and 2-directions were small, averaging <5% for IGF-1 samples and <2% for TGF-β1 samples (data not shown), indicating that IGF-1 induced expansion primarily in the 3-direction. Tissue water content was higher in IGF-1 samples than TGF-β1 samples for both S and M layers (p<0.01, Fig. 1C), but was similar to d0 for all treatment groups with the exception of S layer samples cultured with TGF-β1 (−3.7%, p<0.001).
Figure 1.
Change in (A) wet weight and (B) thickness for superficial and middle cartilage explants cultured for 12 days with IGF-1 or TGF-β1. (C) Water content of day 0 (d0) and cultured explants. For each tissue layer, * indicates a culture group is different from zero (i.e. no change) for wet weight and thickness changes and from d0 for water content (p<0.05), and ◆ indicates d12 IGF-1 is different from d12 TGF-β1 (p<0.05). Mean ± SE; n=12–18.
Compressive Biomechanical Properties
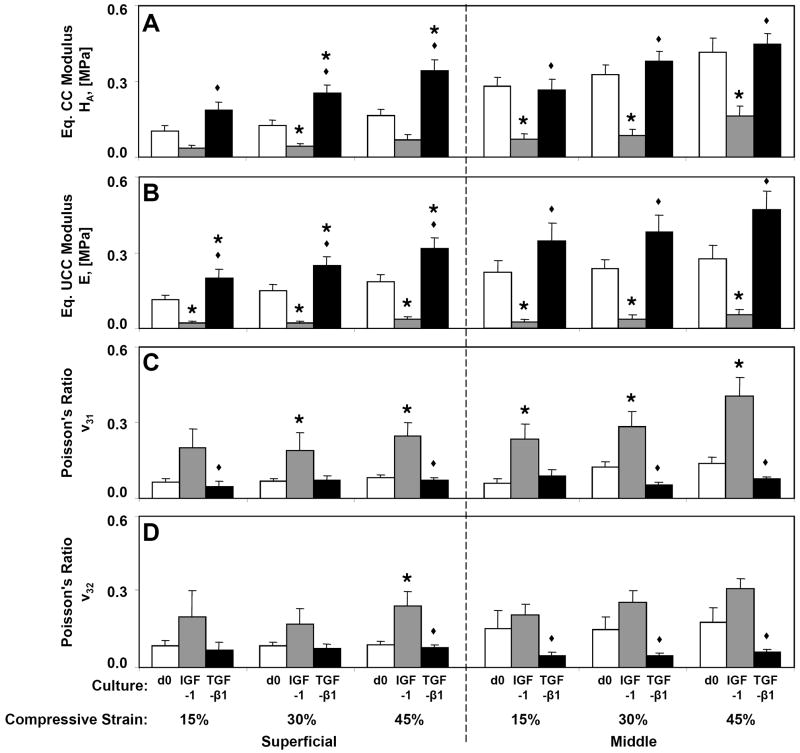
Biomechanical properties of cartilage measured in CC were differentially affected by culture with IGF-1 and TGF-β1. HA (Fig 2A) was significantly reduced by culture with IGF-1 compared to d0 at 30% compressive strain in the S layer (−65%, p<0.05) and at all strains in the M layer (−61% to −74%, p<0.05), but was increased by culture with TGF-β1 at 30% and 45% strain in the S layer (103% and 108%, p<0.01). For all strain levels and layers, treatment with TGF-β1 resulted in greater HA than IGF-1 treated samples (p<0.05). Similar differences were observed in the estimated HA0 (Table 1) with IGF-1 treated samples being softer in both layers (p<0.05) and TGF-β1 treated samples being stiffer in the S layer than d0 controls (p<0.01). The estimated permeability, kp0, and permeability strain-dependence parameter, M, did not vary with culture condition for either layer (p=0.67 and 0.42, respectively, for S and p=0.15 and 0.57 for M).
Figure 2.
Compressive properties of superficial and middle cartilage explants at day 0 (d0) or following 12 days culture with IGF-1 or TGF-β1. (A) Equilibrium confined compression (CC) modulus, HA, (B) equilibrium unconfined compression (UCC) modulus, E, and Poisson’s ratios, (C) ν31, and (D) ν32 were measured at three compressive offset strains of 15, 30, and 45%. For each strain level and tissue layer, * indicates a culture group is different from d0 (p<0.05), and ◆ indicates d12 IGF-1 is different (p<0.05) from d12 TGF-β1. Mean ± SE; n=6–15.
Table 1.
Equilibrium confined compression modulus, HA [MPa], and hydraulic permeability, kp [m2/(Pa·s)], were estimated at the free-swelling thickness. The permeability strain-dependence parameter, M, was also determined.
| d0 |
d12 IGF-1 |
d12 TGF-β1 |
||||
|---|---|---|---|---|---|---|
| Superficial | Middle | Superficial | Middle | Superficial | Middle | |
| HA0 | 0.096 ± 0.014 | 0.233 ± 0.031 | 0.033 ± 0.008* | 0.068 ± 0.017* | 0.188 ± 0.024*,◆ | 0.253 ± 0.024◆ |
| log10 kp0 | −14.49 ± 0.19 | −14.98 ± 0.13 | −14.57 ± 0.18 | −14.57 ± 0.28 | −14.34 ± 0.19 | −14.51 ± 0.16 |
| M | 8.6 ± 1.3 | 6.8 ± 0.6 | 7.4 ± 0.9 | 7.7 ± 1.4 | 9.1 ± 0.8 | 8.2 ± 0.7 |
For each tissue layer,
indicates a culture group is different from d0 (p<0.05), and
indicates d12 IGF-1 is different from d12 TGF-β1 (p<0.05). Mean ± SE; n=9–15.
Biomechanical properties measured in UCC also varied with culture condition (Fig. 2B–D). Compared to d0, E was reduced by culture with IGF-1 for all strain levels and layers (−80% to −89%, p<0.05), but was increased by culture with TGF-β1 in the S layer (65% to 77%, p<0.05). Culture with TGF-β1 resulted in greater E than IGF-1 treated samples for all strain levels and layers (p<0.05). Poisson’s ratios did not vary between directions for either the S layer (p>0.34) or M layer (p>0.13); however, culture condition was a significant factor (p<0.05) for most comparisons. In general, Poisson’s ratios increased from d0 by culture with IGF-1 (198% for S layer and 97% for M layer, averaged across strain levels and directions), but were maintained by culture with TGF-β1.
In a majority of samples (65%), HA>E at corresponding strains, as expected for materials with ν>0 (as measured in all samples) tested under ideal conditions. Deviations might have arisen from imperfect confinement, non-frictionless boundaries, tissue inhomogeneity/testing different portions of tissue in CC and UCC, and errors in determining the zero strain thickness due in part to a curvilinear articular surface. For samples with HA>E, estimates of ν were also calculated from the two moduli at each strain according to a linear isotropic model (Armstrong, et al., 1984). Estimates of ν (e.g. 0.02 ± 0.01 for d0 S-layer and 0.10 ± 0.04 for d0 M-layer at 15% compression) were generally similar in magnitude to the optically measured ν31 and ν32 (Fig. 2C, D).
Biochemical Composition
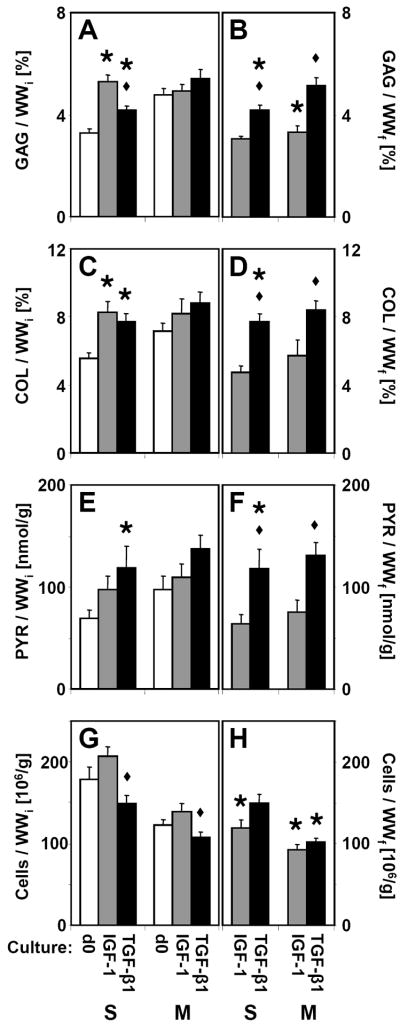
Total cartilage matrix and chondrocyte content varied with culture condition (Fig 3A, C, E, G). In the S layer, GAG and COL contents were increased following treatment with IGF-1 (62%, p<0.05 and 50%, p<0.01, respectively) and with TGF-β1 (27%, p<0.05 and 40%, p<0.01). PYR crosslink content was increased by culture with TGF-β1 (72%, p<0.05), but not with IGF-1 (p=0.31). However, when PYR was normalized to COL (Fig. 4), there were no differences in the extent of COL crosslinking for either layer (p=0.60 for S and p=0.19 for M). Changes in cartilage matrix content were less pronounced in M layer explants. Cell contents did not differ from d0 in either culture condition (p>0.25), but TGF-β1 treated samples had slightly fewer cells than IGF-1 treated samples for both layers (p<0.05).
Figure 3.
(A, B) Glycosaminoglycan, (C, D) collagen, (E, F) pyridinoline crosslinks, and (G, H) cellularity of superficial and middle cartilage explants analyzed on day 0 (d0) or following 12 days culture with IGF-1 or TGF-β1. Data are normalized to (A, C, E, G) initial wet weight (WWi) to indicate constituent content or (B, D, F, H) final wet weight (WWf) to indicate constituent concentration. For d0 samples, WWi = WWf, and the data is presented once. For each tissue layer, * indicates a culture group is different from d0 (p<0.05), and ◆ indicates d12 IGF-1 is different from d12 TGF-β1 (p<0.05). Mean ± SE; n=11–18.
Figure 4.
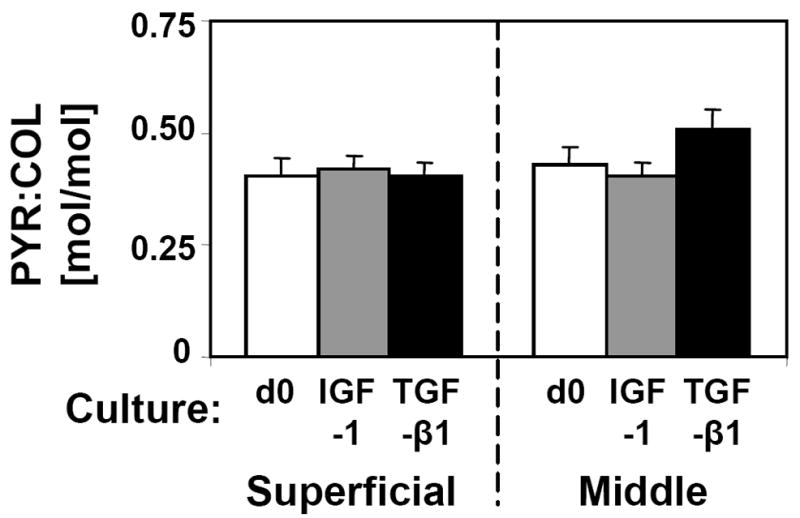
The ratio of pyridinoline crosslinks to collagen molecules for superficial and middle cartilage explants analyzed on day 0 (d0) or following 12 days culture with IGF-1 or TGF-β1. Mean ± SE; n=7–15.
After accounting for changes in wet weight during culture, the final concentrations of matrix components strongly varied with culture condition (Fig 3B, D, F, H). GAG, COL, and PYR concentrations in samples cultured with IGF-1 were similar to those of d0 samples, except for a reduction of GAG concentration in M layer samples (−31%, p<0.01). Increases in GAG (27%, p<0.01), COL (40%, p<0.001), and PYR (72%, p<0.05) concentrations were observed in S layer samples cultured with TGF-β1 compared to d0. Final concentrations of GAG, COL, and PYR were higher in TGF-β1 treated samples than IGF-1 treated samples for both layers (p<0.05).
Composition-Function Analyses
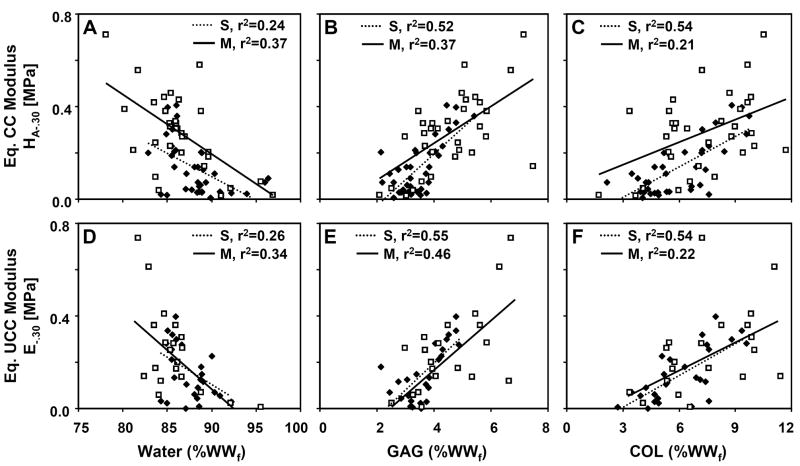
Univariate regression analysis indicated significant relationships between certain biomechanical properties and matrix constituents (Fig. 5). HA-.30 was negatively related to water (r2=0.24 for S, r2=0.37 for M), and positively related to GAG (r2=0.52 for S, r2=0.37 for M) and COL concentrations (r2=0.54 for S, r2=0.21 for M). Similarly, E-.30 was negatively related to water (r2=0.26 for S, r2=0.34 for M), and positively related to GAG (r2=0.55 for S, r2=0.46 for M), COL (r2=0.54 for S, r2=0.22 for M), and PYR concentrations (r2=0.23 for M). Poisson’s ratios at 30% strain were weakly related to matrix constituents, with ν31 negatively related to GAG (r2=0.37 for M) and ν32 negatively related to GAG (r2=0.43 for M) and COL (r2=0.22 for S, r2=0.20 for M).
Figure 5.
Regression of (A–C) equilibrium CC modulus and (D–F) equilibrium UCC modulus at 30% offset strain on matrix constituent concentrations (% final WW), including (A, D) water, (B, E) GAG, and (C, F) COL. Data points correspond to individual d0, d12 IGF-1, and d12 TGF-β1 cartilage explants from the S layer (◆, dotted line) and the M layer (□, solid line). Coefficients of determination (r2) are indicated for significant relationships (p<0.05).
For some cases, multiple regression analysis significantly improved the strength of association between composition and function. HA-.30 was related to GAG and COL in the S layer with regression coefficients of 0.0618 and 0.0283, respectively, and a constant of −0.248 (R2=0.63), and to GAG and water in the M layer with regression coefficients of 0.0518 and −0.0164, respectively, and a constant of 1.47 (R2=0.48). Similarly, E-.30 was related to GAG and COL in the S layer by coefficients of 0.0744 and 0.0289, respectively, and a constant of −0.299 (R2=0.70); however, the univariate regression of E-.30 on GAG in the M layer with a coefficient of 0.106 and constant of −0.246 (r2=0.46) was not improved by addition of other variables.
Discussion
In vitro stimulation of immature cartilage with IGF-1 and TGF-β1 differentially regulated the compressive moduli, Poisson’s ratios, size, and matrix composition of the tissue. Culture with TGF-β1 produced a functional improvement in the S layer, measured as increased compressive moduli (Fig. 2A, B) and GAG, COL, and PYR concentrations (Fig. 3), but was nearly homeostatic with respect to Poisson’s ratios (Fig. 2C, D) and tissue size (Fig. 1). In contrast, culture with IGF-1 was characterized by a functional impairment of mechanical properties, indicated by softening (Fig. 2A, B) and increased Poisson’s ratios (Fig. 2C, D), and a prominent volumetric expansion (Fig. 1). Thus the propensities for immature cartilage to resist axial load and maintain lateral dimensions during compressive loading were modulated differentially by IGF-1 and TGF-β1.
Consideration was given to a number of factors in designing the experiments reported here. Immature articular cartilage from bovine calf patellofemoral grooves was selected as the model system based on previous characterization studies of in vivo development and in vitro serum-supplemented growth (Ficklin, et al., 2007, Williamson, et al., 2001). Explants from two depths (S and M layers) were also used similar to previous work (Asanbaeva, et al., 2008), which helps validate the control data with previously determined depth-dependent compressive properties in CC and UCC for fresh calf articular cartilage (Chahine, et al., 2004, Klein, et al., 2007). Accordingly, the biochemical and mechanical properties of d0 control cartilage generally agree with those of preceding studies. In addition, the growth factor concentrations chosen for this study (50 ng/ml IGF-1 and 10 ng/ml TGF-β1) represent near maximal stimulation of calf cartilage metabolism. For calf articular cartilage, stimulation of DNA, GAG, and hydroxyproline synthesis and deposition by IGF-1 saturate between 30–300 ng/ml (Sah, et al., 1994), while stimulation of GAG synthesis by TGF-β1 saturates between 5–20 ng/ml (Morales and Roberts, 1988). Moreover, the selected growth factor concentrations have been shown to produce distinct effects versus basal culture controls (i.e. no growth factor supplementation) with respect to explant metabolism, matrix composition, and mechanical function (Asanbaeva, et al., 2008).
Measurements of compressive properties extend previous findings that culture of calf cartilage with IGF-1 decreased tensile stiffness and strength, while culture with TGF-β1 maintained tensile properties (Asanbaeva, et al., 2008). The increased lateral expansion of cartilage cultured with IGF-1during UCC testing may be another indicator of a loss of tensile integrity and a reduced ability of the COL network to restrain PG swelling. This finding provides further support for an inverse relationship between cartilage tensile modulus and compressive Poisson’s ratios (Ficklin, et al., 2007, Jurvelin, et al., 1997, Kiviranta, et al., 2006). Additionally, the changes in HA, E, ν31, and ν32 and maintenance of kp0 and M during culture with IGF-1 resemble those that occur during the culture of calf cartilage with 20% fetal bovine serum (Ficklin, et al., 2007). This observation is consistent with IGF-1 being the primary component of serum responsible for stimulating cartilage PG synthesis (Mcquillan, et al., 1986) and the two treatments similarly altering matrix composition (Asanbaeva, et al., 2008).
Marked differences in volumetric expansion as well as altered matrix composition in cartilage stimulated with IGF-1 and TGF-β1 support the concept that in vitro cartilage growth can result from deposition of PG in excess of COL (Asanbaeva, et al., 2008). The resulting dynamic imbalance between the swelling pressure of PG and restraint provided by the COL network produces expansion of the tissue (Klisch, et al., 2003, Maroudas, 1976). In the S-layer cultures with IGF-1 where volumetric expansion tended to be largest, the increase in GAG content exceeded that of COL on a percentage basis. The opposite occurred in S-layer cultures with TGF-β1 where size was generally maintained. In general, changes in the size and biochemical composition of cartilage explants stimulated with IGF-1 and TGF-β1 agree with previous studies (Asanbaeva, et al., 2008, Morales and Roberts, 1988, Sah, et al., 1994).
The observed relationships between mechanical properties and matrix constituent concentrations of immature cartilage grown in vitro further implicate GAG, COL, and PYR as having pivotal roles in determining cartilage mechanical functions, specifically compressive Poisson’s ratios and moduli. These composition-function relationships are consistent with those of articular cartilage observed over a span of in vivo development from fetus to young adult (Williamson, et al., 2001) or following in vitro growth with FBS supplemented medium (Ficklin, et al., 2007). The relationships suggest that differences in compressive moduli between the softer IGF-1 cultured cartilage and stiffer TGF-β1 cultured samples can largely be explained by the lower concentrations of GAG, COL, and PYR in the IGF-1 group. However, potential effects of altered collagen network architecture or chondrocyte phenotype (e.g. relative expression of different collagen types or proteoglycans) on compressive properties cannot be excluded and remain to be examined in future studies.
The compressive properties measured in this study along with complementary tensile properties of immature cartilage cultured with IGF-1 or TGF-β1 (Asanbaeva, et al., 2008) could be used to develop more accurate constitutive relationships. These relationships and corresponding measures of cartilage composition can help refine and validate cartilage growth mixture models, which describe the evolving biochemical and mechanical properties of cartilage during growth and remodeling (Klisch, et al., 2008). A translational goal of this in vitro work and related growth modeling is to elucidate specific conditions and underlying mechanisms for manipulating the biomechanical maturity and size of cartilaginous tissues. This goal aligns with a broad interest in using anabolic factors, such as IGF-1 and TGF-β1, to promote cartilage repair or enhance the formation of engineered cartilage constructs (Elisseeff, et al., 2001, Fortier, et al., 1999, Madry, et al., 2001). The ability to specify cartilage growth, maturation, or homeostasis may ultimately aid the development of cartilage repair techniques or the fabrication and storage of transplantable grafts of desired sizes and properties for the treatment of synovial joint injury and disease.
Acknowledgments
This work was supported by grants from the National Institutes of Health (SJH, RLS, SMK), the National Science Foundation (RLS, SMK), the Howard Hughes Medical Institute through the HHMI Professors Program (to UCSD supporting RLS) and the Donald E. Bently Center for Engineering Innovation (SMK). Individual support (to GMW) was provided through an NSF Graduate Research Fellowship and an NIH Ruth L. Kirchstein Pre-Doctoral Fellowship.
Footnotes
Conflict of Interest Statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong CG, Lai WM, Mow VC. An analysis of the unconfined compression of articular cartilage. J Biomech Eng. 1984;106:165–73. doi: 10.1115/1.3138475. [DOI] [PubMed] [Google Scholar]
- Asanbaeva A, Masuda K, Thonar EJMA, Klisch SM, Sah RL. Regulation of immature cartilage growth by igf-i, tgf-beta1, bmp-7, and pdgf-ab: Role of metabolic balance between fixed charge and collagen network. Biomech Model Mechanobiol. 2008;7:263–76. doi: 10.1007/s10237-007-0096-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine NO, Wang CC, Hung CT, Ateshian GA. Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J Biomech. 2004;37:1251–1261. doi: 10.1016/j.jbiomech.2003.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlebois M, McKee MD, Buschmann MD. Nonlinear tensile properties of bovine articular cartilage and their variation with age and depth. J Biomech Eng. 2004;126:129–137. doi: 10.1115/1.1688771. [DOI] [PubMed] [Google Scholar]
- Chen AC, Bae WC, Schinagl RM, Sah RL. Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J Biomech. 2001;34:1–12. doi: 10.1016/s0021-9290(00)00170-6. [DOI] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of igf-i and tgf-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Grypnas MD, Shapiro FD, Creasman CM. Mature crosslink formation and molecular packing in articular cartilage collagen. Sem Arthritis Rheum. 1981;11:46–47. [Google Scholar]
- Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- Ficklin T, Thomas G, Barthel JC, Asanbaeva A, Thonar EJMA, Masuda K, Chen AC, Sah RL, Davol A, Klisch SM. Articular cartilage mechanical and biochemical property relations before and after in vivo growth. J Biomech. 2007;40:3607–14. doi: 10.1016/j.jbiomech.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier LA, Lust G, Mohammed HO, Nixon AJ. Coordinate upregulation of cartilage matrix synthesis in fibrin cultures supplemented with exogenous insulin-like growth factor-i. J Orthop Res. 1999;17:467–74. doi: 10.1002/jor.1100170403. [DOI] [PubMed] [Google Scholar]
- Herbage D, Bouillet J, Bernengo JC. Biochemical and physicochemical characterization of pepsin-solubilized type-ii collagen from bovine articular cartilage. Biochem J. 1977;161:303–312. doi: 10.1042/bj1610303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker EB, Kapfinger E, Geiss J. The structural architecture of adult mammalian articular cartilage evolves by a synchronized process of tissue resorption and neoformation during postnatal development. Osteoarthritis Cartilage. 2007;15:403–13. doi: 10.1016/j.joca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of poisson’s ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30:235–41. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Fluorometric assay of dna in cartilage explants using hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- Kiviranta P, Rieppo J, Korhonen RK, Julkunen P, Toyras J, Jurvelin JS. Collagen network primarily controls poisson’s ratio of bovine articular cartilage in compression. J Orthop Res. 2006;24:690–699. doi: 10.1002/jor.20107. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Chaudhry M, Bae WC, Sah RL. Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J Biomech. 2007;40:182–90. doi: 10.1016/j.jbiomech.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Klisch SM, Asanbaeva A, Oungoulian SR, Masuda K, Thonar EJ-MA, Davol A, Sah RL. A cartilage growth mixture model with collagen remodeling: Validation protocols. J Biomech Eng. 2008;130:031006-1–11. doi: 10.1115/1.2907754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch SM, Chen SS, Sah RL, Hoger A. A growth mixture theory for cartilage with applications to growth-related experiments on cartilage explants. J Biomech Eng. 2003;125:169–179. doi: 10.1115/1.1560144. [DOI] [PubMed] [Google Scholar]
- Madry H, Zurakowski D, Trippel SB. Overexpression of human insulin-like growth factor-i promotes new tissue formation in an ex vivo model of articular chondrocyte transplantation. Gene Ther. 2001;8:1443–9. doi: 10.1038/sj.gt.3301535. [DOI] [PubMed] [Google Scholar]
- Maroudas A. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature. 1976;260:808–9. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Katz EP, Wachtel EJ, Mizrahi J, Soudry M. Physico-chemical properties and functional behavior of normal and osteoarthritic human cartilage. In: Kuettner K, Schleyerbach R, Hascall VC, editors. Articular cartilage biochemistry. Raven Press; New York: 1986. [Google Scholar]
- McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of dna in human articular and septal cartilage using picogreen and hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–7. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-i in cultured bovine articular cartilage. Biochem J. 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales TI, Hascall VC. Transforming growth factor-β1 stimulates synthesis of proteoglycan aggregates in calf articular organ cultures. Arch Biochem Biophys. 1991;286:99–106. doi: 10.1016/0003-9861(91)90013-9. [DOI] [PubMed] [Google Scholar]
- Morales TI, Roberts AB. Transforming growth factor-β regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- Mow VC, Gu WY, Chen FH. Structure and function of articular cartilage and meniscus. In: Mow VC, Huiskes R, editors. Basic orthopaedic biomechanics and mechano-biology. Lippincott Williams & Wilkins; Philadelphia: 2005. p. 720. [Google Scholar]
- Pal S, Tang LH, Choi H, Habermann E, Rosenberg L, Roughley P, Poole AR. Structural changes during development in bovine fetal epiphyseal cartilage. Collagen Rel Res. 1981;1:151–76. doi: 10.1016/s0174-173x(81)80017-9. [DOI] [PubMed] [Google Scholar]
- Roemhildt ML, Coughlin KM, Peura GD, Fleming BC, Beynnon BD. Material properties of articular cartilage in the rabbit tibial plateau. J Biomech. 2006;39:2331–7. doi: 10.1016/j.jbiomech.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah RL, Chen AC, Grodzinsky AJ, Trippel SB. Differential effects of bfgf and igf-i on matrix metabolism in calf and adult bovine cartilage explants. Arch Biochem Biophys. 1994;308:137–147. doi: 10.1006/abbi.1994.1020. [DOI] [PubMed] [Google Scholar]
- Sah RL, Trippel SB, Grodzinsky AJ. Differential effects of serum, insulin-like growth factor-i, and fibroblast growth factor-2 on the maintenance of cartilage physical properties during long-term culture. J Orthop Res. 1996;14:44–52. doi: 10.1002/jor.1100140109. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J, Joosten LAB, van den Berg WB, van Wyk JJ, van de Putte LBA. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 1989;32:66–71. doi: 10.1002/anr.1780320111. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. WH Freeman and Co; New York: 1995. [Google Scholar]
- Uebelhart D, Thonar EJMA, Pietryla DW, Williams JW. Elevation in urinary levels of pyridinium cross-links of collagen following chymopapin-induced degradation of articular cartilage in the rabbit knee provides evidence of metabolic changes in bone. Osteoarthritis Cartilage. 1993;1:185–92. doi: 10.1016/s1063-4584(05)80090-1. [DOI] [PubMed] [Google Scholar]
- Williams GM, Chan EF, Temple-Wong MM, Bae WC, Masuda K, Bugbee WD, Sah RL. Shape, loading, and motion in the bioengineering design, fabrication, and testing of personalized synovial joints. J Biomech. 2010;43:156–165. doi: 10.1016/j.jbiomech.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Masuda K, Thonar EJMA, Sah RL. Tensile mechanical properties of bovine articular cartilage: Variations with growth and relationships to collagen network components. J Orthop Res. 2003;21:872–880. doi: 10.1016/S0736-0266(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Williamson AK, Chen AC, Sah RL. Compressive properties and function-composition relationships of developing bovine articular cartilage. J Orthop Res. 2001;19:1113–21. doi: 10.1016/S0736-0266(01)00052-3. [DOI] [PubMed] [Google Scholar]
- Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wong M, Ponticiello M, Kovanen V, Jurvelin JS. Volumetric changes of articular cartilage during stress relaxation in unconfined compression. J Biomech. 2000;33:1049–54. doi: 10.1016/s0021-9290(00)00084-1. [DOI] [PubMed] [Google Scholar]