Abstract
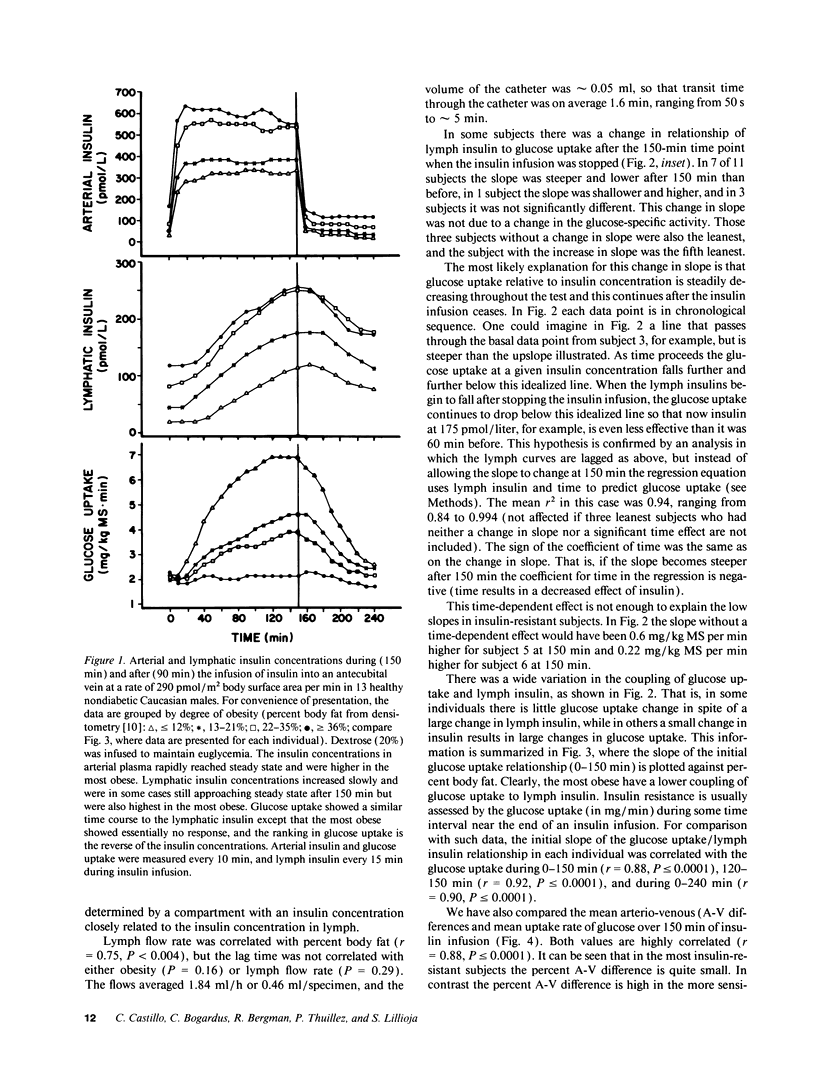
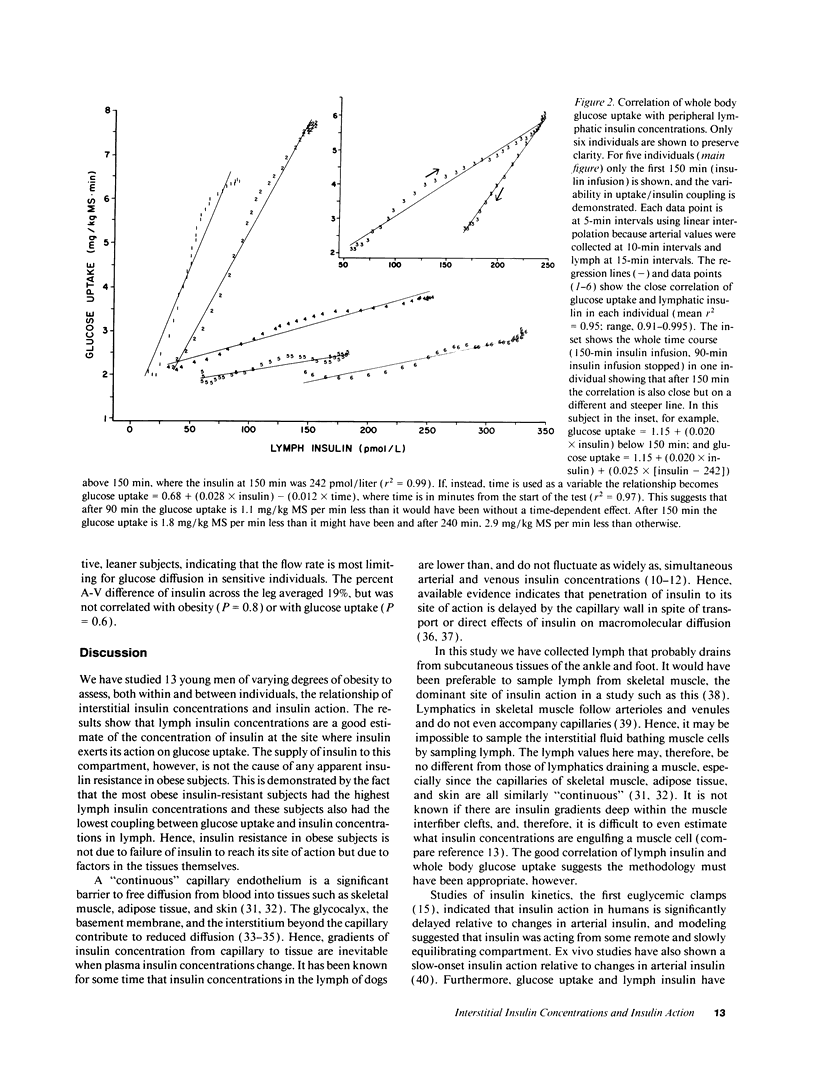
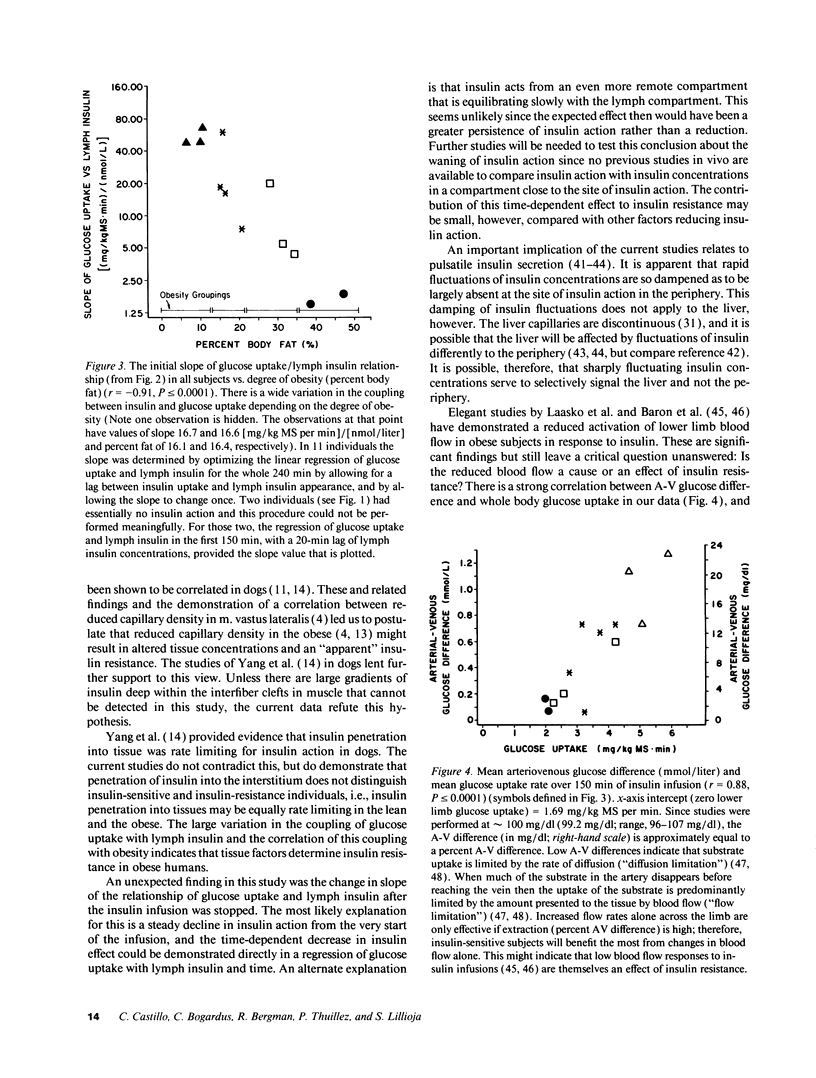
Insulin action and obesity are both correlated with the density of muscle capillary supply in humans. Since the altered muscle anatomy in the obese might affect interstitial insulin concentrations and reduce insulin action, we have cannulated peripheral lymphatic vessels in lean and obese males, and compared peripheral lymph insulin concentrations with whole body glucose uptake during a euglycemic, hyperinsulinemic clamp. Lymph insulin concentrations in the lower limb averaged only 34% of arterial insulin concentrations during 150 min of insulin infusion. Obese subjects had the highest arterial (P < or = 0.0001) and lymph insulin (P < 0.005) concentrations, but the lowest glucose uptake rates (P < 0.002). In contrast to the initial steep rise then plateau of arterial insulins, both lymph insulin and whole body glucose uptake rates rose slowly and did not consistently reach a plateau. In each individual, the glucose uptake closely correlated with peripheral lymphatic insulin concentrations (mean r2 = 0.95). The coupling between glucose uptake and lymph insulin (glucose uptake/pmol insulin) was much steeper in lean subjects than in the obese (P < or = 0.0001). These results indicate that even if insulin diffusion into tissues is rate limiting for insulin action, a tissue defect rather than an insulin diffusion defect causes insulin resistance in obese subjects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977 Sep;270(3):677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A. D., Laakso M., Brechtel G., Edelman S. V. Reduced capacity and affinity of skeletal muscle for insulin-mediated glucose uptake in noninsulin-dependent diabetic subjects. Effects of insulin therapy. J Clin Invest. 1991 Apr;87(4):1186–1194. doi: 10.1172/JCI115117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. H. The diagnosis of diabetes: new international classification and diagnostic criteria. Annu Rev Med. 1983;34:295–309. doi: 10.1146/annurev.me.34.020183.001455. [DOI] [PubMed] [Google Scholar]
- Bergman R. N., Hope I. D., Yang Y. J., Watanabe R. M., Meador M. A., Youn J. H., Ader M. Assessment of insulin sensitivity in vivo: a critical review. Diabetes Metab Rev. 1989 Aug;5(5):411–429. doi: 10.1002/dmr.5610050501. [DOI] [PubMed] [Google Scholar]
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S. Where all the glucose doesn't go in non-insulin-dependent diabetes mellitus. N Engl J Med. 1990 Jan 25;322(4):262–263. doi: 10.1056/NEJM199001253220409. [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain P. R., Komjati M., Waldhäusl W. K. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986 Aug;35(8):922–926. doi: 10.2337/diab.35.8.922. [DOI] [PubMed] [Google Scholar]
- Camu F., Rasio E. Peripheral glucose uptake in relation to physiological levels of plasma and lymph insulin. Eur J Clin Invest. 1972 Mar;2(3):188–194. doi: 10.1111/j.1365-2362.1972.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Castillo C. E., Lillioja S. Peripheral lymphatic cannulation for physiological analysis of interstitial fluid compartment in humans. Am J Physiol. 1991 Oct;261(4 Pt 2):H1324–H1328. doi: 10.1152/ajpheart.1991.261.4.H1324. [DOI] [PubMed] [Google Scholar]
- Chernick S. S., Gardiner R. J., Scow R. O. Restricted passage of insulin across capillary endothelium in perfused rat adipose tissue. Am J Physiol. 1987 Nov;253(5 Pt 1):E475–E480. doi: 10.1152/ajpendo.1987.253.5.E475. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Joyner W. L., Rutledge J. C. Graded modulation of frog microvessel permeability to albumin using ionophore A23187. Am J Physiol. 1990 Feb;258(2 Pt 2):H587–H598. doi: 10.1152/ajpheart.1990.258.2.H587. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Doeden B., Rizza R. Use of a variable insulin infusion to assess insulin action in obesity: defects in both the kinetics and amplitude of response. J Clin Endocrinol Metab. 1987 May;64(5):902–908. doi: 10.1210/jcem-64-5-902. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Hilsted J., Christensen N. J. Dual effect of insulin on plasma volume and transcapillary albumin transport. Diabetologia. 1992 Feb;35(2):99–103. doi: 10.1007/BF00402539. [DOI] [PubMed] [Google Scholar]
- Hom F. G., Goodner C. J. Insulin dose-response characteristics among individual muscle and adipose tissues measured in the rat in vivo with 3[H]2-deoxyglucose. Diabetes. 1984 Feb;33(2):153–159. doi: 10.2337/diab.33.2.153. [DOI] [PubMed] [Google Scholar]
- Ingjer F. Effects of endurance training on muscle fibre ATP-ase activity, capillary supply and mitochondrial content in man. J Physiol. 1979 Sep;294:419–432. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D. E., Jenkins A. B., Kraegen E. W. Heterogeneity of insulin action in individual muscles in vivo: euglycemic clamp studies in rats. Am J Physiol. 1985 May;248(5 Pt 1):E567–E574. doi: 10.1152/ajpendo.1985.248.5.E567. [DOI] [PubMed] [Google Scholar]
- Johnson K. H., O'Brien T. D., Betsholtz C., Westermark P. Islet amyloid, islet-amyloid polypeptide, and diabetes mellitus. N Engl J Med. 1989 Aug 24;321(8):513–518. doi: 10.1056/NEJM198908243210806. [DOI] [PubMed] [Google Scholar]
- KEYS A., BROZEK J. Body fat in adult man. Physiol Rev. 1953 Jul;33(3):245–325. doi: 10.1152/physrev.1953.33.3.245. [DOI] [PubMed] [Google Scholar]
- King G. L., Johnson S. M. Receptor-mediated transport of insulin across endothelial cells. Science. 1985 Mar 29;227(4694):1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- Komjati M., Astner-Kremsmayr H., Waldhäusl W., Reitgruber W., Breitenecker F., Troch I. Interaction of sympathomimetics and insulin with hepatic glucose production by isolated perfused rat livers: effects of continuous versus pulsatile infusion. Endocrinology. 1988 Oct;123(4):1798–1807. doi: 10.1210/endo-123-4-1798. [DOI] [PubMed] [Google Scholar]
- Laakso M., Edelman S. V., Brechtel G., Baron A. D. Decreased effect of insulin to stimulate skeletal muscle blood flow in obese man. A novel mechanism for insulin resistance. J Clin Invest. 1990 Jun;85(6):1844–1852. doi: 10.1172/JCI114644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton B., Cooper G. J. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature. 1988 Oct 13;335(6191):632–635. doi: 10.1038/335632a0. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C., Mott D. M., Kennedy A. L., Knowler W. C., Howard B. V. Relationship between insulin-mediated glucose disposal and lipid metabolism in man. J Clin Invest. 1985 Apr;75(4):1106–1115. doi: 10.1172/JCI111804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S., Bogardus C. Obesity and insulin resistance: lessons learned from the Pima Indians. Diabetes Metab Rev. 1988 Aug;4(5):517–540. doi: 10.1002/dmr.5610040508. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Mott D. M., Zawadzki J. K., Young A. A., Abbott W. G., Bogardus C. Glucose storage is a major determinant of in vivo "insulin resistance" in subjects with normal glucose tolerance. J Clin Endocrinol Metab. 1986 May;62(5):922–927. doi: 10.1210/jcem-62-5-922. [DOI] [PubMed] [Google Scholar]
- Lillioja S., Young A. A., Culter C. L., Ivy J. L., Abbott W. G., Zawadzki J. K., Yki-Järvinen H., Christin L., Secomb T. W., Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987 Aug;80(2):415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry C. V., Kimmey J. S., Felder S., Chi M. M., Kaiser K. K., Passonneau P. N., Kirk K. A., Lowry O. H. Enzyme patterns in single human muscle fibers. J Biol Chem. 1978 Nov 25;253(22):8269–8277. [PubMed] [Google Scholar]
- McGuire E. A., Tobin J. D., Berman M., Andres R. Kinetics of native insulin in diabetic, obese, and aged men. Diabetes. 1979 Feb;28(2):110–120. doi: 10.2337/diab.28.2.110. [DOI] [PubMed] [Google Scholar]
- Michel C. C. Capillary permeability and how it may change. J Physiol. 1988 Oct;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski W. L., Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am J Physiol. 1980 Dec;239(6):H775–H783. doi: 10.1152/ajpheart.1980.239.6.H775. [DOI] [PubMed] [Google Scholar]
- Olszewski W., Engeset A., Jaeger P. M., Sokolowski J., Theodorsen L. Flow and composition of leg lymph in normal men during venous stasis, muscular activity and local hyperthermia. Acta Physiol Scand. 1977 Feb;99(2):149–155. doi: 10.1111/j.1748-1716.1977.tb10365.x. [DOI] [PubMed] [Google Scholar]
- Paolisso G., Scheen A. J., Verdin E. M., Luyckx A. S., Lefebvre P. J. Insulin oscillations per se do not affect glucose turnover parameters in normal man. J Clin Endocrinol Metab. 1986 Aug;63(2):520–525. doi: 10.1210/jcem-63-2-520. [DOI] [PubMed] [Google Scholar]
- Peter J. B., Barnard R. J., Edgerton V. R., Gillespie C. A., Stempel K. E. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972 Jul 4;11(14):2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Prager R., Wallace P., Olefsky J. M. In vivo kinetics of insulin action on peripheral glucose disposal and hepatic glucose output in normal and obese subjects. J Clin Invest. 1986 Aug;78(2):472–481. doi: 10.1172/JCI112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Rasio E. A., Mack E., Egdahl R. H., Herrera M. G. Passage of insulin and inulin across vascular membranes in the dog. Diabetes. 1968 Nov;17(11):668–672. doi: 10.2337/diab.17.11.668. [DOI] [PubMed] [Google Scholar]
- Rasio E. The capillary barrier to circulating insulin. Diabetes Care. 1982 May-Jun;5(3):158–161. doi: 10.2337/diacare.5.3.158. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Kramer K. J., Tobin J. D., Insel P. A., Liljenquist J. E., Berman M., Andres R. A model of the kinetics of insulin in man. J Clin Invest. 1974 May;53(5):1481–1492. doi: 10.1172/JCI107697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalak T. C., Schmid-Schönbein G. W., Zweifach B. W. New morphological evidence for a mechanism of lymph formation in skeletal muscle. Microvasc Res. 1984 Jul;28(1):95–112. doi: 10.1016/0026-2862(84)90032-3. [DOI] [PubMed] [Google Scholar]
- Storlien L. H., Jenkins A. B., Chisholm D. J., Pascoe W. S., Khouri S., Kraegen E. W. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991 Feb;40(2):280–289. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- Tesch P. A., Thorsson A., Kaiser P. Muscle capillary supply and fiber type characteristics in weight and power lifters. J Appl Physiol Respir Environ Exerc Physiol. 1984 Jan;56(1):35–38. doi: 10.1152/jappl.1984.56.1.35. [DOI] [PubMed] [Google Scholar]
- Thomason D. B., Baldwin K. M., Herrick R. E. Myosin isozyme distribution in rodent hindlimb skeletal muscle. J Appl Physiol (1985) 1986 Jun;60(6):1923–1931. doi: 10.1152/jappl.1986.60.6.1923. [DOI] [PubMed] [Google Scholar]
- Watson P. D. The interstitial matrix as a barrier in blood-to-lymph solute movement. Physiologist. 1980 Feb;23(1):86–89. [PubMed] [Google Scholar]
- Weigle D. S. Pulsatile secretion of fuel-regulatory hormones. Diabetes. 1987 Jun;36(6):764–775. doi: 10.2337/diab.36.6.764. [DOI] [PubMed] [Google Scholar]
- Yang Y. J., Hope I. D., Ader M., Bergman R. N. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989 Nov;84(5):1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H., Consoli A., Nurjhan N., Young A. A., Gerich J. E. Mechanism for underestimation of isotopically determined glucose disposal. Diabetes. 1989 Jun;38(6):744–751. doi: 10.2337/diab.38.6.744. [DOI] [PubMed] [Google Scholar]



