Abstract
During midlife, physical functioning limitations emerge and depressive symptoms are highly prevalent. We examined the relationship between physical functioning and depressive symptoms in the Michigan Study of Women's Health Across the Nation (SWAN) cohort of midlife women (n=377). Seven performance-based physical functioning measures quantifying strength, balance, coordination, flexibility and range of motion and perceived physical functioning, assessed with the SF-36 physical functioning sub-score, were included. The Center for Epidemiological Studies Depression Scale (CES-D) identified concurrent depressive symptom trajectory from 2000/01 through 2005/06 and history of depressive symptoms from 1996/7 through 1999/00. Longitudinal mixed-effects regression modeling was used to evaluate relationships. Median age of participants was 50 years. As age increased, higher CES-D scores were associated with performance-based functions including slower timed walk sit-to-stand, and stair climb after adjusting for five-year history of depressive symptoms and relevant covariates. As age increased, those with higher CES-D scores were more likely to have perceived limitations in physical functioning, though the association was weak. History of depressive symptoms was not significant in any model. These findings suggest that higher concurrent depressive symptoms are modestly associated with slower movement and a perception of poorer functioning. In contrast, history of depressive symptoms played little or no role in current physical functioning of mid-life women. When evaluating physical function, women's current mental health status should be considered.
Keywords: physical functioning, depression, women, midlife, USA, age
Limitations in physical functioning have been defined as restrictions in performing fundamental physical actions used in daily life by one's own age-sex group (Vebrugge & Jette, 1994). Typical performance-based physical functioning measures include timed walk, stair climb and measures that reflect strength, aerobic capacity, flexibility, balance and/or coordination. Alternatively, respondents are asked to rate their perceived difficulty or limitations in specific activities such as climbing stairs or walking several blocks. The midlife, between 40 to 60 years, has been identified as a time when women become increasingly vulnerable to diminishing physical functioning (Sowers, Pope, Welch, Sternfeld, & Albrecht, 2001; Pope, Sowers, Welch, & Albrecht, 2001).
Elevated depressive symptoms and depressive disorder are highly prevalent among women at mid-life, occurring about twice as frequently in women compared to men (Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993). The reported frequency of high depressive symptoms was 26% in midlife women in the Seattle Midlife Women's Health cohort (Woods, & Mitchell, 1997) and 23% in the Study of Women's Health Across the Nation (SWAN) cohort of midlife women (Bromberger et al., 2007). Women aged 45- 54 may have a higher prevalence of depressive symptoms than younger or older women (Freeman, Sammel, Lin, & Nelson, 2006; Burt & Stein, 2002).
Limitations in physical functioning in the elderly have been associated with depressive symptoms (Penninx, Deeg, van Eijk, Beekman, & Guralnik, 2000; Lenze at al., 2005). Among the 5,888 Cardiovascular Health Study participants aged 65 and older (Lenze et al., 2005), physical functioning declined over a three-year period except among participants with consistently fewer depressive symptoms -- they did not experience a decline.
Links between depressive symptoms and decline in physical functioning are likely complex and probably at least in part, bidirectional. Potential effects of depressive symptoms on physical functioning outcomes may exist via immune system pathways (Cesari et al., 2004; Taaffe, Harris, Ferrucci, Rowe, & Seeman, 2000), which could directly impact muscle and strength (Goodman, 1994; Goodman, 1991; Schaap, Pluijm, Deeg, & Visser, 2006). Additionally, long-term activation of the hypothalamic-pituitary-adrenal (HPA) axis, associated with chronic depression, could lead to compromised physical function (Penninx et al., 2009) through its impact on abdominal obesity and related sequelae (Björntorp & Rosmond, 2000). Finally, in addition to the characteristic motor slowing effects of depressive symptoms (Marin, Firinciogullari, & Biedrzycki, 1993; Pier, Hulstijn & Sabbe, 2004; Sachdev, & Aniss, 1994), other potential mechanisms could include increased pain associated with inflammation and higher sensitivity to it in depressive states (Bair, Robinson, Katon, & Kroenke, 2003).
Few studies have examined the association between depressive symptoms and physical functioning in persons younger than age 65. Using longitudinal data from the Michigan SWAN cohort of midlife women, aged 46-56, we hypothesized that greater evidence of depressive symptoms would be associated with poorer performance-based and perceived physical functioning outcome measures. While self-report physical functioning measures capture an individual's perception of their ability, performance-based measures may identify early deficits in functioning (sometimes referred to as “pre-clinical disability”) which may be particularly pertinent in midlife when substantial deficits in functioning are not common.
Methods
Sample
Participants are from the Michigan site of the Study of Women's Health Across the Nation (SWAN). SWAN is a multisite, multiethnic longitudinal study of women at midlife established to characterize the health of women as they approach and traverse menopause. The study design and recruitment for SWAN have been described (Sowers et al., 2000). Briefly, a screening survey of women assessed eligibility for SWAN between November 1995 and October 1997. Eligibility criteria included being aged 42-52, having an intact uterus, having had at least one menstrual period and no use of reproductive hormones in the previous 3 months, and self-identifying with a site's designated race/ethnic groups. All women were either premenopausal or early perimenopausal. Each site recruited white women and a sample of a predetermined minority group. In 1995, African American and white women in two suburban communities near Detroit, Michigan were recruited via a household census of two nearby suburban communities. The Michigan site of the SWAN longitudinal cohort study enrolled 543 premenopausal women in 1996 and the participation rate at baseline among women eligible for the cohort study was 59%. Forty percent were Caucasian and 60% were African-American, with sampling probabilities to reflect the underlying population.
At the fourth follow up visit in 2000/01, this site added a broad range of physical functioning measurements to the standard SWAN battery of measurements. At this visit, six women had died and a total of 384 women were still participating in the study. This report includes physical functioning data from 2000/01 through 2005/06. A total of 377 of these 384 participants contributed physical functioning data at one or more time points and comprise the analytical sample for this report. Approval for conducting the study was obtained from the University of Michigan Institutional Review Board, and written informed consent was obtained from participants.
Measurements
The timing of data collection for each variable is described in Table 1. Annual data on performance-based physical functioning and covariates were available beginning in 2000/2001, and perceived physical functioning data were available biannually starting in 2000/2001. Depressive symptoms trajectory, our primary exposure, included annual measurements from 2000/01 through 2005/06. History of depressive symptoms based on annual data collected in SWAN core from 1996/97 - 1999/00 was included as a covariate in all models.
Table 1.
Timing of data collection for dependent and independent variables
| 96/97 | 97/98 | 98/99 | 99/00 | 00/01 | 01/02 | 02/03 | 03/04 | 04/05 | 05/06 | |
|---|---|---|---|---|---|---|---|---|---|---|
| |
||||||||||
| Physical functioning | ||||||||||
| Performance-based physical functionsa | X | X | X | X | X | X | ||||
| Leg strength (also a performance-based function) | X | X | X | X | X | |||||
| SF-36 PF score | X | X | X | |||||||
| Depressive symptoms, based on CES-D score | ||||||||||
| History of depressive symptoms | X | X | X | X | ||||||
| Trajectory of depressive symptoms | X | X | X | X | X | X | ||||
| Additional covariates (e.g., demographic variables, pain) | X | X | X | X | X | X | ||||
Grip strength, forward reach, 2-lb lift, stair climb, timed walk, sit to stand
b Data from the CES-D administered 4 times prior to the physical performance measures data collection were used to create a variable representing history of depressive symptoms.
Performance-based physical functioning measures
Physical functioning, our outcome of interest, was assessed with seven performance-based measures. Research staff were trained according to a standard protocol and retrained annually to ensure that measured results were consistent across staff and over time.
Strength
Quadriceps strength was measured with a portable isometric chair which replicates the chair designed for the Dynamics of Health, Aging and Body Composition Study (Goodpaster et al., 2001). Quadriceps strength was quantified as torque (Nm), the product of force and torque arm length. Results from three successful trials were averaged. To measure grip strength (kg), a participant was seated in a chair with her forearm at a 90- degree elbow bend and hands placed with fingers and thumb parallel to her body. Three efforts from the dominant hand were averaged for each participant. Higher values suggest greater grip strength. Grip strength has been shown to have good discrimination across the range of physical function, including in younger, high-functioning persons (Curb et al., 2006).
Timed walk and timed stair climb
Each participant was timed (in seconds) in two purposeful walks down a 40-foot carpeted corridor, and the average time was used for the timed walk variable. For the stair climb, participants were timed (in seconds) during ascent and descent of three standardized stairs. Timing began with the toe-off of the leading leg at the start of ascent and ended with final foot contact of the trailing leg following descent. Slower stair climb is reflected in a longer time to complete the task. This measure is intended to capture a variety of physical functioning domains including strength, balance, and range of motion (Terwee, Mokkink, Steultjens, & Dekker, 2006).
Timed sit-to-stand
Participants were asked to rise from a standard height, armless chair with their arms folded over their chest. This variable captured movement time (in seconds) from onset of trunk motion on the chair to an upright standing position in five repetitions; slower sit-to-stand is captured by a longer time to complete the task. Chair rise has been shown to have good discrimination across the range of physical function, including in younger persons (Curb et al., 2006).
Flexibility and range of motion
In the forward reach, a measure of flexibility and range of motion (Duncan, Weiner, Chandler, & Studenski, 1990), participants were asked to stand straight with their dominant arm extended parallel to the floor and reach the greatest distance (cm) possible forward while maintaining their arm in the same horizontal plane. Greater number of cm suggests greater flexibility and range of motion. In the 2-lb lift, the number of seconds required to lift a 2-lb box from the floor to a plane with the waist was recorded; slower times are reflected in a longer time to complete the task. The 2-lb lift was designed to capture dynamic balance ability, joint coordination and initial posture for lifting, which have been related to functional status in mid-life women (Buhr, 1998).
Perceived physical functioning was assessed using responses to a 10-question subscale of the Medical Outcomes Study Short-Form 36 (SF-36; Ware, 1995; Sherman & Reuben, 1998). Participants indicated whether they were not limited, limited a little or limited a lot in walking, climbing stairs, bending, carrying groceries, moderate and vigorous athletic activities, bathing and dressing. Scores ranged from 0 – 100 with higher scores representing better functioning. This outcome was categorized as a three-level ordinal variable. We classified women with a score of less than 50 points as having substantial limitations; such individuals could have reported no limitations on five or less of the ten activities. Those with a score of 50-85 were classified as having moderate limitations. A woman with 85 points could have reported no limitations on, at most, eight of ten activities, thus allowing for some limitations in vigorous and moderate activities). Women with 86–100 points were considered not limited. While no standard cutoff has been developed, these ordinal classifications have been used previously (Tomey, Sowers, Crandall, Johnston, Jannausch, & Yosef, 2008).
Depressive Symptoms were assessed using the Center for Epidemiologic Studies Depression (CES-D) Scale, a 20-item questionnaire querying frequency of depressive symptoms during the previous week. Each item is scored on a 4-point scale of 0 (rarely) to 3 (most or all of the time) for a possible range of 0-60 points (Radloff, 1977). Though the CES-D score does not represent clinical depression, it has been shown as valid and reliable in African-American and Caucasian populations (Jones-Webb, & Snowden, 1993). The standard ‘cutoff’ score of 16 or more points was used in characterizing participants as having depressive symptoms (Boyd, Weissman, Thompson, & Myers, 1982).
The CES-D score was used to define two variables for regression analysis. Our primary exposure is depressive symptoms trajectory, characterizing level of depressive symptoms with age. This variable includes annual evaluations from 2000/01-2005/06 and the possible range at each time point is 0-60 points (Table 1). The second variable is a history of elevated depressive symptoms in the prior five years, used as a covariate. The variable has three levels, including those who did not have a CES-D score of > 16 at any time points in the previous five years, those with an elevated score at one time point, and those with an elevated score at two or more time points from 1996/97 – 1999/2000 (Table 1; the time period preceding the initial assessment of performance-based measures of physical functioning).
Measures of Pain were also considered as pain may mediate the relationship between depression and physical functioning (Stubbs, D., Krebs, E., Bair, M., Damush, T., Wu, J., Sutherland, J., et al. 2010). Pain was based on two questions from the MOS SF-36 Bodily Pain index about severity of pain and interference with normal work (outside the home and housework). Designation of pain status was based on a score with a maximum of 100 points; participants were classified into one of three pain categories at each time point. Those with a score of 100 points were classified as having “no pain,” while others in the upper half of the score distribution were designated as having “some pain” (a score of 61 – 99 points), and those in the lower half of the distribution were classified as having “most pain” (a score of 0-60 points).
Other covariates
Race was self-designated. Economic stress was characterized by responses about whether it was very hard, somewhat hard, or not hard at all to pay for basics such as food, housing, and health care. Body mass index (BMI) was calculated as weight (kg)/height (m)2; weight and height were measured by use of calibrated scales and stadiometers, respectively. Cigarette smoking was measured via questionnaire and participants were classified as current smokers, past smokers, or those with no history of smoking. Heart disease was self-reported (yes/no) via questionnaire, diabetes status was based on self-reported medication use or a fasting glucose value of ≥126 mg/dL, and arthritis was based on self-reported medication use or self-reported presence of arthritis or osteoarthritis. Trouble sleeping was assessed with a questionnaire item asking participants if they had problems sleeping in the past 2 weeks; participants were classified as having problems sleeping 1-2 times per week or less or 3-4 times per week or more.
Statistical analyses
Univariate statistics including frequencies, medians, and interquartile ranges (IQRs) were computed as appropriate to describe participant demographics, health conditions, depressive symptoms and physical functioning abilities. For regression modeling, variables with highly skewed distributions were transformed for analysis and back-transformed to their original scale for presentation. The SF-36 perceived physical functioning questionnaire score was categorized into an ordinal, 3-level variable as described above because it was highly skewed in this mid-life population and could not be successfully transformed to approximate a normal distribution.
Two mixed-effect modeling approaches were used to evaluate associations between depressive symptoms and each physical functioning measure separately. Linear mixed regression models (PROC MIXED) were used to evaluate relationships with annual continuous performance-based physical functioning measures, such as hand grip strength or timed walk, across visits. Mixed-effect multinominal logistic regression (PROC NLMIXED) models were used to evaluate the association between depressive symptoms and the ordinal 3-level variable perceived limitations in physical functioning.
The use of mixed models allowed us to include repeated measures of depressive symptoms and physical functioning from the same individual and to ask to what extent initial level and subsequent change in physical functioning outcomes differed according to trajectory of CES-D. We also asked whether physical functioning differed based on prior history of having elevated levels of depressive symptoms. This approach accounted for within-woman correlation in successive measurements across time, and allowed flexible parameterization of the nature of these correlations. Additionally this approach allowed us to analyze data collected at unequally-spaced time intervals (slightly greater or less than annually), and to include persons with missing data at one or more visits (Fitzmaurice, Laird, & Ware, 2004; Verbecke & Molenberghs, 2000).
In both the linear and logistic models, random intercepts were used in every model and random slopes were included based on evaluations of the likelihood ratio test as well as comparisons of AIC and BIC. Goodness of fit of the models was assessed by the AIC criterion. All continuous covariates were centered at their grand mean values to facilitate interpretation of the intercept and beta coefficients.
In the linear mixed models, age was used to define the repeated measures variance structure to account for the within-person dependence in observations across time. There is some debate about whether it is more appropriate to use calendar time or age to define the repeated measures variance structure (Jacobs, Hannan, Wallace, Liu, Williams, & Lewis, 1999; Holford, 1992); age was justified here due to the small age range of women (eleven years), because age and calendar time represented the same incremental increase in time (approximately one year) and because of the similarity in fit and results of preliminary models using age vs. calendar time as the repeated measures variance structure.
In the non-linear mixed models, comparisons were made based on depressive symptom status between those with substantial or moderate limitations vs. those with no limitations as well as between those with substantial limitations vs. those with moderate or no limitations. The assumptions of proportionality of associations between both of these comparisons were examined using the likelihood ratio test and Akaike Information Criterion (AIC) for goodness of fit. Because these assumptions were met in all models, results from the proportional odds mixed-effect model summarize both comparisons.
Covariates included, race, economic stress, as well as time-varying variables for cigarette smoking, logBMI, pain and age. Additionally, cumulative heart disease, diabetes and arthritis, as well as the baseline value of each physical functioning outcome variable were included as covariates. Trouble sleeping was tested but not included in final models because it was not significantly associated with physical functioning variables in models. Five-year history of depressive symptoms was included as a covariate in all models. Interactions between depressive symptom trajectory and five-year history of depressive symptoms were tested in each model. Additionally, interactions between depressive symptom trajectory and age were evaluated, along with those between history of depressive symptoms and age in both linear and non-linear models.
Average annual percent change was calculated for the continuous primary exposure variable CESD score, as well as for selected physical functioning outcomes. These values and the accompanying figures were estimated from regression models adjusted for all covariates; the estimates were averaged over the mean levels of the exposure and covariates. The confidence intervals are symmetric on the log scale, on which models were fit; however transformation back to the original scale yields asymmetric confidence intervals.
We used three approaches to provide information about whether missing data due to loss to follow up may have biased our results. These included assessment of 1) whether having missing depressive symptom data at the most recently available visit was associated with each physical functioning variable at the initial measurement; 2) whether there was a significant difference in history of depressive symptoms between those who were lost to follow up and those who continued to participate, evaluated with a chi-square test; and 3) whether the most recently observed CESD scores were predictive of the likelihood of being lost to follow up.
Data from the 377 individuals with physical functioning data at one or more time points were used in modeling. In the analytic sample, 364 women have physical functioning data at 2 or more time points, 346 women have data at 3 or more time points, and 325 women have data at 4 or more time points. Women may have missed any given visit because of illness, time constraints, or because of loss to follow-up.
The probability of significant associations occurring by chance alone was expressed with p-values based on two-sided tests as well as 95% confidence intervals (CI). Associations with p-values < 0.05 were considered statistically significant. Analyses were undertaken using SAS 9.1 (SAS Institute Inc., Cary, NC).
Results
Descriptive characteristics of overall study sample
In 2000/01, there were 336 women with physical functioning data. The median (IQR) age of participants was 50 (5) years with a range of 45-56 years (Table 2). Median (IQR) body mass index (kg/m2) was 32.1 (12.7) with a range of 16.0 - 56.3 kg/m2 and median (IQR) CES-D score was 8.0 (12) with a range of 0 – 49 points.
Table 2.
Descriptive characteristics in 2000/01 by depressive symptom status (Michigan site)
| Total Sample at 2000/01 time point (n=336) | Without depressive symptoms in 2000/01 (CESD score < 16) n=258 (77%) | With depressive symptoms in 2000/01 (CESD score ≥ 16) n=78 (23%) | |
|---|---|---|---|
|
Median (Interquartile Range) |
|||
| Age (years) | 50 (5) | 50 (4) | 50 (4) |
| Body mass index (kg/m2) | 32.1 (12.7) | 32.6 (12.5) | 31.4 (11.6) |
| CES-D scorea | 8.0 (12) | 5.0 (7) | 23.0 (11) |
| Performance-based physical functioning measures | |||
| Hand grip strength (kg) | 28.0 (8.4) | 28.2 (7.3) | 27.3 (9.3) |
| Leg strength (Nm) | 79.6 (32.6) | 80.8 (30.1) | 78.4 (40.4) |
| Forward reach distance (cm) | 34.8 (9.5) | 34.6 (9.5) | 34.8 (9.2) |
| 2-lb lift from the floor (seconds) | 1.9 (0.9) | 1.9 (0.8) | 2.0 (1.4) |
| Sit-to-stand (seconds) | 1.2 (0.7) | 1.2 (0.7) | 1.2 (0.7) |
| Stair climb (seconds)b | 17.9 (6.6) | 17.6 (6.4) | 19.0 (7.7) |
| 40-foot timed walk (seconds) | 8.8 (2.4) | 8.8 (2.1) | 8.9 (3.4) |
| Frequency (%) |
|||
| Five year depressive symptom historya,c | |||
| CES-D score ≥ 16 at no time points | 128 (38) | 128 (50) | 0 |
| CES-D score ≥ 16 at one time point | 105 (31) | 89 (34) | 16 (21) |
| CES-D score ≥ 16 at two or more time points | 103 (31) | 41 (16) | 62 (79) |
| Perceived physical functioning based on SF-36 PF scorea | |||
| Not limited (≥ 86 points) | 140 (42.8) | 122 (48.2) | 18 (24.3) |
| Moderately limited (50-85 points) | 137 (41.9) | 99 (39.1) | 38 (51.4) |
| Substantially limited (<50 points) | 50 (15.3) | 32 (12.7) | 18 (24.3) |
| Race | |||
| Caucasian | 183 (45.5) | 111 (43.0) | 36 (46.2) |
| African American | 153 (54.5) | 147 (57.0) | 42 (53.9) |
| Current cigarette smokera | |||
| Yes | 80 (23.8) | 50 (19.4) | 30 (37.5) |
| Economic stressa | |||
| Very difficult to pay for basics | 40 (11.9) | 20 (7.8) | 20 (25.6) |
| Somewhat difficult to pay for basics | 106 (31.6) | 75 (29.2) | 31 (39.7) |
| Not difficult to pay for basics | 189 (56.4) | 162 (63.0) | 27 (34.6) |
| Diabetes | 52 (15.5) | 42 (16.3) | 10 (12.8) |
| Heart diseaseb | 30 (8.9) | 15 (5.8) | 15 (19.2) |
| Arthritisb | 80 (23.8) | 50 (19.4) | 30 (38.5) |
| Pain (SF-36 Bodily Pain index)a | |||
| Most pain (<61 points) | 34 (10.1) | 100 (38.8) | 47 (60.3) |
| Some pain (61 – 99 points) | 155 (46.1) | 126 (48.8) | 29 (37.2) |
| No pain (100 points) | 147 (43.8) | 32 (12.4) | 2 (2.6) |
P<0.001 based on Wilcoxon test for continuous data, or chi-square test for categorical data.
P<0.05 based on Wilcoxon test for continuous data, or chi-square test for categorical data.
This is a sum of the number of annual visits a person scored at least 16 points on the CESD from 1996/97 – 1999/2000.
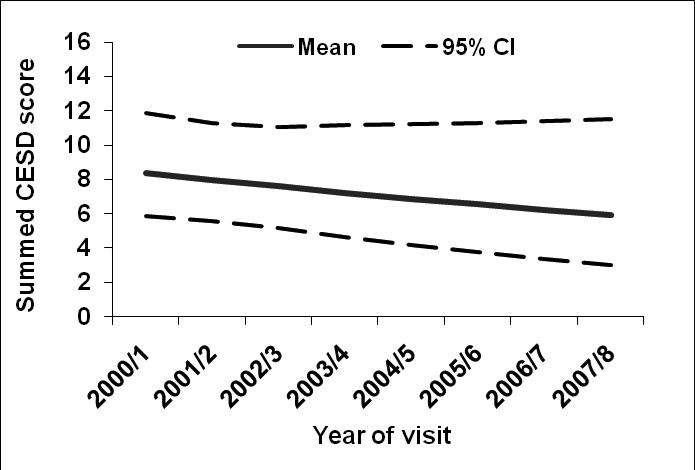
Figure 1 shows the estimated mean values and 95% confidence interval bands for CES-D score over the five years of follow-up, adjusted for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value. Adjusted CES-D scores declined 0.4 points per year after adjustment (P value= 0.005).
Figure 1.
Estimated mean values and 95% confidence bands for CES-D score across time, adjusted for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value.
At baseline, twelve percent of participants indicated it was very difficult to pay for basics like food and housing, 32% said it was somewhat difficult and 56% said it was not difficult. Consistent with the study design, 46% participants were Caucasian and 54% African American. Twenty-four percent of participants were current cigarette smokers.
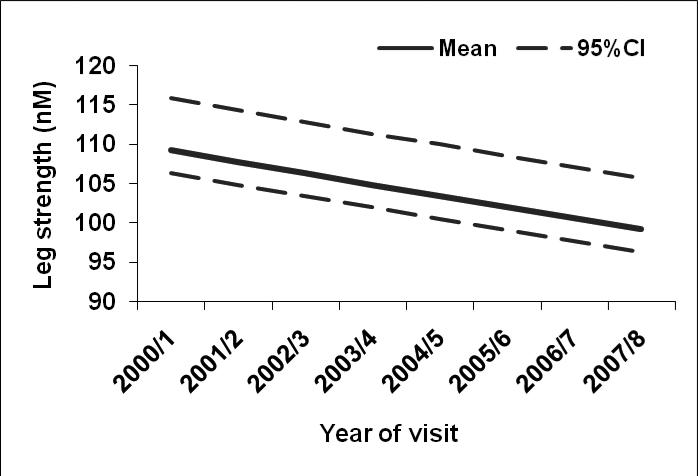
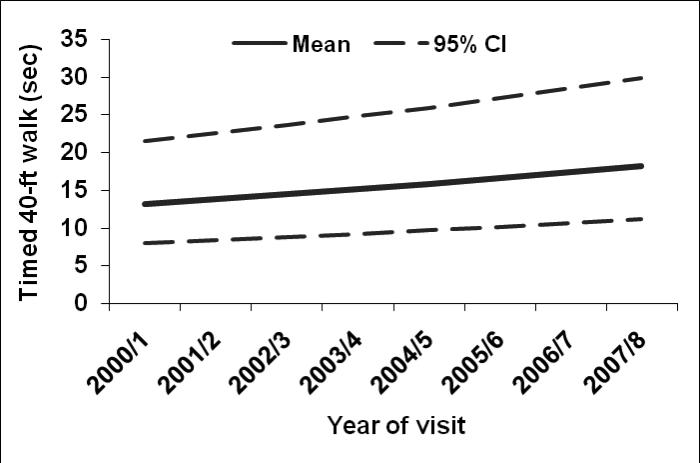
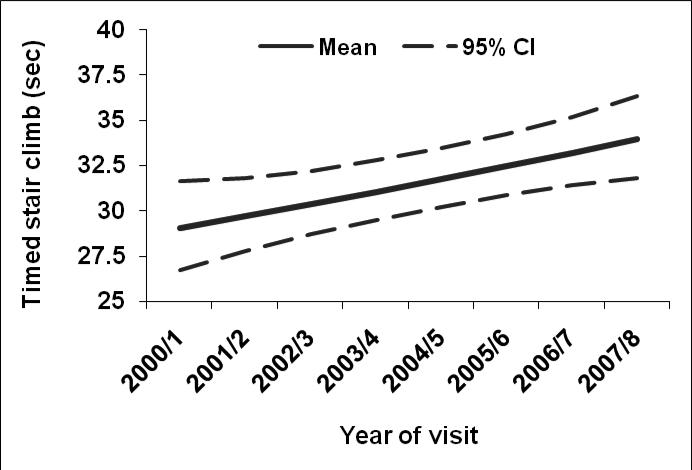
Overall, participants had median (IQR) hand grip strength of 28.0 (8.4) kg, and leg strength was 79.6 (32.6) Nm. Median (IQR) forward reach distance in the total sample was 34.8 (9.5) cm. Additionally, median (IQR) of timed measures included 2-lb lift [1.9 (0.9) seconds], sit-to-stand [1.2 (0.7) seconds], stair climb [17.9 (6.6) seconds] and 40-foot timed walk [8.8 (2.4) seconds]. The estimated mean trajectories and 95% confidence interval bands across time for leg strength, stair climb, and timed walk respectively are shown in Figures 2-4, adjusting for covariates. Leg strength (Figure 2) declined 1% per year (P value =0.01), while average stair climb time increased 1.1% per year (P value= 0.01), and average walking time increased 4.8% per year (P value <0.0001; Figures 3-4, respectively).
Figure 2.
Estimated mean values and 95% confidence bands for leg strength in nM across time after adjusting for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value.
Figure 4.
Estimated mean values and 95% confidence bands for timed walk in seconds across time after adjusting for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value.
Figure 3.
Estimated mean values and 95% confidence bands for timed stair climb in seconds across time after adjusting for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value.
Participant characteristics based on depressive symptom status
Twenty-three percent of participants were classified as having depressive symptoms in 2000/01 (Table 2). Median CES-D score, as expected, was much lower in those classified as having no depressive symptomatology [median (IQR) score 5.0 (7) vs. 23.0 (11) in those without and with depressive symptoms, respectively, p <0.001].
Those with a CES-D score of at least 16 points in 2000/01 tended to have poorer average physical functioning compared to those without high depressive symptoms though most of these differences were not statistically significant (Table 2). Participants with high depressive symptoms had a significantly slower stair climb time [median (IQR) 17.6 (6.4) vs. 19.0 (7.7) seconds, p value <0.05].
Perceived physical functioning was considerably worse in those with higher depressive symptom scores. Those with depressive symptoms had nearly double the prevalence of being classified as substantially limited [24% vs. 13%, respectively], and were about half as likely to be not limited [24% vs. 48%, respectively], p<0.001.
There were large differences in covariates based on depressive symptom status. More participants classified as with vs. without depressive symptoms were smokers (38% vs. 19% respectively, p value <0.001). While 26% of those with depressive symptoms reported that it was very difficult to pay for basics, only 8% of those without depressive symptoms reported this difficulty, p value <0.001. Those with depressive symptoms were more also likely than those without depressive symptoms to have heart disease (19% vs. 6% respectively, p value <0.05), arthritis (39% vs. 19% respectively, p value <0.05) and “most” pain (60% vs. 39% respectively, p value <0.001).
Association of depressive symptom trajectory and physical functioning outcomes
In those whom depressive symptoms increased with age, our measure of longitudinal change, performance diminished on many of the physical functioning outcomes. Results from separate mixed effect models for each outcome show that a higher CES-D score was significantly related to slower 2-lb lift [β (SE) 0.003 (0.001); p value <0.01] and stair climb [β (SE) 0.003 (0.0007); p value <0.0001], after adjusting for covariates including history of depressive symptoms (Table 3). These parameter estimates reflect a 6% faster time for 2-lb lift and stair climb in those with a CESD score of 5.0 vs. 23.0 points, the median scores in those with and without depressive symptoms in 2000/01 respectively, holding covariates constant. Self-reported arthritis was a significant covariate in the model for 2-lb lift [β (SE) 0.06 (0.02), p value <0.01], as well as the model for timed stair climb [β (SE) 0.03 (0.01) p value <0.05].
Table 3.
Associations between trajectory of CES-D score and performance-based physical functioning (n=377)a,b
| CES-D score trajectory | ||
|---|---|---|
| β (SE) | P-value of β | |
| |
||
| Hand grip strength (kg) | -0.02 (0.02) | NS |
| logLeg strength (nM) | -0.001 (0.01) | NS |
| Forward reach distance (cm) | -0.009 (0.02) | NS |
| log2-lb lift from the floor (s) | 0.003 (0.001) | <0.01 |
| logSit-to-stand (s) | 0.004 (0.001) | <0.01 |
| logStair climb (s) | 0.003 (0.0007) | <0.0001 |
| log40-foot timed walk (s) | 0.002 (0.001) | <0.001 |
A separate model was performed for each physical functioning outcome.
Longitudinal mixed-effects linear regression models were adjusted for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value.
Higher CES-D score was significantly related to slower sit-to-stand [β (SE) 0.004 (0.001); p value <0.01], or a 7% faster time in those with a CESD score of 5.0 vs. 23.0 points holding covariates constant. ‘Some pain’ was borderline significant covariate in this model [β (SE) 0.04 (0.02), p=0.05], however ‘more pain’ was not significant. Arthritis was significant in this model [β (SE) 0.051 (0.02) p value <0.05].
Higher CES-D score with advancing age was also significantly related to slower 40-foot timed walk [β (SE) 0.002 (0.001); p value <0.001], such that those with a CESD score of 5.0 points walked 4% faster than women with a CES-D score of 23 points, holding covariates constant.
We observed a weak association between higher CES-D score with advancing age and worse perceived physical functioning limitations, after adjusting for covariates including history of depressive symptoms [OR (95% CI) 1.05 (1.02, 1.07)] for perceived moderate or substantial limitations vs. no limitations and also for substantial vs. moderate or no limitations (Table 4). In other words, those with increased concurrent depressive symptom scores were 5% more likely to be classified as substantially limited compared to those with no/moderate perceived limitations. Likewise, those with increased concurrent depressive symptom scores were 5% more likely to have substantial or moderate limitations compared to those with no perceived limitations. Pain was a significant covariate [OR (95% CI) 2.7 (1.2, 6.4) and 6.3 (2.6, 15.5) for some pain and more pain, respectively].
Table 4.
Summary odds ratio describing associations between trajectory of CES-D score and perceived physical functioninga
| Odds Ratio (95% Confidence Interval)b |
|
|---|---|
| Perceived physical functioning (SF-36 PF) | 1.05 (1.02, 1.07) |
Longitudinal mixed-effects non-linear regression model was adjusted for five-year history of depressive symptoms, age, race/ethnicity, economic stress, smoking, BMI, pain, heart disease, diabetes, arthritis, and baseline (or earliest non-missing) physical function value.
The odds ratio summarizes the likelihood of having moderate or substantial limitations vs. no limitations based on depressive symptom trajectory, and also for the likelihood of having substantial vs. moderate or no limitations based on depressive symptom trajectory.
A five-year history of depressive symptoms prior to functional assessment was not significant in any model. No significant interactions were observed between depressive symptom trajectory and five-year history of depressive symptoms. Additionally, no significant interactions were observed between depressive symptom trajectory and age, our measure of longitudinal change, or five-year history of depressive symptoms and age.
There was no significant association between having missing depressive symptom data at the most recently available visit and physical functioning status at the initial measurement. There was no significant difference in history of depressive symptoms between participants who were lost to follow-up and the women who continued to participate. The most recently observed CESD scores were not associated with being lost to follow-up.
Discussion
In our sample of mid-life women, annual changes in physical function were modest for leg strength and stair climb time, however average walking time increased 4.8% per year. Though clinically meaningful changes in these performance-based measures have not been established, comparable declines have been reported for similar measures in high-functioning older persons. In a sample of approximately 1,000 high functioning older persons aged 70-79 years, average declines for 10-foot walk were 3% annually, and declines in chair stands were 1% per year in a study that followed participants for 3 years (Seeman et al., 1994).
We observed modest but consistent associations between measures of diminishing physical functioning and the depressive symptom trajectory after adjusting for covariates, including five-year history of depressive symptoms and baseline level of physical functioning. Penninx et al. (2000), reported similar results in a sample of 2121 community-dwelling persons aged 55-85 years. Odds of substantial decline in physical functioning performance in participants who transitioned from depressed to non-depressed status during the three-year study follow up using the CESD to classify depression were protective but not significant [OR (95% CI) 0.66 (0.41, 1.09)]; however, those who transitioned from non-depressed to depressed had 76% increased odds of substantial decline in physical functioning performance [OR (95% CI) 1.76 (1.23, 2.51)]. Those with consistent depressive symptoms also had increased odds of substantial physical functioning decline [OR (95% CI) 1.67 (1.09, 2.58)].
Recent research has highlighted the immune system as playing a role in poor physical functioning (Cesari et al., 2004; Taaffe et al., 2000). Depressive symptoms have been linked to activation of inflammatory pathways, including increases in C-reactive protein, greater abdominal adiposity, and related changes (Danner, Kasl, Abramson, & Vaccarino, 2003; Ford & Erlinger, 2004; Miller, Stetler, Carney, Freedland & Banks, 2002; Vogelzangs et al., 2008), and higher inflammation has been linked to compromised functioning (Cesari et al., 2004; Tomey, Sowers, Zheng, & Jackson 2009).
The HPA axis may be involved in such pathways. It has been postulated that long-term activation of the HPA axis associated with chronic depression (and/or stress) is associated with greater abdominal obesity, and this greater abdominal fat accumulation could be linked through elevated cortisol, particularly in combination with low sex steroid hormones (Björntorp & Rosmond, 2000). Excess abdominal obesity has been related to poorer physical functioning (Penninx et al., 2009) as well as conditions such as insulin resistance, cardiovascular and other conditions that frequently co-exist with compromised physical functioning (Lean, 2000).
Another mechanism may be related to the catabolic effect of inflammatory markers on muscle. Experimental studies of rats have reported that administration of IL-6 or TNF-α causes muscle breakdown (Goodman, 1994; Goodman, 1991), and prospective findings in humans suggest that higher levels of IL-6 and CRP are associated with muscle strength loss after adjusting for covariates including BMI (Schaap et al., 2006). It remains unclear whether inflammation precedes or follows depressive symptoms in such a context (Dunn, Swiergiel & de Beaurepaire, 2005). In our study, though depressive symptoms were associated with some physical functions that rely in part on strength, such as the stair climb and sit-to-stand, other measures that rely on strength such as grip strength and leg strength were not associated with depressive symptoms.
Pain, considered a key comorbidity in compromised physical functioning, in depression, and with inflammation, played a role in our observed associations. Self-reported arthritis, often linked to pain, also played a role. These variables helped to explain variation in sit-to-stand and 2-lb lift, which require bending at the waist, as well as timed stair climb and perceived physical functioning. Evidence shows that depressed persons are more sensitive to pain, and those who report pain are more likely to be depressed (Bair et al., 2003). The term “depression-pain syndrome” has been used to characterize this complex interrelationship (Bair et al., 2003).
Additionally, associations with timed measures could relate to the motor slowing effect of depressive symptoms. This slowing, fatigue and apathy have been described and may be partly responsible for significant associations with performance-based functions, all of which were timed (Marin, Firinciogullari, & Biedrzycki, 1993; Pier, Hulstijn, & Sabbe, 2004; Sachdev, & Aniss, 1994). Psychomotor retardation is frequently observed in depressed individuals and in fact, decreased motor activity is a diagnostic criterion for major depression (Futterman & Tryon, 1994).
Lack of sleep has been associated with both depression and poor physical functioning (Habte-Gabr et al., 1991; Newman, Enright, Manolio, Haponik, & Wahl, 1997), and poorer functioning may be in part due to fatigue from lack of sleep. In this study, sleep problems reported by participants were by definition recent (the timeframe for the report was the past two weeks), although these problems could have been more persistent than the time frames allowed by the interview. Nevertheless, report of sleep problems was not a significant confounder or mediator in the association of physical functioning and depressive symptoms.
In our study, associations with some performance-based functioning measures were stronger compared to associations with perceived physical functioning. In contrast to our results, some authors have found a greater association with perceived versus performance-based measures (Lenze et al, 2001; Cress et al., 1995; Kempen, Steverink, Ormel, & Deeg, 1996). It has been postulated that this differential may occur because those who are depressed may give an overly pessimistic view of their functioning levels (Lenze et al, 2001) and that performance-based measures would be less influenced by personality, cognition, and mood (Cress et al., 1995; Kempen et al., 1996). Direct comparisons to this study are challenging however, because those studies evaluated elderly participants, many of whom were frail and lived in nursing homes. In addition to experiencing a greater number of comorbidities, these populations experienced more substantial physical functioning and depression problems compared to our younger mid-life sample.
The factors and dynamics impacting depressive symptoms and physical functioning are complex, and conceptual models attempt to explain these dynamics in describing development of physical functioning limitations. Classic conceptual models, including those proposed by Saad Nagi (1991), and the National Center for Medical Rehabilitation Research (Jette, 1994) infer that the process of functional decline begins with an active pathology or pathophysiology. Nagi (1991) notes that initiating pathology may result from infection, trauma, metabolic imbalance, degenerative disease processes or other etiology.
While the current study was not designed to address the causal processes hypothesized in these classic models, our observed associations between depressive symptoms and physical functioning endpoints do not appear to fit into these conceptualizations. The conceptual models are predicated on pathology progressing to impairment and functional limitation; however, five-year history of depressive symptoms was not associated with physical functioning in any model. Instead, the depressive symptom trajectory exposure was consistently associated with performance-based and perceived physical functioning outcomes, even after adjusting for historical symptoms. Though our performance-based outcomes do not represent “functional limitations” per se, our findings highlight the importance of accounting for the timing of depressive symptoms.
It remains to be elucidated as to how psychomotor and fatigue-related pathways could fit into this scheme, particularly since some trials have shown improvement in physical functioning with depression treatment (Hunkeler et al., 2006; Callahan et al., 2005). If these observed relationships are confirmed, future conceptual models should address the role of depressive symptom trajectory and apparent lack of longer-term effect of depressive symptoms on physical functioning limitations.
Our study includes several strengths including the longitudinal nature of the data, inclusion of two races with a wide distribution in BMI values, the ability to statistically adjust for relevant demographic and health variables, and inclusion of a five-year history of depressive symptoms. In our study, although some participants were lost to follow up, having missing depressive symptom data at the most recently available visit was not associated with physical functioning status at the initial measurement. There was no significant difference in history of depressive symptoms between participants who were lost to follow-up and the women who continued to participate. Further, the most recently observed CESD scores were not predictive of the likelihood of being lost to follow-up.
Our analyses had limitations. Although our statistical models adjusted for potential confounders (Bruce, Seeman, Merrill, & Blazer, 1994), residual confounding may have been present which could result in an overestimate of the associations between depressive symptoms and physical functioning measures. Variables such as pain may not have been fully captured, and other conditions such as subclinical cardiovascular disease, cognitive limitation or other conditions that may occur in this mid-life sample of women either were not assessed or occurred too infrequently to consider in this analysis. Finally, while the analysis of selection bias provides some assurance of a minimal impact, loss to follow-up in this study remains a potential limitation.
It is important to note that history of depressive symptoms was characterized on a different scale compared to the concurrent depressive symptom trajectory variable. The former was an ordinal summary variable that quantified cumulative exposure to high levels of depressive symptoms versus the latter which was a continuous scale representing level of depressive symptomatology. This could have contributed to some of the differences we observed in strength of associations with the two depressive symptom variables.
While it is difficult to disentangle the direction of the relationship between depression and decline in physical functioning, evidence supports a reciprocal relationship in older populations where most of the research exists (Ormel, Rijsdijk, Sullivan, van Sonderen, & Kempen, 2002). The notion that a fairly moderate decline in physical functioning in mid-life would cause depressive symptoms is less likely than in an older population where deficits may become more severe. Nevertheless, the impact of depressive symptoms on physical functioning is important and treatment of these symptoms could mitigate this decline. Our findings suggest that in a clinical setting, evaluation of physical functioning in midlife women should include consideration of current depressive symptom status. It remains to be determined if the reductions in physical performance observed with depression have a long-term effect in quality of life and if treatment of depression and pain at the midlife changes the trajectory of physical performance decline with aging (Hunkeler et al., 2006; Callahan et al., 2005; Weigl, Cieza, Cantista, Reinhardt, & Stucki, 2008).
Acknowledgments
The Michigan site of the Study of Women's Health Across the Nation (SWAN) was funded through a grant from the National Institute on Aging [AG017104]. SWAN also has grant support from the National Institutes of Health, Department of Health and Human Service, the National Institute on Aging, the National Institute of Nursing Research and the National Institutes of Health Office of Research on Women's Health [Grants NR004061; nursing AG012505, aging AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495].The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristin Tomey, University of Michigan Ann Arbor, MI UNITED STATES, ktea@umich.edu.
MaryFran R Sowers, University of Michigan School of Public Health.
Sioban Harlow, University of Michigan School of Public Health.
Mary Jannausch, University of Michigan School of Public Health.
Huiyong Zheng, University of Michigan School of Public Health.
Joyce Bromberger, University of Pittsburgh.
References
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Archives of Internal Medicine. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S80–5. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample. Understanding the discrepancies between depression symptom and diagnostic scales. Archives of General Psychiatry. 1982;39:1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN). Journal of Affective Disorders. 2007;103:267–72. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptomatology on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994;84:1796–9. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr TA. Ph.D. Dissertation. Biomedical Engineering Department, The University of Michigan; 1998. Effects of leg function constraints on a lifting task examined through inverse cinematics analyses, direct dynamics modeling, and electromyographic biomfeedback techniques. [Google Scholar]
- Burt VK, Stein K. Epidemiology of depression throughout the female life cycle. The Journal of Clinical Psychiatry. 2002;63(supp 7):9–15. [PubMed] [Google Scholar]
- Callahan CM, Kroenke K, Counsell SR, Hendrie HC, Perkins AJ, Katon W, et al. Treatment of depression improves physical functioning in older adults. Journal of the American Geriatrics Society. 2005;53:367–73. doi: 10.1111/j.1532-5415.2005.53151.x. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Cress ME, Schechtman KB, Mulrow CD, Fiatarone MA, Gerety MB, Buchner DM. Relationship between physical performance and self-perceived physical function. Journal of the American Geriatrics Society. 1995;43:93–101. doi: 10.1111/j.1532-5415.1995.tb06372.x. [DOI] [PubMed] [Google Scholar]
- Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- Danner M, Kasl SV, Abramson JL, Vaccarino V. Association between depression and elevated C-reactive protein. Psychosom Med. 2003;65:347–356. doi: 10.1097/01.psy.0000041542.29808.01. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192–197. doi: 10.1093/geronj/45.6.m192. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, de Beaurepaire R. Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev. 2005;29:891–909. doi: 10.1016/j.neubiorev.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. John Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]
- Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–1014. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Archives of General Psychiatry. 2006;63:375–82. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- Futterman CS, Tryon WW. Psychomotor retardation found in depressed outpatient women. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:41–8. doi: 10.1016/0005-7916(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol. 1991;260(pt 1):E727–E730. doi: 10.1152/ajpendo.1991.260.5.E727. [DOI] [PubMed] [Google Scholar]
- Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205:182–185. doi: 10.3181/00379727-205-43695. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. Journal of Applied Physiology. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- Habte-Gabr E, Wallace RB, Colsher PL, Hulbert JR, White LR, Smith IM. Sleep patterns in rural elders: demographic, health, and psychobehavioral correlates. Journal of Clinical Epidemiology. 1991;44:5–13. doi: 10.1016/0895-4356(91)90195-f. [DOI] [PubMed] [Google Scholar]
- Holford TR. Analysing the temporal effects of age, period and cohort. Stat Methods Med Res. 1992;1:317–37. doi: 10.1177/096228029200100306. [DOI] [PubMed] [Google Scholar]
- Hunkeler EM, Katon W, Tang L, Williams JW, Jr., Kroenke K, Lin EH, et al. Long term outcomes from the IMPACT randomised trial for depressed elderly patients in primary care. British Medical Journal. 2006;332:259–63. doi: 10.1136/bmj.38683.710255.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs DR, Jr., Hannan PJ, Wallace D, Liu K, Williams OD, Lewis CE. Interpreting age, period and cohort effects in plasma lipids and serum insulin using repeated measures regression analysis: the CARDIA Study. Stat Med. 1999;18:655–79. doi: 10.1002/(sici)1097-0258(19990330)18:6<655::aid-sim62>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Jette AM. Physical disablement concepts for physical therapy research and practice. Physical Therapy. 1994;74:380–6. doi: 10.1093/ptj/74.5.380. [DOI] [PubMed] [Google Scholar]
- Jones-Webb RJ, Snowden LR. Symptoms of depression among blacks and whites. American Journal of Public Health. 1993;83:240–44. doi: 10.2105/ajph.83.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempen GI, Steverink N, Ormel J, Deeg DJ. The assessment of ADL among frail elderly in an interview survey: self-report versus performance-based tests and determinants of discrepancies. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 1996;51:P254–60. doi: 10.1093/geronb/51b.5.p254. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Lean ME. Pathophysiology of obesity. Proc Nutr Soc. 2000;59:331–6. doi: 10.1017/s0029665100000379. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Rogers JC, Martire LM, Mulsant BH, Rollman BL, Dew MA, et al. The association of late-life depression and anxiety with physical disability: a review of the literature and prospectus for future research. The American Journal of Geriatric Psychiatry. 2001;9:113–35. [PubMed] [Google Scholar]
- Lenze EJ, Schulz R, Martire LM, Zdaniuk B, Glass T, Kop WJ, et al. The course of functional decline in older people with persistently elevated depressive symptoms: longitudinal findings from the Cardiovascular Health Study. Journal of the American Geriatrics Society. 2005;53:569–75. doi: 10.1111/j.1532-5415.2005.53202.x. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. Journal of Affective Disorders. 1993;28:7–14. doi: 10.1016/0165-0327(93)90072-r. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. Am J Cardiol. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Nagi S. Disability concepts revisited: implication for prevention. In: Pope A, Tarlov A, editors. Disability in America: Toward a National Agenda for Prevention. National Academy Press; Washington, DC: 1991. pp. 309–27. [Google Scholar]
- Newman AB, Enright PL, Manolio TA, Haponik EF, Wahl PW. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. Journal of the American Geriatrics Society. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Ormel J, Rijsdijk FV, Sullivan M, van Sonderen E, Kempen GI. Temporal and reciprocal relationship between IADL/ADL disability and depressive symptoms in late life. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2002;57:P338–47. doi: 10.1093/geronb/57.4.p338. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Deeg DJ, van Eijk JT, Beekman AT, Guralnik JM. Changes in depression and physical decline in older adults: a longitudinal perspective. Journal of Affective Disorders. 2000;61:1–12. doi: 10.1016/s0165-0327(00)00152-x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Nicklas BJ, Newman AB, Harris TB, Goodpaster BH, Satterfield S, et al. Metabolic syndrome and physical decline in older persons: results from the Health, Aging And Body Composition Study. J Gerontol A Biol Sci Med Sci. 2009;64:96–102. doi: 10.1093/gerona/gln005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier MP, Hulstijn W, Sabbe BG. Psychomotor retardation in elderly depressed patients. Journal of Affective Disorders. 2004;81:73–7. doi: 10.1016/j.jad.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Pope SK, Sowers MF, Welch GW, Albrecht G. Functional limitations in women at midlife: the role of health conditions, behavioral and environmental factors. Womens Health Issues. 2001;11:494–502. doi: 10.1016/s1049-3867(01)00089-5. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Sachdev P, Aniss AM. Slowness of movement in melancholic depression. Biological Psychiatry. 1994;35:253–62. doi: 10.1016/0006-3223(94)91256-4. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Charpentier PA, Berkman LF, Tinetti ME, Guralnik JM, Albert M, Blazer D, Rowe JW. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol. 1994;49:M97–108. doi: 10.1093/geronj/49.3.m97. [DOI] [PubMed] [Google Scholar]
- Sherman SE, Reuben D. Measures of functional status in community-dwelling elders. Journal of General Internal Medicine. 1998;13:817–23. doi: 10.1046/j.1525-1497.1998.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers M, Pope S, Welch G, Sternfeld B, Albrecht G. The association of menopause and physical functioning in women at midlife. Journal of the American Geriatrics Society. 2001;49:1485–92. doi: 10.1046/j.1532-5415.2001.4911241.x. [DOI] [PubMed] [Google Scholar]
- Sowers MF, Crawford S, Sternfeld B, Morgenstein D, Gold E, Greendale G, et al. Design, survey sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. Academic Press; New York: 2000. pp. 175–88. [Google Scholar]
- Stubbs D, Krebs E, Bair M, Damush T, Wu J, Sutherland J, et al. Sex Differences in Pain and Pain-Related Disability among Primary Care Patients with Chronic Musculoskeletal Pain. Pain Med. doi: 10.1111/j.1526-4637.2009.00760.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–715. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- Terwee CB, Mokkink LB, Steultjens MP, Dekker J. Performance-based methods for measuring the physical function of patients with osteoarthritis of the hip or knee: a systematic review of measurement properties. Rheumatology (Oxford) 2006;45:890–902. doi: 10.1093/rheumatology/kei267. [DOI] [PubMed] [Google Scholar]
- Tomey K, Sowers MR, Crandall C, Johnston J, Jannausch M, Yosef M. Dietary intake related to prevalent functional limitations in midlife women. Am J Epidemiol. 2008;167:935–43. doi: 10.1093/aje/kwm397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomey K, Sowers M, Zheng H, Jackson EA. Physical functioning related to C-reactive protein and fibrinogen levels in mid-life women. Exp Gerontol. 2009;44:799–804. doi: 10.1016/j.exger.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. Springer-Verlag; New York: 2000. [Google Scholar]
- Verbrugge LM, Jette AM. The disablement process. Social Science & Medicine. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Kritchevsky SB, Beekman AT, Newman AB, Satterfield S, Simonsick EM, et al. Depressive symptoms and change in abdominal obesity in older persons. Arch Gen Psychiatry. 2008;65:1386–93. doi: 10.1001/archpsyc.65.12.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE., Jr. The status of health assessment 1994. Annual Review of Public Health. 1995;16:327–54. doi: 10.1146/annurev.pu.16.050195.001551. [DOI] [PubMed] [Google Scholar]
- Weigl M, Cieza A, Cantista P, Reinhardt JD, Stucki G. Determinants of disability in chronic musculoskeletal health conditions: a literature review. European Journal of Physical and Rehabilitation Medicine. 2008;44:67–79. [PubMed] [Google Scholar]
- Woods NF, Mitchell ES. Pathways to depressed mood for midlife women: observations from the Seattle Midlife Women's Health Study. Research in Nursing & Health. 1997;20:119–29. doi: 10.1002/(sici)1098-240x(199704)20:2<119::aid-nur4>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]