Abstract
The present study tested whether aging sensitizes hippocampal microglia to a pro-inflammatory challenge ex vivo. Hippocampal microglia from 3 and 24 mo old male F344 × BN F1 rats were exposed to LPS (0, 0.1, 1, 10 and 100 ng/ml) ex vivo. 2 h post-LPS challenge, gene expression of microglial activation markers and cytokines were assessed. 24 mo old animals exhibited a potentiated pro-inflammatory cytokine (IL-1β and IL-6) response to LPS and increased levels of CD11b, Iba-1 and MHCII irrespective of LPS treatment. The present results demonstrate that aging sensitizes hippocampal microglia to pro-inflammatory challenges.
Keywords: aging, neuroinflammation, sensitization, priming, o-inflammatory cytokines, microglia
1. Introduction
In normal aging, the CNS exhibits a sensitized neuroinflammatory response to peripheral as well as central administration of proinflammatory agents (Abraham et al., 2008; Abraham and Johnson, 2009; Barrientos et al., 2009; Barrientos et al., 2006; Chen et al., 2008; Godbout et al., 2005; Henry et al., 2008; Henry et al., 2009; Huang et al., 2007). Microglia are critical to the mediation of neuroinflammation (Kreutzberg, 1996) and it has been suggested that aging “primes” microglia so that they produce exaggerated levels of inflammatory mediators, such as IL-1β, when stimulated (Dilger and Johnson, 2008), although direct evidence is lacking. We (Barrientos et al., 2009) and others (Abraham and Johnson, 2009) have focused on the age-related vulnerability of the hippocampus as this CNS structure appears particularly susceptible to exaggerated pro-inflammatory responses in the face of a peripheral immune challenge. Thus, for example, hippocampal IL-1β increases following peripheral infection are potentiated in aging subjects (Barrientos et al., 2009), but there is no direct evidence that the hippocampal microglia are actually sensitized. In the present study, we determined whether hippocampal microglia are indeed sensitized by aging by rapidly separating microglia from hippocampus using a procedure that we have previously developed (Frank et al., 2006b) and stimulating them ex vivo.
2. Materials and Methods
2.1. Subjects
3 and 24 mo old male F344×BN F1 rats (N = 4/group) were utilized. Animals were obtained from the National Institute on Aging (Bethesda, MD). The animal colony was maintained at 22 C° on a 12-h light/dark cycle (lights on at 07:00 h). All rats were allowed free access to food and water and were given 1 week to acclimate to colony conditions before experimentation began. All experiments were conducted in accordance with protocols approved by the University of Colorado Animal Care and Use Committee.
2.2 Tissue collection
Animals were given a lethal dose of sodium pentobarbital and transcardially perfused with ice-cold saline (0.9%) for 3 min to remove peripheral immune leukocytes from the CNS vasculature. Brain was rapidly extracted and placed on ice and hippocampus dissected for microglia cell isolation.
2.3. Ex vivo immune stimulation of hippocampal microglia with LPS
Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006b). In the present experiments, immunophenotype and purity of microglia was assessed using real time RT-PCR. Microglia were suspended in DMEM+10% FBS and microglia concentration determined by trypan blue exclusion. Cell number did not significantly differ between 3 and 24 mo animals. Microglia concentration was adjusted to a density of 5 × 103 cells/100 µl and 100 µl added to individual wells of a 96-well v-bottom plate. LPS was utilized to challenge microglia ex vivo as we have previously determined the optimal in vitro conditions under which LPS stimulates a microglia pro-inflammatory cytokine response (Frank et al., 2006b). Cells were incubated with LPS (0.1, 1, 10, and 100 ng/ml) or media alone for 2 h at 37° C, 5% CO2. The plate was centrifuged at 1000 × g for 10 min at 4 °C to pellet cells and cells washed 1× in ice cold PBS and centrifuged at 1000 × g for 10 min at 4 °C. Cell lysis/homogenization, Dnase treatment, and cDNA synthesis were performed using the SuperScript III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA).
2.4. Real time RT-PCR measurement of gene expression
A detailed description of the PCR amplification protocol has been published previously (Frank et al., 2006b). cDNA sequences were obtained from Genbank at the National Center for Biotechnology Information (NCBI; www.ncbi.nlm.nih.gov). Primer sequences were designed using the Qiagen Oligo Analysis & Plotting Tool (oligos.qiagen.com/oligos/toolkit.php?) and tested for sequence specificity using the Basic Local Alignment Search Tool at NCBI (Altschul et al., 1997). Primers were obtained from Invitrogen. Primer specificity was verified by melt curve analysis. All primers were designed to exclude amplification of genomic DNA. Primer sequences are as follows: β-Actin, F- TTCCTTCCTGGGTATGGAAT, R- GAGGAGCAATGATCTTGATC; CD11b, F- CTGGTACATCGAGACTTCTC, R- TTGGTCTCTGTCTGAGCCTT; CD163, F- GTAGTAGTCATTCAACCCTCAC, R- CGGCTTACAGTTTCCTCAAG; Glial Fibrillary Acid Protein (GFAP), F- AGATCCGAGAAACCAGCCTG, R- CCTTAATGACCTCGCCATCC; Ionized Calcium Binding Adapter Protein (Iba-1), F- GGCAATGGAGATATCGATAT, R- AGAATCATTCTCAAGATGGC; IL-1β, F- CCTTGTGCAAGTGTCTGAAG, R- GGGCTTGGAAGCAATCCTTA; IL-6, F- AGAAAAGAGTTGTGCAATGGCA, R- GGCAAATTTCCTGGTTATATCC; IL-10, F- GGACTTTAAGGGTTACTTGGG, R- AGAAATCGATGACAGCGTCG; Major Histocompatibility Complex (MHC)II, F- AGCACTGGGAGTTTGAAGAG, R- AAGCCATCACCTCCTGGTAT.
PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA). Formation of PCR product was monitored in real time using the MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA). Relative gene expression was determined by taking the expression ratio of the gene of interest to β-Actin.
2.5. Statistical analysis and data presentation
All data are presented as mean + SEM. Statistical analyses consisted of ANOVA followed by t tests with a Bonferroni correction. Threshold for statistical significance was set at α = .05.
3. Results
3.1. PCR phenotyping of microglia
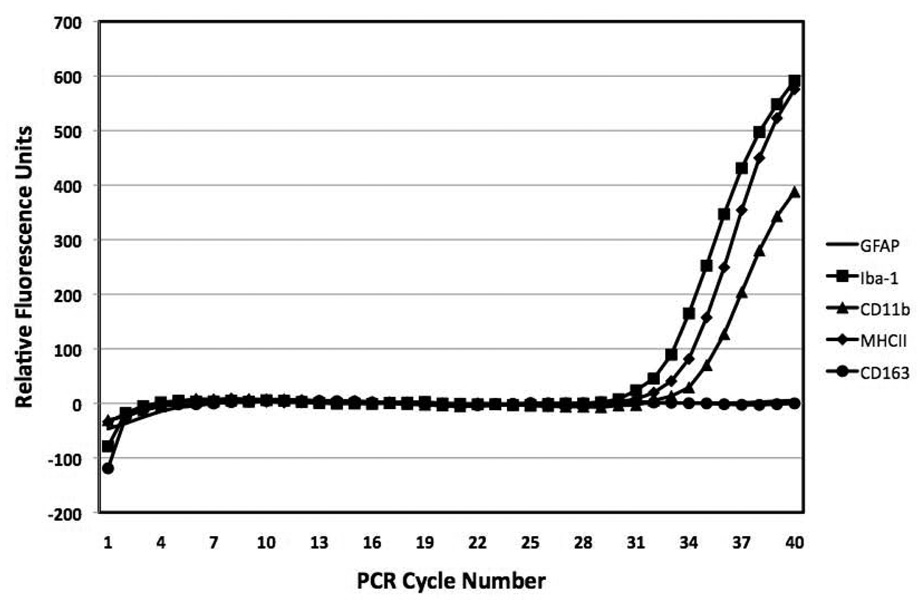
Consistent with our prior work, analysis of cDNA from hippocampal microglia showed robust expression of the microglia markers CD11b, Iba-1 and MHCII, whereas the perivascular/meningeal macrophage marker CD163 failed to amplify by 40 cycles of PCR. Likewise, the astrocyte marker GFAP failed to amplify by 40 cycles of PCR in all samples indicating that the microglia isolation procedure yielded a highly pure microglia population devoid of other CNS macrophages as well as astrocytes. Figure 1 is a representative amplification plot of each gene through 40 cycles of PCR.
Figure 1.
PCR phenotyping of microglia activation. Hippocampal microglia were isolated and gene expression of several microglia antigens (Iba-1, MHCII, CD11b), perivascular macrophage antigens (CD163) and astrocyte antigens (GFAP) were assayed. Through 40 cycles of PCR, only Iba-1, MHCII, and CD11b amplified, whereas CD163 and GFAP failed to amplify.
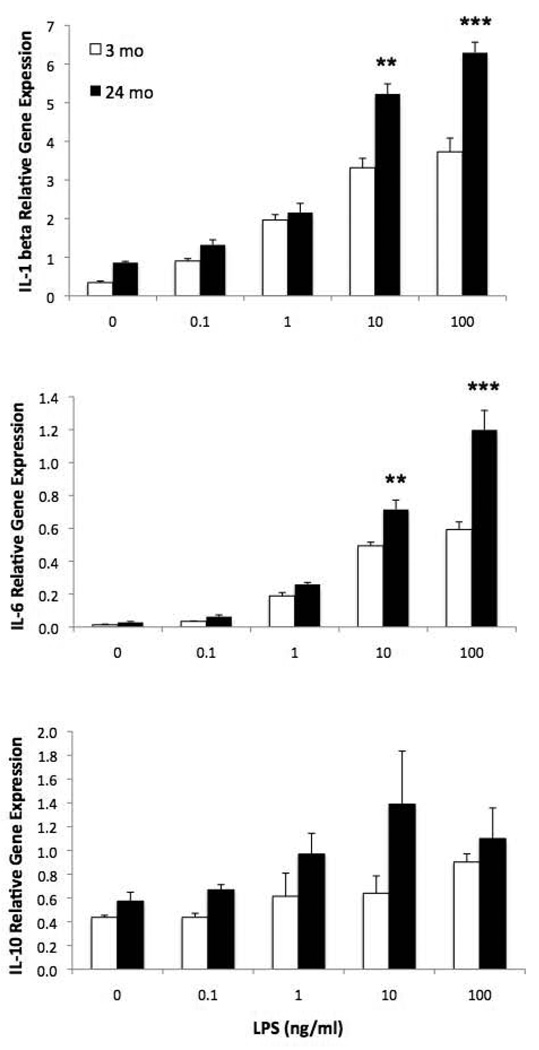
3.2. Microglia from 24 mo animals exhibit a potentiated pro-inflammatory cytokine response to LPS
Hippocampal microglia from 3 and 24 mo old animals were exposed to LPS ex vivo to directly test whether aging sensitizes microglia to pro-inflammatory stimuli. Microglia from 24 mo animals exhibited a potentiated IL-1β (F=176.9, 4,30, p < .0001) and IL-6 (F = 139.0, 4, 30, p < .0001) cytokine response to LPS, whereas IL-10 expression, an anti-inflammatory cytokine, was not potentiated in 24 mo animals (Fig. 2). For IL-1β and IL-6, 24 mo animals showed a greater increase in cytokine expression at 10 ng/ml (p < .01) and 100 ng/ml (p < .001) LPS compared to 3 mo animals. IL-10 expression was significantly higher in 24 mo animals compared to 3 mo animals (F = 10.94, 1, 30, p < .01) and LPS increased IL-10 expression irrespective of age (F = 4.54, 4, 30, p < .01).
Figure 2.
Aging-induced sensitization of the hippocampal microglia response to LPS ex vivo. Hippocampal microglia were isolated from 3 and 24 mo old animals and exposed to LPS ex vivo. 2 h post-LPS exposure, gene expression levels of cytokines were measured. 24 mo old animals exhibited a potentiated IL-1β and IL-6 response to LPS compared to 3 mo old animals at 10 ng/ml and 100 ng/ml LPS, **p < .01, ***p < .001. IL-10 was increased in 24 mo animals compared to 3 mo animals (p < .01) and LPS induced an overall increase in IL-10 (p < .01).
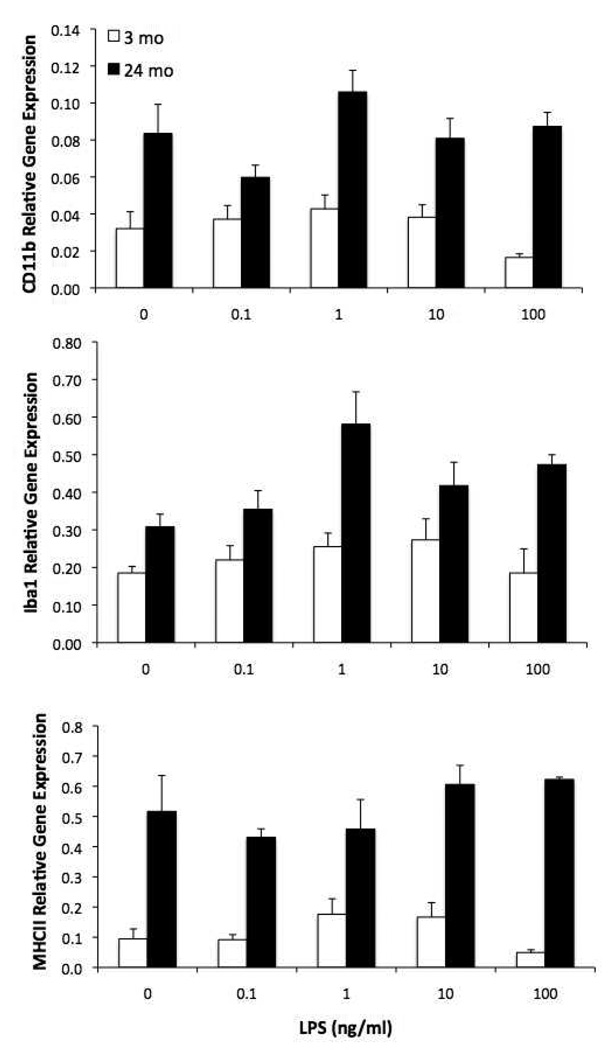
3.3. 24 mo animals exhibit increased expression of hippocampal microglia activation markers
To confirm prior reports that microglia activation markers are upregulated with aging, we assessed the expression level of 3 commonly measured antigens. Expression of CD11b (F = 74.41, 1, 30, p < .0001), Iba-1 (F = 37.09, 1, 30, p < .0001) and MHCII (F = 42.13, 1, 30, p < .0001) was significantly increased in 24 mo old animals compared to 3 mo animals (Fig. 3). The main effect of LPS was not significant for any activation marker.
Figure 3.
Aging increases expression of microglia activation antigens. Hippocampal microglia were isolated from 3 and 24 mo old animals and exposed to LPS ex vivo. 2 h post-LPS exposure, gene expression levels of microglia activation antigens were measured. Expression of CD11b, Iba-1, and MHCII were all increased in 24 mo animals compared to 3 mo animals irrespective of LPS treatment.
4. Discussion
When exposed to a pro-inflammatory immune challenge, aged animals exhibit an exaggerated neuroinflammatory response. It is known that aging increases various microglial activation markers in the hippocampus (Frank et al., 2006a) as well as the hippocampal IL-1β response to both peripherally (Barrientos et al., 2009) and centrally administered LPS (Huang et al., 2008). However, these findings do not conclusively document that aging exaggerates the microglial inflammatory response to stimulation. Even if microglia were the sole source of IL-1β in the CNS, a risky assumption, it could be that in the above studies administered LPS acted at some other cell type to deliver an exaggerated signal to the microglia in the aging subjects. In the present study, hippocampal microglia from 24 mo animals showed a potentiated pro-inflammatory cytokine response to LPS suggesting that aging-induced sensitization of microglia does occur and so may underlie the potentiated neuroinflammatory responses observed in aged animals. Here, there were no other cell types for LPS to act on (the cultures are typically >95% pure microglia), and so it must be microglia that were sensitized by age.
It is important to note that the present results do not exclude the possibility that other CNS immune competent cells (i.e. astrocytes, perivascular macrophages) are sensitized with age. As already noted, prior studies have demonstrated that LPS administered in vivo results in a potentiated pro-inflammatory response in aged animals (Dilger and Johnson, 2008; Sierra et al., 2007). However, since LPS was given in vivo, conclusions regarding cellular sensitization are precluded as LPS can act upon multiple innate immune cell types both peripherally and centrally.
Consistent with the present findings, cultures of hippocampal mixed glia from aged animals exhibited a potentiated pro-inflammatory response to LPS (Xie et al., 2003), however the use of mixed glial cultures does not permit conclusions regarding aging-induced microglia sensitization. Of note, these effects of aging on the mixed glial response to LPS were selective to the hippocampus and cortex, which points to the importance of brain regional variability in both microglia activation and responsiveness to pro-inflammatory challenges.
Aging is characterized by a progressive shift towards a pro-inflammatory microenvironment, most notably an increase in the microglia activation state (Dilger and Johnson, 2008). Activated microglia exhibit a shift in immunophenotype such as up-regulation of MHCII, but basal levels of pro-inflammatory cytokines are not necessarily increased with age. We have previously shown that MHCII in whole hippocampus is up-regulated in aged animals compared to young animals, but pro-inflammatory cytokine levels do not differ between age groups (Barrientos et al., 2006; Frank et al., 2006a). Consistent with these prior findings, the present results show that hippocampal microglia from aged animals exhibited a basal up-regulated expression of Iba-1, CD11b, and MHCII, but not up-regulation of cytokines indicating an aging-induced activation of hippocampal microglia. Importantly, a shift in microglia activation state does not necessarily reflect a primed or sensitized state. Macrophage populations show a high degree of functional plasticity depending on macrophage immunophenotype and the cytokine microenvironment, which underscores the difficulty in relating immunophenotype to functional outcomes (Mosser and Edwards, 2008). The present results show that an immune challenge (LPS) triggered a potentiated pro-inflammatory cytokine response in aged microglia suggesting that aging-induced sensitization or priming of microglia to pro-inflammatory challenges may be a consequence of the age-related shift in the activation state of microglia.
Acknowledgements
The present work was supported by an NIH grant (AG028271) to R.M.B. and S.F.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham J, Jang S, Godbout JP, Chen J, Kelley KW, Dantzer R, Johnson RW. Aging sensitizes mice to behavioral deficits induced by central HIV-1 gp120. Neurobiol Aging. 2008;29:614–621. doi: 10.1016/j.neurobiolaging.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham J, Johnson RW. Central inhibition of interleukin-1beta ameliorates sickness behavior in aged mice. Brain Behav Immun. 2009;23:396–401. doi: 10.1016/j.bbi.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain Behav Immun. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Higgins EA, Biedenkapp JC, Sprunger DB, Wright-Hardesty KJ, Watkins LR, Rudy JW, Maier SF. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2008;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006a;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J Neurosci Methods. 2006b;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne A, Hanke M, Himler J, Bailey MT, Sheridan JF, Godbout JP. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2008;29:1744–1753. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Xie Z, Morgan TE, Rozovsky I, Finch CE. Aging and glial responses to lipopolysaccharide in vitro: greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp Neurol. 2003;182:135–141. doi: 10.1016/s0014-4886(03)00057-8. [DOI] [PubMed] [Google Scholar]