Abstract
Memory (M) impairments have been suggested in pediatric Obstructive Sleep Apnea along with attention and executive (AE), language (L) and visuospatial (V) dysfunctions. NEPSY assessment of children aged 5–9-years who were either healthy (n= 43), or who had OSA without L, V, AE (OSA−, n= 22) or with L (n=6), V (n=1), AE (n=3) (OSA+, n=10) dysfunctions revealed no gross memory problems in OSA; however, over the 3 learning trials of cross-modal association learning of name with face, the OSA− progressively improved performance, whereas the OSA+ failed to progress. No within-group differences between immediate and delayed memory tasks were apparent. The data suggest the presence of slower information processing, and/or secondary memory problems, in the absence of retrieval or recall impairments among a subset of children with OSA. We hypothesize that inefficient/insufficient encoding may account for the primary deficit.
Keywords: obstructive sleep apnea, memory, neurodevelopment, learning
INTRODUCTION
In the last decade, studies on the neurocognitive consequences of childhood sleep-disordered breathing (SDB) have been widely performed and published (Beebe, 2006; Blunden, Lushington, Kennedy, Martin, & Dawson, 2000; Giordani, et al., 2008; Gottlieb, et al., 2003; Halbower, et al., 2006; Kaemingk, et al., 2003; Kurnatowski, Putynski, Lapienis, & Kowalska, 2006; Mulvaney, et al., 2006; O'Brien, Mervis, Holbrook, Bruner, Klaus, et al., 2004; O'Brien, Mervis, Holbrook, Bruner, Smith, et al., 2004; Rhodes, et al., 1995) following our initial observation on the impact of SDB on school performance (Gozal, 1998). However, the overt neurodevelopmental morbidity profile of the child with SDB has not yet been consistently delineated.
Childhood encompasses the peak period of wakefulness, i.e., the period during which the brain is maximally apt to learn and perform, and therefore disruption of such normal processes may adversely impact on brain development and function. Consequently, cumulative evidence increasingly suggests that SDB may challenge this learning capacity via pathophysiological mechanisms triggered by sleep fragmentation and gas exchange abnormalities during sleep. Conversely, sleep promotes and enhances memory and learning (Wagner, Kashyap, Diekelmann, & Born, 2007). Accordingly, disruption of sleep may potentially impact on any stage of memory formation namely, acquisition and encoding, consolidation, integration, recall and recognition, reconsolidation, and forgetting (Hill, Hogan, & Karmiloff-Smith, 2007). Depending on the memory network involved during a specific task, different sleep stages may be preferentially implicated. For example, explicit or declarative memory involving the hippocampus has been associated with non-rapid eye movement sleep, particularly slow-wave sleep, whereas hippocampus-independent implicit or nondeclarative memory is associated with rapid eye movement sleep (Hill, et al., 2007; Wagner, et al., 2007). Several studies have reported differences in memory ‘index’ findings among children with SDB, but such findings have been remarkably inconsistent (Beebe, 2006). Surprisingly, no studies have specifically explored memory or learning processes, rather than a cumulative outcome memory index, among children with SDB. This is all the more surprising considering the fact that encoding, consolidation, storage, and/or retrieval of information are unmistakably fundamental in the childhood behavioral repertoire and are decisive for adaptive behaviors. For example, a child’s social learning environment is based on recognizing friends and making new friends in the playground, a social behavior already crucial since infancy with respect to parental ‘facial’ recognition. Intact neural networks are therefore a prerequisite to normal development with sleep imposing a critical modulatory role in the function of such networks. Obviously, children with deficient neural networks (Diomedi, et al., 1999) or compromised health (Rhodes, et al., 1995) will more heavily rely on good quality sleep for daytime functioning.
Considering that the anterior brain regions are generally viewed as the last cerebral regions to become myelinated, and given that the frontal lobes, and in particular the prefrontal area, orchestrate behavior (i.e. dependent on other cerebral areas for input) the suggested neurobehavioral correlates of these frontal lobe cerebral processes are known to be speed of response, attentional abilities, and information processing capacity. Maturation of memory and learning abilities subsumed by midline structures including the hippocampus, which can be defined by modality-specific systems or in terms of processes such as encoding (e.g. models by Milner, Baddeley and Cowan), may parallel such maturational processes (Richmond & Nelson, 2007) as well, as reflected by the development of information-processing skills. Dusek and Eichenbaum (Dusek & Eichenbaum, 1997) further suggested that parahippocampal cortex subsumes simple representations of individual items, but hippocampus involvement is necessary for the flexible use of such knowledge. The interrelationships of sleep and neurodevelopment has been broadly accepted, but just recently explored in more detail. In the context of SDB, executive dysfunction behaviors (Beebe & Gozal, 2002; Bruni & Ferri, 2009; Gottlieb, et al., 2003; Karpinski, Scullin, & Montgomery-Downs, 2008; Richmond & Nelson, 2007) have been associated with impaired frontal lobe networks in children with SDB. Similarly, problematic behaviors, such as aggression, impulsivity, and hyperactivity (Mitchell & Kelly, 2006; Mulvaney, et al., 2006; Spruyt, et al., 2006) are remarkably frequent among children with SDB, suggesting an underlying executive function problem. In addition, interconnected brain regions, such as the medial temporal lobe (e.g. hippocampus) which are involved in auditory processing and memory, display evidence of possible neuronal injury in the context of pediatric SDB (Halbower, et al., 2006), with both reversible and irreversible alterations in brain structure and function becoming increasingly apparent among animal models of SDB (Bartlett, et al., 2004; Cooper, 1999; Gozal, et al., 2003; Kheirandish, Gozal, Pequignot, Pequignot, & Row, 2005).
Considering that pediatric memory impairments may often be secondary in nature, and associated with executive, language and visuospatial dysfunction, which would further compromise information processing, and considering that SDB may adversely affect interconnected limbic structures and frontal brain regions involved in emotion, memory, and autonomic functions, we hypothesized that memory underperformance in children with SDB might be exacerbated or more readily apparent when increased levels of processing are required. The aim of the present study was to assess memory and learning abilities of children with SDB children with and without co-morbid cognitive dysfunctions, and in particular, to evaluate their learning processes.
METHODS
Participants
Otherwise healthy elementary school children within the age range of 5–9-years were invited to participate in a sleep study followed by neurocognitive testing the following morning. Exclusionary criteria for participation in the study included chronic medical conditions, genetic or craniofacial syndromes, developmental delays, a current Individual Education Plan (IEP) at school, indicative of significant learning or other difficulties, current use of psychotropic medications, the presence of an acute infection, or a past adenotonsillectomy. The study was approved by the University of Louisville Human Research Committee, and informed consent was obtained from the legal caregiver of each participant, with assent being obtained from children older than 7 years of age.
Measures
Neuropsychological assessment
Luria conceptualized the working brain as organized in ‘blocks of the brain’ that operate dependently of each other. Namely, a dynamic interaction between areas is assumed, with any weakness in one area of the brain interacting and affecting the functioning of the other areas. Designed along Luria’s principles, the NEPSY (version 1)(Korkman, 1998), a neuropsychological battery test, assesses neurodevelopment in 5 domains; i.e. attention and executive functions (AE), language (L), sensorimotor functions (S), visuospatial processing (V) and memory and learning (M). The memory and learning abilities subtests administered include memory for faces (MF), memory for names (MN), sentence repetition (SR) and narrative memory (NM).
Memory for faces tests the ability to recognize faces. Immediately after initial presentation, the child is asked to identify the face seen before while being presented in a set of 3; i.e. immediate memory of faces. Similarly, delayed memory for faces is assessed 30 minutes later, after the initial presentation, whereby the child is requested to pick the target face presented along with two distracters. For both subtests credit is given for a correct answer, and summated total score indicates face recognition. Memory for names involves learning paired-associations. The psychometrician names each drawn children’s face, giving the child time to repeat and memorize the association. This subtest has three learning trials and also a delayed recall after 30 minutes. Again credit is given for correct recalls on each trial, and summated with the delayed recall score indicates visual-verbal paired-associate learning, or cross-modal learning. Both these subtests assessing an ability to reliably recognize faces, and recall the names, are fundamental for a child’s daytime behavioral repertoire. Narrative memory or the ability to retell a story is assessed through free and cued recall. A cued recall effect can be calculated as the number of details recalled regardless of the recall condition. Because of the age range of our cohort, only the expanded subtest, Sentence repetition which assesses memory span and short-term memory was administered. Here, the child is requested to recall sentences that increase in length and complexity.
Polysomnographic assessment
Overnight polysomnography (NPSG) was performed and sleep parameters were scored as previously described (Gozal, Capdevila, & Kheirandish-Gozal, 2008; Montgomery-Downs, O'Brien, Gulliver, & Gozal, 2006). Sleep architecture was evaluated by standard techniques (Rechtschaffen, 1968; Rechtschaffen A, 1968). Diagnostic criteria for OSA included preceding night total sleep duration ≥7 hours, and the presence of an obstructive apnea-hypopnea index (OAHI) ≥2/h total sleep time (TST), a nadir oxygen saturation (SaO2) value during sleep <90% (Montgomery-Downs, et al., 2006; Salyer, 2003; Urschitz, et al., 2003), and the report of snoring at least on 3 nights per week as assessed by Gozal (Gozal, 1998) sleep questionnaire. Control children were defined as children with an OAHI <2/h TST, a nadir SaO2 value ≥90%, arousal index < 20/hrTST, as well as total sleep duration ≥7 hours in children born at full-term, whom were reported to never or rarely snore during sleep in the questionnaire.
Relevant cognitive and sleep measures
The School-Age or Preschool Form of the Differential Ability Scales (DAS I)(Elliott, 1990), designed to assess the cognitive ability and achievement of children, is part of the standard protocol of neurocognitive testing at our sleep center. Only children with a General Cognitive Ability score of ≥85 were included.
Disability on either of the NEPSY subtests AE, L, V was defined as a standard score of ≤85. The parental sleep questionnaire which incorporates all pertinent demographic data was used to extract other relevant information (Gozal, 1998).
PROCEDURES
Study Recruitment and Participant Information
The morning after NPSG, neurocognitive functioning was assessed and 75 elementary school children were retained (6.6±0.5yr; Caucasian (76.4%), African American (20.8%), and other (2.8%) ethnicity). The sample included 57.3% boys, and intelligence scores fell within normal range for all children. Three groups were formed based on the sleep study findings and corresponding neuropsychological performance: control group (n=43), i.e. children with normal sleep study and without any evidence of neurodevelopmental disabilities. Children diagnosed with OSA were further subdivided into 2 groups, namely OSA without co-morbid problems in the domains of attention and executive (AE), language (L) and visuospatial (VS) functioning [OSA−, n= 22; i.e. standardized domain scores above 85] as assessed by NEPSY, and OSA with disability in either one or more of these domains [OSA+, n=10; 6 children with L, 1 with VS, and 3 with AE].
Data Analyses and Statistical procedures
StatSoft, Inc. (2008). STATISTICA (data analysis software system), version 8.0 was used for analyses. Data are presented as mean ± sd unless otherwise indicated. Non-parametric Kruskal-Wallis [H(df,N)] Anova, with multiple comparisons (z’) were performed to compare sleep and neurodevelopmental performance between groups. Wilcoxon matched pairs test (Z) or Friednman Anova [χ2(N,df)], allowed for analyses of within-group differences in neurodevelopment. Finally, Spearman correlations expressed the relationship between sleep parameters and neurodevelopment within groups. Importantly, due to the multiple comparisons, an overall Bonferroni correction of alpha (i.e. 0.001) was chosen as criterion for statistical significance. Furthermore for clinical relevance, Cohen’s d effect sizes (with .2<d<.5 denoting a small effect, .5<d<.8 denoting a medium effect, and d>.8 denoting a large effect) (Cohen, 1988) were calculated.
RESULTS
Demographics
The 3 groups were similar in age [control: 6.7±0.4yrs, OSA− :6.6±0.6, OSA+:6.4±0.6; H(2, 75) =3.7, p =.16], gender [control: 27 boys: 16 girls, OSA−: 12:10, OSA+: 4:6; χ2(2)=1.8, p=0.40], and ethnicity [control: 36 Caucasian: 4 African American: 2 Other, OSA−: 11:9:0, OSA+: 8:2:0; χ2(2)=11.3, p=0.08]. There no differences in duration of gestation [χ2(2)=6.1, p=0.05, control (term): 100% (n=43), OSA−:85.7% (n=18), OSA+:90%(n=9)], parentally reported ADHD [χ2(2)=0.9, p=0.64, control (yes): 19.05% (8), OSA−:21.05% (4), OSA+:33.33% (3)], vision [χ2(2)=0.8, p=0.67, control (yes): 4.76%(2), OSA−:10%(2), OSA+:11.11%(1)) or hearing problems [χ2(2)=5.3, p=0.070, control (yes): 4.76%(2), OSA−:0%(0), OSA+:20%(2)) of the child, snoring of the father [χ2(2)=3.7, p=0.16, control (snore): 51.16%(22), OSA−:71.43%(15), OSA+:77.78%(7)], BMI z score [H (2,58) =2.8, p =0.25, control : 0.56±0.9, OSA−:0.74±1.3, OSA+:1.63±1.2). Likewise, no differences were found for parental education [regarding father: χ2(4)=11.9, p=0.02, control: high school (HS) :27.9%, college (COL): 41.9%, graduate (GRAD):30.2%, OSA−: HS :60%, COL: 25%, GRAD:15%, OSA+: HS :77.8%, COL: 0%, GRAD:22.2%, and regarding mother: χ2(2)=14.8, p=0.005, control: HS :13.9%, COL: 38.1%, GRAD:19.1%, OSA−: HS:70%, COL: 20%, GRAD:10%], smoking habits of the father (χ2(2)=8.6, p=0.01, control (smoke): 14.2%, OSA−:38.9%, OSA+:55.6) and mother [χ2(2)=1.6, p=0.46, control (smoke): 13.95%, OSA−:21.05%, OSA+:30%]. However, more OSA+ children’s mothers were likely to be snorers than the other 2 groups [χ2(2)=14.6, p=0.00068, control (snore): 23.26%, OSA−:57.14%, OSA+:80%].
Control children snored never (67.44%) to rarely (32.56%), whereas OSA− snored frequently (9.09%) to almost always (90.91%) and OSA+ respectively snored frequently (30%) to almost always (70%).
Sleep study
NPSG findings were subjected to Bonferroni correction of alpha (Table 1, in bold), and indicated that respiratory indices, saturation, and respiratory arousal index differed between the groups. However, the OSA subgroups were not different from each other with respect to sleep measures.
Table 1.
Overnight sleep study findings
| parameter | control(1) mean±sd [range] |
OSA−(2) mean±sd [range] |
OSA+(3) mean±sd [range] |
Kruskal-Wallis Anova |
Multiple comparison z’ | ||
|---|---|---|---|---|---|---|---|
| H (2,75) = | p-value | groups | p-value | ||||
| Sleep Efficiency Index (%) | 92.3±4.7 | 92.1±5.8 | 90.7±3.3 | 1.78 | 0.4096 | ||
| Sleep Onset Latency (min) | 18±17.2 | 18.8±22.2 | 22.5±21.9 | 0.29 | 0.8633 | ||
| REM latency (min) | 129.3±59.2 | 127±39.3 | 146.9±55.3 | 1.34 | 0.5126 | ||
| Number of awakenings | 5.5±5.6 | 4.5±4.88 | 3.9±2.8 | 0.59 | 0.7431 | ||
| Total sleep time (hr) | 8.2±0.5 | 8.1±0.6 | 7.8±0.5 | 4.59 | 0.1006 | ||
| Stage 1 (% TST) | 6.7±4.9 | 10.7±8.3 | 8.8±8.6 | 4.84 | 0.0890 | ||
| Stage 2 (% TST) | 45.8±7.3 | 46.1±9 | 46.6±12.3 | 0.19 | 0.9099 | ||
| Stage 3 (% TST) | 4.6±2.0 | 6.3±2 | 7.1±3 | 13.50 | 0.0012 | 1 vs. 2: 2.9 1 vs. 3: 2.9 |
0.01 0.01 |
| Stage 4 (% TST) | 16.8±5.1 | 12.9±7.3 | 15.8±7 | 5.56 | 0.0620 | ||
| NREM (% TST) | 18.5±1.7 | 19±1.33 | 19.6±2 | 1.98 | 0.3715 | ||
| REM (% TST) | 25.6±6.9 | 24±5.3 | 21.7±8.1 | 1.51 | 0.4705 | ||
| Apnea Hypopnea Index | 0.56±0.4 [0 – 1.54] |
6.02±2.87 [3.01 – 12.14] |
6.03±2.04 [3.31 – 8.79] |
54.35 | 0.0000 | 1 vs. 2: 6.5 1 vs. 3: 5 |
0.00 0.00 |
| Apnea Index | 0.5±0.38 [0 – 1.54] |
1.72±1.95 [0 – 9.27] |
1.39±1.28 [0 – 3.97] |
17.47 | 0.0002 | 1 vs. 2: 4 | 0.00001 |
| Obstructive Apnea Index | 0.03±0.06 [0 – 0.24] |
1.21±2.13 [0 – 9.15] |
1.11±1.34 [0 – 3.84] |
30.36 | 0.0000 | 1 vs. 2: 4.2 1 vs. 3: 3.6 |
0.00001 0.00001 |
| Hypopnea Index | 0.06±0.09 [0 – 0.26] |
4.3±2.42 [0.45 – 9.87] |
4.64±2 [2.68 – 8.52] |
58.16 | 0.0000 | 1 vs. 2: 6.4 1 vs. 3: 5.2 |
0.000 0.0000001 |
| Obstructive Apnea Hypopnea Index | 0.08±0.1 [0 – 0.38] |
5.5±2.8 [2.29 – 11.92] |
5.75±2.08 [2.93 – 8.79] |
56.22 |
0.0000 |
1 vs. 2: 6.4 1 vs. 3: 5.1 |
0.000 0.0000001 |
| Nadir SaO2 | 94.3±1.5 | 87±6.7 | 83.4±7 | 45.13 |
0.0000 |
1 vs. 2: 6.6 1 vs. 3: 4.9 |
0.000 0.0000001 |
| Spontaneous Arousal Index (%TST) | 8.2±3.8 | 7.3±4 | 6.3±4.6 | 2.06 | 0.3575 | ||
| Respiratory Arousal Index (%TST) | 0.6±0.9 | 5.6±3.9 | 4.3±3.8 | 27.38 |
0.0000 |
1 vs. 2: 4.9 1 vs. 3: 2.8 |
0.0000001 0.02 |
| Total Arousal Index (%TST) | 9.2±3.6 | 13.4±5.6 | 11.2±5.8 | 8.40 | 0.0150 | 1 vs. 2: 2.9 | 0.01 |
| Sleep Pressure Score | 0.05±0.14 | 0.25±0.26 | 0.24±0.22 | 21.70 | 0.0000 | 1 vs. 2: 4.2 1 vs. 3: 2.9 |
0.0001 0.01 |
Italicized: significant without Bonferonni correction.
Bold: significant with Bonferonni correction.
Memory and learning abilities (Table 2)
Table 2.
Neurodevelopmental performance
| control mean±sd |
OSA− mean±sd |
OSA+ mean±sd |
Kruskal-Wallis Anova | Cohen’s d | ||||
|---|---|---|---|---|---|---|---|---|
| p-value | 1 vs 2 | 2 vs 3 | 1 vs 3 | |||||
|
DAS General Cognitive Ability (GCA) |
106.7±11.1 | 104±10.1 | 99±6.1 | H (2,75) =3.92 | 0.1407 | 0.25 | 0.56 | 0.75 |
|
NEPSY Domain score | ||||||||
|
Memory and learning ability |
113.5±15.1 | 108.8±11.3 | 110±12.3 | H (2,75) =1.04 | 0.5938 | 0.34 | −0.10 | 0.24 |
|
NEPSY Subtest score | ||||||||
| Memory for face (MF) | 12±2.6 | 12.5±2.6 | 12.6±3.0 | H (2,75) =1.18 | 0.5532 | −0.19 | −0.04 | −0.22 |
| Immediate recognition | 12.3±2.7 | 12.3±3.4 | 12.2±4.0 | H (2,73) =0.09 | 0.9576 | 0 | 0.03 | 0.03 |
| Delayed recognition | 11.3±2.4 | 12.4±2.6 | 12.7±2.1 | H (2,73) =4.50 | 0.1048 | −0.45 | −0.12 | −0.60 |
| Memory for Names (MN) | 11.1±3.3 | 10.1±2.3 | 10.6±2.8 | H (2,75) =1.07 | 0.5847 | 0.32 | −0.20 | 0.16 |
| Trial 1 (raw score) | 3.1±1.8 | 2.7±1.5 | 2.9±1.7 | H (2,73) =0.24 | 0.8859 | 0.24 | −0.13 | 0.11 |
| Trial 2 (raw score) | 4.2±2.2 | 3.8±1.6 | 4.0±1.2 | H (2,73) =0.30 | 0.8607 | 0.20 | −0.14 | 0.10 |
| Trial 3 (raw score) | 4.9±1.9 | 4.4±1.8 | 4.2±1.6 | H (2,73) =1.32 | 0.5172 | 0.27 | 0.12 | 0.38 |
| Trial learning score | 11±3.2 | 10.5±2.5 | 11.1±3.7 | H (2,73) =0.75 | 0.6870 | 0.17 | −0.21 | −0.03 |
| Delayed recall | 11.5±3.5 | 9.8±3.1 | 11.1±2.2 | H (2,73) =2.30 | 0.3174 | 0.50 | −0.46 | 0.12 |
| Narrative Memory (NM) | 12.7±3.4 | 11.1±2.6 | 10.9±3.3 | H (2,75) =4.31 | 0.1160 | 0.50 | 0.07 | 0.53 |
| Free recall | 18.8±8.3 | 16±7.6 | 13.4±6.9 | H (2,73) =4.62 | 0.0994 | 0.35 | 0.35 | 0.67 |
| Cued recall | 4.4±2.5 | 5.1±3.2 | 6.4±2.4 | H (2,73) =4.87 | 0.0875 | −0.21 | −0.44 | −0.81 |
| Cued recall effect | 13.8±2.5 | 13.1±2.2 | 13.1±2.6 | H (2,73) =1.66 | 0.4366 | 0.29 | 0 | 0.28 |
| Sentence Repetition (SR) | 11.1±2.9 | 11.6±2.7 | 9.9±1.6 | H (2,75) =2.28 | 0.3201 | −0.18 | 0.72 | 0.45 |
DAS = Differential Ability Scales I.
Scores in table are standardized score unless otherwise specified. Mean NEPSY Domain score is 100±15, whereas mean Subtest score is 10±3.
NEPSY revealed no gross memory and learning problems in children with OSA, with nearly all standard scores falling within normal range. However, supplemental score analyses were suggestive of subtle memory impairments.
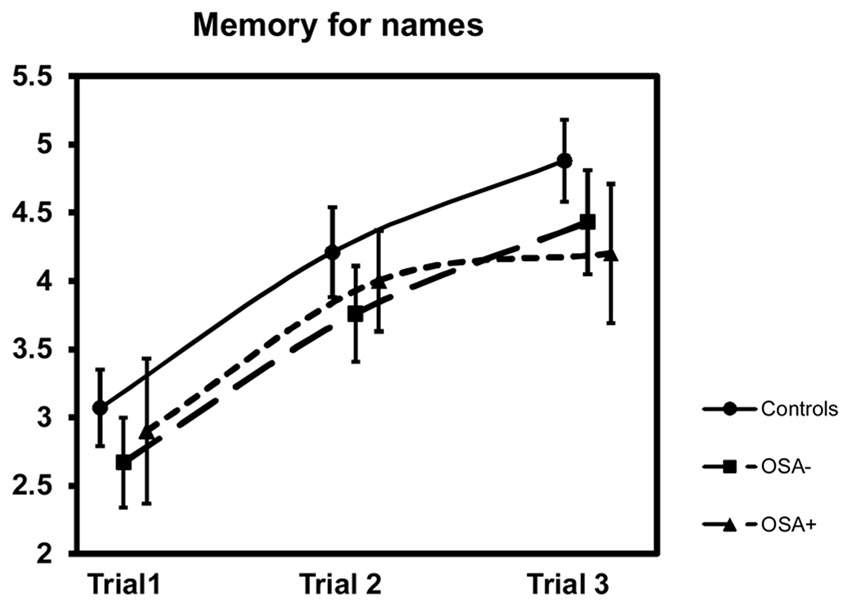
Face recognition performance was similar between and within groups; for the latter i.e. control: Z=2.6, p=0.01, OSA−: Z=0.39, p=0.70, OSA+: Z=0.47, p=0.64 (see table for means). Likewise the subtest as well as the trial learning scores for cross-model association learning between visual and semantic information in the Memory for Names subtest were not different between groups, yet subtle differences became apparent. Specifically, as shown in figure 1, control children’s learning ability steadily increased with each learning trial (Anova χ2(42, 2)=35.5, p=0.00000) as shown by multiple comparisons (Bonferroni corrected p-value); i.e. Trial 1 vs. Trial 2: Z=3.88, p=0.0003, Trial 2 vs. Trial 3: Z=2.59, p=0.029, and Trial 1 vs. Trial 3: Z=4.82, p=0.000003. OSA− learned the same trials with greater difficulty (Anova χ2(21, 2)=19. 7, p=0.00005) with Trial 1 vs. Trial 2: Z=2.79, p=0.016, Trial 2 vs. Trial 3: Z=1.92, p=0.165 and Trial 1 vs. Trial 3: Z=3.29, p=0.003. However, OSA+ showed reduced learning ability (Anova χ2(10, 2)=7.8, p=0.02). In fact, in the realm of the Bonferroni corrections, OSA+ did not exhibit significant learning improvements over the learning trials [for the multiple comparisons: Trial 1 vs. Trial 2: Z=2.37, p=0.054, Trial 2 vs. Trial 3: Z=0.49, p=1.87, Trial 1 vs. Trial 3: Z=2.02, p=0.13]. In the recall or delayed performance on the Memory for Names subtest, no group differences emerged. Likewise, retrieval ability was similar within groups; i.e. control: Z=0.52,p=0.60, OSA−: Z=0.54, p=0.59, OSA+: Z=0.84, p=0.40 (see table for means). Remembering a story was done equally well by both OSA groups similar to control children, although a tendency towards weaker performance for the OSA+ group might be hypothesized based on the subtest score and effect sizes. Furthermore, since only the items initially ‘forgotten’ during the free recall condition were being cued, a separate cued recall effect needs to be calculated to infer the cuing benefit in retrieval. This cuing benefit was similar among the groups, and appropriate for age (i.e. when average value is compared in c2 Table of NEPSY 1 manual p. 337 and when their distribution over the cumulative percentages of the standardized sample is analyzed). Finally, overall sentence repetition was performed adequately by all participants independent of group allocation. Therefore no memory span or short-term memory problems were identified.
Figure 1.
Learning Trials Of Memory For Names
OSA−: OSA children without comorbidities
OSA+: OSA children with comorbidities in language, visuospatial and executive function domain of the NEPSY.
Data are presented as means±SEM.
Interestingly, complex visual-verbal paired-associate learning (MN) and Apnea Index were inversely related only in the OSA+ group (r=−0.91, p<0.001), and a strong positive correlation (r=0.96, p<0.001) for Memory for Names trial 2 with Total Arousal Index also occurred in this group. No other significant relationships between sleep and neurodevelopment were found within groups (detailed data not shown).
DISCUSSION
This study supports the hypothesis that the neurodevelopment of children with OSA is challenged by the underlying sleep disorder, and that problems with acquisition of complex information can be readily identified in a subset of children with OSA. Learning a visual-verbal paired-association is more difficult in OSA children, and this is particularly apparent when novel information encoding is reduced. However, and of practical importance is that all learning and memory abilities remain within the normal range. Therefore, current findings are suggestive of slower information processing, and/or of secondary memory problems. Under such conceptual construct, the deficits in information processing could reflect impaired integrity of myelination of the neural circuitry in functionally relevant brain regions, such that the presence or absence of structural damage to interconnected limbic structures could potentially affect intellectual and school performance in children with OSA.
Before discussing our findings, some important limitations need to be addressed. First, the sample size of OSA+ children was relatively small and precluded sufficient power for more advanced statistical analyses. Nevertheless, this was a rather homogeneous group consisting of mainly children with lower language domain scores on the NEPSY, in the presence of normal intelligence. If a larger sample was available, then NEPSY scores on language domain could also be contrasted to other scores on NEPSY subtest, such as Speeded Naming, and potentially disclose dysnomic problems or error prone speeded responding time. Secondly, the Bonferroni correction is a rather conservative approach towards interpretation of the results, and particularly on a small sample it could lead to Type II errors. In addition, performance assessments using NEPSY version 1 are slightly overestimated (Strauss, 2006) (note that this issue has now been addressed in version 2). Our approach to the core domain interpretation, which consisted in subdividing the OSA sample, and our subtest-level analyses, should at least partially compensate this interpretability flaw. Our evaluation of component processes, an important aspect within the NEPSY neuropsychological framework, between and within groups attends to the clinical relevance of the findings. Noteworthy, all children were randomly recruited from a community sample and not from clinically referred patients. Testing was performed in the morning after documented evidence of adequate sleep duration in the preceding night. However, although our neuropsychological assessments included several breaks and were pre-arranged in a child-friendly fashion, no objective fatigue determinations were available at the time of testing.
In our OSA sample of school-aged children with normal cognitive abilities, 22 children had no neuropsychological co-morbidity as assessed by NEPSY, and 10 had evidence of such morbidity. Since the earliest publication reviewing the impact of SDB (Guilleminault, Korobkin, & Winkle, 1981), findings of neurodevelopmental performance remain highly diverse and inconsistent (Beebe, 2006; Blunden, et al., 2000). Notwithstanding such heterogeneity of findings, memory, learning and executive dysfunction, and behavioral ADHD-like patterns are more frequently and likely in children with SDB of varying severity. Based on current findings, the wide range of language test execution issues, phonological and auditory processing difficulties, sustained and selective attention deficits, alertness and scholastic performance problems, as well as the executive function issues more frequently reported in children with SDB might be rooted in the presence of upstream encoding difficulties (Beebe, 2006; Karpinski, et al., 2008; O'Brien, Mervis, Holbrook, Bruner, Smith, et al., 2004; Ziliotto, et al., 2006). Encoding is assumed to be the first step in memory information processing or acquisition (Kolb, 2008; Naegele, et al., 2006; Strauss, 2006). Associations between increased cerebral blood flow velocity among children with SDB and deficient processing speed have been reported recently (Hill, et al., 2006), and suggest compromised levels of processing, from simple to elaborative tasks, that in fact involve encoding, consolidation, storage, and/or retrieval of information. This is not surprising considering that neural pathways or networks that mediate these learning and memory processes are potentially affected by hypoxia in a rather diffuse fashion (Halbower, et al., 2006). Therefore, inefficient or insufficient encoding may yield reduced learning strategies, learning ability and subsequently impaired knowledge, which in turn will further impact future developmental progress. Sequentially challenged acquisition of knowledge may then translate into reduced short-term and long-term neurobehavioral and cognitive consequences (Gozal & Pope, 2001). Thus, despite inherent brain plasticity, the cumulative learning debt may lead to a neuropsychological and/or intellectual deficit, if such learning debt is of sufficient magnitude (Gottlieb, et al., 2003; Michel, 2001). Therefore, when inadequate reserve is present, i.e., the OSA+ child group, a more widespread adverse impact on performance and behavior will emerge.
Current results support previous findings whereby memory performance was at the lower end of the normal range (Gottlieb, et al., 2004) with comorbidities possibly increasing the reduction in neurodevelopmental performance. This is of particular interest since sleep was similar among the 2 OSA sub-groups. Thus, the discussion on the existence of an association between OSA severity and cognitive dysfunction will need to incorporate consideration of co-morbidities in overall performance as a modulatory factor, and the latter may not be apparent in the widely heterogeneous groups reported thus far in the literature. In other words, variances in overall performance may promote inconsistency in the delineation of a SDB-specific neurodevelopmental profile, and this issues may further reduce comparability among studies (Lumeng & Chervin, 2008).
In one of the few studies in adults involving face recognition as a control task in post-sleep learning, an association between sleep spindles and verbal memory consolidation was reported, whereas accuracy of retention of learned faces was associated with non-rapid eye movement sleep (Clemens, Fabo, & Halasz, 2005). In our study, despite the absence of any difference in sleep parameters within the OSA− and OSA+ groups, only the latter displayed significant correlations between sleep measures and aspects of neurodevelopmental performance. Indeed, as OAHI increased, complex visual-semantic learning was slower. In addition, even though control children greatly benefitted from presentation of a complex task during a second trial, the total arousal index correlated only with performance among OSA+ children. This might be interpreted in the realm of selective attention theories positing that individuals have a tendency to orient themselves toward, or process information from only one part of the environment, with the exclusion of other parts, and that such exclusion is governed by our arousal level and shares neural inputs between interconnected limbic structures and autonomic functions. In other words, selective attention involves the process by which we attend to certain information, but not to all the information available to us. Under such circumstances, the complexity of the task may trigger selection across modalities, and thus selective encoding. In children with OSA and comorbidities efficient acquisition will be hampered. Literally, the brain works harder to complete the more complex task, but does not have the resources available as in the otherwise healthy child. Consequently, the child with SDB should theoretically benefit from increased cuing (as in narrative memory performance), decreased complexity (sentence repetition), or executive function aids, all of which may improve performance, and thus compensate for the underlying encoding deficits.
Our proposition whereby the inability to encode is present in OSA can also be derived from reflecting upon the other memory and learning subtest scores, even if these are within the normal range. Free recall storytelling assessments should deliver evidence that (verbal) information is primarily encoded, and that it can be secondarily accessed. Although not significant, children with OSA tended to perform at a weaker level in the free recall. This hypothetically might support the presence of inefficient or insufficient encoding during a complex task. Also, the increasing amount of verbal information, presented during storytelling and sentence repetition relies on episodic memory, and predominantly the (verbal) information is presented just once. It would be of interest in future studies to perform qualitative analyses of testing behavior comparing simple to complex processing tasks. Blunden et al (Blunden, et al., 2000), and others (Beebe, 2006; Gottlieb, et al., 2004; Rhodes, et al., 1995), suggested the possibility of an attentional capacity deficit, with its underlying neurological substrates, hampering learning. However none of these studies reported on performance over the learning trials or detailed the memory indexes with respect to information processing (Ziliotto, et al., 2006). One study (Kaemingk, et al., 2003) has compared the level of learning, i.e., measured by recall of a list of words across 5 learning trials, between snoring children and using an OAHI of 5 as the cut-off and no control group. Results were indicative of a decreased level of learning among those children with AHI ≥ 5/hrTST especially across trials 3, 4 and 5. Even though the authors did not perform within group analyses, they hypothesized a performance pattern suggestive of difficulties in acquisition or recalling of new information across those learning trials instead of attention or recalling difficulties when information was presented only once. Clinically this profile of altered encoding could mimic the patterns found among ADHD children in whom struggles with acquisition of new information reflect superficial encoding of information over trials (Korkman, 1998).
In summary, while normal children with undisrupted sleep significantly benefit from recurrent presentation of information, and more particularly benefit from a single repetition, children with OSA display reduced benefit from repetition during learning, and in fact when co-morbid cognitive deficits are present, underperformance emerges. Thus, the importance of good quality sleep cannot be emphasized enough in the context of ecological tests of cross-modal learning, such as linking face and name. Our findings further buttress the possibility that either inefficient or insufficient encoding may constitute the primary deficit associated with OSA, and that such deficit may then interact with other neuropsychological functions, and thus promote the wide range and rather inconsistent neurodevelopmental profile performances thus far reported among children affected with OSA.
Acknowledgments
Funding Sources: This study was supported by NIH grant HL-65270, The Children’s Foundation Endowment for Sleep Research, and by the Commonwealth of Kentucky Challenge for Excellence Trust Fund.
Contributor Information
Karen Spruyt, Division of Pediatric Sleep Medicine, Department of Pediatrics, and Kosair Children’s Hospital Research Institute, University of Louisville, Louisville, Kentucky.
Oscar Sans Capdevila, Division of Pediatric Sleep Medicine, Department of Pediatrics, and Kosair Children’s Hospital Research Institute, University of Louisville, Louisville, Kentucky.
Leila Kheirandish-Gozal, Department of Pediatrics, Pritzker School of Medicine, Comer Children's Hospital, University of Chicago, 5721 S. Maryland Avenue, MC 8000, Suite K-160, Chicago, IL 60637.
David Gozal, Department of Pediatrics, Pritzker School of Medicine, Comer Children's Hospital, University of Chicago, 5721 S. Maryland Avenue, MC 8000, Suite K-160, Chicago, IL 60637.
REFERENCES
- Bartlett DJ, Rae C, Thompson CH, Byth K, Joffe DA, Enright T, et al. Hippocampal area metabolites relate to severity and cognitive function in obstructive sleep apnea. Sleep Medicine. 2004;5(6):593–596. doi: 10.1016/j.sleep.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. Journal of Sleep Research. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- Blunden S, Lushington K, Kennedy D, Martin J, Dawson D. Behavior and neurocognitive performance in children aged 5–10 years who snore compared to controls. Journal of Clinical and Experimental Neuropsychology. 2000;22(5):554–568. doi: 10.1076/1380-3395(200010)22:5;1-9;FT554. [DOI] [PubMed] [Google Scholar]
- Bruni O, Ferri R. Neurocognitive deficits in pediatric obstructive sleep apnea: A multifaceted pathogenetic model. Sleep Medicine. 2009;10(2):161–163. doi: 10.1016/j.sleep.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Clemens Z, Fabo D, Halasz P. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience. 2005;132(2):529–535. doi: 10.1016/j.neuroscience.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2 ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cooper CE. In vivo measurements of mitochondrial function and cell death following hypoxic/ischaemic damage to the new-born brain. Biochemical Society Symposium. 1999;66:123–140. doi: 10.1042/bss0660123. [DOI] [PubMed] [Google Scholar]
- Diomedi M, Curatolo P, Scalise A, Placidi F, Caretto F, Gigli GL. Sleep abnormalities in mentally retarded autistic subjects: Down's syndrome with mental retardation and normal subjects. Brain & Developement. 1999;21(8):548–553. doi: 10.1016/s0387-7604(99)00077-7. [DOI] [PubMed] [Google Scholar]
- Dusek Jeffery A, Eichenbaum Howard. The hippocampus and memory for orderly stimulusrelations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(13):7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott C, editor. Differential Ability Scales. The Psychological Corporation; 1990. [Google Scholar]
- Giordani B, Hodges EK, Guire KE, Ruzicka DL, Dillon JE, Weatherly RA, et al. Neuropsychological and behavioral functioning in children with and without obstructive sleep apnea referred for tonsillectomy. Journal of the International Neuropsychological Society. 2008;14(4):571–581. doi: 10.1017/S1355617708080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DJ, Chase C, Vezina RM, Heeren TC, Corwin MJ, Auerbach SH, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. The Journal of Pediatrics. 2004;145(4):458–464. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- Gottlieb DJ, Vezina RM, Chase C, Lesko SM, Heeren TC, Weese-Mayer DE, et al. Symptoms of sleep-disordered breathing in 5-year-old children are associated with sleepiness and problem behaviors. Pediatrics. 2003;112(4):870–877. doi: 10.1542/peds.112.4.870. [DOI] [PubMed] [Google Scholar]
- Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 Pt 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. American Journal of Respiratory and Critical Care Medicine. 2008;177(10):1142–1149. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal D, Pope DW., Jr Snoring during early childhood and academic performance at ages thirteen to fourteen years. Pediatrics. 2001;107(6):1394–1399. doi: 10.1542/peds.107.6.1394. [DOI] [PubMed] [Google Scholar]
- Gozal D, Row BW, Gozal E, Kheirandish L, Neville JJ, Brittian KR, et al. Temporal aspects of spatial task performance during intermittent hypoxia in the rat: evidence for neurogenesis. The European Journal of Neuroscience. 2003;18(8):2335–2342. doi: 10.1046/j.1460-9568.2003.02947.x. [DOI] [PubMed] [Google Scholar]
- Guilleminault C, Korobkin R, Winkle R. A review of 50 children with obstructive sleep apnea syndrome. Lung. 1981;159(5):275–287. doi: 10.1007/BF02713925. [DOI] [PubMed] [Google Scholar]
- Halbower AC, Degaonkar M, Barker PB, Earley CJ, Marcus CL, Smith PL, et al. Childhood obstructive sleep apnea associates with neuropsychological deficits and neuronal brain injury. PLoS Medicine. 2006;3(8):e301. doi: 10.1371/journal.pmed.0030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Hogan AM, Karmiloff-Smith A. To sleep, perchance to enrich learning? Archives of Disease in Childhood. 2007;92(7):637–643. doi: 10.1136/adc.2006.096156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CM, Hogan AM, Onugha N, Harrison D, Cooper S, McGrigor VJ, et al. Increased cerebral blood flow velocity in children with mild sleep-disordered breathing: a possible association with abnormal neuropsychological function. Pediatrics. 2006;118(4):e1100–e1108. doi: 10.1542/peds.2006-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaemingk KL, Pasvogel AE, Goodwin JL, Mulvaney SA, Martinez F, Enright PL, et al. Learning in children and sleep disordered breathing: findings of the Tucson Children's Assessment of Sleep Apnea (tuCASA) prospective cohort study. Journal of the International Neuropsychological Society. 2003;9(7):1016–1026. doi: 10.1017/S1355617703970056. [DOI] [PubMed] [Google Scholar]
- Karpinski AC, Scullin MH, Montgomery-Downs HE. Risk for sleep-disordered breathing and executive function in preschoolers. Sleep Medicine. 2008;9(4):418–424. doi: 10.1016/j.sleep.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Kheirandish L, Gozal D, Pequignot JM, Pequignot J, Row BW. Intermittent hypoxia during development induces long-term alterations in spatial working memory, monoamines, and dendritic branching in rat frontal cortex. Pediatr Research. 2005;58(3):594–599. doi: 10.1203/01.pdr.0000176915.19287.e2. [DOI] [PubMed] [Google Scholar]
- Kolb B, Wishaw IQ. Fundamentals of Human Neuropsychology. 2008:763. [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: a developmental neuropsychological assessment. San Antonio TX: The Psychological Corporation; 1998. [Google Scholar]
- Kurnatowski P, Putynski L, Lapienis M, Kowalska B. Neurocognitive abilities in children with adenotonsillar hypertrophy. International Journal of Pediatric Otorhinolaryngology. 2006;70(3):419–424. doi: 10.1016/j.ijporl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel GF. A developmental-psychobiological approach to developmental neuropsychology. Developmental Neuropsychology. 2001;19(1):11–32. doi: 10.1207/S15326942DN1901_2. [DOI] [PubMed] [Google Scholar]
- Mitchell RB, Kelly J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. International Journal of Pediatric Otorhinolaryngology. 2006;70(3):395–406. doi: 10.1016/j.ijporl.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, O'Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- Mulvaney SA, Goodwin JL, Morgan WJ, Rosen GR, Quan SF, Kaemingk KL. Behavior problems associated with sleep disordered breathing in school-aged children--the Tucson children's assessment of sleep apnea study. Journal of Pediatric Psychology. 2006;31(3):322–330. doi: 10.1093/jpepsy/jsj035. [DOI] [PubMed] [Google Scholar]
- Naegele B, Launois SH, Mazza S, Feuerstein C, Pepin JL, Levy P. Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep. 2006;29(4):533–544. doi: 10.1093/sleep/29.4.533. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Klaus CJ, Rutherford J, et al. Neurobehavioral implications of habitual snoring in children. Pediatrics. 2004;114(1):44–49. doi: 10.1542/peds.114.1.44. [DOI] [PubMed] [Google Scholar]
- O'Brien LM, Mervis CB, Holbrook CR, Bruner JL, Smith NH, McNally N, et al. Neurobehavioral correlates of sleep-disordered breathing in children. Journal of Sleep Research. 2004;13(2):165–172. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen . A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. Vol. 204. Washington DC: National Institutes of Health; 1968. p. 4. [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring systems for sleep stages of human subject. Vol. 204. Washington DC: National Institutes of Health; 1968. p. 4. [Google Scholar]
- Rhodes SK, Shimoda KC, Waid LR, O'Neil PM, Oexmann MJ, Collop NA, et al. Neurocognitive deficits in morbidly obese children with obstructive sleep apnea. The Journal of pediatrics. 1995;127(5):741–744. doi: 10.1016/s0022-3476(95)70164-8. [DOI] [PubMed] [Google Scholar]
- Richmond J, Nelson CA. Accounting for change in declarative memory: A cognitive neuroscience perspective. Developmental Review. 2007;27(3):349–373. doi: 10.1016/j.dr.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyer JW. Neonatal and pediatric pulse oximetry. Respiratory Care. 2003;48(4):386–396. discussion 397-388. [PubMed] [Google Scholar]
- Spruyt K, O'Brien LM, Macmillan Coxon AP, Cluydts R, Verleye G, Ferri R. Multidimensional scaling of pediatric sleep breathing problems and bio-behavioral correlates. Sleep Medicine. 2006;7(3):269–280. doi: 10.1016/j.sleep.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Strauss E, Sherman EMS, Spreen O, editors. A Compendium of Neuropsychological Tests. 3 ed. Oxford: Oxford University Press; 2006. [Google Scholar]
- Urschitz MS, Guenther A, Eggebrecht E, Wolff J, Urschitz-Duprat PM, Schlaud M, et al. Snoring, intermittent hypoxia and academic performance in primary school children. American Journal of Respiratory and Critical Care Medicine. 2003;168(4):464–468. doi: 10.1164/rccm.200212-1397OC. [DOI] [PubMed] [Google Scholar]
- Wagner U, Kashyap N, Diekelmann S, Born J. The impact of post-learning sleep vs. wakefulness on recognition memory for faces with different facial expressions. Neurobiology of learning and memory. 2007;87(4):679–687. doi: 10.1016/j.nlm.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Ziliotto KN, dos Santos MF, Monteiro VG, Pradella-Hallinan M, Moreira GA, Pereira LD, et al. Auditory processing assessment in children with obstructive sleep apnea syndrome. Brazilian Journal of Otorhinolaryngology. 2006;72(3):321–327. doi: 10.1016/S1808-8694(15)30963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]