Abstract
A key to understanding the functioning of the immune system is to define the mechanisms that facilitate directed lymphocyte migration to and within tissues. The recent development of improved imaging technologies, most prominently multi-photon microscopy, has enabled the dynamic visualization of immune cells in real-time directly within intact tissues. Intravital imaging approaches have revealed high spontaneous migratory activity of T cells in secondary lymphoid organs and inflamed tissues. Experimental evidence points towards both environmental and cell-intrinsic cues involved in the regulation of lymphocyte motility in the interstitial space. Based on these data, several conceptually distinct models have been proposed in order to explain the coordination of lymphocyte migration both at the single cell and population level. These range from “stochastic” models, where chance is the major driving force, to “deterministic” models, where the architecture of the microenvironment dictates the migratory trajectory of cells. In this review, we focus on recent advances in understanding naïve and effector T cell migration in vivo. In addition, we discuss some of the contradictory experimental findings in the context of theoretical models of migrating leukocytes.
Keywords: Imaging, Migration, Lymphocytes, Tumor immunology
Introduction
T lymphocytes represent a major cellular constituent of the immune system that coordinately recognize and target infected or transformed cells. In order to perform these tasks effectively, T cells possess a high migratory potential. Thus, they have the capacity to traffic actively between different compartments within organisms [70, 72], and these trafficking pathways are influenced by their differentiation state. A key property of naïve T cells is to circulate continuously between the lymphoid and blood compartments, which optimizes their chance of encountering cells presenting cognate antigen. Effector T cells reprogram their expression of adhesion molecules and chemokine receptors enabling them to migrate to peripheral organs [32]. Extensive research has identified a multi-step molecular cascade that regulates organ-specific homing of blood-borne T cells across the endothelial barrier [68, 70].
In contrast to these well-defined intravascular events, the behavior of T cells in the extravascular space has remained insufficiently characterized. Nevertheless, elucidation of this critical final step of the immune response at the cellular and molecular level is important for understanding how T cells manage to destroy target cells, for example, during infection, in tumors, or autoimmune disease. Undoubtedly, the introduction of multi-photon microscopy into immunologic research has enormously expanded our knowledge of the patterns of T lymphocyte locomotion in the interstitial space, and we are now beginning to understand the underlying molecular cues that direct lymphocytes within tissues [13, 28, 55, 69]. The use of (near) infrared laser light in multi-photon microscopy facilitates significantly improved tissue penetration as compared to traditional confocal microscopy, which relies on wavelengths in the visible light range. Thus, cellular behavior can now be observed up to several hundred micrometers below tissue surfaces. Imaging experiments in live experimental mice or intact explanted tissues therefore allow for the direct visualization of migrating T cells in the context of their natural environments. Seminal initial studies on naïve T cells within lymph nodes (LN) have revealed that T cells, in the absence of cognate antigen, show very active migration, which appeared to be random [49]. Further studies have highlighted the impact of environmental cues, such as the presence of cognate antigen on naïve T cell behavior [10, 45, 48]. In addition, the use of genetic approaches has provided first insights into the molecular basis of the migratory pathways used by T cells in intact tissues [51, 76]. Finally, mathematical modeling has provided a theoretical framework of migrating T cells at the population level [6, 16, 65, 74]. Together, this has led to several partially conflicting models of T cell migration. The major focus of this review is to relate the proposed T cell migration models to supporting experimental evidence. Other immune cell types, for example, dendritic cells (DC), are also mentioned; however, readers are also referred to published reviews [9, 15].
Concepts of interstitial leukocyte migration
The migratory cycle
In order to understand cell migration within three-dimensional tissues, it is useful to take a look at the basic characteristics of migrating cells. Based on dynamic imaging of isolated cells, such as fibroblasts, on artificial substrates, a paradigm of cellular migration defined by coordinate execution of distinct steps has been proposed (Fig. 1) [42]. These steps comprise the formation of cellular protrusions, preferentially at the existing or prospective leading edge. These membrane extensions can be wire-like and thin (filopodia) or exhibit a sheet-like architecture (lamellipodia). The next step of the cycle is mediated by fastening of these protrusions to the substratum by biochemical bonds. In fibroblasts, integrins fulfill this function by forming stable interactions with the underlying substratum. Contraction of the cell body, as a result of cytoskeletal rearrangements, completes the cycle. Due to adhesion at the leading edge, this contraction leads to forward pull of the trailing edge towards the leading edge. Repeated performance of these steps leads to effective forward migration.
Fig. 1.
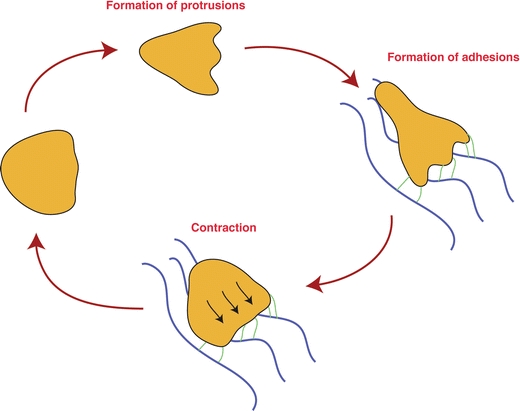
Schematic depiction of the classical migratory cycle. Depicted here is a simplified version of the classical model, explaining cellular migration as a series of individual steps. Step 1: a cell forms protrusions at the leading edge, probing the environment. Step 2: under suitable conditions, receptors at the leading edge (symbolized by green lines) form adhesive bonds with the environment (symbolized by blue lines), providing mechanical traction. Step 3: due to the established mechanical traction, cellular contraction now leads to forward pull towards the leading edge. Repetition of these steps leads to cellular migration. Note that for didactic reasons, the individual steps are depicted as consecutive steps, while in fact they may occur simultaneously. In this scenario, migrating cells would appear to “slide” on the substrate, while continuously maintaining a polarized shape
It is apparent that active cell migration is characterized by polarized cell shape, which is accompanied by asymmetric distribution of adhesion and signaling molecules [42]. Polarity pathways, such as the Par3 and Scribble complexes, represent the molecular machinery responsible for inducing cellular asymmetry (reviewed in [31, 40, 44]). Importantly, generation of a longitudinal anterior–posterior axis through the cell body may cause a directional bias of migrating cells (discussed further below), indicating that polarity pathways may act as critical coordinators of cellular locomotion.
While the migratory model was initially derived from observations of epithelial cells and fibroblasts, migrating leukocytes show, at the morphological level, a similar migration cycle. However, recent studies have revealed that in three-dimensional matrices, certain leukocyte subsets, such as T cells and DC, have the capacity to migrate in the absence of integrins [24, 41]. Thus, rather than being a sine qua non for interstitial migration, the actual usage of integrins may depend on the context of the environment [33, 63] (discussed in more detail below). To achieve integrin-independent motility, migrating leukocytes may generate traction by extending cell protrusions into pockets within the extracellular matrix (ECM), a strategy that has been dubbed “biophysical migration” (Fig. 2) [22].
Fig. 2.
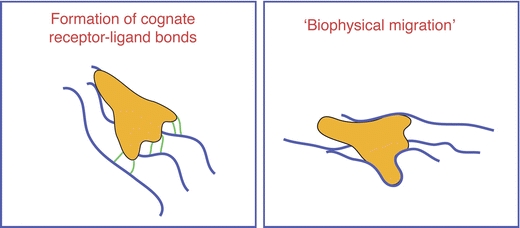
Establishment of mechanical traction. To succeed in forward propulsion, cells need to generate mechanical traction. This can be achieved by conceptually distinct approaches. Left panel: cells may form interactions with the surrounding environment via cognate receptor–ligand bonds (cellular receptors: symbolized by green lines, environmental ligands: symbolized by blue lines). Right panel: as an alternative approach, cells may use biophysical constraints such as pores and tissue-pockets as a non-specific mechanical substrate to establish traction
This simplified outline of the migratory cycle illustrates the basis for the analysis of migratory patterns and structural cues that guide leukocytes through tissues.
Higher-level coordination of lymphocyte migration
While definition of individual steps of the migratory cycle of cells is important for the understanding of interstitial leukocyte migration, it is equally important to develop a basic framework for understanding the behavior of migrating cells in the context of their natural environment. A key question is whether this process is stochastic or deterministic, i.e., to what degree cell-intrinsic or environmental cues regulate lymphocyte locomotion within distinct environments. Such information provides insight into the guidance principles of cells within a given tissue, for example, the influence of chemokine gradients, the role of ECM fibers, and the effect of other cellular components such as antigen-presenting cells on lymphocyte migration. Based on experimental data generated with two-photon microscopy, various fundamental scenarios that may underlie the overall coordination of leukocyte migration have been proposed (Fig. 3).
Fig. 3.
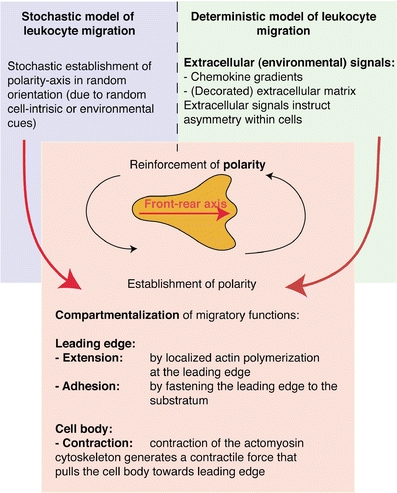
Overview of basic conceptual models explaining coordination of cellular migration
Deterministic models: guidance by the microenvironment
Active guidance by the microenvironment
Mechanical structures, most prominently ECM fibers, may function as guidance scaffolds and direct tissue-infiltrating cells by haptotaxis [24]. Intravital imaging has recently provided evidence for crawling of leukocytes along ECM components, which is consistent with active guidance of leukocytes by the environment [4, 52, 73] (Fig. 4a). Random branching of such “roads” may also add a stochastic element to this model (Fig. 4a, see also below). In addition, chemokine gradients may direct cellular migration by coordinating the cytoskeletal architecture of individual cells [36, 64], and a role of chemokine gradients in the navigation of cells within intact tissues has been surmised [2, 59]. A variety of cells, including stromal cells, leukocytes, or tumor cells, may be the source of chemoattractants giving rise to distinct micro-gradients within a given microenvironment. For instance, a role for CCR7 ligands in the navigation of naïve T cells within the cortex of peripheral LN has recently been demonstrated [3, 77].
Fig. 4.
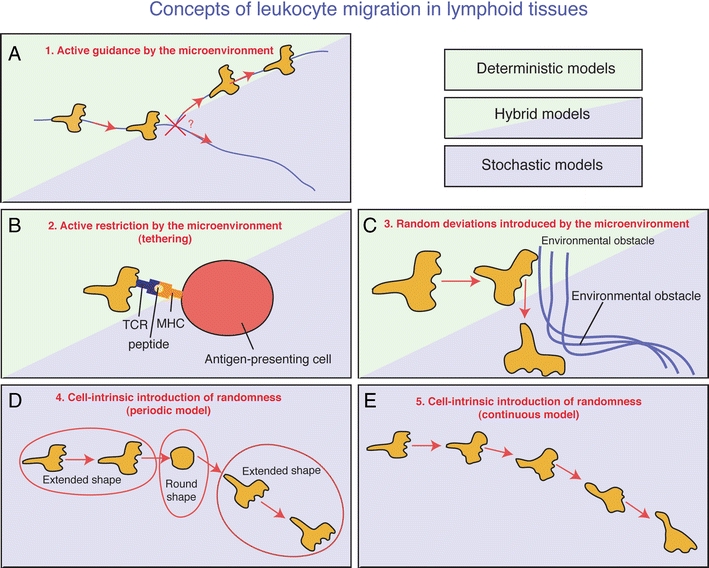
Possible models explaining leukocyte migration within intact biological tissues. a Extracellular fibrous structures (blue lines) provide haptotactic and/or chemotactic stimuli, which promote crawling along the fibers. When encountering an intersection of multiple fibers (cross), the decision as to which branch to follow may be the result of a stochastic process or determined by an unknown instructive environmental signal. b T cells encounter environmental cells presenting cognate antigen in the context of their MHC. This leads to establishment of stable TCR–peptide–MHC interactions. This interaction functions as a “tether,” effectively restricting the migratory potential of the now entrapped T cell. c T cells migrate actively and highly directionally due to conservation of cell-intrinsic polarity pathways and cytoskeletal architecture. Minor environmental obstacles can be circumnavigated without apparent loss of directionality. Only when encountering major environmental obstacles (e.g., dense strands of fibrous tissue or cells with an extended size) the cell is forced to re-orientate and pursue navigation in an alternate directionality. d T cells migrate highly directionally due to cell-intrinsic capacity to conserve cell polarity. Over time, cells lose polarity and revert to a rounded shape. After reestablishment of polarity in a new random direction, the cell proceeds to migrate along the novel polarity axis. Random walk is performed by continuously switching between extended and round shapes. e The cells form cellular protrusions at random positions, either at the existing leading edge or laterally. By following those randomly formed protrusions, the cell performs a random walk
Cell entrapment and cell–cell interactions
Rather than actively guiding and directing leukocytes, the environment may also impose antagonistic effects resulting in cellular entrapment (Fig. 4b). This may be due to mechanical obstacles in the interstitium, such as strands of ECM fibers or vessels, or molecular interactions between migrating cells and their surroundings. For example, the presentation of cognate antigen facilitates prolonged physical interactions between T cells and target cells, such as DC or tumor and infected cells, leading to a migratory stop [10, 35, 45, 47, 49, 52, 57].
Stochastic models: from chaos to fine-tuning of intrinsic migratory properties
Random collisions with environmental obstacles
As an alternative to the deterministic approach, it is possible that migration of cells within tissues is independent of external cues. Instead, the cells could use their intrinsic migratory machinery to maintain directionality for prolonged periods of time. The assumption of such an intrinsic directionality is based on the observation that various cell types have a tendency to migrate straight ahead for particular periods of time, even in the absence of environmental directional cues. For example, cultured fibroblasts show directional persistence for approximately 2 h [27]. In various cell types, there appears to be a preference to form new protrusions at the pre-existing leading edge, a behavior that is partly the result of cell-autonomous signaling pathways [60]. This spatial restriction of the formation or maintenance of the leading edge could impose conservation of cellular directionality. Thus, interstitially migrating cells may maintain directionality until they randomly hit an environmental obstacle. In silico simulations have revealed that such a model may represent the basis of the random migration behavior of naive T cells in LN (Fig. 4c) [6].
Cell-intrinsic generation of randomness
A strictly “leading edge-driven” model of migration would predict that tissue-infiltrating leukocytes migrate in a highly directional manner for infinite periods of time. However, it has long been appreciated that the intrinsic directionality of migrating cells decays spontaneously after prolonged time periods, even in the absence of environmental obstacles [27]. Thus, a cell-autonomous “imprecision” in maintaining directionality may contribute to the observed random trajectories of cells. One means by which this “imprecision” could occur is by periodic switching between directional and non-directional behavior, consistent with a “stop-and-go” mode of migration (Fig. 4d) [71]. The directional state may correspond to a polarized shape, facilitating migration in the direction of the front-rear axis. Conversely, reversion to a round, non-polarized shape could erase spatial memory, thereby randomizing the orientation of the next directional step. As an alternative to periodic switching between directional and non-directional states, migrating cells could form protrusions from lateral portions of the cell body while maintaining polarity, providing a continuous cell-intrinsic mechanism to achieve random directionality (Fig. 4e) [60].
Heterogeneous and homogeneous behavior at the cell or population level
It is important to point out that a stochastic basis of migration at an individual cell level does not necessarily equal chaotic or uncoordinated migration at the population level. While the future position of an individual, stochastically migrating cell cannot be predicted precisely, it is possible to predict the likelihood of a particular behavior. By measuring a sufficient number of real-time migration events, “migration libraries” containing the probability distributions for the performance of all possible migratory behaviors can be created. Pertinent parameters include, but are not limited to, the probability to deviate from the direction of a previous segment with a particular angle (“turning angle”) or to move with a particular speed (“instantaneous speed”). Such libraries can then be exploited for in silico modeling. Figure 5 shows an example of how to calculate the probabilities for the turning behavior.
Fig. 5.
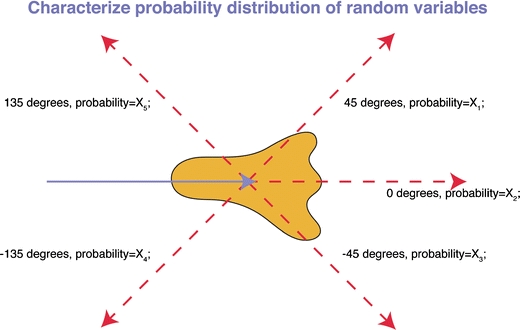
Determination of the probabilities of migratory parameters. Here, we illustrate how to obtain the probability distribution of a particular migratory behavior, the turning angle. The figure depicts the velocity vector of a “present” reference segment of the trajectory (blue arrow). Several potential follow-up velocity vectors are drawn with a red dashed line. The deviation of this future segment in relation to the present segment can be measured. By performing a sufficiently large number of measurements, the probability distribution of this particular behavior can be determined. This approach can be extended to other parameters (for example, speed) to generate a comprehensive library, containing the probability distributions of various migratory behaviors. If T cell migration is indeed a stochastic process, a library of generated probability distributions should allow simulation and prediction of T cell migration
In order to predict the migratory behavior of leukocytes at the population level, it is further necessary to determine whether individual cells display homogeneous migratory characteristics within some random distribution of behaviors or whether a population is composed of mixture of cells with heterogeneous migratory properties. The “stop–start” behavior of lymphocytes provides an example of this: When individual cells are considered over a short time interval, we might consider them motile or resting depending on whether they are in “stop” or “start” mode [49] (Fig. 6). However, over longer periods of time, we might conclude that cells behave in a more homogenous manner but were just caught in different phases of their motion (Fig. 6). Figure 6 exemplifies representative homogeneous and heterogeneous population behaviors of migrating cells. These considerations are corroborated by studies using fibroblasts, which exhibit homogeneous migratory behavior when migrating spontaneously [27] but show individually distinct responsiveness to chemokines [66]. Moreover, evidence for heterogeneous migratory behavior of a genetically identical cell population was recently provided by detailed measurements of keratocyte shape and migration characteristics [37]. Studies focusing on lymphocyte migration often do not address this issue. A more direct analysis, using statistical mathematical tools, which could differentiate between homo- or heterogeneous nature of interstitially migrating leukocytes, should provide a better understanding of the regulation of this process. Cell population heterogeneity would have to be incorporated into the “migration library” by assigning individual cells with a unique migration signature. Once a comprehensive library of the probabilities of distinct migratory parameters has been established, it should be possible to accurately simulate (and predict) the behavior of randomly migrating T cells at the population level.
Fig. 6.
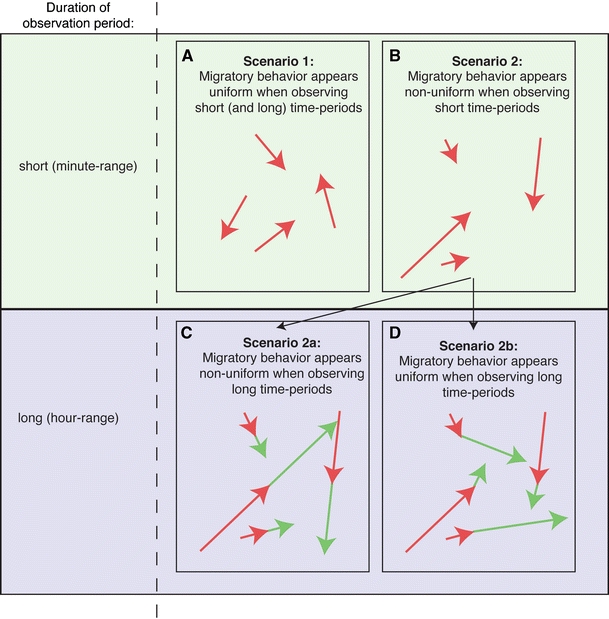
Migratory characteristics of a cell population. Visual inspection of migrating cells over short time periods can reveal various distinct apparent phenotypes. First, the migrating cells can show homogeneous behavior with only minor variability due to random fluctuations (a, arrows symbolize displacement vectors over short time periods). Alternatively, distinct cells could show qualitatively distinct behavior, e.g., some cells appearing restricted, while others exhibit a pronounced forward momentum (b). This latter scenario could arise for various reasons. One possible explanation is that the apparent variability in behavior is due to the presence of cells with truly distinctive migratory properties. In that scenario, the observed migration properties of individual cells at one particular time point should remain constant during consecutive time points. Therefore, under these circumstances, measurement of migratory properties for individual cells over longer time periods would also reveal a population of individual cells with highly variant behavior (c, red and green arrows symbolize displacement vectors of consecutive segments of the trajectory). Alternatively, individual cells may periodically switch between different behavior types (e.g., “stop” and “go” behavior). In this scenario, the measured motility differences would be averaged out over time, and this measurement would then reveal a relatively homogeneous population (d, red and green arrows symbolize displacement vectors of consecutive segments of the trajectory over short time periods). Thus, careful measurement of cellular migration over distinct time intervals can yield profound insights into the regulatory basis of T cell migration
Altogether, dynamic imaging of intact tissues has revealed unprecedented insights into the locomotion of leukocytes and has inspired partially conflicting migration models. In the following sections, we will review the experimental findings in more detail in the context of the proposed theoretical migration models. The key findings of the various original papers are also summarized in Tables 1, 2, 3, 4, and 5.
Table 1.
Stochastic model
| Selected key papers | Investigated tissue | Investigated cell type | Experimental approach |
|---|---|---|---|
| Miller et al., Science [49] | Lymph node | Adoptively transferred naïve T cells | Mathematical modeling (square root of time vs. displacement analysis) |
| Miller et al., Proc Natl Acad Sci USA [48] | Lymph node | Adoptively transferred naïve T cells | Mathematical modeling (distribution of velocity vectors/binomial model) |
| Kawakami et al., J Exp Med [35] | Spinal cord slices, rat experimental autoimmune encephalitis model | Adoptively transferred effector T cells | Mathematical modeling (velocity vector sum) |
| Mrass et al., J Exp Med [52] | Lymph node, explanted tumor tissue/intravital experiments | Endogenous/adoptively transferred effector T cells | Mathematical modeling (square root of time vs. displacement analysis) |
| Worbs et al., J Exp Med [77] | Lymph node | Adoptively transferred T cells | Mathematical modeling (square root of time vs. displacement analysis) |
| Kim et al., Nature [38] | Brain, LCMV-infection model | Adoptively transferred T cells | Mathematical modeling (square root of time vs. displacement analysis) |
| Bartholomäus et al., Nature [5] | Rat experimental autoimmune encephalitis model, intravital (spinal window) | Adoptively transferred T cells after infiltrating neuronal parenchyma | Analysis of T cell distribution within tissue |
Table 2.
Environment-mediated entrapment
| Selected key papers | Investigated tissue | Investigated cell type; cell/structure that causes entrapment | Experimental approach |
|---|---|---|---|
| Mempel et al., Nature [45]; Miller et al., J Exp Med [47] | Lymph node (intravital imaging) | Adoptively transferred naïve T cells; subcutaneously injected cognate dendritic cells | Image analysis: comparing the speed of T cells in the presence and absence of antigen-pulsed dendritic cells; direct visualization of interactions |
| Kawakami et al., J Exp Med [35] | Spinal cord slices, rat experimental autoimmune encephalitis model | Adoptively transferred cognate and non-cognate effector T cells | Manual image analysis: comparison of migratory activity of T cells that recognize or do not recognize brain-associated peptides |
| Mrass et al., J Exp Med [52] | Lymph node, explanted tumor tissue/intravital experiments | Endogenous/adoptively transferred effector T cells/tumor cells | Manual image analysis: comparing the migratory properties of cognate and non-cognate T cells; direct visualization and measurement of interactions |
| Kim et al., Nature [38] | Brain, LCMV-infection model | Adoptively transferred T cells; antigen-presenting cells within the brain | Manual image analysis: comparison of migration in the presence and absence of anti-class I MHC antibody |
| Kim et al., Nature [38] | Brain, LCMV-infection model | Adoptively transferred T cells | Mathematical modeling (square root of time vs. displacement analysis) |
| Bartholomäus et al., Nature [5] | Rat experimental autoimmune encephalitis model, intravital (spinal window) | Adoptively transferred T cells after infiltrating neuronal parenchyma | Analysis of T cell distribution within tissue |
Table 3.
Active environmental guidance model
| Selected key papers | Investigated tissue | Investigated cell type | Experimental approach/investigated environmental cue |
|---|---|---|---|
| Bajenoff et al., Immunity [4] | Lymph node | Adoptively transferred naïve T cells, B cells | Manual image analysis; genetically tagged reticular network |
| Mrass et al., J Exp Med [52] | Tumor tissue | Endogenous/adoptively transferred effector T cells | Manual image analysis; extracellular matrix detected by second-harmonic generation |
| Castellino et al., Nature [14] | Lymph node | Adoptively transferred T cells | Chemokine-secreting dendritic cells; measurement of probability to perform fast movement towards dendritic cell |
| Boissonnas et al., J Exp Med [8] | Tumor tissue | Adoptively transferred T cells | Manual image analysis: blood vessels |
| Wilson et al., Immunity [73] | Brain slices, toxoplasma infection model | Endogenous/adoptively transferred effector T cells | Manual image analysis: extracellular matrix detected by second-harmonic generation |
| Bartholomäus et al., Nature [5] | Rat experimental autoimmune encephalitis model, intravital (spinal window) | Adoptively transferred T cells immediately before and after extravasation | Analysis of T cells crawling along interior and exterior vascular wall |
Table 4.
Environmental collision model and other simulation-based models
| Key paper | Investigated tissue | Investigated cell type | Experimental approach |
|---|---|---|---|
| Beltman et al., J Exp Med [6] | Lymph node | T cells | Computer simulation (cellular Potts model) |
| Riggs et al., J Theor Biol [65] | Lymph node | T cells | Computer simulation (agent-based model): evidence that chemotaxis has a negative impact on T cell activation and expansion |
Table 5.
Molecular cues that regulate cell migration
| Selected key papers | Investigated tissue | Mechanism/regulatory effect | |
|---|---|---|---|
| Mrass et al., J Exp Med [52] | Tumor tissue | Adoptively transferred effector T cells | Stimulation of cell-intrinsic migratory activity by TCR-mediated signaling |
| Fischer et al., PNAS [20] | Lymph node | Adoptively transferred naïve T cells | Tonic stimulation of TCR by self-antigens is required to maintain active T cell migration |
| Worbs et al., J Exp Med [77] | Lymph node | Adoptively transferred naïve T cells | Stimulation of T cell-expressed CCR7 by environmental CCL19 and CCL21 is required for rapid T cell migration |
| Asperti-Boursin et al., J Exp Med [3] | Lymph node | Naïve T cells “overlaid” on lymph node slices | CCR7 expression by T cells is required for optimal motility |
| Nombela-Arrieta et al., J Exp Med [56] | Lymph node | Adoptively transferred naïve T cells | DOCK2 (an activator of G-protein coupled signaling) needs to be expressed by T cells to ensure effective forward locomotion within lymph node |
| Mrass et al., Immunity [51] | Tumor tissue | Adoptively transferred effector T cells | CD44 expression by T cells is required to effectively maintain stable polarity; this leads to optimized tumor cell scanning and improved tumor-rejection capacity |
| Lämmermann et al., Nature [41] | Lymph node, dermis | Injected dendritic cells | Normal migration occurs independently of integrins |
Visualization of T cells in lymphoid tissues
The behavior of naïve T cells within LN has been studied in great detail over the past several years [4, 34, 45, 49, 52, 67, 77]. T cells have been tracked both under steady-state conditions and during the early priming phase of the immune response to model antigens, as well as in LN draining sites of pathogen-infected skin [18, 34]. Both explanted tissues and LN in vivo have been used, and experiments in both systems have shown largely overlapping results.
The multi-photon technology also enables the visualization of structural tissue components. In particular, second-harmonic generation signals allow the identification of ECM components, such as collagen fibers [25, 50, 52]. Furthermore, transgenic mouse models that enable co-visualization of migrating T cells and the fibroblastic reticular cells (FRC) within LN have been developed [4]. These features have made it possible to correlate T cell migration in relation to underlying tissue structures.
Regulation of lymphocyte migration in lymph nodes
Evidence for random naïve T cell migration in the steady state
The recent advances in intravital imaging have enabled addressing conceptual questions relating to the role of putative exogenous and endogenous directional cues involved in T cell migration. Observation of naïve T cells has revealed highly active migration within peripheral LN [48, 49], which is in contrast with low migratory activity of isolated, unstimulated, naïve T cells in vitro [17]. This suggests that intact LN provide a promigratory environment for T cells, which supports their search for rare cognate antigen-presenting cells during immune responses. Based on the patterns of chemokines expressed in specific compartments within LN, it was proposed that lymphocyte migration may be regulated by an intricate network of chemokine trails [19]. However, at the population level, no apparent evidence of higher-level coordination of naïve T cell migration was found [49]. Furthermore, mathematical modeling of migrating cells supported a random walk model, similar to particles exhibiting Brownian movement [7]. From these observations, it was concluded that naïve T cell behavior in intact LN is consistent with a stochastic cell migration model [71].
Evidence for active guidance of naïve T cells by the FRC network
Germain's group employed multi-photon microscopy to specifically define the role of environmental structures in the guidance of naïve lymphocytes within LN [4]. T cells labeled with the red fluorescent dye SNARF-1 were adoptively transferred into mice in which the FRC network was marked with GFP. Confocal and multi-photon microscopy showed that migrating T cells were associated with FRC fibers. This was further supported by electron microscopy, where cytoplasmic T cell extensions (microvilli) contacted the FRC network. By correlating directional turns of T cells with the presence or absence of GFP-signals, they further found that the majority of sharp turns was associated with the presence of underlying FRC. Based on these results, it was proposed that the environment within the LN is the major determinant of T cell migration. Since T cells reaching fiber bifurcations randomly decide on which branch to continue their journey, this may provide an explanation for the observed randomness of the naïve T cell population termed “guided randomness” of T cell migration, [46]. Furthermore, as the FRC network is absent from B cell follicles, but rather contains a distinct follicular DC network, this could contribute to the segregation of B cells and T cells within LN [4].
While the model of guided randomness is very attractive, the analysis only included cells performing sharp turns, i.e., turning angles in excess of 40°, leaving out a large fraction of the migration events [45, 52]. Thus, it remains to be determined whether cells are in contact with the FRC under all conditions. Second, in areas of high FRC density, T cells may contact FRCs merely by chance due to their close physical proximity. Thus, even when an enrichment of turning behavior by T cells that contact the FRC can be determined, this may in fact be coincidental. The existence of a correlation between contacts with the FRC and specific cellular migratory behavior could be more rigorously tested by generating digital maps of the FRC network together with images of T cell shapes and trajectories. The dependence of the cell shape and the direction of cellular motion on the proximity of cells to FRCs could then be analyzed by automated image analysis. Images of comparable fiber density, but with a “scrambled” orientation, could be used as a control. Such an analysis would clarify the basic mechanism of T cell navigation within LN that would then need to be further refined and extended to other microenvironments.
Stop-and-go behavior of migrating lymphocytes
As mentioned above, tracking of individual naïve T cells within LN has highlighted a “stop-and-go” mode, in which the migratory status of individual cells fluctuates between active locomotion and periods of low speed [49, 71]. Thus, periods of directional migration occur for only limited periods of time and are followed by a migratory stop in which cells exhibit a round shape and subsequently continue migration at a random angle relative to their previous paths. Based on these findings, it was proposed that rearrangement of the cytoskeleton during migrational pauses may lead to the formation of a new front-rear axis resulting in a random change in directionality. Alternatively, migrating cells may hit obstacles, such as ECM fibers or cells, in their path. It has been shown that naïve T cells engage in short-term encounters with DC, even in the absence of cognate antigen [10, 45, 47]. In addition, some arrested cells exhibit elevations in their intracellular Ca2+ levels, which is consistent with physical contact with antigen-presenting cells [3]. The relative contributions of these factors to the heterogeneous movement of naïve T cells require further experimental validation.
In silico modeling has recently been used to understand the basis of the stop-and-go mode of naïve T cells [6]. Based on computer simulations, these authors concluded that the experimentally observed velocity fluctuations could be the consequence of collisions of migrating T cells with environmental obstacles, rather than a cell-intrinsic program of migratory changes. In other words, it was proposed that migrating T cells move at a certain average speed, which fluctuates because of the heterogeneity of the environment. This interpretation was based on the analysis of the autocorrelation function of speed fluctuations around the mean in simulated and experimental migrating cells. The analysis shows to what extent the speed deviates from the average at a given follow-up time point from the (arbitrary) initial time point. If the “stop-and-go” motion had intrinsic periodicity, this parameter would exhibit time-periodic behavior irrespective of the length of the time series used in calculation. However, measurement of long speed sequences in experimental T cells has shown that the autocorrelation function of speed fluctuations approaches zero with time more smoothly when longer time series is used in calculation, although it might seem periodic when calculated from a short sequence of speed fluctuations. This was taken to indicate that after a relaxation period, the speed becomes independent of the behavior at the initial time point, and that the periods of restricted motion are randomly distributed. However, although the apparent time periodicity becomes somewhat less observable, it is not extinguished even after a time series of speeds of 30–40 min. Thus, while these data argue against a scenario where T cells switch between distinct states of motility after a very narrowly defined time interval, they do not eliminate the possibility that T cells indeed switch between “stop” and “go” states due to an intrinsic mechanism, but do that in time intervals selected from a broad probability distribution with a well-defined median. In addition, even if the time interval between “stop” and “go” modes is indeed random and consistent with an “environmental collision model,” it could be also explained as a result of complete randomness in the cell-intrinsic mechanism. Furthermore, it needs to be pointed out that Beltman et al. visualized a polyclonal T cell population which, for example by different T cell receptor (TCR) affinities with self antigen, may mask a cell-intrinsic motility program. In other words, while their finding supports a stochastic model of migration, it does not definitively distinguish between the randomness introduced by the environment or a cell-intrinsic mechanism.
While simulations are very useful in the development of new migration models, it is still necessary to carefully analyze experimental data in order to conclusively validate these models. In order to confirm the role of the environmental components as “obstacles,” it will be necessary to develop tools enabling measurement of this phenomenon as captured by dynamic microscopy in an automated, non-biased manner. This could be achieved by continuous tracking of migrating cells, along with an automated recognition of the putative obstacles (e.g., DC or FRC). Measurement of whether the probability of migrating T cells that actively turn is increased after DC contacts would lend further evidence for the collision model in a real tissue.
Modulation of naïve T cell migration by chemokines
Chemoattractant signals have long been suspected to orchestrate lymphocyte migration within tissues in vivo and thereby to contribute to the sequestration of B and T cells to specific regions within LN, such as B cell follicles and T cell zones, respectively. Indeed, knockout studies of chemokines/chemokine receptors, such as CCR7 or CXCR5 and their ligands, have shown profound disorganization of the LN microenvironment, indicating a role for these molecules in lymphocyte homing as well as positioning within these organs [21, 54].
What then are the signals that regulate the spontaneous steady-state migration of T cells within the paracortex of LN? Several groups have investigated the effects of exposure of naïve T cells to pertussis-toxin (PTX), an inhibitor of Gαi-coupled receptors, including chemokine and sphingosine-1-phosphate receptors. PTX-treated naïve T cells exhibited significantly reduced migratory speed and displacement as well as sharper turning angles when compared to controls [3, 30, 58]. In addition, Okada and Cyster found that PTX treatment led to a misplacement of naïve T cells within the T cell zones, suggesting that their internal positioning depended upon Gαi-coupled receptor signaling. These data were further corroborated by studies using T cells from mice deficient in DOCK2, a downstream mediator of chemokine-induced signaling. DOCK2-deficient T cells showed a severe reduction in interstitial migration [56]. The speed of T cell migration was markedly reduced and, more strikingly, the motility coefficient approached a value close to zero. This result shows that while DOCK2-deficient cells retain a basal capacity to wiggle back and forth, they are incapable of achieving sustained forward propulsion.
CCR7 represents the primary candidate chemokine receptor in the regulation of interstitial migration within intact LN since it is highly expressed by naïve T cells, and the corresponding ligands, CCL19 and CCL21, are present in the T cell zones of LN [21, 54]. Indeed, time-lapse microscopy has revealed that CCR7-deficient naïve T cells in wild-type LN or wild-type T cells in plt/plt mice, which lack expression of CCR7 ligands in LN, migrate at significantly reduced speed [3, 77]. Notably, the magnitude of this reduction was less pronounced than after treatment with PTX, indicating that chemoattractants other than CCR7 ligands may be involved in the regulation of naïve T cell motility [3, 30]. Mathematical analysis of the migratory behavior of CCR7-deficient T cells revealed that while the speed of the migrating cells was reduced, the overall directionality was unaffected, i.e., cells maintained their random walk behavior [77]. This suggests that contact-dependent chemokines on the FRC act at a small tissue scale to promote motility of naïve T cells, but that directionality, at least in the steady state, depends on other cues such as the cell-intrinsic migratory machinery.
The situation may change during an ensuing immune response. Indeed, by analyzing the motility of T cells in the proximity of DC, Castellino et al. found that CD8+ T cells showed a directional bias towards DC that were contacted previously by stimulated CD4+ T cells [14]. This directional bias was dependent on CCR5, which was upregulated by CD8+ T cells in the inflamed LN. Together, these results suggest that under particular circumstances, chemokines may establish medium-ranged gradients within the LN that facilitate initiation of cytotoxic T cell priming.
Role of T cell receptor signaling in naïve T cell migration
Fischer et al. tested the role of signals delivered through the TCR on lymphocyte motility within the LN environment [20]. CD4+ OT-II transgenic T cells were transferred into wild-type mice or mice deficient for MHC-II. This setup allowed comparison of T cell migration in the presence or absence of tonic stimulation by self-ligands presented by MHC-II. Multi-photon microscopy performed on LN isolated at various time points after adoptive transfer revealed that while in the control group motility remained stable, naïve T cells continuously decreased their migratory activity in the absence of MHC-II. In addition, the cells showed a reduced ability to interact with antigen-presenting DC. The absence of continuous TCR stimulation was associated with decreased Rap1 and Rac1 activity, small GTPases involved in cytoskeletal remodeling [20]. These results suggest that self-ligand stimulation through the TCR represents a prerequisite for the spontaneous migration of naïve T cells. The precise mechanisms underlying these interesting observations require further studies.
The role of integrins during migration of leukocytes in the interstitial space
Studies of mesenchymal cells or tumor cells have demonstrated an important role of surface adhesion receptors, such as integrins or CD44, in the migratory cycle by establishing physical bonds with the substratum (Fig. 1). The role of these molecules in interstitial migration of leukocytes is less clear. Experiments using function-blocking antibodies against beta-1, -2, -3, and alpha-V integrins alone or in combination did not influence the migration of CD4+ T cells in collagen lattices in vitro [23]. Furthermore, it was reported that chemokine-mediated activation of integrins occurs only when cells are exposed to sheer stress and therefore within the vasculature, but not in extravascular sites [75]. Consistently, interstitial migration of T cells in LN, an environment that is protected from shear stress, was only marginally affected in T cells lacking LFA-1 or after blockade of VLA-4 [75]. This provides a potential explanation for the fact that chemokines present in the T cell zones of LN are promigratory but do not induce inappropriate sticking of migrating cells to other cells in the interstitial space. However, time-lapse imaging of in vitro cultured lymphocytes has shown that decoration of antigen-presenting cells with chemokines induces tethering of contacting T cells in an integrin-dependent manner, indicating that integrins may play a subtle regulatory function that is overlooked when focusing on track-based parameters [26].
Recent genetic studies have further advanced our understanding of integrins in this process. Lammermann et al. generated DC devoid of integrins [41] and monitored migration of integrin-deficient DC after their injection into the skin of wild-type animals. Strikingly, their migration to the draining LN was unaffected. Furthermore, multi-photon microscopy revealed that interstitial migration of integrin-deficient DCs in lymphoid tissue occurred with similar efficiency as compared to wild-type controls. Similarly, by utilizing VavNULL mice, which have a defect in downstream integrin-mediated signaling, it was revealed that directional migration of neutrophils within intact tissues was unaffected [29]. These results show that integrins do not represent an absolute requirement for interstitial leukocyte migration and suggest that leukocytes generate mechanical traction by alternative means [22].
Nevertheless, the observation that integrin-signaling-deficient VavNULL neutrophils exhibit a reduction of speed and polarity [29] may indicate that that integrins do impact on the quality of interstitial leukocyte migration. Furthermore, a recent in vitro study using DC suggested a switch from an integrin-dependent to an integrin-independent mode of cellular migration (see above) [63]. Blocking signal transduction from the integrin receptor to the actin cytoskeleton leads to an increased rate of actin polymerization, which appears to function as backup mechanism that ensures active migration even under conditions that do not support integrin ligation. Consistent with this notion, a recent in vitro study showed that T cells display qualitatively distinct migratory behavior on high-adhesive and low-adhesive substrates [33]. While compelling, it still needs to be determined whether facultative ligation of integrin receptors plays a similar role in shaping leukocyte migration within intact tissues.
Migratory behavior of effector T cells in the periphery
Following antigenic activation, naïve T cells differentiate into effector cells, which are capable of trafficking into peripheral organs in order to fight pathogens or tumor cells. Several intravital imaging models of inflammatory tissues have been established, including tumor tissues as well as central nervous system (CNS) tissues with autoimmune and infectious diseases [52, 55, 73]. Tracking of effector T cells at such sites relies on the same principles as described for lymphoid tissues, including maintenance of a physiologic environment.
Migratory behavior of tumor-infiltrating T cells
Several intravital multi-photon microscopy models for tracking tumor-infiltrating T cells (TIL) have been described in the past few years, which either rely on the induction of an endogenous anti-tumor associated antigen (TAA) immune response or the adoptive transfer of TAA-specific T cells [8, 11, 51, 52]. Since both approaches have certain advantages over the other, ideally they should be combined.
The advantage of the endogenous immune response model is that it more closely resembles physiologic conditions, which may, for example, be achieved in patients. However, the quality of the anti-tumor immune response may differ between individual animals making reproducibility of experiments more challenging. In addition, tracking of T cells is usually based on transgenic expression of fluorophores by T cell-specific promoters, for example, regulatory elements of the CD4 locus [52]. In this case, different T cell subsets, such as effector or regulatory cells, cannot be distinguished. We have previously made use of a vaccination approach, where animals bearing subcutaneously injected TC-1 tumors, which express the human papilloma virus type 16 (HPV-16) E7 antigen, were serially vaccinated with human serotype 5 adenovirus expressing E7 (AdHu5E7; [39]). This results in a robust polyclonal anti-E7 cytotoxic T cell response capable of rejecting TC-1 tumors [52]. T cells were visualized by virtue of transgenic GFP expression, and their behavior was compared in progressing (mock-vaccinated animals) versus regressing (vaccinated animals) tumors.
The advantages of the adoptive transfer model are that titrated numbers of naïve or effector T cells of known specificity are injected, which facilitates highly reproducible conditions. In addition, genetically modified T cells, for example, T cells deficient in certain adhesion or cytotoxic molecules, can be used. Moreover, retroviral transduction allows for the expression of fluorescently tagged molecules within the cells, thereby allowing their tracking within T cells at the subcellular level in vivo. We and others have employed transgenic, ovalbumin-specific CD8+ T cells from TCR transgenic OT-I mice [8, 11, 52]. The behavior of these cells was then studied in animals carrying EL4 or E.G7-OVA thymoma cells, which do not or do express ovalbumin, respectively. Under these conditions, effector T cells home to the same extent into both tumors; however, regression is only induced in E.G7-OVA tumors. Thus, similar to the above models, T cell behavior can be studied in both regressing and progressing tumors. Analysis of migratory parameters in those distinct settings has revealed diverse requirements, such as exposure to TCR signals, for optimal T cell migration (see below). A caveat with adoptive transfer of high numbers of TCR-transgenic effector T cells into mice is that this may represent a non-physiological setting. For example, the T cells are stimulated ex vivo, and their TCR has a uniform affinity towards model antigens. Therefore, a combination of endogenous and adoptive transfer models will provide an optimal experimental strategy.
Guided random migration of TIL?
Similar to naïve T cells within LN, analysis of OT-I TIL tracks within EL4 tumors, i.e., in the absence of cognate antigen, revealed a highly migratory phenotype, consistent with an active screening mode of effector CTL [8, 52]. Visual inspection of the tracks did not reveal an apparent high-level organization of the trajectories, arguing against guidance by long-range chemokine gradients. This was further confirmed by mathematical modeling using plots of the dependence of mean displacement on square root of time, revealing apparently random migration of TIL [52]. These results support a stochastic model of T cell migration at a peripheral site, at least in the absence of cognate antigen.
Not surprisingly, the migratory characteristics of TIL within E.G7-OVA tumors or after vaccination of animals carrying TC-1 tumors were different. Under these conditions, a proportion of TIL was restricted in their motility, and co-visualization of T cells and tumor cells revealed long-term interactions between antigen-specific TIL and tumor cells [52]. Coinciding with tumor cell destruction, the proportion of restricted TIL decreased with time, and cells resumed migration. Analysis of non-restricted, migratory cells at all time points revealed random migratory patterns also in regressing tumors.
Of note, time-course analysis of adoptively transferred cytotoxic T cells that cannot recognize TAA revealed that their migratory activity decreased over time both in regressing and non-regressing tumors, which is in contrast to antigen-specific cells that increase their speed in regressing tumors [52]. This suggests that signals delivered through the TCR activate a promigratory program in effector T cells similar to that acting on naïve T cells in LN [20]. These results indicate that cognate antigen, as a component of the environment, influences the migratory behavior of TIL.
While the observed random directionality of the measured T cell trajectories challenges the notion that the environment, i.e., ECM components, actively dictates the directionality of tissue-infiltrating effector T cells similarly to naïve T cells in LN, it is still possible that at least over short periods of time, active guidance plays a role in TIL navigation. Indeed, some TIL were found to crawl along ECM fibers as detected by second-harmonic generation signals [8, 52]. In addition, migrating TIL sometimes followed blood vessels. These cells displayed increased polarity indices, i.e., a more elongated morphology, as compared to T cells that were not in contact with the vasculature [8]. However, neither of these latter studies systematically analyzed the relationship of ECM fibers, blood vessels, and TIL in an automated manner. This will be of importance, in particular, in areas of high fiber density where visual inspection of image sequences is insufficient to obtain an unbiased quantification of the association of migrating cells with environmental cues.
The role of CD44 in TIL migration
To gain further insight into the question of guided migration of TIL, we hypothesized that, if indeed cell–matrix interactions were important, specific molecules may regulate this process. In this context, CD44 appeared to be an interesting candidate due to its dual function as receptor for ECM components and, intracellularly, as a regulator of the cytoskeleton. Specifically, the extracellular domain has been shown to mediate adhesion to hyaluronic acid, osteopontin, and fibronectin [61, 62], while the intracellular portion of CD44 contains several docking sites for signaling molecules such as the ezrin–radixin–moesin (ERM) family members that may regulate the cytoskeleton [12]. CD44 has also been implicated in promoting cellular polarity [1, 43]. Thus, CD44 appeared ideally suited to integrate environmental information and relay this information to the migratory machinery of cells.
To pursue this idea further, we analyzed the migratory behavior of CD44-deficient effector T cells in our adoptive transfer model. Importantly, CD44-deficient cytotoxic T cells were indistinguishable from their wild type counterparts with regard to activation in response to cognate antigen, effector T cell generation, and tissue homing capacity [51]. Therefore, it was possible to use this system to address the role of CD44 in interstitial migration of effector CTL. Using dynamic multi-photon imaging, analysis of migratory parameters, including speed, confinement ratio, and displacement, revealed that the motility of CD44-deficient TIL was significantly reduced both in the presence and absence of cognate antigen. Reconstitution of the cells with CD44 rescued the migratory defect, indicating that the effect was a direct consequence of the absence of CD44.
To test whether this promigratory effect of CD44 was due to its interactions with the ECM, we tested the binding of known ligands to CTL in vitro. Hyaluronic acid bound to freshly stimulated (day 2 after activation) CD8+ wild type, but not CD44-deficient T cells. However, CD44 expressed by terminally differentiated effector cells (>day 7 of culture) failed to bind hyaluronic acid and a variety of other potential ligands [51], arguing that CD44 mediates its promigratory effect independently of its receptor function. To more specifically address this question, we generated CD44 deletion mutants lacking either the extra- or intracellular domain, and then introduced these mutants into CD44-deficent T cells. Analysis of T cell migration in collagen matrices, where CD44-deficient T cells also showed a migratory defect, revealed that as expected, full-length CD44 rescued the migratory defect of CD44-deficient cells [51]. In contrast, the CD44 mutant devoid of the intracellular domain did not restore motility. Strikingly, the CD44 mutant lacking the extracellular domain retained the capacity to rescue the migratory defect. Together, these results corroborated the notion that CD44 acted independently of extracellular cues.
A potential mechanism for cell-autonomous promotion of T cell migration by CD44 is its effect on cell polarity [1]. Quantification of the percentage of effector T cells displaying a uropod, a key feature of polarized T cells, revealed a significant decrease in polarized T cells in the absence of CD44 both in vitro and in intact tumors [51]. Dynamic analysis of polarized cell shapes further revealed that while wild-type cells with a uropod maintained an extended shape for prolonged periods of time, in the absence of CD44, the phenotype of individual cells changed frequently between extended and round shapes. Thus, CD44-deficient T cells successfully initiated cell polarization but were defective in the capacity to maintain polarity.
To genetically address a potential role of CD44 in the regulation of polarity, CD44 mutants lacking the ERM-binding domain were generated. Recruitment of phosphorylated ERM to the membrane is involved in the generation of a polarized shape in a T cell line [43]. Indeed, CD44 deletion mutants lacking the ERM-binding domain failed to rescue the migration defect [51].
The effects of CD44 deficiency within the intact tumor microenvironment not only resulted in reduced migration but also in decreased scanning efficiency of tumor cells by TIL [51]. Nevertheless, once physical contacts were established between CD44-deficient TIL and tumor cells, the interaction times were the same as observed for wild-type cells. At the macroscopic level, initial reduction of the tumor volume by adoptively transferred TIL was achieved to a similar degree by wild-type and CD44-deficient T cells. However, at later time points, animals treated with CD44-deficient T cells showed a significantly accelerated outgrowth of tumors. This may be explained by the fact that in the early phases of tumor rejection, TIL migration may not be as important as later on, since tumor cell density is very high, and TIL need not transverse large distances to find target cells. In contrast, in regressing tumors, the numbers of tumor cells decrease, and migration-deficient TIL may simply overlook residual cells in the tumor bed.
Effector T cell behavior in the inflamed central nervous system
The CNS represents an immuno-privileged site that is protected from inflammatory insults by the blood–brain barrier [53]. Nevertheless, this barrier can be breached under particular conditions, such as during autoimmune and certain infectious diseases. We and others have recently investigated the behavior of effector T cells in the inflamed brain during Toxoplasma gondii or lymphocytic choriomeningitis virus (LCMV) infections as well as in the spinal cord during experimental allergic encephalitis [35, 38, 55, 73].
CD4+ T cell behavior in experimental autoimmune encephalomyelitis
Kawakami et al. adoptively transferred fluorescently labeled, myelin basic protein-specific CD4+ T cells into recipient Lewis rats, which leads to the induction of experimental autoimmune encephalomyelitis after 3–4 days [35]. Live spinal cord slices were then imaged by multi-photon microscopy. Analysis of the migratory behavior of T cells revealed several interesting insights. First, the observed adoptively transferred migratory population was heterogeneous, with the displacement of some cells being restricted to a circumscribed area, while other cells moved forward effectively. In contrast, T cells of unrelated specificity showed unrestricted motility. Together, similarly to tumor tissue, these results indicate that recognition of cognate antigen leads to a restriction of T cell motility in the inflamed CNS.
Visual inspection of the migrating cells did not reveal an apparent global coordination of their trajectories. Indeed, computation of the vector sum of the velocities of the observed cells revealed a value approaching zero. This is consistent with an equal probability of moving in any direction and argues against long-range chemotactic guidance of tissue-infiltrating T cells but, conversely, suggests a random walk behavior. Further analysis showed that active migration of T cells was followed by stop periods [35], reminiscent of the behavior of naïve T cells in LN. This behavior was observed both in the presence and absence of cognate antigen, indicating that the stop periods are not related to antigen recognition. These data would support a cell-intrinsic model of migration resulting in stochastic directionality decision by CD4+ effector T cells in the periphery. Further studies are required to dissect the mechanistic basis of this behavior and to determine whether molecular cues similar to CD8+ effector CTL, such as CD44, regulate helper T cells.
In a subsequent study, Flügel's group used intravital microscopy to directly explore the events during and after T cell homing to the inflamed CNS [5]. They found that effector T cells used pial blood vessels as primary pathways to enter the brain parenchyma. Intriguingly, vessel-associated T cells crawled along the vasculature, both preceding and following diapedesis, indicative of environmental-guided migration at this particular stage. After extravasation, T cells engaged in cognate interactions with perivascular meningeal phagocytes, followed by active migration into the parenchyma of the CNS. To dissect the influence of antigen recognition on brain-infiltrating T cells more directly, the behavior of OT-I T cells in the presence and absence of phagocytes loaded with cognate antigen was compared. As expected, long-term T cell–phagocyte interactions depended on the presence of specific antigen. Interestingly, exposure to cognate antigen was also required to optimize tissue infiltration after extravasation, consistent with the requirement of T cell restimulation to activate promigratory signal-transduction pathways. These results corroborate the role of environmental factors, as well as cognate antigen, as two critical cues in the regulation of interstitial T cell migration.
Evidence for environmentally determined migration during T. gondii-induced encephalitis infection
We have recently visualized the T cell response in the brain of toxoplasma parasite-infected mice [73]. In this model, recipient mice are infected intra-peritoneally with toxoplasma tachyzoites, which results in brain inflammation approximately 4 weeks later. We tracked both endogenous polyclonal T cells in infected DPEGFP mice, as well as adoptively transferred antigen-specific OT-I cells in mice infected with T. gondii expressing ovalbumin.
Analysis of the endogenous T cells revealed a subset of cells that was highly migratory, as well as a subset that was highly restricted, suggestive of cognate interactions. In the adoptive transfer model, a large proportion OT-I T cells initially showed limited displacement, while at later time points, coinciding with reduced levels of toxoplasma parasites, the fraction of freely moving cells was increased, reminiscent of the findings in the tumor studies. At very late time points, the migratory activity again decreased, which correlated with a re-emergence of the pathogen load. Together, these findings support a model, where the presence of cognate antigen within the infected target site limits effector T cell migration due to tethering by T cell–target cell interactions.
To obtain further insights into cell-extrinsic cues regulating T cell migration, we used second-harmonic generation signals to visualize ECM structures. We discovered a cerebral reticular fiber network that formed in the course of infection. Comparison of the location of ECM fibers and T cells revealed a high degree of colocalization. Similarly, correlation analysis of T cell turns with the presence of underlying fibers revealed that most turns occurred in the context of fibrous structures. Together, these findings raise the possibility that the inflammatory process leads to the remodeling of the neural microenvironment in a manner that makes it more conducive for T cell migration. As in the other aspects of migratory behavior discussed above, automated non-biased mathematical approaches are required to extend the analysis of effector T cell–ECM correlations. In addition, the de novo formation of ECM networks as migratory support structures should be explored in other inflammatory microenvironments.
Migratory behavior of killer T cells in LCMV-infected brain
Kim et al. analyzed the migratory behavior of cytotoxic T cells in the CNS after infection with LCMV [38]. They transferred fluorescently tagged P14 transgenic T cells, which express a TCR specific for an LCMV-associated peptide, into recipient mice. This was followed by intracerebral inoculation with LCMV 24 h later. T cell behavior was visualized by multi-photon microscopy when mice became symptomatic. Analysis revealed that a large fraction of the T cells was restricted in their migration, which was reduced after administration of an antibody that interferes with MHC–TCR interactions. These results extend previous findings and indicate that migratory restriction by cognate antigen represents a general regulatory mechanism for the behavior of T cells within intact inflammatory tissues.
Conclusions
The development of microscopic tools enabling dynamic visualization of cells in their native environments has led to the characterization of the motility of tissue-infiltrating leukocytes. In addition, advanced mathematical models are emerging that complement and expand these real-time observations. A key finding of these studies is the generally highly active motility of lymphocytes, both in lymphoid and inflammatory tissues, which maximizes their capability to find antigen-presenting cells. Somewhat unexpectedly, mathematical analysis supports a random walk model of spontaneously migrating T cells, thereby negating the involvement of long-range chemoattractant gradients within tissues in the regulation of T cell migration, and rather pointing to a stochastic model of interstitial lymphocyte navigation. Conflicting evidence has been generated with regard to whether this stochastic behavior is due to spontaneous fluctuations of a cell-intrinsic motility state or is primarily caused by interactions with the environment, such as ECM fibers. Recent findings that T cells may indeed follow fibrous components within tissues support the concept of “guided randomness,” whereby cells stochastically decide which branch of bifurcating fibers to follow, unless guided by chemokine signals secreted, for instance, by activated DC. While this concept is intriguing, more robust automated image analysis will be required to test its validity within a variety of different tissue compartments.
At the molecular level, it appears obvious that T cell motility within tissues is regulated by certain signals, one of which is delivered by MHC–peptide complex interactions with the TCR. This, on the one hand, leads to direct cell–cell contacts of a range of durations and, on the other hand, is important for keeping T cells in a “migratory state.” In addition, PTX-sensitive signals regulate naïve T cell migration in the LN microenvironment. Furthermore, cell-intrinsic regulators, most prominently CD44, have taken center stage in modulating T cell migration in the interstitial space, while the role of other cell adhesion receptors, such as integrins, still needs to be validated.
Future studies will focus on the elucidation of T cell migration in distinct microcompartments of tissues under distinct disease conditions. The fact that effector cells may temporarily use guidance structures for early orientation within tissues, as demonstrated in viral meningitis, or utilize newly formed ECM networks in the inflamed brain points towards a plethora of possible regulators of T cells behavior. Deciphering the molecular basis of these processes should enable the development of new concepts of disease mechanisms and highlight potential new ways of preventing pathology.
In conclusion, dynamic imaging of intact tissues represents a powerful approach to understand the behavior of leukocytes in intact tissues and has already led to significant conceptual advances. However, this is a fledgling field of research, and much more work is required to obtain a more comprehensive understanding of this fascinating process. A multidisciplinary approach, combining dynamic imaging with genetic, molecular, and computational approaches, holds a promise of more surprises and answers.
Acknowledgements
We thank Drs. Ichiko Kinjyo, Sioh-Yang Tan, Lois Cavanagh, Ben Roediger, Nital Sumaria, and Saparna Pai for critical reading of the manuscript. This work was supported by grants from the NHMRC and the New South Wales Cancer Institute. P.M. is recipient of the Career Development and Support Fellowship, Cancer Institute, New South Wales.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This article is published as part of the Special Issue on Immunoimaging of Immune System Function.
Contributor Information
Paulus Mrass, Phone: +61-2-95656247, FAX: +61-2-95656101, Email: p.mrass@centenary.org.au.
Wolfgang Weninger, Phone: +61-2-95156861, FAX: +61-2-96561048, Email: w.weninger@centenary.org.au.
References
- 1.Alstergren P, Zhu B, Glogauer M, Mak TW, Ellen RP, Sodek J. Polarization and directed migration of murine neutrophils is dependent on cell surface expression of CD44. Cell Immunol. 2004;231:146–157. doi: 10.1016/j.cellimm.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Ansel KM, Cyster JG. Chemokines in lymphopoiesis and lymphoid organ development. Curr Opin Immunol. 2001;13:172–179. doi: 10.1016/S0952-7915(00)00201-6. [DOI] [PubMed] [Google Scholar]
- 3.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J Exp Med. 2007;204:1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, et al. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 6.Beltman JB, Maree AF, Lynch JN, Miller MJ, de Boer RJ. Lymph node topology dictates T cell migration behavior. J Exp Med. 2007;204:771–780. doi: 10.1084/jem.20061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg HC. Random walks in biology. Princeton: Princeton University Press; 1993. [Google Scholar]
- 8.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol. 2008;8:675–684. doi: 10.1038/nri2379. [DOI] [PubMed] [Google Scholar]
- 10.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 11.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 13.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 15.Cavanagh LL, Weninger W. Dendritic cell behaviour in vivo: lessons learned from intravital two-photon microscopy. Immunol Cell Biol. 2008;86:428–438. doi: 10.1038/icb.2008.25. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty AK, Dustin ML, Shaw AS. In silico models for cellular and molecular immunology: successes, promises and challenges. Nat Immunol. 2003;4:933–936. doi: 10.1038/ni1003-933. [DOI] [PubMed] [Google Scholar]
- 17.Chang TW, Celis E, Eisen HN, Solomon F. Crawling movements of lymphocytes on and beneath fibroblasts in culture. Proc Natl Acad Sci USA. 1979;76:2917–2921. doi: 10.1073/pnas.76.6.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chtanova T, Han SJ, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 20.Fischer UB, Jacovetty EL, Medeiros RB, Goudy BD, Zell T, Swanson JB, Lorenz E, Shimizu Y, Miller MJ, Khoruts A, et al. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. Proc Natl Acad Sci USA. 2007;104:7181–7186. doi: 10.1073/pnas.0608299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 22.Friedl P, Borgmann S, Brocker EB. Amoeboid leukocyte crawling through extracellular matrix: lessons from the Dictyostelium paradigm of cell movement. J Leukoc Biol. 2001;70:491–509. [PubMed] [Google Scholar]
- 23.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 24.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 25.Friedl P, Wolf K, von Andrian UH, and Harms G (2007) Biological second and third harmonic generation microscopy. Curr Protoc Cell Biol Chapter 4, Unit 4 15 [DOI] [PubMed]
- 26.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 27.Gail MH, Boone CW. The locomotion of mouse fibroblasts in tissue culture. Biophys J. 1970;10:980–993. doi: 10.1016/S0006-3495(70)86347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 29.Graham DB, Zinselmeyer BH, Mascarenhas F, Delgado R, Miller MJ, Swat W. ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo. PLoS ONE. 2009;4:e4652. doi: 10.1371/journal.pone.0004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, et al. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178:7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- 31.Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Iparraguirre A, Weninger W. Visualizing T cell migration in vivo. Int Arch Allergy Immunol. 2003;132:277–293. doi: 10.1159/000074896. [DOI] [PubMed] [Google Scholar]
- 33.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 34.John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, Mrass P, Roos DS, Dzierszinski F, Weninger W, et al. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009;5:e1000505. doi: 10.1371/journal.ppat.1000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawakami N, Nagerl UV, Odoardi F, Bonhoeffer T, Wekerle H, Flugel A. Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med. 2005;201:1805–1814. doi: 10.1084/jem.20050011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kay RR, Langridge P, Traynor D, Hoeller O. Changing directions in the study of chemotaxis. Nat Rev Mol Cell Biol. 2008;9:455–463. doi: 10.1038/nrm2419. [DOI] [PubMed] [Google Scholar]
- 37.Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, Theriot JA. Mechanism of shape determination in motile cells. Nature. 2008;453:475–480. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JV, Kang SS, Dustin ML, McGavern DB. Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature. 2009;457:191–195. doi: 10.1038/nature07591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowalczyk DW, Wlazlo AP, Blaszczyk-Thurin M, Xiang ZQ, Giles-Davis W, Ertl HC. A method that allows easy characterization of tumor-infiltrating lymphocytes. J Immunol Meth. 2001;253:163–175. doi: 10.1016/S0022-1759(01)00356-8. [DOI] [PubMed] [Google Scholar]
- 40.Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- 41.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 42.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Katakai T, Hara T, Gonda H, Sugai M, Shimizu A. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J Cell Biol. 2004;167:327–337. doi: 10.1083/jcb.200403091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- 45.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 46.Mempel TR, Junt T, von Andrian UH. Rulers over randomness: stroma cells guide lymphocyte migration in lymph nodes. Immunity. 2006;25:867–869. doi: 10.1016/j.immuni.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 50.Mohler W, Millard AC, Campagnola PJ. Second harmonic generation imaging of endogenous structural proteins. Methods. 2003;29:97–109. doi: 10.1016/S1046-2023(02)00292-X. [DOI] [PubMed] [Google Scholar]
- 51.Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity. 2008;29:971–985. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, et al. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mrass P, Weninger W. Immune cell migration as a means to control immune privilege: lessons from the CNS and tumors. Immunol Rev. 2006;213:195–212. doi: 10.1111/j.1600-065X.2006.00433.x. [DOI] [PubMed] [Google Scholar]
- 54.Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–135. doi: 10.1034/j.1600-065X.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 55.Ng LG, Mrass P, Kinjyo I, Reiner SL, Weninger W. Two-photon imaging of effector T-cell behavior: lessons from a tumor model. Immunol Rev. 2008;221:147–162. doi: 10.1111/j.1600-065X.2008.00596.x. [DOI] [PubMed] [Google Scholar]
- 56.Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martinez AC, Fukui Y, von Andrian UH, Stein JV. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Odoardi F, Kawakami N, Klinkert WE, Wekerle H, Flugel A. Blood-borne soluble protein antigen intensifies T cell activation in autoimmune CNS lesions and exacerbates clinical disease. Proc Natl Acad Sci USA. 2007;104:18625–18630. doi: 10.1073/pnas.0705033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J Immunol. 2007;178:2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 59.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O'Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–549. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 62.Pure E, Cuff CA. A crucial role for CD44 in inflammation. Trends Mol Med. 2001;7:213–221. doi: 10.1016/S1471-4914(01)01963-3. [DOI] [PubMed] [Google Scholar]
- 63.Renkawitz J, Schumann K, Weber M, Lammermann T, Pflicke H, Piel M, Polleux J, Spatz JP, Sixt M. Adaptive force transmission in amoeboid cell migration. Nat Cell Biol. 2009;11:1438–1443. doi: 10.1038/ncb1992. [DOI] [PubMed] [Google Scholar]
- 64.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 65.Riggs T, Walts A, Perry N, Bickle L, Lynch JN, Myers A, Flynn J, Linderman JJ, Miller MJ, Kirschner DE. A comparison of random vs. chemotaxis-driven contacts of T cells with dendritic cells during repertoire scanning. J Theor Biol. 2008;250:732–751. doi: 10.1016/j.jtbi.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samadani A, Mettetal J, van Oudenaarden A. Cellular asymmetry and individuality in directional sensing. Proc Natl Acad Sci USA. 2006;103:11549–11554. doi: 10.1073/pnas.0601909103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 69.Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21:315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 70.von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 71.Wei SH, Parker I, Miller MJ, Cahalan MD. A stochastic view of lymphocyte motility and trafficking within the lymph node. Immunol Rev. 2003;195:136–159. doi: 10.1034/j.1600-065X.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 72.Weninger W, von Andrian UH. Chemokine regulation of naive T cell traffic in health and disease. Semin Immunol. 2003;15:257–270. doi: 10.1016/j.smim.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, et al. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Witt C, Raychaudhuri S, Chakraborty AK. Movies, measurement, and modeling: the three Ms of mechanistic immunology. J Exp Med. 2005;201:501–504. doi: 10.1084/jem.20050197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 76.Worbs T, Bernhardt G, Forster R. Factors governing the intranodal migration behavior of T lymphocytes. Immunol Rev. 2008;221:44–63. doi: 10.1111/j.1600-065X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 77.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]


