Abstract
Objective
Vascular smooth muscle cells respond to mechanical injury via tightly orchestrated series of gene regulatory events. Kruppel Like Factor 15 (KLF15) is a zinc finger transcriptional factor that is expressed in vascular smooth muscle cells (VSMCs), however its role in vascular biology is unknown.
Methods and Results
KLF15 is broadly expressed in both arterial and venous vascular beds in a VSMCs restricted fashion. KLF15 expression is markedly reduced by both pharmacologic and mechanical stimuli. To examine the specific role of KLF15 in the vascular response to injury, we performed femoral artery wire injury in KLF15−/− and wild type (WT) mice. KLF15−/− mice develop exaggerated neointimal growth, with evidence of increased SMC proliferation and migration within the neointima. In concordance, gain and loss of function studies in isolated VSMCs demonstrate that KLF15 can directly inhibit both SMC proliferation and migration.
Conclusions
These data are first to identify KLF15 as a novel inhibitor of VSMCs proliferation and migration and implicate this factor as a critical regulator of the vascular response to injury.
Keywords: Kruppel, KLF15, smooth muscle, vascular injury, proliferation, migration
INTRODUCTION
In response to injury the vascular smooth muscle cells (VSMCs) undergoes marked phenotypic changes characterized by enhanced cellular proliferation, migration, and extracellular response that contribute to disease processes such as restenosis or transplant arteriopathy. The transcriptional events leading to the highly orchestrated process of VSMCs activation, proliferation, migration, and neointimal formation have been the subject of intense study, but remain incompletely defined1–4.
Kruppel Like Factors are a subclass of the zinc-finger family of transcriptional regulators that are key regulators of gene expression, cell growth, and differentiation5, 6. Our laboratory identified KLF15 as a novel member of this family that plays critical roles in cardiomyocyte, adipocyte and hepatocyte biology 6, 7. However, its role in VSMCs has yet to be determined. In the current study, we report that KLF15 is robustly expressed in VSMCs across arterial and venous beds. Furthermore, our in vitro and in vivo observations identify KLF15 as a novel and essential regulator of VSMC response to injury.
MATERIALS & METHODS
KLF15−/− mouse was generated as previously described7. Mouse femoral artery wire injury was performed in WT and KLF15−/− mice (n=17 in each group) and histopathologic analyses performed after two weeks. RNA isolation, Real-time PCR and Northern blot were performed as previous described7. VSMCs migration was examined by Boyden chamber assay and DNA synthetic rate was determined by [3H]-thymidine incorporation assay. Data is expressed as mean ± SD. Differences between experimental groups were evaluated for statistical significance by using the Student’s t test for unpaired data. P<0.05 was considered statistically significant.
RESULTS
KLF15 regulates the biological response to vascular injury
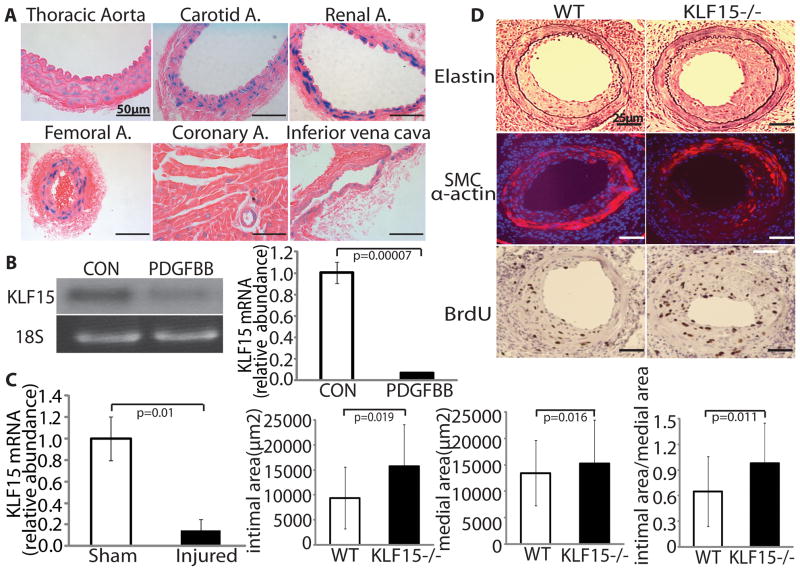
Using a KLF15+/− reporter mouse in which the mouse KLF15 locus was targeted using a nuclear-localized lacZ cassette 7, we examined vascular KLF15 expression using in situ X-gal staining. KLF15 is abundantly expressed in the VSMCs layer of both arterial and venous (Figure 1A). To gain initial insights into KLF15 function, we assessed its expression following pathologic stimuli in vitro and in vivo. KLF15 mRNA expression was markedly reduced in cultured rat aortic smooth muscle cells (RASMC) in response to platelet-derived growth factor-BB (PDGF-BB), a potent inducer of SMC proliferation and migration (Figure 1B left panel, representative Northern blot of 4 independent experiments; Figure 1B right panel, Real-time PCR of KLF15/18S rRNA, n=3 in each group). Similarly, we observed a marked reduction in KLF15 mRNA at 5 days of wire-injured femoral arteries (~80%) (n=7 in Sham and n=5 in injured group) (Figure 1C).
Figure 1.
KLF15 regulates the vascular response to injury. A, Vascular expression pattern of KLF15. B&C, Northern blot and Real-time PCR analysis for KLF15 expression following treatment with PDGF-BB (B) and in mouse femoral artery wire injury samples(C). D, WT and KLF15−/− mice were subjected to femoral artery wire injury. Neointima was characterized by elastic, smooth muscle cell α-actin, and BrdU staining.
To determine whether KLF15 directly modulates the biological response to vascular injury, we performed femoral artery wire injury in KLF15−/− mice and WT littermate controls. Fourteen days following wire injury, KLF15−/− mice developed enhanced intimal growth with a 170% increase (P=0.019) in intimal area (Figure 1D). Intimal: medial area ratio was increased significantly in KLF15−/− compared to WT mice (0.98 ± 0.47 vs. 0.65 ± 0.41; P=0.011). At 14d, SMC α-actin and leukocyte CD45 staining confirmed that the dominant cellular contributor to the neointima was VSMC (Figure 1D and data not shown for CD45 staining). Intimal cellular proliferation was assessed by incorporation of BrdU and was increased by 167% (P=0.045) in KLF15−/− mice (Figure 1D). Quantitative morphometry data are summarized in Supplementary Table 1.
KLF15 regulates VSMC proliferation and migration
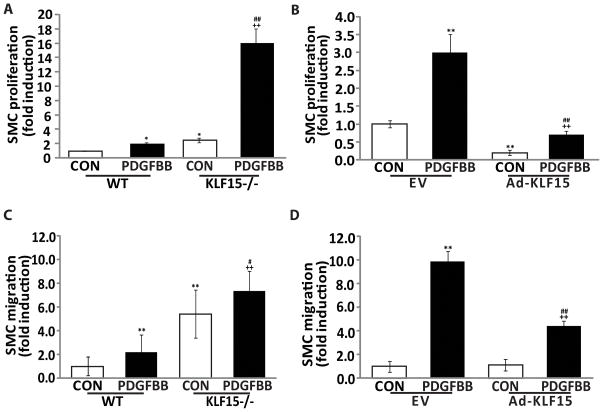
Since KLF15−/− femoral arteries demonstrated excess neointimal growth (Fig. 1D), we hypothesized that KLF15 might directly regulate VSMCs proliferation and migration. KLF15−/− mouse aortic smooth muscle cells (MASMCs) exhibited significantly increased proliferation and migration, both at baseline and in response to PDGF-BB stimulation (Figures 2A & 2C). Conversely, adenoviral overexpression of KLF15 in RASMCs strongly inhibited PDGF-BB induced cellular proliferation and migration (Figures 2B & 2D).
Figure 2.
KLF15 regulates VSMCs proliferation and migration. A&B, VSMCs proliferation was assessed using 3H thymidine incorporation assay. C&D, VSMCs migration was measured using the Boyden chamber assay. *P<0.05 vs. WT or EV control group; **P<0.01 vs. WT or EV control group; #P<0.05 vs. PDGF-BB treated WT or EV group; ## P<0.01 vs. PDGF-BB treated WT or EV group; ++ P<0.01 vs. KLF15−/−or Ad-KLF15 control group.
DISCUSSION
The data presented herein are first to identify KLF15 as an essential regulator of the biological response to vascular injury. Our studies indicate that KLF15 is a novel inhibitor of SMC migration and proliferation. Consistent with these observations, KLF15 −/− mice exhibited enhanced neointimal formation following wire injury. Our data also indicate that the effects observed are likely due to a cell-intrinsic defect as evidenced by the fact that KLF15−/− VSMCs exhibit augmented proliferation and migration. Taken together, these studies suggest that KLF15 positively regulates the vascular response to injury.
Our observations add to an increasing appreciation of the importance of Kruppel Like Factors in vascular smooth muscle cell biology. To date two members of this gene family have been shown to critically regulate the SMC response to injury. KLF5 is minimally expressed in the vasculature under basal conditions, but is robustly induced following injury. This factor promotes SMC proliferation and, consistent with this observation, hemizygous deficiency of KLF5 results in reduced neointimal formation8. Similarly, KLF4 is also expressed at low levels in quiescent VSMCs and induced rapidly following injury in VSMCs9. However, conditional deletion of KLF4 accelerates neointimal formation suggesting that its post-injury induction is likely a negative feedback response1. By contrast to KLF4 and KLF5, KLF15 is robustly expressed in VSMC in vivo under basal conditions and significantly reduced following injury. Given that KLF15 inhibits VSMCs proliferation/migration, this high basal expression may serve as a means to prevent unrestrained SMC activation. Taken together, our work coupled with previous observations highlight a critical role for the Kruppel Like factor gene family in the regulating vascular response to injury.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grant HL072952, HL075427, HL076754, HL084154 to MKJ, HL086614 to SH, HL094660 to DJ, DK080742 to SG, and HL85816 and HL57506 to DIS.
References
- 1.Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102:1548–1557. doi: 10.1161/CIRCRESAHA.108.176974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki T, Sawaki D, Aizawa K, Munemasa Y, Matsumura T, Ishida J, Nagai R. Kruppel-like factor 5 shows proliferation-specific roles in vascular remodeling, direct stimulation of cell growth, and inhibition of apoptosis. J Biol Chem. 2009;284:9549–9557. doi: 10.1074/jbc.M806230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grabski AD, Shimizu T, Deou J, Mahoney WM, Jr, Reidy MA, Daum G. Sphingosine-1-phosphate receptor-2 regulates expression of smooth muscle alpha-actin after arterial injury. Arterioscler Thromb Vasc Biol. 2009;29:1644–1650. doi: 10.1161/ATVBAHA.109.191965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meredith D, Panchatcharam M, Miriyala S, Tsai YS, Morris AJ, Maeda N, Stouffer GA, Smyth SS. Dominant-negative loss of PPARgamma function enhances smooth muscle cell proliferation, migration, and vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:465–471. doi: 10.1161/ATVBAHA.109.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieker JJ. Kruppel-like factors: three fingers in many pies. J Biol Chem. 2001;276:34355–34358. doi: 10.1074/jbc.R100043200. [DOI] [PubMed] [Google Scholar]
- 6.Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, Cui L, Kumar A, Lin Z, Sen-Banerjee S, Das H, Petersen CA, Mende U, Burleigh BA, Zhu Y, Pinto YM, Liao R, Jain MK. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci U S A. 2007;104:7074–7079. doi: 10.1073/pnas.0701981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray S, Feinberg MW, Hull S, Kuo CT, Watanabe M, Sen-Banerjee S, DePina A, Haspel R, Jain MK. The Kruppel-like factor KLF15 regulates the insulin-sensitive glucose transporter GLUT4. J Biol Chem. 2002;277:34322–34328. doi: 10.1074/jbc.M201304200. [DOI] [PubMed] [Google Scholar]
- 8.Shindo T, Manabe I, Fukushima Y, Tobe K, Aizawa K, Miyamoto S, Kawai-Kowase K, Moriyama N, Imai Y, Kawakami H, Nishimatsu H, Ishikawa T, Suzuki T, Morita H, Maemura K, Sata M, Hirata Y, Komukai M, Kagechika H, Kadowaki T, Kurabayashi M, Nagai R. Kruppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med. 2002;8:856–863. doi: 10.1038/nm738. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Sinha S, McDonald OG, Shang Y, Hoofnagle MH, Owens GK. Kruppel-like factor 4 abrogates myocardin-induced activation of smooth muscle gene expression. J Biol Chem. 2005;280:9719–9727. doi: 10.1074/jbc.M412862200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.