Abstract
The mammalian inner ear forms from a thickened patch of head ectoderm called the otic placode. The placodal ectoderm invaginates to form a cup whose edges cinch together to establish a fluid-filled sac called the otic vesicle or otocyst. The progenitor cells lining the otocyst lumen will give rise to sensory and non-sensory cells of the inner ear. These formative stages of inner ear development are initiated during the first week of postimplantation embryonic development in the mouse. The inaccessibility of the inner ear in utero has hampered efforts to gain insight into the molecular mechanisms regulating essential developmental processes. An experimental embryological method to misexpress genes in the developing mammalian inner ear is presented. Expression plasmid encoding a gene of interest is microinjected through the uterine wall into the lumen of the otocyst and electroporated into otic epithelial progenitor cells. Downstream analysis of the transfected embryonic or postnatal inner ear is then conducted to gain insight into gene function.
Keywords: inner ear, transuterine microinjection, beveled microinjection pipette, in vivo electroporation, square wave pulse train, mouse experimental embryology, mouse survival surgery, in utero gene transfer
1. Introduction
The first developmental biologists were experimental embryologists who physically manipulated embryos to describe gross mechanisms of development (1). Dye injection, carbon particle transfer, extirpation, and tissue transplantation were the methods of choice (2). Indeed, our first insights into neural plate induction (3), optic nerve regeneration (4), vertebrate limb formation (5), and neural crest migration (6,7) were gleaned through experimental embryological studies in lower vertebrates. Modern molecular embryologists who use the mouse as a model system are constrained by the inaccessibility of the embryo in vivo. In the case of the embryonic inner ear, accessibility is further complicated because the otic epithelium that will give rise to the vestibular and auditory sensory structures develops within a membranous labyrinth cloistered in the head mesenchyme (8). The elucidation of experimental embryological techniques that permit manipulation of gene expression in the developing mouse inner ear may advance our mechanistic understanding of how the inner ear forms.
A solution to the challenge of gene transfer to the mammalian inner ear in utero is conceptually straightforward: gain access to the embryo; atraumatically introduce a reagent capable of transducing a bioactive signal in otic epithelial progenitor cells; and maintain a healthy pregnancy postoperatively. An experimental embryological approach to misexpress a gene in the developing mammalian inner ear is presented in three procedural stages: 1) sodium pentobarbital-based anesthesia for mouse survival surgery; 2) ventral laparotomy to expose the uterine horns; and 3) transuterine microinjection and electroporation of expression plasmid to transfect otic epithelial progenitors.
2. Materials
2.1 General Anesthesia for Mouse Ventral Laparotomy
50 mg/mL Nembutal (pentobarbital sodium solution, United States Pharmacopeia (USP); this is a controlled substance requiring a prescription or license to obtain).
Magnesium sulfate heptahydrate (MgSO4) dissolved in water at 65 mg/mL. Sterile filter and store in 1mL aliquots at −20°C (see Note 1).
Propylene glycol, USP. Sterile filter and store in 1 mL aliquots at room temperature.
Ethanol, absolute, 200 proof for molecular biology (Sigma-Aldrich, St. Louis, MO). Aliquot in 1.5 mL sterile tubes and store at room temperature.
Becton Dickinson (BD) Allergy Syringe Tray 0.5 mL, 27G, 3/8” Testing (cat. no. 305536, Becton Dickinson, Franklin Lakes, NJ).
Sterile ophthalmic ointment (non-prescription item available from any drug store).
T/Pump (Gaymar Industries, Inc, Orchard Park, NY) connected to a Hallowell EMC (Pittsfield, MA) Heated Hard Pad.
Oster (Shelton, CT) Grooming Shears with a #40 (fine) blade.
2.2 Ventral Laparotomy
Surgical instruments (Fine Science Tools, Foster City, CA): needle driver (cat. no. 12502-12), ball-tipped scissors (cat. no. 14109-09), ring forceps (cat. no. 11106-09) (see Note 2).
Suture: 6-0 (0.7 metric) Polysorb braided lactomer 9-1, 30″ (75 cm) violet, CV-11, taper (cat. no. GL-889, Syneture, United States Surgical, Norwalk, CT).
Sterile disposable supplies: surgical drape, cotton balls, and cotton tipped applicators.
Disinfection solutions: 70% ethanol and 10% povidone iodine (10% Betadine®, Purdue Pharma, L. P., Stamford, CT) solution.
Lactated Ringer’s, Injection USP (Baxter 2B2323, Deerfield, IL). This is a sterile, 500 mL intravenous (IV) bag of lactated Ringer’s solution whose precise electrolyte composition is described under the USA National Drug Code 0338-0117.
Non-di-ethylhexyl phthalate (DEHP) IV Fat Emulsion Administration Set (cat. no. 2C1145, Baxter). This is a sterile IV tube set used to aseptically dispense lactated Ringer’s solution from the IV bag.
Dedicated mouse survival surgical area (see Note 3)
2.3 Transuterine Microinjection and In Vivo Electroporation
Leica stereofluorescence dissecting microscope (MZ10F with PLAN 0.8X long working distance objective, 10X eyepieces, and GFP2 filter set).
Borosilicate glass capillaries with filament (cat. no. GC150F-10, Harvard Apparatus, Holliston, MA).
CUY21 Electroporator with foot pedal external trigger (Protech International Inc., San Antonio, TX).
Tweezers-style electrode with 5 mm diameter platinum disks (cat. no. CUY650P5, Protech International Inc.).
Micropipette puller: Flaming/Brown Model P-97 (Sutter Instrument Co., Novato, CA).
Micropipette beveler: K.T. Brown Type with 104C abrasive plate (Sutter Instrument Co.).
PicoSpritzer III (Parker Hannifin Corporation, General Valve Operation, Fairfield, NJ) with 60 psi regulator and foot pedal external trigger. Source gas is compressed nitrogen (>99% purity).
M33 roller bearing micromanipulator (Stoelting Co., Wood Dale, IL).
Articulated arm on a magnetic base (Stoelting Co.).
Accublock digital dry bath (LabNet International, Woodbridge, NJ) with aluminum block to hold five sterile, 50 mL conical centrifuge tubes.
Phosphate buffered saline: 137 mM NaCl, 2.7 mM KCl, 9.9 mM Na2HPO4, 2 mM KH2PO4, pH 7.2.
Fast green, crystalline (Sigma-Aldrich).
Timed-pregnant mouse (dam) whose embryos are at embryonic day 11.5 (E11.5). Noontime on the day a vaginal plug is detected is considered E0.5 (i.e., E11.5 is 11 days after the day the plug is detected).
3. Methods
3.1 General Anesthesia for Mouse Ventral Laparotomy
Assemble a fresh working solution of the anesthetic mixture each day from stock solutions: Mix 320 μL of the 65 mg/mL magnesium sulfate stock, with 100 μL of absolute ethanol, 400 μL of propylene glycol, and 180 μL of 50 mg/mL pentobarbital sodium solution. Vortex the solution and briefly spin down.
Weigh the dam to the nearest 0.1 g.
Administer 7.2 μL of anesthetic mixture per gram body weight by intraperitoneal injection with the BD allergy syringe. Example: A 25.0 g mouse receives 7.2 μL of the anesthetic mixture per gram body weight for a total of 0.18 mL. (see Note 4).
Place the injected mouse in a warmed cage for 4-6 min.
Assess responses to tail/toe pinches and the intactness of the ocular reflex. Proceed only after the mouse is unresponsive to these noxious stimuli and the reflex is absent.
Apply a thin layer of sterile ophthalmic ointment over the corneas of each eye.
Proceed with ventral laparotomy.
3.2 Ventral Laparotomy on the Anesthetized Dam
Shave the fur from the suprapubic region to just beneath the rib cage with fine (#40 blade) shears (see Note 5).
- Disinfect the shaved skin by alternating ethanol and povidone iodine:
- 70% ethanol: gentle swipes from rostral to caudal with ethanol-dampened surgical cotton ball (see Note 6).
- 10% povidone iodine: gentle swipes from rostral to caudal with an iodine-saturated cotton tipped applicator.
- 70% ethanol: repeat as indicated above.
- Place the mouse in a supine position on a sterile drape and allow the skin to air dry.
Make a 2-3 mm ventral midline cut through the skin only with scissors.
Validate the location of the linea alba, an avascular connective tissue band extending from the xiphoid process past the navel. The small skin incision provides a viewing portal to search for the linea alba. Gently slide the incised skin from side to side to identify the precise location of the linea alba if it is not visible beneath the initial incision.
Extend the initial midline incision by cutting the skin that overlies the linea alba. This incision may involve a slight course correction to align it with the underlying linea alba. The total length of the skin incision is 12-15 mm.
Gently grasp the connective tissue overlying the abdominal wall with blunt forceps and lift it away from the abdominal organs. This maneuver will pull the abdominal wall away from the underlying abdominal viscera.
Make a ventral midline scissor nick through the abdomen on the linea alba at the level of the navel. Since the linea alba is avascular, there should be no bleeding post-incision (see Note 7).
Insert the ball-tipped scissor through the abdominal slit and incise the linea alba rostrally first and then caudally. The total abdominal incision length is 10-14 mm. The ball tip prevents accidental nicking of any abdominal visera. If the bowel is nicked, euthanize the dam. Do not attempt a surgical repair.
Immediately irrigate the abdomen with sterile, 37°C lactated Ringer’s solution. Ringer’s solution is kept at a set temperature in a sterile, 50 mL conical centrifuge tube resting in the tapered, aluminum heating block. Dispense Ringer’s solution with a sterile Pasteur pipette (see Note 8).
Gently displace the intestines and inguinal fat that obstruct access to the right uterine horn with blunt forceps.
Gently lift the right horn of the uterus through the abdominal incision with ring forceps and rest it on the abdomen.
Validate that the entire uterine horn is externalized by identification of the ovary/oviduct rostrally and the cervical region of the uterus caudally. The oviduct appears as a coiled, muscular tube and the ovary looks like a small granular white cluster of tissue embedded in fat. The cervical region of the uterus is the pronounced, Y-shaped bifurcation point of the two horns.
Perform the transuterine microinjection and in vivo electroporation procedures.
3.3 Transuterine Microinjection and In Vivo Electroporation
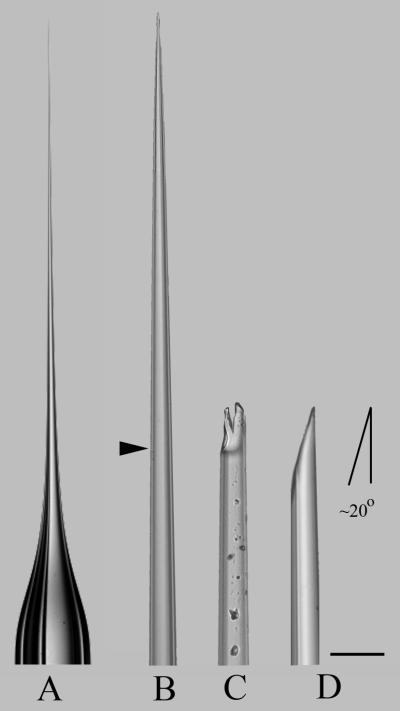
1. Load a 1.5 mm outer diameter by 0.86 mm inner diameter borosilicate glass capillary pipette into the Sutter P-97 micropipette puller outfitted with a 3 mm box filament that is 3 mm wide. Conduct the ramp test as indicated in the P-97 instruction manual. Program the instrument as follows: Pressure = 200; Heat = ramp value plus 3 units; Pull = 0; Velocity = 46; and Time = 110. The capillary pipette produced is then manually broken with forceps to approximately 14 μm outer diameter and beveled at 20 degrees on the BV-10 micropipette beveler fitted with the Sutter 104C (gold) abrasive plate (Fig. 1) (see Note 9).
2. Backfill the beveled capillary pipette with 3 μg/μL of expression plasmid in sterile phosphate buffered saline (see Note 10).
3. Attach the pipette to its holder and secure the shaft firmly to avoid ejection of the pipette from the holder during a pressure injection cycle (see Note 11).
4. Set the Picospritzer to 20 ms injection duration and 15 psi injection pressure. Test the ejection of plasmid by injecting into a drop of sterile lactated Ringer’s solution. Confirm that all air bubbles are purged from the plasmid solution within the pipette (see Note 12).
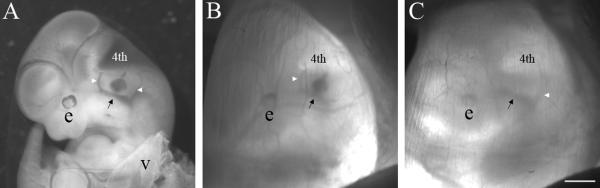
5. Transillumination of the uterus enables visual identification of the gross features of the E11.5 mouse embryo. An implantation site housing the embryo is indicated by a bulge in the uterus. Gently grasp the uterine horn with latex-gloved thumb and forefinger and press the output end of the fiber optic light guide against the freshly irrigated uterine wall. Center the light on a uterine bulge that houses an embryo. Gently knead the uterus between the thumb and forefinger to reposition the embryo, so that the left side of the embryo is parallel to the surface of the dissecting scope objective (the embryo is oriented as though it is lying on its right side; see Fig. 2B). Search for the hindbrain which looks like an angular white notch in the caudal most region of the cephalic neural tube. Ventral to the hindbrain and dorsal to the branchial arches, identify a blood vessel pattern that looks like the uprights of an American football goalpost: a thick base with rostral and caudal branches that are finer (Fig. 2). The otocyst is superficially set within the periotic mesenchyme midway between the uprights, though it is not possible to see the fluid-filled otocyst directly. The location of the otocyst is, therefore, interpolated using the flanking vasculature as an instructive anatomical landmark.
6. Advance the beveled microinjection pipette through the uterine wall along a trajectory that will place the tip of the pipette in the head mesenchyme between the uprights. Pulse the Picospritzer to eject some fast green-tinged plasmid solution as an aid to define pipette tip position. If the pipette tip is located too lateral with respect to the otocyst, dye will collect in either the exocoelomic or amniotic cavities. If the pipette is advanced through the otocyst, dye will collect in the medial periotic mesenchyme or neural tube. When the otocyst is properly targeted, the vestibule and endolymphatic duct fill with dye and the borders of the otocyst become clearly outlined (Fig. 2A,B). The number of injection pulses required to fill the otocyst is typically 10-15 with an 18-24 μm outer diameter micropipette at 15 psi injection pressure and 20 ms injection duration per pulse. Release finger pressure on the uterus and then remove the pipette in one quick reverse stroke of the micromanipulator (see Note 13).
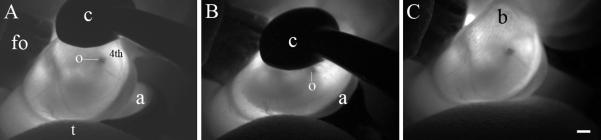
7. Immediately irrigate the uterus and the tweezer-style electrode paddles with pre-warmed lactated Ringer’s solution. Position the negative electrode on the surface of the uterus that is adjacent to the lateral wall of the injected, left otocyst (Fig. 3A). Position the positive electrode on the surface of the uterus that is adjacent to the lateral wall of the right, uninjected otocyst (Fig. 3B). Gently compress the uterus to securely hold and center the otocyst between the electrode paddles (Fig. 2B) and trigger the electroporator by actuating the foot pedal. When the 5-pulse train is completed, release the tweezer-style electrode grip and immediately irrigate the uterus with pre-warmed, lactated Ringer’s solution (see Note 14).
8. Inject and electroporate 2-3 embryos per uterine horn for a total of 4-6 injected embryos per dam. Document which embryos were injected (right horn, embryo 1 is the embryo closest to the right ovary); the quality of the otocyst injections (a weak fill fails to demonstrate the endolymphatic duct and a strong fill does); and the current transferred to each embryo during the electroporation (see Note 15).
9. Liberally irrigate the uterus and any other externalized abdominal viscera and return them to their native positions within the abdominal cavity. Irrigate the abdominal cavity with 4-8 mL of lactated Ringer’s solution, allowing the excess to spill out of the incision site. Approximately 2 mL of lactated Ringer’s solution should remain in the abdominal cavity to facilitate rehydration (see Note 16).
10. Carefully place the dam on a fresh, dry sterile drape and begin the two stage closure with 6-0 resorbable suture. A running stitch consisting of alternating a conventional throw and a locking stitch is ideal for both the abdominal wall and the skin closures.
11. Remove excess moisture from the dam’s fur by gently blotting with sterile, absorbent paper towel. Clear any bedding away from the bottom of the heated recovery cage, tuck the dam into a folded, sterile paper towel, and set her down on the floor to maximize heat transfer. The dam’s eyes should be fully shielded from direct light (see Note 17).
13. Conduct postoperative monitoring of the dam every 30 min until conscious by assessing the surgical site for bleeding or exudate; the quality of respiration (unlabored breathing); and the time of ambulation. The dam should take initial, unsteady steps within 1.5-2 hours and begin grooming to remove the ophthalmic ointment from her eyes. Record these observations in the mouse survival surgery log book. Construct a calendar to record the dam’s identification number, the bioactive reagent electroporated, and the date of embryo or pup harvest.
14. Re-evaluate the dam the morning after surgery and assess the quality of respirations, the surgical site, and evidence of eating (moist feces) and drinking (pick the dam up by the tail and look for stress-induced urination). Return the dam to the main mouse colony in the same cage (see Note 18).
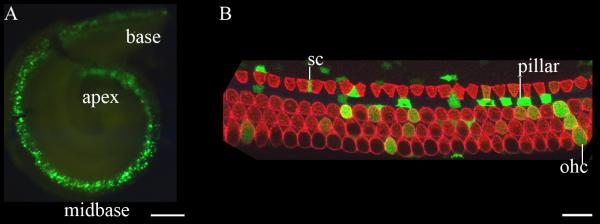
15. Harvest electroporated inner ears at a relevant downstream embryonic or postnatal time point and analyze the phenotype. Figure 4 presents a representative analysis of an E17.5 inner ear that was transfected at E11.5 with a construct encoding enhanced green fluorescent protein.
Fig. 1.
Fabrication of a transuterine microinjection pipette. A pipette was pulled with the P-97 micropipette puller using the Pressure:Heat:Pull:Velocity:Time settings are indicated in Subheading 3.3, step 1. (A) The outer diameter of the unpulled shaft is 1.5 mm and the length of the tapered part is ~12 mm. The tip of this pipette was imaged in 3 successive stages of preparation (B-D). (B) The approximate location of the manual break point at the tip of the pipette is indicated by the arrowhead. The pipette tip was broken by pinching the glass with biologie #5 forceps (Fine Science Tools). (C) The broken pipette presents a bifurcated, coarse tip with particulate debris on the outside of the glass. (D) The pipette tip was beveled with the BV-10 Beveler to replace the jagged leading edge and establish a 20 degree tip. The final outer diameter of the injection pipette is ~20 μm (target range is 18-24 μm). Scale bar = 50 μm (applies to B-D).
Fig. 2.
Transuterine microinjection into the embryonic day 11.5 mouse otocyst (A) An aqueous solution (0.1%) of fast green dye was microinjected into the left otocyst through the uterine wall at E11.5. The embryo was dissected free of the uterus and the extraembryonic membranes and its left side was imaged. The otocyst is located superficially in the lateral head mesenchyme, dorsal and slightly medial to the main trunk of the primary head vein (black arrow) and approximately midway between its rostral and caudal branches (white arrowheads). The trunk of the primary head vein forms the base of an American football goalpost and its rostral and caudal branches form the uprights. The otocyst displays a clamshell-shaped vestibule and tapered dorsal endolymphatic duct. Abbreviations: e, left eye; 4th, nascent 4th ventricle; v, visceral yolk sac. (B) The same embryo imaged in panel A immediately after transuterine microinjection of dye into the left otocyst. The uterus and extraembryonic membranes are intact. The 4th ventricle, eye, and rostral branch (white arrowhead) of the primary head vein (black arrow) are visible. The caudal branch of the primary head vein is not apparent. (C) The left side of the E11.5 mouse embryo prior to transuterine microinjection into the otocyst. The transillumination scheme in this image highlights the caudal branch (white arrowhead) of the primary head vein (black arrow), but the rostral branch is not clearly visible. Since the otocyst cannot be visually identified at E11.5, its location is inferred by interpretation of the anatomical landmarks provided by the primary head vein vasculature. Scale bar = 1 mm and applies to all panels.
Fig. 3.
In vivo electroporation of the embryonic day 11.5 mouse otocyst. (A) The uterus was transilluminated with light from a fiber optic cable whose output end (fo) was directly in contact with the irrigated uterus. The left otocyst (o) of the E11.5 mouse embryo was injected with fast green solution and its gross morphology is discernable beneath the nascent 4th ventricle (4th) in the caudal hindbrain. The insulated surface of the cathode (c) and the reflective, platinum surface of the anode (a) were grossly positioned around the uterus to flank the embryo. (B) Gentle compression of the uterus with the tweezer-style electrodes forced a counter-clockwise rotation of the embryo, placing the injected otocyst toward the center of the 5 mm cathode-anode field. The goal is to drive negatively charged DNA into ventral progenitors within the otic vesicle. (C) Bubbles (b) on the surface of the uterus after execution of the 5-pulse train appear as graininess in the image. Light pressure from the thumb (t) reorients the embryo within the uterus to facilitate placement of the electrodes (panel A). Both the fiber optic light head and the thumb are removed from contact with the uterus prior to triggering the square wave pulse train. Scale bar = 1 mm and applies to all panels.
Fig. 4.
Electroporation-mediated transfer of an expression plasmid encoding green fluorescent protein transfects progenitors that give rise to the organ of Corti. An expression plasmid encoding green fluorescent protein (GFP) driven by the human elongation factor 1-α promoter (EF1-α) was electroporated into the E11.5 mouse otocyst. The inner ear was harvested six days later at E17.5 and fixed in 4% paraformaldehyde in PBS for 12 hours. The cartilaginous otic capsule and the cochlear lateral wall were removed and the whole mount preparation was imaged in panel A. GFP expression is detectable in the base, midbase, and proximal apex of the cochlea. (B) Laser confocal microscopy of a representative 100 μm section of the EF1-α /GFP-transfected cochlea immuostained with an antibody against myosin VIIa (red) to identify the single row of inner hair cells and three rows of outer hair cells. Several supporting cells (sc), pillar cells (pillar), and outer hair cells (ohc) express GFP. These data indicate that progenitors giving rise to supporting cells and hair cells of the organ of Corti were transfected and expression of the transgene was maintained in differentiated cell types of the maturing cochlea. Scale bar in A = 100 μm and in B = 10 μm.
4. Notes
All stock solutions are prepared in sterile, pyrogen-free water with a resistivity of 18.2 MΩ-cm unless otherwise indicated. The concentration of magnesium sulfate administered does not induce paralysis but merely relaxes uterine tone. This enables the embryos to be repositioned for microinjection by gentle uterine palpation with the thumb and index finger.
Instruments suitable for mouse ventral laparotomy should be of surgical grade stainless steel since the rigors of repeated sterilization will dull cutting surfaces and corrode hinge points of lower quality scissors and needle drivers. Surgical grade instruments are expensive, though the veterinary staff of your home institution may be able to purchase these items at reduced cost.
Animal care and use guidelines of the home institution should be consulted regarding mouse survival surgery requirements. The use of a horizontal laminar flow hood is encouraged to prevent exposure of the dam to potentially infectious agents. Scrupulous attention to asepsis in the surgical arena is essential. A dedicated mouse survival surgery suite or station is highly recommended.
Intraperitoneal injection in a robustly pregnant mouse requires practice, since it is fairly easy to misplace the needle in abdominal viscera that are displaced by enlarged uterine horns. The Becton Dickinson allergy syringe has an intradermal bevel designed for presentation of antigen beneath the human skin. This syringe is ideal for intraperitoneal injections of pregnant mice because the needle is short; the bevel pierces the skin atraumatically and virtually all of the anesthetic mixture is transferred to the abdominal cavity (i.e., the syringe has an insignificant volume of dead space). The dam should lose consciousness within 3-5 min after a successful intraperitoneal injection of the sodium pentobarbital anesthesia mixture. The intraperitoneal injection was unsuccessful if the dam remains ambulatory after 5-7 min. If this occurs, do not redose the dam and try again with another dam. Redosing with sodium pentobarbital correlates with reduced embryonic survival.
Complete fur removal facilitates disinfection and promotes the rapid healing of the skin incision. At least 30% of individuals who work with mice will eventually develop an allergy to them. Shave the fur in a chemical fume hood to keep the free-fur and dander contained.
Surgical cotton balls are less likely to shed fibers and contaminate the surgical field compared with cosmetic cotton balls. Store the cotton balls in a closed container of 70% ethanol. Aggressively squeeze the residual ethanol out of the cotton ball before contact with the abdominal integument. A thin film of ethanol should coat the skin to achieve disinfection. Care should be exercised to avoid excessive superficial ethanol exposure, because excessive evaporative cooling can induce physiological stress.
The presence of active blood flow from the incised abdominal wall indicates that the incision extended from the linea alba to the adjacent musculature. Identify the precise location of the bleeding by stereomicroscopic inspection and apply direct pressure with forceps for 1-2 min to stop the hemorrhage. Do not proceed with the laparotomy until all bleeding vessels are managed. The observation of vascular compromise is fortunately rare during linea alba incision and when it is encountered direct pressure stops the bleeding.
The compromise of the abdominal cavity necessitates aggressive irrigation of the abdominal organs. The small and large intestines, and the inguinal fat pads in particular, will suffer from desiccation that will affect the progression of the pregnancy. Irrigation with sterile, pre-warmed (37°C) lactated Ringer’s solution is therefore critical. The heat block temperature is set a few degrees Celsius above 37°C to account for cooling of the lactated Ringer’s solution during pipette transfer from the culture tube to the abdomen.
Carefully fabricated micropipettes are critical to the success of the transuterine microinjection procedure. An appropriately crafted pipette enables accurate, atraumatic injection into the otocyst. The recommended glass is thick-walled with a filament to provide durability during targeting and to facilitate back-filling with plasmid, respectively. Pipettes should be broken under a suitable microscope with the aid of a reticule that enables estimation of outer diameter after the break. Beveling is normally conducted in an aqueous environment with a surfactant added. For in vivo applications, bevel the pipettes in water alone to avoid the possibility of introducing chemical contaminants to the embryo in utero. However, this method will most certainly reduce the lifespan of the 104C abrasive plate, an acceptable tradeoff for enhanced embryonic survival. The P-97 Pipette Cookbook (2006, revision C, Sutter Instrument Co.) is an excellent discourse on the theory underlying micropipette fabrication. Refer to the Pipette Cookbook for a comprehensive discussion of all issues related to microinjection pipette fabrication with the P-97 puller and beveling with the BV-10 beveler.
Expression plasmids are isolated and purified with the Qiagen HiSpeed Plasmid Maxi Kit (cat. no. 12662, Qiagen, Valencia, CA) according to manufacturer’s instructions. The plasmid is sterile filtered prior to standard ethanol precipitation. The plasmid is resuspended in sterile phosphate buffered saline, diluted to 3 μg/μL final concentration, and stored at −20°C in 8 μL aliquots. Prior to use, crystalline fast green is added to the thawed plasmid solution, which is then triturated 50 times, spun at 10,000 g for 15 s, and backfilled into the beveled microinjection pipette.
The Picospritzer contains high performance pneumatics that reliably deliver pressurized nitrogen to the injection pipette. The pipette is secured to the pipette holder with a screw-type fitting that compresses a gasket in contact with the pipette shaft. Wear of the pipette holder gasket can result in a weak seal that permits gas leakage and inefficient ejection of plasmid DNA. Replace the gasket prophylactically every 3 months or sooner if performance issues arise. Moreover, an inexperienced operator may be unfamiliar with the requisite torque needed to secure the pipette firmly in the holder. In this case, the pipette can be ejected from the holder with considerable force. As a general precaution, the operator’s fingers and hands should never be placed in the putative trajectory path of the pipette.
Pull micropipettes in advance and store them unbroken in a plastic, covered Petri dish by lengthwise insertion into a thin band of modeling clay. Break and bevel 3 pipettes prior to administering anesthesia to the dam. Pipettes broken and beveled the day before may become clogged as the residual water within the pipette evaporates and deposits glass fines inside the pipette lumen.
Transuterine microinjection into the E11.5 mouse otocyst is challenging at several levels. Identification of gross embryonic anatomy by transillumination requires practice. Start with finding the beating heart, the pigmented epithelium of the eye, the limb buds, and brain vesicles. Then find the hindbrain notch and the goalpost vasculature. Experiment with various lighting intensities and orientations since individuals vary tremendously in what “looks good” to them. It is certainly best to use the minimum intensity of illumination necessary. Once the target area cloistering the otocyst is identified, microinjection should be conducted with an “outside-in” progression. Advance through the uterus and pulse to view the tracer dye as an indication of approximate pipette tip position. Advance under micrometer control and pulse again to track pipette position, working from lateral to medial or outside-in with respect to the embryo. It may be possible to see a slight “toggle” when the pipette tip contacts the lateral side of the embryo’s head. Typically, 3-5 pulses executed in rapid succession are required to fill the otocyst with enough dye to discern that indeed the pipette is properly positioned. Thereafter, fill the otocyst fully so that the endolymphatic duct and the vestibule proper swell with injected DNA solution. Freshly irrigate the uterus and abdominal viscera immediately before and after the targeting/microinjection procedures, to ensure that these tissues do not desiccate. Replace the sterile drape that is beneath the mouse when it becomes saturated with lactated Ringer’s solution.
The 5 mm electrode paddles must be coupled to the uterus with lactated Ringer’s solution, an efficient charge carrier. Poor hydration of the uterus will reduce current delivered to the embryo and focally heat the uterine vasculature. Bubbles will be generated on the surface of the hydrated uterus during the electroporation cycle and their presence is a dynamic indication of current flow (Fig. 3C). Compressing the uterus with too much force will rupture the amnion and/or the visceral yolk sac resulting in embryonic lethality. The CUY21 will report the current transferred during the last pulse of the 5-pulse train. Typically, current transfer of 50-100 mA per pulse is sufficient to transfect the otic epithelium.
Develop a mouse survival surgery log book with the guidance of your institutional animal care and use committee and veterinary staff. This resource serves as a permanent record of preoperative, operative, and post-operative animal care and should be included in your animal care and use protocol. Subtle observations during targeting, injection, and electroporation are recorded for each embryo manipulated, and these data are correlated with transfection efficiency after tissue harvest. Follow postoperative recovery for at least 24 hours. Ventral laparotomy and transuterine microinjection are extremely well tolerated. Minor, temporary vaginal bleeding can occur and likely results from focal compromise of embryonic or uterine vasculature during an injection. When present, active blood flow ceases while the dam is still under anesthesia in her recovery cage. Euthanize the dam if blood flow does not cease during this time. Perform a necropsy to determine the source of the bleeding and try to correlate the outcome with a causal procedural step. To avoid microinjection-induced bleeding, concentrate on fabrication of beveled pipettes of the appropriate diameter and geometry.
Mice experiencing ventral laparotomy will tend to drink and eat less immediately after surgery. Filling the abdominal cavity with lactated Ringer’s solution prior to suturing is, therefore, vitally important to ensure normal fluid homeostasis. Post-operative supplemental fluid administration is not necessary, following ventral laparotomy, provided that sufficient care is taken to load the abdominal cavity with Ringer’s solution prior to closing. Food placed on the bottom of the cage facilitates access and obviates the need for stretching to reach food in an overhead hopper. Dams recovering from ventral laparotomy benefit from the community effect provided by co-housing. Up to 4 post-surgical dams may be co-housed through the 24 hour post-operative recovery period. House a post-surgical dam alone rather than pairing her with a mouse that has not undergone surgery.
It is essential to provide 10-12 hours of accessory heating for the dam immediately after surgery to reduce physiological stress and promote healing. The entire recovery cage is efficiently warmed with a Gaymar T/Pump that recirculates water through a Hallowell hard pad. Use a funnel to replace evaporated water in the T/Pump fluid reservoir, since water spilled during refill will track directly to sensitive electrical components in the pump and cause a short circuit that is expensive to repair.
Housing mice overnight in any location other than the animal facility will require justification in the animal care protocol.
6. Acknowledgments
We thank Tomomi Fukuchi-Shimogori and Elizabeth Grove for demonstrating transuterine microinjection techniques and Donna M. Fekete for inspired postdoctoral mentorship (to JB). Funding from the National Institute on Deafness and Other Communication Disorders and the McKnight Fund for Neuroscience is gratefully appreciated.
5. References
- 1.Schoenwolf GC. Cutting, pasting and painting: experimental embryology and neural development. Nat. Rev. Neurosci. 2001;2:763–771. doi: 10.1038/35097549. [DOI] [PubMed] [Google Scholar]
- 2.Hamburger V. The rise of experimental neuroembryology: A personal reassessment. Int. J. Devl. Neurosci. 1990;8:121–131. doi: 10.1016/0736-5748(90)90002-j. [DOI] [PubMed] [Google Scholar]
- 3.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int. J. Dev. Biol. 2001;45:13–38. [PubMed] [Google Scholar]
- 4.Sperry R. Restoration of vision after crossing of optic nerves and after contralateral transplantation of eye. J. Neurophysiol. 1945;8:15–28. [Google Scholar]
- 5.Hamburger V. The development and innervation of transplanted limb primordia of chick embryos. J. Exp. Zool. 1939;80:347–389. [Google Scholar]
- 6.Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 1974;41:162–84. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 7.Le Douarin N. A biological cell labeling technique and its use in expermental embryology. Dev. Biol. 1973;30:217–22. doi: 10.1016/0012-1606(73)90061-4. [DOI] [PubMed] [Google Scholar]
- 8.Kelley MW. Regulation of cell fate in the sensory epithelia of the inner ear. Nat. Rev. Neurosci. 2006;7:837–849. doi: 10.1038/nrn1987. [DOI] [PubMed] [Google Scholar]