Abstract
While it has been shown in several publications that the serine-threonine kinase PKCθ is required for efficient activation of mature T lymphocytes, the role of PKCθ in T cell development in the thymus is somewhat controversial. In this study, using knockout mice, we show that PKCθ is important in positive selection. The thymus of PKCθ−/− animals contains significantly less mature single positive T cells compared to wild-type controls. Biochemically, PKCθ deficient thymocytes show defective activation of the transcription factors AP-1, NFAT and NFκB as well as impaired phosphorylation of the MAP kinase ERK after T cell receptor stimulation in vitro. Together, these results reveal a crucial role of PKCθ in positive selection of thymocytes in a pathway leading to the activation of ERK, AP-1, NFAT, and NFκB.
Keywords: Positive selection, PKCθ, NFκB, NFAT, AP-1
1. Introduction
T cells represent a major component of the adaptive immune system that allows the organism to fight various pathogens. Activation and subsequent differentiation of T lymphocytes crucially depend on the interaction of the T cell receptor (TCR) with a cognate antigen-MHC-complex which is provided on the surface of antigen presenting cells (APCs). This dependence upon self-MHC molecules is known as “MHC restriction”.
The formation of a TCR during T cell maturation is the consequence of random rearrangements of the TCR loci giving rise to a great diversity of T lymphocytes with distinct antigen specificities. In a current model of T cell development, thymocytes undergo a twofold selection process [1]: in the thymic cortex, CD4+ CD8+ double positive (DP) thymocytes interact with epithelial cells expressing MHC class I and MHC class II on their surface. DP cells bearing TCRs with low to moderate affinity to MHC/antigen complexes receive a survival signal in a process called positive selection. However, the great majority (around 90%) of DP thymocytes without proper TCRs die by neglect. Positively selected DP cells then migrate towards the corticomedullary junction, where negative selection occurs: high-affinity interaction of TCRs with self-MHC/self-peptide complexes destines the thymocytes for apoptosis. Subsequently, selected thymocytes downregulate either CD4 or CD8 and go through several rounds of division in the medulla before they leave the thymus as fully matured pheripheral T cells.
Since signaling through the TCR can have completely different outcomes (positive vs. negative selection), the biochemical events following TCR engagement on thymocytes are of special interest. A number of effector molecules that drive T cell maturation have been defined so far. It was shown that sustained signaling through the MAP kinase ERK is required for positive selection, whereas strong but transient ERK activation promotes negative selection [2,3]. Negative selection is also regulated by Bim, a proapoptotic member of the BH3-only Bcl-2 family [4]. Interestingly, it was demonstrated that ERK can mediate the phosphorylation of Bim [5] which leads to its ubiquitination and proteasomal degradation [6,7]. In several studies, the calcium-dependent phosphatase calcineurin has been shown to be important for positive selection of thymocytes [8–11]. This result suggests that the downstream transcriptional effector of calcineurin, i.e. NFAT (nuclear factor of activated T cells) may be involved in the transcriptional program required for positive selection. Indeed, loss of function of nfatc3 leads to a defect in thymocyte selection which resembles the phenotype of calcineurinAβ knockout mice [8,12]. Most recently, PKCθ has been linked to the maturation of thymocytes during positive selection [13]. This serine-threonine kinase was shown to be crucial for productive NFAT activation in peripheral T cells [14–17]. In the present study we provide evidence that PKCθ indeed plays an important role in the signaling events leading to positive selection of thymocytes. The numbers of mature single positive T cells in the thymus were reduced in PKCθ−/− mice. Biochemically, phosphorylation of the MAP kinase ERK1/2 was impaired in thymocytes of PKCθ knockout animals compared to wild-type controls. PKCθ deficient thymocytes also showed a severe activation defect, reflected in decreased proliferation and IL-2 secretion. Most importantly, the present work for the first time demonstrates that PKCθ is crucial for the activation of the transcription factors NFAT, AP-1 and NFκB in immature thymocytes.
2. Materials and methods
2.1. Mice and reagents
PKCθ knockout mice were described elsewhere [15]. γ32P-ATP was purchased from Amersham. The antibodies used for T cell stimulation were anti-CD28 monoclonal antibody (mAb) (clone 28.2) and the CD3-specific mAb 2C11 (mouse). The anti-hamster immunoglobulin G1 antibody (clone HIG-632) was used for crosslinking the CD3 antibodies during short-term stimulation.
2.2. Analysis of proliferative responses and cytokine release
For anti-CD3-mediated stimulation, 5 × 105 thymocytes in 200 μl of proliferation medium [RPMI supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, and 50 units/ml penicillin/streptomycin] were added in duplicates to 96-well plates precoated with 10 μg/ml anti-CD3 antibody. Soluble anti-CD28 antibody (1 μg/ml) was also added where indicated. Cells were harvested at 64 h after a 16 h pulse with 1 μCi [3H]-thymidine per well; [3H]-thymidine incorporation was measured with a Matrix 96 direct β counter system. IL-2 produced from the cultures was measured via the IL-2-dependent indicator cell line CTLL-2. Supernatants of cultures were added to CTLL-2 cells (104/well) and cultured for additional 48 h and pulsed for 6 h with 1 μCi [3H]-thymidine per well. A standard curve was established by using recombinant IL-2.
2.3. Gel mobility shift assays
Thymocytes were either co-stimulated with 10 μg/ml anti-CD3 and 1 μg/ml anti-CD28 antibodies for 20 h or with 10 μg/ml anti-CD3 or 10 ng/ml phorbol 12,13-dibutyrate (PdBu) alone for 8 h.
Nuclear extracts were harvested from 2 × 107 cells according to standard protocols. Briefly, thymocytes were washed in PBS, and resuspended in 10 mM Hepes, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, and protease inhibitors. Cells were incubated on ice for 15 min. NP-40 was then added to a final concentration of 0.6%, the cells were vigorously mixed, and the mixture was centrifuged for 5 min at 2300 × g. The nuclear pellets were washed twice and resuspended in 20 mM Hepes, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and protease inhibitors, and the tubes were rocked for 30 min at 4 °C. After centrifugation for 10 min at 15,000 × g, the supernatants were collected. 2 μg extract proteins were incubated in binding buffer with end-labeled, double-stranded oligonucleotide probes (NF-κB: 5′-CTG GGG ACT TTC CGC T-3′; NFAT: 5′-GCC CAA AGA GGA AAA TTT GTT TCA TAC AG-3′). 3 × 105 cpm of labeled probe was used in each reaction and band shifts were resolved on 4% and 5% polyacrylamide gels. Where indicated, 0.5 μg of supershift antibody was added. All experiments were performed at least three times with similar outcomes.
2.4. Flow cytometry
Single-cell suspensions were prepared and incubated for 30 min on ice in staining buffer (PBS containing 2% FCS) with the appropriate fluoresceinisothiocyanate-, phycoerythrin-, or allophycocyanin-conjugated antibodies. The presence of surface markers was analyzed with a FACS Calibur™ cytometer (BD Biosciences) and CellQuest™ software according to standard protocols. Antibodies against murine CD4 and CD8 were obtained from Caltag Laboratories. Antibodies against CD5 and CD69 were obtained from BD Pharmingen.
2.5. Western blotting analysis of ERK activation
Thymocytes were stimulated with 1 μg/ml of hamster anti-CD3 antibody and 1 μg/ml of hamster anti-CD28 antibody together with 2 μg/ml of anti-hamster crosslinking antibody at 37 °C for indicated time periods. Cells were lysed in ice-cold lysis buffer (5 mM NaPP, 5 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 50 mM NaCl, 50 mM Tris, pH 7.3, 1% NP-40, 50 μg/ml each of aprotinin and leupeptin) and centrifuged at 15,000 × g for 15 min at 4 °C. Protein lysates were subjected to Western blotting analysis with antibodies against phospho-ERK1/2 (Thr202/Tyr204) and ERK1/2 (Cell Signaling Technology).
3. Results
3.1. PKCθ−/− thymocytes show diminished positive selection
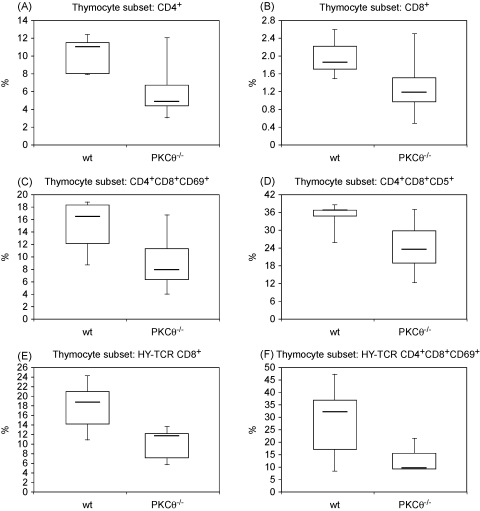
To examine whether PKCθ is of importance in the maturation of thymocytes, we compared the numbers of single positive T cells in the thymi of wild-type and PKCθ deficient mice. The loss of PKCθ led to a strong reduction of CD4 single positive T cells in the thymus (4.9% vs. 11.1%) (Fig. 1A). The number of CD8 positive T cells was also reduced, albeit less pronounced (1.2% vs. 1.9%) (Fig. 1B). Consequently, the double positive fraction was enhanced from 79% to 85%, whereas total thymocyte numbers were comparable between wild-type and PKCθ−/− mice (not shown). The numbers of mature T lymphocytes in lymph nodes and spleens were also not altered between genotypes (not shown), indicating that migration of T cells from the thymus into the periphery is not influenced by the loss of PKCθ. To investigate whether the reduction of mature T cells in the thymi of PKCθ−/− mice was due to a suboptimal positive selection, we analyzed the expression of CD69 and CD5. The upregulation of these surface proteins at the double positive stage has been associated with positive selection of developing thymocytes and can therefore serve as marker molecules [18,19]. Interestingly, CD69+ double positive thymocytes were diminished by 50% in the PKCθ knockout mice (7.9% vs. 16.4%) (Fig. 1C), and CD5 expression was reduced by one third (23.7% vs. 36.8%) (Fig. 1D). These results strongly indicate that PKCθ is important for efficient positive selection of thymocytes that occurs during the transition from CD4+CD8+ double positive to either CD4+ or CD8+ single positive T cells.
Fig. 1.
PKCθ deficiency leads to impaired maturation of thymocytes. Wild-type and PKCθ−/− thymocytes from non-transgenic (A–D) or HY TCR transgenic (E–F) mice were stained with PE-anti-CD4, APC-anti-CD8, and FITC-anti-CD5 or FITC-anti-CD69. Percentages of indicated subsets are shown as boxplots. p < 0.01 (A–E); p = 0.015 (F). p values were calculated with Student's t-test.
3.2. H-Y TCR transgenic PKCθ−/− thymocytes show diminished positive selection
As the CD8+ single positive subset comprises only about 2% of whole thymocytes, a defect in the maturation of this lineage is difficult to detect. In order to investigate whether PKCθ is important in the positive selection of the CD4−CD8+ subset, we introduced the H-Y T cell receptor transgene into mice deficient in PKCθ. Consistent with the results obtained with TCR non-transgenic mice, PKCθ deficient H-Y TCR transgenic mice showed a clear defect in the maturation of CD8+ single positive thymocytes (11.7% vs. 18.6%) (Fig. 1E). This developmental defect is most probably due to a suboptimal positive selection as the number of CD69+ double positive thymocytes was decreased by 70% in PKCθ deficient compared to wild-type mice (9.6% vs. 32%) (Fig. 1F). Total thymocyte numbers were not altered between genotypes, and male H-Y TCR mice deficient in PKCθ exhibited the same subset distribution as their wild-type counterparts (not shown), indicating that negative selection was not affected by the loss of PKCθ.
3.3. PKCθ deficient thymocytes lack significant ERK signaling
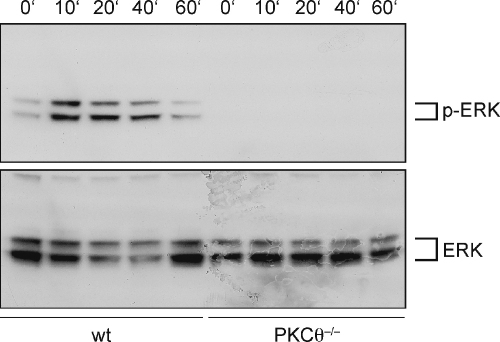
Since ERK activation was suggested to be required for positive selection [3,20], we investigated whether PKCθ deficiency influenced ERK activation, quantified as phosphorylation of ERK1/2 on Thr202 and Tyr204. After CD3/CD28 stimulation of wild-type thymocytes, ERK phosphorylation was readily detected and prolonged over at least 40 min. By contrast, in PKCθ−/− thymocytes virtually no ERK phosphorylation was detectable over a time period of 60 min (Fig. 2).
Fig. 2.
Activation of ERK is strongly diminished in PKCθ deficient thymocytes. Wild-type and PKCθ−/− thymocytes were stimulated with anti-CD3 and anti-CD28 antibodies for indicated time periods and whole cell extracts were prepared. Phosphorylation of ERK1/2 on Thr202 and Tyr204 was detected by immunoblotting with a phospho-specific antibody. panERK1/2 served as a loading control.
3.4. PKCθ−/− thymocytes exhibit diminished proliferation and IL-2 responses
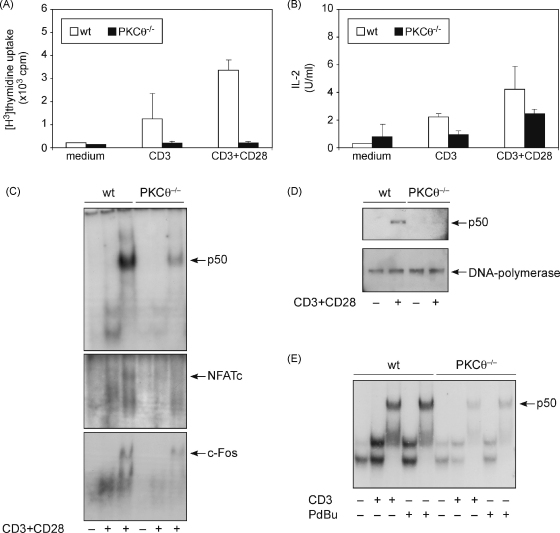
It was shown previously that proliferation and IL-2 secretion of mature T cells crucially depend on PKCθ [14,15,21]. To assess whether this holds also true for immature T cells, we stimulated thymocytes with anti-CD3 and anti-CD28 antibodies and measured proliferative and IL-2 responses. PKCθ deficient thymocytes showed profound defects in proliferation as well as IL-2 secretion (Fig. 3A and B) which suggests that PKCθ is not only important for TCR signaling in mature but also immature T cells.
Fig. 3.
PKCθ deficient thymocytes show severe defects in proliferation, IL-2 secretion and transcription factor activation. (A and B) Thymocytes were stimulated with anti-CD3 and anti-CD28 antibodies, as indicated. Cellular proliferation was determined by measurement of the incorporation of [3H]thymidine (A), and the amount of IL-2 secreted under each condition was measured via the IL-2-dependent indicator cell line CTLL-2 (B). (C and D) Thymocytes were stimulated with 10 μg/ml anti-CD3 and 1 μg/ml anti-CD28 antibodies for 20 h. Nuclear extracts were analyzed by electrophoretic mobility shift assay (EMSA) to assess DNA binding (C) or by Western blotting to measure nuclear entry (D). Equal protein amounts were used for EMSA analysis and Western blotting. DNA polymerase δ in the nuclear extracts served as a loading control. (E) EMSA with nuclear extracts from thymocytes, stimulated with 10 μg/ml anti-CD3 or 10 ng/ml PdBu for 8 h. In lanes 3, 5, 8, and 10 the DNA/protein complex was supershifted.
3.5. PKCθ−/− thymocytes show defective activation of the transcription factors NFAT, AP-1, and NFκB
IL-2 expression after TCR/CD28 engagement is contingent on a number of gene regulatory proteins, the most prominent being NFAT, AP-1, and NFκB [22,23]. The activation of these transcription factors in mature peripheral T cells is crucially dependent on PKCθ [14,15,21]. In light of these findings and considering the IL-2 secretion defect of PKCθ deficient thymocytes, we examined whether the activation of these transcription factors is also PKCθ-dependent in developing immature T cells. Wild-type and PKCθ−/− thymocytes were stimulated with anti-CD3 and anti-CD28 antibodies and nuclear extracts were analyzed in band shift assays. The activation of NFAT and AP-1 was strongly diminished in PKCθ deficient thymocytes (Fig. 3C). Interestingly and in contrast to previous reports [13,21], NFκB activation was also severely impaired in PKCθ−/− thymocytes (Fig. 3C). This defect in NFκB activation was reproducibly observed and confirmed by analyzing the nuclear translocation of p50, a NFκB subunit (Fig. 3D). To exclude that this differing result was simply due to different stimulation conditions, we treated thymocytes in accordance with Sun et al. either with anti-CD3 alone or with phorbol 12,13-dibutyrate (PdBu) alone for 8 h. Still, PKCθ deficient thymocytes showed a severe defect in NFκB activation (Fig. 3E).
4. Discussion
It has been shown in several publications that PKCθ is required for efficient activation of mature T lymphocytes [14–17,21]. Particularly, PKCθ is a positive regulator of pathways that activate the transcription factors NFAT, AP-1, and NFκB and ultimately converge at the induction of IL-2 expression in peripheral T cells. PKCθ also mediates LFA-1/ICAM-1 adhesion and stabilizes the immunological synapse [24]. We could demonstrate recently that activation of PKCθ leads to the degradation of the negative regulator Cbl-b, thereby contributing to full T cell activation [14]. While the significance of PKCθ in the activation of mature peripheral T lymphocytes is well established [25], the role of PKCθ in T cell development in the thymus is somewhat controversial [13,15,21]. Our study now confirms a previous one in which PKCθ was shown to be required for efficient positive selection [13]. Albeit inconsistent with an earlier publication by our laboratory which concentrated on mature peripheral T cells [15], a more thorough investigation now revealed clearly that the loss of PKCθ led to a strong reduction of mature T cells in the thymus and a concomitant decrease of markers for positive selection, i.e. CD5 and CD69 (Fig. 1). However, while Morley et al. used CD4-restricted TCR transgenic mice, we employed the CD8-restricted HY specific TCR, thereby complementing previous data (Fig. 1E and F). The H-Y specific and MHC class I restricted TCR recognizes a peptide from the male H-Y antigen presented on H-2Db. As a consequence, thymocytes expressing the H-Y TCR are positively selected in female H-2b mice and negatively selected in male H-2b mice [26]. Therefore, in female mice, the thymocyte development is shifted towards the CD8+ lineage.
Several studies reported that ERK signaling was required for efficient positive selection [3,20]. We here show that activation of ERK1/2 was strongly impaired in PKCθ deficient thymocytes (Fig. 2). Thus, as opposed to the situation in mature T cells [15], ERK activation in thymocytes is strictly dependent on PKCθ, and impaired ERK activity could contribute to the defective positive selection of PKCθ−/− thymocytes. Moreover, PKCθ deficient thymocytes showed profound defects in proliferation as well as IL-2 secretion (Fig. 3A and B) which confirms that PKCθ is not only important for TCR signaling in mature but also immature T cells.
NFAT was previously implicated in positive selection of thymocytes: The calcineurin inhibitors cyclosporin A and FK506 inhibit positive selection in vivo [27–29]. Mice deficient in the predominant calcineurin catalytic isoform in T cells, i.e. calcineurin Aβ [30], show defective T cell development, reflected in fewer total CD3 cells and reduced CD4 and CD8 single positive cells [8]. Moreover, it was shown that the calcineurin regulatory subunit, CnB1, is essential for positive selection during thymocyte development [10]. In addition, it was reported that AP-1 is also involved in thymocyte maturation [31], and Ras activation, which is upstream of AP-1, is necessary for positive selection [32,33]. As we now demonstrate that PKCθ is necessary for efficient activation of NFAT as well as AP-1 in thymocytes (Fig. 3C), it is likely that the defective positive selection of PKCθ deficient thymocytes is at least in part due to impaired NFAT and/or AP-1 activation.
Surprisingly, since in striking contrast to previous reports [13,21], NFκB activation was also reproducibly affected by the loss of PKCθ (Fig. 3C–E). The reasons for these conflicting results are not known, but the different mouse strains used in the studies are one possible explanation for the varying outcomes. Whether our observed NFκB activation defect contributes to impaired positive selection is not known so far. At least one publication stated that NFκB was dispensable for positive selection [34], whereas two groups reported that it plays a role in apoptosis of double positive thymocytes [35,36].
Collectively, we could demonstrate that in our system PKCθ is required for full activation of NFAT, AP-1, and NFκB in thymocytes. In our hands, PKCθ proved to be upstream of key transcription factors not only in mature [15] but also in immature T cells.
Despite the fundamental role PKCθ obviously plays in positive selection, no complete block in thymocyte maturation was observed. To examine whether other PKC isoforms partially compensate for the loss of PKCθ, we generated mice that were additionally deficient in either PKCalpha, beta or epsilon (PCKθ−/−/alpha−/−, PKCθ−/−/beta−/−, PKCθ−/−/epsilon−/−). None of these double-deficient mouse lines exhibited a more severe positive selection defect than did the PKCθ-deficient alone (not shown). This indicates that there is no functional redundancy between these PKC isoforms during the maturation of thymocytes.
Conflict of interest
The authors have no financial conflict of interest.
Acknowledgements
This work was supported by the FWF Austrian Science Fund (grants SFB-021 and P19505-B05) and the Hertha Firnberg Fellowship T264-B13. We are grateful to G. Böck from Innsbruck for FACS analysis and W. Ellmeier for providing HY TCR transgenic animals. All experiments comply with the current laws of Austria.
References
- 1.Ashton-Rickardt P.G., Bandeira A., Delaney J.R., Van Kaer L., Pircher H.P., Zinkernagel R.M. Evidence for a differential avidity model of T cell selection in the thymus. Cell. 1994;76:651–663. doi: 10.1016/0092-8674(94)90505-3. [DOI] [PubMed] [Google Scholar]
- 2.Mariathasan S., Zakarian A., Bouchard D., Michie A.M., Zuniga-Pflucker J.C., Ohashi P.S. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 3.McNeil L.K., Starr T.K., Hogquist K.A. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci USA. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouillet P., Purton J.F., Godfrey D.I., Zhang L.C., Coultas L., Puthalakath H. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 5.Harada H., Quearry B., Ruiz-Vela A., Korsmeyer S.J. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA. 2004;101:15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ley R., Balmanno K., Hadfield K., Weston C., Cook S.J. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 7.Luciano F., Jacquel A., Colosetti P., Herrant M., Cagnol S., Pages G. Phosphorylation of Bim-EL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 8.Bueno O.F., Brandt E.B., Rothenberg M.E., Molkentin J.D. Defective T cell development and function in calcineurin A beta-deficient mice. Proc Natl Acad Sci USA. 2002;99:9398–9403. doi: 10.1073/pnas.152665399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gallo E.M., Winslow M.M., Cante-Barrett K., Radermacher A.N., Ho L., McGinnis L. Calcineurin sets the bandwidth for discrimination of signals during thymocyte development. Nature. 2007;450:731–735. doi: 10.1038/nature06305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neilson J.R., Winslow M.M., Hur E.M., Crabtree G.R. Calcineurin B1 is essential for positive but not negative selection during thymocyte development. Immunity. 2004;20:255–266. doi: 10.1016/s1074-7613(04)00052-4. [DOI] [PubMed] [Google Scholar]
- 11.Wang C.R., Hashimoto K., Kubo S., Yokochi T., Kubo M., Suzuki M. T cell receptor-mediated signaling events in CD4+CD8+ thymocytes undergoing thymic selection: requirement of calcineurin activation for thymic positive selection but not negative selection. J Exp Med. 1995;181:927–941. doi: 10.1084/jem.181.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oukka M., Ho I.C., de la Brousse F.C., Hoey T., Grusby M.J., Glimcher L.H. The transcription factor NFAT4 is involved in the generation and survival of T cells. Immunity. 1998;9:295–304. doi: 10.1016/s1074-7613(00)80612-3. [DOI] [PubMed] [Google Scholar]
- 13.Morley S.C., Weber K.S., Kao H., Allen P.M. Protein kinase C-theta is required for efficient positive selection. J Immunol. 2008;181:4696–4708. doi: 10.4049/jimmunol.181.7.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gruber T., Hermann-Kleiter N., Hinterleitner R., Fresser F., Schneider R., Gastl G. PKC-theta modulates the strength of T cell responses by targeting Cbl-b for ubiquitination and degradation. Sci Signal. 2009;2:ra30. doi: 10.1126/scisignal.2000046. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifhofer C., Kofler K., Gruber T., Tabrizi N.G., Lutz C., Maly K. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J Exp Med. 2003;197:1525–1535. doi: 10.1084/jem.20020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altman A., Kaminski S., Busuttil V., Droin N., Hu J., Tadevosyan Y. Positive feedback regulation of PLCgamma1/Ca(2+) signaling by PKCtheta in restimulated T cells via a Tec kinase-dependent pathway. Eur J Immunol. 2004;34:2001–2011. doi: 10.1002/eji.200324625. [DOI] [PubMed] [Google Scholar]
- 17.Manicassamy S., Sadim M., Ye R.D., Sun Z. Differential roles of PKC-theta in the regulation of intracellular calcium concentration in primary T cells. J Mol Biol. 2006;355:347–359. doi: 10.1016/j.jmb.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 18.Brandle D., Muller S., Muller C., Hengartner H., Pircher H. Regulation of RAG-1 and CD69 expression in the thymus during positive and negative selection. Eur J Immunol. 1994;24:145–151. doi: 10.1002/eji.1830240122. [DOI] [PubMed] [Google Scholar]
- 19.Dutz J.P., Ong C.J., Marth J., Teh H.S. Distinct differentiative stages of CD4+CD8+ thymocyte development defined by the lack of coreceptor binding in positive selection. J Immunol. 1995;154:2588–2599. [PubMed] [Google Scholar]
- 20.Fischer A.M., Katayama C.D., Pages G., Pouyssegur J., Hedrick S.M. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z., Arendt C.W., Ellmeier W., Schaeffer E.M., Sunshine M.J., Gandhi L. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 22.Northrop J.P., Ho S.N., Chen L., Thomas D.J., Timmerman L.A., Nolan G.P. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994;369:497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro V.S., Truitt K.E., Imboden J.B., Weiss A. CD28 mediates transcriptional upregulation of the interleukin-2 (IL-2) promoter through a composite element containing the CD28RE and NF-IL-2B AP-1 sites. Mol Cell Biol. 1997;17:4051–4058. doi: 10.1128/mcb.17.7.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Letschka T., Kollmann V., Pfeifhofer-Obermair C., Lutz-Nicoladoni C., Obermair G.J., Fresser F. PKC-theta selectively controls the adhesion-stimulating molecule Rap1. Blood. 2008;112:4617–4627. doi: 10.1182/blood-2007-11-121111. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi K., Altman A. Protein kinase C theta (PKCtheta): a key player in T cell life and death. Pharmacol Res. 2007;55:537–544. doi: 10.1016/j.phrs.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Boehmer H. Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol. 1990;8:531–556. doi: 10.1146/annurev.iy.08.040190.002531. [DOI] [PubMed] [Google Scholar]
- 27.Gao E.K., Lo D., Cheney R., Kanagawa O., Sprent J. Abnormal differentiation of thymocytes in mice treated with cyclosporin A. Nature. 1988;336:176–179. doi: 10.1038/336176a0. [DOI] [PubMed] [Google Scholar]
- 28.Hollander G.A., Fruman D.A., Bierer B.E., Burakoff S.J. Disruption of T cell development and repertoire selection by calcineurin inhibition in vivo. Transplantation. 1994;58:1037–1043. doi: 10.1097/00007890-199411150-00011. [DOI] [PubMed] [Google Scholar]
- 29.Urdahl K.B., Pardoll D.M., Jenkins M.K. Cyclosporin A inhibits positive selection and delays negative selection in alpha beta TCR transgenic mice. J Immunol. 1994;152:2853–2859. [PubMed] [Google Scholar]
- 30.Jiang H., Xiong F., Kong S., Ogawa T., Kobayashi M., Liu J.O. Distinct tissue and cellular distribution of two major isoforms of calcineurin. Mol Immunol. 1997;34:663–669. doi: 10.1016/s0161-5890(97)00054-0. [DOI] [PubMed] [Google Scholar]
- 31.Rincon M., Flavell R.A. Regulation of AP-1 and NFAT transcription factors during thymic selection of T cells. Mol Cell Biol. 1996;16:1074–1084. doi: 10.1128/mcb.16.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alberola-Ila J., Hogquist K.A., Swan K.A., Bevan M.J., Perlmutter R.M. Positive and negative selection invoke distinct signaling pathways. J Exp Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dower N.A., Stang S.L., Bottorff D.A., Ebinu J.O., Dickie P., Ostergaard H.L. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 34.Jost P.J., Weiss S., Ferch U., Gross O., Mak T.W., Peschel C. Bcl10/Malt1 signaling is essential for TCR-induced NF-kappaB activation in thymocytes but dispensable for positive or negative selection. J Immunol. 2007;178:953–960. doi: 10.4049/jimmunol.178.2.953. [DOI] [PubMed] [Google Scholar]
- 35.Hettmann T., DiDonato J., Karin M., Leiden J.M. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145–158. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang F., Kitaura Y., Jang I., Naramura M., Kole H.H., Liu L. Establishment of the major compatibility complex-dependent development of CD4+ and CD8+ T cells by the Cbl family proteins. Immunity. 2006;25:571–581. doi: 10.1016/j.immuni.2006.08.021. [DOI] [PubMed] [Google Scholar]