Abstract
A common HIV/AIDS risk reduction strategy among men who have sex with men (MSM) is to limit their unprotected sex partners to those who are of the same HIV status, a practice referred to as serosorting. Decisions to serosort for HIV risk reduction are based on personal impressions and beliefs, and there is limited guidance offered on this community derived strategy from public health services. This paper reviews research on serosorting for HIV risk reduction and offers an evidence-based approach to serosorting guidance. Following a comprehensive electronic and manual literature search, we reviewed 51 studies relating to the implications of serosorting. Studies showed that HIV negative MSM who select partners based on HIV status are inadvertently placing themselves at risk for HIV. Infrequent HIV testing, lack of HIV status disclosure, co-occurring STIs, and acute HIV infection impede the potential protective benefits of serosorting. Public health messages should continue to encourage reductions in numbers of sexual partners and increases in condom use. Risk reduction messages should also highlight the limitations of relying on one’s own and partner’s HIV status in making sexual risk decisions.
Keywords: serosorting, acute infection, HIV testing, prevention messages
Introduction
Sexual transmission accounts for the vast majority of HIV infections and men who have sex with men (MSM; CDC, 2007) consistently account for a majority of HIV infections in North America and Western Europe. In response to the continued risk of HIV infection among MSM, partner selection strategies to reduce the likelihood of HIV infection have emerged (Parsons et al., 2005). One such strategy is limiting unprotected sexual partners to those who have the same HIV status or serosorting (Clatts, Goldsamt, & Yi, 2005; Elford, Bolding, & Hart, 2007; Mao, 2006; Xia, 2006). Serosorting is a common HIV prevention practice, with 21% to 62% of both HIV positive and HIV negative MSM reporting serosorting to reduce HIV transmission risks (Eaton et al., 2007; Golden et al., 2007; Mao et al., 2006; Xia et al., 2006).
Serosorting practices stem from multiple motivations, most salient of which appear to be intentions to maintain a sense of personal safety while avoiding condom use (Ostrow, 2008; Stolte et al., 2006). Serosorting may also allow for an escape from stigma related to sexual orientation or HIV status and the opportunity to experience sex as a natural behavior. As such, changing community norms and risk perceptions have facilitated the use of protective alternatives in place of condoms. The perceived threat of HIV has decreased with increased HIV treatment optimism and beliefs that HIV treatments eliminate the risk for HIV transmission (Sullivan, Drake, & Sanchez, 2007; Kalichman et al., 2007).
In theory, selecting sex partners of the same HIV status should offer protection against transmission. However, in practice, the protective value of serosorting may be questionable. In this paper we focus on the nuances of partner selection strategies that fall under the rubric of serosorting and explicate the necessary assumptions for serosorting to be effective. To frame our review we define serosorting and its risks differently for (1) people who have tested HIV positive and seek HIV positive partners and (2) for people who test HIV negative and seek HIV negative sex partners. For HIV positive persons, who can be certain of their HIV status, serosorting can provide benefits. However, unprotected sex between HIV infected persons carries risks, namely HIV superinfection and sexually transmitted infections (STI), that should be considered when making sexual decisions. In contrast, serosorting for persons who test HIV negative does risk new HIV infections. For HIV negative persons, the necessary features of effective serosorting are hinged on accurate knowledge of one’s own and partner’s HIV status. It is these features that make up the nuances of serosorting that are the focus of this review.
Literature Reviewed
We conducted a comprehensive literature search in August 2008 using several search engines and manual searches of journals, with key terms serosorting, HIV status, partner selection, and sexual risk behaviors. Studies differed in their operational definitions of serosorting. For example, some studies defined serosorting by participants explicitly stating that they intentionally limit their unprotected sex partners to those who are of the same HIV status. In contrast, other studies defined serosorting based on sexual behaviors, namely persons who exclusively report having same HIV status unprotected partners regardless of whether or not they are motivated to select partners based on HIV status. For the purposes of this review, we included studies that employed either an identity-based or behavioral definition of serosorting.
In total, 51 studies were included in the review. These studies either directly investigated serosorting as a preventive practice or provided key information on whether or not people have accurate knowledge of own and partner’s HIV status. Overall, we identified 25 studies on the prevalence and/or specific implications of serosorting, 8 studies on HIV status disclosure in relation to serosorting, 10 studies on HIV testing related to serosorting, 7 studies addressing issues related to STI/HIV coinfection, 6 studies on HIV superinfection, 4 studies detailing the impact of acute HIV infection, with several of these studies offering data on more than one aspect of serosorting.
Serosorting among HIV Positive MSM
Serosorting as a partner selection strategy has likely occurred largely undefined for many years among HIV positive men and only more recently has been labeled as such and addressed in research. HIV positive men may be motivated to seek out same status partners due to several factors including: altruism - not wanting to further spread the virus (O’Dell et al., 2008), legal concerns around HIV disclosure – the criminalization of HIV transmission (Galletly & Pinkerton, 2006), and psycho-social factors – stigma, fear of rejection, and safe sex fatigue (Remien & Mellins, 2007). Serosorting can therefore serve an important function in the relationships of many HIV positive MSM. Serosorting allows HIV positive men to remain sexually active and avoid condom use without the risk of infecting others. Moreover, HIV seroconcordance has possibly slowed the number of incident HIV infections (Truong et al., 2006b).
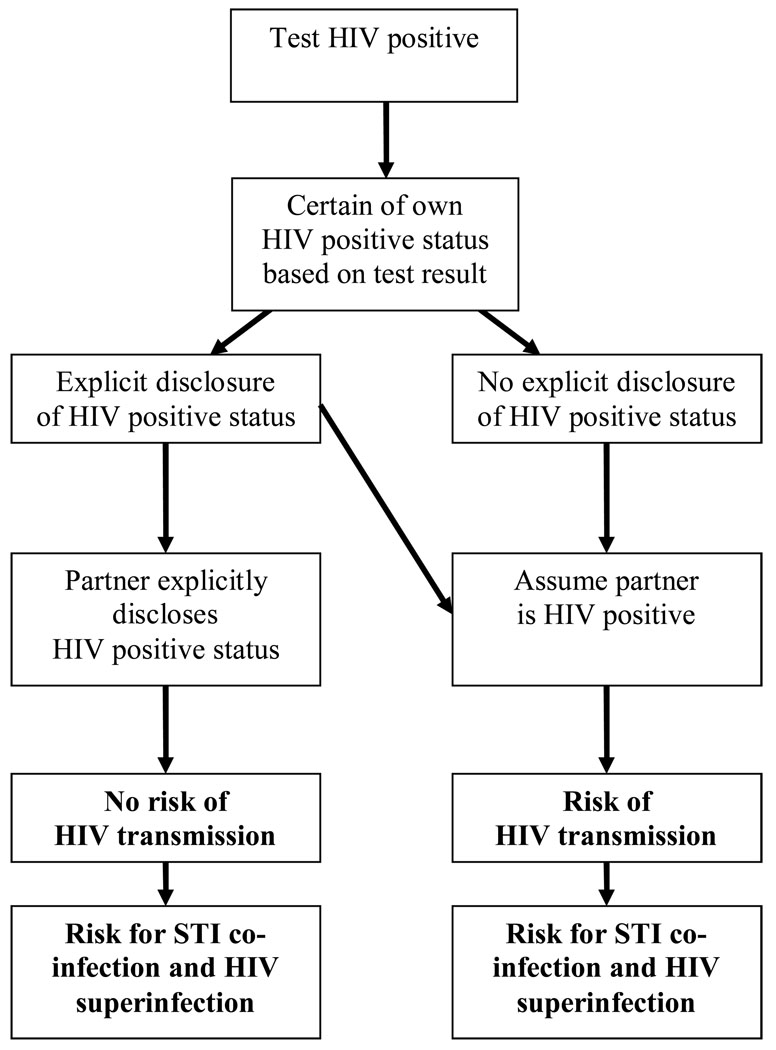
Although serosorting among HIV positive MSM can potentially protect against transmitting the virus to uninfected men, nuances of serosorting practices can threaten the health of HIV positive MSM. Because serosorting involves unprotected sexual acts, it is necessary to consider the implications of all factors related to condomless sex that may affect HIV transmission risks or HIV disease progression, particularly HIV status disclosure, STI coinfection, and HIV superinfection (Colfax et al., 2004). The potential risks and benefits posed by serosorting for HIV positive persons are summarized in Figure 1.
Figure 1.
Paths for HIV transmission and knowledge of one’s own and partner’s HIV status among HIV positive MSM who serosort.
Serosorting and HIV Status Disclosure
Accurately knowing the HIV serostatus of sexual partners ultimately determines the protective value of serosorting among people who have tested HIV positive. There are, however, barriers to serostatus disclosure such as stigma and lack of communication skills that seriously impede the effectiveness of serosorting among HIV positive men. It is common for people to make implicit assumptions about their sexual partner’s HIV status as opposed to relying on explicit, verbal discussions in which HIV status is disclosed. As many as one in three HIV positive MSM engage in unprotected anal intercourse (UAI) without ever disclosing their HIV status to their partners (Ciccarone et al., 2003; Grov et al., 2007; Marks & Crepaz, 2001; Wolitski, 1998).
Some studies have shown that a majority of HIV positive MSM report assuming that the HIV status of their sex partners is HIV positive (Parsons et al., 2006). Niccolai et al. (2002) found that the percent agreement between partner’s reported HIV status and partner’s actual HIV status was less than would be expected by chance (kappa = −.06). Inconsistencies between partner’s reported HIV status and actual HIV status may result from a confirmation of status bias: individuals assume, based on non-verbal cues, that their partner’s HIV status is the same as their own (Suarez & Miller, 2001b).
STI Coinfection
HIV positive men who serosort risk coinfection with STIs, which increases infectiousness and can potentially accelerate HIV disease progression. The extent to which STIs affect HIV disease progression differs for specific pathogens. Repeated exposure to ejaculate during UAI is associated with slight declines in specific immune markers, particularly CD4 cell counts, and is most likely due to STI coinfection (Wiley et al., 2000). Syphilis has also been linked to both increased blood HIV load and decreased CD4 cells (Buchacz et al., 2004). Although evidence is somewhat mixed, genital ulcer diseases have been linked to decreased CD4 cells and increased HIV viral load in blood and genital secretions (Dyer et al., 1998; Kalichman et al., 2007; Duffus et al., 2005; Cachay et al., 2007). In general, non-HIV viral infections appear to hasten HIV viral replication by compromising the immune system (White, 2006).
HIV Superinfection
Once considered only a theoretical risk, the potential for HIV superinfection has now been confirmed (Smith et al., 2004). HIV superinfection occurs following reinfection with previously unexposed variants of HIV, resulting in recombinant genetic processes. HIV superinfection is of particular concern because it is associated with antiretroviral drug resistance, increased HIV virulence (Cohen, 1998), acceleration of HIV disease, and increased HIV infectiousness (Blackard, 2002). However, HIV superinfection occurs most frequently among HIV positive persons with more recent HIV infection and even among those who are recently infected, superinfection appears to be a rare event (Grant et al., 2004; Diaz et al., 2005, Gross et al., 2004).
Serosorting among HIV negative MSM
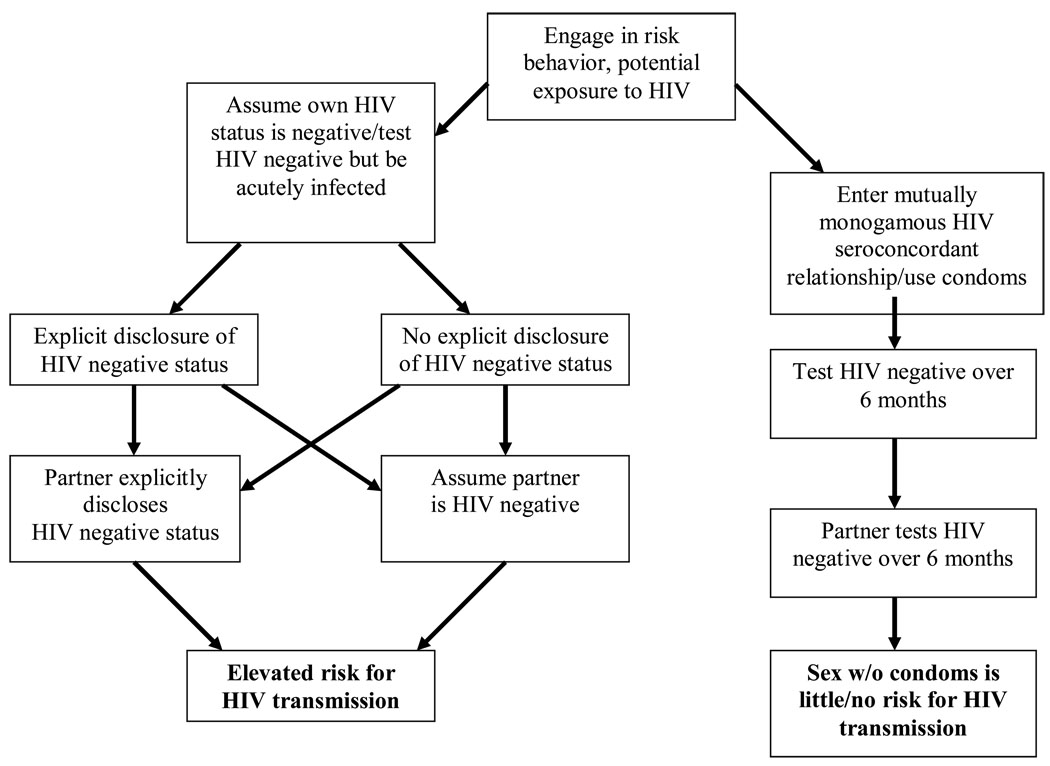
Although some factors related to serosorting among HIV negative MSM are similar to those of HIV positive MSM, such as the central role of HIV status disclosure in partner selection, the behavioral health implications of serosorting are considerably different. Unlike serosorting among persons who have tested HIV positive, serosorting as a risk reduction practice among HIV negative persons engenders considerable risk for new HIV infections. With the potential for acute infection and an overall lack of HIV testing and explicit HIV status disclosure, serosorting alone can not be relied on for preventing HIV infection. Furthermore, co-occurrence of other STI during possible exposure to HIV further undermines the potential protective value of serosorting for HIV negative men. Figure 2 summarizes the potential risks for HIV transmission among persons who test HIV negative and serosort.
Figure 2.
Paths for HIV transmission and knowledge of one’s own and partner’s HIV status among HIV negative MSM who serosort.
Frequency of HIV testing
The effectiveness of serosorting in reducing HIV transmission risks depends on accurately knowing one’s own HIV status and the HIV status of one’s sex partners. Knowing one’s own HIV status requires individuals who engage in unprotected sexual behaviors to frequently test for HIV. Unfortunately, studies have shown that MSM who engage in risk behaviors do test repeatedly but infrequently (Eaton et al., 2007). The studies summarized in Table 1 suggest that it is nearly impossible for persons who engage in high risk behaviors to ever be certain of their HIV status in part because they do not test often enough. HIV testing is even infrequent among persons at risk who receive services where HIV testing is readily available. For example, in a study of 52,260 STI clinic patients HIV infection was greater among unlinked HIV tests conducted on routinely collected blood specimens than it was among individuals who received voluntary counseling and testing notification (Weinstock et al., 2002)..
Table 1.
Reporting HIV negative status and risk of infection for HIV negative MSM
| Authors, Year | Participants | Study Design | Major Findings |
|---|---|---|---|
| Golden et al., 2008 | 8,314 MSM attending an STD clinic in Seattle, WA | Routine clinical assessments were collected between 2001–2007 |
|
| Eaton et al., 2007 | 628 self reported HIV negative MSM from Atlanta, GA | Cross sectional surveys administered at a gay pride festival |
|
| Jin et al., 2007 | 102 recently diagnosed men reporting UAI as event that led to HIV infection living in Sydney and Melbourne, AU | Nurse administered survey collected between 2003–2006 |
|
| Reitmiejer et al., 2007 | 1,400 MSM from the Denver Metro Health Clinic | Cross sectional surveys administered to clients at an outpatient HIV clinics at an STI clinic |
|
| Koblin et al., 2006 | 4,295 HIV negative MSM from 6 US cities | Longitudinal data from EXPLORE-randomized behavioral intervention assessing participants semi- annually up to 48 mo |
|
| MacKellar et al., 2006b | 1,701 MSM from six US cities | YMS, MSM completed surveys, had HIV test taken, and were provided with counseling when needed. Studied occurred between 1998–2000 |
|
| MacKellar et al., 2006a | 2,797 MSM aged 23–29 attending MSM identified venues | Cross-sectional data including HIV testing and counseling on men denying knowledge of HIV positive status |
|
| Golden, 2006 | MSM attending STD clinic | Cross-sectional data were collected by clinicians as part of routine clinical encounters |
|
| Buchbinder et al., 2005 | 3,257 MSM in six US cities from 1995–1997 | HIVNET longitudinal study, participants seen every 6 mo for 18 mos. Eligible participants were HIV negative and reported AI at baseline |
|
| CDC, 2005 | 2,261 men attending MSM identified venues | NHBS collected cross sectional data from five US cities between 2004–2005 |
|
| MacKellar et al., 2005 | 5,649 MSM aged 15–29 from six US cities | YMS using cross-sectional survey at MSM identified venues between 1994–2000 |
|
| Suarez et al., 2001a | 472 MSM in Milwaukee, WI, who reported being HIV negative | Cross-sectional self administered surveys at gay pride festival in 1999. Participants were asked to rate risk associated with behavior |
|
| Golden et al., 2004 | 2,032 MSM who attended an STD clinic in Seattle, WA between 2001–2003 | Cross-sectional data were collected by clinicians as part of routine clinical encounters |
|
| Niccolai et al., 2002 | 76 HIV positive individuals, 24% MSM | Interview administered questionnaires at an STD clinic. Participants provided identifying information about their sexual partners. |
|
| CDC, 2002 | 920 Black MSM aged 15–22 attending an MSM identified venue between 1994–1998 | CDC's Young Men's Survey. Men completed surveys, had HIV test taken, and were provided with counseling when needed |
|
For men at elevated risk, the rate of HIV infection among those who are not aware of their HIV status is alarming. Several studies, including those with large representative samples such as the Young Men’s Survey and the National Behavioral Surveillance system, have shown that HIV infected MSM are often unaware of their HIV positive status (CDC, 2002; Golden et al., 2004; MacKellar et al., 2006a; MacKellar et al., 2006b). The CDC’s (2005) study of HIV/AIDS in MSM found that among men who were HIV infected and unaware of their HIV status, 42% perceived themselves at low risk for currently being HIV infected or becoming infected. In this same study among men who reported anal intercourse in the past six months, 52% reported not using condoms. The most common reasons for not using condoms included either “knowing” they were HIV negative, “knowing” their partner was HIV negative, or believing their partner was at low risk for HIV transmission.
As many as half of all persons recently diagnosed with HIV deny having engaged in risk behaviors with any HIV positive or HIV unknown status partners before testing HIV positive (Golden, 2006). In a retrospective study of recently HIV infected and diagnosed MSM who reported UAI, one in five were certain their sex partner, who was the source of their infection, was HIV negative (Jin et al., 2007). Increased risk for HIV infection is also associated with engaging in sex with HIV negative partners in longitudinal studies (Koblin et al., 2006). Misrepresenting HIV status, or falsely disclosing, may also be an important factor in explaining these findings, with one study reporting that among HIV positive individuals, one in five reported telling a sex partner they were HIV negative since being informed of their HIV positive diagnosis (Golden et al., 2007).
Acute HIV Infection and Infectiousness
Viral replication during the acute infection stage of HIV disease is significantly higher than during chronic HIV infection (Pilcher et al., 2004). Acute HIV infection has been linked to increased likelihood of HIV transmission due to increased infectiousness during this period (Wawer et al., 2005). Further complicating the transmission risk during acute HIV infection is that standard enzyme immunoassays do not detect HIV antibodies during the acute infection phase (Pilcher et al., 2005; Truong et al., 2006a). When acute infection is considered, individuals who are most infectious may test HIV antibody negative, mistakenly disclose they are HIV negative, and engage in unprotected sex believing that they are not placing their partners at risk (Butler & Smith, 2007).
Exposure to STI and Increased Risk for HIV infection
An abundance of research indicates that the presence of both ulcerative (HSV, syphilis) and non-ulcerative (Chlamydia, gonorrhea) STIs is associated with increased risks of HIV infection (Mehta et al., 2006; Koblin et al., 2006; Engels et al., 2007) with one review finding a two to five fold increase in HIV transmission risk as a result of STI (Fleming et al., 1999). The presence of an STI leads to an increased likelihood of becoming HIV infected due to exposure of susceptible cells and portal of entry to the immune system. Thus, men who serosort place themselves at risk for STI which can ultimately increase their susceptibility to HIV infection (CDC, 1999; Kalichman et al., 2008).
Conclusions and Implications
The implications of partner selection strategies for HIV prevention differ for persons who have tested HIV positive versus those who have tested HIV negative. For HIV positive persons and their HIV positive partners, who are able to openly and accurately disclose their HIV status, serosorting under these circumstances eliminates the risks for new HIV infections. However, it is essential that disclosure be explicit rather than implicit, and that both HIV positive partners be aware of the risks to their health posed by co-infection with other STI and potentially superinfection. Furthermore, condom use remains the most effective option for protecting one’s health. For individuals who have tested HIV negative, there is a protective value in serosorting when it is practiced under limited conditions. For couples who test HIV negative within the context of a mutually monogamous relationship, engaging in unprotected intercourse poses little or no risk for HIV infection.
However, for HIV negative MSM who have concurrent or multiple sex partners, serosorting possesses limitations that impede its potential for risk reduction. HIV status of sex partners is often assumed rather than openly discussed, HIV testing is typically infrequent, and considerable risk for HIV since last test is commonly reported. It is important to highlight that HIV testing is insufficient for providing protective knowledge of HIV status when risk behavior continues. Given that risk behaviors may actually increase with serosorting coupled with the biological sequelae of acute infections, it is unlikely that HIV negative MSM who serosort could ever test for HIV frequently enough for testing to provide protection against HIV infection.
Although serosorting appears to be flawed for preventing HIV infection among HIV negative MSM, combing this practice with other measures of prevention may offer opportunities for HIV risk reduction. By serosorting MSM are clearly seeking to meet sexual needs while protecting themselves from HIV. Serosorting combined with strategies such as condom use, strategic positioning, early withdrawal, and negotiated safety may assist MSM in taking rational and calibrated risks, a concept referred to as seroadapting (Le Talec and Jablonski, 2008). Although, with the exception of condom use, these methods alone are probably not highly effective, together they may lead to overall reductions in risk.
Content for interventions that focus on serosorting should emphasize the necessity of explicit HIV status disclosure discussions, including emphasizing errors in assuming own or partner’s HIV status. In particular, stressing that implicit disclosure is not disclosure. For HIV negative MSM who have multiple partners, condom use continues to be the most viable option for preventing HIV transmission and its importance needs to remain a clear message in behavioral interventions. HIV negative individuals who choose serosorting and not condom use as their means for reducing risks for HIV infection must recognize the importance of mutually monogamous relationships for protection against HIV. Additionally, within an intervention context, consideration needs to be given to the fact that some MSM who engage in serosorting will not be familiar with the term or even identify with the term.
From this review, we posit that public health messages pertaining to serosorting include clear statements regarding the difficulty of men who maintain high risk practices to ever test often enough for HIV to know they are not infected. HIV testing must be considered a medical diagnostic rather than an HIV prevention strategy. The ineffectiveness of HIV negative serosorting alone for HIV prevention further illustrates the pressing need for effective risk reduction counseling.
Limitations to this review include the challenges associated with summarizing studies from multiple literature domains. However, this process was necessary to fully appreciate and explore the many characteristics of serosorting that affect its effectiveness as an HIV prevention strategy. These challenges include having to cover a broad spectrum of research in areas relating to public health, psychology, and biology, and identifying information most relevant to serosorting. Moreover, given that serosorting occurs within a broader context of risk reduction strategies and risk behavior, it is difficult to ascertain the absolute effectiveness of serosorting. Finally, to better understand risk taking among ethnic minority MSM, further studying of their partner selection strategies is warranted.
Given the continued high rates of HIV infection among MSM, it is critical that public health service providers and the prevention messages they deliver continue to promote condom use when engaging in anal intercourse or alternatives to UAI. Serosorting among HIV negative men should be discouraged as the sole means of reducing HIV transmission risks. Addressing the limitations of using serosorting for protection against HIV is critical and may prevent further spread of HIV infection among MSM.
Acknowledgments
National Institute of Mental Health (NIMH) Grants RO1-MH71164 and T32- MH074387 supported this research.
References
- Blackard JT, Cohen DE, Mayer KH. Human immunodeficiency virus superinfection and recombination: current state of knowledge and potential clinical consequences. Clinical Infectious Diseases. 2002;34:1108–1114. doi: 10.1086/339547. [DOI] [PubMed] [Google Scholar]
- Buchacz K, Patel P, Taylor M, Kerndt PR, Byers RH, Holmberg SD, Klausner JD, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18:2075–2079. doi: 10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DM, Smith AM. Serosorting can potentially increase HIV transmission. AIDS. 2007;21:1218–1220. doi: 10.1097/QAD.0b013e32814db7bf. [DOI] [PubMed] [Google Scholar]
- Cachay ER, Frost SD, Poon AF, Looney D, Rostami SM, Pacold ME, et al. Herpes simplex virus type 2 infection does not influence viral dynamics during early HIV-1 infection. Journal of Infectious Diseases. 2007;195:1270–1277. doi: 10.1086/513568. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. HIV/AIDS statistics and surveillance. 2007 http://www.cdc.gov/hiv/topics/surveillance/basic.htm#hivaidsexposure.
- Centers for Disease Control and Prevention. HIV prevalence, unrecognized infection, and HIV testing among men who have sex with men---five U.S. cities, June 2004—April 2005. Morbidity and Mortality Weekly Report. 2005;54:597–601. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young black men who have sex with men-six U.S. cities, 1994–1998. Morbidity and Mortality Weekly Report. 2002;51:733–736. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Increases in unsafe sex and rectal gonorrhea among men who have sex with men—San Francisco, California, 1994–1997. Morbidity and Mortality Weekly Report. 1999;48:45–48. [PubMed] [Google Scholar]
- Ciccarone DH, Kanouse DE, Collins RL, Miu A, Chen JL, Morton SC, Stall R. Sex without disclosure of positive HIV serostatus in a US probability sample of persons receiving medical care for HIV infection. American Journal of Public Health. 2003;93:949–954. doi: 10.2105/ajph.93.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatts MC, Goldsamt LA, Yi H. An emerging HIV risk environment: a preliminary epidemiological profile of an MSM POZ party in New York City. Sexually Transmitted Infections. 2005;81:373–376. doi: 10.1136/sti.2005.014894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen OJ, Fauci AS. Transmission of multidrug-resistant human immunodeficiency virus-the wake-up call. New England Journal of Medicine. 1998;339:341–343. doi: 10.1056/NEJM199807303390511. [DOI] [PubMed] [Google Scholar]
- Colfax GN, Guzman R, Wheeler S, Mansergh G, Marks G, Rader M, Buchbinder SP. Beliefs about HIV reinfection (superinfection) and sexual behavior among a diverse sample of HIV-positive men who have sex with men. JAIDS. 2004;36:990–992. doi: 10.1097/00126334-200408010-00017. [DOI] [PubMed] [Google Scholar]
- Diaz RS, Pardini R, Catroxo M, Operskalski EA, Mosley JW, Busch MP. HIV-1 superinfection is not a common event. Journal of Clinical Virology. 2005;33:328–330. doi: 10.1016/j.jcv.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Duffus WA, Mermin J, Bunnell R, Byers RH, Odongo G, Ekwaru P, Downing R. Chronic herpes simplex virus type-2 infection and HIV viral load. International Journal of STDs & AIDS. 2005;16:733–735. doi: 10.1258/095646205774763298. [DOI] [PubMed] [Google Scholar]
- Dyer JR, Eron JJ, Hoffman IF, Kazembe P, Vernazza PL, Nkata E, Costello Daly C, Fiscus SA, Cohen MS. Association of CD4 cell depletion and elevated blood and seminal plasma human immunodeficiency virus type (HIV-1) RNA concentrations with genital ulcer disease in HIV-1-infected men in Malawi. Journal of Infectious Diseases. 1998;177:224–227. doi: 10.1086/517359. [DOI] [PubMed] [Google Scholar]
- Eaton LA, Kalichman SC, Cain DN, Cherry C, Stearns HL, Amaral CM, Flanagan JA, Pope HL. Serosorting sexual partners and risk for HIV among men who have sex with men. American Journal of Preventive Medicine. 2007;33:479–485. doi: 10.1016/j.amepre.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford J, Bolding G, Hart G. No evidence of an increase in serosorting with casual partners among HIV-negative gay men in London, 1998–2005. AIDS. 2007;21:243–245. doi: 10.1097/QAD.0b013e3280118fdb. [DOI] [PubMed] [Google Scholar]
- Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, Cohn S, Whitby D, Goedert JJ. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. Journal of Infectious Diseases. 2007;196:199–207. doi: 10.1086/518791. [DOI] [PubMed] [Google Scholar]
- Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sexually Transmitted Infections. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly CL, Pinkerton SD. Conflicting messages: how criminal HIV disclosure laws undermine public health efforts to control the spread of HIV. AIDS and Behavior. 2006;10:451–461. doi: 10.1007/s10461-006-9117-3. [DOI] [PubMed] [Google Scholar]
- Golden MR, Stekler J, Hughes JP. HIV serosorting in men who have sex with men: is it safe? JAIDS. 2008;49:212–218. doi: 10.1097/QAI.0b013e31818455e8. [DOI] [PubMed] [Google Scholar]
- Golden MR, Wood RW, Buskin SE, Fleming M, Harrington RD. Ongoing risk behavior among persons with HIV in medical care. AIDS and Behavior. 2007;11:726–735. doi: 10.1007/s10461-007-9244-5. [DOI] [PubMed] [Google Scholar]
- Golden M. HIV serosorting among men who have sex with men: implications for prevention. 13th Conference on Retroviruses and Opportunistic Infections.2006. [Google Scholar]
- Golden MR, Brewer DD, Kurth A, Holmes KK, Handsfield HH. (2004). Importance of sex partner HIV status in HIV risk assessment among men who have sex with men. JAIDS. 2004;36:734–742. doi: 10.1097/00126334-200406010-00011. [DOI] [PubMed] [Google Scholar]
- Grant RM, McConnell, et al. “No superinfection among seroconcordant couples after well defined exposure. XV International AIDS Conference.2004. [Google Scholar]
- Gross KL, Porco TC, Grant RM. HIV-1 superinfection and viral diversity. AIDS. 2004;18:1513–1520. doi: 10.1097/01.aids.0000131361.75328.47. [DOI] [PubMed] [Google Scholar]
- Grov C, DeBusk JA, Bimbi DS, Golub SA, Nanin JE, Parsons JT. Barebacking, the internet, and harm reduction: An intercept survey with gay and bisexual men in Los Angeles and New York City. AIDS and Behavior. 2007;11:527–536. doi: 10.1007/s10461-007-9234-7. [DOI] [PubMed] [Google Scholar]
- Jin F, Prestage GP, Ellard J, Kippax SC, Kaldor JM, Grulich AE. How homosexual men believe they became infected with HIV. JAIDS. 2007;46:245–247. doi: 10.1097/QAI.0b013e3181565db5. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, DiBerto G, Eaton L. HIV Viral Load in Blood Plasma and Semen: Review and Implications of Empirical Findings. Sexually Transmitted Diseases. 2008;35:55–60. doi: 10.1097/olq.0b013e318141fe9b. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Eaton L, Cain D, Cherry C, Fuhrel A, Kaufman M, Pope H. Changes in HIV treatment beliefs and sexual risk behaviors among gay and bisexual men, 1997–2002. Health Psychology. 2007;26:650–656. doi: 10.1037/0278-6133.26.5.650. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, Barresi PJ, Coates TJ, Chesney MA, Buchbinder S. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20:731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- Le Talec J-Y, Jablonski O. Seroadapting instead of serosorting: a broader concept and a more precise process model. XVII International AIDS Conference; Mexico City, Mexico. 2008. Conference abstract. [Google Scholar]
- MacKellar DA, Valleroy LA, Anderson JE, Behel S, Secura GM, Bingham T, Celentano DD, Koblin BA, LaLota M, Shehan D, Thiede H, Torian LV, Janssen RS. Recent HIV testing among young men who have sex with men: correlates, contexts, and HIV seroconversion. Sexually Transmitted Diseases. 2006a;33:183–192. doi: 10.1097/01.olq.0000204507.21902.b3. [DOI] [PubMed] [Google Scholar]
- MacKellar DA, Valleroy LA, Behel S, Secura GM, Bingham T, Celentano DD, Koblin BA, LaLota M, Shehan D, Thiede H, Torian LV. Unintentional HIV exposures from young men who have sex with men who disclose being HIV-negative. AIDS. 2006b;20:1637–1644. doi: 10.1097/01.aids.0000238410.67700.d1. [DOI] [PubMed] [Google Scholar]
- MacKellar DA, Vallerory LA, Secura GM, Behel S, Bingham T, Celentano DD, et al. Unrecognized HIV infection, risk behaviors, and perceptions of risk among young men who have sex with men: opportunities for advancing HIV prevention in the third decade of HIV/AIDS. JAIDS. 2005;38:603–614. doi: 10.1097/01.qai.0000141481.48348.7e. [DOI] [PubMed] [Google Scholar]
- Mehta SD, Ghanem KG, Rompalo AM, Erbelding EJ. HIV seroconversion among public sexually transmitted disease clinic patients: analysis of risks to facilitate early identification. JAIDS. 2006;42:116–122. doi: 10.1097/01.qai.0000200662.40215.34. [DOI] [PubMed] [Google Scholar]
- Mao L, Crawford JM, Hospers HJ, Prestage GP, Gruhlich AE, Kaldor JM, Kippax SC. ‘Serosorting’ casual anal sex of HIV-negative gay men is noteworthy and is increasing in Sydney, Australia. AIDS. 2006;20:1204–1205. doi: 10.1097/01.aids.0000226964.17966.75. [DOI] [PubMed] [Google Scholar]
- Marks G, Crepaz N. HIV-positive men’s sexual practices in the context of self-disclosure of HIV status. JAIDS. 2001;27:79–85. doi: 10.1097/00126334-200105010-00013. [DOI] [PubMed] [Google Scholar]
- Niccolai LM, Farley TA, Ayoub MA, Magnus M, Kissinger PJ. HIV-infected persons’ knowledge of their sexual partners’ HIV status. AIDS Education and Prevention. 2002;14(3):183–189. doi: 10.1521/aeap.14.3.183.23893. [DOI] [PubMed] [Google Scholar]
- O’Dell BL, Rosser BR, Miner MH, Jacoby SM. HIV prevention altruism and sexual risk behavior in HIV-positive men who have sex with men. AIDS and Behavior. 2008;12:713–720. doi: 10.1007/s10461-007-9321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow DG, Silverberg MJ, Cook RL, Chmiel JS, Johnson L, Li X, Jacobson LP. Prospective study of attitudinal and relationship predictors of sexual risk in the multicenter AIDS cohort study. AIDS and Behavior. 2008;12:127–138. doi: 10.1007/s10461-007-9223-x. [DOI] [PubMed] [Google Scholar]
- Parsons JT, Severino J, Nanin J, Punzalan JC, von Sternberg K, Missildine W, Frost D. Positive, negative, unknown: assumptions of HIV status among HIV-positive men who have sex with men. AIDS Education and Prevention. 2006;18:139–149. doi: 10.1521/aeap.2006.18.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Schrimshaw EW, Wolitski RJ, Halkitis PN, Purcell DW, Hoff CC, Gomez CA. Sexual harm reduction practices of HIV-seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. AIDS Education and Prevention. 2005;18:139–149. doi: 10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, Ashby R, O’Dowd JO, McPherson JT, Stalzer B, Hightow L, Miller WC, Eron JJ, Cohen MS, Leone PA. Detection of acute infections during HIV testing in North Carolina. New England Journal of Medicine. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Chuan Tien H, Eron JJ, Vernazza PL, Leu S, Stewart PW, Goh L, Cohen MS. Brief but efficient: acute HIV infection and the sexual transmission of HIV. Journal of Infectious Diseases. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- Remien RH, Mellins CA. Long-term psychosocial challenges for people living with HIV: let’s not forget the individual in our global response to the pandemic. AIDS. 2007:S55–S63. doi: 10.1097/01.aids.0000298104.02356.b3. [DOI] [PubMed] [Google Scholar]
- Rietmiejer CA, Lloyd LV, McLean C. Discussing HIV serostatus with prospective partners: A potential HIV prevention strategy among high-risk men who have sex with men. Sexually Transmitted Diseases. 2007;43:215–219. doi: 10.1097/01.olq.0000233668.45976.a1. [DOI] [PubMed] [Google Scholar]
- Smith DM, Wong JK, Hightower BA, Ignacio BS, Koelsch KK, Daar ES, Richman DD, Little SJ. Incidence of HIV superinfection following primary infection. JAMA. 2004;292:1177–1178. doi: 10.1001/jama.292.10.1177. [DOI] [PubMed] [Google Scholar]
- Stolte IG, de Wit JB, Kolader M, Fennema H, Coutinho RA, Dukers NH. Association between ‘safer sex fatigue’ and rectal gonorrhea is mediated by unsafe sex with casual partners among HIV-positive homosexual men. Sexually Transmission Disease. 2006;33:201–208. doi: 10.1097/01.olq.0000194596.78637.8e. [DOI] [PubMed] [Google Scholar]
- Suarez T, Kelly J, Pinkerton SD, Stevenson YL, Hayat M, Smith MD, Ertl T. Influence of partner’s HIV serostatus, use of highly active antiretroviral therapy, and viral load on perceptions of sexual risk behavior in a community sample of men who have sex with men. JAIDS. 2001a;28:471–477. doi: 10.1097/00042560-200112150-00011. [DOI] [PubMed] [Google Scholar]
- Suarez T, Miller J. Negotiating risks in context: a perspective on unprotected anal intercourse and barebacking among men who have sex with men-where do we go from here? Archives of Sexual Behaviors. 2001b;30:287–300. doi: 10.1023/a:1002700130455. [DOI] [PubMed] [Google Scholar]
- Sullivan PS, Drake AJ, Sanchez TH. Prevalence of treatment optimism-related risk behavior and associated factors among men who have sex with men in 11 states, 2000–2001. AIDS and Behavior. 2007;11:123–129. doi: 10.1007/s10461-006-9100-z. [DOI] [PubMed] [Google Scholar]
- Truong HM, Grant RM, McFarland W, Kellogg T, Kent C, Louie B, Wong E, Klausner JD. Routine surveillance for the detection of acute and recent HIV infections and transmission of antiretroviral resistance. AIDS. 2006;20:2193–2197. doi: 10.1097/01.aids.0000252059.85236.af. [DOI] [PubMed] [Google Scholar]
- Truong HM, Kellogg T, Klausner JD, Katz MH, Dilley J, Knapper K, Chen S, Prabhu R, Grant RM, Louie B, McFarland W. (2006). Increases in sexually transmitted infections and sexual risk behavior without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting. Sexually Transmitted Infection. 2006;82:461–466. doi: 10.1136/sti.2006.019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankarnbo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, et al. Rates of HIV-1 tranmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. Journal of Infectious Diseases. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- Weinstock H, Dale M, Linley L, Gwinn M. Unrecognized HIV infection among patients attending sexually transmitted disease clinics. American Journal of Public Health. 2002;92:280–283. doi: 10.2105/ajph.92.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MK, Gorrill TS, Khalili K. Reciprocal transactivation between HIV-1 and other human viruses. Virology. 2006;352:1–13. doi: 10.1016/j.virol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Wiley DJ, Visscher BR, Grosser S, Hoover DR, Day R, Gange S, Chmiel JS, Mitsuyasu R, Detels R. Evidence that anoreceptive intercourse with ejaculate exposure is associated with rapid CD4 cell loss. AIDS. 2000;14:707–715. doi: 10.1097/00002030-200004140-00010. [DOI] [PubMed] [Google Scholar]
- Wolitski RJ, Rietmeijer CAM, Goldbaum GM, Wilson RM. HIV serostatus disclosure among gay and bisexual men in four American cities: general patterns and relation to sexual practices. AIDS Care. 1998;10:599–610. doi: 10.1080/09540129848451. [DOI] [PubMed] [Google Scholar]
- Xia Q, Molitor F, Osmond DH, Tholandi M, Pollack LM, Ruiz JD, Catania JA. Knowledge of sexual partner’s HIV serostatus and serosorting practices in a California population-based sample of men who have sex with men. AIDS. 2006;20:2081–2089. doi: 10.1097/01.aids.0000247566.57762.b2. [DOI] [PubMed] [Google Scholar]