Abstract
Multiple sclerosis (MS) has been suggested to be an autoimmune demyelinating disease of the central nervous system (CNS), whose primary target is either myelin itself, or myelin-forming cells, the oligodendrocytes. Although axonal damage occurs in MS, it is regarded as a secondary event to the myelin damage. Here, the lesion develops from the myelin (outside) to the axons (inside) “Outside-In model”. The Outside-In model has been supported by an autoimmune model for MS, experimental autoimmune (allergic) encephalomyelitis (EAE). However, recently, 1) EAE-like disease has also been shown to be induced by immune responses against axons, and 2) immune responses against axons and neurons as well as neurodegeneration independent of inflammatory demyelination have been reported in MS, which can not be explained by the Outside-In model. Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease (TMEV-IDD) is a viral model for MS. In TMEV infection, axonal injury precedes demyelination, where the lesion develops from the axons (inside) to the myelin (outside) “Inside-Out model”. The initial axonal damage could result in the release of neuroantigens, inducing autoimmune responses against myelin antigens, which potentially attack the myelin from outside the nerve fiber. Thus, the Inside-Out and Outside-In models can make a “vicious” immunological cycle or initiate an immune cascade.
Keywords: Apoptosis, Autoimmunity, Microglia, Mouse Wld protein, Picornaviridae infections, Wallerian degeneration, CD4-Positive T-Lymphocytes, CD8-Positive T-Lymphocytes
Introduction; anti-myelin autoimmunity in multiple sclerosis, the Outside-In model
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) [1–3]. In the United States, MS affects greater than 350,000 people with a prevalence rate of 85/100,000 persons and a ratio of women to men of 2.6:1 [4]. Although the precise etiology of MS is unknown, MS has been thought to be an immune-mediated disease, in which autoimmune responses against myelin antigens lead to production of inflammatory cytokines and chemokines, and upregulation of adhesion molecules, contributing to the pathogenesis of MS [5–9]. The autoimmune etiology of MS has been supported by an animal model for MS, experimental autoimmune (allergic) encephalomyelitis (EAE) [10].
In EAE, demyelination is induced by anti-myelin autoimmune responses, where both cellular (CD4+ and CD8+ T cells) and humoral immune responses play pathogenic roles (Table 1). CD4+ T cells recognize antigens presented by major histocompatibility complex (MHC) class II on antigen presenting cells (APCs). In most EAE models, CD4+ T helper (Th) 1 cells initiate CNS inflammation via delayed-type hypersensitivity (DTH) responses to myelin antigens in the presence or absence of epitope (determinant) spreading [11–14]. Myelin antigen-specific Th17 cells, a novel subset of CD4+ T cells, also play an important role in the induction of EAE [15–17]. Interactions between these CD4+ T cells and CNS APCs (i.e., microglia and macrophages) most likely damage myelin sheaths and myelin forming cells, oligodendrocytes, indirectly by production of cytotoxic factors, such as proinflammatory cytokines, since oligodendrocytes do not express MHC class II molecules [18]. Interferon (IFN)-γ and interleukin (IL)-17 are the major effector cytokines of Th1 and Th17 cells, respectively.
Table 1.
Immune-mediated primary demyelination in EAE: possible patho-mechanisms in the Outside-In model
| Effector | Patho-mechanism | References |
|---|---|---|
| CD4+ T cell | Th1 and Th17 cells against myelin antigens damage myelin sheaths | [11,12,17] |
| Anti-myelin specific Th1 cells induced by epitope spreading | [13,14] | |
| CD8+ T cell | CTLs against myelin antigens attack MHC class I + oligodendrocytes | [3,19,20] |
| B cell | Anti-myelin antibodies exacerbate demyelination | [22,23] |
CTLs, cytotoxic T lymphocytes; EAE, experimental autoimmune (allergic) encephalomyelitis; MHC, major histocompatibility complex; Th, T helper
In some EAE models, MHC class I-restricted myelin-specific CD8+ cytotoxic T lymphocytes (CTLs) have been shown to induce an EAE-like disease [3,19,20]. In these models, myelin sheaths could be damaged by CD8+ T cells either directly or indirectly. Oligodendrocytes can express MHC class I molecules during inflammation, whereas resting oligodendrocytes do not express MHC class I molecules [18,21]. Anti-myelin antibodies have also been shown to play a key role in some EAE models of primary progressive (PP-MS) and secondary progressive MS (SP-MS), where antibody deposition in the CNS and serum anti-myelin antibody responses were associated with disease progression [22]. Co-transfer of auto-antibodies with myelin-specific autoreactive T cells could also exacerbate EAE [23].
Since antibodies against myelin-specific antigens as well as autoreactive T cells have also been identified in MS patients [5,24], CNS lesions in MS have been hypothesized to be induced by autoimmune responses against myelin sheaths as shown in EAE. In this theory, the primary target in MS is myelin itself (myelinopathy) or the oligodendrocytes (oligodendrogliopathy). Axonal degeneration, which is demonstrated in MS and EAE, is regarded as secondary damage following myelin destruction [25–27]. In this process, the lesion develops from the myelin (outside) to the axons (inside) “Outside-In model” [28,29]. In this Outside-In autoimmune model, immune responses against myelin and oligodendrocytes are initiators of CNS damage (Table 1).
Anti-axon autoimmunity in MS and EAE, the Inside-Out model
Recently, gray matter involvement and axonal damage in normal-appearing white matter (NAWM) have been demonstrated in MS [26,29–33]. Magnetic resonance spectroscopy (MRS) has been used to detect the decreased N-acetylasparate (NAA) signal, indicating axonal and neuronal injury in the NAWM [34]. In addition, auto-antibodies against axonal and neuronal antigens, including contactin-2/transiently expressed axonal glycoprotein 1 (TAG-1), neurofilament light chain (NF-L), and neurofascin, have been identified in the serum and cerebrospinal fluid (CSF) of MS patients [5,35–40]. Derfuss et al. [41] showed contactin-2/TAG-1 as an auto-antigen for both Th1/Th17 cells and antibodies in MS patients. In MS, CD8+ CTLs may also damage axons and neurons directly or indirectly; axons and neurons in demyelinating lesions have been shown to express MHC class I by some, but not all, research groups [18,42].
In animals, NF-L- and neurofascin-specific autoreactive T cells and autoantibodies have also been shown to induce axonal degeneration and gray matter inflammation with mild demyelination (Table 2) [40,41,43,44]. Interestingly, mice immunized with axonal and neuronal antigens demonstrated different lesion distribution, compared with those immunized with myelin proteins. For example, immunization with NF-L preferentially induced lesions in the dorsal funiculus of the spinal cord, whereas immunization with myelin oligodendrocyte glycoprotein (MOG) preferentially led to lesions in the lateral and ventral funiculi of the spinal cord [41,45]. Therefore, both in MS and EAE, autoimmune responses against axonal and neuronal antigens could lead to primary axonal and neuronal damage in the CNS, resulting in secondary demyelination. In this theory, the lesion develops from the axons (inside) to the myelin (outside) “Inside-Out model” [28,29]. The Inside-Out model can explain early neurodegeneration, including gray matter involvement and axonal degeneration in NAWM, in some patients with MS and as well as in animal models.
Table 2.
Immune-mediated secondary demyelination in EAE: possible patho-mechanisms in the Inside-Out model
| Effector | Patho-mechanism | References |
|---|---|---|
| CD4+ T cell | NF-L- or contactin-2/TAG-1-specific Th1 and Th17 cells attack axons and neurons | [41,43,44] |
| CD8+ T cell | CTLs attack axons and neurons directly or indirectly | [42] |
| Bcell | Anti-axon and neuron antibodies attack axons and neurons | [40,43] |
NF-L, neurofilament light chain; TAG, transiently expressed axonal glycoprotein
Immune-mediated demyelination in TMEV infection
Environmental factors, particularly viral infections, have been associated with induction or exacerbation of MS [46–49]. Viruses, including human endogenous retrovirus (HERV), Epstein-Barr virus, and human herpesvirus 6, have been linked with MS pathogenesis [50–54]. A mouse model of MS, Theiler's murine encephalomyelitis virus (TMEV) infection, has been widely used to elucidate this possible viral etiology [10,55]. TMEV is a non-enveloped, positive sense, single stranded RNA virus [56]. TMEV belongs to the genus Cardiovirus, family Picornaviridae, and is divided into two subgroups, GDVII and Theiler's original (TO), based on neurovirulence in mice [56]. Intracerebral infection with the GDVII subgroup leads to an acute fatal polioencephalomyelitis in all mouse strains. Infected mice show weight loss and encephalitic signs, including a hunched back and ruffled fur, and die within 10 days [57].
On the other hand, infection with the TO subgroup, such as Daniels (DA) and BeAn strains, causes a biphasic disease [57]. During the acute phase, 1 week postinfection, TMEV infects neurons, and infected mice develop acute polioencephalomyelitis pathologically. But most mice are clinically asymptomatic, and the pathological changes resolve by 2 weeks after infection [57]. Thereafter, virus clearance or persistence depends on the strain of mice. Resistant mouse strains, such as BALB/c and C57BL/6 mice, develop little or no chronic disease, since virus is eradicated from the CNS [58,59]. In contrast, susceptible mouse strains, such as SJL/J mice, develop a chronic inflammatory demyelinating disease in the white matter of the spinal cord with virus persistence in glial cells and macrophages, 1 month after infection (chronic phase) [60].
In TMEV infection, immunopathology (immune-mediated tissue injury) has been shown to play a pathogenic role in the CNS (Table 3). Cellular immune responses seem to play both protective and pathogenic roles in TMEV infection. CD4+ T cells have a vital protective role in the early stage postinfection; mice depleted of CD4+ T cells prior to infection with TMEV die within 3–5 weeks [61]. However, during the early chronic phase, CD4+ T cell-mediated DTH responses against virus in the CNS have been proposed to damage myelin sheaths in a “bystander” fashion [62]. During the late chronic phase, CD4+ T cell responses against myelin antigens, such as myelin proteolipid protein (PLP), can be induced by epitope spreading, and this autoimmunity has been suggested to exacerbate demyelination [63,64].
Table 3.
Virus- and/or immune-mediated demyelination in TMEV infection: possible patho-mechanisms in the Outside-In model
| Effector | Patho-mechanism | References |
|---|---|---|
| CD4+ T cell | DTH responses against virus antigens damage myelin sheaths in a "bystander" fashion | [61,62] |
| Anti-myelin specific Th1 cells induced by epitope spreading | [63,64] | |
| CD8+ T cell | Anti-virus CTLs kill virus-infected and uninfected oligodendrocytes | [3,65–68] |
| B cell | Anti-virus antibodies cross-reacts with myelin lipid, galactocerebroside | [62,70] |
| Virus | Direct virus infection of oligodendrocytes | [74] |
DTH, delayed type hypersensitivity; TMEV, Theiler's murine encephalomyelitis virus
CD8+ T cell responses have also been associated with both pathogenesis and viral clearance in TMEV infection [65,66]. CD8+ MHC class I-restricted virus-specific CTLs have been shown to contribute to viral clearance. However, TMEV infection can result in induction of autoreactive CTLs that recognize both virus and host antigens, which can potentially lead to CNS pathology [66–68]. Similar to cellular immune responses, humoral immune responses against TMEV can play dual roles. Although anti-TMEV antibody contributes to viral clearance [69], anti-TMEV antibody has been demonstrated to cross-react with a major myelin lipid component, galactocerebroside, and passive transfer of anti-TMEV antibody results in augmentation of demyelination in mice with EAE [62,70].
TMEV persistently infects macrophage/microglia lineage cells, oligodendrocytes and astrocytes during the chronic phase [56,71]. Macrophages have been suggested to play an effector role in demyelination, since 1) depletion of macrophages ameliorates TMEV-induced demyelination and 2) intracerebral inoculation with a TMEV-infected macrophage cell line induces acute focal demyelination [57,72,73].
Oligodendrocyte death in TMEV infection
On the other hand, virus-induced pathology (direct infection of neuronal cells) can also play a role in demyelination during TMEV infection. Since TMEV infects oligodendrocytes, the myelin-forming cells, both in vivo (during the chronic phase) and in vitro, direct lytic infection of oligodendrocytes could result in demyelination in the absence of immune cells. Roos et al. demonstrated that nude mice, which have a limited T cell response, developed demyelinating lesions following TMEV infection [74]. While the precise mechanism of oligodendrocyte death in vivo is not clear, TMEV leader (L) protein, but not capsid proteins, has been shown to trigger apoptosis in mammalian cells in vitro [75]. Further studies on the role of the L protein as well as other regions of the TMEV genome, such as IRES, will clarify the mechanism of oligodendrocyte apoptosis in vivo (see Box. 1).
Although death of oligodendrocytes induced by direct viral infection can lead to demyelination, autoimmune responses and other patho-mechanisms may also lead to death of oligodendrocytes. Oligodendroglial apoptosis is observed in several demyelinating diseases, including MS and EAE, by terminal deoxynucleotidyl-transferase-mediated dUTP-biotin nick-end labeling (TUNEL) [10,76–80]. During the chronic phase of TMEV infection, TUNEL-positive nuclei were double-stained with both oligodendrocyte and macrophage/microglia markers, but not with viral antigen or the astrocyte marker, glial fibrillary acidic protein (GFAP) [80,81]. Interestingly, oligodendrocyte apoptosis was detected in the white matter of the spinal cord, as early as 1 week after infection with either GDVII or DA virus, without infection seen in oligodendrocytes [82]. Since 1) the apoptotic oligodendrocytes were detected adjacent to degenerated axons during the acute phase of TMEV infection, and 2) the distribution of axonal damage present during the early phase of TMEV infection corresponds to regions where subsequent demyelination occurs during the chronic phase [82], these results suggest that there is an association between oligodendrocyte death and axonal degeneration (discussed below).
Axonal degeneration induced by TMEV infection
Historically, as in EAE and MS studies, most TMEV studies had focused on how oligodendrocytes and myelin sheaths are damaged; axonal degeneration had not drawn much attention until recently. Dal Canto and Lipton first demonstrated axonal damage in susceptible SJL/J mice during the chronic phase of DA virus infection [83]. Axonal degeneration caused by TMEV infection has been investigated by two groups; Tsunoda et al. conducted a time course study of damaged axons from 1 week to the early chronic phase, while Rodriguez et al. investigated axonal loss during the late chronic phase (Table 4) [82,84–86].
Table 4.
Virus- and/or immune-mediated neurodegeneration in TMEV infection: possible patho-mechanisms in the Inside-Out model
| Effector | Patho-mechanism | References |
|---|---|---|
| Virus | Direct virus infection in neurons leads to wallerian degeneration | [55,82,87] |
| Virus/microglia | Axonal degeneration leads to oligodendrocyte apoptosis by the disruption of cross-talk between axons and oligodendrocytes or by locally activated microglia | [10,95,101] |
| Inflammatory cells, microglia/macrophages | Axonal injury recruits inflammatory cells at the site of wallerian degeneration, leading to inflammatory demyelination | [90,93] |
| CD8+ T cell | Perforin+ CTLs damage axons and neurons | [3,88] |
Tsunoda et al. have demonstrated that axonal damage heralds demyelination in TMEV infection [55,82,87]. Damaged axons were detected in the white matter of the spinal cord, using immunohistochemistry against non-phosphorylated neurofilaments, as early as 1 week after DA virus infection. At 1 week postinfection, spinal axonal damage in the white matter was accompanied by neither T cell infiltration nor virus-infected cells; T cells and infected cells were mainly present in the gray matter of the brain. During the subclinical phase (2 to 3 weeks after infection), the extent and distribution of axonal damage in the white matter of the spinal cord were associated with microglia and macrophage activation, but minimal or no inflammation and no viral antigens were detected [82]. Thus, neither T cell nor direct viral attack of axons seems to be required for the induction of this early axonal degeneration. Since obvious demyelination is not observed until 4–5 weeks post-infection, these results demonstrate that axonal degeneration precedes demyelination in TMEV infection (Inside-Out lesion development) (Table 4). In addition, during the early chronic phase, the level of axonal loss is greater than the level of demyelination, which further supports the Inside-Out model in TMEV infection (Fig. 2).
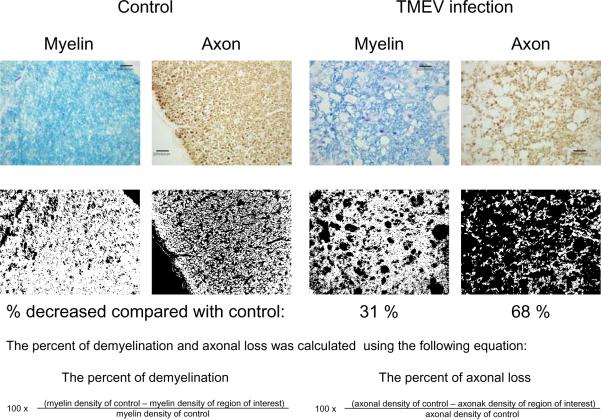
Fig. 2.
The percent of demyelination (myelin loss) and axonal loss during the chronic phase of TMEV infection. Normal axons were immunostained using an antibody cocktail against phosphorylated neurofilament, SMI 312, with diaminobenzidine (DAB) as the chromogen. Myelin was stained using Luxol fast blue. Densities of myelin sheaths and axons were compared in the white matter of the spinal cord between controls and TMEV-infected mice, using Image PloPlus. Sections of TMEV-infected spinal cord showed 31% myelin loss and 68% axonal loss, compared with control sections. This result supports the Inside-Out model of lesion development, whereby axonal damage heralds demyelination in TMEV infection. Scale bars = 20 μm.
During the late chronic phase, Rodriguez et al., using plastic-embedded 1-μm sections, observed spinal cord atrophy in the ventral and lateral funiculi, but not in the dorsal funiculus [84–86]. A significant reduction in spinal cord areas was observed at 195–220 days after TMEV infection, including a 25% reduction in the ventral and lateral funiculi, compared with a 12% area reduction seen at 45 and 92–100 days postinfection. Since spinal cord atrophy was not observed in resistant C57BL/10 mice infected with TMEV, the authors speculated that spinal cord atrophy occurred following demyelination during the chronic phase, but not neuronal infection during the acute phase, since acute neuronal infection occurs in both resistant and susceptible mice. Interestingly, however, the same research group demonstrated that a significant decrease in medium to large myelinated axons in normally myelinated areas occurred on days 195–220 post TMEV infection. They also found increased intra-axonal mitochondria, an indicator of axonal injury, in normally myelinated, remyelinated, and demyelinated axons [86]. Therefore, axonal degeneration may also be independent of demyelination in some areas during the late chronic phase.
The precise mechanism of axonal damage, particularly in NAWM, in TMEV infection in susceptible SJL/J mice, remains unclear. Deb et al. demonstrated that perforin-producing CD8+ T cells play a central role in the induction of axonal degeneration during the late chronic phase, 6 months after virus infection, in mice with a resistant C57BL/6 genetic background (Table 4) [88]. In another viral model for MS, mouse hepatitis virus (MHV) infection, axonal loss seems to occur due to direct attack, rather than occurring secondary to demyelination [89].
Axonal degeneration itself can contribute to secondary neuropathology
In general, axonal degeneration is considered to be an end product in most neuropathological insults. However, experimental and clinical findings demonstrate that axonal degeneration itself can trigger secondary patho-mechanisms, 1) recruitment of inflammatory cells and 2) induction of oligodendrocyte apoptosis. In TMEV infection, axonal damage in the CNS has been shown to contribute to the recruitment of inflammatory cells into the site of axonal degeneration, exacerbating lesion development [90]. In TMEV infection, the distribution of damaged axons observed during the early phase corresponds to regions, where subsequent inflammatory demyelination occurs during the chronic phase [82]. This suggests that axonal degeneration triggers recruitment of T cells and macrophages into the CNS, leading to subsequent loss of myelin. If this is the case, axonal injury recruits inflammatory cells into sites of wallerian degeneration, leading to inflammatory demyelination.
To prove the hypothesis, Tsunoda et al. used an approach for induction of wallerian degeneration in the CNS, which involves injecting Ricinus communis agglutinin (RCA) I (a toxic lectin) into the peripheral nervous system (PNS) [90]. In this experimental system, RCA I, which is injected into the sciatic nerve, is transported axonally (retrogradely), causing cell death of dorsal root ganglion cells and wallerian degeneration of the dorsal funiculus in the spinal cord. Three weeks after TMEV infection, RCA I was injected into the sciatic nerve of SJL/J mice. Neuropathologically, control mice that received TMEV alone, but no RCA I, had inflammatory demyelinating lesions only in the ventral and lateral funiculi, while the other control mice receiving RCA I alone had wallerian degeneration without inflammatory demyelination only in the dorsal funiculus. In contrast, RCA I injection in TMEV-infected mice induced inflammatory demyelinating lesions not only in the ventral and lateral funiculi but also in the dorsal funiculus. This suggests that axonal degeneration itself contributes to recruitment of inflammatory cells into the CNS, targeting lesion development. In this case, lesions are triggered from the axons (inside) to the myelin (outside) “Inside-Out model” (Table 4) [28,90,91].
Similarly, in a passive EAE model, Konno et al demonstrated that adoptive transfer of encepahlitogenic immune cells resulted in inflammatory demyelinating lesion development in accord with the distribution of artificially induced axonal degeneration with activated MHC class II+ microglia; i.e., in the ipsilateral thalamus after cortical cryoinjury, and in the ipsilateral optic nerve, the contralateral optic tract and superior colliculus after unilateral eye ball enucleation [92,93]. Here, the EAE locus may be targeted by axonal degeneration. In MS, the NAWM damage has been correlated with abnormalities in connected gray matter atrophy [94]. These results suggested that, in these regions, there may be a link between the pathological processes occurring in the two compartments.
Axonal degeneration has also been shown to induce oligodendrocyte apoptosis. For example, spinal cord injury (SCI) induces axonal (wallerian) degeneration in the part of the spinal cord distal to the transection site; wallerian degeneration is defined as the changes, such as fragmentation, occurring distal to the site of transaction of a nerve fiber. Then, apoptosis of a substantial number of oligodendrocytes occurs along the fiber tracts undergoing wallerian degeneration, even extending into regions remote from the lesion [28,95,96]. The exact mechanisms of oligodendrocyte apoptosis following SCI remain unclear and are still controversial. Oligodendrocytes have been shown to express apoptosis-related molecules p53, p21, Bcl-2, and Bax, as early as 30 min after experimental SCI [97]. Since axonal degeneration itself can activate resident microglia, oligodendrocyte apoptosis may be caused by soluble toxic factors, such as inflammatory cytokines, produced from activated microglia. Alternatively, oligodendrocyte apoptosis may be induced by glutamate-mediated excitotoxicity [98]. Glutamate receptors are expressed not only on neurons but also on oligodendrocytes and these cells are vulnerable to glutamate mediate excitotoxicity [99]. Glutamate spillover from injured axons has been suggested to damage oligodendrocytes [100].
An alternative mechanism for oligodendrocyte apoptosis occurring as a result of axonal degeneration is the disruption of cross-talk between axons and oligodendrocytes; a failure of cell communication between axons and oligodendrocytes has been proposed in both axonal and oligodendroglial pathology (Table 4) [101]. The survival of the oligodendrocytes depends on the presence of axons, at least during development [102]. Oligodendrocyte apoptosis occurs selectively in transected neonatal optic nerves in which the axons degenerate. The cell death does not occur in optic nerves, if the same experiment is performed in C57BL/Wld (wallerian degeneration slow mutant) (Wld) mice, which are a substrain of C57BL/6 mice and have prolonged survival of the distal stumps of transected axons (see Box 2). Purified neurons, but not neuron-conditioned culture medium, promote the survival of purified oligodendrocytes in vitro [103]. Thus, the cell-to-cell contact between axons and oligodendrocytes is required for the survival of the oligodendrocytes. This supports the hypothesis that oligodendrocytes compete for axon-derived survival signals, helping to adjust the number of oligodendrocytes to the number of axons that require myelination.
Axonal degeneration leads to demyelination: the Inside-Out model
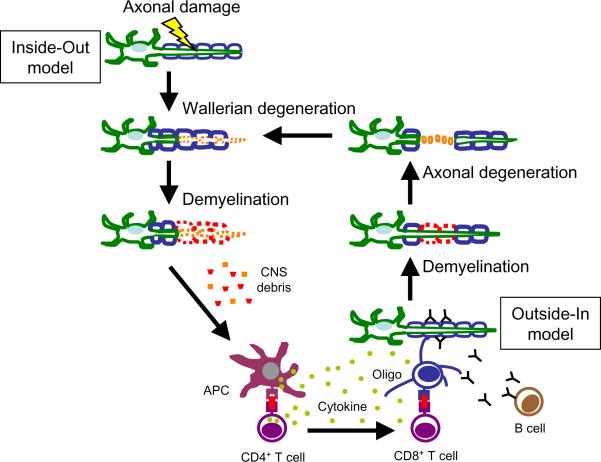
Based on the above findings, we propose a possible patho-mechanism of demyelination and neurodegneration (axonal degeneration) in TMEV infection, i.e. the Inside-Out model, in which the lesion develops from the axons (inside) to the myelin (outside) (Fig. 3). First, TMEV infects neurons in the gray matter of the CNS and damage axons. This leads to degeneration of the distal stumps of axons in the white matter of the spinal cord. Oligodendrocyte apoptosis is induced either by axonal degeneration itself or by direct virus infection [74]; TMEV is able to traffic from the axon into the surrounding myelin [104]. Axonal degeneration and/or (infected) oligodendrocyte apoptosis activate local microglia and macrophages. Activated microglia and macrophages can induce demyelination immunopathologically by secreting inflammatory cytokines, such as tumor necrosis factor (TNF)-α. Activated microglia and macrophages phagocytose degenerated oligodendrocytes (infected or uninfected), myelin and axons, resulting in persistent infection as well as viral antigen presentation by microglia and macrophages [28]. Activated microglia can also produce chemokines and up-regulate adhesion molecules in the CNS, leading to transvascular migration of T cells to areas of axonal degeneration.
Fig. 3.
In TMEV infection, axonal degeneration precedes demyelination “Inside-Out model”; lesions develop from the axons (inside) to the myelin (outside). Virus first infects neurons and damages axons, which lead to wallerian degeneration of the distal stumps of axons, and activation of microglia and macrophages. Then, the axonal degeneration itself as well as virus-infected glial cells and activated macrophages recruit inflammatory cells to the site of wallerian degeneration, leading to demyelination. Damaged CNS debris is captured by microglia and macrophages, which function as APCs, activating CD4+ T cells. This leads to production of inflammatory cytokines, antibody production by B cells, and CD8+ T cells that attack oligodendrocytes (Oligo). At this time point, the autoimmune cells can attack myelin sheaths from the outside, leading to secondary axonal degeneration, “Outside-In model”. Here, the Inside-Out and Outside-In models can collaborate in a “vicious” cycle, initiating a reinforcing cascade.
These activated microglia and macrophages act as APCs, which present neuroantigens and viral antigens to CD4+ T cells. CD4+ T cells mediated DTH response against virus and/or myelin, leading to further demyelination [28]. CD4+ T cells produce cytokines, helping anti-myelin antibody production by B cells as well as killing of oligodendrocytes by CD8+ CTLs. These autoimmune T cells and auto-antibodies may attack myelin according to the Outside-In mechanism. At this time point, the lesion develops from the outside (myelin) to the inside (axons) (Fig. 3), although the initial step of demyelination developed from the inside to the outside. Here, the Inside-Out and Outside-In models are not mutually exclusive but act in synergy, resulting in a reinforcing immune cascade reaction and further disease progression.
Conclusion
Axonal pathology may precede or be independent of demyelination in some patients with MS and EAE models. We reviewed findings that support axonal degeneration preceding demyelination in TMEV infection, “Inside-Out model”. We then discussed the process whereby the Inside-Out and Outside-In models can collaborate to create a “vicious” immunopathological cycle, initiating a reinforcing cascade of events, leading to disease progression. Theoretically, therapeutic strategies targeting each step (axonal degeneration, inflammation, and demyelination), in these models may interfere with or arrest this cascade reaction. We believe that the concept of the Inside-Out and Outside-In models will be helpful in designing therapeutic strategies to prevent disease progression, particularly in primary and secondary progressive MS.
Box 1 A possible role of IRES in TMEV infection.
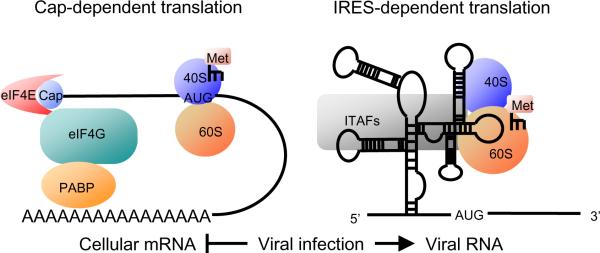
Most of the cellular protein synthesis is initiated by cap-dependent translation which is mediated by the binding of the mRNA 5'-end cap structure and eukaryotic translation initiation factors (eIFs) [105] (Fig. 1 left). During infection with some eukaryotic viruses, host cell mRNA translation is selectively inhibited. A unique viral RNA structure, internal ribosomal entry site (IRES), which allows efficient translation when the cap-dependent translation is inhibited, is identified in 5' UTR of TMEV genome. The IRES structure recruits ribosomes without the binding of cap structure and eIFs, thereby bypassing the cap-dependent translation [106] (Fig. 1 right).
IRES was first discovered in several picornaviruses, whose RNA are naturally uncapped [107]. Later, IRES has also been identified in other viral families and is known as a critical mechanism to maintain viral activity while protein synthesis was shut off in viral-infected host cells [108]. Recently, IRES-dependent translation has also been found even in some cellular mRNAs, such as XIAP, Apaf-1, and Bcl-2, which are involved in inhibition of apoptosis [109–111]. These IRES-dependent gene expressions can help host cell survival when global cap-dependent protein synthesis is inhibited. If the viral infection itself switches the translation mechanism from cap-dependent to IRES-dependent, the virus might obtain advantages by producing simultaneously both viral protein and cellular proteins that are involved in cell survival.
Efficient IRES-dependent translation requires auxiliary cellular proteins, IRES trans-acting factors (ITAFs). These proteins bind to the IRES structure and stimulate the recruitment of ribosomes. TMEV induces apoptosis in neurons during the acute phase and in oligodendrocytes during the chronic phase in vivo. Fan et al. demonstrated that TMEV leader (L) protein triggers apoptosis in mammalian cells in vitro [75]. On the other hand, TMEV mutant virus, H101, which has mutations in the 5'UTR sequence with no mutations in L protein, has been shown to induce apoptosis in vivo only in the meninges, and not in neurons or oligodendrocytes [112]. These findings suggest that the structural difference of 5'UTR might cause alteration of the IRES-dependent translation of L protein, which is mediated by the binding of ITAFs, and that these changes influence induction of apoptosis in different cell types. Research in this field may elucidate the mechanism whereby some neurons seem to survive even after extensive neuronal infection with TMEV.
Box 2 Axonal degeneration as a self destructive defense mechanism against virus infection.
A novel role of axonal degeneration has been proposed from findings of TMEV infection in Wld mice, which are a substrain of C57BL/6 (B6) mice and have prolonged survival of the distal stumps of transected axons [113–115]. Transected axons from Wld mice survive for up to 4 weeks, support action potentials for at least 2 weeks, and continue anterograde and retrograde transport of proteins for similar amounts of time [116–118]. TMEV-infected B6 and Wld mice had similar neuropathology and virus replication in the CNS at 1 week postinfection [119]. However, at 3 weeks after infection, only Wld mice showed clinical signs, substantial inflammation, and viral persistence. Since TMEV is known to infect neurons and spread using axonal flow, delayed axonal degeneration in Wld mice could favor virus transport and persistence in the CNS. In contrast, rapid induction of axonal degeneration in B6 mice prevents viral dissemination in the CNS [119]. Since axonal degeneration has been shown to be a self-destructive physiological process during the development, axonal degeneration in TMEV infection may be a self-destructive defense mechanism that protects from the transport of toxic substances, including virus, in the CNS [87,114].
Fig. 1.
Cap-dependent translation and IRES dependent translation. (Left) eIF4E binds to the cap structure at the 5' end of cellular mRNA. This binding initiates recruitment of ribosomes and methionin-loaded initiator tRNAs on mRNA. (Right) Viral RNAs do not have cap structure. IRES structures recruit the ribosomes and methionin-loaded initiator tRNAs without eIFs. Viral infection can switch the translation mechanism from cap-dependent to IRES-dependent. eIF: eukaryotic translation initiation factor, PABP: poly (A) binding protein, 40S: 40S ribosomal subunit, 60S: 60S ribosomal subunit, Met: methionin-loaded initiator tRNA.
Acknowledgements
The authors thank J. Steven Alexander, PhD, Tomoko Tanaka, MD, and Stephen L. Jaffe, MD for many helpful discussions, and Daniel Doty and Krystal D. Porter BS for excellent technical assistance. This work was supported by the National Institutes of Health (R21NS059724).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Correale J, Fiol M. BHT-3009, a myelin basic protein-encoding plasmid for the treatment of multiple sclerosis. Curr. Opin. Mol. Ther. 2009;11:463–470. [PubMed] [Google Scholar]
- [2].Kira J. Neuromyelitis optica and asian phenotype of multiple sclerosis. Ann. N. Y. Acad. Sci. 2008;1142:58–71. doi: 10.1196/annals.1444.002. [DOI] [PubMed] [Google Scholar]
- [3].Johnson AJ, Suidan GL, McDole J, Pirko I. The CD8 T cell in multiple sclerosis: Suppressor cell or mediator of neuropathology? Int. Rev. Neurobiol. 2007;79:73–97. doi: 10.1016/S0074-7742(07)79004-9. [DOI] [PubMed] [Google Scholar]
- [4].Schwendimann RN, Alekseeva N. Gender issues in multiple sclerosis. Int. Rev. Neurobiol. 2007;79:377–392. doi: 10.1016/S0074-7742(07)79017-7. [DOI] [PubMed] [Google Scholar]
- [5].Luque FA, Jaffe SL. Cerebrospinal fluid analysis in multiple sclerosis. Int. Rev. Neurobiol. 2007;79:341–356. doi: 10.1016/S0074-7742(07)79015-3. [DOI] [PubMed] [Google Scholar]
- [6].Jaffe SL, Minagar A. Demyelinating pseudotumor. Arch. Neurol. 2005;62:1466–1467. doi: 10.1001/archneur.62.9.1466. [DOI] [PubMed] [Google Scholar]
- [7].Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult. Scler. 2003;9:540–549. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- [8].García-Meríno A, Barcenilla H, Diaz D, Monserrat J, Prieto A, Álvarez-Mon M. IFNβ therapy progressively normalizes the increased ex vivo T lymphocyte apoptosis observed in active patients with multiple sclerosis. Clin. Immunol. 2009;132:195–202. doi: 10.1016/j.clim.2009.04.006. [DOI] [PubMed] [Google Scholar]
- [9].Berger JR. Paradoxically aggressive multiple sclerosis in the face of natalizumab therapy. Mult. Scler. 2008;14:708–710. doi: 10.1177/1352458507087135. [DOI] [PubMed] [Google Scholar]
- [10].Tsunoda I, Fujinami RS. Two models for multiple sclerosis: Experimental allergic encephalomyelitis and Theiler's murine encephalomyelitis virus. J. Neuropathol. Exp. Neurol. 1996;55:673–686. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- [11].Tsunoda I, Kuang L-Q, Tolley ND, Whitton JL, Fujinami RS. Enhancement of experimental allergic encephalomyelitis (EAE) by DNA immunization with myelin proteolipid protein (PLP) plasmid DNA. J. Neuropathol. Exp. Neurol. 1998;57:758–767. doi: 10.1097/00005072-199808000-00005. [DOI] [PubMed] [Google Scholar]
- [12].Takacs K, Chandler P, Altmann DM. Relapsing and remitting experimental allergic encephalomyelitis: a focused response to the encephalitogenic peptide rather than epitope spread. Eur. J. Immunol. 1997;27:2927–2934. doi: 10.1002/eji.1830271127. [DOI] [PubMed] [Google Scholar]
- [13].Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- [14].McRae BL, Vanderlugt CL, Dal Canto MC, Miller SD. Functional evidence for epitope spreading in the relapsing pathology of experimental autoimmune encephalomyelitis. J. Exp. Med. 1995;182:75–85. doi: 10.1084/jem.182.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- [16].Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Klemann C, Raveney BJ, Klemann AK, Ozawa T, von Horsten S, Shudo K, Oki S, Yamamura T. Synthetic retinoid AM80 inhibits Th17 cells and ameliorates experimental autoimmune encephalomyelitis. Am. J. Pathol. 2009;174:2234–2245. doi: 10.2353/ajpath.2009.081084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pender MP. An introduction to neuroimmunology. In: Pender MP, McCombe PA, editors. Autoimmune Neurological Disease. Cambridge University Press; Melbourne: 1995. pp. 14–25. Chapter 2. [Google Scholar]
- [19].Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman J. A pathogenic role for myelin-specific CD8(+) T cells in a model for multiple sclerosis. J. Exp. Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J. Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- [21].Suzumura A, Silberberg DH. MHC antigen expression on glial cells. Ann. N. Y. Acad. Sci. 1988;540:495–497. doi: 10.1111/j.1749-6632.1988.tb27148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsunoda I, Kuang L-Q, Theil DJ, Fujinami RS. Antibody association with a novel model for primary progressive multiple sclerosis: Induction of relapsingremitting and progressive forms of EAE in H2s mouse strains. Brain Pathol. 2000;10:402–418. doi: 10.1111/j.1750-3639.2000.tb00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- [24].Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat. Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- [25].Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- [26].Matthews PM, De Stefano N, Narayanan S, Francis GS, Wolinsky JS, Antel JP, Arnold DL. Putting magnetic resonance spectroscopy studies in context: axonal damage and disability in multiple sclerosis. Semin. Neurol. 1998;18:327–336. doi: 10.1055/s-2008-1040884. [DOI] [PubMed] [Google Scholar]
- [27].Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- [28].Tsunoda I, Fujinami RS. Inside-Out versus Outside-In models for virus induced demyelination: axonal damage triggering demyelination. Springer. Semin. Immunopathol. 2002;24:105–125. doi: 10.1007/s00281-002-0105-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Geurts JJG, Stys PK, Minagar A, Amor S, Zivadinov R. Gray matter pathology in (chronic) MS: Modern views on an early observation. J. Neurol. Sci. 2009;282:12–20. doi: 10.1016/j.jns.2009.01.018. [DOI] [PubMed] [Google Scholar]
- [30].Bjartmar C, Kinkel RP, Kidd G, Rudick RA, Trapp BD. Axonal loss in normal-appearing white matter in a patient with acute MS. Neurology. 2001;57:1248–1252. doi: 10.1212/wnl.57.7.1248. [DOI] [PubMed] [Google Scholar]
- [31].Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000;123:308–317. doi: 10.1093/brain/123.2.308. [DOI] [PubMed] [Google Scholar]
- [32].Sharma R, Narayana PA, Wolinsky JS. Grey matter abnormalities in multiple sclerosis: proton magnetic resonance spectroscopic imaging. Mult. Scler. 2001;7:221–226. doi: 10.1177/135245850100700402. [DOI] [PubMed] [Google Scholar]
- [33].Pirko I, Lucchinetti CF, Sriram S, Bakshi R. Gray matter involvement in multiple sclerosis. Neurology. 2007;68:634–642. doi: 10.1212/01.wnl.0000250267.85698.7a. [DOI] [PubMed] [Google Scholar]
- [34].Gonzalez-Toledo E, Kelley RE, Minagar A. Role of magnetic resonance spectroscopy in diagnosis and management of multiple sclerosis. Neurol. Res. 2006;28:280–283. doi: 10.1179/016164106X98161. [DOI] [PubMed] [Google Scholar]
- [35].Newcombe J, Gahan S, Cuzner ML. Serum antibodies against central nervous system proteins in human demyelinating disease. Clin. Exp. Immunol. 1985;59:383–390. [PMC free article] [PubMed] [Google Scholar]
- [36].Terryberry JW, Thor G, Peter JB. Autoantibodies in neurodegenerative diseases: antigen-specific frequencies and intrathecal analysis. Neurobiol. Aging. 1998;19:205–216. doi: 10.1016/s0197-4580(98)00049-9. [DOI] [PubMed] [Google Scholar]
- [37].Bartos A, Fialova L, Soukupova J, Kukal J, Malbohan I, Pit'ha J. Elevated intrathecal antibodies against the medium neurofilament subunit in multiple sclerosis. J. Neurol. 2007;254:20–25. doi: 10.1007/s00415-006-0185-0. [DOI] [PubMed] [Google Scholar]
- [38].Rawes JA, Calabrese VP, Khan OA, DeVries GH. Antibodies to the axolemma-enriched fraction in the cerebrospinal fluid and serum of patients with multiple sclerosis and other neurological diseases. Mult. Scler. 1997;3:363–369. doi: 10.1177/135245859700300601. [DOI] [PubMed] [Google Scholar]
- [39].Lily O, Palace J, Vincent A. Serum autoantibodies to cell surface determinants in multiple sclerosis: a flow cytometric study. Brain. 2004;127:269–279. doi: 10.1093/brain/awh031. [DOI] [PubMed] [Google Scholar]
- [40].Mathey EK, Derfuss T, Storch MK, Williams KR, Hales K, Woolley DR, Al-Hayani A, Davies SN, Rasband MN, Olsson T, Moldenhauer A, Velhin S, Hohlfeld R, Meinl E, Linington C. Neurofascin as a novel target for autoantibody-mediated axonal injury. J. Exp. Med. 2007;204:2363–2372. doi: 10.1084/jem.20071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Derfuss T, Parikh K, Velhin S, Braun M, Mathey E, Krumbholz M, Kumpfel T, Moldenhauer A, Rader C, Sonderegger P, Pollmann W, Tiefenthaller C, Bauer J, Lassmann H, Wekerle H, Karagogeos D, Hohlfeld R, Linington C, Meinl E. Contactin-2/TAG-1-directed autoimmunity is identified in multiple sclerosis patients and mediates gray matter pathology in animals. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8302–8307. doi: 10.1073/pnas.0901496106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hoftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H. Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions. Brain Pathol. 2004;14:43–50. doi: 10.1111/j.1750-3639.2004.tb00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Huizinga R, Heijmans N, Schubert P, Gschmeissner S, `t Hart BA, Herrmann H, Amor S. Immunization with neurofilament light protein induces spastic paresis and axonal degeneration in Biozzi ABH mice. J. Neuropathol. Exp. Neurol. 2007;66:295–304. doi: 10.1097/nen.0b013e318040ad5c. [DOI] [PubMed] [Google Scholar]
- [44].Huizinga R, Hintzen RQ, Assink K, van Meurs M, Amor S. T-cell responses to neurofilament light protein are part of the normal immune repertoire. Int. Immunol. 2009;21:433–441. doi: 10.1093/intimm/dxp011. [DOI] [PubMed] [Google Scholar]
- [45].Huizinga R, Gerritsen W, Heijmans N, Amor S. Axonal loss and gray matter pathology as a direct result of autoimmunity to neurofilaments. Neurobiol. Dis. 2008;32:461–470. doi: 10.1016/j.nbd.2008.08.009. [DOI] [PubMed] [Google Scholar]
- [46].Antony JM, van Marle G, Opii W, Butterfield DA, Mallet F, Yong VW, Wallace JL, Deacon RM, Warren K, Power C. Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 2004;7:1088–1095. doi: 10.1038/nn1319. [DOI] [PubMed] [Google Scholar]
- [47].Kennedy PG. Neurological aspects of lentiviral infections in animals. Baillieres. Clin. Neurol. 1992;1:41–59. [PubMed] [Google Scholar]
- [48].Kennedy PG, Steiner I. On the possible viral aetiology of multiple sclerosis. Qjm. 1994;87:523–528. doi: 10.1093/oxfordjournals.qjmed.a068963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Opsahl ML, Kennedy PG. Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain. 2005;128:516–527. doi: 10.1093/brain/awh390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cermelli C, Jacobson S. Viruses and multiple sclerosis. Viral Immunol. 2000;13:255–267. doi: 10.1089/08828240050144590. [DOI] [PubMed] [Google Scholar]
- [51].Stüve O, Racke M, Hemmer B. Viral pathogens in multiple sclerosis: An intriguing (hi)story. Arch. Neurol. 2004;61:1500–1502. doi: 10.1001/archneur.61.10.1500. [DOI] [PubMed] [Google Scholar]
- [52].Christensen T. The role of EBV in MS pathogenesis. Int. MS. J. 2006;13:52–57. [PubMed] [Google Scholar]
- [53].Berger JR, Tornatore C, Major EO, Bruce J, Shapshak P, Yoshioka M, Houff S, Sheremata W, Horton GF, Landy H. Relapsing and remitting human immunodeficiency virus-associated leukoencephalomyelopathy. Ann. Neurol. 1992;31:34–38. doi: 10.1002/ana.410310107. [DOI] [PubMed] [Google Scholar]
- [54].Saresella M, Rolland A, Marventano I, Cavarretta R, Caputo D, Marche P, Perron H, Clerici M. Multiple sclerosis-associated retroviral agent (MSRV)-stimulated cytokine production in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 2009;15:443–447. doi: 10.1177/1352458508100840. [DOI] [PubMed] [Google Scholar]
- [55].Tsunoda I, Fujinami RS. Neuropathogenesis of Theiler's murine encephalomyelitis virus infection, a viral model for multiple sclerosis. J. Neuroimmune Pharmacol. doi: 10.1007/s11481-009-9179-x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Tsunoda I, Fujinami RS. Theiler's murine encephalomyelitis virus. In: Ahmed R, Chen ISY, editors. Persistent Viral Infections. John Wiley & Sons Ltd.; Chichester, West Sussex: 1999. pp. 517–536. [Google Scholar]
- [57].Tsunoda I, Iwasaki Y, Terunuma H, Sako K, Ohara Y. A comparative study of acute and chronic diseases induced by two subgroups of Theiler's murine encephalomyelitis virus. Acta Neuropathol. 1996;91:595–602. doi: 10.1007/s004010050472. [DOI] [PubMed] [Google Scholar]
- [58].Melvold RW, Jokinen DM, Knobler RL, Lipton HL. Variations in genetic control of susceptibility to Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell beta-chain constant gene on chromosome 6. J. Immunol. 1987;138:1429–1433. [PubMed] [Google Scholar]
- [59].Rodriguez M, Leibowitz J, David CS. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J. Exp. Med. 1986;163:620–631. doi: 10.1084/jem.163.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Roussarie JP, Ruffie C, Brahic M. The role of myelin in Theiler's virus persistence in the central nervous system. PLoS Pathog. 2007;3:e23. doi: 10.1371/journal.ppat.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Borrow P, Welsh CJ, Nash AA. Study of the mechanisms by which CD4+ T cells contribute to protection in Theiler's murine encephalomyelitis. Immunology. 1993;80:502–506. [PMC free article] [PubMed] [Google Scholar]
- [62].Tsunoda I, Fujinami RS. TMEV and neuroantigens: Myelin genes and proteins, molecular mimicry, epitope spreading, and autoantibody-mediated remyelination. In: Lavi E, Constantinescu CS, editors. Experimental Model of Multiple Sclerosis. Springer; New York, NY: 2005. pp. 593–616. Chapter B2. [Google Scholar]
- [63].Olson JK, Miller SD. The role of T cells and the innate immune system in the pathogenesis of Theiler's virus demyelinating disease. In: Lavi E, Constantinescu CS, editors. Experimental Model of Multiple Sclerosis. Springer; New York, NY: 2005. pp. 645–657. [Google Scholar]
- [64].Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- [65].Rodriguez M, Sriram S. Successful therapy of Theiler's virus-induced demyelination (DA strain) with monoclonal anti-Lyt-2 antibody. J. Immunol. 1988;140:2950–2955. [PubMed] [Google Scholar]
- [66].Tsunoda I, Kobayashi-Warren M, Libbey JE, Fujinami RS. Central nervous system degeneration caused by autoimmune cytotoxic CD8+ T cell clones and hybridomas following virus infection. In: Binder MD, Hirokawa N, Windhorst U, editors. Encyclopedia of Neuroscience. Springer-Verlag GmbH; Berlin Heidelberg: 2009. pp. 619–625. [Google Scholar]
- [67].Tsunoda I, Kuang L-Q, Fujinami RS. Induction of autoreactive CD8+ cytotoxic T cells during Theiler's murine encephalomyelitis virus infection: Implications for autoimmunity. J. Virol. 2002;76:12834–12844. doi: 10.1128/JVI.76.24.12834-12844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Tsunoda I, Kuang L-Q, Kobayashi-Warren M, Fujinami RS. Central nervous system pathology caused by autoreactive CD8+ T-cell clones following virus infection. J. Virol. 2005;79:14640–14646. doi: 10.1128/JVI.79.23.14640-14646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fujinami RS, Rosenthal A, Lampert PW, Zurbriggen A, Yamada M. Survival of athymic (nu/nu) mice after Theiler's murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J. Virol. 1989;63:2081–2087. doi: 10.1128/jvi.63.5.2081-2087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yamada M, Zurbriggen A, Fujinami RS. Monoclonal antibody to Theiler's murine encephalomyelitis virus defines a determinant on myelin and oligodendrocytes, and augments demyelination in experimental allergic encephalomyelitis. J. Exp. Med. 1990;171:1893–1907. doi: 10.1084/jem.171.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Lipton HL, Twaddle G, Jelachich ML. The predominant virus antigen burden is present in macrophages in Theiler's murine encephalomyelitis virus-induced demyelinating disease. J. Virol. 1995;69:2525–2533. doi: 10.1128/jvi.69.4.2525-2533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Rossi CP, Delcroix M, Huitinga I, McAllister A, van Rooijen N, Claassen E, Brahic M. Role of macrophages during Theiler's virus infection. J. Virol. 1997;71:3336–3340. doi: 10.1128/jvi.71.4.3336-3340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rodriguez M, Quddus J. Effect of cyclosporin A, silica quartz dust, and protease inhibitors on virus-induced demyelination. J. Neuroimmunol. 1986;13:159–174. doi: 10.1016/0165-5728(86)90062-7. [DOI] [PubMed] [Google Scholar]
- [74].Roos RP, Wollmann R. DA strain of Theiler's murine encephalomyelitis virus induces demyelination in nude mice. Ann. Neurol. 1984;15:494–499. doi: 10.1002/ana.410150516. [DOI] [PubMed] [Google Scholar]
- [75].Fan J, Son KN, Arslan SY, Liang Z, Lipton HL. Theiler's murine encephalomyelitis virus leader protein is the only nonstructural protein tested that induces apoptosis when transfected into mammalian cells. J. Virol. 2009;83:6546–6553. doi: 10.1128/JVI.00353-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lucchinetti CF, Bruck W, Rodriguez M, Lassmann H. Distinct patterns of multiple sclerosis pathology indicates heterogeneity on pathogenesis. Brain Pathol. 1996;6:259–274. doi: 10.1111/j.1750-3639.1996.tb00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain. 1994;117:1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- [78].Vartanian T, Li Y, Zhao M, Stefansson K. Interferon-gamma-induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol. Med. 1995;1:732–743. [PMC free article] [PubMed] [Google Scholar]
- [79].Pender MP, Nguyen KB, McCombe PA, Kerr JF. Apoptosis in the nervous system in experimental allergic encephalomyelitis. J. Neurol. Sci. 1991;104:81–87. doi: 10.1016/0022-510x(91)90219-w. [DOI] [PubMed] [Google Scholar]
- [80].Tsunoda I, Kurtz CIB, Fujinami RS. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology. 1997;228:388–393. doi: 10.1006/viro.1996.8382. [DOI] [PubMed] [Google Scholar]
- [81].Rose JW, Hill KE, Wada Y, Kurtz CIB, Tsunoda I, Fujinami RS, Cross AH. Nitric oxide synthase inhibitor, aminoguanidine, reduces inflammation and demyelination produced by Theiler's virus infection. J. Neuroimmunol. 1998;81:82–89. doi: 10.1016/s0165-5728(97)00162-8. [DOI] [PubMed] [Google Scholar]
- [82].Tsunoda I, Kuang L-Q, Libbey JE, Fujinami RS. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 2003;162:1259–1269. doi: 10.1016/S0002-9440(10)63922-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Dal Canto MC, Lipton HL. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab. Invest. 1975;33:626–637. [PubMed] [Google Scholar]
- [84].McGavern DB, Murray PD, Rivera-Quinones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123:519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].McGavern DB, Murray PD, Rodriguez M. Quantitation of spinal cord demyelination, remyelination, atrophy, and axonal loss in a model of progressive neurologic injury. J. Neurosci. Res. 1999;58:492–504. doi: 10.1002/(sici)1097-4547(19991115)58:4<492::aid-jnr3>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Sathornsumetee S, McGavern DB, Ure DR, Rodriguez M. Quantitative ultrastructural analysis of a single spinal cord demyelinated lesion predicts total lesion load, axonal loss, and neurological dysfunction in a murine model of multiple sclerosis. Am. J. Pathol. 2000;157:1365–1376. doi: 10.1016/S0002-9440(10)64650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tsunoda I. Axonal degeneration as a self-destructive defense mechanism against neurotropic virus infection. Future Virol. 2008;3:579–593. doi: 10.2217/17460794.3.6.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Deb C, Lafrance-Corey RG, Zoecklein L, Papke L, Rodriguez M, Howe CL. Demyelinated axons and motor function are protected by genetic deletion of perforin in a mouse model of multiple sclerosis. J. Neuropathol. Exp. Neurol. 2009;68:1037–1048. doi: 10.1097/NEN.0b013e3181b5417e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Das Sarma J, Kenyon LC, Hingley ST, Shindler KS. Mechanisms of primary axonal damage in a viral model of multiple sclerosis. J. Neurosci. 2009;29:10272–10280. doi: 10.1523/JNEUROSCI.1975-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tsunoda I, Tanaka T, Saijoh Y, Fujinami RS. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am. J. Pathol. 2007;171:1563–1575. doi: 10.2353/ajpath.2007.070147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends. Neurosci. 2006;29:518–527. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]
- [92].Konno H, Yamamoto T, Iwasaki Y, Suzuki H, Saito T, Terunuma H. Wallerian degeneration induces Ia-antigen expression in the rat brain. J. Neuroimmunol. 1989;25:151–159. doi: 10.1016/0165-5728(89)90132-x. [DOI] [PubMed] [Google Scholar]
- [93].Konno H, Yamamoto T, Suzuki H, Yamamoto H, Iwasaki Y, Ohara Y, Terunuma H, Harata N. Targeting of adoptively transferred experimental allergic encephalitis lesion at the sites of wallerian degeneration. Acta Neuropathol. 1990;80:521–526. doi: 10.1007/BF00294613. [DOI] [PubMed] [Google Scholar]
- [94].Bodini B, Khaleeli Z, Cercignani M, Miller DH, Thompson AJ, Ciccarelli O. Exploring the relationship between white matter and gray matter damage in early primary progressive multiple sclerosis: an in vivo study with TBSS and VBM. Hum. Brain Mapp. 2009;30:2852–2861. doi: 10.1002/hbm.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Abe Y, Yamamoto T, Sugiyama Y, Watanabe T, Saito N, Kayama H, Kumagai T. Apoptotic cells associated with Wallerian degeneration after experimental spinal cord injury: a possible mechanism of oligodendroglial death. J. Neurotrauma. 1999;16:945–952. doi: 10.1089/neu.1999.16.945. [DOI] [PubMed] [Google Scholar]
- [96].Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- [97].Saito N, Yamamoto T, Watanabe T, Abe Y, Kumagai T. Implications of p53 protein expression in experimental spinal cord injury. J. Neurotrauma. 2000;17:173–182. doi: 10.1089/neu.2000.17.173. [DOI] [PubMed] [Google Scholar]
- [98].Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends. Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- [99].Carlson NG, Hill KE, Tsunoda I, Fujinami RS, Rose JW. The pathologic role for COX-2 in apoptotic oligodendrocytes in virus induced demyelinating disease: implications for multiple sclerosis. J. Neuroimmunol. 2006;174:21–31. doi: 10.1016/j.jneuroim.2006.01.008. [DOI] [PubMed] [Google Scholar]
- [100].Li S, Mealing GA, Morley P, Stys PK. Novel injury mechanism in anoxia and trauma of spinal cord white matter: glutamate release via reverse Na+-dependent glutamate transport. J. Neurosci. 1999;19:RC16. doi: 10.1523/JNEUROSCI.19-14-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Allt G, Ghabriel MN. Demyelination: a failure of cell communication? J. Neurol. Sci. 1982;57:287–290. doi: 10.1016/0022-510x(82)90035-1. [DOI] [PubMed] [Google Scholar]
- [102].Barres BA, Raff MC. Axonal control of oligodendrocyte development. J. Cell Biol. 1999;147:1123–1128. doi: 10.1083/jcb.147.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Raff MC, Barres BA, Burne JF, Coles HS, Ishizaki Y, Jacobson MD. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- [104].Roussarie JP, Ruffie C, Edgar JM, Griffiths I, Brahic M. Axon myelin transfer of a non-enveloped virus. PLoS One. 2007;2:e1331. doi: 10.1371/journal.pone.0001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gebauer F, Hentze MW. Molecular mechanisms of translational control. Nat. Rev. Mol. Cell. Biol. 2004;5:827–835. doi: 10.1038/nrm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell. Biol. 2005;6:318–327. doi: 10.1038/nrm1618. [DOI] [PubMed] [Google Scholar]
- [107].Agol VI. Picornavirus genome: An overview. In: Semler BL, Wimmer E, editors. Molecular Biology of Picornaviruses. AMS Press; Washington, D.C.: 2002. pp. 127–148. [Google Scholar]
- [108].I.R.E.S. Database . Inserm. The French Institute of Health and Medical Research; http://www.rangueil.inserm.fr/IRESdatabase/ [Google Scholar]
- [109].Holcik M, Lefebvre C, Yeh C, Chow T, Korneluk RG. A new internal-ribosome-entry-site motif potentiates XIAP-mediated cytoprotection. Nat. Cell. Biol. 1999;1:190–192. doi: 10.1038/11109. [DOI] [PubMed] [Google Scholar]
- [110].Coldwell MJ, Mitchell SA, Stoneley M, MacFarlane M, Willis AE. Initiation of Apaf-1 translation by internal ribosome entry. Oncogene. 2000;19:899–905. doi: 10.1038/sj.onc.1203407. [DOI] [PubMed] [Google Scholar]
- [111].Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J. Biol. Chem. 2004;279:29066–29074. doi: 10.1074/jbc.M402727200. [DOI] [PubMed] [Google Scholar]
- [112].Tsunoda I, McCright IJ, Kuang LQ, Zurbriggen A, Fujinami RS. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J. Neuropathol. Exp. Neurol. 1997;56:1302–1313. doi: 10.1097/00005072-199712000-00005. [DOI] [PubMed] [Google Scholar]
- [113].Coleman MP, Perry VH. Axon pathology in neurological disease: a neglected therapeutic target. Trends. Neurosci. 2002;25:532–537. doi: 10.1016/s0166-2236(02)02255-5. [DOI] [PubMed] [Google Scholar]
- [114].Gillingwater TH, Ribchester RR. Compartmental neurodegeneration and synaptic plasticity in the Wld(s) mutant mouse. J. Physiol. 2001;534:627–639. doi: 10.1111/j.1469-7793.2001.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Perry VH, Lunn ER, Brown MC, Cahusac S, Gordon S. Evidence that the Rate of Wallerian Degeneration is Controlled by a Single Autosomal Dominant Gene. Eur. J. Neurosci. 1990;2:408–413. doi: 10.1111/j.1460-9568.1990.tb00433.x. [DOI] [PubMed] [Google Scholar]
- [116].Glass JD, Griffin JW. Neurofilament redistribution in transected nerves: evidence for bidirectional transport of neurofilaments. J. Neurosci. 1991;11:3146–3154. doi: 10.1523/JNEUROSCI.11-10-03146.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Glass JD, Griffin JW. Retrograde transport of radiolabeled cytoskeletal proteins in transected nerves. J. Neurosci. 1994;14:3915–3921. doi: 10.1523/JNEUROSCI.14-06-03915.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Watson DF, Glass JD, Griffin JW. Redistribution of cytoskeletal proteins in mammalian axons disconnected from their cell bodies. J. Neurosci. 1993;13:4354–4360. doi: 10.1523/JNEUROSCI.13-10-04354.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tsunoda I, Tanaka T, Terry EJ, Fujinami RS. Contrasting roles for axonal degeneration in an autoimmune versus viral model of multiple sclerosis: When can axonal injury be beneficial? Am. J. Pathol. 2007;170:214–226. doi: 10.2353/ajpath.2007.060683. [DOI] [PMC free article] [PubMed] [Google Scholar]