Abstract
Adipocyte complement-related protein (30 kDa) (Acrp30), a secreted protein of unknown function, is exclusively expressed in differentiated adipocytes; its mRNA is decreased in obese humans and mice. Here we describe novel pharmacological properties of the protease-generated globular head domain of Acrp30 (gAcrp30). Acute treatment of mice with gAcrp30 significantly decreased the elevated levels of plasma free fatty acids caused either by administration of a high fat test meal or by i.v. injection of Intralipid. This effect of gAcrp30 was caused, at least in part, by an acute increase in fatty acid oxidation by muscle. As a result, daily administration of a very low dose of gAcrp30 to mice consuming a high-fat/sucrose diet caused profound and sustainable weight reduction without affecting food intake. Thus, gAcrp30 is a novel pharmacological compound that controls energy homeostasis and exerts its effect primarily at the peripheral level.
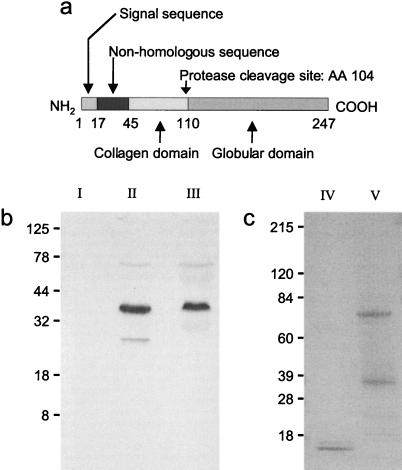
Adipocyte complement-related protein (30 kDa) (Acrp30) is a secreted protein expressed exclusively in differentiated adipocytes (1). Primary sequence analysis reveals four main domains (Fig. 1A): a cleaved amino-terminal signal sequence, a region without homology to known proteins, a collagen-like region, and a globular segment at the carboxy terminus. The globular domain forms homotrimers (2), and additional interactions between Acrp30 collagenous segments cause the protein to form higher order structures. Acrp30 is also known as AdipoQ (3), and its human homolog has been designated independently as apm-1 (4) and GBP28 (5).
Figure 1.
Characterization of Acrp30, gAcrp30, and a fragment of apm-1 in human plasma. (a) Acrp30 has four domains; the C-terminal globular head region (aa 110–247) makes up the majority. (b) A cleavage product of apm-1, the human homolog of Acrp30, was detected in human plasma with the use of a globular head-specific anti-serum for immunoprecipitation as well as for subsequent Western blotting. The apparent molecular mass of this truncated form is 27kDa, corresponding to about 70% of the complete form of apm-1 (Lane II). This truncated form was not detected when an anti-serum specific for the human nonhomologous region (HDQETTTQGPGVLLPLPKGA) of the protein was used for immunoprecipitation (Lane III), followed by immunoblotting with the globular head-specific anti-serum. Preimmune serum of the same animal did not detect any protein (Lane I). (c) Purification of Acrp30 and gAcrp30 shown by SDS-PAGE. Lane V shows the complete form of purified Acrp30. The apparent molecular mass is 37kDa (including an amino-terminal non-Acrp30 region derived from the plasmid). An incompletely reduced dimer (1) of Acrp30 can also be seen. Lane IV shows the proteolytic cleavage product gAcrp30.
Acrp30 protein shares sequence homology with a family of proteins showing a modular design containing a characteristic C-terminal complement factor C1q-like globular domain (6). In addition to C1q, members of this family include the human type VIII and X collagens (6), precerebellin (7, 8), and the hibernation-regulated proteins hib 20, 25, and 27 (9). Other than C1q, little is known regarding the function of the C-terminal globular regions of these proteins. In active and hibernating animals members of the hib family are differentially expressed in liver, suggesting a role in energy storage or mobilization (10). A similar function has been suggested for Acrp30 because the three-dimensional structure of its C-terminal globular domain is strikingly similar to that of tumor necrosis factor-α (TNFα) (2), even though there is no homology at the primary sequence level. Among its various biological effects TNFα regulates several aspects of energy homeostasis (11). Furthermore, Acrp30 mRNA is decreased in adipose tissue from both obese ob/ob mice and obese humans (3). However, thus far no specific function for Acrp30 has been described.
Protein cleavage is a common feature in the complement cascade activation, and the Acrp30 homolog precerebellin is proteolytically cleaved into the active cerebellin peptide (6, 8). In addition, the hib family of proteins is found in blood as a complex that includes a homolog of α1-antitrypsin (12), suggesting regulation by protease. We speculate that Acrp30 circulates in plasma as an inactive precursor of a regulatory protein. Here we show that a fragment of human Acrp30 that includes the C-terminal globular domain circulates in human plasma at low abundance. In addition, the bacterially expressed and purified C-terminal globular domain of Acrp30 is pharmacologically active and induces free fatty acid (FFA) oxidation in muscle and weight reduction in mice. Our evidence suggests that Acrp30 is one of the long-sought hormones that carry a signal from adipose tissue to muscle (13, 14).
Materials and Methods
Male C57BL/6J mice were obtained from the Jackson Laboratory and housed under controlled temperature (23°C) and lighting (12 h light, 0600–1800h; 12 h dark, 1800–0600h) with free access to water and standard mouse chow (type 7011C; Harlan Teklad, Madison, WI). In some experiments animals were put on a high-fat/sucrose diet provided by Research Diets (New Brunswick, NJ) (type D12331, 58 kcal% fat from coconut oil + 28 kcal% sucrose). All procedures were approved by the Biological Test Center Animal Care and Use Committee (Irvine, CA). Experiments studying effects on postprandial lipemia were conducted in animals fasted for 3 h. A high-fat and high-sugar test meal (6 g butter, 6 g sunflower oil, 10 g nonfat dry milk, 10 g sucrose, 12 ml distilled water prepared fresh) was given to the animals by intragastric gavage (vol. = 1% of body weight), and blood samples were taken over the subsequent study period. In other experiments, instead of a high-fat meal, “Intralipid 20%” (Clintec Nutrition, Deerfield, IL) was injected i.v. (tail vein) to generate a sudden rise in plasma FFAs. Plasma concentrations of triglycerides, glucose, and FFAs were determined with commercial kits (triglycerides and glucose, Sigma; FFA, Wako Biochemicals, Osaka). Insulin and leptin were determined by RIA with kits from Linco Research (St. Charles, MO). The glucagon RIA test kit was from ICN.
Recombinant Protein Production and Protein Characterization.
Recombinant Acrp30 (GenBank U37222) was produced by cloning Acrp30 cDNA in pTRC His B (Invitrogen) between the BamHI and XhoI sites and was maintained in Escherichia coli DH5-α. The Acrp30 sequence contains no predicted glycosylation sites. The N-terminal His6-tagged fusion protein was produced in E. coli, isolated from the lysed bacterial pellet by FPLC with Probond resin (Invitrogen), and eluted with imidazole-containing buffer.
The globular region of Acrp30 was purified from the complete form after enzymatic cleavage. Briefly, purified Acrp30 was incubated with acetylated trypsin-type V-S from bovine pancreas (E.C. 3.4.21.4; Sigma) in PBS at 400 units/mg protein at 25°C for 10 min. The reaction was stopped by running the sample over a PolyPrep Column (Bio-Rad) containing immobilized trypsin inhibitor (Pierce) at 4°C. Fractions with proteins were then dialyzed extensively against saline with the use of dialysis tubing with a molecular weight cutoff of 10,000. The globular head domain of Acrp30 (gAcrp30) was further concentrated on an Amicon Centricon Filter (Millipore) with a molecular weight cutoff of 10,000 and stored under sterile conditions in frozen aliquots. The purity and efficiency of cleavage were checked by SDS/PAGE. Additional degradation products, all smaller than 10 kDa, were also generated from the C-terminal region. However, these were removed by the filtration steps on semipermeable membranes as described above. The actual cleavage site was identified by N-terminal sequencing as lysine at position 104. ActiClean Etox affinity columns (Sterogene Bioseparations, Carlsbad, CA) were used to remove potential endotoxin contaminations. Endotoxin levels were determined by Endosafe (Charleston, SC).
Detection of Secondary Product of apm-1 in Human Plasma After Immunoprecipitation.
To detect apm-1 cleavage products, preimmune serum or serum raised against the globular head domain or human nonhomologous region (HDQETTTQGPGVLLPLPKGA) was cross-linked to protein A (Sigma) with the use of dimethylpimelimidate dihydrochloride (Sigma) and used to immunoprecipitate apm-1 from human serum. After washing (0.2 M salt), proteins were eluted from protein A, separated by SDS-PAGE, and transferred to nitrocellulose membrane (Schleicher & Schuell) by standard procedures. Apm-1 products were visualized with the use of globular head domain antibodies labeled with biotin, horseradish peroxidase conjugated to streptavidin, and a CN/DAB substrate kit (Pierce) according to the manufacturer's instructions.
Oleate Oxidation in Isolated Muscles and in Cultured Cells.
Oleate oxidation in isolated muscle was measured as previously described (15). Briefly, mice were killed by cervical dislocation, and soleus and extensor digitorum longus (EDL) were rapidly isolated from the hind limbs. All incubations were carried out at 30°C in 1.5 ml of Krebs–Henseleit bicarbonate buffer (118.6 mM NaCl/4.76 mM KCl/1.19 mM KH2PO4/1.19 mM MgSO4/2.54 mM CaCl2/25 mM NaHCO3,/10 mM Hepes, pH 7.4) supplemented with 4% FFA-free BSA (fraction V, RIA grade; Sigma) and 5 mM glucose (Sigma). The concentration of oleate (Sigma) throughout the experiment was 0.25 mM. All media were oxygenated and hydrated (95% O2/5% CO2) throughout incubation. Muscles were first rinsed for 30 min and then transferred to fresh medium (1.5 ml) in the presence of 1 μCi/ml [1-14C]oleic acid (American Radiolabeled Chemicals, St. Louis). The incubation vials containing this medium were sealed with a rubber septum from which a center well carrying a piece of Whatman paper was suspended. After an initial incubation period of 10 min with constant oxygenation, gas circulation was removed to close the system to the outside environment, and the muscles were incubated for 90 min. Subsequently, 0.45 ml of Solvable (Packard) was injected onto the Whatman paper in the center well. After 5 min on ice, the muscle was removed from the medium. The vials were closed again, and 1 ml of 35% perchloric acid was injected with a syringe into the medium. After a 90-min collection period, the Whatman paper was removed from the center well, and the amount of 14C radioactivity was determined by liquid scintillation counting. The rate of oleate oxidation was expressed as nmol oleate produced in 90 min/g muscle. To test the effect of gAcrp30 or Acrp30 on oleate oxidation, these proteins were added to the medium at a final concentration of 2.5 μg/ml and maintained in the medium throughout the procedure.
Cultured C2C12 skeletal muscle cells and Hepa-1–6 hepatocytes (CRL-1830; American Type Culture Collection) were maintained according to the supplier's instructions. The oleate oxidation assay was performed as previously described (16). Briefly, nearly confluent myocytes were kept in differentiation medium (DMEM, 2.5% horse serum) for 7 days, at which time formation of myotubes became maximal. Hepatocytes were kept in the same DMEM supplemented with 10% FCS for 2 days. One hour before the experiment the medium was removed and 1 ml of preincubation medium (MEM/3 mM glucose/4 mM glutamine/25 mM Hepes/1% FFA-free BSA/0.25 mM oleate/5 μg/ml gentamycin) was added. At the start of the oxidation experiment [1-14C]oleic acid (1 μCi/ml; American Radiolabeled Chemicals) was added, and cells were incubated for 90 min at 37°C in the absence/presence of 2.5 μg/ml gAcrp30. After the incubation period 0.75 ml of the medium was removed and assayed for 14CO2 as described above.
Statistical Analysis.
Data are expressed as means ± SEM, a P value less than 0.05 was considered statistically significant. Statistical analysis was done by unpaired Student's t test unless otherwise indicated, with the use of sas software, Version 6.12.
Results
To determine whether Acrp30 can be processed into smaller fragments, we immunoprecipitated human plasma followed by Western blotting, with the use of anti-sera specific for either the globular domain or the nonhomologous sequence, as shown in Fig. 1A. A smaller form of apm-1, the human homolog of Acrp30, was detected with the use of a globular head-specific anti-serum for immunoprecipitation as well as for immunoblotting (Fig. 1B, Lane II. The apparent molecular mass of this truncated form was 27 kDa, corresponding to about 70% of the complete form of apm-1. This truncated form was not detected by immunoprecipitation with a different antibody directed against the human nonhomologous region of apm-1 when the globular domain-specific anti-serum was used for Western analysis (Fig. 1B, Lane III). As shown in Fig. 1A, this domain is located at the amino-terminal end of the full-length protein, outside of the globular domain. By immunoprecipitation, both the globular and the nonhomologous domain anti-sera identified the full-length form of the protein, as well as a low-abundance dimer with an apparent molecular mass of 74 kDa (1) (Fig. 1B, Lanes II and III).
We produced a recombinant form of Acrp30 protein that has an apparent molecular mass of 37 kDa and forms a dimer of 74 kDa (Fig. 1C, Lane V). With the use of acetylated trypsin we generated a proteolytic fragment of Acrp30 that contains the entire globular head region (gAcrp30) and migrates with an apparent molecular mass of approximately 16 kDa (Fig. 1C, Lane IV). “ActiClean Etox” affinity columns were used to remove potential endotoxin contaminations, and both protein preparations (Acrp30 and gAcrp30) were free of detectable endotoxin. As determined by amino-terminal sequencing of purified gAcrp30, the site of cleavage was just before amino acid 104.
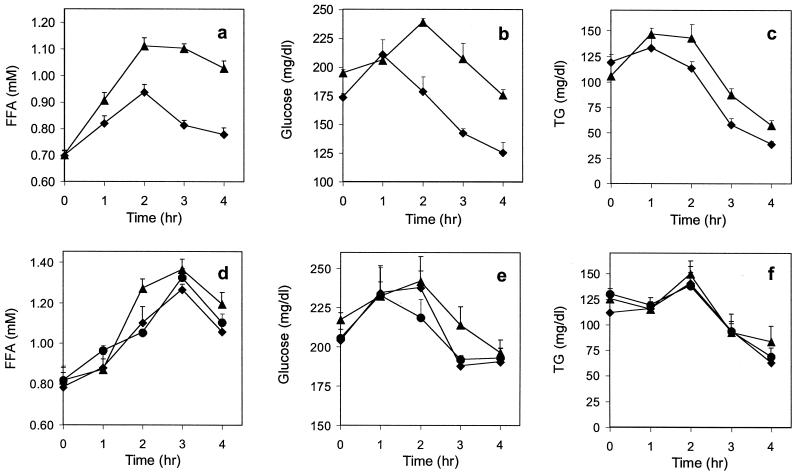
To investigate the physiological role of Acrp30, we fed normal C57BL/6J mice a high-fat/sucrose meal and followed the postprandial response of plasma FFAs, glucose, and triglycerides (Fig. 2). Saline-injected animals showed the expected rise in plasma FFAs, glucose, and triglycerides after the meal (Fig. 2 a–c). In contrast, injection of gAcrp30 (25 μg) at the time of gavage and again at 45 and 105 min significantly decreased the levels of plasma FFAs, glucose, and triglycerides (Fig. 2 a–c). This regulatory effect of gAcrp30 was most pronounced on FFAs and on glucose. Treatment with gAcrp30 also lowered triglyceride plasma levels significantly between 2 and 4 h. A second set of animals was treated with the complete form of Acrp30, following the same schedule of injections (Fig. 2 d–f). The dose given was either the same as used with gAcrp30 (Fig. 2 d–f, filled diamonds) or 2-fold higher (Fig. 2 d–f, filled circles) to achieve equimolar amounts. Only transient effects on plasma FFAs (at 2 h) and on glucose at 3 h (Fig. 2 d and e) were observed in mice injected with the full-length protein. No significant effect on plasma triglycerides was detectable (Fig. 2F). The results of this experiment were confirmed in a second study performed 1 week later with the same animals, but for which the treatment groups were reversed.
Figure 2.
Effect of complete Acrp30 and globular region of Acrp30 (gAcrp30) on plasma FFA, glucose, and triglyceride levels in C57BL/6J mice after a high-fat meal. (a–c) Two groups of animals (▴, control; ⧫, gAcrp30-treated; n = 8, each) were fasted for 3 h before the experiment before a baseline blood sample was taken. A high-fat/sucrose meal was given by gavage (vol. = 1% of body weight). Saline or 25 μg gAcrp30 was injected immediately after the high-fat meal and again at 45 min and at 1 h 45 min. Treatment with gAcrp30 resulted in a significant reduction of plasma FFAs at 1–4 h and of glucose and triglycerides at 2–4 h (P < 0.05). (d–f) A similar experiment was conducted in three groups of animals (▴, control, n = 6; ⧫, 3 × 25 μg; ●, 3 × 50 μg full-length Acrp30 treated, n = 4 each). In contrast to the globular head protein, treatment with Acrp30 showed only very small effects, and a significant reduction of plasma FFAs was only seen at 2 h, and a reduction of glucose was seen at 3 h (P < 0.05). All control animals were injected with saline. Blood samples were immediately put on ice; plasma was prepared and kept at −20°C; and triglycerides, FFAs, and glucose were determined within 24 h.
We questioned whether these effects of gAcrp30 on plasma FFAs, glucose, and triglycerides were direct or resulted from secondary effects of insulin, leptin, or glucagon. As shown in Table 1, treatment of mice with gAcrp30 following the protocol described above did not significantly affect leptin, insulin, or glucagon levels, whereas it significantly reduced the rise in plasma FFA levels seen in saline-treated control animals.
Table 1.
Effect of gAcrp30 on plasma leptin, insulin, and glucagon in C57BL/6J mice
| 0 h | 2 h | Change (%) | P value | ||
|---|---|---|---|---|---|
| FFA, mM | Saline | 0.74 ± 0.05 | 1.08 ± 0.04 | 46 ± 4 | |
| gAcrp30 | 0.81 ± 0.04 | 0.87 ± 0.06 | 8 ± 7 | <0.01 | |
| Leptin, ng/ml | Saline | 4.67 ± 0.12 | 3.81 ± 0.38 | −18 ± 10 | |
| gAcrp30 | 4.38 ± 0.58 | 3.80 ± 0.24 | −13 ± 6 | 0.59 | |
| Insulin, ng/ml | Saline | 0.32 ± 0.03 | 0.68 ± 0.04 | 112 ± 11 | |
| gAcrp30 | 0.45 ± 0.06 | 1.17 ± 0.09 | 160 ± 16 | 0.18 | |
| Glucagon, pg/ml | Saline | n.d. | 163 ± 12 | ||
| gAcrp30 | n.d. | 181 ± 7 | 0.20 |
Two groups of animals (n = 8 each) were treated with saline or 25 μg gAcrp30, and a high-fat/sucrose test meal was given as described in Fig. 2. Blood samples were taken at 0 and 2 h after the test meal, and plasma FFAs, leptin, insulin, and glucagon were determined. Treatment with gAcrp30 significantly reduced the level of FFAs at the peak of the postprandial response (2 h). The treatment had no effect on leptin; insulin and glucagon levels were both only marginally higher at 2 h. The P values (two-tailed t test) shown were calculated for the normalized values, except for glucagon levels, in which case plasma concentration was measured at the 2-h time point only. Values are expressed as mean ± SEM.
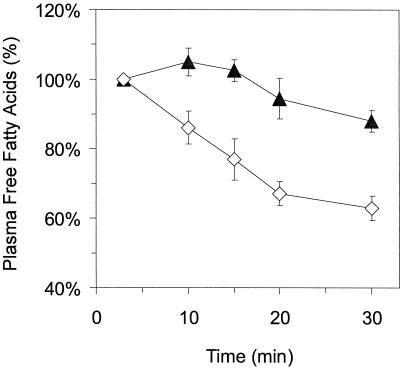
To investigate whether gAcrp30-induced decreases in plasma FFA levels were caused by a decreased rate of nutrient absorption, we injected mice i.v. with a bolus of Intralipid, an i.v. fat emulsion used in nutritional therapy, thus by-passing intestinal absorption. The effect of gAcrp30 on the decay of plasma FFAs after the peak induced by Intralipid injection was then monitored. As shown in Fig. 3, gAcrp30 accelerates the removal of FFAs from plasma after Intralipid injection. Thus gAcrp30 accelerates the clearance of FFAs without interfering with intestinal absorption.
Figure 3.
Treatment with gAcrp30 accelerates the removal of plasma FFAs after Intralipid injection. Two groups of mice (n = 5 each) were injected i.v. with 30 μl of Intralipid-20%. A treated group (◊, gAcrp30-treated) was injected with gAcrp30 (25 μg) at 30 and 60 min before Intralipid was given, and control animals (▴, control) received saline. Plasma was isolated, and FFAs were measured as described in Materials and Methods. Three minutes after Intralipid injection, FFA levels rose from 1.07 ± 0.06 to 1.43 ± 0.2 in control animals and from 1.00 ± 0.08 to 1.62 ± 0.15 in gAcrp30-treated animals. Clearance of FFA from plasma measured at later time points was normalized to FFA levels at 3 min after Intralipid injection (100%). Excursion of plasma FFA between saline- and gAcrp30-treated mice was significantly different at P < 0.05 by repeated measure ANOVA with Fisher's probable least-squares difference for post hoc analysis.
The reduction in plasma FFA concentration cannot be explained by inhibition of lipoprotein lipase, as this would cause an increase in plasma triglycerides, and a decrease of plasma triglycerides is actually observed (Fig. 2). In vitro testing for inhibition of hormone-sensitive lipase activity in isolated adipocytes showed no evidence of any inhibitory effect of Acrp30 or of gAcrp30 (data not shown). Thus, the simplest explanation is that gAcrp30 causes increased removal of FFAs from the circulation by promoting cellular uptake.
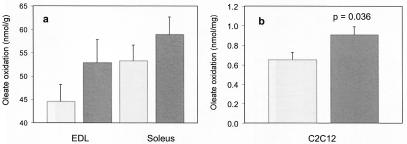
To investigate the effect of gAcrp30 on muscle FFA oxidation, we isolated intact hind limb muscles from C57BL/6J mice and measured FFA oxidation with oleate as substrate (17). Two muscles of different oxidative capacity (soleus and EDL) were tested. To directly compare the effects within the same animal, the muscles isolated from the left and right hind legs of previously untreated animals were incubated under control or treatment conditions. Fig. 4A shows that incubation of EDL and soleus muscles for 90 min in medium containing 2.5 μg/ml gAcrp30 leads to a statistically significant increase in oleate oxidation (P = 0.0041, repeated measures ANOVA, univariate tests of hypotheses for within-subject effects) in both muscle types. In agreement with previous reports, soleus has higher FFA oxidative activity than does EDL (17), but both muscle types showed a significant response to gAcrp30. The relative increase in FFA oxidation was 19% and 11% for EDL and soleus, respectively. At first analysis, such changes might not be considered dramatic. However, in average humans muscle represents 25% of body weight. Therefore, even a moderate increase in FFA oxidation can have quantitatively important consequences for overall energy utilization.
Figure 4.
Effect of gAcrp30 treatment on fatty acid metabolism in isolated muscle and in C2C12 cells. (a) EDL and soleus muscles were isolated from both legs of normal C57BL/6J mice (n = 17). One muscle of each pair was incubated in medium with 2.5 μg/ml gAcrp30 (dark gray) and one in medium without gAcrp30 (control, light gray). This experimental design allowed us to compare oleate oxidation in pairs of muscles isolated from the same animal. [1-14C]Oleate oxidation was determined over 90 min. Incubation with gAcrp30 led to a statistically significant increase in oleate oxidation in both muscles (P = 0.0041, repeated measures ANOVA, univariate tests of hypotheses for within-subject effects). (b) Oleate oxidation was also measured in differentiated skeletal muscle C2C12 cells. The formation of 14CO2 was determined in untreated control cells (light gray) and in cells incubated for 90 min in the presence of 2.5 μg/ml gAcrp30 (dark gray). Each experiment was performed in triplicate.
Further experiments were carried out to confirm the stimulatory effects of gAcrp30 on FFA metabolism selectively in skeletal muscle. We tested the effect of gAcrp30 on the rate of oleate oxidation in differentiated cultured C2C12 murine skeletal muscle cells and in the Hepa1-6 hepatocyte cell line. Oleate oxidation in C2C12 cells determined over 90 min increased significantly (39%; P = 0.036, two-tailed t test) in cells treated with gAcrp30 (Fig. 4B). In contrast, no detectable increase in the rate of FFA oxidation was seen in hepatocytes incubated with gAcrp30 (data not shown).
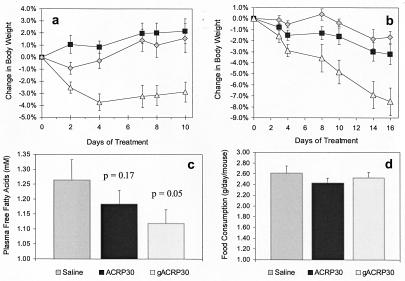
The impact of gAcrp30-induced lipid metabolism alteration on overall energy homeostasis was examined in two independent studies. In the first, 10-week-old male C57BL/6J mice were put on a high-fat/sucrose diet for 19 days. The mice were then surgically implanted with an osmotic pump (Alzet, Newark, DE) delivering 2.5 μg gAcrp30/day, 5 μg Acrp30/day, or physiological saline. The mice were continued on the high-fat diet, and their body weight was recorded over the following 10-day period. Mice treated with saline or 5 μg full-length Acrp30/day continued to gain weight at an average daily rate of 0.16% and 0.22%, respectively. In contrast, mice treated with gAcrp30 experienced a significant weight reduction (−3.7%, P = 0.002) during the first 4 days, and then their weight remained constant (Fig. 5A). Thus, in this inbred strain of normal mice, a continuous infusion of a daily low dose of gAcrp30 can prevent weight gain caused by high-fat/sucrose feeding, in a sustainable way.
Figure 5.
Effects of long-term treatment with gAcrp30 on mice fed a high-fat diet. (a) Ten-week-old male C57BL/6J mice were put on a high-fat/sucrose diet for 19 days. The average body weights at this time were 29.0 ± 2.4, 29.3 ± 2.2, and 32.0 ± 2.6 g for control, Acrp30, and gAcrp30 groups, respectively. The mice were then treated by continuous infusion with either 2.5 μg gAcrp30/day (▵), 5 μg Acrp30/day (■), or physiological saline (◊). The mice were continued on the high-fat diet, and their body weight was recorded. Mice treated with gAcrp30 experienced a significant weight reduction (−3.7%, P = 0.002) during the first 4 days. This reduction in body weight remained significant throughout the study. (b) C57BL/6J mice were fed a high-fat diet for more than 6 months before treatment. The average body weights at this time were 51.2 ± 4.5, 51.9 ± 4.1, and 54.3 ± 3.2 g for control, Acrp30, and gAcrp30 groups, respectively. Three groups of mice (n = 8, each) were injected twice daily with gAcrp30 (▵, 25 μg per injection), Acrp30 (■, 25 μg per injection), or saline (◊). Body weights were recorded at the indicated time points. Treatment with gAcrp30 led to significant (P < 0.05) weight loss at day 3. This effect became even more significant as the study continued. The animals had lost 7.5% of their initial body weight at day 16 (P = 0.001). (c) This effect was paralleled by a drop in plasma FFAs, which reached statistical significance (P < 0.05 vs. saline) at day 3 and persisted throughout the study. Shown is the plasma FFA level at day 16 of the study. The initial FFA plasma concentration was the same in all three groups. (d) Daily food intake averaged over the course of the study was not significantly different in either treatment group when compared with saline-injected animals.
This result was confirmed and extended in a second study performed in mature 9-month-old male obese C57BL/6J mice that had been on the same high-fat/sucrose diet for 6 months. The average body weight when the study began was 52.5 ± 0.8 g. Three groups of eight mice were treated with saline, Acrp30, or gAcrp30 for 16 days. Animals in the treated group received 25 μg of protein s.c. twice daily. During the 16-day study period the obese C57BL/6J mice that received gAcrp30 lost 7.5% (P = 0.001) of their initial body weight despite the fact that they were maintained on a high-fat/sucrose diet (Fig. 5B). Saline-treated animals showed only marginal fluctuations in their body weight (P = n.s.). Animals treated with the full-length Acrp30 but at a 10-fold higher dose than that used in the first experiment also lost significant weight (−3.2%, P = 0.025). Interestingly, mice treated with the gAcrp30 continued to lose weight at a steady rate during the 16-day study period, whereas the rate of weight reduction in those treated with the full-length Acrp30 decreased during the later phase of the study. Treatment with gAcrp30 caused a significant reduction in the concentration of plasma FFAs (Fig. 5C). This effect was significant after 3 days of treatment and continued throughout the complete study period. It should be noted, however, that despite this reduction, the plasma FFA concentration of these massively obese animals remains about 40–60% higher than that of normal mice. Food consumption in gAcrp30-treated animals was not significantly different from that of saline- or Acrp30-treated animals (Fig. 5D). A blood chemistry analysis (including determination of serum glutamine-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, urea, creatinine, or bilirubin) performed on the terminal blood samples did not reveal any abnormal plasma parameters (data not shown).
Discussion
Here we show that the purified globular C-terminal domain of Acrp30 exhibits novel pharmacological properties for regulation of body weight and lipid metabolism. Acrp30 is exclusively expressed in and secreted by adipose tissue, and its effects on energy homeostasis are mediated, to a large extent, by a rapid increase in FFA oxidation by muscle. Since the discovery of leptin and TNFα, adipose tissue has been recognized as an important site of synthesis of hormones that regulate whole-body metabolism. Our results suggest that Acrp30 is another adipose tissue-derived hormone that affects fat and perhaps glucose metabolism.
Our results demonstrate that gAcrp30 may regulate energy balance by stimulating muscle FFA oxidation by mitochondria. The molecular mechanism through which gAcrp30 achieves this effect is currently unknown but may involve increased expression of enzymes consisting of the β-oxidation pathway and/or oxidative phosphorylation. It remains to be determined whether gAcrp30 can also promote increased uptake of FFA into muscle cells. Increased FFA uptake may be achieved through stimulation of function or expression of members of a newly identified family of fatty acid transporters or of the CD36 scavenger receptor (18, 19).
Because gAcrp30 is much more potent than full-length Acrp30, we presume that Acrp30 undergoes proteolysis to generate the C-terminal fragment. Immunoprecipitation experiments in this study revealed the presence in plasma of a small amount of a fragment of the human homolog, apm-1, containing the globular domain of the protein. Whether the smaller apm-1 fragment is generated from proteolytic cleavage of full-length apm-1 or from alternative splicing of human apm-1 mRNA remains to be determined.
Interestingly, the three-dimensional structure of the C-terminal globular domain of Acrp30 has homology to that of TNFα (2). However, the physiological effects of the two proteins are very different from each other, and some of the effects of TNFα are in fact the opposite of those of gAcrp30 (11, 20).
The effect of gAcrp30 on weight reduction is most likely a result of its ability to stimulate lipid oxidation. Previous attempts at increasing FFA oxidation in muscle to control body weight have been reported (21, 22). The experimental strategy in these studies was to increase muscle FFA delivery by selectively overexpressing lipoprotein lipase in muscle tissues through a transgenic approach. Both groups of investigators reported significant improvements in the control of body weight in mice placed on either a normal or a high-fat/sucrose diet. However, both groups observed severe muscle and cardiac toxicity in those animals with extremely high lipoprotein lipase activity (>24-fold that of normal) (22). In contrast, gAcrp30 exerts its pharmacological effects differently. The main difference between gAcrp30 administration and lipoprotein lipase overexpression lies in the fact that the latter caused a dramatic increase in muscle FFA content, whereas the former did not (Fruebis et al., unpublished data).
In lean mice subjected to a high-fat test meal, gAcrp30 also significantly lowered plasma glucose levels. Analysis of the time course (Fig. 2) indicates that the glucose-lowering effect of gAcrp30 occurs with a slight delay compared with the effect on plasma FFA concentration. This effect occurred without a significant increase in insulin levels or a decrease in glucagon. Furthermore, gAcrp30 causes lowering of FFA after i.v. injection of Intralipid and stimulates FFA oxidation in the absence of insulin in cultured muscle cells in vitro as well as in isolated muscles. It has been shown that FFA causes impairment of insulin signaling (20, 23). It is thus tempting to speculate that either the decrease in plasma FFA concentration observed in gAcrp30-treated lean mice or a possible reduction in FFA metabolites due to accelerated FFA oxidation might reduce the inhibitory effect of FFA on insulin signaling and hence might increase glucose uptake. We cannot currently rule out the possibility that gAcrp30 directly stimulates Glut-4.
The discovery of gAcrp30 and the possibility of pharmacologically increasing FFA oxidation in muscle offer an important tool for the control of energy homeostasis. Of the tissues that can significantly remove lipids from circulation and cause FFA oxidation, muscle is quantitatively the most important. Thus, gAcrp30 can control energy expenditure by increasing FFA oxidation at the main site of utilization, muscle cells. It remains to be determined whether gAcrp30 also affects exercise performance. At this stage, gAcrp30 provides a unique and novel pharmacological tool that allows for body weight control without interfering with food intake, but through peripheral regulation of lipid partitioning toward oxidation.
Acknowledgments
We thank Drs. J. Bogan, A. Sirotkin, P. Bougnere, and M. Krieger for insightful discussions. This work was supported in part by National Institutes of Health Grant R37 DK47618 (to H.F.L.), by Genset Corp., and by Grant Unit 391 from the Institut National de la Santé et de la Recherche Médicaleto (to B.E.B.). T.-S.T. was supported by an Ares-Serono Foundation postdoctoral fellowship.
Abbreviations
- Acrp30
30-kDa adipocyte complement-related protein
- gAcrp30
globular head domain of Acrp30
- TNFα
tumor necrosis factor-α
- FFA
free fatty acid
- EDL
extensor digitorum longus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.041591798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.041591798
References
- 1.Scherer P E, Williams S, Fogliano M, Baldini G, Lodish H F. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro L, Scherer P E. Curr Biol. 1998;8:335–338. doi: 10.1016/s0960-9822(98)70133-2. [DOI] [PubMed] [Google Scholar]
- 3.Hu E, Liang P, Spiegelman B M. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 4.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 5.Nakano Y, Tobe T, Choi-Miura N H, Mazda T, Tomita M. J Biochem (Tokyo) 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 6.Kishore U, Reid K B. Immunopharmacology. 1999;42:15–21. doi: 10.1016/s0162-3109(99)00011-9. [DOI] [PubMed] [Google Scholar]
- 7.Kavety B, Morgan J I. Brain Res Mol Brain Res. 1998;63:98–104. doi: 10.1016/s0169-328x(98)00264-2. [DOI] [PubMed] [Google Scholar]
- 8.Satoh F, Takahashi K, Murakami O, Totsune K, Ohneda M, Mizuno Y, Sone M, Miura Y, Takase S, Hayashi Y, et al. J Endocrinol. 1997;154:27–34. doi: 10.1677/joe.0.1540027. [DOI] [PubMed] [Google Scholar]
- 9.Kondo N, Kondo J. J Biol Chem. 1992;267:473–478. [PubMed] [Google Scholar]
- 10.Takamatsu N, Ohba K, Kondo J, Kondo N, Shiba T. Mol Cell Biol. 1993;13:1516–1521. doi: 10.1128/mcb.13.3.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peraldi P, Spiegelman B. Mol Cell Biochem. 1998;182:169–175. [PubMed] [Google Scholar]
- 12.Takamatsu N, Kojima M, Taniyama M, Ohba K, Uematsu T, Segawa C, Tsutou S, Watanabe M, Kondo J, Kondo N, et al. Gene. 1997;204:127–132. doi: 10.1016/s0378-1119(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 13.Ross S R, Graves R A, Spiegelman B M. Genes Dev. 1993;7:1318–1324. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- 14.Moitra J, Mason M M, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, Feigenbaum L, Lee E, Aoyama T, Eckhaus M, et al. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Marchand-Brustel Y, Jeanrenaud B, Freychet P. Am J Physiol. 1978;234:E348–E358. doi: 10.1152/ajpendo.1978.234.4.E348. [DOI] [PubMed] [Google Scholar]
- 16.Muoio D M, Seefeld K, Witters L A, Coleman R A. Biochem J. 1999;338:783–791. [PMC free article] [PubMed] [Google Scholar]
- 17.Peters S J, Dyck D J, Bonen A, Spriet L L. Am J Physiol. 1998;275:E300–E309. doi: 10.1152/ajpendo.1998.275.2.E300. [DOI] [PubMed] [Google Scholar]
- 18.Rigotti A, Acton S L, Krieger M. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch D, Stahl A, Lodish H F. Proc Natl Acad Sci USA. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotamisligil G S, Peraldi P, Budavari A, Ellis R, White M F, Spiegelman B M. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 21.Jensen D R, Schlaepfer I R, Morin C L, Pennington D S, Marcell T, Ammon S M, Gutierrez-Hartmann A, Eckel R H. Am J Physiol. 1997;273:R683–R689. doi: 10.1152/ajpregu.1997.273.2.R683. [DOI] [PubMed] [Google Scholar]
- 22.Levak-Frank S, Radner H, Walsh A, Stollberger R, Knipping G, Hoefler G, Sattler W, Weinstock P H, Breslow J L, Zechner R. J Clin Invest. 1995;96:976–986. doi: 10.1172/JCI118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roden M, Price T B, Perseghin G, Petersen K F, Rothman D L, Cline G W, Shulman G I. J Clin Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]