Abstract
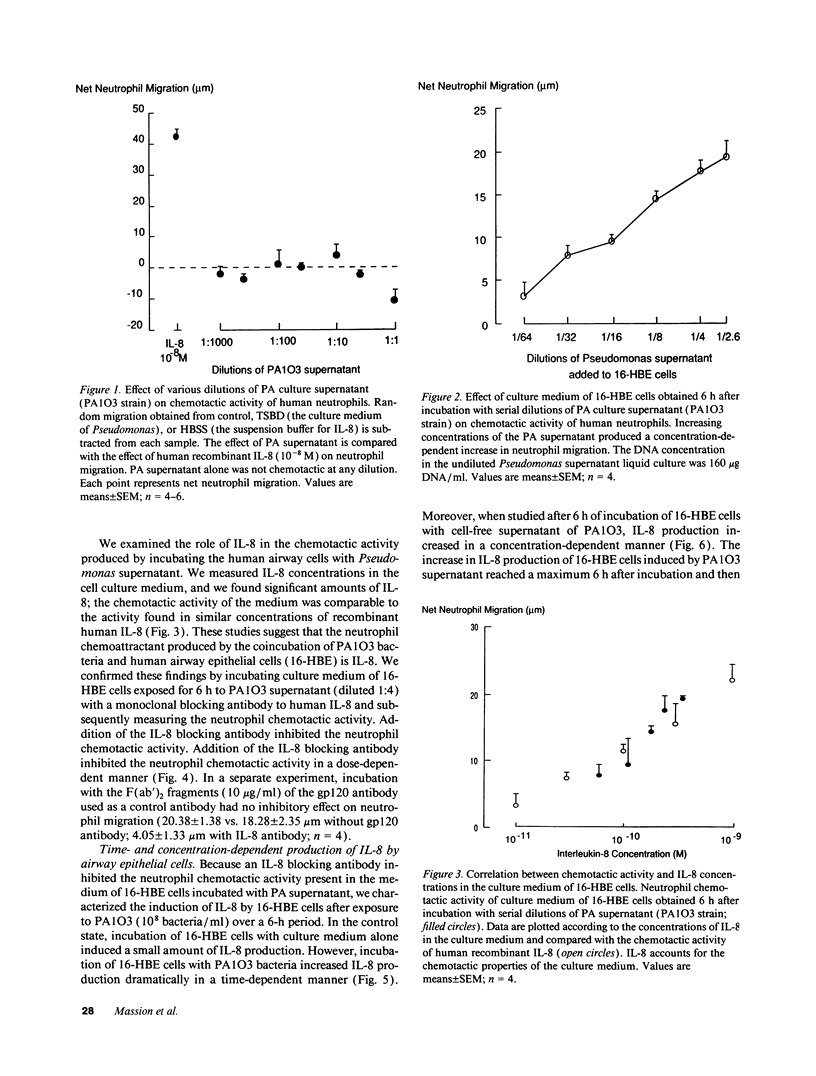
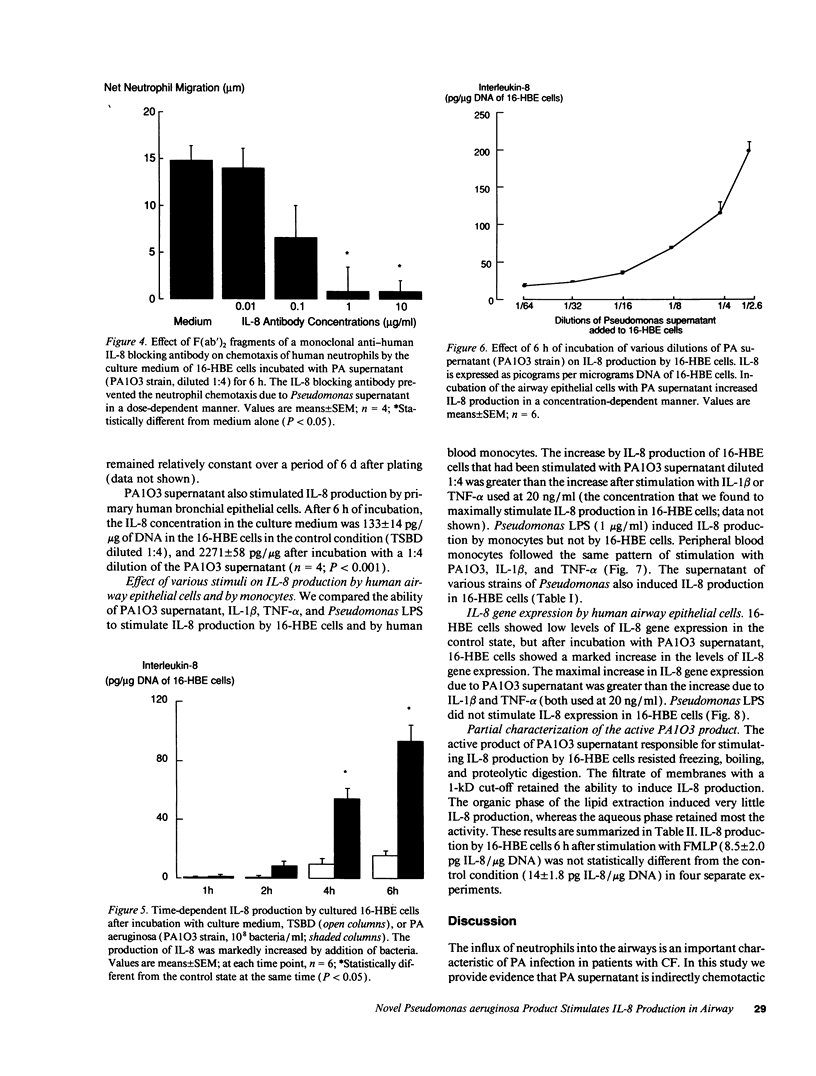
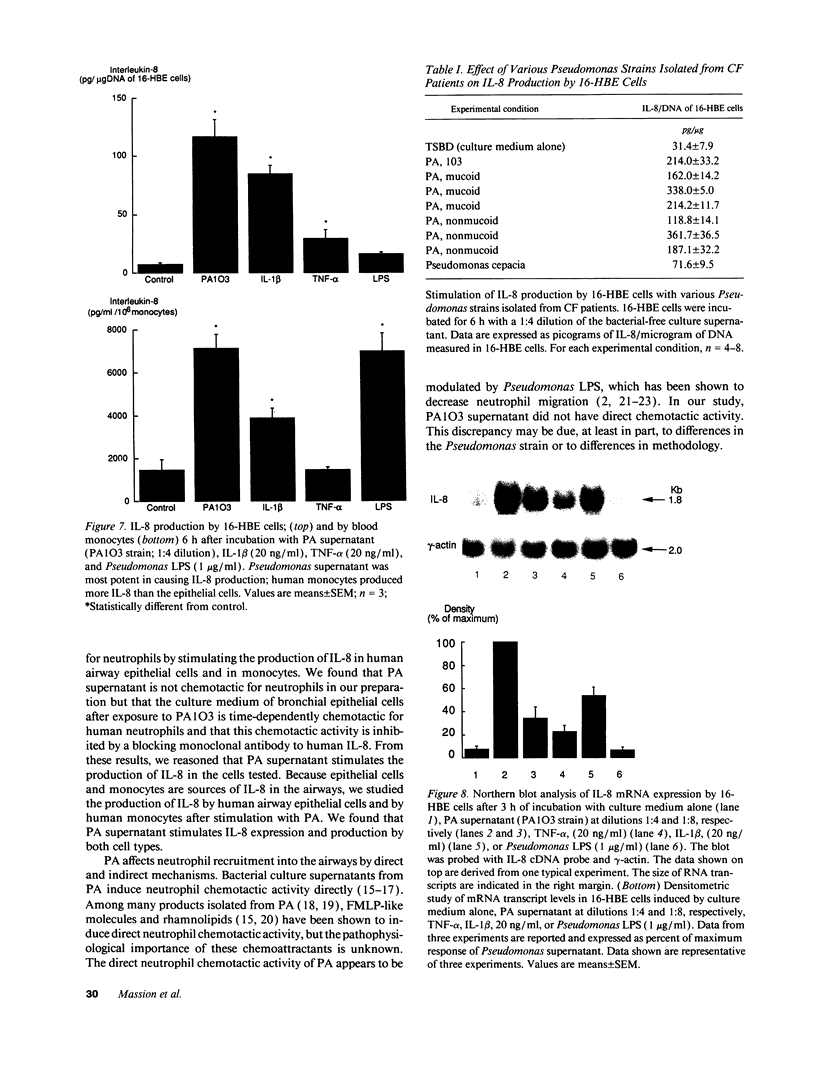
Because high concentrations of IL-8 are found in the sputum of cystic fibrosis patients, we hypothesized that Pseudomonas aeruginosa (PA) induces the production of IL-8 in airway epithelial cells and in monocytes. Therefore, we incubated the supernatant from PA culture with human transformed bronchial epithelial cells (16-HBE) or with monocytes. The culture medium of 16-HBE cells that had been incubated with PA supernatant for 6 h had chemotactic activity that was inhibited by an antibody to human IL-8. The PA supernatant induced IL-8 production by primary bronchial epithelial cells, by 16-HBE cells, and by monocytes. After incubation with PA supernatant, 16-HBE cells showed a marked increase in the levels of IL-8 gene expression. The PA product responsible for IL-8 production resisted freezing, boiling, and proteolysis. This product was not lipid extractable and was present in a 1-kD filtrate. We conclude that a small molecular mass product of PA stimulates IL-8 production by 16-HBE cells and by monocytes, and that the chemotactic activity produced by 16-HBE cells after exposure to PA is due principally to IL-8.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carré P. C., Mortenson R. L., King T. E., Jr, Noble P. W., Sable C. L., Riches D. W. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis. A potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Invest. 1991 Dec;88(6):1802–1810. doi: 10.1172/JCI115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesarone C. F., Bolognesi C., Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979 Nov 15;100(1):188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- Dahinden C., Galanos C., Fehr J. Granulocyte activation by endotoxin. I. Correlation between adherence and other granulocyte functions, and role of endotoxin structure on biologic activity. J Immunol. 1983 Feb;130(2):857–862. [PubMed] [Google Scholar]
- Fontán P. A., Amura C. R., García V. E., Cerquetti M. C., Sordelli D. O. Preliminary characterization of Pseudomonas aeruginosa peptide chemotactins for polymorphonuclear leukocytes. Infect Immun. 1992 Jun;60(6):2465–2469. doi: 10.1128/iai.60.6.2465-2469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata J. S., Fick R. B., Jr Pseudomonas aeruginosa and the airways disease of cystic fibrosis. Clin Chest Med. 1988 Dec;9(4):679–689. [PubMed] [Google Scholar]
- Jackowski J. T., Szepfalusi Z., Wanner D. A., Seybold Z., Sielczak M. W., Lauredo I. T., Adams T., Abraham W. M., Wanner A. Effects of P. aeruginosa-derived bacterial products on tracheal ciliary function: role of O2 radicals. Am J Physiol. 1991 Feb;260(2 Pt 1):L61–L67. doi: 10.1152/ajplung.1991.260.2.L61. [DOI] [PubMed] [Google Scholar]
- Kharami A., Bibi Z., Nielsen H., Høiby N., Döring G. Effect of Pseudomonas aeruginosa rhamnolipid on human neutrophil and monocyte function. APMIS. 1989 Dec;97(12):1068–1072. [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Mattoli S., Marini M., Fasoli A. Expression of the potent inflammatory cytokines, GM-CSF, IL6, and IL8, in bronchial epithelial cells of asthmatic patients. Chest. 1992 Mar;101(3 Suppl):27S–29S. doi: 10.1378/chest.101.3_supplement.27s. [DOI] [PubMed] [Google Scholar]
- McFaul S. J. A method for isolating neutrophils from moderate volumes of human blood. J Immunol Methods. 1990 Jun 12;130(1):15–18. doi: 10.1016/0022-1759(90)90293-5. [DOI] [PubMed] [Google Scholar]
- Nadel J. A. Protease actions on airway secretions. Relevance to cystic fibrosis. Ann N Y Acad Sci. 1991;624:286–296. doi: 10.1111/j.1749-6632.1991.tb17027.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Yoshimura K., Jaffe H. A., Crystal R. G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991 Oct 15;266(29):19611–19617. [PubMed] [Google Scholar]
- Nicas T. I., Iglewski B. H. The contribution of exoproducts to virulence of Pseudomonas aeruginosa. Can J Microbiol. 1985 Apr;31(4):387–392. doi: 10.1139/m85-074. [DOI] [PubMed] [Google Scholar]
- Ras G., Wilson R., Todd H., Taylor G., Cole P. Effect of bacterial products on neutrophil migration in vitro. Thorax. 1990 Apr;45(4):276–280. doi: 10.1136/thx.45.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell N. B., Taylor G. W., Somerville M., Todd H., Wilson R., Cole P. J. Characterisation of Pseudomonas rhamnolipids. Biochim Biophys Acta. 1990 Jul 16;1045(2):189–193. doi: 10.1016/0005-2760(90)90150-v. [DOI] [PubMed] [Google Scholar]
- Richman-Eisenstat J. B., Jorens P. G., Hébert C. A., Ueki I., Nadel J. A. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993 Apr;264(4 Pt 1):L413–L418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- Sibille Y., Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Sordelli D. O., Cerquetti M. C., Morris Hooke A., Bellanti J. A. Effect of chemotactins released by Staphylococcus aureus and Pseudomonas aeruginosa on the murine respiratory tract. Infect Immun. 1985 Aug;49(2):265–269. doi: 10.1128/iai.49.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiver H. G., Zachidniak K., Speert D. P. Inhibition of polymorphonuclear leukocyte chemotaxis by the mucoid exopolysaccharide of Pseudomonas aeruginosa. Clin Invest Med. 1988 Aug;11(4):247–252. [PubMed] [Google Scholar]
- Strieter R. M., Chensue S. W., Basha M. A., Standiford T. J., Lynch J. P., Baggiolini M., Kunkel S. L. Human alveolar macrophage gene expression of interleukin-8 by tumor necrosis factor-alpha, lipopolysaccharide, and interleukin-1 beta. Am J Respir Cell Mol Biol. 1990 Apr;2(4):321–326. doi: 10.1165/ajrcmb/2.4.321. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Iglewski B. H. Toxins of Pseudomonas aeruginosa: new perspectives. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S715–S722. doi: 10.1093/clinids/5.supplement_4.s715. [DOI] [PubMed] [Google Scholar]





