Abstract
Endoscopic ultrasonography (EUS) is well suited for assessment of the pancreas due to its high resolution and the proximity of the transducer to the pancreas, avoiding air in the gut. Evaluation of chronic pancreatitis (CP) was an early target for EUS, initially just for diagnosis but later for therapeutic purposes. The diagnosis of CP is still accomplished using the standard scoring based on nine criteria, all considered to be of equal value. For diagnosis of any CP, at least three or four criteria must be fulfilled, but for diagnosis of severe CP at least six criteria are necessary. The Rosemont classification, more restrictive, aims to standardize the criteria and assigns different values to different features, but requires further validation. EUS-fine needle aspiration (EUS-FNA) is less advisable for diagnosis of diffuse CP due to its potential side effects. Elastography and contrast-enhanced EUS are orientation in differentiating a focal pancreatic mass in a parenchyma with features of CP, but they cannot replace EUS-FNA. The usefulness of EUS-guided celiac block for painful CP is still being debated with regard to the best technique and the indications. EUS-guided drainage of pseudocysts is preferred in non-bulging pseudocysts or in the presence of portal hypertension. EUS-guided drainage of the main pancreatic duct should be reserved for cases in which endoscopic retrograde cholangiopancreatography has failed owing to difficult cannulation of the papilla or difficult endotherapy. It should be performed only by highly skilled endoscopists, due to the high rate of complications.
Keywords: Endoscopic ultrasonography, Pancreatic neoplasms, Chronic pancreatitis, Contrast agents, Nerve block, Pancreatic pseudocyst, Drainage, Elastography, Main pancreatic duct
INTRODUCTION
Chronic pancreatitis (CP) is an irreversible and progressive inflammatory process featuring pathological modifications of fibrosis, inflammatory infiltration, and destruction of exocrine and endocrine tissue, resulting in characteristic morphological changes in the parenchyma and pancreatic ducts. These modifications vary in intensity and distribution (diffuse or patchy). This has several consequences: (1) Biopsy specimens are difficult to obtain and not always relevant, because they do not fully display the signs of CP; moreover, duct biopsy is usually avoided due to the risk of acute pancreatitis; (2) Most imaging methods reflects only partially the CP modifications, especially those typical for late stages of the disease; some methods, such as endoscopic retrograde cholangiopancreatography (ERCP) and magnetic retrograde cholangiopancreatography (MRCP) detect only the ductal features of CP; and (3) The findings of pancreatic function tests are not modified until a late stage in the natural history of the disease. Endoscopic ultrasonography (EUS) accomplishes the quality of being an imaging method able to detect both early and late changes in the parenchyma and pancreatic ducts.
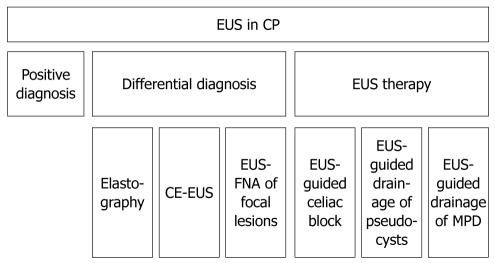
The pancreas is well assessed by EUS due to the method’s high resolution and the proximity of the transducer to the pancreas with the possibility of avoiding air in the gut. In patients with CP, EUS was performed initially for diagnosis, then for differential diagnosis, and later for therapeutic purposes (Figure 1).
Figure 1.
Flowchart of the endoscopic ultrasonography utility in chronic pancreatitis. EUS: Endoscopic ultrasonography; CP: Chronic pancreatitis; CE-EUS: Contrast enhanced endoscopic ultrasonography; MPD: Main pancreatic duct.
POSITIVE DIAGNOSIS
Despite its advantage of assessing the pancreas at very close range, EUS, being operator dependent, is still imperfect in establishing the diagnosis of chronic pancreatitis. The various pathological aspects of the disease are shown as different EUS features, and the same importance for diagnosis has been attributed to all of them. There have been several attempts to define the disease on ductal and parenchymal criteria, initially embracing 11 criteria[1,2], then focusing on nine factors corresponding to histopathological changes[3]: five parenchymal criteria (hyperechoic foci, hyperechoic strands, parenchymal lobularity, cysts, calcifications) and four ductal criteria (pancreatic duct dilatation, pancreatic duct irregularity, hyperechoic pancreatic duct walls, visible pancreatic side branches) (Figure 2). Very rarely are all these manifestations present simultaneously. Some of these features have been found also in elderly people[4], males (OR = 1.8, 95% CI: 1.3-2.55), persons with a history of alcohol consumption abuse (OR = 5.1, 95% CI: 3.1-8.5), smokers (OR = 1.7, 95% CI: 1.2-2.4), and those with history of acute pancreatitis[5-9]. Some features, like gland atrophy or lobularity aspect, can impede the complete assessment of all features (e.g. visualization of side branches of pancreatic ducts).
Figure 2.
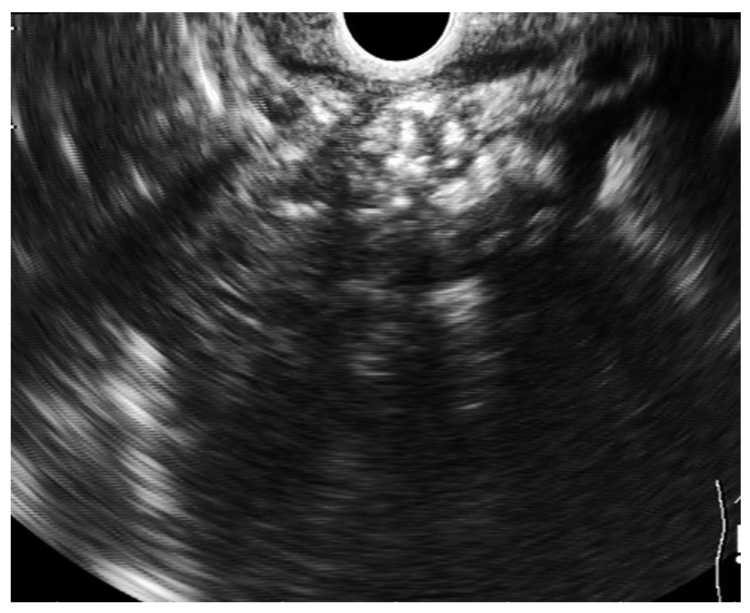
Chronic pancreatitis. Parenchymal and ductal pancreatic stones as hyperechoic structures with shadowing and stenosis of the main pancreatic duct.
The interobserver agreement in one study using these criteria was moderate (k = 0.45), with good agreement only for duct dilatation and lobularity; the main drawback of the study was the limited experience of some examiners with pancreatic EUS. The most important criterion for the diagnosis was considered by all experts to be pancreatic stones, followed by visible side branches and lobularity, and the least significant was main pancreatic duct (MPD) dilatation[9]. In an EUS study in which both digital linear and radial echo endoscopes were employed, the interobserver variability also moderate (k = 0.50 and 0.61 respectively); the best concordance between the two methods was found for detection of cysts, calcifications, and visible side branches[10].
Because histological evaluation of the pancreas is usually difficult, different gold standards have been used to establish the optimum number of EUS criteria for diagnosis of CP. The secretin direct pancreatic test has 85% sensitivity and 85% specificity for CP diagnosis, and the false-negative results are due to preserved pancreatic exocrine function[11]. Using one or two criteria for mild pancreatitis, three to five for moderate pancreatitis, and more than five for severe forms, the agreement with the secretin test as gold standard was 100% for normal parenchyma and severe disease, 50% for moderate forms, and 13% for mild disease[2]. On comparison of both EUS radial and linear assessment with the endoscopic secretin test during the same procedure, the best EUS accuracy was obtained for a cut-off point of more than four criteria (accuracy of 84% and 74%, respectively)[10]. The same group obtained lower sensitivity and specificity for diagnosis using four EUS criteria when cholecystokinin was used instead of secretin to test pancreatic function[12]. Comparison of assessment by non-blinded EUS (three to five criteria for diagnosis) and endoscopic retrograde cholangiopancreatography (ERCP; Cambridge classification) showed quite similar sensitivity (72% vs 68%) and specificity (76% vs 79%) for either mild or severe chronic pancreatitis, with the secretin endoscopic direct pancreatic test as the reference. However, the odds ratio for exocrine insufficiency was higher for EUS assessment than for ERCP[13]. To obtain the best specificity and the best negative predictive value for diagnosis, six criteria were needed, however, the sensitivity was only 26%[8,14]. Secretin-stimulated EUS detected the features of CP better than EUS without secretin (12/13 patients) and the sensitized EUS seemed to be able to predict a favorable outcome or success of endoscopic treatment[15] (Table 1).
Table 1.
Diagnostic value of endoscopic ultrasonography in chronic pancreatitis
| Author | No. of pts | No. of EUS criteria | Threshold for CP diagnosis | Comparison | Sn | Sp | PPV | NPV | Acc |
| Wiersema et al[4] | 69 | 11 | > 3 = dg | EUS vs ERCP | 100 | 79 | |||
| EUS vs ERCP + secretin test | 70 | 33 | |||||||
| EUS vs ERCP + history | 90 | 66 | |||||||
| Catalano et al[2] | 80 | 11 | 1-2 mild | EUS vs secretin test | 84 | 78 | |||
| 3-5 moderate | EUS vs ERCP | 86.1 | 95.4 | ||||||
| > 5 severe | EUS vs ERCP + secretin test | 84.2 | 97.6 | ||||||
| Sahai et al[8] | 126 | 9 | > 2 for any CP | EUS vs ERCP | > 85 | < 85 | |||
| < 3 = fibrosis | |||||||||
| > 6 = severe | > 85 | ||||||||
| Conwell et al[14] | 56 | 9 | 4-5 = equivocal | EUS vs ePFT | 36 | 94 | 93 | 41 | |
| > 6 = definite | 26 | 100 | 100 | 39 | |||||
| Stevens et al[13] | 83 | 9 | 3-5 = dg | EUS vs ERCP | 68 | 79 | 83 | 62 | |
| 6-9 = severe | |||||||||
| Stevens et al[10] | 100 | 9 | > 4 | Radial EUS vs ePFT | 68 | 95 | 84 | ||
| Linear EUS vs ePFT | 44 | 95 | 74 | ||||||
| Stevens et al[12] | 50 | 9 | > 4 | EUS vs secretin ePFT | 71 | 92 | |||
| EUS vs CCK ePFT | 63 | 85 | |||||||
| Zimmermann et al[23] | 21 | 9 | > 4 | EUS vs histology (surgery) | 78 | 73 | |||
| Varadarajulu et al[24] | 21 | 9 | > 4 | EUS vs histology1 (surgery) | 90 | 85.7 | 88.1 | ||
| Chong et al[25] | 71 | 9 | > 3 = dg | EUS vs histology1 (surgery) | 83.3 | 80 | |||
| > 4 = severe fibrosis | |||||||||
| Bhutani et al[22] | 11 | 9 | > 3 | EUS vs histology (autopsy) |
Non-calcific chronic pancreatitis. ePFT: Endoscopic pancreatic function test; EUS: Endoscopic ultrasonography; ERCP: Endoscopic retrograde cholangiopancreatography; Sn: Sensitivity; Sp: Specificity; Acc: Accuracy; CCK: Cholecystokinin; PPV: Positive predictive value; NPV: Negative predictive value.
Using ERCP as gold standard, more than two criteria or three criteria, respectively, were found to be optimal for diagnosis[4,8]. The EUS sensitivity for diagnosis varied between 68% and 100% and the specificity was 78%-97% when ERCP was considered the gold standard (Table 1). The overall agreement with ERCP was k = 0.51, but the concordance for mild forms on EUS was only 83%. The factors most predictive for abnormal ERCP were ductal stones and parenchymal calcifications[4]. Among patients with a normal pancreatogram, 84.2% were found to have parenchymal changes of CP (accentuation of lobular pattern, focal areas of reduced echogenicity, hyperechoic foci) or increased ductal wall echogenicity. During follow-up (median 18 mo), 68% of patients with initially normal findings on ERCP progressed to an abnormal pancreatogram, supporting the importance of EUS description for early CP. However, this evolution was not confirmed in a second study of alcoholic chronic cirrhosis and CP[16,17]. Evaluation of images can be improved by computer-assisted image analysis[18].
The patient’s history may be suggestive of CP. More than five features of CP were seen in 49.9% of 156 patients with persistent or non-specific dyspepsia[19]. Another study showed that there were more criteria for CP in the group with pain and steatorrhea than in the group with pain but no steatorrhea, so they concluded that history can be helpful in diagnosing CP[20].
Pathologic diagnosis, the ideal gold standard, is rarely obtained from surgical specimens, EUS fine needle aspiration (EUS-FNA) or Tru-Cut core biopsies. The correspondence of EUS criteria to pathologic changes is shown in Table 2[21,22]. One recent paper showed that in postmortem pancreatic specimens the presence of more than three EUS standard criteria of CP correlated with the histologic diagnosis, but these features were also present in elderly persons dying of diseases other than CP[22] and in 59% of asymptomatic alcohol abusers[5].
Table 2.
Correspondence between standard endoscopic ultrasonography criteria and pathologic features in chronic pancreatitis (adapted from Sahai AV 2002[21])
| Standard EUS criteria | Pathologic features |
| Parenchymal criteria | |
| Hyperechoic foci | Small calcifications |
| Hyperechoic strands | Fibrosis |
| Lobularity | Edema or fibrosis |
| Cysts | Pseudocysts |
| Calcifications | Calcifications |
| Ductal criteria | |
| MPD dilatation | MPD dilatation |
| MPD irregularity | MPD irregular |
| Hyperechoic MPD walls | Ductal fibrosis or edema |
| Visible side branches | Dilated secondary branches |
EUS: Endoscopic ultrasonography; MPD: Main pancreatic duct.
Comparing the EUS standard criteria with the histologic findings from specimens obtained during surgery, fulfillment of five or more criteria was associated with sensitivity of 60% and specificity of 83%, compared with 87% and 64% respectively when three criteria were used[23]. Good correlation with histology obtained during surgery of non-calcific CP was also found for the presence of four pancreatic features and for EUS findings of foci, stranding, lobulation, or ductal modifications. A limitation of this study was its use of surgical specimens secondary to neoplastic pancreatic disease[24]. Using surgical specimens obtained after preoperative EUS, three criteria were shown to differentiate abnormal from normal pancreatic tissue, but four criteria represented the limit for identification of severe fibrosis[25]. Again, the use of four EUS criteria compared with the association of ERCP, surgical pathology, and/or long-term clinical follow-up showed that EUS was more sensitive than MRCP but equally specific, and when both tests were abnormal the specificity was 100%[26]. Therefore, three or four criteria seems to suffice to rule out CP, but to establish the diagnosis at least six criteria are necessary[27].
The diagnosis of autoimmune pancreatitis is based on the same criteria, but for early stages (corresponding to Cambridge grade 0 to 2) the characteristic criteria are lobularity and hyperechoic pancreatic duct walls[28]. One study found diffuse hypoechoic areas, diffuse enlargement of the parenchyma, focal hypoechoic areas, and bile duct wall thickening as supplementary features characterizing autoimmune pancreatitis; these manifestations resolved after steroid treatment and were helpful in differentiation from ductal adenocarcinomas[29]. EUS-FNA is able to show a stromal structure with high lymphoid cellularity[30]. Lymphoplasmocytic sclerosing pancreatitis can be more accurately detected in tissue samples obtained by Tru-Cut biopsy[31]. With regard to the assessment of severity, preliminary data have pointed to significant diagnostic EUS features: hyperechoic foci for mild CP; hyperechoic foci, visible side branches, and duct dilatation for moderate CP; and visible side branches, duct dilatation, duct irregularity, and calcifications for severe CP[32].
Because the different pathological characteristics of CP vary in importance, the nine-criteria scheme assigning each criterion the same importance is insufficiently reliable and its diagnostic accuracy doubtful. The Rosemont classification, elaborated by international consensus, uses parenchymal and ductal criteria divided into major and minor features (Table 3). On this basis the findings are classified as “consistent with CP”, “suggestive of CP”, “indeterminate for CP”, or “normal” (Table 4)[33]. This system, quite complicated and more restrictive in diagnosing CP, proved to agree with the diagnostic classification of the nine-criteria scheme in 74% of cases, increasing to 84% when “suggestive of CP” was included[34,35]. Using this system, the findings were similar for radial and linear EUS, with good results for parenchymal criteria (cysts 100%, hyperechoic foci 98%, lobularity/dilated ducts 94%) and modest results for dilated side branch, irregular pancreatic duct and hyperechoic wall of MPD[36]. In a recent multicenter study, 14 experts evaluated 50 recorded videos using the standard nine EUS criteria (diagnostic: > 4 criteria) and the Rosemont criteria (diagnostic: suggestive of CP or consistent with CP). They obtained substantial interobserver agreement for the Rosemont classification (k = 0.65) and moderate agreement for the standard classification (k = 0.54); the difference was not significant. The best agreement was noted for calcifications (standard scoring), pancreatic duct calcifications (Rosemont classification) and pancreatic duct dilation (both systems). The least agreement was seen for lobularity without honeycomb (Rosemont classification). This study used computed tomography (CT) and endoscopic pancreatic function test (ePFT) as gold standard, without histology. The patients were correctly classified as “definite CP” in 91.2% of cases (standard scoring) and 83.5% (Rosemont scoring); as “mild CP” in 50% (standard scoring) and 42.9% (Rosemont scoring); and “no CP” in 83.3% and 95.2% of cases respectively[37]. Further validation of the Rosemont classifications is needed.
Table 3.
Rosemont consensus definitions
| Rank | Features | Definition | Location | |
| Parenchymal features | ||||
| 1 | Major A | Hyperechoic foci with shadowing | Echogenic structures ≥ 2 mm in length and width that shadow | Body and tail only |
| 2 | Major B | Lobularity with honeycombing | Well-circumscribed, ≥ 5 mm structures with enhancing rims and relatively echo-poor centers, with ≥ 3 lobules | Body and tail only |
| Minor | Lobularity with honeycombing | Well-circumscribed, ≥ 5 mm structures with enhancing rims and relatively echo-poor centers, with non-contiguous lobules | Body and tail only | |
| 3 | Minor | Hyperechoic foci without shadowing | Echogenic structures ≥ 2 mm in length and width with no shadowing | Body and tail only |
| 4 | Minor | Cysts | Anechoic, rounded/elliptical structures with or without septations | Head, body and tail only |
| 5 | Minor | Stranding | Hyperechoic lines ≥ 3 mm in length in at least two different directions with respect to the imaged plane | Body and tail only |
| Ductal features | ||||
| 1 | Major A | MPD calculi | Echogenic structures within the MPD with acoustic shadowing | Head, body and tail only |
| 2 | Minor | Irregularity of MPD contour | Uneven or irregular outline and ectatic course | Body and tail only |
| 3 | Minor | Dilated side branches | 3 or more tubular anechoic structures each measuring ≥ 1 mm in width, budding from MPD | Body and tail only |
| 4 | Minor | MPD dilation | ≥ 3.5 mm in body or > 1.5 mm in tail | Body and tail only |
| 5 | Minor | Hyperechoic duct margin | Echogenic, distinct structure greater than 50%of the entire MPD | Body and tail only |
MPD: Main pancreatic duct.
Table 4.
Rosemont diagnostic stratification
| Stratum | Criteria |
| Consistent with CP | 1 major feature A + ≥ 3 minor features |
| 1 major feature A + major feature B | |
| 2 major feature | |
| Suggestive of CP | 1 major feature A + < 3 minor features |
| 1 major feature B + ≥ 3 minor features | |
| ≥ 5 minor features (any) | |
| Indeterminate for CP | 3 or 4 minor features major feature B alone or with < 3 minor features |
| Normal | ≤ 2 minor features1 |
Excludes cysts, dilated main pancreatic duct, hyperechoic non-shadowing foci, dilated side branch. CP: Chronic pancreatitis.
Using EUS-FNA for diffuse CP, the negative predictive value increased to 100% against 75% for EUS, the specificity increased to 67% vs 60%, with higher concordance for severe disease than for mild CP[38]. Tru-Cut biopsy should not be recommended for non-focal CP because of complications[39], but its utility has been proved in autoimmune pancreatitis[31,40].
DIFFERENTIAL DIAGNOSIS
If focal hypoechoic lesion are found in the pancreatic parenchyma, the differential diagnosis includes primary or secondary pancreatic tumor, focal CP, and autoimmune pancreatitis. Several methods have been developed for this purpose.
Elastography
Elastography evaluates tissue strain resulting from compression and that strain is smaller in harder tissue than in softer tissue. Different tissue elasticity patterns are marked supplementary on the grey-color scale with different colors (blue for hard tissue and red for soft tissue). EUS elastography in CP shows a honeycomb aspect with predominantly hard strands, corresponding to fulfillment of four standard diagnostic criteria. The sensitivity, specificity, and accuracy were found to be 66%, 57% and 60%, respectively, and the method was considered useful in cases of equivocal EUS (three criteria or fewer)[41,42]. Further studies overcame the limitations of qualitative image analysis by means of digital image quantification, which helps to differentiate benign (normal pancreas and chronic pseudotumoral pancreatitis) from malignant lesions (pancreatic cancer and neuroendocrine tumors) with higher sensitivity, specificity, and accuracy (91.4%, 87.9% and 89.7%, respectively)[43]. Using a scoring system based on different color patterns in the images, the differentiation between benign and malignant pancreatic masses had sensitivity of 92.3% and specificity of 80%[44]. However, another study concluded that elastography did not allow complete delineation of the border of lesions greater than 35 mm in diameter or of lesions situated at some distance from the transducer, yielding poor sensitivity (41%), specificity (53%), and accuracy (45%) for predicting the nature of pancreatic focal lesions[45]. Because elastographic images are still difficult to obtain and interpret, although interobserver agreement is good (k = 0.725)[44], further improvement of the equipment with the possibility of quantification is expected. EUS elastography could have a special role in autoimmune pancreatitis, where the whole pancreas shows a typical, unique homogeneous stiffness, distinct from the circumscribed mass lesion in ductal adenocarcinoma[46].
Contrast-enhanced EUS
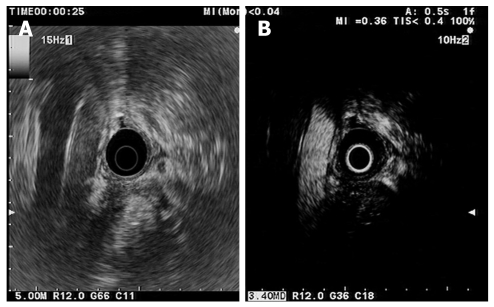
Ultrasound contrast agents increases the signal from the blood and improves the detectability of small vessels flow during ultrasound examinations. Before and after injection of Sonovue® (Bracco), the focal pancreatitis shows no detectable vascularization or the vessels appear regular over a distance of at least 20 mm, with detection of both arterial and venous vessels in the contrast-enhanced phase[47] (Figure 3). Based on the perfusion characteristics of microvessels, contrast-enhanced US facilitates differential diagnosis between inflammatory lesions and ductal adenocarcinoma. The specificity of the discrimination between benign and malignant focal pancreatic lesions was found to be 93.3% using power Doppler contrast-enhanced EUS (CE-EUS) compared with 83.3% for conventional EUS[47]. The hypovascular aspect of lesions under power Doppler CE-EUS seemed highly sensitive and specific (91.1% and 93.3%, respectively) for adenocarcinoma[48]. During power Doppler CE-EUS examinations the ultrasound frequency returned to the transducer is the same with that transmitted, but the method is associated with artifacts resulting from turbulent flow (blooming and overpainting). The use of contrast agents is preferred using harmonic frequencies which result from non-linear and non-symmetrical oscillation of the microbubbles. This yields an image with complete “subtraction” of the tissue-derived signal, optimized by using a low mechanical index, which allows continuous real-time assessment of the microvascularization during contrast medium uptake. Harmonic CE-EUS shows an iso vascular homogeneous pattern of CP[49] or, in severe forms, a hypovascular pattern, due to extensive fibrosis[50] (Figure 4A). Our results confirmed that severe CP may be hypovascular on harmonic CE-EUS, and quantitative assessment of images can improve differentiation between adenocarcinomas and chronic pancreatitis (accuracy of 86%) (unpublished data), but, similar to elastography, cannot replace the use of EUS-FNA.
Figure 3.
Mass resembling chronic pancreatitis. A: Conventional endoscopic ultrasonography (EUS). Hypoechoic inhomogeneous mass in the pancreatic head. Aorta and inferior caval vein are also seen; B: Contrast-enhanced harmonic-EUS. During the arterial phase (25 s after contrast injection) the abdominal aorta becomes hyperechoic and the mass is hypovascular compared with surrounding parenchyma.
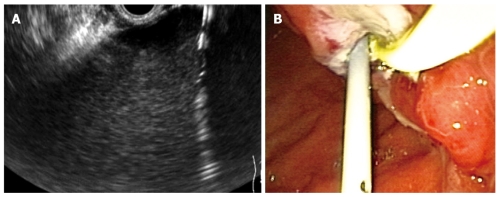
Figure 4.
Endoscopic ultrasonography-guided pseudocyst drainage. A: The cystostomy is seen as a hyperechoic parallel structure inside the hypoechoic well-delineated pseudocyst; B: Endoscopic view of a stent and a nasocystic drainage placed transgastric into a pseudocyst.
EUS-FNA of focal lesions
The EUS sensitivity for detection of suspected pancreatic mass in a parenchyma with CP modifications was 100%, but the positive predictive value of pancreatic malignancy in these situations was only 60%, because some malignant masses present internal or peripheral calcifications, similar to focal CP[51]. The sensitivity of EUS-FNA for malignancy in parenchymal masses with features of CP is only 54%-74%, compared with 89% when the surrounding parenchyma is normal[51-55]. However, in the event of high suspicion of malignancy with negative EUS-FNA, repeated FNA yields a positive diagnosis in 84% of cases, whereas half of the failures of first biopsies are attributed to the presence of CP[56]. Kras mutation and allele deletion of the microsatellite or of the tumor suppressors can be reliably detected in EUS-FNA samples from pancreatic masses, improving the diagnostic accuracy[57,58]. The search for codon-12 Kras mutation revealed no cases in patients with pseudotumor CP, in contrast to the adenocarcinoma group, although 6%-12% of patients with diffuse CP and PanIN lesions had presented Kras mutations in a previous meta-analysis[59,60].
EUS THERAPY
EUS-guided celiac block
One of the therapeutic uses of EUS in CP is celiac plexus blockade, i.e. temporary inhibition of the celiac plexus using a combination of local anesthesia and steroids, with the aim of reducing pain and improving the quality of life[61]. This guidance is preferred to CT-guided blockade because the details of the region are better appreciated and the side effects are fewer and less severe[62]. Frequently the celiac ganglia can be seen as a unique or concatenate hypoechoic structure, less well delineated, with some whitish strands inside[63].
Some issues regarding EUS-guided celiac block remain to be resolved. The indication is pain in CP, but some studies included pain accompanying moderate pancreatitis[64] or patients with pain that had not responded to other forms of treatment[65]. Another unclarified issue is the technique of injection (central or bilateral) and the quantity of steroid needed. The majority of studies used the bilateral injection technique, considered equal in safety to central injection, but the results of the two techniques concerning the alleviation of pain were close and contradictory[64,66], showing the need for a placebo-controlled trial[67]. Direct injection of triamcinolone within the celiac ganglia (13 patients) compared with alcohol injection (5 patients) yielded disappointing results in respect of pain alleviation for steroid use (38% vs 80%)[68]. A comparative study of results between the celiac region injection and celiac ganglia injection for EUS-guided celiac block is still lacking.
The question of cost-effectiveness remains unresolved. Some studies followed up the patients for only 1-4 wk[66,68]. The only study with an extended follow-up period showed duration of pain relief of up to 673 d. This raises the question of whether the natural course of the disease may have been responsible, because there were no data indicative of the level of severity of CP: duration of disease from onset of pain, presence of diabetes, or calcifications[64].
In many studies, the alleviation of pain varied from 55% to 70% with a short duration of follow-up[64-66,69]. Persistence of pain alleviation for as long as 24 wk was seen in no patients[65] or in only 10% of patients[69]. Two meta-analyses showed efficacy in managing chronic abdominal pain in 51.46%[70] and 59.45%[71] of patients respectively. The rate of major complications seemed very low (0.6%), being represented by retroperitoneal abscess[72].
EUS-guided drainage of pseudocysts
Therapeutic intervention in patients with chronic pancreatic pseudocysts is indicated when at least one complication is present (compression of large vessels, obstruction of duodenum, stomach, or common bile duct, infection, hemorrhage into pancreatic pseudocyst, pancreatico-pleural fistula) or when symptoms occur (satiety, pain, nausea or vomiting, upper gastrointestinal bleeding)[73,74]. Since 1996, several series of EUS-guided drainage have been reported, especially for collections without bulging onto the gut wall or with parietal vessels due to portal hypertension[75-77]. The main limitation is location of the fluid collection further than 1 to 1.5 cm from the gut wall[78-80] (Figure 4).
This method is preferred to surgical drainage, which is associated with a high rate of mortality and morbidity[81]. However, a non-randomized case-control study showed the same rates of treatment success, complications, and reinterventions for surgical and EUS-guided drainage, but with lower costs and shorter hospital stay for the EUS-guided procedure[82].
Conventional endoscopic drainage and EUS-guided drainage were compared in four papers. In a prospective non-randomized study the two approaches seemed equally safe and effective[83], but this was not confirmed in a second non-randomized study, where EUS represented a salvage method in the case of failure of conventional endoscopic drainage owing to non-bulging pseudocysts or location in the tail of the organ, but was a more time-consuming procedure[84]. The conclusion of this second study was that EUS should be reserved for pseudocysts located in the tail of the pancreas, because these are unlikely to cause luminal compression or are technically difficult to access. Also, EUS assessment would identify a tumor in 5% of pseudocysts[84]. A third randomized clinical trial showed a significantly better success rate for EUS- than for conventional endoscopic-guided drainage (100% vs 33%), despite the small number of patients, even after statistical adjustment for luminal compression[85]. A fourth randomized study confirmed also a significant advantage for EUS over conventional endoscopic drainage (94% vs 72%); both were considered first-line methods for treatment of bulging pseudocysts, but the authors recommended that EUS-guided drainage should be preferred for non-bulging pseudocysts[86].
Several aspects of EUS-guided drainage remain to be elucidated. First among these is the issue of the means used to create the communication between gut and pseudocyst. There are two major techniques for obtaining this communication: (1) balloon dilatation of a previous puncture site, with a 93%-100% success rate[83,84,87-89]; and (2) coagulation of the communication site by means of a cystostomy (success rate of 95% when two procedures per patient were performed[90] and 71%-82% with one procedure per patient[91,92]), a Giovannini needle (success rate of 94%[93,94], but only 84% after the first attempt[86]), or a needle-knife, with the same success rate as balloon dilatation but a higher perforation rate[88,89,95,96]. Larger comparative studies will be necessary to assess the best device with the highest success rate and the lowest complication rate. The prototype “transluminal balloon accessotome”, which combines a needle-knife and a dilating balloon, will probably allow easier drainage in one single step, reducing the exchange of accessories and simplifying the procedure[97]. Moreover, the use of the prototype three-layer puncture kit, which allows the simultaneous insertion of two guidewires at the initial puncture in one step, or the use of a larger working channel in the echo-endoscope, would allow safer and faster drainage[98]. Furthermore, the use of a forward-viewing echoendoscope seems promising for drainage of pseudocysts, even those inaccessible with a conventional therapeutic side-viewing EUS endoscope[99].
A further issue to be resolved is that of the morphological or biological factors that predict therapeutic success. Knowledge of such factors would facilitate selection of patients suitable for direct surgery. Moreover, to avoid pseudocyst relapse, described in 4%-17% of cases after 6-9 mo follow-up[94,96,100], communication with a secondary pancreatic duct, should be assessed very carefully.
EUS-guided drainage of main pancreatic duct
EUS-guided drainage of the MPD is a second-line procedure indicated when ERCP is unsuccessful owing to inability to cannulate the MPD (severe inflammation, previous surgery, postsurgical stricture) or difficult endotherapy (tight stenosis, large stone, MPD rupture, pancreas divisum). In practice, there are only few cases in which ERCP cannot be successfully performed by an experienced endoscopist, and recent studies suggests the superiority of surgery in managing pain. Thus, only a very small number of patients, namely those in whom ERCP fails and surgery cannot be performed safely, are good candidates for this procedure[101]. Using the transluminal approach or the transpapillary rendezvous approach, EUS-guided drainage of the MPD remains technically challenging because of difficulty in orienting the endoscope along the axis of the duct, difficult dilatation of the transmural tract due to pancreatic fibrosis, or the acute angle of the needle in relation to the MPD. Despite success rates of 68%-71%, the complication rates were important in all four series published (5%-43%); the complications included perforations, bleeding, pancreatitis, fever, and postprocedural pain[102-105]. EUS-guided drainage of the MPD should continue to be confined to tertiary care centers and very experienced endoscopists.
CONCLUSION
The diagnosis of CP is still accomplished using the standard scoring based on nine criteria each considered as having the same value. For diagnosis of any CP, at least three or four of these criteria must be present, but for diagnosis of severe CP more than six criteria must be fulfilled. The more restrictive Rosemont classification aims to standardize the criteria and assigns different values to different features, but requires further validation. EUS-FNA is less advisable for diagnosis of diffuse CP due to the possible side effects. Elastography and contrast-enhanced EUS are orientation in differentiating focal pancreatic mass, but they cannot replace EUS-FNA. The utility of EUS-guided celiac block for painful CP is still a matter of debate with regard to best technique and the indications. EUS-guided drainage of pseudocysts is preferred especially in non-bulging pseudocysts or presence of portal hypertension. EUS-guided drainage of the MPD should be reserved for cases of unsuccessful ERCP caused by difficult cannulation of the papilla or difficult endotherapy. It should be performed only by highly skilled endoscopists, due to the high risk of complications.
Footnotes
Supported by A National Grant from the Education Ministry PANGEN PNII 42110/2008
Peer reviewers: Henning Gerke, MD, Associate Clinical Professor, Medical Director, Diagnostic and Therapeutic Unit, Digestive Disease Center, Endoscopic Ultrasound, Division of Gastroenterology-Hepatology, University of Iowa Hospitals and Clinics, 200 Hawkins Drive, Iowa City, IA 52246, United States; Dr. Rupjyoti Talukdar, Department of Gastroenterology and Hepatology, Nemcare Hospital and Research Center, Guwahati 781024, India
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
References
- 1.Wiersema MJ, Wiersema LM. Endosonography of the pancreas: normal variation versus changes of early chronic pancreatitis. Gastrointest Endosc Clin N Am. 1995;5:487–496. [PubMed] [Google Scholar]
- 2.Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48:11–17. doi: 10.1016/s0016-5107(98)70122-1. [DOI] [PubMed] [Google Scholar]
- 3.The International Working Group for Minimum Standard Terminology for Gastrointestinal Endosonography. Reproduction of minimal standard terminology in gastrointestinal endosonography. Dig Endosc. 1998;10:158–188. [Google Scholar]
- 4.Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25:555–564. doi: 10.1055/s-2007-1010405. [DOI] [PubMed] [Google Scholar]
- 5.Bhutani MS. Endoscopic ultrasonography: changes of chronic pancreatitis in asymptomatic and symptomatic alcoholic patients. J Ultrasound Med. 1999;18:455–462. doi: 10.7863/jum.1999.18.7.455. [DOI] [PubMed] [Google Scholar]
- 6.Yusoff IF, Sahai AV. A prospective, quantitative assessment of the effect of ethanol and other variables on the endosonographic appearance of the pancreas. Clin Gastroenterol Hepatol. 2004;2:405–409. doi: 10.1016/s1542-3565(04)00126-0. [DOI] [PubMed] [Google Scholar]
- 7.Rajan E, Clain JE, Levy MJ, Norton ID, Wang KK, Wiersema MJ, Vazquez-Sequeiros E, Nelson BJ, Jondal ML, Kendall RK, et al. Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointest Endosc. 2005;61:401–406. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 8.Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, Hawes RH, Hoffman BJ. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48:18–25. doi: 10.1016/s0016-5107(98)70123-3. [DOI] [PubMed] [Google Scholar]
- 9.Wallace MB, Hawes RH, Durkalski V, Chak A, Mallery S, Catalano MF, Wiersema MJ, Bhutani MS, Ciaccia D, Kochman ML, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc. 2001;53:294–299. doi: 10.1016/s0016-5107(01)70401-4. [DOI] [PubMed] [Google Scholar]
- 10.Stevens T, Zuccaro G Jr, Dumot JA, Vargo JJ, Parsi MA, Lopez R, Kirchner HL, Purich E, Conwell DL. Prospective comparison of radial and linear endoscopic ultrasound for diagnosis of chronic pancreatitis. Endoscopy. 2009;41:836–841. doi: 10.1055/s-0029-1215061. [DOI] [PubMed] [Google Scholar]
- 11.Heij HA, Obertop H, van Blankenstein M, ten Kate FW, Westbroek DL. Relationship between functional and histological changes in chronic pancreatitis. Dig Dis Sci. 1986;31:1009–1013. doi: 10.1007/BF01300251. [DOI] [PubMed] [Google Scholar]
- 12.Stevens T, Dumot JA, Zuccaro G Jr, Vargo JJ, Parsi MA, Lopez R, Kirchner HL, Purich E, Conwell DL. Evaluation of duct-cell and acinar-cell function and endosonographic abnormalities in patients with suspected chronic pancreatitis. Clin Gastroenterol Hepatol. 2009;7:114–119. doi: 10.1016/j.cgh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Stevens T, Conwell DL, Zuccaro G Jr, Vargo JJ, Dumot JA, Lopez R. Comparison of endoscopic ultrasound and endoscopic retrograde pancreatography for the prediction of pancreatic exocrine insufficiency. Dig Dis Sci. 2008;53:1146–1151. doi: 10.1007/s10620-007-9975-1. [DOI] [PubMed] [Google Scholar]
- 14.Conwell DL, Zuccaro G, Purich E, Fein S, Vargo JJ, Dumot JA, VanLente F, Lopez R, Trolli P. Comparison of endoscopic ultrasound chronic pancreatitis criteria to the endoscopic secretin-stimulated pancreatic function test. Dig Dis Sci. 2007;52:1206–1210. doi: 10.1007/s10620-006-9469-6. [DOI] [PubMed] [Google Scholar]
- 15.Catalano MF, Lahoti S, Alcocer E, Geenen JE, Hogan WJ. Dynamic imaging of the pancreas using real-time endoscopic ultrasonography with secretin stimulation. Gastrointest Endosc. 1998;48:580–587. doi: 10.1016/s0016-5107(98)70039-2. [DOI] [PubMed] [Google Scholar]
- 16.Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc. 2002;55:507–511. doi: 10.1067/mge.2002.122610. [DOI] [PubMed] [Google Scholar]
- 17.Hastier P, Buckley MJ, Francois E, Peten EP, Dumas R, Caroli-Bosc FX, Delmont JP. A prospective study of pancreatic disease in patients with alcoholic cirrhosis: comparative diagnostic value of ERCP and EUS and long-term significance of isolated parenchymal abnormalities. Gastrointest Endosc. 1999;49:705–709. doi: 10.1016/s0016-5107(99)70286-5. [DOI] [PubMed] [Google Scholar]
- 18.Miyakawa H, Suga T, Okamura K. Usefulness of endoscopic ultrasonography for the diagnosis of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:85–89. doi: 10.1007/s00535-006-1935-6. [DOI] [PubMed] [Google Scholar]
- 19.Sahai AV, Mishra G, Penman ID, Williams D, Wallace MB, Hadzijahic N, Pearson A, Vanvelse A, Hoffman BJ, Hawes RH. EUS to detect evidence of pancreatic disease in patients with persistent or nonspecific dyspepsia. Gastrointest Endosc. 2000;52:153–159. doi: 10.1067/mge.2000.107910. [DOI] [PubMed] [Google Scholar]
- 20.Gardner TB, Janec EM, Gordon SR. Relationship between patient symptoms and endosonographic findings in chronic pancreatitis. Pancreatology. 2009;9:398–403. doi: 10.1159/000181178. [DOI] [PubMed] [Google Scholar]
- 21.Sahai AV. EUS and chronic pancreatitis. Gastrointest Endosc. 2002;56:S76–S81. doi: 10.1016/s0016-5107(02)70091-6. [DOI] [PubMed] [Google Scholar]
- 22.Bhutani MS, Arantes VN, Verma D, Moezzi J, Suryaprasad S, Kapadia AS, Gopalswamy N. Histopathologic correlation of endoscopic ultrasound findings of chronic pancreatitis in human autopsies. Pancreas. 2009;38:820–824. doi: 10.1097/MPA.0b013e3181b2bc1a. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann MJ, Mishra G, Lewin DN, Hawes RH, Coyle W, Adams DA, Hoffman B. Comparison of EUS findings with histopathology in chronic pancreatitis. Gastrointest Endosc. 1997;45:AB185. [Google Scholar]
- 24.Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointest Endosc. 2007;66:501–509. doi: 10.1016/j.gie.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of EUS for chronic pancreatitis: a comparison with histopathology. Gastrointest Endosc. 2007;65:808–814. doi: 10.1016/j.gie.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Pungpapong S, Wallace MB, Woodward TA, Noh KW, Raimondo M. Accuracy of endoscopic ultrasonography and magnetic resonance cholangiopancreatography for the diagnosis of chronic pancreatitis: a prospective comparison study. J Clin Gastroenterol. 2007;41:88–93. doi: 10.1097/MCG.0b013e31802dfde6. [DOI] [PubMed] [Google Scholar]
- 27.Wallace MB. Chronic pancreatitis. Gastrointest Endosc. 2009;69:S117–S120. doi: 10.1016/j.gie.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Kubota K, Kato S, Akiyama T, Fujita K, Yoneda M, Takahashi H, Ogawa M, Inamori M, Abe Y, Kirikoshi H, et al. A proposal for differentiation between early- and advanced-stage autoimmune pancreatitis by endoscopic ultrasonography. Dig Endosc. 2009;21:162–169. doi: 10.1111/j.1443-1661.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 29.Hoki N, Mizuno N, Sawaki A, Tajika M, Takayama R, Shimizu Y, Bhatia V, Yamao K. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44:154–159. doi: 10.1007/s00535-008-2294-2. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande V, Mino-Kenudson M, Brugge WR, Pitman MB, Fernandez-del Castillo C, Warshaw AL, Lauwers GY. Endoscopic ultrasound guided fine needle aspiration biopsy of autoimmune pancreatitis: diagnostic criteria and pitfalls. Am J Surg Pathol. 2005;29:1464–1471. doi: 10.1097/01.pas.0000173656.49557.48. [DOI] [PubMed] [Google Scholar]
- 31.Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H, et al. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742–750. doi: 10.1007/s00535-009-0062-6. [DOI] [PubMed] [Google Scholar]
- 32.Irisawa A, Katakura K, Ohira H, Sato A, Bhutani MS, Hernandez LV, Koizumi M. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:90–94. doi: 10.1007/s00535-006-1916-9. [DOI] [PubMed] [Google Scholar]
- 33.Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez LV, Sahai A, Brugge WR, Wiersema MJ, Catalano MF. Standardized weighted criteria for EUS features of chronic pancreatitis: the Rosemont classification. Gastrointest Endosc. 2008;67:AB96–AB97. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 35.Catalano MF, Hernandez LV, Kaul V, Guda NM, Pezanoski JE, Ramasamy D, Samavedy R, Geenen JE. EUS diagnosis of chronic pancreatitis: comparison of the current criteria vs the new” Rosemont criteria” developed by an international consensus conference. Gastrointest Endosc. 2008;67:AB215. [Google Scholar]
- 36.Catalano MF, Kaul V, Hernandez LV, Pezanoski JP, Guda NM, Ramasamy D, Samavedy R, Geenen JE. Diagnosis of chronic pancreatitis (CP) by endoscopic ultrasound (EUS) - radial vs. linear endosonography (EUS) Gastrointest Endosc. 2008;67:AB208. [Google Scholar]
- 37.Stevens T, Lopez R, Adler DG, Al-Haddad MA, Conway J, Dewitt JM, Forsmark CE, Kahaleh M, Lee LS, Levy MJ, et al. Multicenter comparison of the interobserver agreement of standard EUS scoring and Rosemont classification scoring for diagnosis of chronic pancreatitis. Gastrointest Endosc. 2010;71:519–526. doi: 10.1016/j.gie.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 38.Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33:824–831. doi: 10.1055/s-2001-17337. [DOI] [PubMed] [Google Scholar]
- 39.DeWitt J, McGreevy K, LeBlanc J, McHenry L, Cummings O, Sherman S. EUS-guided Trucut biopsy of suspected nonfocal chronic pancreatitis. Gastrointest Endosc. 2005;62:76–84. doi: 10.1016/s0016-5107(05)00504-3. [DOI] [PubMed] [Google Scholar]
- 40.Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, Pearson RK, Rajan E, Topazian MD, Yusuf TE, et al. EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointest Endosc. 2005;61:467–472. doi: 10.1016/s0016-5107(04)02802-0. [DOI] [PubMed] [Google Scholar]
- 41.Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–978. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 42.Micames CG, Gress FG. EUS elastography: a step ahead? Gastrointest Endosc. 2007;65:979–981. doi: 10.1016/j.gie.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 43.Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–1094. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 44.Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587–1593. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirche TO, Ignee A, Barreiros AP, Schreiber-Dietrich D, Jungblut S, Ott M, Hirche H, Dietrich CF. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–917. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 46.Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718–720. doi: 10.1055/s-0029-1214866. [DOI] [PubMed] [Google Scholar]
- 47.Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246–250. doi: 10.3748/wjg.v12.i2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–597.e1. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 49.Kitano M, Sakamoto H, Matsui U, Ito Y, Maekawa K, von Schrenck T, Kudo M. A novel perfusion imaging technique of the pancreas: contrast-enhanced harmonic EUS (with video) Gastrointest Endosc. 2008;67:141–150. doi: 10.1016/j.gie.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 50.Seicean A, Badea R, Stan-Iuga R, Gulei I, Pop T, Pascu O. The added value of real-time harmonics contrast-enhanced endoscopic ultrasonography for the characterisation of pancreatic diseases in routine practice. J Gastrointestin Liver Dis. 2010;19:99–104. [PubMed] [Google Scholar]
- 51.Fritscher-Ravens A, Brand L, Knöfel WT, Bobrowski C, Topalidis T, Thonke F, de Werth A, Soehendra N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–2775. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 52.Barthet M, Portal I, Boujaoude J, Bernard JP, Sahel J. Endoscopic ultrasonographic diagnosis of pancreatic cancer complicating chronic pancreatitis. Endoscopy. 1996;28:487–491. doi: 10.1055/s-2007-1005528. [DOI] [PubMed] [Google Scholar]
- 53.Ardengh JC, Lopes CV, Campos AD, Pereira de Lima LF, Venco F, Módena JL. Endoscopic ultrasound and fine needle aspiration in chronic pancreatitis: differential diagnosis between pseudotumoral masses and pancreatic cancer. JOP. 2007;8:413–421. [PubMed] [Google Scholar]
- 54.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736; quiz 751, 753. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 55.Krishna NB, Mehra M, Reddy AV, Agarwal B. EUS/EUS-FNA for suspected pancreatic cancer: influence of chronic pancreatitis and clinical presentation with or without obstructive jaundice on performance characteristics. Gastrointest Endosc. 2009;70:70–79. doi: 10.1016/j.gie.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 56.Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567–570. doi: 10.1111/j.1440-1746.2007.05119.x. [DOI] [PubMed] [Google Scholar]
- 57.Khalid A, Nodit L, Zahid M, Bauer K, Brody D, Finkelstein SD, McGrath KM. Endoscopic ultrasound fine needle aspirate DNA analysis to differentiate malignant and benign pancreatic masses. Am J Gastroenterol. 2006;101:2493–2500. doi: 10.1111/j.1572-0241.2006.00740.x. [DOI] [PubMed] [Google Scholar]
- 58.Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M, Strnad R, Traboulsi E, Minarik M. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714–3720. doi: 10.3748/wjg.v13.i27.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bournet B, Souque A, Senesse P, Assenat E, Barthet M, Lesavre N, Aubert A, O’Toole D, Hammel P, Levy P, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy coupled with KRAS mutation assay to distinguish pancreatic cancer from pseudotumoral chronic pancreatitis. Endoscopy. 2009;41:552–557. doi: 10.1055/s-0029-1214717. [DOI] [PubMed] [Google Scholar]
- 60.Löhr M, Klöppel G, Maisonneuve P, Lowenfels AB, Lüttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Michaels AJ, Draganov PV. Endoscopic ultrasonography guided celiac plexus neurolysis and celiac plexus block in the management of pain due to pancreatic cancer and chronic pancreatitis. World J Gastroenterol. 2007;13:3575–3580. doi: 10.3748/wjg.v13.i26.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gress F, Schmitt C, Sherman S, Ikenberry S, Lehman G. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus block for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900–905. doi: 10.1111/j.1572-0241.1999.01042.x. [DOI] [PubMed] [Google Scholar]
- 63.Levy M, Rajan E, Keeney G, Fletcher JG, Topazian M. Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol. 2006;101:1787–1791. doi: 10.1111/j.1572-0241.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 64.LeBlanc JK, DeWitt J, Johnson C, Okumu W, McGreevy K, Symms M, McHenry L, Sherman S, Imperiale T. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain. Gastrointest Endosc. 2009;69:835–842. doi: 10.1016/j.gie.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 65.Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, Ramchandani M, Guda NM. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:979–984. doi: 10.1111/j.1365-2036.2009.03963.x. [DOI] [PubMed] [Google Scholar]
- 66.Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–329. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]
- 67.Sahai AV, Wyse J. EUS-guided celiac plexus block for chronic pancreatitis: a placebo-controlled trial should be the first priority. Gastrointest Endosc. 2010;71:430–431; author reply 431. doi: 10.1016/j.gie.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Levy MJ, Topazian MD, Wiersema MJ, Clain JE, Rajan E, Wang KK, de la Mora JG, Gleeson FC, Pearson RK, Pelaez MC, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct Ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98–103. doi: 10.1111/j.1572-0241.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 69.Gress F, Schmitt C, Sherman S, Ciaccia D, Ikenberry S, Lehman G. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: a prospective single center experience. Am J Gastroenterol. 2001;96:409–416. doi: 10.1111/j.1572-0241.2001.03551.x. [DOI] [PubMed] [Google Scholar]
- 70.Kaufman M, Singh G, Das S, Concha-Parra R, Erber J, Micames C, Gress F. Efficacy of endoscopic ultrasound-guided celiac plexus block and celiac plexus neurolysis for managing abdominal pain associated with chronic pancreatitis and pancreatic cancer. J Clin Gastroenterol. 2010;44:127–134. doi: 10.1097/MCG.0b013e3181bb854d. [DOI] [PubMed] [Google Scholar]
- 71.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 72.O’Toole TM, Schmulewitz N. Complication rates of EUS-guided celiac plexus blockade and neurolysis: results of a large case series. Endoscopy. 2009;41:593–597. doi: 10.1055/s-0029-1214868. [DOI] [PubMed] [Google Scholar]
- 73.Raj M, Chen RY. Interventional applications of endoscopic ultrasound. J Gastroenterol Hepatol. 2006;21:348–357. doi: 10.1111/j.1440-1746.2006.04214.x. [DOI] [PubMed] [Google Scholar]
- 74.Aghdassi A, Mayerle J, Kraft M, Sielenkämper AW, Heidecke CD, Lerch MM. Diagnosis and treatment of pancreatic pseudocysts in chronic pancreatitis. Pancreas. 2008;36:105–112. doi: 10.1097/MPA.0b013e31815a8887. [DOI] [PubMed] [Google Scholar]
- 75.Bhattacharya D, Ammori BJ. Minimally invasive approaches to the management of pancreatic pseudocysts: review of the literature. Surg Laparosc Endosc Percutan Tech. 2003;13:141–148. doi: 10.1097/00129689-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 76.Vosoghi M, Sial S, Garrett B, Feng J, Lee T, Stabile BE, Eysselein VE. EUS-guided pancreatic pseudocyst drainage: review and experience at Harbor-UCLA Medical Center. MedGenMed. 2002;4:2. [PubMed] [Google Scholar]
- 77.Barthet M, Bugallo M, Moreira LS, Bastid C, Sastre B, Sahel J. Management of cysts and pseudocysts complicating chronic pancreatitis. A retrospective study of 143 patients. Gastroenterol Clin Biol. 1993;17:270–276. [PubMed] [Google Scholar]
- 78.Smits ME, Rauws EA, Tytgat GN, Huibregtse K. The efficacy of endoscopic treatment of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:202–207. doi: 10.1016/s0016-5107(95)70092-7. [DOI] [PubMed] [Google Scholar]
- 79.Binmoeller KF, Seifert H, Walter A, Soehendra N. Transpapillary and transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 1995;42:219–224. doi: 10.1016/s0016-5107(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 80.Giovannini M, Bernardini D, Seitz JF. Cystogastrotomy entirely performed under endosonography guidance for pancreatic pseudocyst: results in six patients. Gastrointest Endosc. 1998;48:200–203. doi: 10.1016/s0016-5107(98)70165-8. [DOI] [PubMed] [Google Scholar]
- 81.Seewald S, Ang TL, Kida M, Teng KY, Soehendra N. EUS 2008 Working Group document: evaluation of EUS-guided drainage of pancreatic-fluid collections (with video) Gastrointest Endosc. 2009;69:S13–S21. doi: 10.1016/j.gie.2008.10.061. [DOI] [PubMed] [Google Scholar]
- 82.Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649–655. doi: 10.1016/j.gie.2008.02.057. [DOI] [PubMed] [Google Scholar]
- 83.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 84.Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, Canon CL. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107–1119. doi: 10.1016/j.gie.2007.03.1027. [DOI] [PubMed] [Google Scholar]
- 85.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 86.Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842–848. doi: 10.1055/s-0029-1215133. [DOI] [PubMed] [Google Scholar]
- 87.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 88.Barthet M, Lamblin G, Gasmi M, Vitton V, Desjeux A, Grimaud JC. Clinical usefulness of a treatment algorithm for pancreatic pseudocysts. Gastrointest Endosc. 2008;67:245–252. doi: 10.1016/j.gie.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 89.Will U, Wegener C, Graf KI, Wanzar I, Manger T, Meyer F. Differential treatment and early outcome in the interventional endoscopic management of pancreatic pseudocysts in 27 patients. World J Gastroenterol. 2006;12:4175–4178. doi: 10.3748/wjg.v12.i26.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 91.Ahlawat SK, Charabaty-Pishvaian A, Jackson PG, Haddad NG. Single-step EUS-guided pancreatic pseudocyst drainage using a large channel linear array echoendoscope and cystotome: results in 11 patients. JOP. 2006;7:616–624. [PubMed] [Google Scholar]
- 92.McKay C, Denley S, Carter R. One-step, EUS-guided drainage of pancreatic pseudocysts. Experience in 52 patients. Gastrointest Endosc. 2008;67:AB226. [Google Scholar]
- 93.Krüger M, Schneider AS, Manns MP, Meier PN. Endoscopic management of pancreatic pseudocysts or abscesses after an EUS-guided 1-step procedure for initial access. Gastrointest Endosc. 2006;63:409–416. doi: 10.1016/j.gie.2005.11.047. [DOI] [PubMed] [Google Scholar]
- 94.Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524–529. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 95.Azar RR, Oh YS, Janec EM, Early DS, Jonnalagadda SS, Edmundowicz SA. Wire-guided pancreatic pseudocyst drainage by using a modified needle knife and therapeutic echoendoscope. Gastrointest Endosc. 2006;63:688–692. doi: 10.1016/j.gie.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 96.Antillon MR, Shah RJ, Stiegmann G, Chen YK. Single-step EUS-guided transmural drainage of simple and complicated pancreatic pseudocysts. Gastrointest Endosc. 2006;63:797–803. doi: 10.1016/j.gie.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 97.Reddy DN, Gupta R, Lakhtakia S, Jalal PK, Rao GV. Use of a novel transluminal balloon accessotome in transmural drainage of pancreatic pseudocyst (with video) Gastrointest Endosc. 2008;68:362–365. doi: 10.1016/j.gie.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 98.Seewald S, Thonke F, Ang TL, Omar S, Seitz U, Groth S, Zhong Y, Yekebas E, Izbicki J, Soehendra N. One-step, simultaneous double-wire technique facilitates pancreatic pseudocyst and abscess drainage (with videos) Gastrointest Endosc. 2006;64:805–808. doi: 10.1016/j.gie.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 99.Voermans RP, Eisendrath P, Bruno MJ, Le Moine O, Devière J, Fockens P. Initial evaluation of a novel prototype forward-viewing US endoscope in transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2007;66:1013–1017. doi: 10.1016/j.gie.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 100.Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video) Gastrointest Endosc. 2008;68:656–666. doi: 10.1016/j.gie.2008.03.1091. [DOI] [PubMed] [Google Scholar]
- 101.Ginès A, Varadarajulu S, Napoleon B. EUS 2008 Working Group document: evaluation of EUS-guided pancreatic-duct drainage (with video) Gastrointest Endosc. 2009;69:S43–S48. doi: 10.1016/j.gie.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 102.Tessier G, Bories E, Arvanitakis M, Hittelet A, Pesenti C, Le Moine O, Giovannini M, Devière J. EUS-guided pancreatogastrostomy and pancreatobulbostomy for the treatment of pain in patients with pancreatic ductal dilatation inaccessible for transpapillary endoscopic therapy. Gastrointest Endosc. 2007;65:233–241. doi: 10.1016/j.gie.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 103.Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224–230. doi: 10.1016/j.gie.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 104.Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, Meyer F. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007;14:377–382. doi: 10.1007/s00534-006-1139-8. [DOI] [PubMed] [Google Scholar]
- 105.Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100–107. doi: 10.1016/s0016-5107(03)02300-9. [DOI] [PubMed] [Google Scholar]