Abstract
Introduction
The twelve-lead surface electrocardiogram (ECG) is commonly used as a non-invasive modality to assess for left atrial enlargement (LAE), but data comparing ECG against cardiac computed tomography (CT) for LAE is lacking. We aimed to determine the diagnostic performance of six ECG criteria for LAE as compared to CT left atrial volume (LAV) and index to body surface area (LAVI) as the reference standard.
Materials and Methods
In 339 patients (age 53±12 years, 63% male), we evaluated the quantitative ECG parameters of P duration, P to PR segment ratio, P wave area, and P terminal force (PTFV1). We also assessed qualitatively the morphology of bifid and biphasic P waves. Patients were stratified into top and lowest quartile of LAV and LAVI by CT.
Results
Of the six ECG criteria, patients with P duration>110 ms had a 2½-fold increase likelihood of being in the top quartile of LAV (adjusted odds ratio [OR] OR 2.51, p=0.01) and LAVI (adjusted OR 2.74; p=0.007) as measured by CT. For this ECG criterion, the sensitivity and specificity were 71% and 55% for CT LAE by LAV and 61% and 55% for LAVI. The remaining ECG parameters of LAE assessed (P to PR segment ratio, PTFV1, P wave area, bifid, and biphasic P wave) were not associated with LAE by CT-based LAV or LAVI (all p≥0.20).
Discussion
Only P duration>110 ms was independently associated with LAE based on CT-derived LA volume and index. However, none of the established ECG parameters of LAE have sufficient diagnostic accuracies for predicting volumetric enlargement by CT, thus limiting its clinical utility.
Keywords: left atrium, left atrial volume, left atrial enlargement, computed tomography, electrocardiogram
Introduction
Left atrial enlargement (LAE) is associated with increased risk for cardiovascular diseases.1–2 The twelve-lead electrocardiogram (ECG) is commonly used as a non-invasive modality to assess for LAE. Multiple criteria have been established for the diagnosis of LAE.3–4 Both quantitative (P duration >110 ms in lead II, P to PR segment ratio >1.6 in lead II, P wave area > 4 ms × mV in lead II, and the P terminal force in lead V1 [PTFV1] > 40 ms × mm) and qualitative (bifid P wave in lead II, and biphasic P wave in V1) measures of LAE have been described to be a surrogate marker for LAE on surface ECG.5–9 P duration >110 ms and P to PR segment ratio have both been shown to be correlated with LAE.5–8 The P wave area, defined as amplitude × ½ duration, has a high sensitivity and specificity for diagnosis of LAE in patients with mitral stenosis when the cutoff value of > 4 ms × mV was used.10 PTFV1 and PTFV1 > 40 ms × mm are associated with LAE on echocardiography.8, 11–12 The qualitative morphologic parameters of LAE by ECG include bifid P wave and biphasic P wave and have been reported as being highly specific for LAE.13–14
The diagnostic performance of ECG parameters for LAE has demonstrated low sensitivities and variable specificities for detecting LAE when compared against echocardiography, which uses geometric shape assumptions for volumetric assessments.15–16 In recent years, the increase in use of cardiac CT with high spatial resolution has allowed for information on left atrial volumes to be precisely measured using three-dimensional volumetric analysis using this state-of-the-art technology.17–19 No prior studies to date have assessed these ECG criteria of LAE to volumetric measures from cardiac CT with respect to their diagnostic performance. In the “The Rule Out Myocardial Infarction Using Computer Assisted Tomography” (ROMICAT) trial, both ECG and cardiac CT were acquired within the same day, allowing for direct comparison of these ECG criteria for LAE to CT-based LA volume and index. Thus, we aim to assess the diagnostic performance and utility of these six ECG parameters for LAE with cardiac CT as the reference standard.
Methods
Study population
The ROMICAT trial was a prospective, observational cohort study of 368 consecutive adult patients at low-to-intermediate likelihood of acute coronary syndrome who presented to the emergency department of a tertiary hospital with acute chest pain whose initial ECGs and biomarkers were inconclusive and were awaiting hospital admission. The aim of the trial was to determine the diagnostic accuracy of CT for the detection of acute coronary syndrome. Enrollment period was over a cumulative time frame of 18 months ending May 2007. The details of the study design and results have been previously reported and are notable for exclusion of patients in atrial fibrillation.20 Briefly, all eligible patients who consented underwent ECG-gated contrast enhanced 64-slice CT and received standard of care for evaluation of acute coronary syndrome (ACS) during index hospitalization. Our institutional review board approved the study protocol and all patients provided written informed consent. In this analysis, we included patients who had both ECG and CT scans performed within 24 hours and compared the ECG with adequate quality (97%) and closest temporal proximity to that of the CT scan. We excluded 4 patients with ECGs that were either atrial- or ventricular-paced. We included a total of 339 patients for where there was full visualization of the left atrium on the multi-phase reformatted CT dataset (15 patients excluded due to partially visualized left atrium) and where the ECG was interpretable (10 patients excluded due to inability to evaluate the P wave) and available for review.
CT data acquisition
CT imaging was performed using a standard 64-slice CT coronary angiography (Sensation 64, Siemens Medical Solutions, Forchheim, Germany) protocol that was acquired at end inspiration using a test bolus protocol. If no contraindications, we administered sublingual nitroglycerin (0.6 mg). We also gave intravenous beta-blocker (metoprolol 5–20 mg) if the baseline heart rate was >60 beats per minute. A test bolus protocol of 20 ml contrast agent followed by 40 ml saline at a flow rate of 5 ml/s was used to determine the optimal timing of contrast injection. Contrast agent volume of 80–100 ml (Iodhexodol 320 g/cm3, Visipaque, General Electrics Healthcare, Princeton, NJ, USA) followed by 40 ml saline was injected intravenously at a rate of 5 ml/s. CT images were acquired in spiral mode, gantry rotation time of 330 ms, 64 × 0.6 mm slice collimation, tube voltage of 120 kV, maximum effective tube current of 850 mAs, with ECG-correlated tube current modulation used when possible. The maximum effective tube current was on during the time interval from 470 ms to 140 ms before the next expected R wave and the tube current was reduced by 80% during the remain portion of the cardiac cycle. Reconstructions were performed using retrospectively ECG-gated half-scan algorithm for a temporal resolution of 165 ms. For volumetric analysis, transaxial images were reconstructed for 10 phases, each at 10% of the RR-interval, for the multi-phase reformatted dataset (1.5 mm slice thickness, 1.5 mm increments).
CT measurements
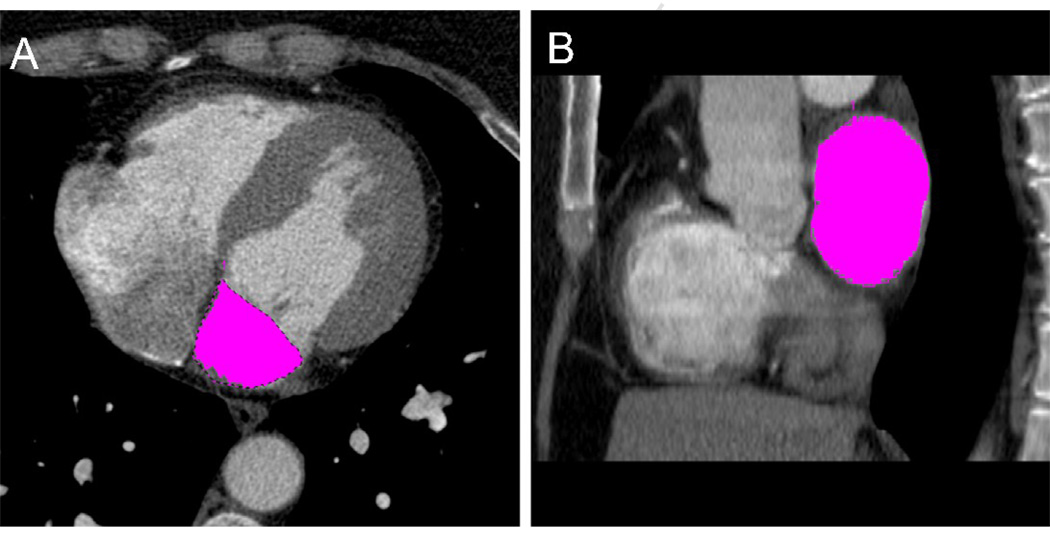
Two experienced readers, blinded to the ECG measurements, performed the CT measurements offline using dedicated cardiac workstations. We used a highly reproducible threshold-based method (Figure 1) for quantifying LA volume three-dimensionally without geometric shape assumptions, as previously validated.18 Briefly, the maximum LA volume was derived by pure volumetric summation of manually traced regions of interests on sequential axial 1.5 mm thick slices with a threshold window width set at 100–1000 Hounsfeld units using a dedicated semi-automated volumetric software program (Volume Viewer, Leonardo, Siemens Medical Solutions, Forchheim, Germany). We measured the maximum LA volume (LAV) from the end-systolic phase just before the mitral valve opening with the largest LA cavity and smallest LV cavity, as determined qualitatively from multiplanar LV short-axis, two-chamber, and four-chamber views. The LAV included the left atrial appendage but excluded the pulmonary veins. The LAV was indexed to body surface area (BSA) for the measurement of LAVI.
Figure 1. Axial (A) and sagittal (B) views of the three-dimensional volumetric threshold-based method for left atrial volume by cardiac CT.
ECG assessments
The twelve-lead ECGs analyzed in our study were recorded at 25 mm/s, 1mV/cm, and 100 Hz standardization. ECGs were measured quantitatively for intervals and qualitatively for morphology. Quantitative assessments were performed using electronic caliper (Cardio Calipers, Iconico, Inc, New York, NY). Intervals were amplified two-fold and measured by a single reader. Qualitative assessments of ECG readings were performed by consensus from two independent cardiologists with disagreement resolved by adjudication from a third cardiologist. Both readers were blinded to the imaging results that corresponded to a given ECG. We assessed six ECG measures of LAE and used this in our analysis of CT measures for LAE. These ECG parameters included P duration >110 ms in lead II, P to PR segment ratio >1.6 in lead II, P wave area > 4 ms × mV in lead II, the PTFV1 > 40 ms × mm in V1, bifid P wave in lead II, and biphasic P wave (defined as P wave in V1 with a width > 0.04 mm and a > 1mm negative deflection).
Covariates of interests
Cardiovascular risk factors and medical history were assessed at the time of subject’s enrollment based on self-report or obtained from the medical records during the index hospitalization. Body mass index (BMI) was defined as weight (kilograms) divided by the height squared (meters). BSA was calculated using the Dubois formula.21 Hypertension was defined as systolic blood pressure of at least 140 mm Hg or diastolic blood pressure of at least 90 mm Hg or current antihypertensive treatment. Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL or treatment with a hypoglycemic agent. Hyperlipidemia was defined as total cholesterol of ≥200 mg/dl or treatment with a lipid lowering medication. Documented history of coronary artery disease (CAD) included previous myocardial infarction or coronary revascularization. Family history of CAD was defined as having a first-degree female (<65 years) or male (<55 years) relative with a documented history of myocardial infarction or sudden cardiac death. Subjects were classified as smokers if they smoked at least 1 cigarette a day currently or in the past. An adjudication panel of 2 physicians, who were blinded to CT, reviewed the medical records and determined the diagnosis of ACS during index hospitalization. ACS was defined as either an acute myocardial infarction or unstable angina, according to the AHA/ACC/ESC guidelines. 22 Disagreement was solved by consensus, which included an additional cardiologist.
Statistical analysis
Descriptive statistics were expressed as mean ± standard deviation (SD) for continuous variables and as frequency and percentages for nominal variables. For continuous variables, the differences in means between groups were determined using Student’s t tests. For binary variables, we compared the difference in proportions using Chi-square or Fisher’s Exact test. We dichotomized the LAV and LAVI into the top quartile versus the lowest quartile. We used logistic regression to evaluate the association between the ECG parameters of LAE to that of CT LAE (top quartile of LAV or LAVI) and utilize to test for effect modification by evaluation the interaction terms for gender and P duration >110 ms for LAE. Multivariable logistic regression models were adjusted for potential confounders and included covariates with p<0.15 from the univariate screen. For both LAV and LAVI models, covariates included were P duration >110 ms, age, gender, diabetes, and history of CAD. For the LAV model, BMI was additionally included. The C statistic,23 which is equivalent to the area under the receiver operating characteristic curve, was determined to evaluate the prognostic discriminatory capacity of the MV models. Testing for collinearity found no violations, and Hosmer and Lemeshow Goodness-of-Fit test (model calibration) for both models demonstrated good model fit (LAV: p=0.21; LAVI: p=0.87). In determining the diagnostic accuracy of the ECG parameters of LAE against the CT reference standard using the top quartile of LAV and LAVI versus the lowest quartile, we calculated the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy. We used intraclass correlation coefficients (ICC) to assess the reproducibility of the quantitative ECG measurements. Two independent readers performed the measurements for inter-observer on 20 randomly selected cases and one reader performed the measurements twice one month apart for intra-observer reproducibility. A 2-tailed p-value of <0.05 was considered to indicate statistical significance. All analyses were performed using SAS (Version 9.1.3, SAS Institute Inc., Cary, North Carolina) and SPSS (Version 16.0, Chicago, Illinois).
Results
For the quantitative ECG measurements, the intra-observer ICC of 20 randomly selected patients was > 0.86 and the inter-observer ICC was > 0.65 for all measures. For the LAV, the intra-observer ICC was 0.996 and inter-observer ICC was 0.98, as previously reported.18
Baseline Characteristics in the ROMICAT cohort
Table 1 depicts the baseline demographics of the 339 patients. Patients were predominantly male (63%), with a mean age of 53 years (range 21– 86 years), and BMI of 29 kg/m2. In this cohort, the LAV by CT was 96.1 ± 25.1 mL and LAVI was 48.6 ± 12.5 mL/m2. Of the ECG parameters, the average P duration was 111±15.8 ms, P to PR segment ratio was 2.2± 0.8, P wave area was 9.4 ± 6.9 ms × mV, and PTFV1 was 42.3 ± 27.7 mm × ms. There were 184 (54%) patients with P duration > 110 ms, 270 (80%) patients with a P to PR segment ratio > 1.6, 275 (81%) of patients with a P wave area > 4 ms × mV, 169 (50%) of patients with PTFV1 > 40mm × ms, 56 (17%) of patients with a bifid P wave and 75 (22%) of patients with biphasic P wave.
Table 1.
Demographics of the study group
| (n = 339) | |
|---|---|
| Age (years) | 53.0 ± 11.8 |
| BSA (m2) | 2.0 ± 0.27 |
| BMI (kg/m2) | 29.1 ± 6.17 |
| Male | 215 (63.4 %) |
| Hypertension | 138 (40.7 %) |
| Diabetes mellitus | 35 (10.3 %) |
| Hyperlipidemia | 140 (41.3 %) |
| History of CAD | 38 (11.2 %) |
| FH of CAD | 83 (24.5 %) |
| Smoking | 173 (51.0 %) |
| ACS | 31 (9.1%) |
BSA denotes body surface area; BMI, body mass index; CAD, coronary artery disease; FH, family history; and ACS, acute coronary syndrome.
Comparison of ECG parameters with CT measures of LA enlargement
Using the entire cohort, patients with P duration >110 ms had larger LAV than those with P duration of ≤ 110 ms (100.3±26.7 mL vs 91.2 ± 22.2 mL, p=0.0007), and trended to have greater LAVI (49.8 ± 12.79 mL/m2 vs 47.2± 12.0 mL/m2, p= 0.06). In contrast, patients with a P to PR segment ratio > 1.6, P wave area > 4 ms × mV, PTFV1 > 40mm × ms, bifid P wave, or biphasic P wave had no significant differences in LAV and LAVI as compared to those who did not meet these ECG criteria for LAE (all p≥0.17).
For evaluation of CT-based LA enlargement, we compared patients in the top quartile (n=84) versus patients in the lowest quartiles (n=84) for the CT-based LAV and LAVI measurements separately. The top quartile LAV ranged from 112.3–184.1 mL as compared to the lowest quartile of 47.0–77.9 mL, while the top quartile of LAVI was 56.0–95.3 mL/m2 and the lowest quartile was 24.0–39.2 mL/m2. Table 2 depicts a comparison of demographics between the top and lowest volume quartiles of LAV and LAVI. Patients in the top quartile of CT LAV were older by 4.4 years than those in the lowest quartile (p=0.02). Similarly, for LAVI, patients in the top volume quartile were 7.7 years older than those in the lowest quartile (p <0.0001). The BMI was greater by 4.4 kg/m2 in patients in the top volume quartile as compared to those in the lowest quartile of LAV. However, after indexing for BSA, there was no difference in BMI between patients in the top versus lowest quartile for LAVI (p=0.28).
Table 2.
Demographics of patients the top (n=84) and lowest quartile (n=84) in the LAV and LAVI cohorts.
| Demographic | LAV | P value | LAVI | P value |
|---|---|---|---|---|
| Age | ||||
| Top quartile | 55.5 ± 12.8 | 0.02 | 58.7 ± 12.8 | <0.001 |
| Lowest quartile | 51.0 ± 11.1 | 51.0 ± 11.5 | ||
| BMI | ||||
| Top quartile | 31.5 ± 6.5 | <0.001 | 28.5 ± 6.4 | 0.28 |
| Lowest quartile | 27.2 ± 5.3 | 29.5 ± 5.9 | ||
| Male | ||||
| Top quartile | 60 (71.4%) | 0.19 | 45 (53.6%) | 0.001 |
| Lowest quartile | 51 (60.7%) | 66 (78.6%) | ||
| HTN | ||||
| Top quartile | 37 (44.1 %) | 0.34 | 39 (46.4%) | 0.64 |
| Lowest quartile | 30 (35.7 %) | 35 (41.7%) | ||
| Diabetes | ||||
| Top quartile | 15 (17.9 %) | 0.06 | 15 (17.9%) | 0.06 |
| Lowest quartile | 6 (7.1 %) | 6 (7.1%) | ||
| Hyperlipidemia | ||||
| Top quartile | 35 (41.7 %) | 0.34 | 37 (44.1%) | 0.53 |
| Lowest quartile | 28 (33.3 %) | 32 (38.1%) | ||
| History of CAD | ||||
| Top quartile | 13 (15.5 %) | 0.04 | 14 (16.7%) | 0.09 |
| Lowest quartile | 4 (4.8 %) | 6 (7.1%) | ||
| FH of CAD | ||||
| Top quartile | 20 (23.8 %) | 1.0 | 18 (21.4%) | 0.37 |
| Lowest quartile | 20 (23.8 %) | 24 (28.6%) | ||
| Smoker | ||||
| Top quartile | 45 (53.6 %) | 1.0 | 47 (56.0%) | 0.75 |
| Lowest quartile | 44 (52.4 %) | 50 (59.5%) | ||
| ACS | ||||
| Top quartile | 11 (13.1%) | 0.19 | 11 (13.1%) | 0.31 |
| Lowest quartile | 5 (6.0%) | 6 (7.1%) | ||
Abbreviations as in Table 2. LAV denotes left atrial volume; and LAVI, left atrial volume index.
Patients in the top quartile of LAV had an increase in P duration compared to the lowest quartile (117.0± 16.6 ms vs 108.4 ± 14.8 ms, p=0.0005), while patients in the top quartile of LAVI trended to have greater P duration than those in the lowest quartile (113.5 ± 17.3 ms vs 109.0 ±15.2 ms, p=0.08). With respect to the P to PR segment ratio, the P wave area, and the PTFV1, there were no differences observed between the top versus lowest quartile of LAV and LAVI (all p≥0.19). Similarly, there was no significant difference in the proportions of patients with bifid or biphasic P wave morphology in those with the lowest versus top quartile (all p≥0.54). Table 3 shows the diagnostic test characteristics of the dichotomized ECG parameters when compared to the CT measurements of LA enlargement, defined as top quartile, by LAV and LAVI.
Table 3.
Diagnostic accuracy of the 6 electrocardiographic criteria for LAE as compared to CT-based LAE by LAV and LAVI.
| LAV | LAVI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity | Specificity | PPV | NPV | Accuracy | Sensitivity | Specificity | PPV | NPV | Accuracy | |
| P duration >110 ms | 71%(60/84) | 55%(46/84) | 61%(60/98) | 66%(46/70) | 63%(106/168) | 61%(51/84) | 55%(46/84) | 57%(51/89) | 58%(46/79) | 58%(97/168) |
| P to PR segment ratio | 81%(68/84) | 27%(23/84) | 53%(68/129) | 59%(23/39) | 54%(91/168) | 81%(68/84) | 21%(18/84) | 51%(68/134) | 53%(18/34) | 51%(86/168) |
| P wave area >4 ms × mV | 73%(61/84) | 19%(16/84) | 47%(61/129) | 41%(16/39) | 46%(77/168) | 75%(63/84) | 20%(17/84) | 48%(63/130) | 45%(17/38) | 48%(80/168) |
| PTFV1 | 49%(41/84) | 54%(45/84) | 51%(41/80) | 51%(45/88) | 51%(86/168) | 48%(40/84) | 51%(43/84) | 49%(40/81) | 49%(43/87) | 49%(83/168) |
| Bifid | 19%(16/84) | 85%(72/84) | 57%(16/28) | 51%(72/140) | 53%(88/168) | 19%(16/84) | 86%(72/84) | 57%(16/28) | 51%(72/140) | 52%(88/168) |
| Biphasic | 26%(22/84) | 76%(64/84) | 52%(22/42) | 51%(64/126) | 51%(86/168) | 26%(22/84) | 77%(65/84) | 54%(22/41) | 51%(65/127) | 52%(87/168) |
Abbreviations as in Table 1 and 3. PPV denotes positive predictive value; and NPV, negative predictive value.
Association of ECG parameters to CT measure of LA enlargement
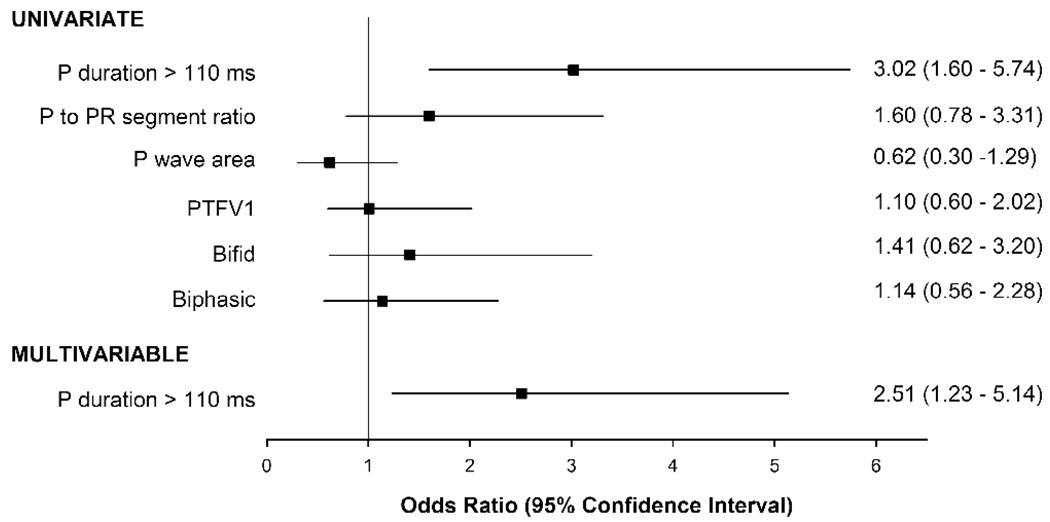
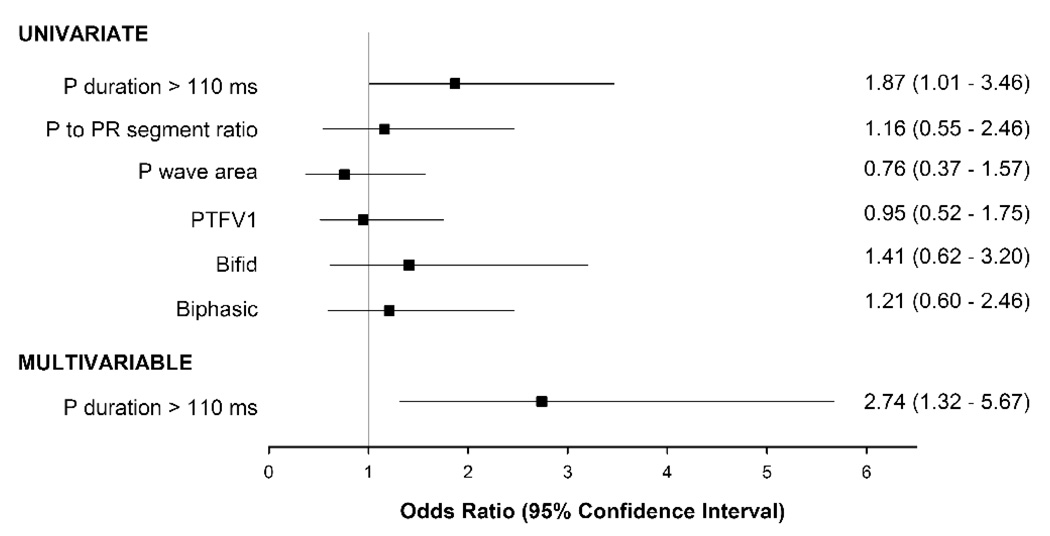
Figure 2 and 3 depict the odds of having LAE and being in the top quartile of LAV and LAVI by CT for each of the 6 ECG parameters. Of the 6 ECG parameters, only P duration >110 ms was significantly associated with an increase risk for LA enlargement by both CT LAV and LAVI. Patients with P duration >110 ms had a 3-fold increase risk of having LA enlargement by CT LAV (odds ratio [OR] 3.02, p=0.0007), and a near 2-fold increase risk by CT LAVI (OR 1.87, p=0.045). There was no gender difference between P duration >110 ms and LA enlargement (LAV: p-interaction=0.33; LAVI: p-interaction=0.24). After multivariable adjustment, P duration >110 ms remained independently associated with and had good discrimination for LA enlargement by CT. There remained over a 2 ½-fold increase in risk for LA enlargement by both CT LAV (OR 2.51, p=0.01; C statistic 0.77) and LAVI (OR 2.74, p=0.007; C statistic 0.80). The other 5 ECG parameters were not associated with LAE by CT LAV or CT LAVI (all p≥0.20).
Figure 2. Odds ratio for various ECG measures of atrial enlargement for detecting LA enlargement by CT LAV.
CT LA enlargement is defined as being in the top quartile of LAV. Multivariable model included P duration > 110 ms and adjusted for age, gender, body mass index, diabetes, and history of coronary artery disease.
Figure 3. Odds ratio for various ECG measures of atrial enlargement for detecting LA enlargement by CT LAVI.
CT LA enlargement is defined as being in the top quartile of LAVI. Multivariable model included P duration > 110 ms and adjusted for age, gender, diabetes, and history of coronary artery disease.
Discussion
In this study, the diagnostic performance of six ECG parameters of LAE was compared to cardiac CT measures of LAV and LAVI. The strength of our study is the large sample size (n=339) and use of both quantitative and qualitative ECG parameters of LAE with direct comparison to CT volumetric measures. When stratifying patients into top and lowest quartile of LAV and LAVI by CT, P duration > 110 ms remained independently associated with patients meeting this ECG criterion having over a 2 ½-fold increase risk in LAE by CT as compared to patients who did not have this ECG criterion. The remaining 5 ECG parameters, including the presence of bifid and biphasic P wave, were not associated with LAE by CT.
While there are multiple non-invasive methods for the assessment of LAE, ECG remains the simplest and cheapest non-invasive clinical tool and is used on a daily basis in identifying LAE.3–4 The ROMICAT trial has offered a unique opportunity to evaluate the findings of both ECG and cardiac CT, which were acquired within 24 hours. Cardiac CT advantages include excellent reproducibility for the assessment of LAV and the ability to perform high-resolution 3-dimensional direct volumetric assessment without the need for geometric shape assumptions.18 The CT volumetric technique which unlike echo allows precise definition of the border of the LA with the inclusion of the left atrial appendage and easily identifiable exclusion of the pulmonary veins. Given the lack of standardized cut-off values for LAE using CT, we used top versus lowest quartile of LAV and LAVI for comparison. LAE has been defined as a LAVI of >55 mL/m2 in a cardiac magnetic resonance (CMR) imaging study, which is an imaging modality similar to cardiac CT where direct volume measurements can be obtained.24 In our study, the definition of LAE was the top quartile of LAVI of >56.0 mL/m2, which is similar to that used in CMR. Thus, the top quartile likely represents those with at least some extent of LAE whereas the lowest quartile likely represents those with normal LA size making a comparison of these two groups valid.
In our study, we compared six ECG parameters of LAE and found that only one parameter (P duration>110 ms) was independently associated with CT-defined LAE. Our findings are in keeping with multiple other studies that demonstrate the diagnostic strength of P wave duration > 110 ms for the determination of LAE. Lee et al. found when compared to other ECG criteria for LAE the sensitivity of P duration > 110 ms was the highest.15 Ariyarajah et al. demonstrated increased P wave duration to be an independent and direct correlate to LA size.6 We described the diagnostic performance of P duration > 110 ms in our study with values similar to those previously documented.13–14 We also demonstrated the independent association of P duration > 110 ms with LAE, after adjusting for age, gender, BMI, diabetes, and history of coronary artery disease. These patients meeting this ECG criterion conferred a 2 ½-fold increase risk of having LAE by CT. Clinically, the presence of LAE predisposes patients to increase risk of CAD, heart failure, atrial fibrillation, stroke, and mortality, thus the presence of this ECG parameter may be helpful for risk stratification.1–2, 25–27
The diagnostic performance of the other five ECGs parameters for LAE has been less consistent.3 Similarly, we found no association between the other five ECG parameters to the CT measurements for LAE, further raising the issue of their clinical utility for the detection of LAE. This may explain the reason why these quantitative parameters, which are burdensome to calculate and derive, are less commonly used. Interestingly, the qualitative morphologic measures of bifid and biphasic P wave, which are frequently used due to their high specificities13–14, 24, were not associated with LAE in our study. In addition, their common application in daily practice may be attributed to their ease in interpretation and analysis on the surface ECG. While we also observed high specificities for these qualitative ECG parameters, their lack of association to CT-based LAE makes us question their clinical usefulness.
Limitations
There are several limitations of our study results. The patient cohort consists of symptomatic patients presenting to the emergency department for chest pain with low to intermediate risk for suspected acute coronary syndrome and the generalizability of our findings may be limited to this patient population. As there are no established definitions for LAE using CT we were unable to grade the severity of LAE. The amplification of the p wave for electronic caliper measurements may not be available for practicing physician. The radiation exposure inherent in the acquisition of CT images should preclude cardiac CT from being performed solely for the evaluation LA volume. We were able to perform our analysis as our CT acquisitions were performed for coronary artery assessment and were acquired using retrospective gating, providing us with data from end-systole. Dose-saving algorithms, such as ECG tube modulation and use of lower tube current and voltage, could still allow for evaluation of LAV at end-systole, though this analysis would not be possible with prospective triggering scans.
Conclusion
Among the six ECG quantitative and qualitative criteria for LAE, only the P duration > 110 ms was found to be independently associated with CT-based LAE. Patients with P duration >110 ms have over 2 ½ -fold increase risk for having LAE by cardiac CT. However, none of the established ECG parameters of LAE have sufficient diagnostic accuracies for predicting volumetric enlargement by CT, thus limiting its clinical utility.
Acknowledgments
Sources of Funding: This work was supported by the NIH R01 HL080053, and in part supported by Siemens Medical Solutions and General Electrics Healthcare. Drs. Blankstein and Truong received support from NIH grant T32HL076136. Dr. Truong also received support from NIH grant L30HL093896.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Conflict of Interests: No conflicts of interest to be disclosed.
REFERENCE
- 1.Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, et al. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS) Am Heart J. 2006;151:412–418. doi: 10.1016/j.ahj.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Laukkanen JA, Kurl S, Eranen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165:1788–1793. doi: 10.1001/archinte.165.15.1788. [DOI] [PubMed] [Google Scholar]
- 3.Alpert MA, Munuswamy K. Electrocardiographic diagnosis of left atrial enlargement. Arch Intern Med. 1989;149:1161–1165. [PubMed] [Google Scholar]
- 4.Surawicz B. Electrocardiographic diagnosis of chamber enlargement. J Am Coll Cardiol. 1986;8:711–724. doi: 10.1016/s0735-1097(86)80207-8. [DOI] [PubMed] [Google Scholar]
- 5.Chirife R, Feitosa G, Frankl W. Electrocardiographic detection of left atrial enlargement. Correlation of P wave with left atrial dimension by echocardiography. Br Heart J. 1975;37:1281–1285. doi: 10.1136/hrt.37.12.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ariyarajah V, Mercado K, Apiyasawat S, Puri P, Spodick DH. Correlation of left atrial size with p-wave duration in interatrial block. Chest. 2005;128:2615–2618. doi: 10.1378/chest.128.4.2615. [DOI] [PubMed] [Google Scholar]
- 7.Macruz R, Perloff JK, Case RB. A method for the electrocardiographic recognition of atrial enlargement. Circulation. 1958;17:882–889. doi: 10.1161/01.cir.17.5.882. [DOI] [PubMed] [Google Scholar]
- 8.Birkbeck JP, Wilson DB, Hall MA, Meyers DG. P-wave morphology correlation with left atrial volumes assessed by 2-dimensional echocardiography. J Electrocardiol. 2006;39:225–229. doi: 10.1016/j.jelectrocard.2005.06.109. [DOI] [PubMed] [Google Scholar]
- 9.Perosio AM, Suarez LD, Torino A, Llera JJ, Ballester A, Roisinblit JM. Reassessment of electrovectorcardiographic signs of left atrial enlargement. Clin Cardiol. 1982;5:640–646. doi: 10.1002/clc.4960051204. [DOI] [PubMed] [Google Scholar]
- 10.Zeng C, Wei T, Zhao R, Wang C, Chen L, Wang L. Electrocardiographic diagnosis of left atrial enlargement in patients with mitral stenosis: the value of the P-wave area. Acta Cardiol. 2003;58:139–141. doi: 10.2143/AC.58.2.2005266. [DOI] [PubMed] [Google Scholar]
- 11.Jin L, Weisse AB, Hernandez F, Jordan T. Significance of electrocardiographic isolated abnormal terminal P-wave force (left atrial abnormality). An echocardiographic and clinical correlation. Arch Intern Med. 1988;148:1545–1549. [PubMed] [Google Scholar]
- 12.Hopkins CB, Barrett O., Jr Electrocardiographic diagnosis of left atrial enlargement. Role of the P terminal force in lead V1. J Electrocardiol. 1989;22:359–363. doi: 10.1016/0022-0736(89)90012-5. [DOI] [PubMed] [Google Scholar]
- 13.Hazen MS, Marwick TH, Underwood DA. Diagnostic accuracy of the resting electrocardiogram in detection and estimation of left atrial enlargement: an echocardiographic correlation in 551 patients. Am Heart J. 1991;122:823–828. doi: 10.1016/0002-8703(91)90531-l. [DOI] [PubMed] [Google Scholar]
- 14.Munuswamy K, Alpert MA, Martin RH, Whiting RB, Mechlin NJ. Sensitivity and specificity of commonly used electrocardiographic criteria for left atrial enlargement determined by M-mode echocardiography. Am J Cardiol. 1984;53:829–832. doi: 10.1016/0002-9149(84)90413-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee KS, Appleton CP, Lester SJ, Adam TJ, Hurst RT, Moreno CA, et al. Relation of electrocardiographic criteria for left atrial enlargement to two-dimensional echocardiographic left atrial volume measurements. Am J Cardiol. 2007;99:113–118. doi: 10.1016/j.amjcard.2006.07.073. [DOI] [PubMed] [Google Scholar]
- 16.Waggoner AD, Adyanthaya AV, Quinones MA, Alexander JK. Left atrial enlargement. Echocardiographic assessment of electrocardiographic criteria. Circulation. 1976;54:553–557. doi: 10.1161/01.cir.54.4.553. [DOI] [PubMed] [Google Scholar]
- 17.Stolzmann P, Scheffel H, Leschka S, Schertler T, Frauenfelder T, Kaufmann PA, et al. Reference values for quantitative left ventricular and left atrial measurements in cardiac computed tomography. Eur Radiol. 2008;18:1625–1634. doi: 10.1007/s00330-008-0939-4. [DOI] [PubMed] [Google Scholar]
- 18.Mahabadi AA, Samy B, Seneviratne SK, Toepker MH, Bamberg F, Hoffmann U, et al. Quantitative assessment of left atrial volume by electrocardiographic-gated contrast-enhanced multidetector computed tomography. J Cardiovasc Comput Tomogr. 2009;3:80–87. doi: 10.1016/j.jcct.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Truong QA, Bamberg F, Mahabadi AA, Toepker M, Lee H, Rogers IS, et al. Left atrial volume and index by multi-detector computed tomography: Comprehensive analysis from predictors of enlargement to predictive value for acute coronary syndrome (ROMICAT study) Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann U, Bamberg F, Chae CU, Nichols JH, Rogers IS, Seneviratne SK, et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: the ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J Am Coll Cardiol. 2009;53:1642–1650. doi: 10.1016/j.jacc.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Archives of Internal Medicine. 1916;17:865–871. [Google Scholar]
- 22.Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, et al. ACC/AHA guideline update for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction--2002: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on the Management of Patients With Unstable Angina) Circulation. 2002;106:1893–1900. doi: 10.1161/01.cir.0000037106.76139.53. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. Jama. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 24.Tsao CW, Josephson ME, Hauser TH, O'Halloran TD, Agarwal A, Manning WJ, et al. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:7. doi: 10.1186/1532-429X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am J Cardiol. 1998;82:2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 26.Ristow B, Ali S, Whooley MA, Schiller NB. Usefulness of left atrial volume index to predict heart failure hospitalization and mortality in ambulatory patients with coronary heart disease and comparison to left ventricular ejection fraction (from the Heart and Soul Study) Am J Cardiol. 2008;102:70–76. doi: 10.1016/j.amjcard.2008.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moller JE, Hillis GS, Oh JK, Seward JB, Reeder GS, Wright RS, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107:2207–2212. doi: 10.1161/01.CIR.0000066318.21784.43. [DOI] [PubMed] [Google Scholar]