Abstract
AIM: To investigate the prevalence of hepatitis C virus (HCV) genotypes and their association with possible transmission routes in the general population of Lahore, as the data exclusively related to this city is limited.
METHODS: Complete data regarding patient’s history, possible route of infection and biochemical tests was collected from the public hospital for 1364 patients. SPSS version 16 windows software was used for data analysis by univariate and multivariate techniques.
RESULTS: Age range ≤ 40 years showed high prevalence of HCV infection. HCV genotype 3a was dominant (55.9%), followed by 1a (23.6%), 4a (12.5%), 3b (3.2%), untypable (2.5%), 4b (1.2%) and mixed type (1.2%). Blood transfusion, dental surgery and barber shops were the main risk factors for HCV transmission. Genotype prevalence was independent of age (P = 0.971) and gender (P = 0.122) while risk factors showed a significant association with age (P = 0.000) and genotypes (P = 0.000). We observed an independent association of risk factors and genotype 3a, while patients with genotype 1 and 4 were mostly infected due to dental surgery blood transfusion and barber shops. Risk factors of intravenous drug use and sexual exposure were exclusively found in ≤ 40 years age group.
CONCLUSION: An increase in genotypes 1a and 4a suggest migration of people, possibly from Balochistan and the northern war-zone area. Government should focus on public education regarding infection routes.
Keywords: Hepatitis C virus, Prevalence, Genotypes, Risk factors, Lahore
INTRODUCTION
Hepatitis C virus (HCV) is a major cause of liver associated diseases all over the world. An estimated 3% of the world’s populations are chronically infected by HCV which is the main cause of liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC) in a substantial number of patients[1,2]. It is an enveloped virus with a single-stranded, positive sense non-segmented RNA genome of approximately 9.6 kb that encodes a poly-protein of approximately 3000 amino acids[3]. To date, at least six major genotypes of HCV, each having multiple subtypes, have been identified worldwide[4]. The different genotypes are relevant to epidemiology, vaccine development, and clinical management of chronic HCV infection[5].
Genotype distribution has been identified in three different patterns[6]. One pattern is genetic diversity, in geographically different areas like West Africa with 1 and 2[7], Central Africa with type 4[8], and Asia with type 3 and 6[9]. The second distribution pattern involves subtypes in specific risk groups e.g. intravenous drug use (IVDU), where the subtype 3a is more common[10]. The third pattern of genotype distribution is the circulation of a single subtype in particular areas such as in Egypt with 4a and South Africa with subtype 5a[11]. Recently, a shift in genotype distribution, mostly comprising an increase of the prevalence of the genotypes 3a, 1a and 4, and decrease of prevalence of other genotypes has been seen in many countries like Serbia, Germany, France and Greece[12-15].
Almost 10 million people in Pakistan are living with HCV. The most prevalent genotype in Pakistan is 3a followed by 3b and 1a[16]. Few studies are available on the distribution of various HCV genotypes in individual cities of Pakistan[17,18]. Unfortunately, there is no national data collection system for evaluation of infection routes and their correlation with genotypes or patients’ demographic data[19]. Lahore is the second largest metropolitan city of Pakistan with more than 7 million population[20]. Data exclusively related to HCV genotype-specific prevalence and route of infection in this city is limited. Although, Ijaz et al[18] showed a high prevalence of HCV 3a genotype (n = 80, 51.61%) followed by 3b (n = 43, 27.74%), 1b (n = 21, 13.54%), untypable (n = 5, 3.22%), mix 3a1b (n = 4, 2.58%), 3a1a (n = 1, 0.64%) and 3b1a (n = 1, 0.64%) in the population of Lahore city, their study utilized a small population size (n = 155) without any association to the mode of transmission[18].
The aim of the present study was to determine the frequency distribution of HCV genotypes, various risk factors prevalence with genotypes and age for its transmission in Lahore.
MATERIALS AND METHODS
Patients
Patients in this study were from Jinnah Hospital, Lahore, which is the only public facility that has HCV patient’s testing service and is the 2nd largest hospital in the area. Therefore, patients visiting at this hospital can be regarded as representative of general population of the Lahore city. The data and samples were collected from March 2007 to September 2009 from the Department of Pathology, Jinnah Hospital, Lahore, Pakistan and data analysis was performed in collaboration with National Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan. In total 1364 adult patients (18-75 years) who were HCV RNA-positive based on HCV antibody (anti-HCV)-positive results were included in this study. A written informed consent was obtained from patients. A complete history with possible route and estimated time of infection, standard biochemical liver function tests and patient’s contact information were collected. This study was approved by the Institutional ethics committee.
Viral investigations
HCV detection and genotyping was performed at the Department of Pathology, Jinnah Hospital, Lahore, Pakistan. RNA was extracted from 140 μL of serum samples using QIAamp viral RNA extraction kit (Qiagen, USA) according to the manufacturer’s protocol. cDNA was synthesized using Moloney murine leukemia virus (MmLV) followed by polymerase chain reaction (PCR) using primers derived from the 5’UTR non-coding region of HCV genome described by Chan et al[21]. For HCV RNA quantification, Qiagen HCV RG RT-PCR assay was used. Quantification was carried out with 10 μL of the extracted RNA on Rotor-gene Real-Time PCR machine (USA) using fluorescent probes to detect amplification after each replicating cycle as described by manufacturer protocol. The lower limit of detection for this assay is 1000 IU/mL HCV and genotyping was carried out using Invader HCV genotyping assay (Third wave technology, USA). Briefly, 100 ng of the HCV RNA was reverse transcribed to cDNA using 200 units of MmLV (Invitrogen, USA). From the amplified product, 2 μL were taken and the genotyping assay was performed for 12 different HCV types.
Statistical analysis
Data was analyzed using a statistical package SPSS version 16 for windows. The data is presented as mean and standard deviations, and categorical variables in absolute numbers and percentages. Student t-test and chi-square tests were applied to evaluate differences in proportions. A P value < 0.05 was considered significant. A multivariate analysis was used to identify variables associated within different genotypes. Bonferroni, Gabriel and LSD tests were performed to evaluate whether significant variables in the univariate analysis could predict differences among genotypes.
RESULTS
Age and gender specific prevalence of HCV
Of 1364 patients, 656 were male while 708 were female, with a median age of 36.8 ± 10.3 years (range 18-75 years). The age of infected patients was taken as a categorical as well as a continuous variable. Patients were divided in two age groups i.e. ≤ 40 years and > 40 years. Distribution of patients’ gender in age groups is given in Table 1. It is clear from Table 1 that people of age group ≤ 40 years (n = 931, 68.3%) were more affected with HCV in comparison to those in the older age group (> 40 years, n = 433, 31.7%).
Table 1.
Patient data according to age and gender
| Parameters |
Age groups |
|
| ≤ 40 yr | > 40 yr | |
| (n = 931, 68.3%) | (n = 433, 31.7%) | |
| Gender | ||
| Male (n = 656) | 468 (50.2%) | 188 (43.3%) |
| Female (n = 708) | 463 (49.8%) | 245 (56.7%) |
| Age (yr) | ||
| mean ± SD (36.8 ± 10.3) | 37.77 ± 10.65 | 46.8 ± 12.38 |
| Age range (18-75) | 18-40 | 41-75 |
Genotype distribution
Based on weighted analysis of patients infected with HCV genotypes 1, 3, 4, mix (4a5a) and untypable genotypes, the most commonly detected genotype in the study was genotype 3 (n = 806, 59.1%), with predominant subtype 3a (n = 763, 55.9%) and 3b (n = 43, 3.2%). Genotype 1 (n = 322, 23.6%,) was exclusively consisted of the subtype 1a, while genotype 4 (n = 186, 13.7%) comprised of the subtypes a (n = 170, 12.5%) and b (n = 16, 1.2%). Mixed inter-genotype 4a5a (n = 16, 1.2%) and untypable (n = 34, 2.5%) genotypes were also detected in 50 (3.66%) patients. The frequency distribution of genotypes revealed that patients of the age group ≤ 40 years were more affected with genotype 1a (71%) and 4a (68%) as compared to patients with the age group > 40 years. However, univariate analysis (Table 2) revealed that prevalence of genotype subtypes within age groups (P = 0.971) and gender (P = 0.122) of the patients were not statistically significant. Figure 1 illustrated the genotype distribution pattern in each gender of patients while Table 3 showed the prevalence of different genotype subtypes in different age groups.
Table 2.
Univariate logistic analysis of genotypes involved in hepatitis C virus infection according to age and gender
| Variables | Type III sum of squares | υ | Mean square | F value | P value |
| Sex | 150.641 | 1 | 150.641 | 2.396 | 0.122 |
| Age groups | 0.084 | 1 | 0.084 | 0.001 | 0.971 |
Figure 1.
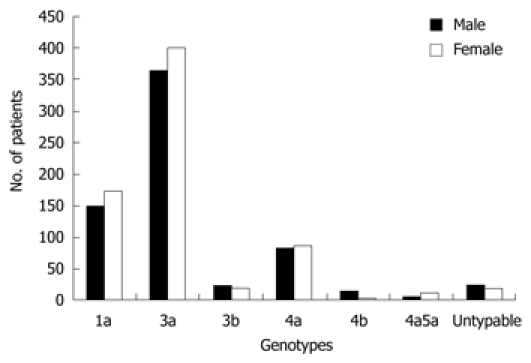
Frequency distribution of different genotypes in gender of patients. The prevalence of hepatitis C virus genotypes in gender was non-significant.
Table 3.
Hepatitis C virus genotype subtypes prevalence in age groups
| Genotype subtypes | Computation |
Age groups |
|
| ≤ 40 yr | > 40 yr | ||
| 1a | Count | 230 | 92 |
| % within age groups | 24.7% | 21.2% | |
| 3a | Count | 507 | 256 |
| % within age groups | 54.5% | 59.1% | |
| 3b | Count | 32 | 11 |
| % within age groups | 3.4% | 2.5% | |
| 4a | Count | 116 | 54 |
| % within age groups | 12.5% | 12.5% | |
| 4b | Count | 13 | 3 |
| % within age groups | 1.4% | 0.7% | |
| 4&5 | Count | 11 | 5 |
| % within age groups | 1.2% | 1.2% | |
| Untypable genotype | Count | 22 | 12 |
| % within age groups | 2.4% | 2.8% | |
Risk assessment for HCV infection
The possible routes of infection were determined by detailed questionnaire. Out of 1364 patients, the possible risk factors for 1183 (86.7%) patients were established, while 13.3% (n = 181) were unaware of their possible route of infection. The major route of HCV infection among patients resides within Lahore city was dental surgery (33.5%), followed by blood transfusion (22.6%), barber shops (12.4%), road accidents (8.4%), sexual exposure (5.9%) and IVDU (3.73%). Statistical analysis illustrated in Table 4 showed that risk factors were dependent on age (P = 0.000) and genotype (P = 0.000) and independent of gender (P = 0.950).
Table 4.
Univariate logistic analysis of risk factors involved in hepatitis C virus infection according to age, gender and genotypes
| Variables | Type III sum of squares | υ | Mean square | F value | P value |
| Sex | 0.014 | 1 | 0.014 | 0.004 | 0.950 |
| Age | 131.744 | 1 | 131.744 | 38.219 | 0.000 |
| Genotype | 457.878 | 6 | 76.313 | 22.139 | 0.000 |
Age-specific distribution of the risk factors
The frequency distribution of different risk factors within age groups is illustrated in Table 5. Dental surgery was the cause of HCV infection in 458 of the 1364 patients (33.6%), and was found more frequently among patients ≤ 40 years (71%) than patients with age > 40 years. Infection due to blood transfusion, road accidents and barber shops was also prevalent in patients ≤ 40 years of age (71.6%, 66.4% and 56.2%, respectively). Infection due to sexual exposure and IVDU was only observed among patients ≤ 40 years age. Our data revealed that infection due to dental surgery was more prevalent in both age groups (age group ≤ 40 years, 34.9%, n = 325; age group > 40 years, 30.7%, n = 133). The second most prevalent infection route in age groups was blood transfusion (age group ≤ 40 years, 23.8%, n = 222; age group > 40 years, 20.3%, n = 88), followed by barber shops (age group ≤ 40 years, 10.2%, n = 95; age group > 40 years, 17.1%, n = 74) and road accidents (age group ≤ 40 years, 8.3%, n = 77; age group > 40 years, 9.0%, n = 39). Multivariate analysis showed a significant association of IVDU (P = 0.000) and sexual exposure (P = 0.000) with age ≤ 40 years (Table 5) whereas route of infection due to dental surgery, blood transfusion, barber shops and road accidents was independent of age groups. Route of infection due to sexual exposure (n = 79, 8.5%) and IVDU (n = 51, 5.5%) was only observed in patients ≤ 40 years.
Table 5.
Distribution and multivariate analysis of risk factors in age groups
| Risk factors | Computation |
Age groups |
95% CI |
P value | ||
| ≤ 40 yr | > 40 yr | Lower | Upper | |||
| Blood transfusion | Count | 222 | 88 | 2.077 | 4.467 | 0.039 |
| % within age groups | 23.8% | 20.3% | ||||
| IVDU | Count | 51 | 0 | 6.414 × 109 | 6.414 × 109 | 0.000 |
| % within age groups | 5.5% | 0.0% | ||||
| Sexual exposure | Count | 79 | 0 | 9.014 × 109 | 9.014 × 109 | 0.000 |
| % within age groups | 8.5% | 0.0% | ||||
| Dental surgery | Count | 325 | 133 | 2.068 | 4.209 | 0.610 |
| % within age groups | 34.9% | 30.7% | ||||
| Road accidents | Count | 77 | 39 | 1.551 | 2.090 | 0.148 |
| % within age groups | 8.3% | 9.0% | ||||
| Barber shops | Count | 95 | 74 | 1.016 | 2.363 | 0.042 |
| % within age groups | 10.2% | 17.1% | ||||
IVDU: Intravenous drug use.
Prevalence of genotypes within risk factors
As risk factors showed a significant association with genotypes, the frequency distribution of various genotypes within risk groups (Table 6) revealed that subtypes 3a (65.2%) and 1a (16.5%) were found more frequently than subtypes 3b (4.2%), 4a (7.4%), 4b (1.3%), mixed (1.3%) and untypable (4.2%) in patients with a past history of blood transfusion. Moreover, subtypes 3a (62.2%), 1a (18.6%) and 4a (13.5%) were observed more frequently than subtypes 3b (2.8%), 4b (1.1%), mixed (0.9%) and untypable (0.9%) in patients infected due to dental surgery. In patients infected through barber shops and road accidents, genotype 3a (52.1% and 58.6%) and 1a (26.6% and 25.9%) were more prevalent than subtypes 3b (4.1% and 3.4%), 4a (11.2% and 9.5%), 4b (0.6% and 0.9%), mixed (3.0% and 0.9%) and untypable (2.4% and 0.9%) respectively. Patients with history of IVDU were mainly infected with genotype 3a (66.7%) and 1a (23.5%), while infection due to sexual exposure was predominantly genotype 3a (67.1%) followed by 1a (17.7%) and 4a (12.7%). These findings indicated that genotype subtype 3a is significantly dominant among all risk groups followed by 1a and 4a. Moreover our results revealed that the genotype with the highest frequency for risk factors is genotype 3a, the highest being in patients with a history of sexual exposure (53, 67.1%) followed by IVDU (34, 66.7%.) and blood transfusion (65.2%). The second most frequent genotype was 1a with the highest frequency in patients possibly infected from barber shops (26.6%), subsequently; genotype 4a was the most frequent in patients experienced the dental surgery (13.5%). Patients (n = 5) with mixed (4a5a) and untypable genotypes (n = 13) were under risk due to barber shops and blood transfusion respectively.
Table 6.
Hepatitis C virus genotype prevalence within risk factors in Lahore - March 2007 - September 2009 (n = 1364) n (%)
| Groups | Total No. | 1a | 3a | 3b | 4a | 4b | Mix | Untypable |
| BT | 310 | 51 (16.5) | 202 (65.2) | 13 (4.2) | 23 (7.4) | 4 (1.3) | 4 (1.3) | 13 (4.2) |
| ≤ 40 yr | 222 | 39 (17.6) | 131 (59.0) | 10 (4.5) | 22 (9.9) | 3 (1.4) | 4 (1.8) | 13 (5.9) |
| > 40 yr | 88 | 12 (13.6) | 71 (80.7) | 3 (3.4) | 1 (1.1) | 1 (1.1) | 0 (0.0) | 0 (0.0) |
| IVDU | 51 | 12 (23.5) | 34 (66.7) | 0 (0.0) | 4 (7.8) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| ≤ 40 yr | 51 | 12 (23.5) | 34 (66.7) | 0 (0.0) | 4 (7.8) | 0 (0.0) | 0 (0.0) | 1 (2.0) |
| > 40 yr | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| SE | 79 | 14 (17.7) | 53 (67.1) | 0 (0.0) | 10 (12.7) | 1 (1.3) | 0 (0.0) | 1 (1.3) |
| ≤ 40 yr | 79 | 14 (17.7) | 53 (67.1) | 0 (0.0) | 10 (12.7) | 1 (1.3) | 0 (0.0) | 1 (1.3) |
| > 40 yr | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| DS | 458 | 85 (18.6) | 285 (62.2) | 13 (2.8) | 62 (13.5) | 5 (1.1) | 4 (0.9) | 4 (0.9) |
| ≤ 40 yr | 325 | 69 (21.2) | 173 (53.2) | 13 (4.0) | 58 (17.8) | 5 (1.5) | 3 (0.9) | 4 (1.2) |
| > 40 yr | 133 | 16 (12.0) | 112 (84.2) | 0 (0.0) | 4 (3.0) | 0 (0.0) | 1 (0.8) | 0 (0.0) |
| RA | 116 | 30 (25.9) | 68 (58.6) | 4 (3.4) | 11 (9.5) | 1 (0.9) | 1 (0.9) | 1 (0.9) |
| ≤ 40 yr | 77 | 30 (39.0) | 46 (59.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.3) |
| > 40 yr | 39 | 0 (0.0) | 22 (56.4) | 4 (10.3) | 11 (28.2) | 1 (2.6) | 1 (2.6) | 0 (0.0) |
| BS | 169 | 45 (26.6) | 88 (52.1) | 7 (4.1) | 19 (11.2) | 1 (0.6) | 5 (3.0) | 4 (2.4) |
| ≤ 40 yr | 95 | 19 (20.0) | 64 (67.4) | 7 (7.4) | 0 (0.0) | 0 (0.0) | 3 (3.2) | 2 (2.1) |
| > 40 yr | 74 | 26 (35.1) | 24 (32.4) | 0 (0.0) | 19 (25.7) | 1 (1.4) | 2 (2.7) | 2 (2.7) |
| UN | 181 | 85 (47.0) | 33 (18.2) | 6 (3.3) | 41 (22.7) | 4 (2.2) | 2 (1.1) | 10 (5.5) |
| ≤ 40 yr | 82 | 47 (57.3) | 6 (7.3) | 2 (2.4) | 22 (26.8) | 4 (4.9) | 1 (1.2) | 0 (0.0) |
| > 40 yr | 99 | 38 (38.4) | 27 (27.3) | 4 (4.0) | 19 (19.2) | 0 (0.0) | 1 (1.0) | 10 (10.1) |
| Total | 1364 | 322 (23.6) | 763 (55.9) | 43 (3.2) | 170 (12.5) | 16 (1.2) | 16 (1.2) | 34 (2.5) |
BT: Blood transfusion; IVDU: Intravenous drug use; SE: Sexual exposure; DS: Dental surgery; RA: Road accidents; BS: Barber shops; UN: Unknown reasons.
Association of HCV risk factors with age and genotype
In patients infected due to blood transfusion, genotype 3a was more prevalent in age group > 40 years (80.7%) than ≤ 40 years (59%), while genotype 4a was more prevalent in age group ≤ 40 years (9.9%) than > 40 years (1.1%) (Table 6). The same prevalence order was observed in patients infected due to dental surgery where genotypes 1a (21.2%) and 4a (17.8%) were more prevalent in the age group ≤ 40 years than the age group > 40 years (12.0% and 3.0%, respectively) and genotype 3a was predominant in the age group > 40 years (84.2%) than the age group ≤ 40 years (53.2%). In patients infected due to barber shops, the prevalence of genotype 1a was higher in the age group > 40 years (35.1%) than the age group ≤ 40 years (20.0%), while genotype 3a was most prevalent in age group ≤ 40 years (67.4%) compared with age group > 40 years (32.4%).
DISCUSSION
HCV is an important cause of chronic liver disease and cirrhosis in Pakistan and accounts for significant morbidity and mortality. It is estimated that about 6% (10 million) of the Pakistani population is infected with HCV[19]. In the present study, we analyzed the relationship between distribution of HCV genotypes and mode of transmission of infection in different age groups and gender. Our results showed a non-significant prevalence of HCV distribution in gender and age which are in accordance to studies carried out by other groups who observed no relation between age, gender and HCV distribution[22,23]. When results were further analyzed keeping age as a categorical variable, people ≤ 40 years of age were more affected with HCV in comparison to those > 40 years of age in Lahore. These results are in conflict with previous data from Muhammad et al[24] in 2005 showing that the prevalence of HCV in Pakistan is greater in old age, however, our results agreed with the findings of Ali et al[25] that HCV prevalence observed was highest among an age group of 13-50 years and similar findings were observed by Shah et al[26]. This could be due to an increasing awareness and early diagnosis of HCV in urban area communities in Pakistan.
HCV shows considerable sequence diversity and sequence comparisons in different parts of the genome. This has led to the classification of the virus into a series of genotypes that show distinct geographical distribution across the world[27]. Genotyping is important because it provides information as to strain variation and potential association with disease severity and a guide about treatment duration and outcomes[28]. Our finding indicating a high prevalence of genotype 3a (55.9%) in Lahore city is consistent with others describing HCV 3a prevalence in Pakistan[17,18], however, an increased prevalence of genotype 1a and 4a (23.6% and 12.5%) found in our study is significantly higher than the previously reported HCV prevalence for these genotypes. Idress et al[17] in 2008 showed that the prevalence of HCV infection due to genotype 4 and 1 is increasing without an increase in the frequency of genotype 3 in various areas of Pakistan mainly NWFP (1a, 6.56% and 4, 2.30%) and Balochistan (1a, 25.80% and 4, 4.03%). They claimed the appearance of genotype 4 in Pakistan for the first time with 1.15% prevalence in the Punjab area which includes Lahore city. A shift in genotype distribution, mostly comprising an increase in the prevalence of the genotypes 3a, 1a and 4, and decrease in prevalence of other genotypes, is also found in many countries largely due to migration[12,23]. Our results indicated an increased prevalence of genotype 4 and 1 in Lahore city may suggest a recent migration of people, possibly from Balochistan and northern areas (active terrorist war-zone area). Although there is evidence of increasing population size for Lahore, recent reports highlighting migration data to the city of Lahore are not available[29]. These results suggest a possible disaster in HCV management for Pakistan in coming years due to poor response against current therapy for genotypes 4 and 1. Furthermore, genotype 4 is reported to be associated with liver cirrhosis[30], therefore, it may potentially lead to an increased risk of liver cirrhosis in Pakistan. The absence of genotype 1b, 2a and 2b in our study indicates that these are rarely present in our population. A similar frequency distribution pattern of genotypes in neighboring countries like India and Iran has been observed where genotype 3 is most prevalent and genotype 2 is very rare[22,31].
As described earlier there is no national database for collection of risk factors involved in HCV transmission, however, studies from different areas of Pakistan revealed that IVDU due to excessive use of unnecessary injections and reuse of needles, dental surgery by using unhygienic practice and unsafe blood transfusion are the major causes of HCV infection[19,32-35], while HCV transmission due to sexual exposure and barber shaving was also reported in Pakistan[36,37]. Kuo et al[38] reported 93% HCV prevalence among IVDUs in Lahore. Although IVDU has been identified as a route of infection in Lahore, it was not significant in our HCV population due to denial by a large number of people. Our results indicate dental surgery to be a leading cause of HCV transmission followed by unsafe blood transfusion. Our results are also in agreement with the results from other countries reporting blood transfusion, unsafe injection, barber shops and dental instruments as the main routes of HCV infection[39].
Data analysis for finding an association of various HCV genotypes with possible routes of transmission indicated blood transfusion and dental surgery to be the leading cause in all genotypes while infection due to IVDU and sexual exposure was predominant in genotype 1 and 3. In the present study, genotype 3 had a high prevalence in patients with a history of blood transfusion and IVDU, similar to USA and Europe[19,34] while genotype 1 was most prevalent in patients infected from barber shops. Genotype 4 has been reported to be related with different routes of infection such as dental surgery, dialysis, barber shops[19,40] and we observed dental surgery as a major route of infection in genotype 4 followed by sexual exposure. Barber shops and blood transfusion were observed as possible routes of infection in mixed and untypable genotype, respectively. We observed a notable variation in the distribution of HCV subtypes in different risk groups within age groups. As patients infected due to blood transfusion and dental surgery were more frequent (57%) than infection from other risk factors, genotype 3a was more prevalent in the age group > 40 years, while genotype 1a and 4a were observed in the age group ≤ 40 years. These findings conclude 3a is the original subtype present in Lahore while the others were introduced at a later date by an increase in population movements like migration and change in mode of risk factors. A high prevalence of genotype 1a and 4a in the age group ≤ 40 years can confirm this epidemiological shift in new generation.
Our results also revealed that the routes of HCV transmission were significantly associated with age groups while risk factors like dental surgery, blood transfusion and road accidents were evenly distributed in both age groups. Sexual exposure was reported in 79 patients and all of them belonged to the age group ≤ 40 years. The major reason for infection due to sexual exposure was non-awareness of people about sexually transmitted diseases and low use of condoms[41]. However, sexual activity cannot be applied as an independent risk factor as Tong et al[42] suggested low transmission of HCV among spouses. Blood transfusion, the most commonly recognized transmission mechanism of HCV, showed a high prevalence (71.6%) for patients ≤ 40 years. This could be due to many reasons - mainly concealing HCV status of donors from their relatives during blood donation and improper screening of blood donors[43]. HCV prevalence due to barber shops was also significantly higher (56.2%) in patients ≤ 40 years. The possible reason could be due to the use of inadequately sterilized instruments, reuse of razors and other shaving kits and transfusion of contaminated blood also seen by others in Pakistan[31,35-37]. The use of illegal drugs by injection is rising especially in young people in Pakistan as the effect of IDVU is more satisfying and intense[19,33]. Although the number of patients with IDVU was small, 100% IVDU patients belonged to the age group ≤ 40 years.
In conclusion, this study used a large HCV population for the first time and reported the HCV genotype-specific prevalence with age and possible risk factors associated with its transmission in Lahore city of Pakistan. Genotype 3 was predominant and patients infected with genotype 4 are increasing along with genotype 1 due to possible inland migration in Pakistan. We observed a maximum prevalence for HCV in the age group ≤ 40 years (68.3%) with involvement of multiple routes of infections. Infection due to sexual exposure and IVDU was exclusively linked to ≤ 40 years age group. Our study revealed that at least 66% of HCV infections are associated with the health-care sector as most of the patients infected with HCV developed the infection as a result of blood transfusion, dental surgery and IVDU. For genotype 3, blood transfusion was the major infection route, while infection attributed to barber shops was associated with genotype 1. The most prevalent risk factor for genotype 4 was found to be dental surgery. This study also highlights future possible HCV disease complications due to an increase in genotypes 4 and 1 and also provides a basis for the government of Pakistan to implement higher health standards.
COMMENTS
Background
Chronic hepatitis C virus (HCV) is one of the major causes of liver fibrosis, with distortion of the hepatic architecture and ultimate progression to cirrhosis. To date, at least six major genotypes of HCV, each having multiple subtypes, have been identified worldwide. The different genotypes are relevant to epidemiology, route of infection, vaccine development, and clinical management of chronic HCV infection.
Research frontiers
In Pakistan, more than 10 million people are infected with chronic HCV. The exact data about the relationship between HCV genotypes, epidemiological factors and route of infection is limited.
Innovations and breakthroughs
The frequency distribution of different HCV genotypes and subtypes and their association with various risk factors in Lahore, the second largest city of Pakistan was investigated in 1364 patients with chronic HCV. The maximum prevalence of HCV was found in the age group ≤ 40 years (68.3%). Genotype 3a was the predominant genotype followed by an increase in 1a and 4a as compared to previous reports suggesting a recent migration of people possibly from Balochistan and northern areas (active terrorist war-zone area). The main risk factors observed in patients infected with HCV were blood transfusion, dental surgery and intravenous drug use. Blood transfusion and barber shops were the major infection route, in genotype 3a and 1a respectively.
Applications
This article reported the prevalence of HCV genotypes in Lahore city. Additionally the relationship between different genotypes and subtypes with age, gender and different possible routes of infection was studied. Shifts in HCV genotype distribution needs to be paid more attention as genotype 1a and 4a are associated with severe cirrhosis.
Peer review
This article describes the prevalence of HCV in Lahore population. It is a good work and should be accepted for publication.
Acknowledgments
The authors are thankful to Dr. Tahir Ali Javaid, Dr. Javaid Akram, Fozia Hameed, Muhammad Tahir Iqbal and Dr. Nousheen Wasim, Jinnah hospital for their enormous help in acquiring data for this study.
Footnotes
Supported by Prime Minister Program for Prevention and Control of Hepatitis in Pakistan (2005-2010) and Grant # 863 by Higher Education Commission
Peer reviewer: Randeep Singh Kashyap, MD, Assistant Professor, University of Rochester, School of Medicine and Dentistry, 601 Elmwood Ave, Box SURG, Rochester, New York, NY 14642, United States
S- Editor Wang JL L- Editor O’Neill M E- Editor Ma WH
References
- 1.Giannini C, Bréchot C. Hepatitis C virus biology. Cell Death Differ. 2003;10 Suppl 1:S27–S38. doi: 10.1038/sj.cdd.4401121. [DOI] [PubMed] [Google Scholar]
- 2.Alter MJ. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. doi: 10.1002/hep.510260711. [DOI] [PubMed] [Google Scholar]
- 3.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 4.Zein NN, Persing DH. Hepatitis C genotypes: current trends and future implications. Mayo Clin Proc. 1996;71:458–462. doi: 10.4065/71.5.458. [DOI] [PubMed] [Google Scholar]
- 5.Liew M, Erali M, Page S, Hillyard D, Wittwer C. Hepatitis C genotyping by denaturing high-performance liquid chromatography. J Clin Microbiol. 2004;42:158–163. doi: 10.1128/JCM.42.1.158-163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmonds P. Genetic diversity and evolution of hepatitis C virus--15 years on. J Gen Virol. 2004;85:3173–3188. doi: 10.1099/vir.0.80401-0. [DOI] [PubMed] [Google Scholar]
- 7.Candotti D, Temple J, Sarkodie F, Allain JP. Frequent recovery and broad genotype 2 diversity characterize hepatitis C virus infection in Ghana, West Africa. J Virol. 2003;77:7914–7923. doi: 10.1128/JVI.77.14.7914-7923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ndjomou J, Pybus OG, Matz B. Phylogenetic analysis of hepatitis C virus isolates indicates a unique pattern of endemic infection in Cameroon. J Gen Virol. 2003;84:2333–2341. doi: 10.1099/vir.0.19240-0. [DOI] [PubMed] [Google Scholar]
- 9.Abid K, Quadri R, Veuthey AL, Hadengue A, Negro F. A novel hepatitis C virus (HCV) subtype from Somalia and its classification into HCV clade 3. J Gen Virol. 2000;81:1485–1493. doi: 10.1099/0022-1317-81-6-1485. [DOI] [PubMed] [Google Scholar]
- 10.Pawlotsky JM, Tsakiris L, Roudot-Thoraval F, Pellet C, Stuyver L, Duval J, Dhumeaux D. Relationship between hepatitis C virus genotypes and sources of infection in patients with chronic hepatitis C. J Infect Dis. 1995;171:1607–1610. doi: 10.1093/infdis/171.6.1607. [DOI] [PubMed] [Google Scholar]
- 11.Chamberlain RW, Adams N, Saeed AA, Simmonds P, Elliott RM. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J Gen Virol. 1997;78(Pt 6):1341–1347. doi: 10.1099/0022-1317-78-6-1341. [DOI] [PubMed] [Google Scholar]
- 12.Svirtlih N, Delic D, Simonovic J, Jevtovic D, Dokic L, Gvozdenovic E, Boricic I, Terzic D, Pavic S, Neskovic G, et al. Hepatitis C virus genotypes in Serbia and Montenegro: the prevalence and clinical significance. World J Gastroenterol. 2007;13:355–360. doi: 10.3748/wjg.v13.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ross RS, Viazov S, Renzing-Köhler K, Roggendorf M. Changes in the epidemiology of hepatitis C infection in Germany: shift in the predominance of hepatitis C subtypes. J Med Virol. 2000;60:122–125. [PubMed] [Google Scholar]
- 14.Savvas SP, Koskinas J, Sinani C, Hadziyannis A, Spanou F, Hadziyannis SJ. Changes in epidemiological patterns of HCV infection and their impact on liver disease over the last 20 years in Greece. J Viral Hepat. 2005;12:551–557. doi: 10.1111/j.1365-2893.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- 15.Payan C, Roudot-Thoraval F, Marcellin P, Bled N, Duverlie G, Fouchard-Hubert I, Trimoulet P, Couzigou P, Cointe D, Chaput C, et al. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millenium: The GEMHEP GenoCII Study. J Viral Hepat. 2005;12:405–413. doi: 10.1111/j.1365-2893.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 16.Idrees M, Riazuddin S. Frequency distribution of hepatitis C virus genotypes in different geographical regions of Pakistan and their possible routes of transmission. BMC Infect Dis. 2008;8:69. doi: 10.1186/1471-2334-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idrees M. Development of an improved genotyping assay for the detection of hepatitis C virus genotypes and subtypes in Pakistan. J Virol Methods. 2008;150:50–56. doi: 10.1016/j.jviromet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Ijaz T, Khan MA, Jafri SA, Ranjha FA, Mehmood KA, Imran M, Shahzad MK. Prevalence of Hepatitis C Virus (HCV) Genotype 3a in the Infected Population of Lahore, Pakistan. Int J Infect Dis. 2008;12 Suppl 1:S421. [Google Scholar]
- 19.Raja NS, Janjua KA. Epidemiology of hepatitis C virus infection in Pakistan. J Microbiol Immunol Infect. 2008;41:4–8. [PubMed] [Google Scholar]
- 20.Batool SA, Chaudhry N, Majeed K. Economic potential of recycling business in Lahore, Pakistan. Waste Manag. 2008;28:294–298. doi: 10.1016/j.wasman.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Chan SW, McOmish F, Holmes EC, Dow B, Peutherer JF, Follett E, Yap PL, Simmonds P. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J Gen Virol. 1992;73(Pt 5):1131–1141. doi: 10.1099/0022-1317-73-5-1131. [DOI] [PubMed] [Google Scholar]
- 22.Altuglu I, Soyler I, Ozacar T, Erensoy S. Distribution of hepatitis C virus genotypes in patients with chronic hepatitis C infection in Western Turkey. Int J Infect Dis. 2008;12:239–244. doi: 10.1016/j.ijid.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Dal Molin G, Ansaldi F, Biagi C, D’Agaro P, Comar M, Crocè L, Tiribelli C, Campello C. Changing molecular epidemiology of hepatitis C virus infection in Northeast Italy. J Med Virol. 2002;68:352–356. doi: 10.1002/jmv.10210. [DOI] [PubMed] [Google Scholar]
- 24.Muhammad N, Jan MA. Frequency of hepatitis “C” in Buner, NWFP. J Coll Physicians Surg Pak. 2005;15:11–14. [PubMed] [Google Scholar]
- 25.Ali M, Kanwal L, Tassaduqe K, Iqbal R. Prevalence of hepatitis C virus (HCV) in relation to its promotive factors among human urban population of Multan, Pakistan. Eur J Gen Med. 2009;6:94–98. [Google Scholar]
- 26.Shah FU, Salih M, Malik IA, Hussain I. Increasing prevalence of chronic hepatitis and associated risk factors. Pak J Med Res. 2002;41:46–50. [Google Scholar]
- 27.Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, Beall E, Yap PL, Kolberg J, Urdea MS. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 28.Kabir A, Alavian SM, Keyvani H. Distribution of hepatitis C virus genotypes in patients infected by different sources and its correlation with clinical and virological parameters: a preliminary study. Comp Hepatol. 2006;5:4. doi: 10.1186/1476-5926-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazhar F, Jamal T. Temporal population growth of Lahore. J Sci Res. 2009;39:53–58. [Google Scholar]
- 30.el-Zayadi A, Simmonds P, Dabbous H, Prescott L, Selim O, Ahdy A. Response to interferon-alpha of Egyptian patients infected with hepatitis C virus genotype 4. J Viral Hepat. 1996;3:261–264. doi: 10.1111/j.1365-2893.1996.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 31.Chowdhury A, Santra A, Chaudhuri S, Dhali GK, Chaudhuri S, Maity SG, Naik TN, Bhattacharya SK, Mazumder DN. Hepatitis C virus infection in the general population: a community-based study in West Bengal, India. Hepatology. 2003;37:802–809. doi: 10.1053/jhep.2003.50157. [DOI] [PubMed] [Google Scholar]
- 32.Hamid S, Umar M, Alam A, Siddiqui A, Qureshi H, Butt J. PSG consensus statement on management of hepatitis C virus infection--2003. J Pak Med Assoc. 2004;54:146–150. [PubMed] [Google Scholar]
- 33.Butt AK, Khan AA, Khan SY, Sharea I. Dentistry as a possible route of hepatitis C transmission in Pakistan. Int Dent J. 2003;53:141–144. doi: 10.1111/j.1875-595x.2003.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 34.Khan AJ, Luby SP, Fikree F, Karim A, Obaid S, Dellawala S, Mirza S, Malik T, Fisher-Hoch S, McCormick JB. Unsafe injections and the transmission of hepatitis B and C in a periurban community in Pakistan. Bull World Health Organ. 2000;78:956–963. [PMC free article] [PubMed] [Google Scholar]
- 35.Ali SA, Donahue RM, Qureshi H, Vermund SH. Hepatitis B and hepatitis C in Pakistan: prevalence and risk factors. Int J Infect Dis. 2009;13:9–19. doi: 10.1016/j.ijid.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aslam M, Aslam J. Seroprevalence of the antibody to hepatitis C in select groups in the Punjab region of Pakistan. J Clin Gastroenterol. 2001;33:407–411. doi: 10.1097/00004836-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Janjua NZ, Nizamy MA. Knowledge and practices of barbers about hepatitis B and C transmission in Rawalpindi and Islamabad. J Pak Med Assoc. 2004;54:116–119. [PubMed] [Google Scholar]
- 38.Kuo I, ul-Hasan S, Galai N, Thomas DL, Zafar T, Ahmed MA, Strathdee SA. High HCV seroprevalence and HIV drug use risk behaviors among injection drug users in Pakistan. Harm Reduct J. 2006;3:26. doi: 10.1186/1477-7517-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medhat A, Shehata M, Magder LS, Mikhail N, Abdel-Baki L, Nafeh M, Abdel-Hamid M, Strickland GT, Fix AD. Hepatitis c in a community in Upper Egypt: risk factors for infection. Am J Trop Med Hyg. 2002;66:633–638. doi: 10.4269/ajtmh.2002.66.633. [DOI] [PubMed] [Google Scholar]
- 41.Saleem NH, Adrien A, Razaque A. Risky sexual behavior, knowledge of sexually transmitted infections and treatment utilization among a vulnerable population in Rawalpindi, Pakistan. Southeast Asian J Trop Med Public Health. 2008;39:642–648. [PubMed] [Google Scholar]
- 42.Tong MJ, Lai PP, Hwang SJ, Lee SY, Co RL, Chien RN, Kuo G. Evaluation of sexual transmission in patients with chronic hepatitis C infection. Clin Diagn Virol. 1995;3:39–47. doi: 10.1016/0928-0197(94)00021-l. [DOI] [PubMed] [Google Scholar]
- 43.Emmanuel F, Attarad A. Correlates of injection use of synthetic drugs among drug users in Pakistan: a case controlled study. J Pak Med Assoc. 2006;56:119–124. [PubMed] [Google Scholar]


