Abstract
Kir2 subunits form channels that underlie classical strongly inwardly rectifying potassium currents. While homomeric Kir2 channels display a number of distinct and physiologically important properties, the functional properties of heteromeric Kir2 assemblies, as well as the stoichiometries and the arrangements of Kir2 subunits in native channels, remain largely unknown. Therefore, we have implemented a concatemeric approach, whereby all four cloned Kir2 subunits were linked in tandem, in order to study the effects of Kir2.1 and Kir2.2 heteromerization on properties of the resulting channels. Kir2.2 subunits contributed stronger to single-channel conductance than Kir2.1 subunits, and channels containing two or more Kir2.2 subunits displayed conductances indistinguishable from that of a Kir2.2 homomeric channel. In contrast, single-channel kinetics was a more discriminating property. The open times were significantly shorter in Kir2.2 channels compared with Kir2.1 channels and decreased nearly proportionally to the number of Kir2.2 subunits in the heteromeric channel. Similarly, the sensitivity to block by barium also depended on the proportions of Kir2.1 to Kir2.2 subunits. Overall, the results showed that Kir2.1 and Kir2.2 subunits exert neither a dominant nor an anomalous effect on any of the properties of heteromeric channels. The data highlight opportunities and challenges of using differential properties of Kir2 channels in deciphering the subunit composition of native inwardly rectifying potassium currents.
Keywords: Inward rectifier potassium channels, Patch-clamp barium, Kinetics, Single channel
Introduction
Members of the Kir2 subfamily (Kir2.1–Kir2.3) are constitutively active channels that underlie classical strongly inwardly rectifying potassium currents, which contribute to stabilization of the resting membrane potential and action potential repolarization in excitable cells [1]. Several studies have shown that Kir2 subunits may form heteromers in exogenously expressing systems [2–4], and it is highly likely that native strongly inwardly rectifying potassium channels may also be heteromers of distinct Kir2 subunits. Since different Kir2 channels possess a number of distinct and physiologically important properties, including different profiles of outward current in the range of physiological membrane potentials [5–8], determining the specific Kir2 subunits that compose native channels is an important goal for better understanding their role in cellular excitability.
Subunit composition of a native channel can potentially be derived from its properties if one knows the contribution of individual subunits to the properties of their possible heteromeric assemblies. The proportional contributions of individual subunits cannot be simply assumed, and previous experimental data pointed to both possible dominant [6] and even anomalous [3] contributions of Kir2 subunits to some properties of Kir2 heteromers.
Previous studies have utilized external blockers of Kir2 channels to determine the molecular composition of native channels. For example, block by Ba2+ was used to compare the properties of cloned Kir2 channels to that of I K1, the major inwardly rectifying potassium channels in the heart [3, 4]. This is a potentially very powerful technique since homomeric Kir2 channels are known to possess significantly different affinities for Ba2+ ions [2, 9]. However, assessment of the Ba2+ sensitivities of heteromeric Kir2 channels has yielded conflicting results. For example, Preisig-Muller et al. [2] showed that channels formed by co-expressed Kir2.1 and Kir2.3 subunits had sensitivities to Ba2+ that were intermediate to those of Kir2.1 or Kir2.3 channels. In contrast, in similar experiments, Schram et al. [3] found that Kir2.1/Kir2.3 heteromers were significantly more sensitive to Ba2+ than Kir2.1 or Kir2.3 channels expressed alone. Several factors may have contributed to this discrepancy. In particular, the relative level of expression of specific Kir2 subunits or their effective participation in the formation of heteromeric channels cannot be controlled in experiments using co-expression of subunits. In addition, in co-expression experiments, presumably random heteromerization of Kir2 subunits would lead to formation of channels with unknown subunit stoichiometries and arrangements, thus significantly complicating the interpretation of the data.
Some suggestions with regard to specific properties of Kir2 heteromers can be made based on known underlying mechanisms. For example, evidence suggests that spermine binding may primarily be determined by the total electronegativity of its binding site inside the channel rather than by the position of specific subunits (non-coordination binding) [10]. In contrast, block by Ba2+ may strongly depend on precise coordination of the ion in the pore of the channel, rather than on the total electronegativity of binding sites. The precise contribution of specific Kir2 subunits to the properties of the heteromeric channels, however, cannot be predicted with certainty and thus must be determined experimentally.
To circumvent the inherent limitations of co-expression studies, we implemented a concatemeric approach whereby all four cloned Kir2 subunits were linked in tandem, which allowed us to assess both single-channel properties and Ba2+ sensitivities of channels with known stoichiometries and arrangements of subunits. In doing so, we were able to delineate Kir2 channels of different subunit stoichiometries based on their functional properties. The data provide essential tools needed for further elucidation of the exact subunit composition of channels underlying native inwardly rectifying potassium currents.
Materials and methods
Cloning of Kir2.x channels and producing concatemeric constructs
Cloning of Kir2.x channels
Kir2.1 and Kir2.2 subunits were cloned from mouse genomic DNA using a PCR-based technique and then sub-cloned into a pIRES-GFP vector (Clontech, USA), as described previously [8]. The Kir2.1-GFP fusion construct was made as described previously [11].
Concatemeric constructs
Concatemeric constructs were generated as described previously [12]. Briefly, Kir2.1/Kir2.2 tetrameric concatemers were made using a PCR-based method, whereby each full subunit was linked in tandem with eight glutamine residues each containing a restriction site in the middle. Each PCR product was digested and ligated into a pBluescript SK(-) vector (Stratagene, USA). Subsequent digestions and ligations were performed in order to introduce each channel component into the tetramer. After the complete tetramer was made in the pBluescript SK(-) vector, it was sub-cloned into a pIRES-EGFP vector for future cell transfections and expression.
HEK293A cell transfection
HEK293A cells were plated on 35-mm dishes, which contained glass coverslips. Cells were transfected (approximately 24 h after plating) with the Lipofectamine 2000 system (Invitrogen, USA) according to the manufacture’s protocol. For single-channel studies, smaller amounts of DNA (0.25–0.5 μg per dish) were used, and transfection times were only 2–3 h in order to produce lower expression. For studies with Ba2+, larger DNA amounts (1–2 μg per dish) were used, and transfection times were 4–6 h. Cells were used for electrophysiological experiments 1–2 days after transfection.
Solutions
Modified Tyrode (mM): 137 NaCl, 5.4 KCl, 0.5 MgCl2, 0.3 CaCl2, 0.16 NaH2PO4, 3 NaHCO3, 5 HEPES, 5 glucose, pH 7.35 with NaOH.
KINT-CaCl2 (mM): 140 KCl, 10 HEPES, 0.3 CaCl2, pH 7.35 with KOH.
FVPP (mM): 95 KCl, 0.1 Na3VO4, 10 K4P2O7, 5 KF, 10 HEPES, 1 EGTA, pH 7.35 with KOH.
Electrophysiology
Ionic currents were recorded in outside-out, inside-out, and cell-attached configurations [13]. The electrophysiological setup was as described previously [8]. Applied voltages were not corrected for liquid junction potentials. FVPP solution was used to slowdown channel rundown in inside-out and outside-out patches as well as during cell-attached recordings. Temperature of the perfusion solution was measured directly in the flow chamber using a small insulated thermocouple probe (K-08113-28, Cole Parmer).
Single-channel recordings
Patch pipettes, made of glass obtained from either Sutter Instruments (no. BF150-110-10; Novato, CA) or A-M Systems (no. 617000; Sequim, WA), were coated with either Sylgard 184 (Dow Corning, USA) or a heated mixture of parafilm and oil, and filled with KINT-CaCl2 solution. No differences were observed in current properties with either glass type. Cells were first superfused with modified Tyrode solution, which was then quickly changed to the high K+ FVPP solution after a stable cell-attached patch was formed. The use of FVPP solution (high K+ concentration) ensures membrane depolarization to ∼0 mV. Recordings were performed at a holding potential of −100 mV (assuming the above uncertainty of membrane potential) and chord conductances calculated as I/100 mV. A 1-s ramp from −90 to +60 mV was used to confirm that the single-channel currents were inwardly rectifying. All recordings were filtered at 1 KHz and digitized at 2–5 KHz. Patches that contained more than four active channels were discarded from single-channel analysis. The single-channel data were corrected for differences in the temperature of experimental solutions. The Q 10 values for single-channel conductance (1.56 ± 0.04; estimated using Kir2.1-GFP; n = 3) and kinetics (2.42 ± 0.14 for open and 2.06 ± 0.31 for closed; estimated using Kir2.1 monomer, n = 3) were measured by increasing the temperature of the bath solution during cell-attached single-channel recordings. For inside-out recordings, the patch was excised into the FVPP solution to washout polyamines and Mg2+ from the intracellular side of the membrane.
Outside-out recordings
Outside-out recordings of macroscopic currents were carried out essentially as described previously [8] with the following modifications: FVPP plus 100 μM spermine (Spm) was used as the pipette solution (intracellular), and KINT-CaCl2 was used as the external solution. Stocks of BaCl2 were diluted into the KINT-CaCl2 to make appropriate concentrations for experiments. Cells were kept in modified Tyrode solution until a stable outside-out configuration could be formed. To study steady-state Ba2+ sensitivity, currents were recorded at a holding potential of −50 mV. The kinetics of block with 10 μM Ba2+ was studied using a 2-s step pulse to −100 mV from a holding potential of +30 mV. The number of sweeps per recording varied from one to 20. Multiple sweeps from the same recording were averaged.
Data analysis
Data analysis was performed using Microsoft Excel (2000) and Clampfit 10.2 (Molecular Devices Co, CA). For single-channel conductance determination, single-channel records were transformed into mean-variance histograms using a homemade software incorporating the model used in Patlak et al. [14]. Conductances obtained using this program matched those obtained by using Clampfit 10.2. Single-channel kinetics was analyzed using Clampfit 10.2. Records were idealized, and events less than 1 ms were excluded. For kinetics analysis, in multi-channel patches, only few long stretches of activity (in the range of minutes and with hundreds to thousands of events in each) with only one level present were used. In a highly unlikely case that those stretches originated from different channels, the outcome will not be affected in any significant way as the situation would be equivalent to averaging the data from two identical Kir2 constructs. From the events data, logarithmic dwell-time histograms (number of events vs Log Dwell time (ms)) were constructed and fit with the predefined ‘exponential log probability’ function in Clampfit 10.2. Note that the above procedure uses linear rather than square-root ordinate for number of events [15]. Dwell-time histograms had 16 bins per decade and had a range of 1–5,000 ms. Open times were fit with single-exponential function. In general, closed times were fit with a four-exponential function. In a few patches, when either fastest or slowest components were not resolved, two- to three-exponential fits were used.
In experiments with Ba2+, the dose–response relations were fit with the following Hill equation:
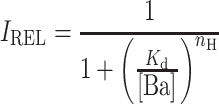 |
where I REL is the current in the presence of Ba2+ relative to the current in zero Ba2+, K d is the concentration at which one half of the current is blocked, [Ba] is the concentration of Ba2+ applied (μM), and n H is the Hill coefficient.
For all analyses of the kinetics of Ba2+ block, the tau of block (τ block) was determined by fitting the current trace with a single-exponential function. The fit began ∼15–30 ms from the beginning of the trace to minimize the contribution of the capacitance current.
Statistics
The data are presented as the mean ± standard error (SE). A two-tailed t test with equal variances was used for estimation of statistical significance of the differences between two means. A paired t test was used to test for statistical significance between single-channel properties in the cell-attached and excised inside-out configurations. For comparisons of multiple means, a one-way ANOVA was used. Asterisks *, **, and *** represent p < 0.05, p < 0.01, and p < 0.001, respectively.
Results
Single-channel conductance of heteromeric Kir2.1/Kir2.2 channels
Figure 1a shows representative cell-attached records of inward currents for several Kir2 channels. All tested channels exhibited strong inwardly rectifying behavior (not shown). In contrast to the study by Picones et al. [16] that revealed a large variability in the single-channel conductance of Kir2.1 channels in a majority of patches, our measurements showed only moderate variability, consistent with a number of previous reports. In a minority of patches, small conductances similar to those described in Picones et al. [16] (Fig. 1b) were also observed in both homomeric and heteromeric Kir2 channels, but their activity was short-lived and channel openings were infrequent when compared with the larger conductance channels. In addition, we also observed sub-conductance levels (Fig.1b), a common phenomenon in various ion channels including Kir2.1 [17]. Sub-conductances and small conductances were still present when the pipette was filled with a CaCl2-free solution containing 1 mM EGTA and 1 mM EDTA, indicating that these sublevels were not being caused by some multivalent cationic contaminant blocking the channel [18]. No such channels (large or small) were observed in untransfected cells (n = 5). For the purposes of this study, we characterized only the main conductance states.
Fig. 1.
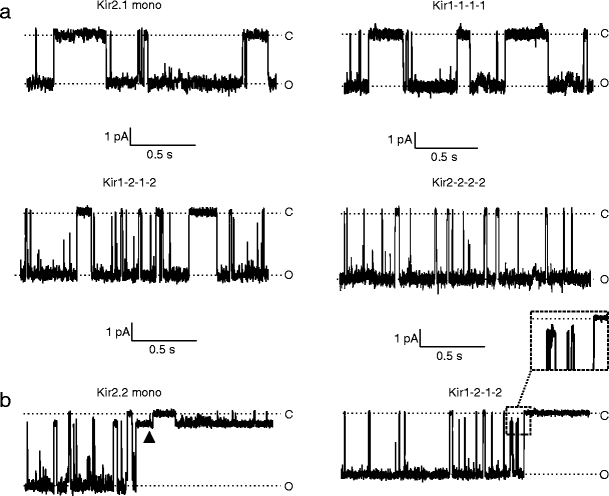
Single-channel recordings of Kir2.1/Kir2.2 channels. a Representative recordings of Kir2 single-channel currents recorded in HEK293 cells at −100 mV in cell-attached mode with high external K+. b Examples of recordings that contain small channels (left) and sub-conductance states (right). Left, a single-channel recording from a patch expressing Kir2.2 monomeric channels. A smaller conductance channel can be observed with a larger conductance channel (black triangle). Right, a single-channel recording from a patch expressing Kir2.1-2-1-2 heteromeric channel. Insert, an enlargement of the section of the recording where sub-conductance states are seen
The conductance increased by 5.7 ± 0.9 pS (n = 4, p < 0.01, Kir2.1-GFP channels were used in this experiment) when the patch was excised and exposed to the polyamine-free bath FVPP solution. Similar results were also observed with Kir2.2 monomeric channels (3.0 ± 0.9 pS; n = 10, p < 0.01). This was not unexpected since it is known that intracellular polyamines may inhibit Kir2 currents and decrease the single-channel conductance of Kir2 channels even at far negative membrane potentials [8, 19]. Effects of free intracellular Mg2+ ions may be similar. It is highly likely that a similar increase in conductance would be observed with any other Kir2 constructs used in this study (this phenomenon was not investigated further). Despite the observed differences between cell-attached and inside-out single-channel conductances, the cell-attached approach employed in this study is not compromised in any way since the composition of intracellular environment is strongly believed to be similar across the cells. In order to minimize channel rundown and to increase the success rate of experiments, single channel measurements were carried out in the cell-attached configuration.
Figure 2 shows conductance values measured at a holding potential of −100 mV for various heteromers. Consistent with past studies [9], Kir2.1 monomeric channels displayed a smaller conductance than Kir2.2 monomeric channels, 30.2 ± 0.77 pS vs 35.8 ± 0.74 pS for Kir2.1 and Kir2.2 channels, respectively (p < 0.01). Importantly, Kir2.1 homo-concatemers, Kir2.1 monomeric channels, and Kir2.1 channels with a C-terminal GFP fusion tag have statistically indistinguishable conductances, 29.9 ± 0.49 pS vs 30.2 ± 0.77 pS vs 30.4 ± 0.7 pS, for 1-1-1-1, Kir2.1 mono, and Kir2.1-GFP, respectively (p = 0.75), indicating that concatemerization or addition of GFP fusion tag has no appreciable effect on K+ permeation. However, hetero-concatemers containing a single Kir2.2 subunit and three Kir2.1 subunits (e.g., 1-1-1-2) display an increased conductance (e.g., 34.4 ± 0.6 pS for 1-1-1-2 concatemer; p < 0.01) when compared to Kir2.1 homomeric channels (Fig. 2). The position of the Kir2.2 subunit does not significantly affect the channel conductance (compare 2-1-1-1 vs 1-1-1-2 vs 1-2-1-1; p = 0.075), and the small observed differences are likely due to experimental variation. Addition of second Kir2.2 subunit leads to further increase in channel conductance, but again, essentially independent of the specific subunit arrangement (p = 0.1 for 1-1-2-2 vs 1-2-1-2). Conductance of channels containing two Kir2.2 subunits was not different from that of monomeric Kir2.2 channels, and concatemers with three or four Kir2.2 subunits displayed somewhat higher conductances. In practical terms, it is clear that assemblies with two or more Kir2.2 subunits form a group of channels with essentially similar conductances.
Fig. 2.
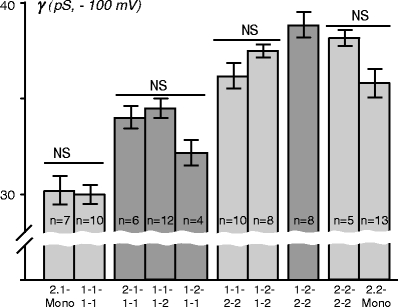
Single-channel conductances of Kir2.1/Kir2.2 channels. Note the break in the vertical axis, which allows for better highlighting the differences in conductances. The single-channel conductance increases with the number of Kir2.2 subunits in the channel, but not directly proportionally. NS no statistical difference
Taken together, the data show that Kir2.1/Kir2.2 heteromerization does not lead to any anomalous single-channel conductances although some stronger contribution of Kir2.2 subunit can be appreciated. Clearly, single-channel conductance property can be useful (but not strongly discriminating) in deciphering the subunit composition of native channels.
Single-channel kinetics of heteromeric Kir2.1/Kir2.2 channels
In contrast to single-channel conductances, kinetics properties might be a significantly more discriminating, in particular, due to relatively larger differences in open times (τ open) between Kir2.1 and Kir2.2 channels (Fig. 4).
Fig. 4.
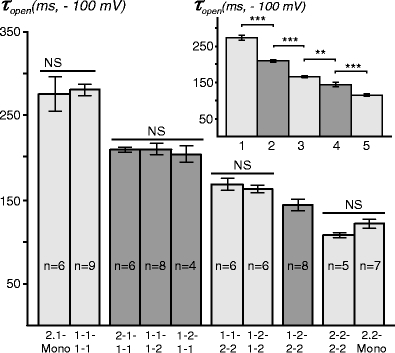
Open times for Kir2.1/Kir2.2 channels. The τ open decreases nearly proportionally to the number of Kir2.2 subunits in the channel. Insert, averaged τ open values for channels of the same stoichiometries: 1, Kir2.1 Mono/1-1-1-1; 2, 2-1-1-1/1-1-1-2/1-2-1-1; 3, 1-1-2-2/1-2-1-2; 4, 1-2-2-2; and 5, Kir2.2 Mono/2-2-2-2. NS no statistical difference
Single-channel recordings in HEK293 cells expressing various Kir2.x concatemers were stable for 3-40 min in the cell-attached configuration. All channels displayed prolonged closed times, sometimes lasting tens of minutes, therefore long-lasting stable cell-attached recordings allowed for both open and closed times to be analyzed. The prolonged closed times were intermittently spaced between high-activity “burst” times, where the channel opened and closed rapidly (Fig. 3a). The mechanism of these prolonged closed states is unknown. These prolonged closed states still occurred even when the pipette was filled with a Ca2+-free solution that contained 1 mM EGTA and 1 mM EDTA, or when the holding potential was changed to +30 mV for ∼1 min and then returned to −100 mV, suggesting that the long closed times are unlikely due to block by an unknown cationic contaminant [18].
Fig. 3.
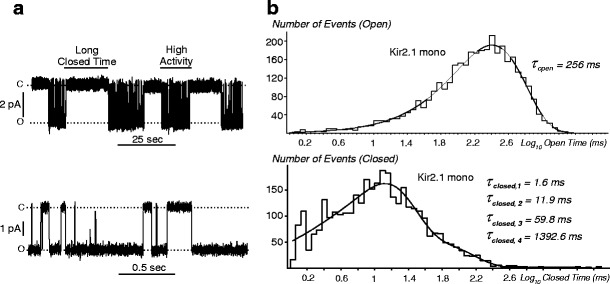
Single-channel kinetics of Kir2.1/Kir2.2 channels. a (top) A representative segment of a single-channel recording in cell-attached mode from a Kir2.1 monomeric channel. Both high activity and long closed portions of the record are indicated. a (bottom) An enlargement of a high-activity portion from the same record. b Dwell-time histograms for open and closed events were plotted from the data in a. The open-time histogram was fit with a single function to obtain the mean open time τ open (top). A four-exponential function was used to fit the closed-time histogram (bottom) in order to obtain four closed times (τ closed)
In all channels, dwell-time histograms for τ open could be well fit with a single-exponential function (see Materials and Methods), indicating the presence of only one open state (Fig. 3b, top). The τ open decreased by 31.4 ± 5.4 ms (n = 4, p = 0.005, Kir2.1-GFP channels) when the patch was excised and exposed to the polyamine-free FVPP bath solution. This change, however, is relatively small compared to the larger values of τ open, and the effect of patch excision on the conclusions will be mostly offset by measuring differences in τ open between different Kir2.1/Kir2.2 channels (see below).
Homo-concatemers and corresponding monomeric channels display statistically indistinguishable τ open, 275.2 ± 20.4 ms vs 279.9 ± 6.0 ms for Kir2.1 and 1-1-1-1, respectively (p = 0.87), vs 121.3 ± 5.1 ms and 106.9 ± 2.5 ms for Kir2.2 and 2-2-2-2, respectively (p = 0.0501), thus justifying the usefulness of concatemerization approach. Kir2.1 monomeric channels tagged with GFP at the C terminus also did not display any significant changes in τ open (262.5 ± 12.8 ms) when compared to Kir2.1 monomers or homo-concatemers. Figure 4 shows τ open for channels of different stoichiometries. A concatemer containing only one Kir2.2 subunit and three Kir2.1 subunits (2-1-1-1) displays a significantly decreased τ open of 209.7 ± 2.0 ms (n = 6) while positioning the Kir2.2 subunit at the C or N terminus or in the middle does not result in any change in τ open (compare 2-1-1-1 vs 1-1-1-2 vs 1-2-1-1; p = 0.82). Adding two Kir2.2 subunits further decreases the τ open, again, independent of the subunit arrangement: 169.3 ± 5.6 ms and 163.2 ± 3.8 ms for 1-1-2-2 and 1-2-1-2 channels, respectively (p = 0.39). Channels with three Kir2.2 subunits can be distinguished from homomeric Kir2.2 as well (Fig. 4).
All together, the data show that channels with the same Kir2.1/Kir2.2 stoichiometries but with different subunit symmetries and arrangements are likely to have same τ open. Accordingly, Fig. 4 (insert) shows the τ open for group-averaged (same stoichiometry) data and highlights the finding that τ open decreases nearly proportionally to the number of Kir2.2 subunits in the heteromeric channel. However, one should not be misled by small error bars in this graph when considering the reverse task of deriving the subunit composition of a channel from a single-channel record.
In contrast to open times, dwell-time histograms for closed times generally required a fit with a four-exponential function (Fig. 3b, bottom; Table 1). Although in some patches (from different constructs), either fastest or slowest component could not be resolved with certainty due to limited time resolution or small amplitude, respectively, the data clearly show the existence of at least four closed states in most Kir2 channels.
Table 1.
Closed times, expressed in milliseconds, for Kir2.1/Kir2.2 channels
| τ closed,1 | τ closed,2 | τ closed,3 | τ closed,4 | Number (n) | |
|---|---|---|---|---|---|
| 2.1 mono | 3.0 ± 0.86 | 15.3 ± 2.0 | 67.9 ± 12.8 | 1961 ± 828 | 6 |
| 1-1-1-1 | 1.6 ± 0.36 | 14.1 ± 0.67 | 60.1 ± 6.8 | 1822 ± 172 | 8–9 |
| 2-1-1-1 | 2.7 ± 1.0 | 11.4 ± 2.1 | 43.4 ± 4.4 | 1821 ± 358 | 5–6 |
| 1-1-1-2 | 3.3 ± 1.0 | 13.9 ± 2.1 | 49.5 ± 3.9 | 1717 ± 331 | 7–8 |
| 1-2-1-1 | 1.7 ± 0.11 | 12.1 ± 0.93 | 57.4 ± 11 | 3070 ± 980 | 4 |
| 1-1-2-2 | 4.3 ± 1.2 | 10.0 ± 1.3 | 38.8 ± 2.0 | 2012 ± 210 | 3–6 |
| 1-2-1-2 | ND | 10.1 ± 1.2 | 50.5 ± 8.3 | 1710 ± 289 | 6 |
| 1-2-2-2 | 2.5 ± 0.54 | 9.5 ± 0.5 | 45.5 ± 5.6 | 2475 ± 307 | 7–8 |
| 2-2-2-2 | ND | 7.0 ± 0.24 | 32.1 ± 5.6 | 2178 ± 582 | 4–5 |
| 2.2 mono | ND | 6.4 ± 0.31 | 33 ± 3.7 | 2376 ± 815 | 7 |
Closed times (mean ± SE) were not significantly different between channels of the same stoichiometry. Closed times τ closed,2 and τ closed,3 in Kir2.1/Kir2.1-1-1-1 channels are significantly longer than those in Kir2.2/Kir2.2-2-2-2 channels (p < 0.001). Shortest (τ closed,1) and longest (τ closed,3) closed times were not different across all channels
ND not determined, this notation is used when the number of patches, where the specific component could be reliably resolved, was less than three
Similar to τ open, some τ closed were significantly different between Kir2.1 and Kir2.2 homomeric channels. In particular, both the τ closed,2 and the τ closed,3 were nearly twice larger in monomeric Kir2.1 than in monomeric Kir2.2 channels (Table 1). Concatemerization of Kir2 subunits in various combinations did not lead to any anomalous values for τ closed. Although there is a clear trend of shortening both τ closed,2 and τ closed,3 as more Kir2.2 subunits are added to the channel, the variability in the data makes individual τ closed less reliable in assigning their values to a specific stoichiometry of Kir2 heteromer.
Steady-state barium sensitivity of Kir2.1/Kir2.2 heteromeric channels
Previous studies examining heteromerization of Kir2 subunits have exploited the distinct sensitivities of Kir2 homomeric channels to extracellular Ba2+ [3, 9] in order to determine subunit composition of native inwardly rectifying potassium currents. However, most investigations have been limited to co-expression experiments of Kir2 subunits, where the subunit stoichiometries of individual channels are unknown. Therefore, in order to advance in this direction, we studied the Ba2+ sensitivity of our Kir2 concatemers.
Experiments were carried out using outside-out patches at high symmetrical K+ in order to fully control the intracellular solution and to easily change the concentration of extracellular Ba2+ (see Materials and Methods). A representative record of a typical experiment is shown in Fig. 5a and Fig. 5b shows dose–response relationships for Ba2+ measured at −50 mV in various Kir2 hetero-concatemers. The Hill coefficients (n H) were close to 1 for every channel studied. Consistent with previous reports, we also find Kir2.2 channels significantly more sensitive to Ba2+ than Kir2.1 channels (Fig. 5) although in somewhat lesser degree: ∼5.4-fold difference in K d compared to ∼6–7-fold in other [3, 9] studies.
Fig. 5.
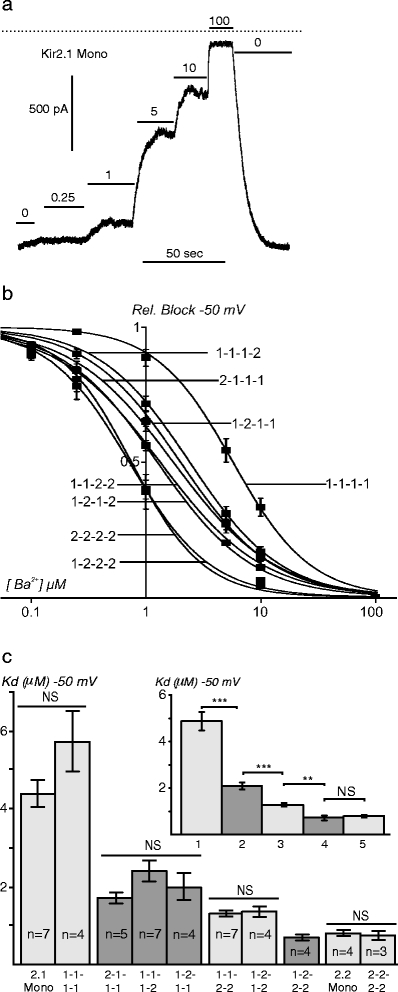
Steady-state Ba2+ sensitivity of Kir2.1/Kir2.2 channels. a A representative outside-out recording for Kir2.1 monomeric channels (macroscopic currents) at a holding potential of −50 mV. Concentrations of 0, 0.25, 1, 5, 10, and 100 μM Ba2+ are indicated. The dashed line represents the zero current level. b Ba2+ dose–response relationships. The data were fit with the Hill equation and the corresponding fits are plotted using smooth lines. c Kd values for Ba2+ block at -50 mV for each construct. c Insert, averaged Kd values for channels with the same stoichiometries: 1, Kir2.1 Mono/1-1-1-1; 2, 2-1-1-1/1-1-1-2/1-2-1-1; 3, 1-1-2-2/1-2-1-2; 4, 1-2-2-2; and 5, Kir2.2 Mono/2-2-2-2
Some of the data on Ba2+ sensitivity can be summarized as follows (Fig. 5c): (1) no significant effect of concatenation of Kir2 subunits per se, (2) no anomalous effects of Kir2 subunits heteromerization, and (3) no effect of specific position of Kir2 subunits in a channel (e.g., p = 0.20 when comparing 2-1-1-1, 1-2-1-1, and 1-1-1-2, and p = 0.66 when comparing 1-1-2-2 and 1-2-1-2). Clearly, Kir2.2 subunits contribute stronger to Ba2+ sensitivity since the addition of the first Kir2.2 subunit to a Kir2.1channel (i.e., 1-1-1-2) leads to a more than two-fold increase in Ba2+ sensitivity. Relative decrease in K d upon consecutive introduction of second and third Kir2.2 subunit is also significant (approximately two-fold) although the absolute (practically important) changes in K d become progressively smaller, and no difference at all (p = 0.74) could be found between K d for Kir2.2 or 2-2-2-2 and 1-2-2-2.
Kinetics of barium block in Kir2.1/Kir2.1 heteromers
In order to further discern the differences in Ba2+ sensitivity between Kir2.1/Kir2.2 heteromers, we examined the kinetics of extracellular Ba2+ block in the presence of 10 μM Ba2+. The recording conditions were the same as those used in steady-state studies except using −100 mV membrane potential. In the absence of Ba2+, currents did not display any measurable decline (data not shown). Figure 6a shows examples of current traces from a 1-1-1-1 homomeric and a 1-1-2-2 heteromeric channels. Both current traces were fit with single-exponential functions, although one can see that each trace would probably be better described by a two-exponential function. A fit with a two-exponential function was surely superior, and we observed fast and slower components (τ block) in most constructs studied. However, for reasons that are not clear, the amplitude and the τ block value of the slower component varied significantly (even for the same construct), and some constructs could be adequately fit with just a single-exponential function. An example in Fig. 6b shows two 2-2-2-2 current traces from two separate outside-out patches, each fit with a single-exponential function. In one patch (top), a single-exponential functional does not fit well the trace, but in the other patch (bottom), a single-exponential function fit was clearly sufficient. Because of this variability in the kinetics of channel block, our practical approach was to use single-exponential fits in all recordings.
Fig. 6.
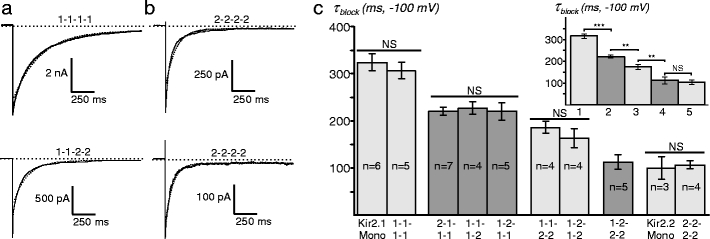
Kinetics of Ba2+ block in Kir2.1/Kir2.2 channels. Kinetics of Ba2+ block was studied in outside-out patches at high symmetrical K+ in the presence of 10 μM Ba2+ and at −100-mV membrane potential. a Examples of current traces for a Kir2.1-1-1-1 channel (top) and a Kir2.1-1-2-2 channel (bottom), both of which are fit (dashed line) with a single-exponential function. For clarity, only the first part of the 2-s current trace is shown. b Two recordings from two separate outside-out patches of Kir2.2-2-2-2 channels. Top, a single-exponential function does not fit well the current trace; however, in the recording from the other patch (bottom), a single-exponential function fits the current trace adequately. c The τ block values for each construct. Insert, averaged τ block values for channels with the same stoichiometries: 1, Kir2.1 Mono/1-1-1-1; 2, 2-1-1-1/1-1-1-2/1-2-1-1; 3, 1-1-2-2/1-2-1-2; 4, 1-2-2-2; and 5, Kir2.2 Mono/Kir2-2-2-2
Figure 6c summarizes the measurements of single-exponential τ block. The Ba2+ block is ∼3.2-fold faster in Kir2.2 than in Kir2.1 monomeric channels (99.8 ± 24.0 ms vs 323.5 ± 16.8 ms; p < 0.01). The τ block in both Kir2.1 and Kir2.2 homo-concatemers are not different from that in corresponding monomeric channels. Sequential addition of one, two, and three Kir2.2 subunits to a Kir2.1 concatemeric channel leads to a progressive decrease in τ block although 1-2-2-2 channels cannot be distinguished from homomeric Kir2.2 based on the rate of Ba2+ block. Importantly, the positioning of single Kir2.2 subunit in a concatemer has no effect on the kinetics of block, and channels with two Kir2.2 subunits but of different symmetries show no differences in kinetics as well. This allows to group and average the data accordingly as shown in Fig. 6c (insert). The addition of only one Kir2.2 subunit to a Kir2.1/1-1-1-1 channel decreases the τ block from 315.8 ± 9.6 ms to 221.4 ± 6.9 ms (p < 0.01), the addition of two Kir2.2 subunits further decreases the τ block to 174.1 ± 11.5 ms (p < 0.01), and the addition of three Kir2.2 subunits decreases the τ block to 112.7 ± 15.8 ms (p < 0.01), which becomes statistically indistinguishable from Kir2.2 homomeric channels (p = 0.62).
Discussion
This study was originated by conflicting data and interpretations regarding Kir2 channel heteromerization and the contribution of individual Kir2 subunits to the properties of native inwardly rectifying potassium channels. A number of attempts were undertaken in order to gain insights into the molecular composition of native strong inward rectifiers, mostly in cardiac tissues, where these channels are highly expressed and play a relatively well-defined roles. The task is twofold: first, to determine the effects of Kir2 heteromerization on a selected property of channels and, second, to use that defined property of distinct heteromers in deciphering the composition of native channels.
Single-channel conductance
Homomeric Kir2 channels display distinct single-channel conductances although significant discrepancies can be found between studies including ours. For example, Picones et al. [16] showed that conductances of Kir2.1 channels expressed in Xenopus oocytes vary from nearly 0 to ∼35 pS, and Liu et al. [9] found that Kir2.1 channels expressed in HEK293 cells display conductances ranging from ∼25 to ∼35 pS. In contrast, we did not see such variation in our recordings of Kir2 channels (only ±1.0–2.5 pS) (although sub-conductance levels were present). Few potential reasons for this conflict may be the differences in the cell type and basic experimental conditions such as differences in solutions composition. However, we also believe that other factors are also critical in order to reach satisfactory precision in comparing various heteromers. In particular, even small drifts in the electrode(s) potential, differences in the intracellular membrane potential, or in the temperature of bath solution may significantly affect the data. For example, a 5-mV drift in the electrode or intracellular potential will account for 1.5 pS of conductance when it is measured at −100 mV or 3 pS if it is measured at −50 mV. Similarly, a 5°C increase in the temperature would lead to increase of the single-channel conductance from ∼30 to ∼37 pS assuming Q 10 = 1.56 as determined in this study. It should be noted that the ∼0–35-pS variation of single-channel conductance of Kir2.1 channels observed by Picones et al. [16] cannot be explained by voltage drifts or temperature variations. In our experiments, we tried to account for all possible variables. With only ∼18% difference in conductance between Kir2.1 and Kir2.2 channels, determining intermediate conductances of Kir2.1/Kir2.2 heteromers may become practically impossible without paying attention to the above detail. Overall, our data show that Kir2.2 subunits exert very strong, although not fully dominant effect, on single-channel conductance such that channels with more than one Kir2.2 subunit could not be distinguished from homomeric Kir2.2 channels. It also follows that in more experimentally challenging situations such as experiments with cardiac myocytes, the use of single-channel conductance alone may be limited and challenging. Nevertheless, an important finding is that Kir2.1/Kir2.2 heteromerization did not lead to any anomalous phenomena (e.g., unusually high or low conductance) in any tested subunit combination.
Single-channel kinetics
Single-channel kinetics proved to be a more useful property for distinguishing between channels containing not only one but two Kir2.2 subunits (and possibly three Kir2.2 subunits). Open-state kinetics had significantly smaller experimental variation compared to that of the four closed states, making the former parameter a more discriminating property. Although some closed times (τ closed,2 and τ closed,3; Table 1) displayed a clear decreasing trend upon sequential addition of Kir2.2 subunits, the experimental errors were too high to allow for a reliable discrimination between channels differing by one Kir2 subunit.
The data show that in a reverse task neither single-channel conductance nor any particular dwell time (e.g., τ closed,2) alone would allow to determine the subunit composition of an underlying channel with great degree of certainty. Nevertheless, since single-channel conductance and all (five) dwell times are independent parameters which can be estimated simultaneously from a single recording these data can be treated together in order to increase the level of confidence. It seems, however, that a better control over the experimental conditions leading to smaller experimental variations would be a more useful approach in future studies. For example, using an open-cell cell-attached configuration (as e.g., in [20]) or excised patches would minimize complications arising from variations in membrane potential and concentration of intracellular K+ as well as other small relevant molecules such as Mg2+ ions and polyamines.
Ba2+ sensitivity
Although differential Ba2+ sensitivity of Kir2 channels can be used in single-channel experiments [9], it is more experimentally challenging compared to macro patch approach employed in our study. On the other hand, the integral currents can only provide a pseudo averaged information on the subunit composition of underlying Kir2 heteromers and thus have more limited use compared to single-channel experiments.
Other functional approaches
Kir2 channels possess other properties which can be employed in deciphering subunit composition of native channels. For example, Kir2 channels display differential sensitivity to intracellular polyamines [8] and H+ [21, 22]. Experiments involving these properties, however, can practically be carried out only in inside-out macro patches and would be even more challenging than those involving Ba2+ ions or single-channel kinetics parameters due to smaller differences in the sensitivities of various Kir2 channels to polyamines and H+ compared.
Limitations
Experiments in this study are limited to only Kir2.1 and Kir2.2 channels although Kir2.3 channels may also be co-expressed in native tissues thus further complicating the task. The situation in this area strongly depends on the species and specific tissues and, as mentioned before, remains quite controversial. For example, using single-channel analysis, Liu et al. [9] provided evidence that Kir2.1, Kir2.2, and Kir2.3 channels are functionally expressed in guinea pig heart, while Dhamoon et al. [6] found no Kir2.2 mRNA and protein in the same tissue. In the mouse heart, Kir2.3 subunits are unlikely to be expressed at a measurable level [12, 23] and thus the data from this study may be most applicable to this species.
The data also clearly show that the application of functional assays like those in this study will be a more challenging task with native channels than that with exogenously expressed ones. Therefore, the deciphering of subunit composition of native channels will require not only a maximal control of possible experimental variables but also additional biochemical and genetic approaches.
Conclusions
The results of this study provide a quantitative assessment of the properties of Kir2.1/Kir2.2 heteromers. In particular, the data show that Kir2.1 and Kir2.2 subunits exert neither dominant nor anomalous but rather proportional effect on all properties of heteromeric channels. This study demonstrates both important opportunities as well as significant challenges of functional assays in deciphering the subunit composition of native Kir2 channels.
Acknowledgments
We thank Dr. Fuzhen Wang for the invaluable assistance with cell culture work in some experiments.
This work was supported by the National Institutes of Health Grant R01-HL-069052 (ANL) and the Systems and Integrative Biology Training Grant T-32GM008322 (BKP).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Brian K. Panama, Phone: +1-416-946-8111, FAX: +1-416-946-8380, Email: brian.panama@utoronto.ca
Anatoli N. Lopatin, Phone: +1-734-615-8903, FAX: +1-734-936-8813, Email: alopatin@umich.edu
References
- 1.Anumonwo JM, Lopatin AN. Cardiac strong inward rectifier potassium channels. J Mol Cell Cardiol. 2010;48:45–54. doi: 10.1016/j.yjmcc.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preisig-Muller R, Schlichthorl G, Goerge T, Heinen S, Bruggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen's syndrome. Proc Natl Acad Sci USA. 2002;99:7774–7779. doi: 10.1073/pnas.102609499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schram G, Pourrier M, Wang Z, White M, Nattel S. Barium block of Kir2 and human cardiac inward rectifier currents: evidence for subunit-heteromeric contribution to native currents. Cardiovasc Res. 2003;59:328–338. doi: 10.1016/S0008-6363(03)00366-3. [DOI] [PubMed] [Google Scholar]
- 4.Zobel C, Cho HC, Nguyen TT, Pekhletski R, Diaz RJ, Wilson GJ, Backx PH. Molecular dissection of the inward rectifier potassium current (IK1) in rabbit cardiomyocytes: evidence for heteromeric co-assembly of Kir2.1 and Kir2.2. J Physiol. 2003;550:365–372. doi: 10.1113/jphysiol.2002.036400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhamoon AS, Jalife J. The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm. 2005;2:316–324. doi: 10.1016/j.hrthm.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JM. Unique Kir2.x properties determine regional and species differences in the cardiac inward rectifier K+ current. Circ Res. 2004;94(10):1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 7.Munoz V, Vaidyanathan R, Tolkacheva EG, Dhamoon AS, Taffet SM, Anumonwo JM. Kir2.3 isoform confers pH sensitivity to heteromeric Kir2.1/Kir2.3 channels in HEK293 cells. Heart Rhythm. 2007;4:487–496. doi: 10.1016/j.hrthm.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panama BK, Lopatin AN. Differential polyamine sensitivity in inwardly rectifying Kir2 potassium channels. J Physiol. 2006;571:287–302. doi: 10.1113/jphysiol.2005.097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin HG, Lu Z. Mechanism of the voltage sensitivity of IRK1 inward-rectifier K+ channel block by the polyamine spermine. J Gen Physiol. 2005;125:413–426. doi: 10.1085/jgp.200409242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLerie M, Lopatin AN. Dominant-negative suppression of I(K1) in the mouse heart leads to altered cardiac excitability. J Mol Cell Cardiol. 2003;35:367–378. doi: 10.1016/S0022-2828(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 12.Panama BK, McLerie M, Lopatin AN. Heterogeneity of IK1 in the mouse heart. Am J Physiol Heart Circ Physiol. 2007;293:H3558–H3567. doi: 10.1152/ajpheart.00419.2007. [DOI] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Patlak JB. Measuring kinetics of complex single ion channel data using mean-variance histograms. Biophys J. 1993;65:29–42. doi: 10.1016/S0006-3495(93)81041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sigworth FJ, Sine SM. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picones A, Keung E, Timpe LC. Unitary conductance variation in Kir2.1 and in cardiac inward rectifier potassium channels. Biophys J. 2001;81:2035–2049. doi: 10.1016/S0006-3495(01)75853-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu T, Ting AY, Mainland J, Jan LY, Schultz PG, Yang J. Probing ion permeation and gating in a K+ channel with backbone mutations in the selectivity filter. Nat Neurosci. 2001;4:239–246. doi: 10.1038/85080. [DOI] [PubMed] [Google Scholar]
- 18.Guo D, Lu Z. Pore block versus intrinsic gating in the mechanism of inward rectification in strongly rectifying IRK1 channels. J Gen Physiol. 2000;116:561–568. doi: 10.1085/jgp.116.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie LH, John SA, Weiss JN. Spermine block of the strong inward rectifier potassium channel kir2.1: dual roles of surface charge screening and pore block. J Gen Physiol. 2002;120:53–66. doi: 10.1085/jgp.20028576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols CG, Lederer WJ. The regulation of ATP-sensitive K+ channel activity in intact and permeabilized rat ventricular myocytes. J Physiol. 1990;423:91–110. doi: 10.1113/jphysiol.1990.sp018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins A, Larson M. Differential sensitivity of inward rectifier K+ channels to metabolic inhibitors. J Biol Chem. 2002;277:35815–35818. doi: 10.1074/jbc.M206032200. [DOI] [PubMed] [Google Scholar]
- 22.Qu Z, Yang Z, Cui N, Zhu G, Liu C, Xu H, Chanchevalap S, Shen W, Wu J, Li Y, Jiang C. Gating of inward rectifier K+ channels by proton-mediated interactions of N- and C-terminal domains. J Biol Chem. 2000;275:31573–31580. doi: 10.1074/jbc.M003473200. [DOI] [PubMed] [Google Scholar]
- 23.Zaritsky J, Redell J, Tempel B, Schwarz T. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


