Abstract
During the last several years, live tissue imaging, in particular using two-photon laser microscopy, has advanced our understanding of leukocyte trafficking mechanisms. Studies using this technique are revealing distinct molecular requirements for leukocyte migration in different tissue environments. Also emerging from the studies are the ingenious infrastructures for leukocyte trafficking, which are produced by stromal cells. This review summarizes the recent imaging studies that provided novel mechanistic insights into in vivo leukocyte migration essential for immunosurveillance.
Electronic supplementary material
The online version of this article (doi:10.1007/s00281-010-0210-3) contains supplementary material, which is available to authorized users.
Keywords: Live imaging, Leukocyte migration, Chemokine, Integrin, Stromal cells
Introduction
Leukocyte migration is fundamental for the immune system function, and understanding of its mechanisms is profoundly beneficial to pathological diagnosis of inflammatory diseases or cancer, and also to clinical regulation of the immune system [1–4]. Although much has been learned about the mechanisms of leukocyte migration from experiments using isolated cells and from static histological analysis, a new approach was needed to understand dynamics of in vivo leukocyte migration. Recent studies using two-photon laser microscopy (TPLM) started to reveal the behavior of lymphocytes and other leukocytes within various tissues [5, 6]. Molecular requirements for leukocyte migration in the native environment can now be directly tested by this imaging technique with high 3D resolution. This review attempts to summarize the mechanisms of in vivo leukocyte migration that have been revealed or proposed by the two-photon imaging studies. The cellular and molecular environments important for leukocyte migration are described. Then the requirements for leukocyte surface and intracellular molecules in their in vivo migration are discussed. Lastly, novel mechanistic insights into in vivo lymphocyte chemotaxis and tissue entry and egress are discussed.
Tissue scaffolds and extracellular cues for leukocyte migration
Cellular and non-cellular scaffolds for leukocyte migration
The first example of leukocyte migration directly observed in the intact tissue by TPLM was lymphocyte migration in the lymph node parenchyma [7]. Naïve T and B cells were observed to travel in the lymph node at about 10 and 6 μm/min, respectively. This rapid migration of lymphocytes is most likely essential for their efficient antigen surveillance [1, 6, 8]. The recent studies also revealed that migration of polyclonal B cells contributes to antigen transport for humoral immune responses [9, 10]. Migration of naïve lymphocytes did not show gross directionality and had characteristics of random walk, although some cells were occasionally observed to take similar migration paths [6, 11]. Later, it was shown that these cells migrated on the network formed by fibroblastic stromal cells, including fibroblastic reticular cells (FRCs) in the T cell zone and follicular dendritic cells (FDCs) in the B cell follicle [12]. The stromal cell network is dense and complex, thereby providing lymphocytes with numerous migration paths. In addition to migrating along the stromal network, lymphocytes are frequently observed to migrate through the network not in parallel directions with any visible stromal fibers. In this case, lymphocytes may move from one fiber to another by simultaneously interacting with multiple fibers, and/or they may migrate on scaffolds that are not visualized, including superfine stromal fibers. Lymphocytes that appear at almost same locations in the network at different time points seem to be able to migrate in different directions. What determines directionality for each lymphocyte at a certain location is currently unknown.
FRC-associated T cell migration was also observed in the periarteriolar lymphoid sheath of the spleen [13]. Similar dynamic interactions between lymphocytes and fibroblasts most likely take place in other lymphoid tissues like Payer’s patches or lamina propria. However, these interactions in the mucosa-associated lymphoid tissue have not been dynamically analyzed. A similar fibroblastic network was observed in the brain after infection with Toxoplasma Gondi, and was suggested to serve as the scaffold for infiltrated effector CD8+ T cells [14]. The lymphoid tissues also contain many motile cells other than T and B cells, including subsets of dendritic cells (DCs) and natural killer cells [15–18]. Their dynamic interactions with FRCs have not been extensively analyzed by TPLM.
In the lymphoid tissue, most of collagen-rich extracellular matrix (ECM) fibers are thought to be ensheathed by fibroblasts which produce collagen fibers and other ECM components [18, 19]. However, in other non-lymphoid tissues such as skin, collagen-rich fibers may be more exposed to infiltrating leukocytes, thereby directly serving as scaffolds for migration. In the delayed hypersensitivity model in the rat skin, CD4+ effector T cells were seen to migrate along bundles of collagen fibers [20]. The ECM is also suggested to serve as the scaffold for T cell migration in the tumor environment [21]. The major migration mode for leukocytes is thought to be amoeboid migration which does not require the remodeling of ECMs [22]. In fact, even within the artificial collagen matrices activated T cell migration was independent of matrix degradation by matrix metalloproteinases (MMPs) or other proteases [23]. Nonetheless, some MMPs including MMP-9 that degrades several types of collagen are upregulated in self-reactive T cells infiltrated into the spinal cord in the rat experimental autoimmune encephalitis model [24]. Thus, effector T cell migration in the non-lymphoid tissue may also employ matrix remodeling depending on the microenvironment.
In addition to fibroblasts and the ECM, other cell types can also serve as the scaffold for leukocyte migration. In the lumens of the blood vessels and lymphatic vessels, leukocytes crawl on endothelial cells. T cells were observed to crawl on the luminal wall of high endothelial venules (HEVs) and other blood vessels before they transmigrate into the lymphoid tissues and inflamed tissues [12, 24, 25]. T cells that have entered lymphatic sinuses in the lymph node cortex crawl in the sinus lumen before they exit the lymph node [26]. Migrating T cells in the tissue also come into direct contact with antigen-presenting cells (APCs) to recognize their antigens. T cell crawling on DCs has been visualized in the lymph node [11, 27]. It is not clear, however, whether T cells crawling on DCs are completely detached from FRCs, because localization of DCs is also associated with the FRC network [18]. T cells that encountered their antigen are stably conjugated onto DCs or other APCs, and the conjugation can last several hours [11, 27–29]. In the early phase of the T-dependent antibody response, helper T cells are conjugated with cognate antigen-presenting B cells in the lymphoid tissues like lymph nodes. The B-T conjugates are motile with B cells migrating ahead of conjugated T cells [29–31], which possibly allows the exchange of partners among conjugates to generate the optimal responses. Movement of the B-T conjugates seems to be, at least in part, associated with the stromal cell network. Figure 1 and Video 1 show that antigen-specific B-T conjugates are migrating on and through the network of radiation-resistant cells in an explants lymph node 36 h after immunization.
Fig. 1.
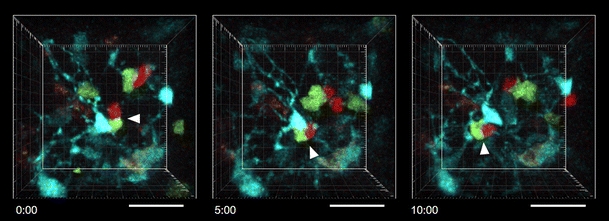
Migration of the B cell-T cell conjugate in the stromal cell network in the lymph node. The time lapse images show dynamics of cognate antigen-specific B cells (green) and helper T cells (red). Relatively sessile structures in cyan are primarily radiation-resistant stromal cells. Rapidly migrating cyan cells are presumed to be radiation-resistant memory T cells based on flow cytometric analysis of cells from the lymph node. Carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled, hen egg lysozyme (HEL)-specific B cells from Hy10 (also known as VDJ9/κ5) mice [109] and 5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR)-labeled, ovalbumin (OVA)-specific CD4 T cells from OT-II mice were adoptively transferred to a β-actin-CFP mouse [109] that had been lethally irradiated and reconstituted with bone marrow cells from wild type mice. One day after transfer, the recipient mouse was subcutaneously immunized with HEL-OVA conjugated protein emulsified in complete Freund’s adjuvant. An inguinal lymph node was excised 36 h after immunization and imaged by TPLM in the perfusion chamber as previously described [29, 109]. The excitation wavelength was 900 nm. The wavelengths of detected emission were 467–499 nm (for CFP+ cells), 520–550 nm (for CFSE-labeled cells), and 565–605 nm (for CMTMR-labeled cells). Arrowheads indicate the same B-T conjugate. Time elapsed from the far left image is shown as minutes/seconds. The images are presented as 60 μm z projections. The scale bars show 30 μm on the nearest x-y plane from the viewpoint. See also supplementary Video 1.
Scaffold-associated molecules sustaining leukocyte migration
The scaffold must provide migrating leukocytes with traction or anchoring, the support for the force generation. Indeed, to analyze in vitro T cell migration on the 2D substrate, coating with adhesion molecules like integrin ligands is often employed. As FRCs in the lymph node express integrin ligands like ICAM-1 and VCAM-1 [18], which are known to play vital roles for leukocyte homing to the tissues, these molecules have been speculated to play essential roles in providing the support for leukocyte migration in the lymphoid tissue. In addition, many of the ECM components including collagen, laminin, and fibronectin are integrin ligands, favoring the possibility of integrin-dependent leukocyte migration in the tissue-like skin and tumor. However, as described in a subsequent section, the imaging studies started to reveal that integrins make rather limited contributions to leukocyte migration in the 3D environment. When sufficiently dense, the FRC-DC network or 3D collagen matrix surrounding leukocytes may be able to provide the physical support for the migration force without using specific adhesion molecules [32]. Yet, the possibility is not excluded that FRCs express specific molecules indispensable for generating traction.
Chemokines, the G-protein coupled receptor (GPCR) ligands known to promote cell migration, are expressed by many types of stromal cells including FRCs, FDCs, and endothelial cells that serve as the migration scaffold [1, 18]. FRCs express CCL19 and CCL21, the ligands for CCR7 expressed on lymphocytes and DCs [1, 33]. FDCs strongly express CXCL13, the ligand for CXCR5 expressed on B cells and subsets of helper T cells [1]. HEV endothelial cells express the ligands for CCR7 and CXCR5 [34, 35]. Other chemokines involved in inflammation and tissue-selective homing of leukocytes are also expressed by various vascular endothelial cells [36]. CXCL12, the ligand for CXCR4, is expressed by pericyte-like cells associated with HEVs and possibly to a lesser extent by FRCs in the lymph node T zone [34, 37]. Lymphatic endothelial cells also express CCL21 [1]. Importantly, chemokines are presented on the surface of the stromal cells. Heparan- and chondroitin-sulfate proteoglycans present on the stromal cells form complexes with chemokines, and other molecules like integrin ligands may also help adsorb chemokines on the stromal cell surface [1, 38]. Indeed, in the immunofluorescence analysis of the lymph node section, CCL21 was observed to be concentrated in the surface area of FRCs [12, 38]. The immobilized chemokines on the substrate are much more potent in enhancing in vitro lymphocyte motility than chemokines in solution [38]. The chemokine-proteoglycan complex is also present on endothelial cells and in the ECM including the basement membrane [39].
Leukocyte intrinsic molecular requirements in in vivo migration
Integrins and CD44
Integrins, a family of surface glycoproteins, play the vital role in leukocyte homing from the blood to the tissues, especially in the step of adhesion to endothelial cells [40]. In addition, integrins provide anchoring necessary for the force transduction in mesenchymal migration of fibroblasts or tumor cells [41, 42]. However, their roles in amoeboid migration of leukocytes in vivo have been elusive. T cell migration in the artificial collagen matrix was shown to be unaltered by the treatment with an antibody cocktail that block multiple integrins [43]. Although the collagen matrix might not be a good model scaffold to study interstitial migration in the lymph node, where collagen fibers are encased by FRCs, the recent two-photon imaging studies also underscored the limited role for integrins in leukocyte interstitial migration in the lymph node. Integrins are heterodimers, each composed of α-subunit and β-subunit. There have been 18 α-subunits, eight β-subunits, and 24 αβ pairs identified in mammals [44]. LFA-1 integrin (the αL-β2 integrin heterodimer that binds ICAMs) is known to be a major player in T cell adhesion to endothelial cells or DCs. CD18 (β2 integrin)-deficient T cells show slightly reduced motility compared to wild-type T cells [38]. On the other hand, antibody blockade of αL integrin did not reduce interstitial motility of T cells in the lymph node [25]. These results seem to be discrepant because CD18 is thought to pair up with only αL integrin on naïve T cells, and the reason for this discrepancy is not clear. The blockade of α4 integrin slightly reduced wild-type T cell motility, but did not show an additive effect on motility of CD18 knockout T cells [38]. The combined in vivo blockade of ICAM-1 and VCAM-1 (a ligand for α4 integrins) also slightly reduced B cell motility in the lymph node [45]. Sixt and colleagues extensively examined the roles for integrins in DC migration by genetically ablating all the known integrin heterodimers, and concluded integrins are dispensable for DC migration in the lymph node and skin [46]. Although some of these studies and another report suggest that integrins play some roles in high-velocity movements of leukocytes [47], all of these studies agree that leukocyte interstitial migration examined so far can be largely sustained without integrins. More studies in the future are needed to test the involvement of integrins in other leukocyte migration in the various tissue environments.
Another cell surface molecule that can interact with the ECM is CD44, which is expressed in many types of leukocytes. In T cells, CD44 is upregulated after activation via the T cell receptor, and memory T cells maintain high CD44 expression. The glycosylated extracellular domain of CD44 can interact with various ECM components, including hyaluronic acid, fibronectin, laminin, collagen, and osteopontin, and the intracellular domain can bind to ERM (Ezrin, Radixin, Moesin) actin-anchoring proteins [48]. Recent studies visualized dynamics of cytotoxic T lymphocytes (CTLs) in the tumor formed by subcutaneously injected thymoma cells [21, 48–50]. Effector CTLs were observed to crawl along the ECM fibers in the tumor [21, 49]. Interestingly, intratumoral migration was impaired by CD44-deficiency in CTLs, resulting in reduced tumor-screening capacity of these cells. However, while the intracellular domain of CD44 that recruits ERM proteins was necessary, the extracellular domain was not required for CD44-dependent motility, disputing the requirement for CD44 binding to the ECM in intratumoral CTL migration [48].
Chemokine receptors and other GPCRs
GPCRs, particularly those coupled to Gαi/βγ heterotrimeric G protein (Gi)-mediated signaling, are known to promote cell migration. Recent imaging studies revealed that Gi signaling is indeed requisite for interstitial migration of lymphocytes. The treatment with pertussis toxin, which inhibits Gi signaling, strongly reduced motility of both naïve B and T cells in the lymph node [51–54]. Among the members of the Gαi subfamily, Gαi2 was shown to be the most important for both B and T cell migration [51, 54]. In addition, B cell migration was significantly enhanced without RGS (G protein Signaling)1, a member of the Regulator of RGS family that attenuates the signaling activity of G proteins [51].
The involvement of the chemokine receptor CCR7 in Gi signaling-dependent T cell motility was recently revealed by three groups using two-photon imaging of the lymph node [52, 54, 55]. The genetic depletion of CCR7-dependent signaling partially reduced interstitial motility of naïve T cells. These studies used different lymph node preparations, lymph node explants [52, 54], and lymph nodes in the live animal [55], but their results were remarkably consistent in quantitative terms. A similar contribution of CCR7 was also shown by another study using conventional epifluorescent microscopy of unfixed lymph node slices although the absolute motility of T cells in this study was lower than that analyzed by TPLM [53]. CCR7-dependent T cell motility could account for less than a half of the Gi-dependent T cell motility, and GPCRs responsible for Gi-dependent, CCR7-independent T cell motility are currently unknown. Pharmacological blockade of CXCR4 did not further reduce motility of CCR7-deficinet T cells [52, 53]. Individual inhibition of other GPCR signaling pathways mediated by the lipids (thromboxanes, sphingosine 1-phosphate (S1P), and lysophosphatidylcholine) or adenosine has so far not resulted in reduced interstitial motility of T cells in the lymph node [54, 56].
CXCR5 is a major chemokine receptor on B cells, and is required for follicular localization of B cells and their capturing of FDC-bound antigen [1, 57]. After capturing cognate antigen and interacting with helper T cells, B cells proliferate and form germinal centers (GCs) within the follicle to mount long-lasting, high affinity antibody responses. Recent two-photon imaging studies revealed the migratory nature of GC B cells [30, 58, 59], and their motility was found to be partially dependent on the CXCR5 ligand, CXCL13 [30]. CXCR5 likely contributes to naïve B cell motility on FDCs as well. Testing this, however, may not be straightforward because CXCR5-deficiency dislocates B cells from the FDC network. Naïve B cells also express CCR7, which may contribute to their motility even when they are in the B cell follicle since CCL21 is present in the T-zone proximal region of the B cell follicle [29, 60]. A recent imaging study showed enhanced follicular migration of LPS-treated B cells, which upregulated CXCR5 and CCR7 along with other chemokine receptors [61].
A few other GPCRs were reported to function for migration of cells with the myeloid origin in vivo. Acutely after induction of tissue damage in the brain cortex, it was observed by TPLM that microglial cells migrated toward the injury site in response to adenosine triphasphate (ATP) released from the site [62]. This ATP-driven migration was found to be mediated by the P2Y12 purinoceptor, which is coupled to the Gi signaling pathways [63]. More recently, it has been reported that S1P can promote migration of monocytoid cells containing osteoblast precursors in the bone marrow, and that this lipid signaling contributes to regulation of the mature osteoblast formation and bone homeostasis [64].
Cytoskeletal molecules and small G proteins
The force generation for amoeboid migration is dependent on the polarized actomyosin cytoskeleton. The protrusion at the front mediated by the actin network expansion is the major part of the force for leukocyte migration [32]. The growth of the actin network requires branching of actin filaments, which is mediated by the Arp2/3 complex. The small GTPases Rac and Cdc42 activate the Arp2/3 complex through the Wiskott-Aldrich syndrome protein family. Rac-deficiency leads to rounding of DCs due to impaired expansion of the actin network, and results in the inability of DCs to migrate from the skin to the lymph node [65]. Cdc42 depletion in DCs severely blocks their migration in the 3D environment by impairing the special coordination of the actin network expansion [66]. Dock2, a guanine nucleotide exchange factor (GEF) for Rac, was shown to be essential for interstitial motility of T and B cells in the lymph node [67], and is most likely important for in vivo neutrophil migration as well [68, 69]. Interestingly, however, DCs may not depend on Dock2 to activate Rac for their migration except for plasmacytoid DCs [70]. Vav family proteins are also the GEF for Rac. Vav1/2/3-deficient neutrophils showed the reduced migration speed in the inflamed dermal tissue, while the directional persistence of their migration was normal [47]. Vav proteins in neutrophils were reported to act downstream of integrins [47]. Consistent with this view, neutrophil migration in the rat mesenteric tissue analyzed by transmitted light microscopy was reported to be impaired after blocking α2β1 integrin function [71]. On the other hand, genetic deletion of all the known integrin heterodimers did not alter neutrophil motility in collagen gels [46]. Effects of genetic ablation of all the integrins on neutrophil interstitial migration in vivo have not been reported.
Rac activation can be mediated by the phosphoinositide 3-kinase (PI3K) signaling pathway. PI3Kγ-deficient T cells but not PI3Kγ-deficient B cells exhibit mildly impaired directional persistence in the lymph node [67]. Basal motility of naïve lymphocytes is also partially dependent on Class IA PI3Ks [72]. Recently, it has been reported that PI3K-produced phosphatidylinositol 3,4,5- trisphosphate and phospholipase D (PLD)-produced phosphatidic acid cooperatively recruit Dock2 to the plasma membrane at the leading edge of chemotaxing neutrophils [68]. It will be important to examine in vivo motility of lymphocytes and neutrophils that lack both PI3Ks and PLD.
The importance of the actin network regulation in leukocyte migration was further underscored by the discovery that defects in the CORO1A gene encoding Coronin 1A severely impair T cell trafficking and interstitial motility [73, 74]. The Coronin family proteins bind F-actin and inhibit the Arp2/3 complex. It is currently unclear how exactly the regulation of branched actin polymerization by Coronin 1A contributes to T cell motility. The CORO1A knockout or mutant mice show striking defects in thymic egress of mature thymocytes [73, 74]. Absence of Coronin 1A caused by deletions in the human CORO1A gene has been also linked to an atypical form of T−B+NK+ severe combined immunodeficiency (SCID) in a patient with the detectable thymus [74, 75]. Mice deficient in Dock2 described above, mDia1, RAPL, or Mst1 described below are also defective in thymocyte emigration, but none of the genes encoding these molecules has been linked to human SCID. Migration of other leukocytes like B cells, NK cells, and neutrophils seems to be less dependent on Coronin 1A, possibly due to redundant expression of other Coronin family members [74–76].
Diaphanous-related formin (DRF) family proteins, which are the effector molecules of the Rho small GTPase subfamily, also stimulate actin polymerization. Unlike Arp2/3, the DRF proteins produce unbranched actin filaments. Deficiency in mDia1, a member of the DRF family, impairs in vitro migration and the in vivo trafficking of T cells [77]. However, interstitial motility of mDia1-deficient T cells has not been directly examined by TPLM. In addition to the formation of unbranched actin filaments, bundling of actin filaments has also been suggested to be important for T cell motility. A recent study using TLPM reported that T cells deficient in an actin-bundling protein L-plastin showed impaired motility in lymph nodes [78]. Another group of Rho effector molecules are the Rho kinases, ROCK1 and ROCK2. Although in vivo trafficking of T cells lacking ROCK1 and/or ROCK2 has not been examined, inhibition of the Rho kinases by the selective inhibitor, Y-27632, significantly suppresses in vitro T cell chemotaxis [79]. The Rho kinases increase phosphorylation of myosin light chains either by directly phosphorylating the light chains or through the inhibition of myosin phosphatases. This stimulates actomyosin contractility, which can promote amoeboid migration depending on the microenvironment [32, 80, 81]. Alternatively, the Rho kinases can also stabilize actin filaments by inactivating actin depolymerizing and severing factors through the phosphorylation of LIM kinases [82].
The signaling pathway involving the small GTPase Rap1 is also important for leukocyte migration. Although in vivo motility of Rap1-deficient leukocytes has not been directly assessed, interstitial motility of B and T cells in the lymph node was found to be dependent on a Rap1 effector molecule, RAPL (also known as an alternative spliced form of Nore1 or Rassf5b) [45]. RAPL binds to the GTP-bound form of Rap1, and this complex was shown to activate the serine-threonine kinase Mst1, a member of the mammalian Ste20-like kinase family [83]. Mst1-deficient mice showed similar lymphocyte trafficking defects to those observed in RAPL-deficient mice, including impaired interstitial motility of B and T cells in the lymph node [84]. The Rap1-RAPL-Mst1 pathway has been best characterized in its role for integrin-dependent adhesion. However, this pathway is also involved in integrin-independent cell polarization most likely through the regulation of the actomyosin system [45, 83–86]. As the dependence of lymphocyte interstitial motility on RAPL or Mst1 seems to be larger than that on integrins, the Rap1-RAP-Mst1 pathway is likely to contribute cell migration both in integrin-dependent and -independent manners.
In vivo chemotaxis and tissue entry/egress of lymphocytes
Chemokine-guided lymphocyte migration in the lymph node parenchyma
The concentration gradient of the environmental cues such as chemokines is thought to be important for guiding leukocyte trafficking. Besides ATP-driven chemotaxis of brain microglia described above, a few studies using TPLM have revealed that lymphocytes undergo directionally biased interstitial migration in the chemokine receptor-dependent manner. In the lymph node, B cells that encountered their antigen in the B cell follicle upregulate CCR7, and directionally migrate toward the T cell zone in the CCR7-dependent manner [29, 87]. This directional migration was observed only in the T cell zone-proximal region of the follicle, approximately where the increasing gradient of CCL21 toward the T zone could be detected. After this directional migration, the antigen-engaged B cells accumulate at the boundary between the follicle and T zone, where they start dynamic interactions with cognate helper T cells [29]. This B cell accumulation at the boundary was shown to be achieved by the balanced responsiveness to the CCR7 ligands in the T cell zone and to CXCL13 in the B cell follicle [1, 87].
Positively selected thymoctes in the thymic cortex were also observed to migrate directionally toward the thymic medulla in the CCR7 dependent manner [88–90]. This CCR7-dependent migration may be important for negative selection of thymocytes in the medulla to prevent the autoimmunity [91]. Unlike antigen-engaged B cells that are accumulated at the border between the B cell follicle and T cell zone, positively selected thymocytes migrate across the cortico-medullary junction to enter into the medulla. The medullary entry of positively selected thymocytes seems to require additional Gi signaling pathways that have not been identified [90].
The chemokine-guided migration was also shown to promote CD4+ T cell help for CD8+ T cells, which involves co-presentation of antigens to CD4+ T cells and CD8+ T cells by the same DCs [92]. In the lymph node, it was observed that polyclonal CD8+ T cells were locally attracted to DCs interacted with antigen-specific CD4+ T cells in the manner dependent on signaling through the chemokine receptor CCR5. This mechanism enables CD8+ T cells to efficiently scan for their antigen and CD4+ T cell help at the same time [92]. Another imaging study also reported that CD8+ T cells are recruited preferentially to the cognate DC-CD4+ T cell interaction sites [93]. Notably, CCR5 signaling blockade reduced the number of memory CD8+ T cells generated after immunization but not primary expansion of CD8+ T cells [92, 94]. Most of polyclonal CD8+ T cells have to migrate away from sites of the antigen-specific DC-CD4+ T cell interaction after they have scanned for their antigen at the sites. An interesting future question is whether desensitization of CCR5 is involved in this migration of CD8+ T cells that is inevitably against the concentration gradient of the CCR5 ligands.
Novel mechanistic insights into tissue entry and egress
For lymphocyte chemotaxis described above, the gradients of chemokine concentration are most likely formed along fibroblastic stromal cells. Concentration gradients of guidance cues can be also formed between the tissue parenchyma and the lumen of blood or lymphatic vessels. A recent imaging study showed that T cells in the lymph node parenchyma extend cell processes into the cortical sinus, a lymphatic structure that is possibly a primary exit site of the lymph node, before they decide to move in or leave the sinus [26]. S1P1 (an S1P receptor)-deficient T cells that are incapable of egressing from the lymph node also extend the processes into the sinus, but fail to migrate into the sinus lumen. These results suggest that T cell sensing of luminal S1P, which is likely to be much more abundant than S1P in the parenchyma, may drive transmigration [26, 95]. It is not clear whether T cells migrate into the cortical sinus through common entry sites, although a preceding report showed that T cells cross the boundary of a medullary sinus-like structure at certain locations [96]. An independent study reported that B cells also entered into the cortical sinus, and that the entry step was inhibited by administration of an immunosuppressant FTY720, which blocks lymphocyte egress from the lymph node [97]. Upon administration, FTY720 is phosphorylated in vivo. Phosphorylated FTY-720 is a potent agonist for S1P1, but can also act as a functional antagonist by desensitizing S1P1 signaling [1]. As B cell egress from the lymph node is also impaired by B cell-intrinsic S1P1 deficiency [98], it is strongly suggested that B cell transmigration into the cortical sinus is also mediated by S1P1.
Recently, DCs were shown to enter initial lymphatic vessels in the skin through preexisting discontinuities in the basement membrane surrounding lymphatic endothelial cells. After migrating through these preformed pores in the basement membrane, DCs were observed to transmigrate into the vessel through lymphatic endothelial junctions that can be ‘flapped’ for entering of DCs [99]. This study visualized DC migration in ex vivo ear sheets with the dermal surface exposed using a spinning disk confocal microscope. DCs added onto the dermal surface migrated into the tissue and accumulated into the lymphatics with striking efficiency in a few hours [46, 99]. This accumulation was shown to be CCR7-dependent, suggesting that DCs are attracted to CCR7 ligands expressed by lymphatic endothelial cells [1, 99, 100].
It is not clear whether leukocyte extravasation from the blood vessels involves concentration gradients of guidance cues across the endothelium. Chemokines immobilized on the apical surface of endothelial cells, together with integrin ligands and application of a shear flow, suffice to promote in vitro T cell transendothelial migration [101]. Apical chemokines and shear stress promote T cells to form numerous dots of high affinity integrins and filopodia that nucleate from the high affinity integrin dots. These filopodia were observed to invade the endothelial cell surface. Rapid turnover of these structures was proposed to allow shear resistant, lateral crawling of T cells on endothelial cells, and may also be required for transendothelial migration [102]. Turnover of these structures most likely requires regulation of integrin affinity, as a recent in vivo imaging of HEVs in the lymph node by TPLM showed that genetically engineered T cells that are unable to down-regulate LFA-1 affinity were slower than wild type cells in both lateral crawling and transendothelial migration [25]. After transendothelial migration, lymphocytes may use discontinuities in the basement membrane surrounding the vessel to leave into the tissue as reported for DCs entering into lymphatics [99]. T cells extravasating from lymph node HEVs were observed to take common paths to leave HEVs into the parenchyma [8, 12].
B cell intravasation into the bone marrow sinusoid has also been visualized by TPLM. Intravital imaging of Rag1 recombinase-expressing B cells in the bone marrow (pro-, pre-, and immature B cells) detected that some of these B cells entered into the bone marrow sinusoids [103]. Flow cytometric analysis of in vivo labeled sinusoidal B cells provided evidence that Rag1-expressing B cells in the sinusoids are immature B cells. After entering into the sinusoids immature B cells were observed to be retained in the sinusoids in a manner dependent on CB2 (a cannabinoid receptor coupled to Gi signaling) and α4 integrin before they are decided to leave the sinusoids into the blood flow. The retention of immature B cells in the sinusoid may contribute to shape the B cell repertoire as CB2-deficiency resulted in reduced lamda light chain-positive B cells in the periphery [103]. The same study also showed that B cell CXCR4, which had been known to provide the retention signal in the bone marrow, serves to retain B cells in the bone marrow parenchyma but not in the sinusoid. Thus CXCR4 inhibits parenchyma-to-sinusoid transmigration of developing and mature B cells that should not leave the bone marrow [103]. The signals responsible for selective transmigration of immature B cells into the sinusoids have not been identified. Other studies showed that adoptively transferred mature B cells could home to the bone marrow parenchyma [104, 105], and survival of these B cells depends on a cytokine called MIF (also known as GIF) produced by bone marrow-resident DCs [104]. It will be intriguing to test the involvement of these DCs in the selection of developing B cells in the bone marrow.
Conclusions and perspectives
The studies described above illustrated that live imaging of intact tissues, in particular by TPLM, is a powerful method to investigate the cell biological and anatomical mechanisms of in vivo leukocyte migration. These studies started to highlight that leukocytes adaptively employ various modes of migration using distinct sets of molecules to regulate actomyosin-dependent force generation and adhesion depending on the nature of the microenvironments. They also revealed the ingenious infrastructures essential for leukocyte migration, which are produced by stromal cells. Importantly, as seen in the B cell-dependent development of the FDC network, leukocytes are also involved in the construction of the migration scaffold for themselves [106]. However, our knowledge is still limited about molecular requirements and mechanisms for individual modes of in vivo leukocyte migration. Future studies will need not only to examine the involvement of many molecules with the issue of functional redundancy in mind, but also to tackle the improvement of visualization ability for subcellular events as well as more precise microstructures in live tissues. Efforts should be also made to clarify the physiological significance of lymphocyte interstitial motility. It has been suggested that even a partial defect in interstitial motility may lead to a significant impairment in tumor cell surveillance by effector CTLs [48]. Future studies are expected to examine the impact of reduced lymphocyte motility on other forms of immunosurveillance, including surveillance by developing lymphocytes for self-antigen in the thymic medulla or in the bone marrow [103, 107]. Another important challenge is the development of the imaging system to study the mouse model for human immunodeficiency caused by leukocyte trafficking defects. For example, in order to fully understand pathological mechanisms underlying SCID linked to Coronin 1A defeciency [75], it will be essential to develop the technique to visualize thymocytes emigration from the thymus in the living animal. Live imaging studies have also started to dissect effects of host-pathogen interactions on leukocyte trafficking. For example, a study using TPLM showed that neutrophil extravasation to the site of Streptococcus pyogenes infection was decelerated by Streptolysin S toxin of the bacteria [108], highlighting the importance of imaging studies using genetically altered pathogens to understand mechanisms of leukocyte trafficking during the infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Migration of the B cell-T cell conjugate in the stromal cell network in the lymph node. The movie shows the 45-min time lapse images recorded every 30 s. Cognate antigen-specific B cells (green) and helper T cells (red) are stably conjugated. In cyan are primarily irradiation-resistant stromal cells. The mouse lymph node was imaged ex vivo by TPLM 36 h after immunization. Elapsed time is shown as hours/minutes/seconds. The image volume is 100 μm (x) × 100 μm (y) × 60 μm (z). The scale bar shows 30 μm on the nearest x-y plane from the viewpoint (MPG 4.71 MB)
Acknowledgment
I thank Jason Cyster for providing Hy10 mice and CFP mice, Yoshikazu Ando for helping figure preparation, and the reviewers for valuable comments. I also acknowledge Masahiro Kitano, Michiyuki Matsudsa, Yasuo Mori, Tomohiro Kurosaki, and Takashi Saito for their help in setting up the two-photon microscope. This work was supported by the Japan Society for the Promotion of Science, the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Uehara Memorial Foundation, the Sumitomo Foundation, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research,
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
This article is published as part of the Special Issue on Immunoimaging of Immune System Function.
References
- 1.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 2.Luster AD, Alon R, von Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6:1182–1190. doi: 10.1038/ni1275. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–1658. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Germain RN, Miller MJ, Dustin ML, Nussenzweig MC. Dynamic imaging of the immune system: progress, pitfalls and promise. Nat Rev Immunol. 2006;6:497–507. doi: 10.1038/nri1884. [DOI] [PubMed] [Google Scholar]
- 6.Cahalan MD, Parker I. Choreography of cell motility and interaction dynamics imaged by two-photon microscopy in lymphoid organs. Annu Rev Immunol. 2008;26:585–626. doi: 10.1146/annurev.immunol.24.021605.090620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 8.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 10.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- 12.Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajenoff M, Glaichenhaus N, Germain RN. Fibroblastic reticular cells guide T lymphocyte entry into and migration within the splenic T cell zone. J Immunol. 2008;181:3947–3954. doi: 10.4049/jimmunol.181.6.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson EH, Harris TH, Mrass P, John B, Tait ED, Wu GF, Pepper M, Wherry EJ, Dzierzinski F, Roos D, Haydon PG, Laufer TM, Weninger W, Hunter CA. Behavior of parasite-specific effector CD8+ T cells in the brain and visualization of a kinesis-associated system of reticular fibers. Immunity. 2009;30:300–311. doi: 10.1016/j.immuni.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- 16.Garrod KR, Wei SH, Parker I, Cahalan MD. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc Natl Acad Sci USA. 2007;104:12081–12086. doi: 10.1073/pnas.0702867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beuneu H, Deguine J, Breart B, Mandelboim O, Di Santo JP, Bousso P. Dynamic behavior of NK cells during activation in lymph nodes. Blood. 2009;114:3227–3234. doi: 10.1182/blood-2009-06-228759. [DOI] [PubMed] [Google Scholar]
- 18.Mueller SN, Germain RN. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat Rev Immunol. 2009;9:618–629. doi: 10.1038/nri2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaldjian EP, Gretz JE, Anderson AO, Shi Y, Shaw S. Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int Immunol. 2001;13:1243–1253. doi: 10.1093/intimm/13.10.1243. [DOI] [PubMed] [Google Scholar]
- 20.Matheu MP, Beeton C, Garcia A, Chi V, Rangaraju S, Safrina O, Monaghan K, Uemura MI, Li D, Pal S, de la Maza LM, Monuki E, Flugel A, Pennington MW, Parker I, Chandy KG, Cahalan MD. Imaging of effector memory T cells during a delayed-type hypersensitivity reaction and suppression by Kv1.3 channel block. Immunity. 2008;29:602–614. doi: 10.1016/j.immuni.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mrass P, Takano H, Ng LG, Daxini S, Lasaro MO, Iparraguirre A, Cavanagh LL, von Andrian UH, Ertl HC, Haydon PG, Weninger W. Random migration precedes stable target cell interactions of tumor-infiltrating T cells. J Exp Med. 2006;203:2749–2761. doi: 10.1084/jem.20060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nat Immunol. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 23.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 24.Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A. Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature. 2009;462:94–98. doi: 10.1038/nature08478. [DOI] [PubMed] [Google Scholar]
- 25.Park EJ, Peixoto A, Imai Y, Goodarzi A, Cheng G, Carman CV, von Andrian UH, Shimaoka M. Distinct roles for LFA-1 affinity regulation during T cell adhesion, diapedesis, and interstitial migration in lymph nodes. Blood. 2009;115(8):1572–1581. doi: 10.1182/blood-2009-08-237917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nat Immunol. 2009;10:58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4:579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 28.Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada T, Miller MJ, Parker I, Krummel MF, Neighbors M, Hartley SB, O’Garra A, Cahalan MD, Cyster JG. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005;3:e150. doi: 10.1371/journal.pbio.0030150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen CD, Okada T, Cyster JG. Germinal-Center Organization and Cellular Dynamics. Immunity. 2007;27:190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21:636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, Cyster JG, Luther SA. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol. 2007;8:1255–1265. doi: 10.1038/ni1513. [DOI] [PubMed] [Google Scholar]
- 34.Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebisuno Y, Tanaka T, Kanemitsu N, Kanda H, Yamaguchi K, Kaisho T, Akira S, Miyasaka M. Cutting edge: the B cell chemokine CXC chemokine ligand 13/B lymphocyte chemoattractant is expressed in the high endothelial venules of lymph nodes and Peyer’s patches and affects B cell trafficking across high endothelial venules. J Immunol. 2003;171:1642–1646. doi: 10.4049/jimmunol.171.4.1642. [DOI] [PubMed] [Google Scholar]
- 36.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 38.Woolf E, Grigorova I, Sagiv A, Grabovsky V, Feigelson SW, Shulman Z, Hartmann T, Sixt M, Cyster JG, Alon R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat Immunol. 2007;8:1076–1085. doi: 10.1038/ni1499. [DOI] [PubMed] [Google Scholar]
- 39.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6:633–643. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 40.Alon R, Ley K. Cells on the run: shear-regulated integrin activation in leukocyte rolling and arrest on endothelial cells. Curr Opin Cell Biol. 2008;20:525–532. doi: 10.1016/j.ceb.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, Sheetz MP. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128:561–575. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26:289–298. doi: 10.1007/s10585-008-9190-2. [DOI] [PubMed] [Google Scholar]
- 43.Friedl P, Entschladen F, Conrad C, Niggemann B, Zanker KS. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol. 1998;28:2331–2343. doi: 10.1002/(SICI)1521-4141(199808)28:08<2331::AID-IMMU2331>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 44.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 45.Ebisuno Y, Katagiri K, Katakai T, Ueda Y, Nemoto T, Inada H, Nabekura J, Okada T, Kannagi R, Tanaka T, Miyasaka M, Hogg N, Kinashi T. Rap1 controls lymphocyte adhesion cascades and interstitial migration within lymph nodes in RAPL-dependent and -independent manners. Blood. 2009;115(4):804–814. doi: 10.1182/blood-2009-03-211979. [DOI] [PubMed] [Google Scholar]
- 46.Lammermann T, Bader BL, Monkley SJ, Worbs T, Wedlich-Soldner R, Hirsch K, Keller M, Forster R, Critchley DR, Fassler R, Sixt M. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature. 2008;453:51–55. doi: 10.1038/nature06887. [DOI] [PubMed] [Google Scholar]
- 47.Graham DB, Zinselmeyer BH, Mascarenhas F, Delgado R, Miller MJ, Swat W. ITAM signaling by Vav family Rho guanine nucleotide exchange factors regulates interstitial transit rates of neutrophils in vivo. PLoS ONE. 2009;4:e4652. doi: 10.1371/journal.pone.0004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mrass P, Kinjyo I, Ng LG, Reiner SL, Pure E, Weninger W. CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity. 2008;29:971–985. doi: 10.1016/j.immuni.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breart B, Lemaitre F, Celli S, Bousso P. Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest. 2008;118:1390–1397. doi: 10.1172/JCI34388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343–354. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 52.Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J Immunol. 2007;178:2973–2978. doi: 10.4049/jimmunol.178.5.2973. [DOI] [PubMed] [Google Scholar]
- 53.Asperti-Boursin F, Real E, Bismuth G, Trautmann A, Donnadieu E. CCR7 ligands control basal T cell motility within lymph node slices in a phosphoinositide 3-kinase-independent manner. J Exp Med. 2007;204:1167–1179. doi: 10.1084/jem.20062079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang JH, Cardenas-Navia LI, Caldwell CC, Plumb TJ, Radu CG, Rocha PN, Wilder T, Bromberg JS, Cronstein BN, Sitkovsky M, Dewhirst MW, Dustin ML. Requirements for T lymphocyte migration in explanted lymph nodes. J Immunol. 2007;178:7747–7755. doi: 10.4049/jimmunol.178.12.7747. [DOI] [PubMed] [Google Scholar]
- 55.Worbs T, Mempel TR, Bolter J, von Andrian UH, Forster R. CCR7 ligands stimulate the intranodal motility of T lymphocytes in vivo. J Exp Med. 2007;204:489–495. doi: 10.1084/jem.20061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Halin C, Scimone ML, Bonasio R, Gauguet JM, Mempel TR, Quackenbush E, Proia RL, Mandala S, von Andrian UH. The S1P-analog FTY720 differentially modulates T-cell homing via HEV: T-cell-expressed S1P1 amplifies integrin activation in peripheral lymph nodes but not in Peyer patches. Blood. 2005;106:1314–1322. doi: 10.1182/blood-2004-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suzuki K, Grigorova I, Phan TG, Kelly LM, Cyster JG. Visualizing B cell capture of cognate antigen from follicular dendritic cells. J Exp Med. 2009;206:1485–1493. doi: 10.1084/jem.20090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwickert TA, Lindquist RL, Shakhar G, Livshits G, Skokos D, Kosco-Vilbois MH, Dustin ML, Nussenzweig MC. In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446:83–87. doi: 10.1038/nature05573. [DOI] [PubMed] [Google Scholar]
- 59.Hauser AE, Junt T, Mempel TR, Sneddon MW, Kleinstein SH, Henrickson SE, von Andrian UH, Shlomchik MJ, Haberman AM. Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26:655–667. doi: 10.1016/j.immuni.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Okada T, Cyster JG. B cell migration and interactions in the early phase of antibody responses. Curr Opin Immunol. 2006;18:278–285. doi: 10.1016/j.coi.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Hwang IY, Park C, Harrison K, Kehrl JH. TLR4 signaling augments B lymphocyte migration and overcomes the restriction that limits access to germinal center dark zones. J Exp Med. 2009;206:2641–2657. doi: 10.1084/jem.20091982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 63.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 64.Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL, Germain RN. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458:524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 66.Lammermann T, Renkawitz J, Wu X, Hirsch K, Brakebusch C, Sixt M. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood. 2009;113:5703–5710. doi: 10.1182/blood-2008-11-191882. [DOI] [PubMed] [Google Scholar]
- 67.Nombela-Arrieta C, Mempel TR, Soriano SF, Mazo I, Wymann MP, Hirsch E, Martinez AC, Fukui Y, von Andrian UH, Stein JV. A central role for DOCK2 during interstitial lymphocyte motility and sphingosine-1-phosphate-mediated egress. J Exp Med. 2007;204:497–510. doi: 10.1084/jem.20061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishikimi A, Fukuhara H, Su W, Hongu T, Takasuga S, Mihara H, Cao Q, Sanematsu F, Kanai M, Hasegawa H, Tanaka Y, Shibasaki M, Kanaho Y, Sasaki T, Frohman MA, Fukui Y. Sequential regulation of DOCK2 dynamics by two phospholipids during neutrophil chemotaxis. Science. 2009;324:384–387. doi: 10.1126/science.1170179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kunisaki Y, Nishikimi A, Tanaka Y, Takii R, Noda M, Inayoshi A, Watanabe K, Sanematsu F, Sasazuki T, Sasaki T, Fukui Y. DOCK2 is a Rac activator that regulates motility and polarity during neutrophil chemotaxis. J Cell Biol. 2006;174:647–652. doi: 10.1083/jcb.200602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gotoh K, Tanaka Y, Nishikimi A, Inayoshi A, Enjoji M, Takayanagi R, Sasazuki T, Fukui Y. Differential requirement for DOCK2 in migration of plasmacytoid dendritic cells versus myeloid dendritic cells. Blood. 2008;111:2973–2976. doi: 10.1182/blood-2007-09-112169. [DOI] [PubMed] [Google Scholar]
- 71.Werr J, Johansson J, Eriksson EE, Hedqvist P, Ruoslahti E, Lindbom L. Integrin alpha(2)beta(1) (VLA-2) is a principal receptor used by neutrophils for locomotion in extravascular tissue. Blood. 2000;95:1804–1809. [PubMed] [Google Scholar]
- 72.Matheu MP, Deane JA, Parker I, Fruman DA, Cahalan MD. Class IA phosphoinositide 3-kinase modulates basal lymphocyte motility in the lymph node. J Immunol. 2007;179:2261–2269. doi: 10.4049/jimmunol.179.4.2261. [DOI] [PubMed] [Google Scholar]
- 73.Foger N, Rangell L, Danilenko DM, Chan AC. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science. 2006;313:839–842. doi: 10.1126/science.1130563. [DOI] [PubMed] [Google Scholar]
- 74.Shiow LR, Roadcap DW, Paris K, Watson SR, Grigorova IL, Lebet T, An J, Xu Y, Jenne CN, Foger N, Sorensen RU, Goodnow CC, Bear JE, Puck JM, Cyster JG. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat Immunol. 2008;9:1307–1315. doi: 10.1038/ni.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shiow LR, Paris K, Akana MC, Cyster JG, Sorensen RU, Puck JM. Severe combined immunodeficiency (SCID) and attention deficit hyperactivity disorder (ADHD) associated with a Coronin-1A mutation and a chromosome 16p11.2 deletion. Clin Immunol. 2009;131:24–30. doi: 10.1016/j.clim.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Combaluzier B, Pieters J. Chemotaxis and phagocytosis in neutrophils is independent of coronin 1. J Immunol. 2009;182:2745–2752. doi: 10.4049/jimmunol.0801812. [DOI] [PubMed] [Google Scholar]
- 77.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bardi G, Niggli V, Loetscher P. Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Lett. 2003;542:79–83. doi: 10.1016/s0014-5793(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 79.Jacobelli J, Chmura SA, Buxton DB, Davis MM, Krummel MF. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat Immunol. 2004;5:531–538. doi: 10.1038/ni1065. [DOI] [PubMed] [Google Scholar]
- 80.Jacobelli J, Bennett FC, Pandurangi P, Tooley AJ, Krummel MF. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J Immunol. 2009;182:2041–2050. doi: 10.4049/jimmunol.0803267. [DOI] [PubMed] [Google Scholar]
- 81.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 82.Morley SC, Wang C, Lo WL, Lio CW, Zinselmeyer BH, Miller MJ, Brown EJ, Allen PM. The actin-bundling protein L-plastin dissociates CCR7 proximal signaling from CCR7-induced motility. J Immunol. 2010;184:3628–3638. doi: 10.4049/jimmunol.0903851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 84.Katagiri K, Katakai T, Ebisuno Y, Ueda Y, Okada T, Kinashi T. Mst1 controls lymphocyte trafficking and interstitial motility within lymph nodes. EMBO J. 2009;28:1319–1331. doi: 10.1038/emboj.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kinashi T, Katagiri K. Regulation of immune cell adhesion and migration by regulator of adhesion and cell polarization enriched in lymphoid tissues. Immunology. 2005;116:164–171. doi: 10.1111/j.1365-2567.2005.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jeon TJ, Lee DJ, Merlot S, Weeks G, Firtel RA. Rap1 controls cell adhesion and cell motility through the regulation of myosin II. J Cell Biol. 2007;176:1021–1033. doi: 10.1083/jcb.200607072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reif K, Ekland EH, Ohl L, Nakano H, Lipp M, Forster R, Cyster JG. Balanced responsiveness to chemoattractants from adjacent zones determines B-cell position. Nature. 2002;416:94–99. doi: 10.1038/416094a. [DOI] [PubMed] [Google Scholar]
- 88.Ueno T, Saito F, Gray DH, Kuse S, Hieshima K, Nakano H, Kakiuchi T, Lipp M, Boyd RL, Takahama Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J Exp Med. 2004;200:493–505. doi: 10.1084/jem.20040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witt CM, Raychaudhuri S, Schaefer B, Chakraborty AK, Robey EA. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 2005;3:e160. doi: 10.1371/journal.pbio.0030160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurobe H, Liu C, Ueno T, Saito F, Ohigashi I, Seach N, Arakaki R, Hayashi Y, Kitagawa T, Lipp M, Boyd RL, Takahama Y. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–177. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 92.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 93.Beuneu H, Garcia Z, Bousso P. Cutting edge: cognate CD4 help promotes recruitment of antigen-specific CD8 T cells around dendritic cells. J Immunol. 2006;177:1406–1410. doi: 10.4049/jimmunol.177.3.1406. [DOI] [PubMed] [Google Scholar]
- 94.Castellino F, Germain RN. Chemokine-guided CD4+ T cell help enhances generation of IL-6RalphahighIL-7Ralpha high prememory CD8+ T cells. J Immunol. 2007;178:778–787. doi: 10.4049/jimmunol.178.2.778. [DOI] [PubMed] [Google Scholar]
- 95.Schwab SR, Cyster JG. Finding a way out: lymphocyte egress from lymphoid organs. Nat Immunol. 2007;8:1295–1301. doi: 10.1038/ni1545. [DOI] [PubMed] [Google Scholar]
- 96.Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD. Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol. 2005;6:1228–1235. doi: 10.1038/ni1269. [DOI] [PubMed] [Google Scholar]
- 97.Sinha RK, Park C, Hwang IY, Davis MD, Kehrl JH. B lymphocytes exit lymph nodes through cortical lymphatic sinusoids by a mechanism independent of sphingosine-1-phosphate-mediated chemotaxis. Immunity. 2009;30:434–446. doi: 10.1016/j.immuni.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- 99.Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 101.Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- 102.Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pereira JP, An J, Xu Y, Huang Y, Cyster JG. Cannabinoid receptor 2 mediates the retention of immature B cells in bone marrow sinusoids. Nat Immunol. 2009;10:403–411. doi: 10.1038/ni.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sapoznikov A, Pewzner-Jung Y, Kalchenko V, Krauthgamer R, Shachar I, Jung S. Perivascular clusters of dendritic cells provide critical survival signals to B cells in bone marrow niches. Nat Immunol. 2008;9:388–395. doi: 10.1038/ni1571. [DOI] [PubMed] [Google Scholar]
- 105.Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, Rose H, Leung H, Cherayil BJ, Russell P, von Andrian U, Pillai S. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity. 2005;23:397–407. doi: 10.1016/j.immuni.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 106.Allen CD, Cyster JG. Follicular dendritic cell networks of primary follicles and germinal centers: phenotype and function. Semin Immunol. 2008;20:14–25. doi: 10.1016/j.smim.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, Chakraborty AK, Robey EA. The impact of negative selection on thymocyte migration in the medulla. Nat Immunol. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lin A, Loughman JA, Zinselmeyer BH, Miller MJ, Caparon MG. Streptolysin S inhibits neutrophil recruitment during the early stages of Streptococcus pyogenes infection. Infect Immun. 2009;77:5190–5201. doi: 10.1128/IAI.00420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;315:528–531. doi: 10.1126/science.1136736. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Migration of the B cell-T cell conjugate in the stromal cell network in the lymph node. The movie shows the 45-min time lapse images recorded every 30 s. Cognate antigen-specific B cells (green) and helper T cells (red) are stably conjugated. In cyan are primarily irradiation-resistant stromal cells. The mouse lymph node was imaged ex vivo by TPLM 36 h after immunization. Elapsed time is shown as hours/minutes/seconds. The image volume is 100 μm (x) × 100 μm (y) × 60 μm (z). The scale bar shows 30 μm on the nearest x-y plane from the viewpoint (MPG 4.71 MB)


