Cellular TF-VIIa proteolytic activity is influenced by thiol modifying reagents [1–3]. TF with a broken allosteric Cys186–Cys209 disulfide bond [4] has low affinity for VIIa and reduced clotting activity [5], but retains normal activity in binary TF-VIIa complex mediated protease activated receptor (PAR) 2 signaling [2]. Cell surface protein disulfide isomerase (PDI) is associated with TF in established cell models of TF-VIIa signaling and modulation of PDI expression alters cell surface TF coagulant activity [2]. Although the choice of cellular models influences the degree by which thiol pathways contribute to the regulation of TF cell surface procoagulant activity [6;7], inhibition of PDI attenuates thrombus formation [8;9], possibly indicating that PDI acts as an important regulator of TF activities in vivo. We showed that procoagulant activity of soluble and micro particle-associated TF is enhanced by bovine liver-derived PDI (bPDI) independent of PDI's reductase and isomerase function [10]. Recombinant PDI from bacterial expression has no TF enhancing activity that is comparable to that observed with PDI from natural sources [11;12](confirmed by us). Kothari et al. [12] showed that proteins known to interact with hydrophobic surfaces (phospholipase C, annexin V) abolished the rate enhancing effects of both, bPDI and mixed phospholipid vesicles containing phosphatidylcholine and phosphatidylserine (PC/PS). Although PS content was not quantified in this study, the authors concluded that contaminating procoagulant phospholipids are the relevant activator of TF activity in bPDI. Experiments presented here document distinct differences between bPDI and PC/PS that are inconsistent with this conclusion.
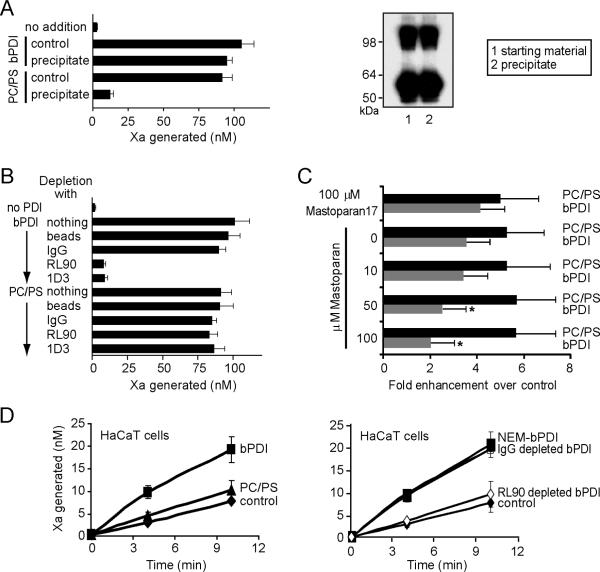
The rate enhancing effect of bPDI obtained from commercial sources (Sigma) [10] was reproducibly observed in subsequently purchased batches and confirmed in independent studies [11;12]. In order to address the presence of potential contaminants in bPDI, we first used the insolubility of proteins in 80% −20°C acetone to recover bPDI in a centrifugation step. Comparison of the starting material with the protein redissolved after precipitation showed no loss of potency in the rate enhancing effect in the soluble TF system. In contrast, no activity was recovered when 10 μM PC/PS vesicles with similar rate enhancing activity as bPDI were subjected to the same acetone precipitation procedure (Fig. 1A). To distinguish between PDI and potential other protein contaminants, two distinct monoclonal antibodies were employed to deplete the bPDI preparation, followed by evaluation of the supernatant in the soluble TF-VIIa mediated factor Xa generation assay. Both specific antibodies completely depleted the rate enhancing effect from the bPDI preparation, but had no effect on the activity of PC/PS vesicles that were treated similarly (Fig. 1B).
Fig. 1. Effects of bPDI and PC/PS on TF procoagulant reactions.
A bPDI or PC/PS were subjected to 80% acetone precipitation at −20°C for 30 minutes, precipitates were recovered by centrifugation for 10 min and redissolved in HBS (10 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM KCl, 1.5 mM CaCl2). The factor Xa generation assay was carried out in HBS, 1 mg/ml BSA with 10 nM VIIa, 500 nM substrate X, and 10 nM soluble TF1–219 with or without 100 nM bPDI or 10 μM PC/PS (70% phosphatidyl choline/30% phosphatidylserine; w/w). Samples were quenched after 45 minutes for chromogenic assay. The efficiency of precipitation was confirmed by Western blotting of non-reduced samples of the starting material and the resuspended acetone precipitate. Note that the dimer content was not changed by the precipitation step and that no other bands were visible on this blot. B RL90 or 1D3 monoclonal anti-PDI antibody (Stressgen, Ann Arbor, MI) or irrelevant IgG control (0.4 μg) were incubated with PDI or PC/PS for 15 minutes followed by immunoprecipitation with Protein G Dynabeads. Controls and depleted supernatants at volumes equivalent to the starting material were added to the soluble TF assay described in A. C TF+ micro particles were recovered from 2 day cultures of adherent HaCaT keratinocytes by a brief wash in 15% isopropanol in HBS which yielded micro particles with properties indistinguishable from those released into serum-free culture supernatant. Cell-free supernatants were subjected to ultracentrifugation and assayed for TF activity under the conditions described above. Rates of Xa generation were calculated from multiple samples over a 20 minute time period. The rate enhancing effects of 100 nM bPDI or 10 μM PC/PS were calculated for the indicated range of mastoparan concentrations or 100 μM inactive control peptide mastoparan 17 (Sigma). Data are mean ± SD (n = 4–5), * p < 0.05. D TF cell surface activity of HaCaT monolayers grown for two days was measured in the absence or presence of 10 μM PC/PS, 100 nM bPDI, 100 nM NEM-blocked bPDI, or 100 nM bPDI subjected to antibody depletion as indicated. Data are mean ± SD (n = 3–5).
Similar to the soluble TF system, 100 nM bPDI or 10 μM PC/PS enhanced by 2- to 3-fold the activity of TF+ micro particles obtained from HaCaT keratinocytes. The wasp venom-derived peptide mastoparan is a well characterized inhibitor of PDI's hydrophobic pocket required for chaperone activity [13]. Mastoparan, but not the inactive control peptide mastoparan 17, dose dependently inhibited the rate enhancing effect of bPDI, but not of PC/PS vesicles (Fig. 1C). These data provide an independent line of evidence for our previous conclusion that bPDI specifically modulates TF's procoagulant activity through chaperone activity. HaCaT keratinocytes are a cell model in which TF activity is regulated by PDI [2]. There was no change in cell surface TF procoagulant activity upon addition of 10 μM PC/PS, but addition of 100 nM bPDI or N-ethylmaleimide (NEM)-blocked bPDI enhanced Xa generation by more than 2-fold (Fig. 1D). Depletion of the bPDI preparation with anti-PDI antibody completely abolished the rate enhancing effect of bPDI. These data demonstrate that bPDI has specific activities on TF cellular activity that are not reproduced by the addition of procoagulant phospholipid.
These experiments in purified and cellular systems argue that bPDI's effects on TF procoagulant activity are distinct from rate enhancing effects of phospholipid. Although we could not detect PS in organic extracts of the commercial bPDI preparation by mass spectrometry, we cannot exclude that bPDI has other structurally bound ligands that are required for its chaperone activity towards the TF initiation complex. Persson [11] further showed that bPDI's effect on TF procoagulant function is modulated by either mutations in the VIIa Gla-domain or changes in divalent metal ions that alter the interaction of the VIIa Gla domain with TF [14]. These data may indicate that PDI not only interacts with TF, as suggested from mutagenesis [10], but also with the VIIa Gla-domain through potential contacts with PDI's hydrophobic pocket. The chaperone activity of PDI should be further considered as a possible mechanism that contributes to the regulation of TF thrombogenic pathways in vivo [8;9]. In future studies, it is imperative to investigate the functional properties and posttranslational modifications of cell surface human PDI from natural and cellular sources.
Acknowledgements
We thank Pablito Tejada for technical assistance and Cheryl Johnson for manuscript preparation. This work was supported by NIH grant HL31950.
Footnotes
Disclosure of Conflict of Interest: The authors have no financial conflicts of interest to declare.
References
- [1].Le D, Rapaport S, Rao LVM. Studies of the mechanism for enhanced cell surface factor VIIa\tissue factor activation of factor X on fibroblast monolayers after their exposure to N-ethylmaleimide. Thromb Haemost. 1994;72:848–55. [PubMed] [Google Scholar]
- [2].Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, Ruf W. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proc Natl Acad Sci USA. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chen VM, Ahamed J, Versteeg HH, Berndt MC, Ruf W, Hogg PJ. Evidence for activation of tissue factor by an allosteric disulfide bond. Biochemistry. 2006;45:12020–8. doi: 10.1021/bi061271a. [DOI] [PubMed] [Google Scholar]
- [4].Chen VM, Hogg PJ. Allosteric disulfide bonds in thrombosis and thrombolysis. J Thromb Haemost. 2006;4:2533–41. doi: 10.1111/j.1538-7836.2006.02236.x. [DOI] [PubMed] [Google Scholar]
- [5].Rehemtulla A, Ruf W, Edgington TS. The integrity of the Cys186–Cys209 bond of the second disulfide loop of tissue factor is required for binding of factor VII. J Biol Chem. 1991;266:10294–9. [PubMed] [Google Scholar]
- [6].Liang HP, Hogg PJ. Critical importance of the cell system when studying tissue factor de-encryption. Blood. 2008;112:912–3. doi: 10.1182/blood-2008-05-158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pendurthi UR, Ghosh S, Mandal SK, Rao LV. Tissue factor activation: is disulfide bond switching a regulatory mechanism? Blood. 2007;110:3900–8. doi: 10.1182/blood-2007-07-101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Reinhardt C, von Bruhl ML, Manukyan D, Grahl L, Lorenz M, Altmann B, Dlugai S, Hess S, Konrad I, Orschiedt L, Mackman N, Ruddock L, Massberg S, Engelmann B. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118:1110–22. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118:1123–31. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282:25416–24. doi: 10.1074/jbc.M702410200. [DOI] [PubMed] [Google Scholar]
- [11].Persson E. Protein disulfide isomerase has no stimulatory chaperone effect on factor X activation by factor VIIa-soluble tissue factor. Thromb Res. 2008;123:171–6. doi: 10.1016/j.thromres.2008.04.012. [DOI] [PubMed] [Google Scholar]
- [12].Kothari H, Sen P, Pendurthi UR, Rao LV. Bovine protein disulfide isomerase-enhanced tissue factor coagulant function: is phospholipid contaminant in it the real culprit? Blood. 2008;111:3295–6. doi: 10.1182/blood-2007-12-129825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Klappa P, Ruddock LW, Darby NJ, Freedman RB. The b' domain provides the principal peptide-binding site of protein disulfide isomerase but all domains contribute to binding of misfolded proteins. EMBO J. 1998;17:927–35. doi: 10.1093/emboj/17.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bajaj SP, Schmidt AE, Agah S, Bajaj MS, Padmanabhan K. High resolution structures of p-aminobenzamidine- and benzamidine-VIIa/soluble tissue factor: unpredicted conformation of the 192–193 peptide bond and mapping of Ca2+, Mg2+, Na+, and Zn2+ sites in factor VIIa. J Biol Chem. 2006 Aug 25;281(34):24873–88. doi: 10.1074/jbc.M509971200. [DOI] [PubMed] [Google Scholar]