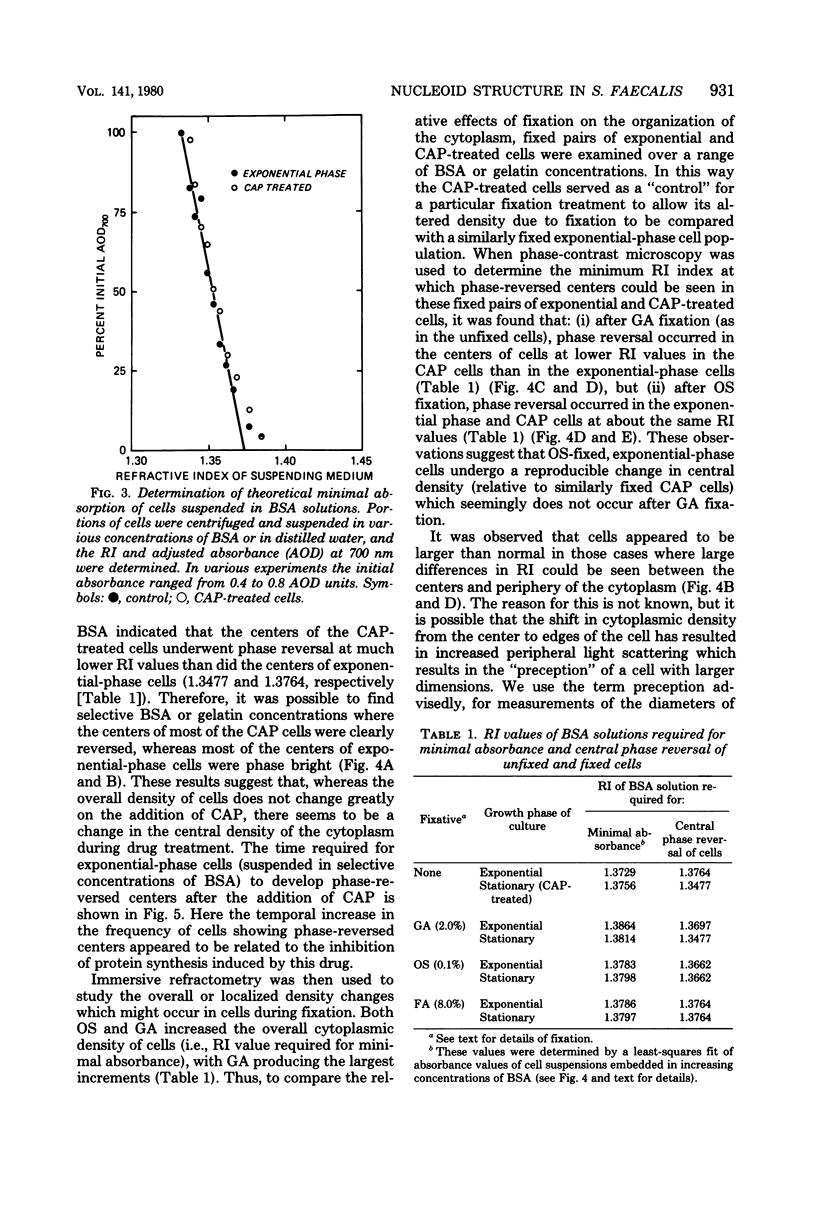
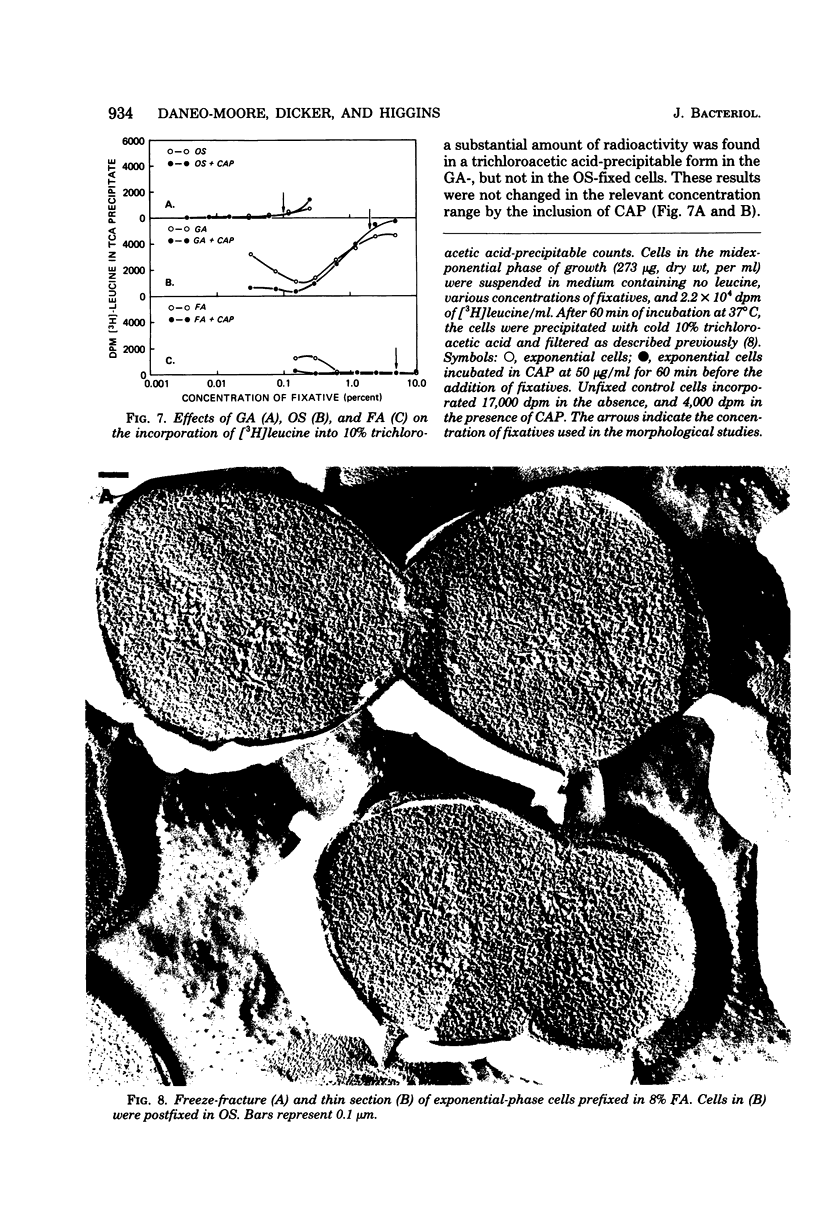
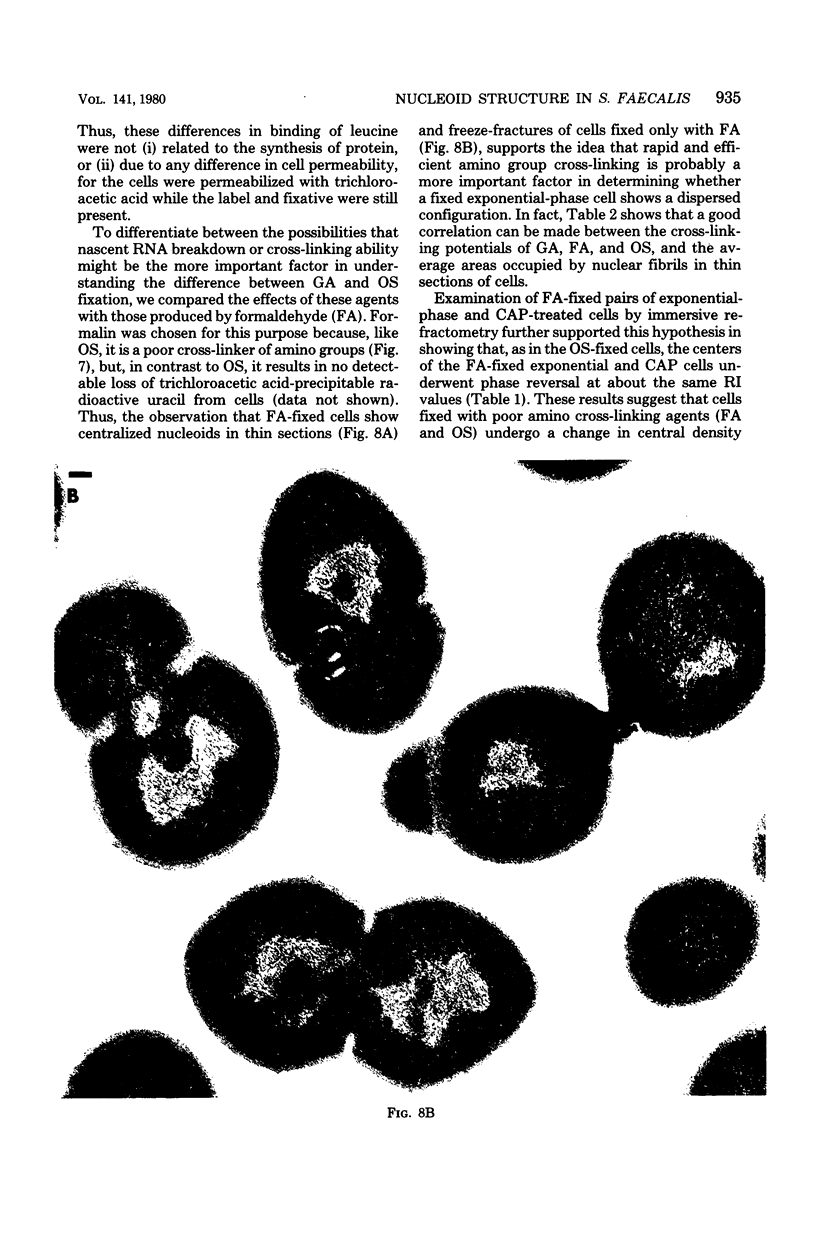
Abstract
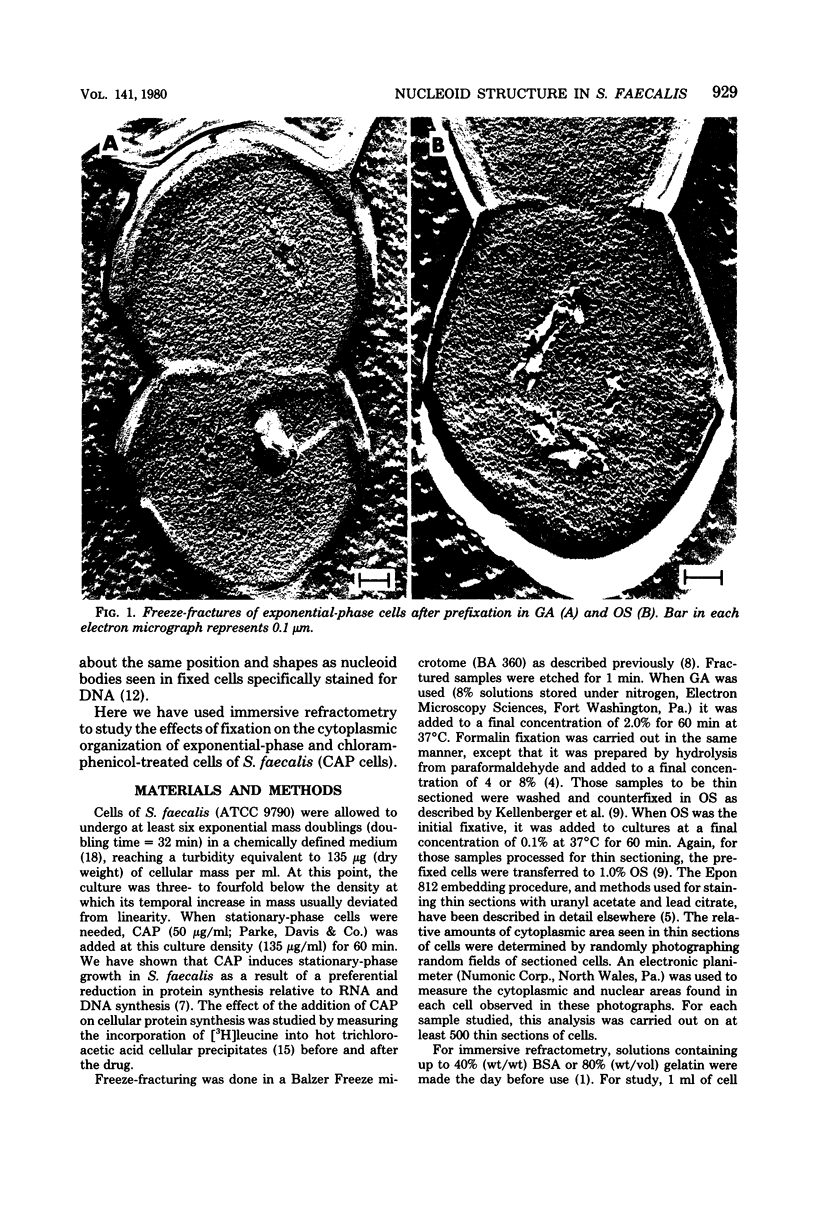
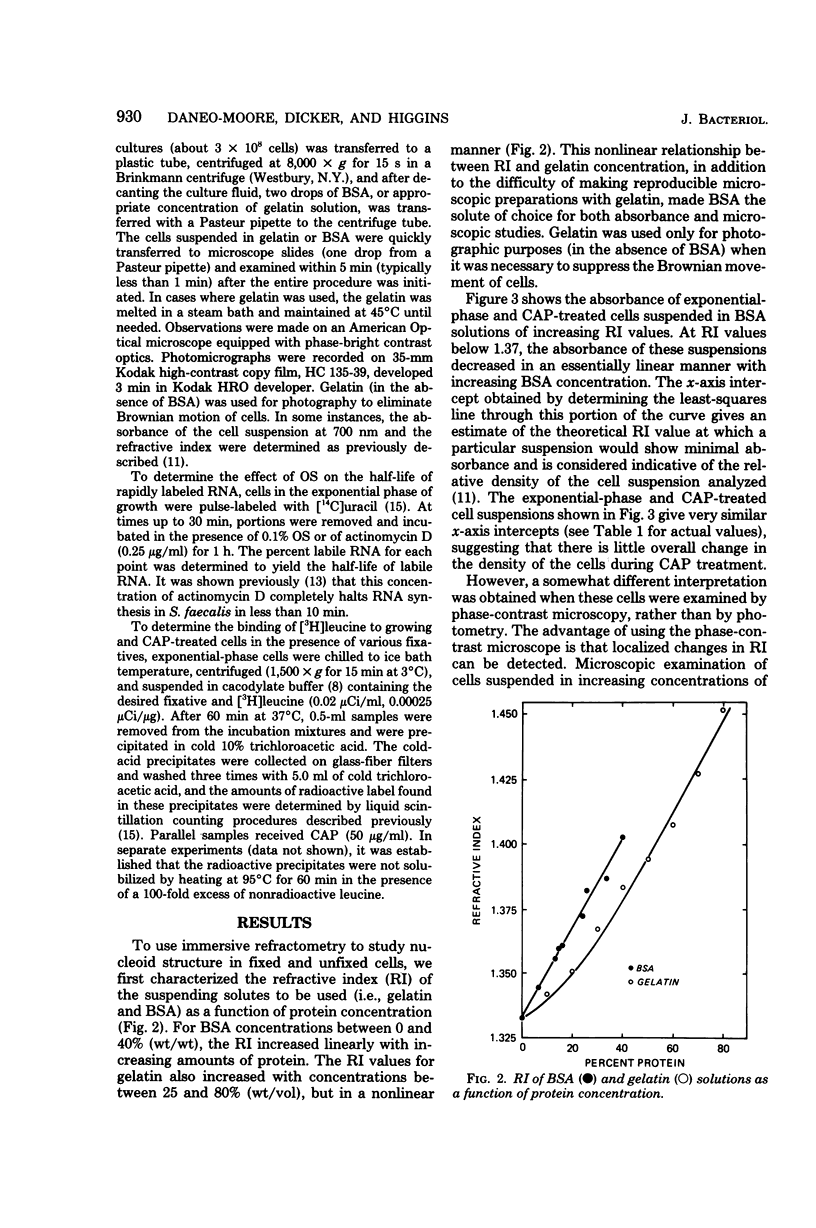
The structure of the nucleoid of Streptococcus faecalis (ATCC 9790) was examined and compared in the unfixed and fixed states by immersive refractometry and electron microscopy. It appears from these studies that the nucleoid structure is much more centralized in unfixed chloramphenicol-treated (stationary-phase) cells than it is in cells in the exponential phase of growth. The more dispersed configuration of the exponential-phase nucleoid could be preserved by fixation in glutaraldehyde, but not in Formalin or in osmium tetroxide. One important factor in explaining these differences in preservation is that glutaraldehyde (but not Formalin or osmium tetroxide) can rapidly cross-link the amino groups of macromolecules in cells. It was also observed that osmium tetroxide resulted in a preferential breakdown of nascent ribonucleic acid. These results are interpreted as indicating that glutaraldehyde is able to stabilize the exponential-phase nucleoid before it assumes the more central appearance seen in osmium tetroxide- and Formalin-fixed cells. These results are discussed in terms of the proposed organization of the exponential-phase nucleoid in unfixed cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daneo-Moore L., Higgins M. L. Morphokinetic reaction of Streptococcus faecalis (ATCC 9790) cells to the specific inhibition of macromolecular synthesis: nucleoid condensation on the inhibition of protein synthesis. J Bacteriol. 1972 Mar;109(3):1210–1220. doi: 10.1128/jb.109.3.1210-1220.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L. Factors influencing the frequency of mesosomes observed in fixed and unfixed cells of Streptococcus faecalis. J Cell Biol. 1974 May;61(2):288–300. doi: 10.1083/jcb.61.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L. Morphokinetic reaction of cells of Streptococcus faecalis (ATCC 9790) to specific inhibition of macromolecular synthesis: dependence of mesosome growth on deoxyribonucleic acid synthesis. J Bacteriol. 1972 Mar;109(3):1221–1231. doi: 10.1128/jb.109.3.1221-1231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Tsien H. C., Daneo-Moore L. Organization of mesosomes in fixed and unfixed cells. J Bacteriol. 1976 Sep;127(3):1519–1523. doi: 10.1128/jb.127.3.1519-1523.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON D. J., POWELSON D. M. Nuclear division as observed in live bacteria by a new technique. J Bacteriol. 1956 Apr;71(4):474–479. doi: 10.1128/jb.71.4.474-479.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., Morgan C., Rosenkranz H. S., Rose H. M. Effect of hydroxyurea on virus development. I. Electron microscopic study of the effect on the development of bacteriophage T4. J Bacteriol. 1966 Feb;91(2):823–833. doi: 10.1128/jb.91.2.823-833.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E. Immersion refractometry of isolated bacterial cell walls. J Bacteriol. 1973 Dec;116(3):1273–1279. doi: 10.1128/jb.116.3.1273-1279.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller O. L., Jr, Hamkalo B. A., Thomas C. A., Jr Visualization of bacterial genes in action. Science. 1970 Jul 24;169(3943):392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- Pettijohn D. E. Prokaryotic DNA in nucleoid structure. CRC Crit Rev Biochem. 1976 Nov;4(2):175–202. doi: 10.3109/10409237609105458. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Daneo-Moore L. Incorporation of radioactive macromolecular precursors into intact cells and osmotically stabilized "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Dec;108(3):980–985. doi: 10.1128/jb.108.3.980-985.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Chang A. Localization of transcribing genes in the bacterial cell by means of high resolution autoradiography. J Mol Biol. 1975 Nov 15;98(4):797–810. doi: 10.1016/s0022-2836(75)80011-8. [DOI] [PubMed] [Google Scholar]
- Séchaud J., Kellenberger E. Electron microscopy of DNA-containing plasms. IV. Glutaraldehyde-uranyl acetate fixation of virus-infected bacteria for thin sectioning. J Ultrastruct Res. 1972 Jun;39(5):598–607. doi: 10.1016/s0022-5320(72)90124-4. [DOI] [PubMed] [Google Scholar]
- WHITFIELD J. F., MURRAY R. G. The effects of the ionic environment on the chromatin structures of bacteria. Can J Microbiol. 1956 May;2(3):245–260. doi: 10.1139/m56-029. [DOI] [PubMed] [Google Scholar]
- Woldringh C. L., Nanninga N. Organization of the nucleoplasm in Escherichia coli visualized by phase-contrast light microscopy, freeze fracturing, and thin sectioning. J Bacteriol. 1976 Sep;127(3):1455–1464. doi: 10.1128/jb.127.3.1455-1464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]