Abstract
Objective
Increased serum phosphate is associated with adverse health outcomes. High intake of inexpensive processed and fast foods is common in impoverished communities and is linked with excessive dietary phosphorus intake and elevated serum phosphate concentrations in chronic kidney disease patients. We examined the impact of socioeconomic status on dietary phosphorus intake and serum phosphate concentrations in the general population.
Design
Cross-sectional study.
Participants
14,261 adult participants in the Third National Health and Nutrition Examination Survey.
Predictors and Outcomes
Poverty to income ratio (PIR; family income indexed to the federal poverty level) was the primary index of socioeconomic status. Serum phosphate was the primary outcome variable.
Results
Although estimated phosphorus intake decreased with decreasing quartiles of PIR (P < 0.001), serum phosphate was inversely associated with PIR (P = 0.003). The relationship between lower PIR and higher serum phosphate remained significant after adjustment for demographic, laboratory, and dietary intake characteristics (P = 0.02). Compared to participants in the highest PIR quartile (income >300% of the federal poverty level), participants in the lowest quartile (income < the federal poverty level) had more than twice the odds of hyperphosphatemia (≥4.4 mg/dl) in unadjusted and multivariable-adjusted logistic regression analyses (OR 2.2, 95%CI 1.5, 3.2).
Conclusions
Although lower income was associated with decreased estimated phosphorus intake, increasing poverty was independently linked with increased serum phosphate and higher likelihood of hyperphosphatemia. These findings may indicate that conventional dietary instruments underestimate phosphorus intake, especially among impoverished individuals. Further studies are needed to explore these possibilities.
Keywords: Phosphate, Poverty, Nutrition
Introduction
Elevated serum phosphate is associated with cardiovascular morbidity and mortality in patients with chronic kidney disease (CKD) and in individuals with normal kidney function.1-5 Experimental data showing that excess phosphate promotes left ventricular hypertrophy, arterial calcification, and renal injury suggest a causal link between increased serum phosphate and adverse outcomes.6-8 Collectively, these results have intensified the focus on identifying potentially modifiable risk factors for elevated serum phosphate in CKD and the general population. Dietary phosphorus intake and the impact of phosphorus-based food additives have received particular attention,9-11 largely because of growing recognition that the phosphorus content of the typical Western diet far exceeds current recommendations for daily intake,12 and the observation that ingesting large dietary phosphorus loads can increase serum phosphate concentrations and cause acute reductions in endothelial function in healthy adults 13, 14.
Poverty is a major public health burden that may promote excess dietary intake of phosphorus. Residents of low income neighborhoods have limited access to food choices that are healthy and affordable, resulting in excessive consumption of inexpensive processed and fast foods that are often rich in highly-absorbable phosphorus additives.15-18 High intake of these foods can nearly double the total dietary phosphorus intake when compared to similar diets with minimal additives,13 and has been associated with increased serum phosphate in patients with CKD.18, 19 Moreover, randomized dietary interventions aimed at decreasing the consumption of these foods significantly lowered serum phosphate concentrations in hemodialysis patients.11 When taken together, these data suggest that low socioeconomic status may lead to increased serum phosphate concentrations by promoting excessive intake of highly absorbable forms of dietary phosphorus in cheap, processed foods.
A recent analysis of adult participants in Third National Health and Nutrition Examination Survey (NHANES III) suggested only a weak correlation between dietary phosphorus intake and serum phosphate concentrations.20 However, like virtually all other currently available dietary instruments that are validated for research, the instrument used to collect dietary nutrient data in NHANES III may not accurately capture the amount of dietary phosphorus contained in food additives since these data are not publicly available and vary widely across different manufacturers and products.21 Further, the specific impact of socioeconomic factors on dietary phosphorus intake and serum phosphate in this population was not examined in detail, which is important given the link between poverty and excess intake of processed and fast foods. Accordingly, we performed a cross-sectional analysis of the relationships between socioeconomic status, estimated dietary phosphorus intake and serum phosphate in NHANES III.
Methods
Study Population
We analyzed participants in NHANES III (1988-1994) who were 20 years of age or older and had complete data on the key study variables of interest, including poverty to income ratio (PIR), dietary phosphorus intake, and serum phosphate concentrations. The design and operation of NHANES has been described in detail elsewhere.22 Briefly, NHANES III provides nationally representative cross-sectional data on the health status of the civilian, non-institutionalized U.S. population. After selection in a complex multi-stage survey design, participants were invited to a mobile examination center (MEC), where they were interviewed and examined, and had blood and urine samples collected for laboratory evaluation. Protocols to recruit and study NHANES III participants were approved by the National Center for Health Statistics Institutional Review Board, and all participants provided informed consent.
Demographic, Clinical and Socioeconomic Variables
Participants self-classified their race-ethnicity as non-Hispanic white, non-Hispanic black, Hispanic, and Other Race. For the purposes of this study, diabetes mellitus was defined as a self-reported physician diagnosis of diabetes or current use of diabetes medications at the time of the MEC interview. PIR was utilized as the primary index of socioeconomic status. This value represents the ratio of the participant's self-reported annual family income to the federal poverty threshold specific for the year of the interview,23 with a ratio of 1 indicating a family at 100% of the federal poverty level. In addition to PIR, highest educational year completed was used as an additional measure of socioeconomic status.
Laboratory and Dietary Variables
Phosphate, calcium, albumin, and creatinine were measured using a Hitachi model 737 multi-channel analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). After extraction with acetonitrile, 25-hydroxyvitamin D concentrations were measured using a radioimmunoassay kit (DiaSorin, Stillwater, MN). Estimated glomerular filtration rate (eGFR) was calculated using the simplified Modification of Diet in Renal Disease formula after calibrating creatinine values to Cleveland Clinic Laboratory reference values.24
Two validated instruments were used to collect dietary intake data from MEC examinees: a 1-month food-frequency questionnaire and a 24-hour dietary recall.25, 26 The food-frequency questionnaire was used to estimate the frequency of consumption of select food items over the 1 month preceding the MEC interview. For the purposes of this study, food items were grouped into general categories (dairy products, meats, vegetables, etc.). 24-hour dietary recall data were collected by a trained dietary interviewer using the NHANES III Dietary Data Collection system.22 These data included nutrients from all foods and beverages consumed over the previous 24-hour time period (midnight to midnight), but did not include nutrients obtained from seasonings added to prepared foods at the table.22 All interviews were reviewed and edited by the National Center for Health Statistics to ensure completeness and reliability of the data. In addition, the U.S. Department of Agriculture Healthy Eating Index (HEI) was used to estimate the overall quality of participants' diets. This index provides a composite measure of how well a participant's diet conformed to recommended nutritional guidelines, with possible scores ranging form 0 to 100.27
Statistical Methods
Because NHANES III utilized a complex, multi-stage probability sample design, procsurvey commands in SAS version 9.1 software (SAS Institute, Cary, NC) were used to account for sampling weights and the complex survey design in all analyses. Serum phosphate concentrations were analyzed on a continuous scale, and dichotomized by presence (≥ 4.4 mg/dl) or absence (< 4.4 mg/dl) of hyperphosphatemia. This threshold was determined by using the 95th percentile of serum phosphate concentrations in the full study sample, as has been done before28 PIR was analyzed as a continuous variable, and categorized in quartiles (< 100% of federal poverty level, 100% – 200%, 201 – 300%, and > 300%). Clinical, demographic, and laboratory data were compared across quartiles of PIR using linear regression for continuous variables and χ2 tests for categorical variables.
Dietary characteristics were compared across quartiles of PIR using linear regression. The association between dietary phosphorus intake and PIR was adjusted for age, gender, race, and ethnicity, all of which may be important confounders of this relationship,20 and total caloric intake, in an effort to account for potential underreporting of overall dietary intake, especially in lower PIR quartiles.29 Linear regression was used to analyze the relationship between serum phosphate concentrations and PIR. We utilized multivariable models to account for potential confounding by age, gender, race, ethnicity, body mass index, eGFR, total caloric intake, dietary phosphorus intake, and diabetes mellitus, all of which have been linked with both poverty and serum phosphate in previous studies.20, 30, 31 In addition, we adjusted for 25-hydroxyvitamin D concentrations since low vitamin D levels decrease gut phosphorus absorption 32 and disproportionately impact racial and ethnic minorities who typically represent a high proportion of impoverished populations in the U.S.23 Because serum phosphate concentrations can be affected by the fasting status of participants at the time of blood draw as well as the time of day of the blood draw,20 we further adjusted for these covariates in the multivariable models. Univariable and multivariable logistic regression models were fit to examine the relationships between socioeconomic status and odds of hyperphosphatemia, adjusted for the same covariates. In order to account for potentially non-linear relationships, we analyzed polynomial terms in these models. In addition, we examined whether the relationship between PIR and serum phosphate was modified by gender, race, ethnicity, dietary phosphorus intake, eGFR ≤ 60, and years of education by including pre-specified interaction terms in the models. Two-tailed P-values < 0.05 were considered statistically significant for all analyses.
Results
Population Characteristics
A total of 16,575 participants 20 years of age or older were available for analysis. Of these participants, 14,261 had complete data on the key study variables of interest and were analyzed. The mean age of the study sample was 45 ± 0.5 years, 52% were women, 10% were black, and 5% were Hispanic. In addition, 6% had diabetes and 6% had an eGFR ≤ 60 ml/min/1.73m2.
Table 1 depicts the demographic, clinical, and laboratory characteristics of the study sample across quartiles of poverty to income ratio (PIR). Decreasing PIR was associated with younger age, higher body mass index, fewer years of education, female gender, black race, Hispanic ethnicity, and higher prevalence of diabetes and eGFR ≤ 60 ml/min/1.73m2, as has been previously reported.23, 30, 33 Although mean serum creatinine concentrations did not differ across PIR quartiles, there was a modest but monotonic increase in mean serum phosphate concentrations with decreasing quartiles of PIR. Similarly, the prevalence of hyperphosphatemia significantly increased with decreasing PIR.
Table 1.
Demographic, clinical and laboratory characteristics of the study sample by quartiles of poverty to income ratio (PIR). Results are expressed as means ± standard errors or frequencies.
| PIR < 100% | PIR 100-200% | PIR 201-300% | PIR > 300% | P | |
|---|---|---|---|---|---|
| No. of Participants (weighted %) | 3259 (12) | 3964 (21) | 2692 (21) | 4346 (45) | |
| Estimated US population (millions)* | 19.2 | 33.5 | 34.1 | 72.4 | |
| Age | 42 ± 0.7 | 46 ± 0.8 | 45 ± 0.6 | 46 ± 0.6 | <0.001 |
| Female (%) | 60 | 55 | 51 | 49 | <0.001 |
| Body mass index (kg/m2) | 27 ± 0.2 | 27 ± 0.2 | 27 ± 0.3 | 26 ± 0.1 | 0.002 |
| Years of education, mean | 10 ± 0.2 | 11 ± 0.1 | 12 ± 0.1 | 14 ± 0.1 | <0.001 |
| Race/Ethnicity | |||||
| Non-Hispanic black (%) | 24 | 14 | 9 | 5 | <0.001 |
| Hispanic (%) | 13 | 7 | 3 | 2 | <0.001 |
| Diabetes (%) | 9 | 8 | 6 | 5 | <0.001 |
| eGFR ≤ 60 ml/min/1.73m2 (%) | 6 | 10 | 6 | 5 | <0.001 |
| Laboratory | |||||
| Creatinine (mg/dl) | 0.84 ± 0.01 | 0.87 ± 0.02 | 0.85 ± 0.00 | 0.85 ± 0.00 | 0.5 |
| Albumin (g/dl) | 4.1 ± 0.02 | 4.1 ± 0.03 | 4.2 ± 0.03 | 4.2 ± 0.02 | 0.004 |
| Calcium (mg/dl) | 9.3 ± 0.02 | 9.3 ± 0.03 | 9.3 ± 0.03 | 9.3 ± 0.02 | 0.87 |
| 25-hydroxyvitamin D (ng/ml) | 27 ± 0.5 | 29 ± 0.4 | 29 ± 0.6 | 31 ± 0.4 | <0.001 |
| Phosphate (mg/dl) | 3.52 ± 0.02 | 3.45 ± 0.01 | 3.44 ± 0.02 | 3.42 ± 0.02 | 0.001 |
| % Hyperphosphatemia (≥ 4.4 mg/dl) | 6 | 4 | 3 | 3 | <0.001 |
eGFR, estimated glomerular filtration rate
U.S. population estimates were derived from application of NHANES-provided survey weights.
Dietary Intake and PIR
Table 2 depicts the 1-month estimated frequency of consumption of select food-group items within each PIR quartile. In general, participants in higher quartiles of PIR more frequently consumed dairy products, seafood, fruits, and vegetables (particularly tossed salad, carrots, and broccoli) than participants in lower quartiles. In addition, more affluent participants on average consumed coffee, diet colas, and alcohol more frequently than less affluent participants. In contrast, participants in lower PIR quartiles more frequently consumed meat products (particularly highly processed items such as hot dogs, bacon, and sausage), eggs, cereals, beans/peanuts, and non-diet colas than participants in higher PIR quartiles.
Table 2.
Estimated frequency of consumption * of selected food-group items according to quartiles of poverty to income ratio (PIR). Results are expressed as means ± standard error.
| PIR < 100% | PIR 100-200% | PIR 201-300% | PIR > 300% | P | |
|---|---|---|---|---|---|
| N | 3259 | 3964 | 2692 | 4346 | |
| Dairy products** | 46 ± 1.7 | 47 ± 1.2 | 50 ± 1.1 | 49 ± 0.8 | 0.04 |
| Meat† | 25 ± 0.9 | 25 ± 0.8 | 25 ± 0.8 | 21 ± 0.4 | <0.001 |
| Seafood‡ | 5 ± 0.2 | 5 ± 0.2 | 6 ± 0.3 | 7 ± 0.2 | <0.001 |
| Poultry (chicken/turkey) | 9 ± 0.3 | 9 ± 0.3 | 9 ± 0.3 | 9 ± 0.2 | 0.2 |
| Eggs | 9 ± 0.4 | 7 ± 0.3 | 7 ± 0.2 | 5 ± 0.1 | <0.001 |
| Fruit§ | 25 ± 0.9 | 26 ± 0.7 | 27 ± 0.8 | 29 ± 0.8 | 0.001 |
| Vegetables‖ | 68 ± 1.6 | 71 ± 1.6 | 71 ± 1.8 | 77 ± 1.1 | <0.001 |
| Cereals¶ | 57 ± 1.4 | 55 ± 1.1 | 52 ± 0.9 | 48 ± 0.6 | <0.001 |
| Beans, peanuts | 13 ± 0.4 | 12 ± 0.4 | 11 ± 0.4 | 10 ± 0.2 | <0.001 |
| Beverages | |||||
| Coffee (regular) | 29 ± 2.2 | 33 ± 1.7 | 36 ± 1.9 | 40 ± 1.4 | <0.001 |
| Diet cola | 7 ± 0.7 | 8 ± 0.7 | 13 ± 1.0 | 15 ± 0.6 | <0.001 |
| Regular cola | 18 ± 1.2 | 18 ± 1 | 16 ± 0.9 | 12 ± 0.7 | <0.001 |
| Alcohol | 7 ± 0.6 | 6 ± 0.5 | 8 ± 0.7 | 11 ± 0.6 | <0.001 |
Cumulative times per month.
Dairy products included milk, yogurt, ice cream, and cheese.
Meat products included bacon, sausage, processed meats (e.g., hot dogs, salami, bologna), liver, beef, pork and ham.
Seafood included shrimp, clams, oysters, crab, lobster and fish.
Fruits included citrus fruits (e.g., oranges, grapefruits, tangerines), melons, peaches, mangos, apples, bananas and nectarines.
Vegetables included carrots, broccoli, brussel sprouts, cauliflower, potatoes, tomatoes, spinach/greens, tossed salad, cabbage, and peppers.
Cereals included bran cereals, cold/hot cereals, white/dark breads, rolls, muffins, and tortillas.
Table 3 depicts average daily nutrient intakes based on 24-hour recall across quartiles of PIR. In general, there was a monotonic decrease in total caloric intake, daily consumption of protein, calcium, and phosphorus, and Healthy Eating Index scores with decreasing quartiles of PIR. When the relationship between PIR and dietary phosphorus intake was adjusted for age, gender, black race, Hispanic ethnicity and caloric intake, decreasing PIR remained independently associated with lower estimated phosphorus intake (P = 0.02).
Table 3.
Nutrient intake characteristics by poverty to income ratio (PIR). Results are expressed as means ± standard error.
| PIR < 100% | PIR 100-200% | PIR 201-300% | PIR > 300% | P | |
|---|---|---|---|---|---|
| N | 3259 | 3964 | 2692 | 4346 | |
| Energy (kilocalories) | 2039 ± 29 | 2079 ± 37 | 2161 ± 24 | 2208 ± 24 | <0.001 |
| % from protein (mean) | 15 ± 0.2 | 15 ± 0.1 | 15 ± 0.1 | 16 ± 0.1 | 0.11 |
| % from carbohydrates (mean) | 51 ± 0.5 | 51 ± 0.4 | 49 ± 0.4 | 49 ± 0.4 | <0.001 |
| % from total fat (mean) | 33 ± 0.3 | 33 ± 0.2 | 34 ± 0.4 | 34 ± 0.3 | 0.001 |
| Protein (g/d) | 78 ± 1.5 | 77 ± 1.5 | 81 ± 1.1 | 84 ± 1.2 | <0.001 |
| Calcium (mg/d) | 751 ± 17 | 764 ± 15 | 818 ± 17 | 840 ± 11 | <0.001 |
| Phosphorus (mg/d) | 1198 ± 19 | 1224 ± 20 | 1292 ± 17 | 1329 ± 16 | <0.001 |
| Healthy Eating Index (0-100) | 60 ± 0.5 | 63 ± 0.5 | 63 ± 0.4 | 66 ± 0.4 | <0.001 |
Serum phosphate and PIR
In unadjusted analysis, serum phosphate was inversely associated with continuous PIR values (β -0.01, P = 0.003). Inclusion of a quadratic term (PIR2) improved model fit (P = 0.02 for PIR2), suggesting a non-linear relationship between phosphate and PIR; inclusion of a cubic term did not improve the model further. The relationship between higher serum phosphate and lower PIR remained significant when adjusted for age, gender, black race, Hispanic ethnicity, body mass index, diabetes, eGFR, 25-hydroxyvitamin D concentrations, total caloric intake and dietary phosphorus intake (P = 0.02). Further adjusting for fasting status and time of day of blood draw did not alter the results (data not shown). There was no significant effect modification by age, gender, race, ethnicity, dietary phosphorus intake or eGFR (P for interaction > 0.1 for all). When PIR was analyzed as a categorical variable, serum phosphate concentrations were significantly though modestly higher in the lowest compared to the highest quartile of PIR in both unadjusted and multivariable- adjusted analyses (Table 4).
Table 4.
Unadjusted and multivariable-adjusted linear regression analyses of the association between serum phosphate concentrations (95% confidence interval) and poverty to income ratio (PIR).
| Δ Serum Phosphate, mg/dl (95% CI)* | P-value | |
|---|---|---|
| Unadjusted Model | ||
| PIR < 100% | 0.10 (0.05, 0.15) | <0.001 |
| PIR 100%-200% | 0.03 (-0.001, 0.07) | 0.05 |
| PIR 201%-300% | 0.03 (-0.006, 0.06) | 0.10 |
| PIR > 300% (reference) | -- | -- |
| Fully-adjusted Model** | ||
| PIR < 100% | 0.08 (0.03, 0.13) | 0.003 |
| PIR 100%-200% | 0.03 (-0.01, 0.06) | 0.1 |
| PIR 201%-300% | 0.03 (-0.005, 0.07) | 0.1 |
| PIR > 300% (reference) | -- | -- |
As compared to the highest income level (PIR > 300%) in both models.
Adjusted for age, gender, black race, Hispanic ethnicity, diabetes, body mass index, estimated glomerular filtration rate, total caloric intake and dietary phosphorus intake.
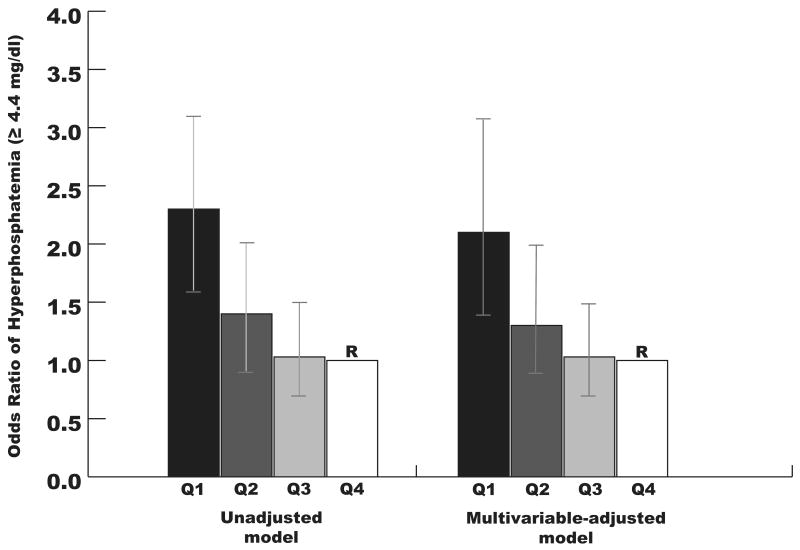
When examined as a continuous variable, decreasing PIR was associated with increasing odds of hyperphosphatemia in unadjusted (P < 0.001) and multivariable-adjusted logistic regression models (P < 0.001). When analyzed as a categorical variable, the lowest quartile of PIR was associated with 2.3-fold higher odds of hyperphosphatemia as compared to the highest quartile (95% CI 1.6, 3.2, P < 0.001, Figure 1). After multivariable adjustment, there was minimal change in the magnitude and strength of this relationship, with the lowest quartile of PIR associated with 2.2-fold higher odds of hyperphosphatemia as compared to the highest (95% CI 1.5, 3.2, P < 0.001, Figure 1).
Figure 1.
Unadjusted and multivariable-adjusted odds ratios of hyperphosphatemia according to quartiles of PIR (quartile 1 < 100% of federal poverty level; quartile 2, 100-200%, quartile 3, 201-300%; quartile 4 > 300%). Quartile 4 was the reference group in both models. The multivariable model was adjusted for age, gender, black race, Hispanic ethnicity, diabetes, body mass index, estimated glomerular filtration rate, total caloric intake and dietary phosphorus intake. The vertical bars represent 95% confidence intervals.
Although serum phosphate was also inversely associated with years of education, the relationship was not significant (P = 0.44). In addition, the association between PIR and serum phosphate was not significantly modified by years of education—the results were qualitatively similar among individuals who had completed post-graduate years of education as compared to individuals with a high school education or less (data not shown).
Discussion
Poverty is a growing public health epidemic that is associated with increased risk of coronary artery disease, stroke, and CKD.34 These associations are largely explained by the close link between poverty and established cardiovascular and renal risk factors, such as obesity, diabetes, hypertension, and smoking.30 However, given the emerging connections between elevated phosphate and cardiovascular and renal disease, the results of this large cohort of over 14,000 U.S adults suggest that increased serum phosphate concentrations and increased odds of hyperphosphatemia among the poor may represent a novel mechanism linking poverty and adverse health outcomes. Although the absolute differences in mean serum phosphate levels across the income groups were relatively small, several prior studies have reported an elevated risk of adverse renal and cardiovascular outcomes in association with comparably small increases in serum phosphate.1-4 Moreover, the greater than two-fold higher likelihood of hyperphosphatemia that we observed in the lowest-income group as compared to the highest-income group support the clinical relevance of these findings, which is further emphasized when extrapolated to the overall US population (Table 1).
Participants in the lowest quartile of PIR had the highest serum phosphate concentrations and the highest prevalence of hyperphosphatemia despite having the lowest estimated daily phosphorus intake. Although this finding may suggest a lack of association between dietary phosphorus intake and serum phosphate concentrations, it may also reflect important limitations in the methods used to estimate nutrient intake in this study. For example, while 24-hour recall is commonly used in national surveys to provide detailed estimates of dietary intake, validation of this method against quantified energy expenditure measurements demonstrated significant underreporting of total caloric intake among individuals who were overweight, had low educational achievement, or reported an annual income below the poverty level.29 Hence, it is possible that total phosphorus intake may have been similarly underreported by participants in lower PIR quartiles in this study, partly accounting for these results.
Alternatively, the discrepancies between estimated dietary phosphorus intake and serum phosphate concentrations may reflect specific imprecision in the estimation of dietary phosphorus intake. One potential source of imprecision that could help explain the association between poverty and higher serum phosphate is incomplete capture of unrecognized phosphorus intake from seasonings or preservatives added to processed foods. Total phosphorus intake is not only dependent on the raw quantities found in “natural” protein sources, but also on the growing amount of phosphorus-rich additives in processed and fast foods.9, 10, 19, 21 These additives can augment phosphorus intake by as much as 1000 mg per day 13 but are often not captured by standard dietary instruments because there are no requirements for the food industry to quantify their amount in product labeling.35, 36 As such, there can be large gaps between estimated phosphorus intake vs. actual phosphorus intake.9, 19, 37 This may be particularly problematic for estimating phosphorus intake in impoverished communities, which have a disproportionately high density of food vendors that provide relatively inexpensive, processed foods with poor nutritional value.15-17 As a result, a plausible explanation for the association between poverty and hyperphosphatemia in this study may be high consumption of largely unaccounted-for sources of phosphorus among impoverished participants. In support of this possibility are previous studies that have shown that diets rich in additive-containing foods can significantly increase serum phosphate in healthy volunteers,13 and that excess consumption of fast foods is associated with increased serum phosphate in hemodialysis patients despite a negligible effect on estimated total phosphorus intake.18 Together, these results underscore the necessity of changing food labeling requirements at the federal level in order to mandate the listing of both the presence and quantity of phosphorus additives in all food labels.
Variable phosphorus bioavailability may also have contributed to these findings. Unlike phosphorus found in processed meats and preservatives, phosphorus found in many plant sources, particularly in the form of phytate, is poorly absorbed by humans.21, 32 Thus, although current estimates suggest that humans on average absorb ∼60% of dietary phosphorus in the gut,32 this percentage may be substantially higher in individuals consuming diets rich in processed foods and lower among individuals consuming diets rich in vegetables and other plant sources.21 Indeed, it is interesting to note that affluent participants in this study more frequently consumed fruits and vegetables, whereas less affluent participants more frequently consumed processed meats and eggs. As such, it is possible that participants in the lower strata of income may have consumed foods with similar total phosphorus content but higher phosphorus bioavailability. Detailed physiological studies and novel nutritional assessment tools that more accurately detail total phosphorus intake and its specific sources are needed to explore these intriguing possibilities.
Our study has limitations. We did not have measurements of either parathyroid hormone (PTH) or fibroblast growth factor 23 (FGF23), both of which increase in response to high phosphorus intake in order to augment urinary phosphate excretion,38-40 and thus, could have obscured larger changes in serum phosphate levels among individuals consuming a high phosphorus diet. Since increases in PTH and FGF23 have emerged as important non-traditional risk factors for cardiovascular morbidity and mortality,41, 42 further studies are needed to assess whether levels of PTH or FGF23 independently differ as a function of socioeconomic status. In addition, we did not have detailed information concerning other factors that might impact the relationship between poverty and serum phosphate, such as decreased access to medical care. Further studies are needed to determine whether these factors may be linked with excess phosphate among the very poor. Finally, although we did not find significant interaction between PIR and eGFR ≤ 60, the relatively low prevalence of decreased eGFR in this study sample may have limited our power to detect effect modification by CKD. Dedicated studies in well-characterized CKD cohorts will need to further explore the relationships between poverty and serum phosphate in patients with kidney disease.
Current practice guidelines have increasingly emphasized the maintenance of normal serum phosphate concentrations in patients with CKD, in large part because of the emerging connections between even mild increases in serum phosphate and adverse outcomes.1-5 Therefore, even though poverty had a relatively modest impact on serum phosphate concentrations in this study, this association could be magnified in patients with limited renal excretory capacity and thereby exacerbate the substantially high rates of cardiovascular events, kidney disease progression, and mortality among impoverished individuals with CKD. Further studies are needed to elucidate the potential mechanisms that may underlie these findings, with the ultimate goal of developing novel therapeutic strategies for minimizing hyperphosphatemia and improving overall health outcomes among CKD patients and the very poor.
Acknowledgments
This study was supported by the American Kidney Fund Clinical Scientist in Nephrology Fellowship (to Dr. Isakova) and grants K23DK081673 (to Dr. Gutiérrez), R01DK076116 and R01DK081374 (to Dr. Wolf) from the National Institutes of Health.
Footnotes
Disclosures: Dr. Gutiérrez reports accepting speaking honoraria from Abbott. Dr. Isakova reports receiving honoraria from Shire. Dr. Wolf reports receiving research support from Shire and honoraria from Abbott, Genzyme, Shire and Davita.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–85. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 2.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–8. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz S, Trivedi B, Kalanter-Zadeh K, Kovesdy C. Association of Disorders of Mineral Metabolism with Progression of Chronic Kidney Disease. Clin J Am Soc Nephrol. 2006;1:825–831. doi: 10.2215/CJN.02101205. [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112:2627–33. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 5.Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol. 2009;20:397–404. doi: 10.1681/ASN.2008020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew S, Tustison KS, Sugatani T, Chaudhary LR, Rifas L, Hruska KA. The mechanism of phosphorus as a cardiovascular risk factor in CKD. J Am Soc Nephrol. 2008;19:1092–105. doi: 10.1681/ASN.2007070760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amann K, Tornig J, Kugel B, et al. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int. 2003;63:1296–301. doi: 10.1046/j.1523-1755.2003.00864.x. [DOI] [PubMed] [Google Scholar]
- 8.Ibels LS, Alfrey AC, Haut L, Huffer WE. Preservation of function in experimental renal disease by dietary restriction of phosphate. N Engl J Med. 1978;298:122–6. doi: 10.1056/NEJM197801192980302. [DOI] [PubMed] [Google Scholar]
- 9.Sherman RA, Mehta O. Phosphorus and potassium content of enhanced meat and poultry products: implications for patients who receive dialysis. Clin J Am Soc Nephrol. 2009;4:1370–3. doi: 10.2215/CJN.02830409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sherman RA, Mehta O. Dietary phosphorus restriction in dialysis patients: potential impact of processed meat, poultry, and fish products as protein sources. Am J Kidney Dis. 2009;54:18–23. doi: 10.1053/j.ajkd.2009.01.269. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301:629–35. doi: 10.1001/jama.2009.96. [DOI] [PubMed] [Google Scholar]
- 12.Calvo MS. Dietary considerations to prevent loss of bone and renal function. Nutrition. 2000;16:564–6. doi: 10.1016/s0899-9007(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 13.Bell RR, Draper HH, Tzeng DY, Shin HK, Schmidt GR. Physiological responses of human adults to foods containing phosphate additives. J Nutr. 1977;107:42–50. doi: 10.1093/jn/107.1.42. [DOI] [PubMed] [Google Scholar]
- 14.Shuto E, Taketani Y, Tanaka R, et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol. 2009;20:1504–12. doi: 10.1681/ASN.2008101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker EA, Schootman M, Barnidge E, Kelly C. The role of race and poverty in access to foods that enable individuals to adhere to dietary guidelines. Prev Chronic Dis. 2006;3:A76. [PMC free article] [PubMed] [Google Scholar]
- 16.Block JP, Scribner RA, DeSalvo KB. Fast food, race/ethnicity, and income: a geographic analysis. Am J Prev Med. 2004;27:211–7. doi: 10.1016/j.amepre.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Powell LM, Chaloupka FJ, Bao Y. The availability of fast-food and full-service restaurants in the United States: associations with neighborhood characteristics. Am J Prev Med. 2007;33:S240–5. doi: 10.1016/j.amepre.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Butt S, Leon JB, David CL, Chang H, Sidhu S, Sehgal AR. The prevalence and nutritional implications of fast food consumption among patients receiving hemodialysis. J Ren Nutr. 2007;17:264–8. doi: 10.1053/j.jrn.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan CM, Leon JB, Sehgal AR. Phosphorus-containing food additives and the accuracy of nutrient databases: implications for renal patients. J Ren Nutr. 2007;17:350–4. doi: 10.1053/j.jrn.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Boer IH, Rue TC, Kestenbaum B. Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2009;53:399–407. doi: 10.1053/j.ajkd.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uribarri J, Calvo MS. Hidden sources of phosphorus in the typical American diet: does it matter in nephrology? Semin Dial. 2003;16:186–8. doi: 10.1046/j.1525-139x.2003.16037.x. [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Plan and operation of the Third National and Nutrition Examination Survey 1988-1994. National Center for Health Statistics. Vital Health Stat. 1994;1 [PubMed] [Google Scholar]
- 23.Martins D, Tareen N, Zadshir A, et al. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;47:965–71. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis. 2007;50:918–26. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr. 1999;69:243–9. doi: 10.1093/ajcn/69.2.243. [DOI] [PubMed] [Google Scholar]
- 26.Karvetti RL, Knuts LR. Validity of the 24-hour dietary recall. J Am Diet Assoc. 1985;85:1437–42. [PubMed] [Google Scholar]
- 27.Kennedy ET, Ohls J, Carlson S, Fleming K. The Healthy Eating Index: design and applications. J Am Diet Assoc. 1995;95:1103–8. doi: 10.1016/S0002-8223(95)00300-2. [DOI] [PubMed] [Google Scholar]
- 28.Foley RN, Wang C, Ishani A, Collins AJ. NHANES III: influence of race on GFR thresholds and detection of metabolic abnormalities. J Am Soc Nephrol. 2007;18:2575–82. doi: 10.1681/ASN.2006121411. [DOI] [PubMed] [Google Scholar]
- 29.Briefel RR, Sempos CT, McDowell MA, Chien S, Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. Am J Clin Nutr. 1997;65:1203S–1209S. doi: 10.1093/ajcn/65.4.1203S. [DOI] [PubMed] [Google Scholar]
- 30.Robbins JM, Vaccarino V, Zhang H, Kasl SV. Socioeconomic status and type 2 diabetes in African American and non-Hispanic white women and men: evidence from the Third National Health and Nutrition Examination Survey. Am J Public Health. 2001;91:76–83. doi: 10.2105/ajph.91.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka H, Hamano T, Fujii N, et al. The impact of diabetes mellitus on vitamin D metabolism in predialysis patients. Bone. 2009;45:949–55. doi: 10.1016/j.bone.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Uribarri J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin Dial. 2007;20:295–301. doi: 10.1111/j.1525-139X.2007.00309.x. [DOI] [PubMed] [Google Scholar]
- 33.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161:1207–16. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 34.Hossain MP, Goyder EC, Rigby JE, El Nahas M. CKD and poverty: a growing global challenge. Am J Kidney Dis. 2009;53:166–74. doi: 10.1053/j.ajkd.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 35.Karalis M. Food and Drug Administration petition on food labeling: an update from the American Dietetic Association and National Kidney Foundation. J Ren Nutr. 2007;17:423–4. doi: 10.1053/j.jrn.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal AR, Sullivan C, Leon JB, Bialostosky K. Public health approach to addressing hyperphosphatemia among dialysis patients. J Ren Nutr. 2008;18:256–61. doi: 10.1053/j.jrn.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oenning LL, Vogel J, Calvo MS. Accuracy of methods estimating calcium and phosphorus intake in daily diets. J Am Diet Assoc. 1988;88:1076–80. [PubMed] [Google Scholar]
- 38.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–9. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 39.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–96. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 40.Calvo MS, Kumar R, Heath H. Persistently elevated parathyroid hormone secretion and action in young women after four weeks of ingesting high phosphorus, low calcium diets. J Clin Endocrinol Metab. 1990;70:1334–40. doi: 10.1210/jcem-70-5-1334. [DOI] [PubMed] [Google Scholar]
- 41.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 42.Gutierrez O, Mannstadt M, Isakova T, et al. Fibroblast Growth Factor 23 and Mortality among Hemodialysis Patients. N Engl J Med. 2008;359:584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]