Abstract
Purpose
It is unclear whether sex hormone profiles obtained in two consecutive months are consistent within females. We prospectively examined month to month consistency in daily, nadir, peak and mean hormone concentrations during the early follicular and luteal phases in recreationally active, young eumenorrheic females.
Methods
60 healthy, non-smoking females who reported normal and consistent menstrual cycles lasting 26–32 days for the past 6 months were followed prospectively to obtain serum samples for the first 6 days of menses, and for 8 days following a positive ovulation test over two consecutive months. Month to month consistency of daily concentrations of estradiol (pg/mL), progesterone (ng/mL), testosterone (ng/dL), SHBG (nmol/L) and FAI were determined using linear mixed models. Month to month consistency in nadir, peak and mean concentrations were then assessed using intraclass correlation coefficients (ICC) and standard error of the measurement (SEM) to more precisely examine intra-individual consistency.
Results
Linear mixed models revealed stable hormone concentrations across cycles and cycles by day. Reliability estimates for nadir, peak, mean menses and mean postovulatory concentrations range from 0.56 – 0.86 for estradiol, 0.44 – 0.91 for progesterone, 0.60 – 0.86 for testosterone, 0.88 – 0.97 for SHBG, and 0.78 – 0.91 for FAI.
Conclusions
Hormone profiles were reproducible over two consecutive months. In order to reduce month to month intra-individual variations and improve measurement consistency, it is recommended that multiple samples be taken over consecutive days as opposed to a single sample.
Key Terms: Reliability, Measurement Accuracy, Estradiol, Progesterone, Testosterone, SHBG, Menstrual cycle variability
INTRODUCTION
Variations in sex hormone concentrations in young, physically active females may be associated with the risk of non-contact anterior cruciate ligament (ACL) injury. Studies report a greater number of injuries than expected during the peri-menstrual[1–4] and peri-ovulatory[5] days, while others generally identified the follicular phase as being the phase of higher risk.[6, 7] These studies collected a single sample (blood or urine) shortly following the injury (range 2 hours[7] to 72 hours[2]), making it difficult to identify the specific time in a particular phase when injury occurred (i.e. whether hormone levels were rising, falling or near their peak). Other work suggests a time delay between when hormone concentrations change and when soft tissues change (e.g. laxity)[8], emphasizing the importance of documenting hormone profiles in the days preceding the injury. Because ACL injuries occur infrequently, retrospective studies are the most practical research design to comprehensively examine hormone profiles associated with injury risk. In order for retrospective studies to be valid, establishing consistency of hormone profiles month to month is both necessary and paramount as a first step. The application of the present data may also be useful in a research setting when projecting hormonal phases for future data collections.
While the typical hormone profile of a 28 day cycle is well established, individual females vary substantially from this typical profile in their cycle length (both follicular and luteal phases); the timing of changes in one hormone relative to another; the day of ovulation; and absolute change in hormone concentrations.[8–12] Although this variability is substantially greater between females than within a female from one month to the next, some variations within a female also occur.[13] Therefore it is important to quantify the magnitude of these intra-individual variations to determine how consistent an individual’s hormone profile will be from one month to the next. We examined the month to month consistency in daily, nadir, peak and mean hormone profiles during the first 6 days of the early follicular phase and the first 8 days of the early luteal phase for two consecutive cycles in young, normal menstruating females. Our expectation was that sex hormone profiles would be highly reproducible from one month to the next.
METHODS
Serum samples were obtained prospectively from 60 females (age=21.7±2.6 yrs, Ht=163.9±6.5 cm and Wt=60.3±8.6 kg), participating in a larger project examining the effects of sex hormone mediated changes in knee laxity on knee joint function. Subjects were included if they self-reported regular physical activity between 2.5 – 10 hours per week for the past 3 months, normal menstrual cycles lasting 26–32 days with consistent cycle lengths varying no more than ± 1 day each month for the past 6 months; no use of oral contraceptives or other hormone stimulating medications for the past 6 months; and no history of pregnancy. Subjects with a body mass index > 30 (BMI = wt/ht2), a previous history of knee ligament injury, or who smoked were excluded. At the time of this study, 67 female subjects had been enrolled in the larger study. However, 7 were excluded due to incomplete data (4 voluntarily withdrew; 3 lacked a positive ovulation test). All participants were informed of the study procedures and signed a consent form approved by the University’s Institutional Review Board for the Protection of Human Subjects.
Procedures
Serum (10cc) was collected daily using standard venipuncture procedures during the first 6 days of the early follicular phase (day 1 identified as the day immediately following the onset of menses per self report; labeled M1-M6) and for the first 8 days of the early luteal phase (day 1 identified as the first day following evidence of ovulation; labeled L1-L8) for two consecutive months. To control for diurnal fluctuations in hormone concentrations, all samples were obtained in the morning hours (0700 – 0900, usually within ± 30 minutes for each participant) prior to physical activity. To estimate the day of ovulation, participants used a commercially available ovulation kit (CVS One Step Ovulation Predictor [sensitivity 20 mIU/ml LH, accuracy 99%]; CVS Corporation, Woonsocket, RI) starting with day 8 of their menstrual cycle. Participants were instructed to maintain normal activity patterns and avoid excessive physical activity for 2 days prior to any testing, and to defer their normal activity until their serum was collected on each test day. Subjects were also instructed to abstain for alcohol consumption for 24 hours prior and throughout each testing block. Participants completed a daily questionnaire to insure study compliance.
Assays
Blood samples were separated and stored at −80°C and shipped to a Ligand Assay and Analysis Core Laboratory to assay serum levels of estradiol, progesterone, testosterone and serum hormone-binding globulin (SHBG). All samples for a given subject were analyzed within the same assay test kit. Estradiol was assayed using a double-antibody RIA Assay (DSL-4400; Beckman Coulter, Webster TX), and progesterone and testosterone concentrations were assayed using Coat-A-Count RIA Assays (TKPG-2 and TKTT-2; Siemens Medical Solutions Diagnostics, Los Angeles CA). SHBG was assayed using the Immulite chemiluminescent technology (LKSH5; Siemens Medical Solutions Diagnostics, Los Angeles CA). Free androgen index (FAI) was calculated based on testosterone and SHBG levels [FAI = total testosterone (nmol/L)/SHBG (nmol/L)]. Mean percent intra-assay and inter-assay coefficient of variations (%CV), respectively, were 5.2% and 10.6% for estradiol, 4.1% and 6.4% for progesterone, 3.4% and 8.1% for testosterone, and 2.4% and 5.8% for SHBG. Assay sensitivities were 10 pg/ml (estradiol), 0.1 ng/mL (progesterone), 10 ng/dL (testosterone), and 0.2 nmol/L (SHBG).
Although estradiol, progesterone, and to a less extent testosterone, have been the primary hormones of interest when describing the hormone profile of a particular female, the capacity of these sex steroids to exert their effect on soft tissues is dependent on the amount of each sex hormone that is freely circulating (i.e. biologically active).[14, 15] Sex hormone binding globulin (SHBG) is considered to be the major regulator of plasma free concentrations for estradiol and testosterone, as it has a high binding affinity for these hormones. While research suggests that little if any change in SHBG concentrations occur across the menstrual cycle[16] (thus the freely circulating concentration of estradiol and testosterone), we felt it was important to confirm the extent to which SHBG concentrations (and similarly a measure of the biologically available fraction of a hormone, i.e FAI in this study) vary from cycle to cycle, as this would have further implications on the ability to reliably predict hormone concentrations retrospectively.
Data Analysis
To examine the general consistency of daily changes in hormone concentrations during the 6 menses days (M1-M6) and the 8 post ovulatory days (L1-L8) from one month to the next, we ran separate linear mixed models (PROX MIXED, Statistical Analysis System version 9.1.2, Cary, NC) for each of the hormones, with the hormone as the dependent variable and cycle, day, and the cycle by day interaction as the independent variables. Significance was set a-priori at p<0.05. To more closely examine the intra-individual month to month consistency of hormone profiles, intraclass correlation coefficient formula 2,1 (ICC2,1)[17] compared cycles 1 and 2 on absolute nadir and peak hormone concentrations obtained during M1-M6 and during L1-L8, respectively. We then used ICC formula 2,k[17] to compare cycles 1 and 2 on mean concentrations obtained across M1-M6 and L1-L8. For each reliability estimate, the standard error of measurement (SEM) was calculated [SD√(1− ICC)].[18]
Once the serum were assayed, 11 females (18%) were found to have anovulatory cycles in one (N=8) or both (N=3) months, defined as a luteal phase progesterone level that did not exceed 3ng/mL.[19, 20] Therefore, we ran all our analyses on both the entire data set (N=60) and then limited analyses to those with two confirmed ovulatory cycles (N=49) to determine the effect of anovulatory cycles on measurement consistency. Our rationale to include anovulatory cycles is that it may not always be possible to confirm the presence or absence of anovulatory cycles in retrospective studies.
RESULTS
Comparison of Daily Hormone Concentrations Between Cycles
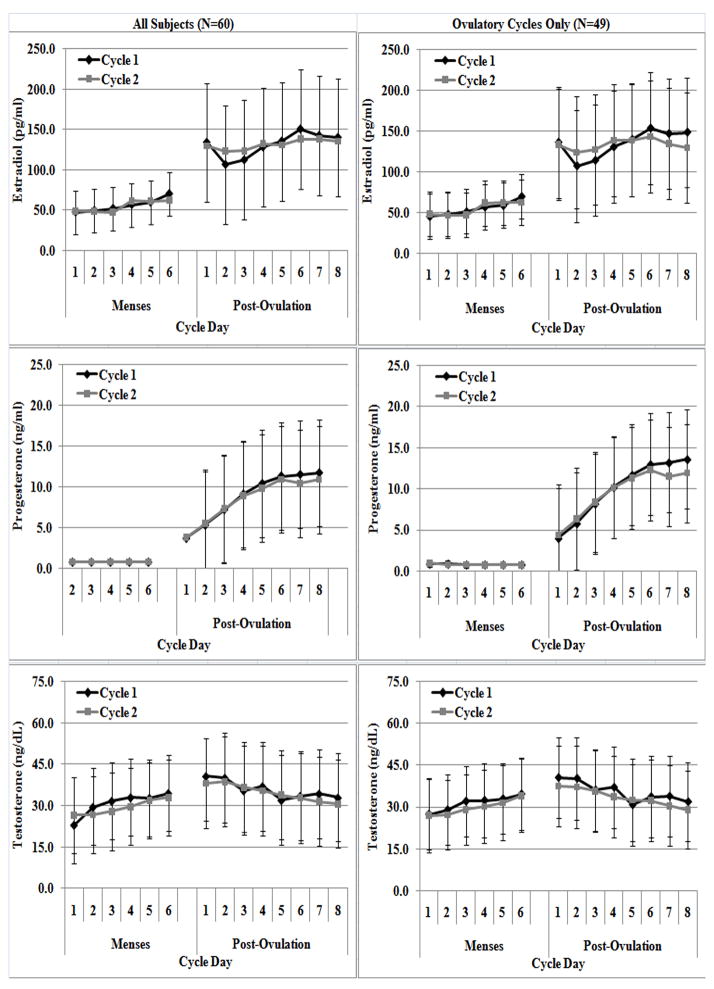
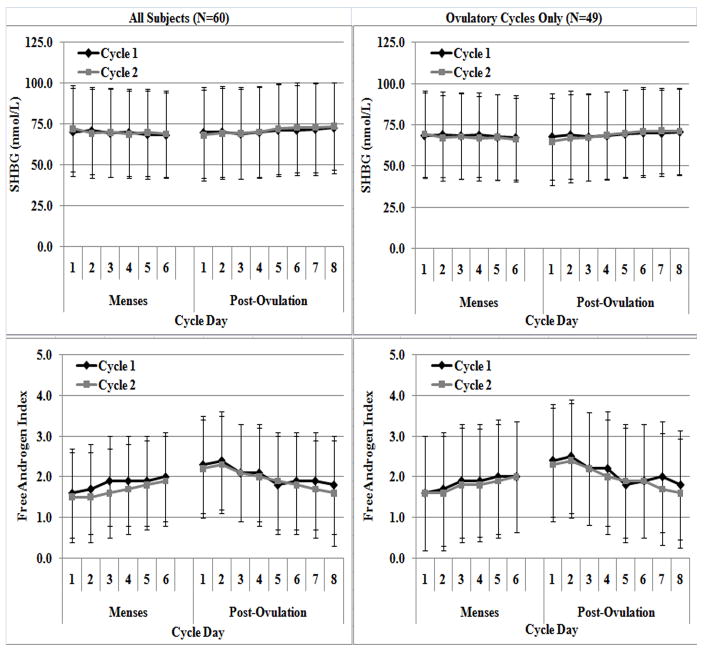
The daily means and standard deviations (SD) of the five hormone concentrations during M1-M6 and L1-L8 over two cycles are shown in Figures 1 and 2. Table 1 reports the linear mixed model results comparing hormone concentrations between cycles, days and cycle by days. During M1-M6, all hormone concentrations differed between individual days, except for SHBG which remained stable (P>0.117). In all cases, daily changes in hormone concentrations were generally consistent between cycles, as no significant differences in hormone concentrations by cycle or cycle by day were observed. When examining L1-L8, all hormone concentrations differed between individual days (all p<0.004), and these differences were also consistent across cycle and cycle by day. Results did not change when anovulatory cycles were removed.
Figure 1.
Daily mean early follicular (M1 – M6) and early luteal (L1 – L8) estradiol (A & B), progesterone (C & D) and testosterone (E & F) concentrations over two consecutive months for all subjects (N=60, left column) and with anovulatory cycles removed (N=49; right column).
Figure 2.
Daily mean early follicular (M1 – M6) and early luteal (L1 – L8) SHBG (A & B) and FAI (C & D) concentrations over two consecutive months for all subjects (N=60; left column) and with anovulatory cycles removed (N=49; right column).
Table 1.
ANOVA results for sex steroid hormones when all subjects were included in the analysis and when analysis included only subjects with 2 ovulatory cycles. Analysis was separated by phase of the menstrual cycle (menses & post ovulatory).
| Effect | All Subjects (N=60) (P-Value) | Ovulatory Cycles (N=49) (P-Value) | ||||
|---|---|---|---|---|---|---|
| Estradiol | Menses | Cycle | 0.691 | 0.880 | ||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.201 | 0.335 | ||||
| Post Ovulatory | Cycle | 0.932 | 0.885 | |||
| Day | 0.004* | 0.003* | ||||
| Cyc × Day | 0.353 | 0.145 | ||||
| Progesterone | Menses | Cycle | 0.480 | 0.391 | ||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.395 | 0.341 | ||||
| Post Ovulatory | Cycle | 0.657 | 0.561 | |||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.842 | 0.247 | ||||
| Testosterone | Menses | Cycle | 0.058 | 0.243 | ||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.597 | 0.807 | ||||
| Post Ovulatory | Cycle | 0.387 | 0.077 | |||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.498 | 0.380 | ||||
| SHBG | Menses | Cycle | 0.749 | 0.518 | ||
| Day | 0.117 | 0.374 | ||||
| Cyc × Day | 0.321 | 0.696 | ||||
| Post Ovulatory | Cycle | 0.820 | 0.874 | |||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.761 | 0.416 | ||||
| FAI | Menses | Cycle | 0.052 | 0.313 | ||
| Day | <0.001* | <0.001* | ||||
| Cyc × Day | 0.760 | 0.834 | ||||
| Post Ovulatory | Cycle | 0.222 | 0.182 | |||
| Day | <0.001* | <.0001* | ||||
| Cyc × Day | 0.485 | 0.415 | ||||
P<.05
Consistency of Nadir, Peak and Mean Hormone Concentrations
Table 2 lists the means, SD, and reliability estimates examining the month to month consistency in absolute nadir and peak hormone concentrations. ICCs for the entire sample ranged from 0.58 – 0.88 for nadir and 0.44 – 0.89 for peak concentrations. While ICC values were consistently high for SHBG and FAI (0.78 – 0.89), estimates were lower for estradiol, progesterone and testosterone levels (0.44–0.71). Analyses of the sources of variance and SEM values for these hormones indicate that the lower ICCs were primarily due to random error rather than systematic differences in concentrations between cycles. When anovulatory cycles were removed, ICC values were similar, except for nadir and peak estradiol levels, where the ICC values decreased somewhat, but the SEMs stayed relatively unchanged.
Table 2.
Mean, standard deviation, ICC and SEM comparing month to month consistency in absolute nadir (from the 6 days of menses) and peak (from the 8 post ovulatory days) sex hormone concentrations for all subjects and with anovulatory cycles removed.
| Cycle 1 Mean (SD) | Cycle 2 Mean (SD) | ICC | SEM | |
|---|---|---|---|---|
| All Subjects (N=60) | ||||
| Nadir (Menses) Values | ||||
| Estradiol (pg/ml) | 37.5 (14.0) | 35.4 (15.2) | 0.58 | 9.8 |
| Progesterone (ng/ml) | 0.6 (0.3) | 0.6 (0.3) | 0.67 | 0.2 |
| Testosterone (ng/dL) | 22.6 (11.4) | 20.2 (10.2) | 0.60 | 7.2 |
| SHBG (nmol/l) | 60.8 (24.2) | 62.9 (23.6) | 0.88 | 8.4 |
| FAI | 1.3 (0.9) | 1.1 (0.8) | 0.78 | 0.4 |
| Peak (Post Ovulatory) Values | ||||
| Estradiol (pg/ml) | 208.7 (92.5) | 209.0 (111.2) | 0.56 | 73.6 |
| Progesterone (ng/ml) | 14.6 (7.8) | 13.9 (7.9) | 0.44 | 5.9 |
| Testosterone (ng/dL) | 49.2 (17.4) | 48.7 (19.6) | 0.71 | 10.5 |
| SHBG (nmol/l) | 80.0 (30.1) | 80.9 (33.8) | 0.89 | 11.4 |
| FAI | 2.9 (1.6) | 2.8 (1.5) | 0.82 | 0.7 |
| Ovulatory Cycles Only (N=49) | ||||
| Nadir (Menses) Values | ||||
| Estradiol (pg/ml) | 36.0 (12.7) | 34.3 (14.7) | 0.48 | 10.6 |
| Progesterone (ng/ml) | 0.6 (0.3) | 0.6 (0.2) | 0.66 | 0.2 |
| Testosterone (ng/dL) | 22.6 (10.9) | 21.0 (9.7) | 0.60 | 6.9 |
| SHBG (nmol/l) | 59.5 (23.6) | 60.7 (22.2) | 0.86 | 8.7 |
| FAI | 1.3 (0.9) | 1.2 (0.8) | 0.82 | 0.4 |
| Peak (Post Ovulatory) Values | ||||
| Estradiol (pg/ml) | 214.1 (83.1) | 211.1 (95.3) | 0.38 | 74.8 |
| Progesterone (ng/ml) | 16.5 (6.7) | 15.7 (6.5) | 0.41 | 5.1 |
| Testosterone (ng/dL) | 48.5 (16.7) | 46.6 (15.6) | 0.73 | 8.6 |
| SHBG (nmol/l) | 77.7 (28.4) | 78.0 (31.5) | 0.87 | 11.3 |
| FAI | 3.0 (1.7) | 2.9 (1.6) | 0.85 | 0.7 |
Table 3 lists the means, SD, and reliability estimates examining the month to month consistency in the mean hormone concentrations collapsed across M1-M6 and across L1-L8. These analyses revealed substantially stronger ICCs and improved precision (SEMs) compared to those obtained for peak and nadir concentrations. ICC values ranged from 0.81 – 0.97 (and were similar with and without anovulatory cycles), with the exception of mean post ovulatory progesterone levels (0.54–0.59), which were more variable month to month.
Table 3.
Mean, standard deviation, ICC (2,k) and SEM examining the consistency of mean sex hormone concentrations during menses (days 1–6) and post ovulation (days 1–8) across two menstrual cycles for all subjects and with anovulatory cycles removed.
| Cycle 1 Mean (SD) | Cycle 2 Mean (SD) | ICC | SEM | |
|---|---|---|---|---|
| All Subjects (N=60) | ||||
| Mean Menses Values | ||||
| Estradiol (pg/ml) | 55.6 (19.7) | 53.7 (18.7) | .86 | 7.5 |
| Progesterone (ng/ml) | 0.8 (0.3) | 0.8 (0.3) | .91 | 0.1 |
| Testosterone (ng/dL) | 31.6 (13.0) | 29.3 (11.4) | .81 | 5.7 |
| SHBG (nmol/l) | 69.4 (26.7) | 69.8 (25.8) | .97 | 4.5 |
| FAI | 1.8 (1.1) | 1.7 (1.0) | .90 | 0.3 |
| Mean Post-Ovulatory Values | ||||
| Estradiol (pg/ml) | 131.0 (46.3) | 131.3 (55.0) | .82 | 23.2 |
| Progesterone (ng/ml) | 8.7 (5.0) | 8.4 (5.6) | .59 | 3.7 |
| Testosterone (ng/dL) | 35.8 (12.9) | 34.7 (14.4) | .86 | 5.3 |
| SHBG (nmol/l) | 61.4 (21.0) | 71.0 (27.9) | .90 | 8.8 |
| FAI | 2.1 (1.2) | 2.0 (1.0) | .91 | 0.4 |
| Ovulatory Cycles Only (N=49) | ||||
| Mean Menses Values | ||||
| Estradiol (pg/ml) | 55.0 (19.2) | 53.2 (17.8) | .83 | 8.0 |
| Progesterone (ng/ml) | 0.8 (0.3) | 0.8 (0.3) | .90 | 0.1 |
| Testosterone (ng/dL) | 31.3 (11.7) | 30.0 (10.9) | .81 | 5.1 |
| SHBG (nmol/l) | 68.3 (25.9) | 67.5 (24.1) | .97 | 4.5 |
| FAI | 1.9 (1.1) | 1.8 (1.0) | .93 | 0.3 |
| Mean Post-Ovulatory Values | ||||
| Estradiol (pg/ml) | 134.4 (40.8) | 133.6 (50.4) | .81 | 22.2 |
| Progesterone (ng/ml) | 10.0 (4.4) | 9.5 (4.8) | .54 | 3.3 |
| Testosterone (ng/dL) | 35.5 (12.5) | 33.6 (11.5) | .88 | 4.1 |
| SHBG (nmol/l) | 59.9 (19.5) | 68.9 (26.6) | .90 | 8.6 |
| FAI | 2.1 (1.3) | 2.0 (1.1) | .94 | 0.3 |
For descriptive purposes only, Table 4 lists the means and SD comparing nadir, peak and mean hormone concentrations between females with 2 ovulatory cycles (OVUL, N=49), 1 ovulatory and 1 anovulatory cycle (OVUL/ANOV, N=8) and 2 Anovulatory cycles (ANOV, N=3). Although difficult to examine statistically given the small number of females with anovulatory cycles, estradiol levels appear to vary considerably when females were stratified based on the consistency of ovulatory vs. anovulatory cycles. Specifically, females with anovulatory cycles had substantially lower luteal phase estradiol levels, with the lowest levels observed in those with consistent anovulatory cycles. SHBG concentrations also appear to vary somewhat, with higher concentrations observed in females with inconsistent ovulatory cycles and somewhat lower concentrations in those who had consistent anovulatory cycles. Related to these differences in SHBG, proportional changes in FAI were observed, suggesting that the free fraction concentrations of these hormones may also differ between these groups.
Table 4.
Means and standard deviations comparing nadir, peak and mean hormone concentrations between females with 2 ovulatory cycles (OVUL, N=49), 1 ovulatory and 1 anovulatory cycle (OVUL/ANOV, N=8) and 2 Anovulatory cycles (ANOV, N=3).
| OVUL (N=49) | OVUL/ANOV (N=8) | ANOV (N=3) | ||
|---|---|---|---|---|
| Mean (SD) | OVUL Cycle Mean (SD) | ANOV Cycle Mean (SD) | Mean (SD) | |
| Nadir and Peak Values | ||||
| Nadir (M1-M6) | ||||
| Estradiol (pg/ml) | 36.0 (12.7) | 50.9 (60.2) | 43.4 (11.1) | 29.4 (18.9) |
| Progesterone (ng/ml) | 0.6 (0.3) | 0.7 (0.4) | 0.6 (0.2) | 0.5 (0.2) |
| Testosterone (ng/dL) | 22.6 (10.9) | 24.2 (11.9) | 16.6 (10.3) | 17.5 (18.2) |
| SHBG (nmol/l) | 59.5 (23.6 | 76.6 (33.0) | 75.2 (26.5) | 53.4 (12.2) |
| FAI | 1.3 (0.9) | 1.0 (0.3) | 0.8 (0.5) | 1.0 (1.1) |
| Peak (L1-L8) | ||||
| Estradiol (pg/ml) | 214.1 (83.1) | 261.8 (181.0) | 197.1 (137.9) | 92.5 (18.2) |
| Progesterone (ng/ml) | 16.5 (6.7) | 14.8 (7.4) | 1.4 (0.5) | 1.4 (0.9) |
| Testosterone (ng/dL) | 48.5 (16.7) | 59.0 (22.0) | 60.8 (33.8) | 42.1 (17.1) |
| SHBG (nmol/l) | 77.7 (28.4) | 105.3 (40.2) | 98.0 (41.0) | 61.8 (18.2) |
| FAI | 3.0 (1.7) | 2.6 (1.0) | 2.4 (0.8) | 2.8 (1.2) |
| Mean Values | ||||
| Menses (M1-M6) | ||||
| Estradiol (pg/ml) | 55.0 (19.2) | 64.5 (22.3) | 62.3 (17.3) | 39.8 (22.1) |
| Progesterone (ng/ml) | 0.8 (0.3) | 0.9 (0.5) | 0.8 (0.3) | 0.7 (0.3) |
| Testosterone (ng/dL) | 31.3 (11.7) | 35.0 (14.6) | 26.7 (13.4) | 26.1 (22.1) |
| SHBG (nmol/l) | 68.3 (25.9) | 85.5 (35.4) | 83.6 (31.3) | 57.8 (13.2) |
| FAI | 1.9 (1.1) | 1.5 (0.3) | 1.1 (0.4) | 1.6 (1.5) |
| Post-Ovulatory (L1-L8) | ||||
| Estradiol (pg/ml) | 134.4 (40.8) | 162.1 (74.1) | 117.1 (59.2) | 61.6 (14.4) |
| Progesterone (ng/ml) | 10.0 (4.4) | 8.0 (5.8) | 1.0 (0.5) | 0.9 (0.5) |
| Testosterone (ng/dL) | 35.5 (12.5) | 40.5 (14.8) | 43.8 (24.2) | 29.3 (16.4) |
| SHBG (nmol/l) | 59.9 (19.5) | 85.6 (32.1) | 79.4 (29.9) | 52.4 (13.0) |
| FAI | 2.1 (1.3) | 1.6 (0.4) | 1.8 (0.8) | 1.9 (1.1) |
DISCUSSION
Much of the literature on hormone repeatability focuses on older premenopausal women (typically 35–50 years of age), to determine whether a single hormone measure can reliably predict hormone exposure over time, thus future disease risk.[21–25] However, in the case of ACL injury where acute hormone exposure is of interest, there is a need to know if hormone profiles obtained post injury can adequately reflect the hormone profiles just prior to injury. As an initial step toward this effort, we quantify the consistency in hormone profiles in young, physically active and normal menstruating females. Our findings in a group of young, recreationally active eumonorrheic women revealed that daily mean hormone concentrations measured during M1-M6 and L1-L8 varied by day as expected, but were generally stable from one month to the next. However, when examining intra-individual month to month consistency in nadir, peak and mean hormone concentrations, reliability estimates varied across the 5 hormones tested. With the exception of nadir and peak estradiol (0.38–0.48 with anovulatory cycles removed) and peak progesterone levels (0.41 and 0.44 with and without anovulatory cycles), reliability estimates for all other nadir and peak hormone values ranged from 0.56 – 0.89. These estimates appear to represent highly reliable measures based on what has been reported in the hormone literature.[22, 24, 25] This is particularly true of SHBG and FAI, suggesting that the proportion of each hormone concentration that is biologically available is very stable from month to month within a female. The lower reliability we observed for nadir and peak estradiol and progesterone levels is consistent with previous studies in premenopausal females where a single measure of estradiol and progesterone were obtained [estradiol (0.38 – 0.53 for follicular measures, 0.06 – 0.45 for luteal phase measures)[13, 21, 22, 24, 25], progesterone (0.29 – 0.54 for luteal phase measures)[22, 24], suggesting there is substantial month to month variations in absolute daily levels of estradiol and progesterone within a female, particularly during the post ovulatory days. However, when concentrations are averaged over multiple days, reliability estimates improved considerably (Table 2 vs. 3). Hence, it may be necessary to take multiple samples in order to gain an adequate representation of a females’ hormone profile, particularly when examining estradiol and progesterone.
To fully appreciate the magnitude of this variability, the SEM provides a unit value of measurement precision that is based on the distribution of measurement error.[26] Specifically, there is a 68% and 95% chance that the participants true hormone value will fall within ±1 or ±2 SEMs, respectively, of the value obtained from a subsequent month. In some cases, particularly for peak estradiol, progesterone and testosterone levels, the SEMs seem rather large and suggest considerable measurement error. When these error variances are compared against the overall deviation and range in concentrations obtained in this cohort, SEM values generally represented less than 15% of the total range in concentrations obtained for nadir (7.0 – 14.5%) and peak (8.2 – 16.3%) and less than 10% of the total range in mean menses (3.6 – 7.5%) and mean luteal (6.2 – 11.7%) values. The only exception was peak progesterone levels, where the SEM represented 18–20% of the range in values for nadir and peak levels, and 10–19% of the range in values for mean menses and luteal values. As epidemiological studies often reduce hormone values to quartiles when classifying the association of a particular hormone with disease[22], the precision of these values may be acceptable. The improved measurement precision when using mean values (Table 3) again indicates the importance of taking multiple samples to enhance the accuracy of determining hormone profiles in a subsequent month.
Our findings also revealed that measurement consistency and precision were relatively robust to the presence of occasional anovulatory cycles. When females with one or both anovulatory cycles were removed (18% of the sample), the ICC and SEM values remained relatively unchanged except for nadir and peak estradiol values (Table 2). As noted in Table 4, females with anovulatory cycles had substantially lower estradiol levels, particularly during the luteal phase. Hence, the lower ICC values for estradiol values when anovulatory cycles were removed may result from a lower proportion of between subject variance. Perhaps most important is that the SEM values for luteal phase estradiol and progesterone did not improve appreciably when the 8 subjects with inconsistent ovulatory cycles (thus inconsistent estradiol and progesterone levels) were removed. This further speaks to the inherent intra-individual variation in estradiol and progesterone levels, even in those with consistent ovulatory cycles.
In summary, sex hormone profiles are in large part reproducible over two consecutive months in young, recreationally active eumonorrheic women. Although intra-individual variations exist, they are substantially smaller than between subject variations. In order to reduce month to month intra-individual variations and improve measurement accuracy, it is recommended that multiple samples be taken over consecutive days as opposed to a single sample to represent a given phase. Further, the inherent stability of SHBG values within a female from one month to the next suggest that while total hormone concentrations may vary somewhat within a female from one cycle to the next, the proportion of these concentrations that are biologically active should change very little within a female from one cycle to the next. While these findings support the feasibility of retrospectively examining relationships between hormone profiles and injury risk, there are important limitations to the current work. Specifically, these findings are limited to healthy physically active females who, by nature of the parent project, were uninjured and reported normal menstrual cycles lasting 26–32 days for the past 6 months and maintained a consistent level of activity throughout the study. Before we can study hormone profiles retrospectively in an injured population, the impact of the trauma and acute changes in exercise status as a result of the injury on hormone profile reproducibility needs to be investigated. For example, one study observed a relationship between physical trauma and surgical stress with irregular cycles post injury[27], however these findings were not based on a young physically active population undergoing surgery for ligament trauma. In regards to exercise, previous research has shown that the intensity of physical activity may also influence menstrual cycle characteristics[28], and therefore a substantial modification in exercise due to injury may affect hormone profiles in some athletes.
These findings are also limited to the reproducibility of sex steroid hormone concentrations. As one report noted a greater than expected risk of ACL injury near ovulation[5], and suggested that it may be important to also account for the gonadatropins that control ovulation and sex steroid secretion (follicle stimulating hormone or luteinizing hormone) when examining injury susceptibility, studies examining the reproducibility of these gonadatropins should also be considered. Finally, it is well accepted that many competitive athletes experience irregular cycles, and we are not aware of studies that have examined hormone profile consistency in oligomenorrheic or amenorrheic female athletes. Hence, this study represents only a first step in understanding the feasibility of examining hormone profiles retrospectively. It is our hope that by first quantifying the natural intra-individual variations in hormone concentrations with these factors controlled, future work can better quantify any additional variability associated with these other factors. Specifically, there is a need to examine the immediate (e.g. subsequent month) and longer term (e.g. 3–6 months later) effects of musculoskeletal trauma and acute exercise changes on hormone reproducibility, to determine the best post injury time frame to obtain an accurate representation of a female’s typical hormone profile. Examining these effects in populations at greatest risk for ligament trauma (e.g. basketball and soccer) is also important.
Information Box.
What is already known on this topic
While there is a growing consensus that the risk of ACL injury varies across the menstrual cycle, the hormonal profile associated with a greater likelihood of injury remains unclear. Because ACL injuries occur infrequently, retrospective studies offer the most practical research design to examine hormone profiles associated with injury risk.
What this study adds
To initially examine the feasibility of retrospectively examining hormone profiles, month to month consistency in hormone concentrations were examined in young, recreationally active eumonorrheic women. Results indicate that sex hormone profiles are in large part reproducible over two consecutive months, particularly when multiple samples are taken over consecutive days.
Acknowledgments
The project described was supported by Grant Number R01- AR53172 NIH-NIAMS, and through a cooperative agreement [NICHD/NIH U54 HD28934] as part of the Specialized Cooperative Centers Program in Reproductive Research. We wish to thank Anh-Dung Nguyen PhD who assisted with collection and processing of the blood samples.
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive license (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd, and its Licensees to permit this article (if accepted) to be published in The British Journal of Sports Medicine and any other BMJPGL products and to exploit all subsidiary rights, as set out in our license.
COMPETING INTEREST
None to declare
References
- 1.Myklebust G, Maehlum S, Holm I, et al. A prospective cohort study of anterior cruciate ligament injuries in elite norwegian team handball. Scan J Med Sci Sports. 1998;8:149–153. doi: 10.1111/j.1600-0838.1998.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 2.Slauterbeck JR, Fuzie SF, Smith MP, et al. The menstrual cycle, sex hormones, and anterior cruciate ligament injury. J Athl Train. 2002;37:275–280. [PMC free article] [PubMed] [Google Scholar]
- 3.Blecher AM, Richmond JC. Transient laxity of an anterior cruciate ligament-reconstructed knee related to pregnancy. J Arthrosc Rel Surg. 1998;14:77–79. doi: 10.1016/s0749-8063(98)70125-2. [DOI] [PubMed] [Google Scholar]
- 4.Myklebust G, Engebretsen L, Braekken IH, et al. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sports Med. 2003;13:71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Wojtys EM, Huston L, Boynton MD, et al. The effect of menstrual cycle on anterior cruciate ligament in women as determined by hormone levels. Am J Sports Med. 2002;30:182–188. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]
- 6.Arendt EA, Bershadsky B, Agel J. Periodicity of noncontact anterior cruciate ligament injuries during the menstrual cycle. Journal of gender specific medicine. 2002;5:19–26. [PubMed] [Google Scholar]
- 7.Beynnon BD, Johnson RJ, Braun S, et al. The relationship between menstrual cycle phase and anterior cruciate ligament injury: A case-control study of recreational alpine skiers. Am J Sports Med. 2006;34:757–764. doi: 10.1177/0363546505282624. [DOI] [PubMed] [Google Scholar]
- 8.Shultz SJ, Sander TC, Kirk SE, et al. Relationship between sex hormones and anterior knee laxity across the menstrual cycle. Med Sci Sports Exerc. 2004;36:1165–1174. doi: 10.1249/01.MSS.0000132270.43579.1A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landgren BM, Unden AL, Deczfalusy E. Hormonal profile of the cycle in 68 normal menstruating women. Acta Endocrinol. 1980;94:89–98. doi: 10.1530/acta.0.0940089. [DOI] [PubMed] [Google Scholar]
- 10.Nestour EL, Marraoui J, Lahlou N, et al. Role of estradiol in the rise in follicle-stimulating hormone levels during the luteal-follicular transition. J Clin Endocrinol Metab. 1993;77:439–442. doi: 10.1210/jcem.77.2.8345049. [DOI] [PubMed] [Google Scholar]
- 11.Rossmanith WG, Schenkel B, Benz R. Role of androgens in the regulation of the human menstrual cycle. Gynecol Endocrinol. 1994;8:151–159. doi: 10.3109/09513599409072449. [DOI] [PubMed] [Google Scholar]
- 12.Smith KD, Rodriguez LJ, Tcholakian RK, et al. The relation between plasma testosterone levels and the lengths of phases of the menstrual cycle. Fert Steril. 1979;32:403–407. doi: 10.1016/s0015-0282(16)44295-0. [DOI] [PubMed] [Google Scholar]
- 13.Lenton EA, Lawrence GF, Coleman RA, et al. Individual variation in gonadotropin and steroid concentrations and in the lengths of the follicular and luteal phases in women with regular menstrual cycles. Clin Reprod Fert. 1983;2:143–150. [PubMed] [Google Scholar]
- 14.Henzyl MR. Norgestimate: From the laboratory to three clinical indications. J Reprod Med. 2001;46:647–661. [PubMed] [Google Scholar]
- 15.Miller KK, Rosner W, Lee H, et al. Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrin Metab. 2004;89:525–533. doi: 10.1210/jc.2003-030680. [DOI] [PubMed] [Google Scholar]
- 16.Hammond GL. Access of reproductive steroids to target tissues. Obstet Gynecol. 2002;29:411–423. doi: 10.1016/s0889-8545(02)00008-6. [DOI] [PubMed] [Google Scholar]
- 17.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psych Bulletin. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 18.Denegar CR, Ball DW. Assessing reliability and precision of measurement: An introduction to intraclass correlation and standard error of measurement. J Sport Rehabil. 1993;2:35–42. [Google Scholar]
- 19.Israel R, Mishell DR, Stone SC, et al. Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112:1043–1046. doi: 10.1016/0002-9378(72)90178-0. [DOI] [PubMed] [Google Scholar]
- 20.Shepard MK, Senturia YD. Comparison of serum progesterone and endometrial biopsy for confirmation of ovulation and evaluation of luteal function. Fert Steril. 1977;28:541–548. doi: 10.1016/s0015-0282(16)42554-9. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad N, Pollard TM, Unwin N. The optimal timing of blood collection during the menstrual cycle for the assessment of endogenous sex hormones: can interindividual differences in levels over the whole cycle be assessed in a single day? Cancer Epidemiol Biomarkers Prev. 2002;11:147–151. [PubMed] [Google Scholar]
- 22.Michaud DS, Manson JE, Spiegelman D, et al. Reproducibility of plasma and urinary sex hormone levels in premenopausal women over a one-year period. Cancer Epidemiol Biomarkers Prev. 1999;8:1059–1064. [PubMed] [Google Scholar]
- 23.Micheli A, Muti P, Pisani P, et al. Repeated serum and urinary androgen measurements in premenopausal and postmenopausal women. J Clin Epidemiol. 1991;44:1055–1061. doi: 10.1016/0895-4356(91)90007-v. [DOI] [PubMed] [Google Scholar]
- 24.Missmer SA, Spiegelman D, Bertone-Johnson ER, et al. Reproducibility of plasma steroid hormones, prolactin and insulin-like growth factor levels among premenopausal women over a 2- to 3-year period. Cancer Epidemiol Biomarkers & Prev. 2006;15:972–978. doi: 10.1158/1055-9965.EPI-05-0848. [DOI] [PubMed] [Google Scholar]
- 25.Muti P, Trevisan M, Micheli A, et al. Reliability of serum hormones in premenopausal and postmenopausal women over a one-year period. Cancer Epidemiol Biomarkers & Prev. 1996;5:917–922. [PubMed] [Google Scholar]
- 26.Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 2. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 27.To WWK, Wong MWN. The relationship of physical trauma and surgical stress to menstrual dysfunction. Aust N Z J Obstet Gynaecol. 2000;40:48–53. doi: 10.1111/j.1479-828x.2000.tb03166.x. [DOI] [PubMed] [Google Scholar]
- 28.Russell JB, Mitchell D, Musey PI, et al. The relationship of exercise to anovulatory cycles in female athletes: hormonal and physical characteristics. Obstet Gynecol. 1984;63:452–456. [PubMed] [Google Scholar]