Abstract
Transfusion-related acute lung injury (TRALI) is the major cause of transfusion related morbidity and mortality, world wide. Efforts to reduce or eliminate this serious complication of blood transfusion are hampered by an incomplete understanding of its pathogenesis. Currently, TRALI is thought to be mediated by donor alloantibodies directed against host leukocytes or the result of two distinct clinical events. For both proposed mechanisms the neutrophil (PMN) is the key effector cell. This paper reviews TRALI pathophysiology, explores the role of the PMN, details practical information for appropriate diagnosis, and promotes further studies into the pathogenesis of TRALI.
PMN DISTRIBUTION, FUNCTION AND PHENOTYPES
PMN distribution
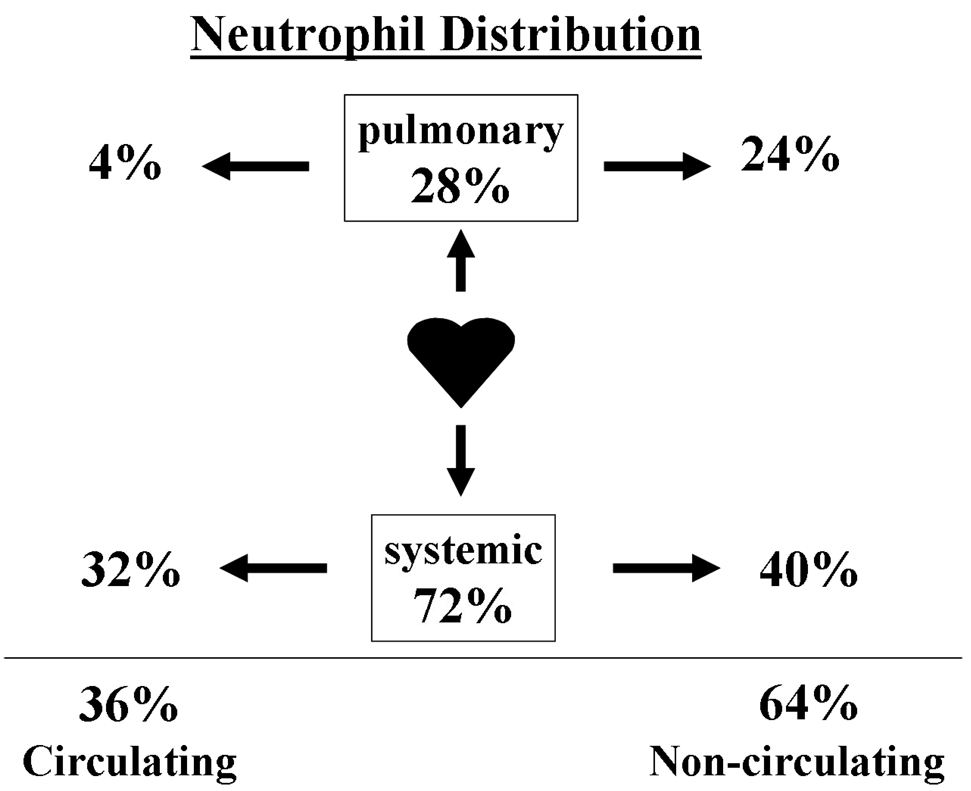
In a healthy adult, 36% of all PMNs reside in the circulating pool and 64% reside in a non-circulating ‘reserve’ pool, in the spleen and the bone marrow (1) (Fig. 1). If one compares the number of PMNs in different anatomical locations, i.e. organ systems, 28% of all PMNs are in the “pulmonary pool”; therefore, a substantial proportion of PMNs are normally located within the lung (1;2). Importantly, the number of PMNs in the pulmonary pool does not stay constant, but increases in systemic inflammatory conditions, including: inflammatory bowel disease, systemic vasculitis and graft versus host disease (1). In these clinical situations the pulmonary accumulation of PMNs is considered pulmonary sequestration because these PMNs 1) remain intravascular, 2) do not migrate into the lung parenchyma, 3) there is no lung edema or acute lung injury (ALI), and 4) the sequestered PMNs clear the pulmonary circulation within 24 hours (1;2).
Fig. 1. Distribution of neutrophils.
In a healthy normal adult, 36% of all PMNs reside in the circulating pool and 64% are in the non-circulating ‘reserve’ pool (1,2). Of the total number of PMNs 28% are in the pulmonary pool, and this consists of both circulating and non-circulating PMNs. Therefore, a substantial proportion of PMNs are normally located in the pulmonary system.
PMN function: the response to infection
The PMN is vital for the clearance of bacterial and fungal pathogens. Central to innate immunity is the ability of PMNs to respond to infections in the tissues and to marginate from the vasculature to the site of an infection. An understanding of the PMN response to infection is critical to comprehending their role in ALI. This response is usually limited via the circulation and the vasculature so that it is confined to the smallest area necessary to appropriately respond to the nidus of infection (3). In addition, the PMN response is usually brief and usually of a magnitude sufficient to resolve the infection or inflammation without damaging host tissue. Loss of control of this response, especially if large numbers of PMNs are positioned in an inappropriate location of the body, can result in collateral damage to host tissue and ALI (3;4). Pulmonary endothelial injury and capillary leak, the hallmark of ALI and especially TRALI, are considered to be the PMN microbicidal response occurring out of context in the pulmonary microcirculation.
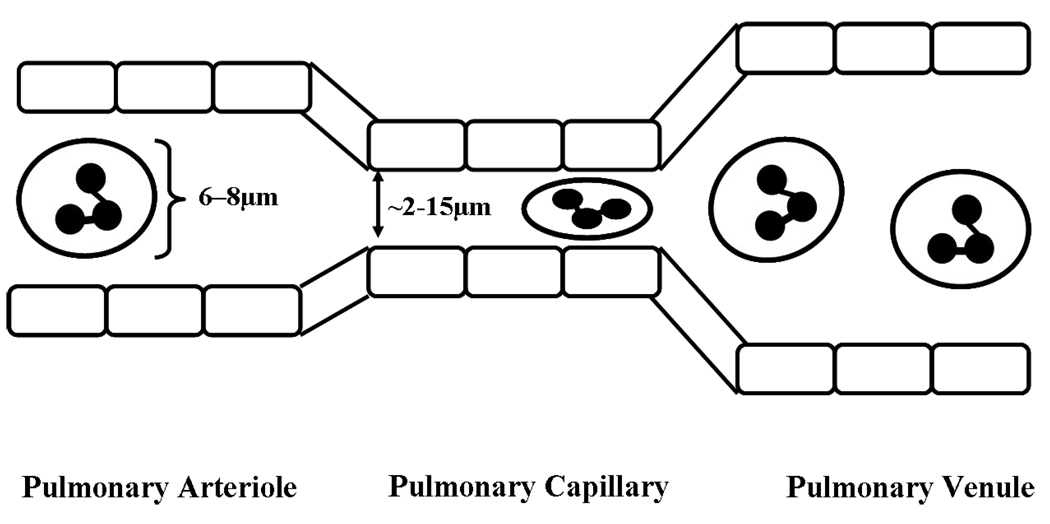
While the large surface area of the lung supports vital gaseous exchange, it concurrently provides an area of direct contact with the external environment. To minimize susceptibility of the host to airborne pathogens, it is critical that this interface thus contains elements of the innate immune system, e.g. a substantial proportion of the circulating PMN population (1). Importantly, migration of PMNs from the pulmonary microvasculature to the lung tissue is different from other vascular beds, as it occurs almost exclusively from the pulmonary capillaries and not in the post-capillary venules (5). The capillaries often have a smaller diameter (2–5 µm) than the PMNs (6–8 µm) themselves (Fig. 2) (6). The recruitment, adhesion and transmigration of PMNs from the pulmonary microvasculature in response to infection in the lung tissue is a sequential, coordinated process involving complex interactions between PMNs and the endothelium (7;8). PMNs are able to transit the narrow capillaries because they can be deformed and squashed into an elliptical shape (Fig. 2) (9;10). This spatial confinement in the pulmonary capillaries appears to obviate the need for selectins normally required to facilitate rolling and tethering of PMNs (6;11) . In the pulmonary microcirculation where PMNs have close contact with endothelial cells (ECs) engagement of selectins does occur (referred to as capture), increasing pulmonary transit times. Firm adhesion via β2-integrins and their obligate ligands appears to be important for effective migration out of the capillaries and into the lung tissue (8;11). Pulmonary transmigration of PMNs across the vascular endothelium may occur via either a CD11/CD18-dependent pathway or a CD11/CD18-independent pathway (8;12). However, selectins and CD11/CD18 on PMNs are not totally redundant, as studies in rabbits have shown that although they are not required for sequestration, they are required for maintaining the sequestered PMNs within the pulmonary capillaries (11). Importantly, the required PMN deformation to traverse the pulmonary circulation, as evidenced by the extended transit time, elicits close contact between PMNs and the vascular endothelium which allows for effective recruitment through pro-inflammatory activation of the vascular EC which may rapidly change a normal quiescent, non-adherent PMN phenotype to a primed, adherent PMN phenotype.
Fig. 2. PMNs deform as they pass through the pulmonary capillaries.
PMNs are 6 – 8 µm in diameter, while the lumen of pulmonary capillaries range from 2 –15 µm (6). Therefore, PMNs are deformed and get squashed into an elliptical shape as they transit the pulmonary capillaries (9,10). This slows down the PMNs and allows them more time to “sense” the surrounding environment.
PMN phenotypes
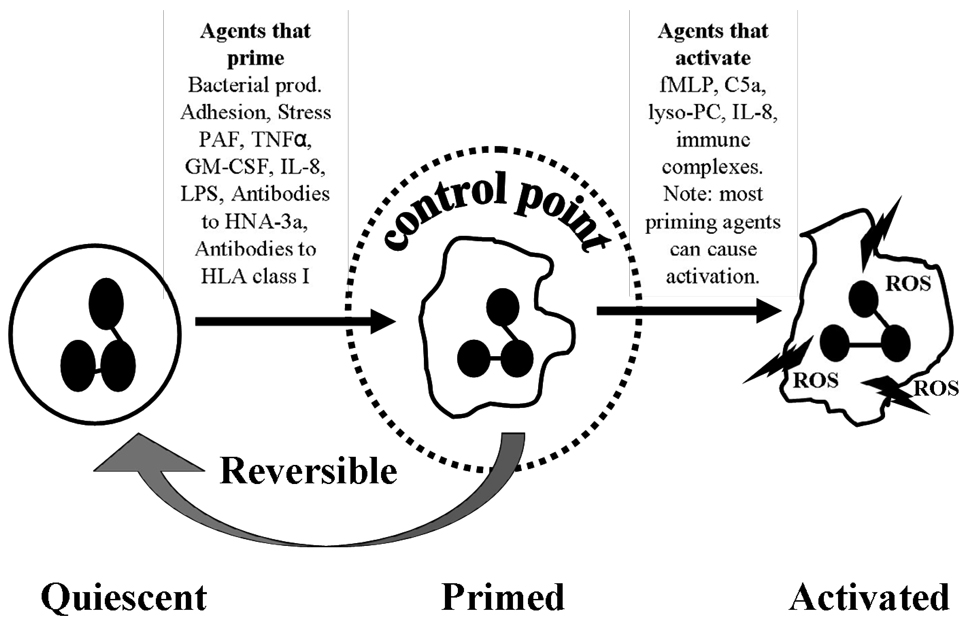
Three PMN functional phenotypes (quiescent, primed and activated) have been described and each phenotype has distinct characteristics which affect the nature and magnitude of the PMN response (Fig. 3) (13). In healthy individuals the circulating PMNs are usually of the quiescent phenotype. Quiescent PMNs are free flowing in the circulation, have an approximate round morphology, are not adhesive, and have minimal membrane ruffling (14). Quiescent PMNs are recruited to the activated endothelium via released chemokines and are tethered, and captured by various selectins. Following selectin-mediated tethering, the β2-integrins on the PMNs firmly adhere to RGD (arginine-glycine-aspartic acid) ligands, mostly ICAM-1, on the surface of the pulmonary vascular endothelium through a rapid conformational change (8;15). When PMNs change from being non-adherent to being firmly adherent to ECs, they are considered to be primed (14;16).
Fig. 3. Functional PMN phenotypes: quiescent, primed and activated.
Activated Complement 5 (C5a); N-formyl-Met-Leu-Phe (fMLP); Granulocyte Monocyte Colony Stimulating Factor (GM-CSF); Interleukin (IL); Lipopolysaccharide (LPS); lysophosphatidylcholines (lyso-PC); Platelet Activating Factor (PAF); Reactive Oxygen Species (ROS); Tissue Necrotising Factor- alpha (TNFα)
There are three distinct PMN phenotypes; quiescent, primed and activated (13). Quiescent PMNs become primed when they are exposed to priming agents such as PAF, TNFα, GM-CSF, IL-8, LPS or bacterial products. Primed PMNs become activated when stimulated with activating agents such fMLP or LTB4, but they can also activated by other priming agents such as HNA-3a or MHC class I antibodies (56, 101). This culminates in the respiratory burst. Priming is reversible as PMNs can deprime if subsequent activation does not occur.
PMN priming is separate from activation as it does not induce release of the PMN microbicidal arsenal to cause ALI. Priming is a physiologic step in the margination of PMNs to the tissues, i.e. the lung proper and in clinical terms results in pulmonary sequestration of PMNs (3;17;18). However, primed PMNs are functionally hyperactive as mediators that normally do not induce release of the microbicidal arsenal, both oxidative and granular proteases, and which may activate primed PMNs. Such “hyperactivity” is important for the efficient eradication of pathogens in the tissues in which they serve as the front line defense against infection. (3;14;19). In addition, priming causes changes in PMN shape so that they become larger and less deformable thus increasing their pulmonary transit times as well as delaying apoptosis, especially in inflammatory states and in injured tissue (20;21). This delay in apoptosis may extend PMN life and increase the reservoir of cells that can participate in the inflammatory response, possibly predisposing the patient to PMN-mediated ALI (22). Lastly, primed PMNs also have the ability to de-prime, or revert to a quiescent phenotype (19;23). Therefore, priming appears to be a key control point, as this will determine if the PMN response is sufficient to deal with pathogen invasion or to participate in the normal, regulated inflammatory response. Priming may also provide a means of avoiding excessive uncontrolled activation of PMNs which may lead to end-organ damage because it is transient (Fig. 3) (19).
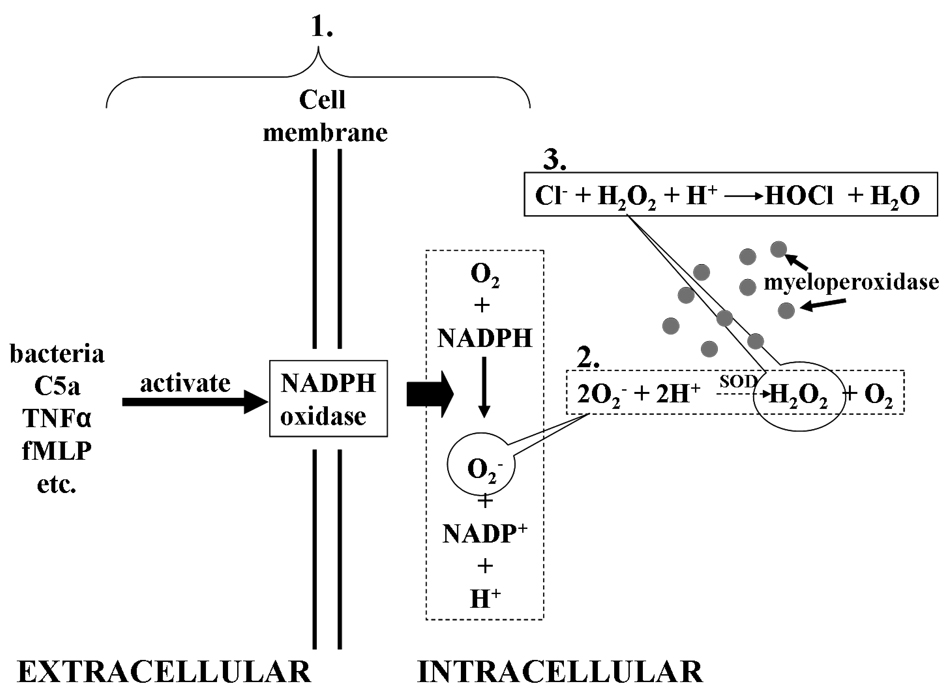
Lastly, activation of PMNs occurs when PMNs engulf bacteria in a phagolysozome and exert their microbicidal function through production of potentially toxic reactive oxygen species (ROS), via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and the release of granular contents focused on the phagocytized invaders to kill them (Fig. 4) (14;24). Priming enhances the release of both oxidative (ROS) and non-oxidative products to a given stimulus to efficiently kill bacteria. In the case of invaders larger than the PMN, for example fungi, the points of firm adhesion of the PMN to fungal hyphae focus the release of oxidative and non-oxidative components to kill these organisms (25).
Fig. 4. Production of O2 − and H2O2 following NADPH oxidase activation.
- Activation of NADPH oxidase catalyses the reduction of oxygen (O2) to superoxide (O2 −). This is a rapid reaction that occurs within 30 to 60 seconds of activation
- Myeloperoxidase uses the Cl−, Br− or I− as reducing substrates to produce hypohalous acid. The oxidation of Cl− produces hypocholorous acid (HOCl).HOCL is a very potent cytotoxic agent for bacteria, virusus, fungi and mycoplasmas.
ANTIBODY-MEDIATED TRALI
Antibody-mediated TRALI most often occurs when donor leukocyte allo-antibodies are infused into recipients who express the cognate antigen on their PMNs (Fig. 5). This antigen-antibody interaction has been proposed to cause PMN sequestration and aggregation in the pulmonary microvasculature. This results in PMN activation causing the release of the microbicidal arsenal, consisting of both reactive oxygen species (ROS) and proteases that can damage the pulmonary vascular endothelium resulting in capillary leak, pulmonary edema, and TRALI.
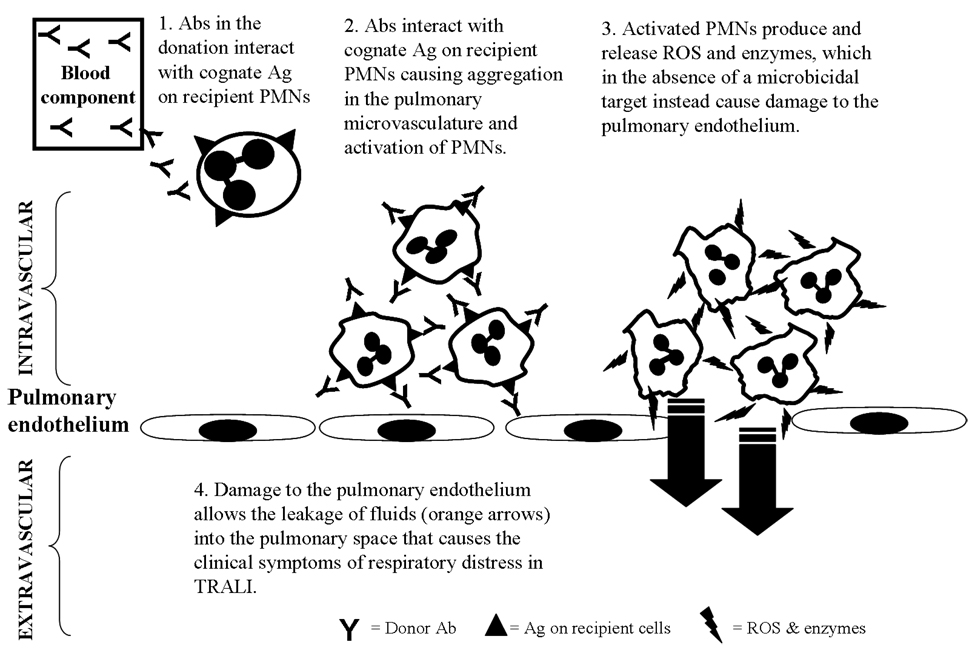
Fig. 5. Antibody mediated TRALI.
Antibodies (Abs) in the transfused donation interacts with its cognate antigen (Ag) on recipient’s PMN, causing aggregation in the pulmonary microvasculature and activation of PMNs (27,28). Activated PMNs produce and release reactive oxygen species (ROS) and enzymes, which in the absence of a microbicidal target instead cause damage to the pulmonary endothelium. The damage to the pulmonary endothelium allows the leakage of fluids into the pulmonary space resulting in the clinical symptoms of the respiratory distress associated with TRALI.
Antibody-mediated TRALI was proposed following the detection of leukoagglutinating and lymphocytotoxic antibodies in sera of transfused blood products in five cases of TRALI (26). Two years later a more comprehensive report of 36 cases detected granulocyte-specific and/or lymphocytotoxic antibody in at least one associated blood product in 89% of the cases (27). Because TRALI appeared clinically identical to acute respiratory distress syndrome (ARDS), Popovsky and Moore postulated that the mechanism for the pathogenesis of TRALI was probably similar to that of ARDS (28). Thus, the passive transfer of leukocyte antibodies from the donor provided a source of either PMN or complement activation that was associated with the occurrence of TRALI (27). The number of reports describing leukocyte antibodies associated with TRALI cases has been steadily growing since those early reports (29–32) . In line with this, it is worth noting that the majority (83%) of TRALI fatalities in the Centre for Biologics Evaluation and Research (CBER) report were associated with a leukocyte antibody (30). As well as case reports, other studies have highlighted the significance of the role of leukocyte antibodies and PMNs in the pathophysiology of TRALI.
Leukocyte alloantibodies can alter the fate of transfused PMNs
The in-vivo effects of antibodies on PMNs have been investigated by the application of PMNs labeled with the 111-Indium isotope (33–36). In the 1980s McCullough et al. (33;35) labeled isolated granulocytes from normal donors with 111-Indium isotope and transfused them into 93 patients (recipients) with localized infection. In the subsequent 24 hours, blood samples were collected from the recipient at intervals to determine intravascular recovery and half-life. Body scans were performed also to monitor the intravascular kinetics and extravascular localization of the transfused granulocytes. To understand the effect of leukocyte antibodies on the fate of the transfused granulocytes, cross-matches were performed subsequently between recipient serum and donor granulocytes and lymphocytes using five different leukocyte antibody techniques. They found that donor-recipient mismatches detected by granulocyte agglutination test (GAT) antibodies in particular were associated with granulocyte immune destruction and reduced granulocyte survival. In some patients where the antibodies were detected by both GAT and granulocyte immunofluorescence test (GIFT), the transfused granulocyte failed to migrate to foci of infection. Antibodies detected by lymphocytotoxicity were associated with reduced recovery and survival only when anti-HLA-A2, B8 or BW44 specificities were involved.
Dutcher et al. performed similar experiments transfusing indium-labeled granulocytes to two patient groups; alloimmunized and non-alloimmunized patients (34;36). The transfused labeled granulocytes localized at sites of infection in all 20 non-alloimmunized patients, but this localization only occurred in 3 of 14 alloimmunized patients. In summary, these studies demonstrated that the immunological mismatch in patients (recipients) with leukocyte alloantibodies receiving transfused granulocytes caused pulmonary sequestration, a significant decrease in circulating granulocyte survival and failure of the granulocytes to localize to sites of infection. Therefore, in cases of TRALI where the recipient has leukocyte antibodies the transfusion of blood products containing mismatched granulocytes may lead to the above mentioned consequences.
Antibodies can precipitate PMN mediated damage to surrounding tissue
To precisely understand the effect of leukocyte allo-antibodies on the pulmonary vasculature Seeger at al. developed an ex-vivo rabbit lung model (37). Human neutrophil antigen (HNA)-3a positive PMNs, together with anti-HNA-3a antibody and human complement were infused into rabbit lungs. This resulted in a significant increase in pulmonary vascular permeability. This model proved that leukoagglutinating antibodies such as anti-HNA-3a had the potential to induce TRALI by causing an increase in lung vascular permeability. However, whether complement or an antibody with leukoagglutinating properties is required for TRALI to occur has since been challenged in an ex-vivo rat lung model (38). TRALI, as measured by an increase in lung vascular permeability, was produced by the transfusion of CD177 (a monoclonal antibody to HNA-2a with no leukoagglutinating properties) in the presence of human HNA-2a positive PMNs. As this was performed in an environment free of foreign plasma, it demonstrated that complement was not required for the induction of TRALI. These results suggested that a specific PMN reactive antibody could activate PMNs directly. It was also noted that the addition of N-formyl-Met-Leu-Phe (fMLP) in the presence of the antibody and PMNs, greatly enhanced the increase in pulmonary vascular permeability. This demonstrated that the PMN response to antibodies can be potentiated by bioactive substances like fMLP. Furthermore, by experimenting with two populations of PMNs (low versus high HNA-2a expression) they showed in the model that the density of the cognate antigen on target PMNs was a determinant of whether TRALI occurred.
Infusion of antibodies can initiate TRALI in healthy individual
Evidence of the adverse effects of infused leukocyte antibodies and their ability to induce TRALI are elegantly described in two unusual human events. In the 1950’s Brittingham investigated the effect of transfusing whole blood or plasma from 10 leukopenic patients to healthy subjects (recipients) (39). One recipient developed a severe illness characterized by vomiting and diarrhea, chills, fever, severe hypotension, severe tachypnea and dyspnea that occurred 45 minutes into the infusion. Chest x-rays taken the next day showed marked bilateral pulmonary infiltrates. The recipient had been transfused with whole blood from a leukopenic patient with hypoplastic anemia, who had previously received 75 transfusions and had a strong leukoagglutinin. A subsequent leucoagglutination test between the recipient’s white cells and the leukopenic patient’s serum confirmed the strength of the antibody with an antibody titer of 1/256 in saline. All clinical abnormalities in the recipient disappeared three days later. This case is significant for a number of reasons: Firstly, it clearly demonstrated that the transfusion of leukoagglutinins into a recipient with a cognate antigen caused a severe adverse reaction. Secondly, because the recipient was normal, the confounding factors of co-morbidities from underlying diseases were excluded. Finally, as the clinical description of this old case fits comfortably within the definition from the 2004 TRALI Consensus Conference, this may have been one of the earliest descriptions of this syndrome (40). However, this report had limitations as there is little information on how the whole blood was prepared, stored and about its age at transfusion; hence we cannot exclude the possible contributions of storage or deterioration factors to the TRALI reaction. Knowledge and technology of the time only allowed a description of the initiating immunological agent as a leukoagglutinin, whereas today we would try to determine the antibody type (e.g. HNA or HLA) and its specificity.
In 1998, there was another unusual report involving a healthy volunteer, when the effects of intravenous administration of experimental monocyte-inhibiting γ-globulin (MIGG) on a human subject was studied (41). The 31 year old healthy male recipient had no history of allergies; was taking no medications and had no febrile illness in the preceding months. Following the infusion of MIGG (0.6g IgG; 0.5mL/min) the volunteer developed a dry cough, malaise, hypotension and had a slightly elevated pulse. This was mistaken as a vagal reaction, and because the volunteer recovered they recommenced the infusion of MIGG after a 50 minute interval. After 20 minutes (80 mL MIGG had been infused) the volunteer began coughing again and became very ill with nausea, vomiting, had a decrease in blood pressure, increase in pulse rate and he produced white foamy sputum that went on to be tinged with blood. Chest radiographs revealed diffuse bilateral pulmonary interstitial infiltration with a normal cardiac silhouette. He was initially treated with intravenous hydrocortisone but deteriorated and was mechanically ventilated. The volunteer remained unstable and thus had therapeutic leukopheresis with plasma exchange, following which he improved, was extubated and had a complete recovery. The MIGG was subsequently found to contain HLA Class I and Class II antibodies and monocyte-reactive antibodies of unknown specificity.
This report was more complete than the previous one, because the infused product contained γ-globulins, the dose was measured and there was a more detailed clinical history for the volunteer. Again, the use of a healthy human recipient removed confounding disease and other possible pathological factors from the equation. Although the MIGG contained a range of different allo-antibodies, its use clearly proved that the infusion of leukocyte antibodies induced serious complications that fit the TRALI definition.
In-vivo mouse model demonstrates that high dose monoclonal antibody cause ALI with a high mortality rate
TRALI as evidenced by interstitial lung leak and lung histology that showed septal thickening, with inflammatory infiltrate consisting mainly of granulocytes was observed in mice transfused with large amounts (4.5 mg / kg) of a murine IgG subclass II (42). PMNs were critical as their removal using a murine granulocyte specific antibody inhibited TRALI. However, these studies indicate a mechanism different from that initially proposed by Popovsky and reinforced by others, in that there was a mortality of 50%, the amounts of antibody infused were supra-physiologic (4.5 mg / kg), and knock-out mice lacking Fc receptors did not show evidence of TRALI. These animal studies implicated immune complexes forming on the surface of the vascular endothelium as being responsible for TRALI. There is a single case report of a lung transplant recipient that reinforces this latter hypothesis in the pathogenesis of TRALI (43). Most cases of TRALI have never been linked to immune complex formation as the cognate leukocyte antigens that were thought to be on the surface of the PMNs have never been demonstrated to be of endothelial origin. Moreover, the mortality of clinical TRALI is 5–10% in most studies (16;44;45), although possible it is unlikely that clinically the antibody concentrations would ever reach the levels seem in this murine models because lower concentrations did not elicit TRALI events in this experimental model (42).
Antibodies to HLA class II antigens and PMN-mediated TRALI
Antibodies to HLA class II antigens have been implicated in TRALI and recipients have often been shown to express the cognate antigen on their leukocytes (46–48). Despite these data, PMNs are not antigen presenting cells and normally do not express these antigens. There are a few reports of prolonged (72 hours) cytokine stimulation of PMNs resulting in PMNs expressing these antigens and both G-CSF and GM-CSF have induced HLA-DR antigen surface expression on granulocytes in-vivo (49;50); however clinically neither PMNs nor pulmonary endothelial cells express class II antigens in any TRALI cases in which antibodies to HLA class II antibodies were implicated. In-vitro data has shown that monocytes can be stimulated to synthesize cytokines intracellularly but such synthesis takes 4 hours and these mediators were not released extracellularly (46). Nishimura et al. have examined co-culture experiments with monocytes and pulmonary endothelial cells which produced cytokines and lipids implicated in ALI, but the concentrations observed were very low and did not reach levels associated with pro-inflammatory changes in PMNs, let alone ALI (51). In-vitro studies show that HLA class II antibodies in the presence of monocytes with the cognate antigen were able to cause dysfunction of lung microvascular endothelium, cause the secretion of inflammatory cytokines and neutrophil-activating chemokines (52) and increase endothelial permeability (53). Therefore, instead of having a direct effect on PMNs, HLA class II antibodies appear to cause the release of proinflammatory mediators which in turn activate PMNs; more akin to the first event in a two event model (32;52). Further in-vitro and in-vivo work clearly is required to define the role of antibodies to HLA class II antigens in the etiology of TRALI.
THE TWO-EVENT MODEL
Since TRALI is a form of ALI and is clinically similar to ARDS, its pathogenesis should be similar to that of ALI/ARDS as initially postulated (27). Animal models of ALI/ARDS as well as the clinical syndrome are the result of at least two distinct clinical events; thus, a similar two event pathogenesis was proposed for TRALI (54;55). The first event is the patients’ clinical condition, which induces pro-inflammatory activation of the pulmonary endothelium and PMN sequestration (Fig. 6). Thus the adherent, primed PMNs are functionally hyper-reactive in that substances that normally do not activate PMNs (i.e. cause the release of the microbicidal arsenal), are able to augment the release of their microbicidal arsenal to a subsequent second stimulus (19;56). The second event is the infusion of a biologic response modifier (BRM) present in the blood product that activates the adherent PMNs in the pulmonary vasculature eliciting PMN-mediated endothelial damage, capillary leak and ALI. These BRMs include antibodies, lipids that are generated during routine storage of cellular blood products, and soluble CD40 ligand which is generated from platelet membranes during the routine storage of platelet concentrates (PCs) (55;57–61). This two-event model attempts to explain both antibody-mediated TRALI and TRALI in which no leukocyte allo-antibodies could be identified. Moreover for it is important to remember that TRALI is a clinical diagnosis with no prerequisites for laboratory information hence leukocyte antibodies are not required for its diagnosis (27;30;62;63).
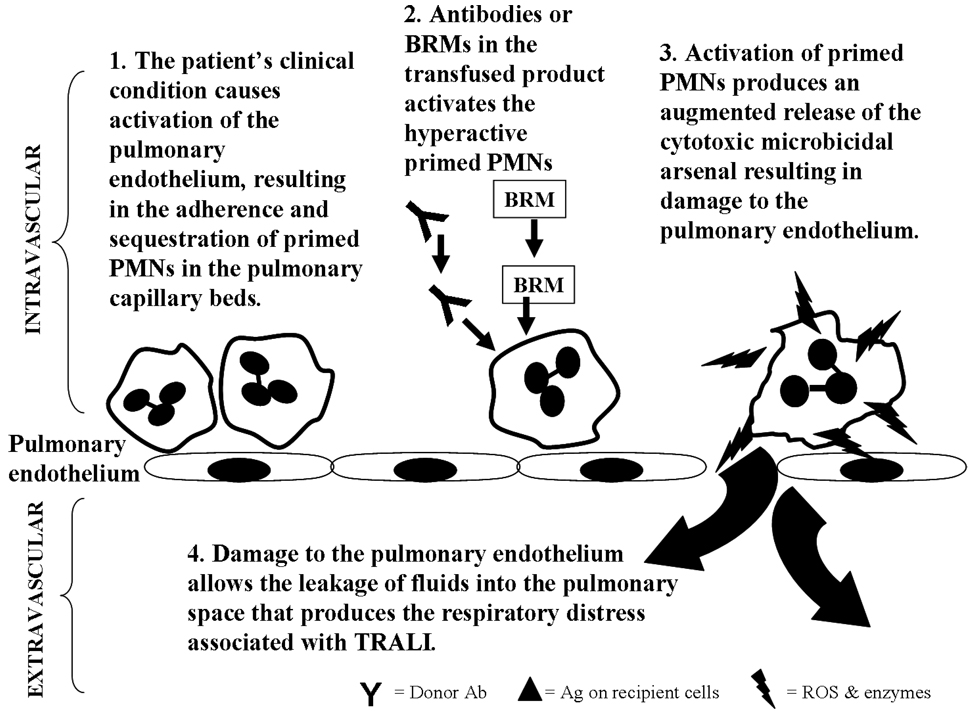
Fig. 6. The Two Event Model.
The patient’s clinical condition causes activation of the pulmonary endothelium, resulting in the adherence and sequestration of primed PMNs in the pulmonary capillary beds (49,50). Antibodies or biological response modifers (BRMs) in the transfused blood product activates the primed PMNs. Activation of primed PMNs produces an augmented release of the cytotoxic microbicidal arsenal resulting in damage to the pulmonary endothelium. Damage to the pulmonary endothelium allows the leakage of fluids into the pulmonary space that produces the respiratory distress associated with TRALI.
Priming activity in stored cellular blood component
The accumulation of lipid priming activity occurs with all stored cellular components and reaches a relative maximum the last day the product may be transfused (18;55;57). This activity was, for the most part, chloroform soluble indicating it was lipid in nature (64). Partial characterization has demonstrated that packed red blood cells (PRBCs), platelet concentrates (PCs) and whole blood (WB) all contain a mixture of lysophosphatidylcholines (lyso-PCs) (64;65). In addition, both PRBCs and WB contain a neutral lipid priming activity that has not been fully characterized to date. Importantly during storage of PCs, sCD40L accumulates which accounts for the non-lipid portion of the priming activity seen in PCs (66;67). Pre-storage leukoreduction does not abolish the generation of lipid priming activity of PRBCs, but in preliminary studies it inhibits the generation of lyso-PCs but has no effect on the accumulation of the neutral lipids (68). Fresh frozen plasma (FFP) does not contain lipid priming activity even when stored under identical conditions to PCs or PRBCs suggesting that the accumulation of the bioactive lipids is due to cellular breakdown for it mimics the storage lesion (55). Serial studies of plasma from aphaeresis platelets and whole blood platelets (WB-Plts) demonstrate that significant amounts of these lipids appear within 24 to 48 hours of storage, respectively (65). Conversely, the accumulation of lipid priming activity in PRBCs and WB occur within 2 weeks of storage (64).
Lipids in stored components and TRALI
The association of lipid priming agents with TRALI was demonstrated in a study that compared blood samples from patients where TRALI was clinically recognized versus samples from a control patient group of uncomplicated febrile non-hemolytic transfusion reactions (FNHTR) or urticarial reactions (69). Samples from TRALI patients contained significantly higher PMN priming activity, compared to both pre-transfusion samples from the same patients and samples from the control group, thereby implicating PMN priming in the pathogenesis of TRALI. Priming alone does not cause PMN activation which is required for PMN-induced ALI. However, as priming agents do have the capacity to activate primed PMNs, the cases were reviewed for underlying clinical conditions that may be associated with PMN priming. All 10 TRALI patients had: 1) surgery within 24 hours, 2) acute active infection, 3) cytokine administration (granulocyte colony stimulating factor (G-CSF) or granulocyte monocyte colony stimulating factor (GM-CSF)), or, 4) were massively transfused, while only 2/10 patients in the control group had similar clinical conditions (69). Furthermore, all of these predisposing conditions have been implicated by other investigators in cases of antibody-negative TRALI (16;28;30;58;70–73)
Two-event in-vitro and animal models demonstrate that lipids from stored blood cause PMN-mediated pulmonary endothelial damage
Using oxidase activation and elastase release as a measure of PMN activation, Wyman et al. confirmed that individually, endotoxin (lipopolysaccharide (LPS)) and lyso-PCs prime but did not activate PMNs; however, lyso-PCs were also able to activate LPS-primed PMNs (15). These data demonstrated that sequential exposure of PMNs to priming agents could activate PMNs. To model TRALI in-vitro, co-culture experiments were performed with human pulmonary microvascular endothelial cells (HMVECs) and PMNs (15). HMVECs were activated with LPS to mimic sepsis as the first event which caused increases in the surface expression of intracellular adhesion molecules (ICAM) such as ICAM-1, synthesis and release of chemokines, and firm adhesion of PMNs to these activated HMVECs. Lyso-PCs were added as the second event to mimic transfusion of stored cellular components which resulted in PMN-mediated HMVEC damage. This model demonstrated that pulmonary endothelial activation was required, PMNs must be present, and lyso-PCs could serve as a second event to activate the primed, adherent PMNs resulting in PMN cytotoxicity. When sCD40L was used in place of lyso-PCs, identical PMN priming activity and PMN-mediated cytotoxicity (second event) were also demonstrated (67).
Ex-vivo rat models also verified that the plasma and the lipids from stored cellular components, both PRBCs and PCs, could cause TRALI as the second event in a two-event ex-vivo animal model (68). Rats treated with LPS exhibited PMN sequestration and the plasma and lipids from stored, but not fresh, PRBCs and PC (both apheresis and WB-derived) caused ALI in LPS –treated animals (68). No ALI was demonstrated in rats who received saline as the first event. Importantly both the plasma and lipids from fresh and stored PRBCs and PCs came from identical units. Recent in-vivo modeling demonstrated similar results in that the plasma from stored but not fresh PRBCs elicited ALI at both day 28 and day 42, and pre-storage leukoreduction did not remove the ability of the plasma from stored PRBCs to induce TRALI in-vivo as a second event (74).
Lipids and Clinical TRALI
Bioactive lipids were also found in clinical TRALI, in a study of some 90 consecutive cases of TRALI secondary to the transfusion of PCs (64). In these studies lipid priming activity was detected in the plasma of patients at a sample drawn when TRALI was clinically recognized. No lipid priming activity was demonstrated in the pre-transfusion patient plasma sample. The lipids found in the patients with TRALI comprised both neutral lipids and lyso-PCs. Antibodies to leukocyte antigens, including HNA and both HLA class I and class II antigens, were ruled out in 97% of these TRALI cases. In addition in a prospective nested case control study patients with hematological malignancy in the induction phase of chemotherapy and patients requiring cardiac surgery were predisposed to TRALI as compared to 225 hospitalized, matched control subjects (71). Both of these two patient groups made up the largest sets of patients with fatal TRALI reported to the FDA in 2004 (30). Lastly, analyses of these TRALI cases also demonstrated that sCD40L appeared to be at least a co-factor, if not a mediator, of clinical TRALI relating to the second clinical event during the transfusion of PCs (67).
The Threshold Model
Like the two event model, the Threshold Model is dependent on two factors i.e. patient predisposition and the strength of transfusion related mediator, with an emphasis on how the gravity of each of these two factors contribute to the severity of the TRALI (75). If we consider the TRALI look-back report involving HNA-3a where only 36% instead of 98% of patients with antigen:antibody pairing; this model would suggest that only 36% of the patients had an underlying clinical condition that could precipitate TRALI and alternatively in the remaining 62% of recipients the extent of the clinical predisposing factors leading to PMN activation “threshold” had not been overcome (76). This look-back report in conjunction with case reports by Brittingham & Dooren et al., affirms the need for research into the immunobiological characteristics of TRALI implicated antibodies (39;41). The requirement of a clinical justification before any transfusion makes a one hit event much less likely but the relative contributions of patient predisposition factors and the age and quality of the transfused blood products cannot be denied and certainly warrants further research.
MERGING THE PATHOGENESIS: THE TWO EVENT MODEL OF ANTIBODY-MEDIATED TRALI
In-vitro and animal models demonstrate that antibodies cause PMN mediated ALI in a two-event model
Antibodies to HNA-3a have been implicated in numerous cases of fatal TRALI and 96% of the population express this antigen on the surface of their PMNs (32;76–80). However, clinical look-back studies have demonstrated that antigen:antibody pairing was not enough to cause TRALI as the majority of people transfused with plasma containing antibodies to HNA-3a did not have evidence of ALI events (76;80). Silliman et al. postulated that antibodies to HNA-3a had PMN priming activity analogous to bioactive lipids and sCD40L and required two events to cause PMN cytotoxicity (61). Antibodies to HNA-3a caused priming of the fMLP-activated oxidase in PMNs that expressed HNA-3a (HNA-3a+) but not in PMNs that did not express HNA-3a (HNA-3a−). Moreover, the HNA-3a antibody alone did not induce PMN mediated damage of HMVECs even when the PMNs did express HNA-3a . However, if the HMVECs were activated with LPS first and then incubated with HNA-3a+ but not HNA-3a− PMNs, followed by the addition of antibodies to HNA-3a, then widespread PMN-mediated cytotoxicity of activated HMVECs was observed. Importantly, these antibody-antigen interactions were specific because blockade of the Fcγ receptors I-III with (Fab’)2 fragments did not block the ability of the antibodies to HNA-3a to prime PMNs or cause PMN-induced cytotoxicity. These studies demonstrated that specific antibodies to HNA-3a alone could prime PMNs, and that the antibodies only mediated cytotoxicity of HMVECs that had already been activated.
Recent in-vivo modeling demonstrated that murine antibodies to rat MHC class I antigens, OX18 and OX 27, did not cause ALI; however, if the animals were first given LPS and then transfused with these antibodies ALI was demonstrated by Evans blue dye leak, increases in total protein and CINC-1 in the bronchoalveolar lavage fluid and by lung histology (74). Furthermore, these studies demonstrated that: 1) the antibodies localized to the surface of the PMNs and did not form immune complexes on the surface of the pulmonary vascular endothelium; 2) that PMNs were required because granulocyte-depleted rats did not show evidence of TRALI; 3) even at supra-physiologic concentrations that these antibodies alone did not elicit ALI; and 4) the antibodies specifically recognized antigens on the PMN surface and primed the fMLP-activated oxidase of rat PMNs (74). Importantly, the murine antibody which caused immune clearance of rat PMNs did not prime PMNs and did not cause ALI as the second event in the model, implying that PMN priming by a pro-inflammatory stimulus was important for the ability of the antigen antibody reaction to elicit ALI (74). The observed transfusion related alloimmune neutropenia (TRAIN) in this rat experiment may model the persistent neutropenia observed in the neonatal case following the transfusion of plasma containing antibodies to HNA-1b (81).
All leukocyte antibodies are not equal in triggering TRALI
Antibodies to HNA-3a and HLA-A2 have been associated with TRALI fatalities (32;76;82–85). In contrast TRALI cases with antibodies to HNA-1a and HNA-2a have had no fatalities (29;86–88) indicating characteristics of each antibody may be a determining factor in whether mild, severe or no TRALI reaction is precipitated. By comparing an anti-HNA-4a sera from an alloimmune neonatal neutropenia (ANN) case with other anti-HNA-4a antibodies in women with no ANN history showed that although all of the sera had the same specificity, their functional characteristics were not identical (89). We have also observed that anti-HNA-2a antibodies from TRALI and ANN cases demonstrate both serological and functional differences (unpublished). Keep in mind that while HNA-1a,b,c and HNA-2a are specific to PMNs, HNA-3a, HNA-4a and HNA-5a are not limited to PMN but common to other cell types (90;91). Hence, antibodies to HNA-3a, HNA-4a and HNA-5a would theoretically have a wider target population as they can react with other cells. Therefore, there is a case to be made for doing detailed research and better profiling of the characteristics of identified plasma/sera antibodies from TRALI implicated donors for both their immune and PMN priming/activating function. Characteristics that are of interest include:
antibody isotype (IgG, IgA, IgE, IgM) and sub types
method of detection. i.e., whether an agglutinating (GAT) or binding antibody (GIFT)
antibody titer
epitope specificity
whether it primes and/or activates PMNs
whether it increases or decreases particular PMN surface functional molecules
Such detailed antibody information may shed more light on the role of allo-antibodies in the TRALI mechanism.
LABORATORY INVESTIGATION OF TRALI
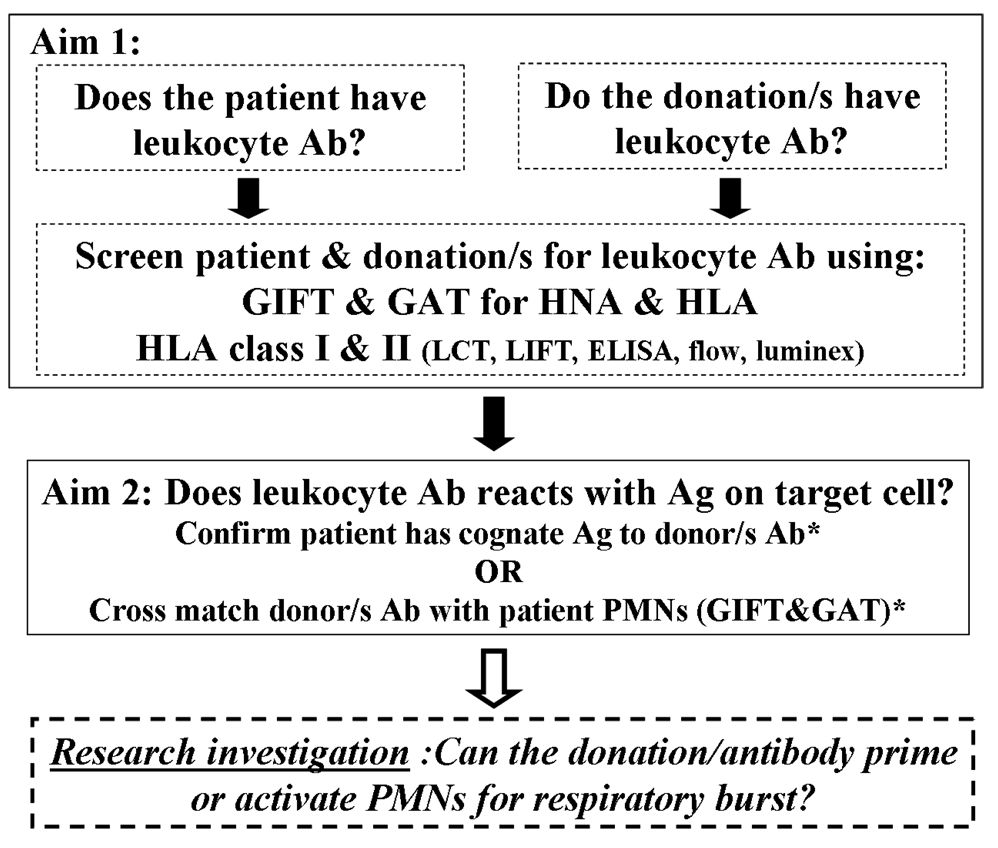
There are two aims to this exercise (Fig. 7) (27;29;62;92):
To detect any leukocyte antibodies (HNA, HLA class I and HLA class II, ) in the patient (transfusion recipient) and the associated donations in all blood products transfused within 6 hours of the recognition of TRALI
To seek corroborative evidence that the detected antibody can react with an available corresponding antigen on the target cell
Fig. 7. Laboratory investigation of TRALI.
Antigen (Ag); Antibody (Ab); Granulocyte Agglutination Test (GAT); Granulocyte Immunofluorescence Test (GIFT); Lymphocytotoxicity Test (LCT); Lymphocyte Immunofluorescence Test (LIFT)
The first aim of the laboratory investigation of TRALI is to detect any leukocyte antibodies (Abs) (HNA, HLA class I and HLA class II) in either the patient (transfusion recipient) or associated donors/donations using well validated assays (27,57,86) . The second aim is to seek corroborative evidence that the detected Ab can react with an available corresponding antigen on the target cell. *In the majority of cases the Ab is of donor/donation origin and this is investigated either by confirming that the patient has a corresponding antigen or by a cross-match of donor sample against the recipient’s PMN. In the rare instance where the Ab is from the patient, a cognate antigen in the donors/donation is sought and the cross-match is between the patient serum/plasma against donor PMNs. If resources are available further investigation includes determining if the Abs detected are able to prime or activate for PMN respiratory burst using assays such as the superoxide dismutase-inhibitable reduction of cytochrome C assay.
Screening for TRALI associated leukocyte antibodies
The results of the International Granulocyte Workshop (IGW) show that the combination of the GIFT and GAT is an effective approach for detecting PMN reactive antibodies in serum or plasma (93;94). These methods have the advantage of detecting not only antibodies to HNA but can also detect antibodies to HLA class I, and possibly HLA class II antibodies (95;96). Results from the IGW confirm that the GAT is an essential technique if antibodies to HNA-3a, which has been implicated in multiple fatal TRALI cases are to be detected (94). GAT provides the best indication as to how the antibodies affect PMNs in-vivo, as the observed agglutination is a result of viable PMNs being sensitized by the antibody, leading to chemotaxis and producing homotypic PMN:PMN agglutinates, as distinct from passive cross-liking via IgM (97;98). Thus, using GAT in combination with GIFT analysis and panels of phenotyped PMNs, the specificity of all PMN reactive antibodies can be elucidated. Since these techniques are often limited to reference laboratories the Granulocyte Working Party (GWP) of the ISBT (www.isbt-web.org) is a necessary reference for identifying appropriate laboratories in different geographic locales.
The laboratory investigation of HLA class I and II antibodies will not be discussed in detail as the associated techniques are widely available and well known. Readers are referred to recent reviews on the topic (99;100). However, it is important to note that many of the assays employed are based upon testing donors and recipients prior to solid organ transplantation, and these techniques are highly sensitive in order to detect the presence of any antibodies in donor or recipient sera that could be clinically relevant. Because HLA antibody screening is widely available it is often the first step in many TRALI investigations when screening patients and associated donor samples. However, two reports reveal that a disproportionately small number of HLA class I antibodies actually induce TRALI (101;102). With this in mind and the high sensitivity of current HLA techniques, one questions the clinical relevance of all of the HLA antibodies detected in TRALI because most of the current flow-based assays are so sensitive that a relatively high percentage of non-transfused males demonstrated antibodies (103;104). Current efforts to develop effective HLA antibody screening methods for blood donors from various testing sites reveal challenges in differentiating background noise and defining suitable cut offs such that when one employs these new normal range of cutoffs some antibodies implicated in TRALI never reach a sufficient titer to be considered relevant (105;106). Furthermore, the clinical effect of transfused HLA antibodies in-vivo may also be attenuated as they are absorbed by both platelets and neutralized by soluble HLA class I molecules which make up 90% of HLA class I antigens in the blood and thereby reduce the likelihood of the antibody triggering a cellular response leading to TRALI (107). Therefore, while HLA class I antibody screening is often easily available its may not be effective if it is the sole first line TRALI investigation screen. One notable exception is HLA-A2which has been implicated in many TRALI cases and thus should be included in any first line TRALI investigation screen. (26;62;83;108).
The flow based assays used to detect antibodies to HLA class II antigens in donors are designed to detect very small amounts of antibodies using a PRA luminex bead assay (One Lambda, Canoga Park, CA). Such detection of antibody presence alone may overestimate the role of antibodies to HLA class II antigens in TRALI because: (i) no titers of antibody concentration are employed, (ii) without sufficient modeling of HLA class II antibody-mediated TRALI it is not known what concentration is applicable and (iii) the role of the antibody has been thought to be PMN-dependent (27;42;109). In this vein, the current Leukocyte Antibody Prevalence Study (LAPS) which is a study done by the Retrovirus Epidemiology Donor Study-II (REDS II) group, has embarked upon a study to determine the prevalence of HLA class I and II antibodies in a substantial donor cohorts of non-transfused males, transfused males, and female donors with and without a history of pregnancy (110;111). Separate curves were generated for both antibodies to HLA class I and class II antigens, and the data was log transformed such that three standard deviations above the mean had an normalized background ratio (NBG) cut-off of 6.9 for the detection of HLA class II antibodies which resulted in 0.9% of transfused males, 0.6% of non-transfused males and 0.8% of never pregnant non-transfused females having HLA class II antibodies (111). Such criteria need to be implemented because prior work that implicated antibodies to HLA class II antigens used a 10% shift (46;112) or shifts as low as 8% (113). The presence of such small changes in flow shifts may be very important for successful organ transplantation but has not been demonstrated by other methods of antibody measurement to be relevant in models of TRALI.
In spite of the number of TRALI cases associated with antibodies to HLA class II, these antibodies have not been demonstrated to directly activate PMNs and instead may lead to the release of pro-inflammatory mediators which in turn activate PMNs (32;52). Given that the expression of HLA class II on PMNs is not normal but dependent on treatment with agents such as G-CSF, interferon-gamma (IFN-γ), GM-CSF and interleukin 3 (IL-3), is transient and donor dependent, investigation of HLA class II antibodies as the first rather than the second event in TRALI may provide further insight into their mechanism of action (50;114).
Identifying TRALI implicated antibodies by cognate recipient antigen or cross-match between the antibody and target cell
The second and probably more important aim of the laboratory investigation of antibody-mediated TRALI is to demonstrate that the detected antibody must recognize an antigen on the transfused host’s leukocytes, since a proportion of healthy blood donors will “naturally” have leukocyte antibodies. There is a high likelihood that many of them will not be involved with the TRALI reaction. Look-back studies have shown that even though the recipient may have the cognate antigen, this does not always lead to a reaction (76;102;115). In addition, such data provides evidence that may be used to confirm that a donor may not have been implicated in the TRALI event and permit them to continue to donate blood (29).
Matching the antibody detected with a cognate antigen
HLA typing is often performed using a molecular techniques with a variety of sequence-specific primers, sequence-specific oligonucleotide probes, and sequence-based typing assays (116). To determine the HNA type, both phenotyping and genotyping is applied. To determine a HNA phenotype of PMNs, well characterized human anti-sera with known specificities are required, as there are no commercial sources of such anti-sera (117). Freshly isolated PMNs are required and normally both the GIFT and GAT techniques are applied. HNA genotyping methods are only available for HNA-1a, 1b, 1c, 4a and 5a, (118–121) but not HNA-2a and 3a. While confirmation of the presence of a cognate antigen implies that the donor antibody has a target or substrate to react with, it does not confirm that the antibody contributed etiologically to the TRALI reaction, for in three look-back reports the minority of confirmed antigen:antibody pairs developed TRALI (76;80;115).
Cross-match between the antibody and target cell
Currently there is great variability in how TRALI cross-matches are performed. Techniques employed include the lymphocytotoxicity test (LCT) (27), GIFT alone or GIFT and GAT combination (29). The LCT is based on lymphocytes which are stable and storable making them investigator friendly, however lymphocytes are not the primary target cell in TRALI and do not express PMN-specific antigens (HNA-1, 2 and 3) and thus will not detect any incompatibilities with these antigens. Therefore, reports using the LCT cross-match alone need to be interpreted with these potential limitations in mind.
In the majority of cases, antibodies implicated in TRALI are donor-derived. Consequently, an appropriate cross-match strategy involves only the testing of the associated donor serum/plasma with the recipient’s cells. In the rare case where the antibody is present in the TRALI-affected transfusion recipient, the cross-match should, in addition, test recipient serum/plasma with donor cells, especially for TRALI linked to granulocyte transfusions (83). In a PMN cross-match, serum or plasma from associated donors should be incubated with the recipient’s PMNs using both the GIFT and GAT techniques (29;83;122). The PMN cross-match is very valuable because positive cross-matches or incompatibilities provide in-vitro evidence of a reaction between recipient PMNs and associated donor serum/plasma antibodies. This is especially useful in cases where antibody specificities cannot be defined, a common phenomenon in PMN serology, because of the very small number of well defined and characterized HNAs.
Progress and sophistication in TRALI antibody identification will need to be complemented by more research into the priming ability of various implicated blood components and TRALI patient samples if the TRALI mechanism is to be elucidated and the risk of this potentially serious transfusion complication reduced or eliminated.
Detecting PMN priming activity in blood components and patient’s samples
PMN priming activity can be investigated by measuring the respiratory burst activity. This assay involves the isolation of PMNs from healthy donors which are incubated (primed) with buffer or serum/plasma samples from patients pre-transfusion and/or patient post-transfusion, or the implicated blood product at 37°C. The PMNs are then activated with fMLP, and the maximal rate of O2 − produced can be measured as the superoxide dismutase inhibitable reduction of cytochrome c at 550 nm (54;123). Priming is defined as the enhancement of the fMLP-activated respiratory burst (64). Importantly, PMN isolation for these procedures need to be performed using pyrogen-free (sterile) solutions and equipment to avoid inadvertently priming or activating the PMNs. LPS levels of as low as 1–10 ng/mL can prime PMNs (124). This approach has demonstrated that stored blood components contain priming activity (55), that post-TRALI samples but not in pre-TRALI patient samples had priming enhancing ability (69) and that that antibodies to HNA-3a primed HNA-3a positive PMNs for an augmented respiratory burst leading to PMN mediated damage of HMVEC (61). An explanation for the occurrence of TRALI where no leukocyte antibody has been detected may be provided by investigating the priming ability of donor and patient samples from these cases. As HLA class II antibodies are less likely then HNA antibodies to activate PMNs, these antibodies should also be investigated for their priming ability.
For research purposes respiratory burst measurements have been used to differentiate phenotypes of circulating PMNs. Altered PMN phenotypes have been reported in torso trauma, major trauma, elective surgery, post coronary artery bypass and systemic inflammatory response syndrome patients (123;125–127). The significance of such periods of increased primeability and the increased number of primed PMNs is that activation of these PMN phenotypes would result in an augmented PMN response leading to multi-organ failure and tissue injury as hypothesized in TRALI (125;126).
CONCLUSIONS
All three mechanisms proposed for TRALI confirm the pivotal role of the PMN and identify three dimensions to the pathogenesis of TRALI namely:
predisposition factors in the recipient
insult or trigger factors in transfused blood products, and
cellular targets (i.e. PMN ) in the recipient
Many countries have begun using predominant male plasma to reduce the risk of immune mediated TRALI (103;128). Based on our current limited understanding of the mechanism for the pathogenesis of TRALI this approach is prudent. However, this approach has some deficiencies. Firstly, blood donors are a rare and valuable resource and limiting the use of female plasma could have significant negative repercussions for blood component supplies. Secondly, as this strategy continues to ignore the non-immune factors in the blood component and the recipient (patient) susceptibility factors that may contribute to TRALI, it will never effectively eliminate all TRALI events. Thirdly, if a positive serology is required as is the case with SHOT data, this makes the reporting of TRALI less likely. Such negative reporting bias will artificially skew reports of using male-only plasma in TRALI elimination without affecting the reporting of those cases which may not be due to the infusion of antibodies, giving falsely low reporting of TRALI events (129;130).
The TRALI problem can only be addressed by a concerted effort of blood providers, hospitals and researchers. There remains a need to increase awareness of TRALI. However, if we aim to minimize or avoid TRALI, systems must be in place to facilitate the thorough investigation of reported TRALI cases. Published literature clearly show that TRALI investigations today consist predominantly of HLA antibody investigations. While this is important it will not detect antibodies against a key effector cell of TRALI, namely the PMNs (131;132). This paper has provided some insights into the role of PMNs in TRALI and also ways of investigating the role of PMNs in TRALI pathogenesis. The ISBT Granulocyte Working Party is a good place to start identifying laboratories within each geographical region with such PMN immunobiology expertise.
Evidence that factors, including antibodies to MHC class I and HNA antigens, in blood components can prime or activate PMNs should be considered carefully and more research in this area is required. Research into this mechanism is important as it may identify alternative component manufacturing procedures to reduce or eliminate these PMN priming / activating factors. Such research has the potential to provide blood providers with alternative strategies for minimizing the risk of TRALI in blood component recipients.
Given that the laboratory investigations of PMNs and TRALI are complex and highly technical, it would be ineffective and unproductive to expect laboratories to acquire such skills quickly. Efficiencies may be gained by having designated specialized TRALI investigation and research laboratories, where each case would be investigated according to a comprehensive standardized protocol (91;92). Such protocols should include the screening of both the recipient and associated donors/donations for leukocyte antibodies and also PMN priming / activating abilities. The TRALI-implicated antibodies need to be better characterized and titering of antibodies to HLA class II antigens has to be completed as well as sufficient modeling of HLA class II-mediated TRALI. Such centralized services could have the extra benefit of holding a national sample archive of all TRALI patients and their associated donor and donation samples. This would facilitate future retrospective sample research studies nationally and internationally. The data generated should also feed into a database that would be useful to direct new strategies to minimize the risk of TRALI and could also be used for tracking intervention strategies to minimize TRALI risk e.g. the use of plasma from males only.
TRALI is a complex syndrome. We will only be able to control the risk of TRALI when we clearly understand its underlying mechanism. Achieving this aim will only be possible with an international collaborative approach.
ACKNOWLEDGEMENTS
The authors thank Dr. Robyn Minchinton for reviewing this manuscript.
This work was supported by the Australian Red Cross Blood Service, the Bonfils Blood Center, and grants GM49222 from NIGMS and HL59355 from NHLBI, NIH (CCS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Peters AM. Just how big is the pulmonary granulocyte pool? Clin Sci (Lond) 1998;94:7–19. doi: 10.1042/cs0940007. [DOI] [PubMed] [Google Scholar]
- 2.Ussov WY, Peters AM, Savill J, et al. Relationship between granulocyte activation, pulmonary granulocyte kinetics and alveolar permeability in extrapulmonary inflammatory disease. Clin Sci (Lond) 1996;91:329–335. doi: 10.1042/cs0910329. [DOI] [PubMed] [Google Scholar]
- 3.Chilvers ER, Cadwallader KA, Reed BJ, et al. The function and fate of neutrophils at the inflamed site: Prospects for therapeutic intervention. J R Coll Physicians Lond. 2000;34:68–74. [PMC free article] [PubMed] [Google Scholar]
- 4.Swain SD, Rohn TT, Quinn MT. Neutrophil priming in host defense: Role of oxidants as priming agents. Antioxid Redox Signal. 2002;4:69–83. doi: 10.1089/152308602753625870. [DOI] [PubMed] [Google Scholar]
- 5.Lien DC, Henson PM, Capen RL, et al. Neutrophil kinetics in the pulmonary microcirculation during acute inflammation. Lab Invest. 1991;65:145–159. [PubMed] [Google Scholar]
- 6.Doerschuk CM. Neutrophil rheology and transit through capillaries and sinusoids. Am J Respir Crit Care Med. 1999;159:1693–1695. doi: 10.1164/ajrccm.159.6.ed08-99. [DOI] [PubMed] [Google Scholar]
- 7.Lee WL, Downey GP. Neutrophil activation and acute lung injury. Curr Opin Crit Care. 2001;7:1–7. doi: 10.1097/00075198-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Doerschuk CM. Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation. 2001;8:71–88. [PubMed] [Google Scholar]
- 9.Doerschuk CM, Beyers N, Coxson HO, et al. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol. 1993;74:3040–3045. doi: 10.1152/jappl.1993.74.6.3040. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y, Doerschuk CM, Kamm RD. Computational modeling of RBC and neutrophil transit through the pulmonary capillaries. J Appl Physiol. 2001;90:545–564. doi: 10.1152/jappl.2001.90.2.545. [DOI] [PubMed] [Google Scholar]
- 11.Kubo H, Doyle NA, Graham L, et al. L- and P-selectin and CD11/CD18 in intracapillary neutrophil sequestration in rabbit lungs. Am J Respir Crit Care Med. 1999;159:267–274. doi: 10.1164/ajrccm.159.1.9709011. [DOI] [PubMed] [Google Scholar]
- 12.Doerschuk CM, Tasaka S, Wang Q. CD11/CD18-Dependent and -Independent Neutrophil Emigration in the Lungs. American Journal of Respiratory Cell and Molecular Biology. 2000;23:133–136. doi: 10.1165/ajrcmb.23.2.f193. [DOI] [PubMed] [Google Scholar]
- 13.Hallett MB, Lloyds D. Neutrophil priming: the cellular signals that say 'amber' but not 'green'. Immunol Today. 1995;16:264–268. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 14.Sheppard FR, Kelher MR, Moore EE, et al. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- 15.Wyman TH, Bjornsen AJ, Elzi DJ, et al. A two-insult in-vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release. Am J Physiol Cell Physiol. 2002;283(6):C1592–C1603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 16.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105(6):2266–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 17.Botha AJ, Moore FA, Moore EE, et al. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J Trauma. 1995;39:411–417. doi: 10.1097/00005373-199509000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Condliffe AM, Kitchen E, Chilvers ER. Neutrophil priming: Pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 1998;94:461–471. doi: 10.1042/cs0940461. [DOI] [PubMed] [Google Scholar]
- 20.Downey GP, Doherty DE, Schwab B, III, et al. Retention of leukocytes in capillaries: role of cell size and deformability. J Appl Physiol. 1990;69:1767–1778. doi: 10.1152/jappl.1990.69.5.1767. [DOI] [PubMed] [Google Scholar]
- 21.Biffl WL, Moore EE, Zallen G, et al. Neutrophils are primed for cytotoxicity and resist apoptosis in injured patients at risk for multiple organ failure. Surgery. 1999;126:198–202. [PubMed] [Google Scholar]
- 22.Murray J, Barbara JA, Dunkley SA, et al. Regulation of neutrophil apoptosis by tumor necrosis factor-alpha: Requirement for TNFR55 and TNFR75 for induction of apoptosis in-vitro. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- 23.Kitchen E, Rossi AG, Condliffe AM, et al. Demonstration of reversible priming of human neutrophils using platelet-activating factor. Blood. 1996;88:4330–4337. [PubMed] [Google Scholar]
- 24.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- 25.Lavigne LM, Albina JE, Reichner JS. Beta-glucan is a fungal determinant for adhesion-dependent human neutrophil functions. J Immunol. 2006;177:8667–8675. doi: 10.4049/jimmunol.177.12.8667. [DOI] [PubMed] [Google Scholar]
- 26.Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. American Reviews of Respiratory Disease. 1983;128:185–189. doi: 10.1164/arrd.1983.128.1.185. [DOI] [PubMed] [Google Scholar]
- 27.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 28.Bernard GR, Artigas A, Brigham KL, et al. Consensus Committee. Report of the American-European Consensus conference on acute respiratory distress syndrome: Definitions, mechanisms, relevant outcomes, and clinical trial coordinations. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 29.Fung YL, Goodison KA, Wong JK, Minchinton RM. Investigating transfusion-related acute lung injury (TRALI) Intern Med J. 2003;33:286–290. doi: 10.1046/j.1445-5994.2003.00352.x. [DOI] [PubMed] [Google Scholar]
- 30.Holness L, Knippen MA, Simmons L, Lachenbruch PA. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Middelburg RA, van Stein D, Briet E, van der Bom JG. The role of donor antibodies in the pathogenesis of transfusion-related acute lung injury: A systematic review. Transfusion. 2008;48:2167–2176. doi: 10.1111/j.1537-2995.2008.01810.x. [DOI] [PubMed] [Google Scholar]
- 32.Reil A, Keller-Stanislawski B, Gunay S, Bux J. Specificities of leucocyte alloantibodies in transfusion-related acute lung injury and results of leucocyte antibody screening of blood donors. Vox Sang. 2008;95:313–317. doi: 10.1111/j.1423-0410.2008.01092.x. [DOI] [PubMed] [Google Scholar]
- 33.McCullough J, Weiblen BJ, Clay ME, Forstrom L. Effect of leukocyte antibodies on the fate in-vivo of indium-111-labeled granulocytes. Blood. 1981;58:164–170. [PubMed] [Google Scholar]
- 34.Dutcher JP, Schiffer CA, Johnston GS, et al. Alloimmunization prevents the migration of transfused indium-111-labeled granulocytes to sites of infection. Blood. 1983;62:354–360. [PubMed] [Google Scholar]
- 35.McCullough J, Clay M, Hurd D, et al. Effect of leukocyte antibodies and HLA matching on the intravascular recovery, survival, and tissue localization of 111-indium granulocytes. Blood. 1986;67:522–528. [PubMed] [Google Scholar]
- 36.Dutcher JP, Riggs CJ, Fox JJ, et al. Effect of histocompatibility factors on pulmonary retention of indium-111-labeled granulocytes. Am J Hematol. 1990;33:238–243. doi: 10.1002/ajh.2830330405. [DOI] [PubMed] [Google Scholar]
- 37.Seeger W, Schneider U, Kreudler B, et al. Reproduction of transfusion-related acute lung injury in an ex-vivo lung model. Blood. 1990;76:1438–1444. [PubMed] [Google Scholar]
- 38.Sachs UJ, Hattar K, Weissmann N, et al. Antibody-induced neutrophil activation as a trigger for transfusion-related acute lung injury in an ex-vivo rat lung model. Blood. 2006;107:1217–1219. doi: 10.1182/blood-2005-04-1744. [DOI] [PubMed] [Google Scholar]
- 39.Brittingham TE. Immunologic studies on leukocytes. Vox Sanguinis. 1957;2:242–248. doi: 10.1111/j.1423-0410.1957.tb03699.x. [DOI] [PubMed] [Google Scholar]
- 40.Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion. 2004;44:1774–1789. doi: 10.1111/j.0041-1132.2004.04347.x. [DOI] [PubMed] [Google Scholar]
- 41.Dooren MC, Ouwehand WH, Verhoeven AJ, et al. Adult respiratory distress syndrome after experimental intravenous gamma-globulin concentrate and monocyte-reactive IgG antibodies. Lancet. 1998;352(9140):1601–1602. doi: 10.1016/s0140-6736(05)61049-5. [DOI] [PubMed] [Google Scholar]
- 42.Looney MR, Su X, Van Ziffle JA, et al. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. J Clin Invest. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dykes A, Smallwood D, Kotsimbos T, et al. Transfusion-related acute lung injury (TRALI) in a patient with a single lung transplant. Br J Haematol. 2000;109:674–676. doi: 10.1046/j.1365-2141.2000.01999.x. [DOI] [PubMed] [Google Scholar]
- 44.Popovsky MA. Transfusion-Related Acute Lung Injury (TRALI) In: Popovsky MA, editor. Transfusion Reactions. Bethesda: AABB Press; 2001. pp. 155–170. [Google Scholar]
- 45.Bux J. Transfusion-related acute lung injury (TRALI): a serious adverse event of blood transfusion. Vox Sang. 2005;89:1–10. doi: 10.1111/j.1423-0410.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 46.Kopko PM, Paglieroni TG, Popovsky MA, et al. TRALI: correlation of antigen-antibody and monocyte activation in donor-recipient pairs. Transfusion. 2003;43:177–184. doi: 10.1046/j.1537-2995.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 47.Win N, Brown C, Navarrete C. TRALI associated with HLA class II antibodies. Transfusion. 2003;43:545–546. doi: 10.1046/j.1537-2995.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 48.Lydaki E, Bolonaki E, Nikoloudi E, et al. HLA class II antibodies in transfusion-related acute lung injury (TRALI). A case report. Transfus Apher Sci. 2005;33:107–111. doi: 10.1016/j.transci.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Zarco MA, Ribera JM, Urbano-Ispizua A, et al. Phenotypic changes in neutrophil granulocytes of healthy donors after G-CSF administration. Haematologica. 1999;84:874–878. [PubMed] [Google Scholar]
- 50.Spagnoli GC, Juretic A, Rosso R, et al. Expression of HLA-DR in granulocytes of polytraumatized patients treated with recombinant human granulocyte macrophage-colony-stimulating factor. Hum Immunol. 1995;4:45–50. doi: 10.1016/0198-8859(94)00131-9. [DOI] [PubMed] [Google Scholar]
- 51.Nishimura M, Mitsunaga S, Ishikawa Y, et al. Possible mechanisms underlying development of transfusion-related acute lung injury: Roles of anti-major histocompatibility complex class II DR antibody. Transfus Med. 2003;13:141–148. doi: 10.1046/j.1365-3148.2003.00434.x. [DOI] [PubMed] [Google Scholar]
- 52.Sakagawa H, Miyazaki T, Fujihara M, et al. Generation of inflammatory cytokines and chemokines from peripheral blood mononuclear cells by HLA Class II antibody-containing plasma unit that was associated with severe nonhemolytic transfusion reactions. Transfusion. 2007;47:154–161. doi: 10.1111/j.1537-2995.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- 53.Wakamoto S, Fujihara M, Sakagawa H, et al. Endothelial permeability is increased by the supernatant of peripheral blood mononuclear cells stimulated with HLA Class II antibody. Transfusion. 2008;48:2060–2080. doi: 10.1111/j.1537-2995.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- 54.Silliman CC, Pitman J, Thurman G, Ambruso D. Neutrophil (PMN) priming agents develop in patients with transfusion related acute lung injury. Blood. 1992;80 Suppl. 1:261. abstr. [Google Scholar]
- 55.Silliman CC, Thurman G, Ambruso DR. Stored blood components contain agents that prime the neutrophil NADPH oxidase through the platelet-activating-factor receptor. Vox Sanguinis. 1992;63:133–136. doi: 10.1111/j.1423-0410.1992.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 56.Silliman CC. The two-event model of transfusion-related acute lung injury. Crit Care Med. 2006;34(5 Suppl):S124–S131. doi: 10.1097/01.CCM.0000214292.62276.8E. [DOI] [PubMed] [Google Scholar]
- 57.Silliman CC, Johnson CA, Clay KL, Thurman GW, Ambruso DR. Compounds biologically similar to platelet activating factor are present in stored blood components. Lipids. 1993;28(5):415–418. doi: 10.1007/BF02535939. [DOI] [PubMed] [Google Scholar]
- 58.Silliman CC. Transfusion-Related Acute Lung Injury. Transfusion Medicine Reviews. 1999;13:177–186. doi: 10.1016/s0887-7963(99)80031-5. [DOI] [PubMed] [Google Scholar]
- 59.Zallen G, Moore EE, Ciesla DJ, et al. Stored red blood cells selectively activate human neutrophils to release IL-8 and secretory PLA2. Shock. 2000;13(1):29–33. doi: 10.1097/00024382-200013010-00006. [DOI] [PubMed] [Google Scholar]
- 60.Biffl WL, Moore EE, Offner PJ, et al. Plasma from aged stored red blood cells delays neutrophil apoptosis and primes for cytotoxicity: abrogation by poststorage washing but not prestorage leukoreduction. J Trauma. 2001;50:426–431. doi: 10.1097/00005373-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Silliman CC, Curtis BR, Kopko PM, et al. Donor antibodies to HNA-3a implicated in TRALI reactions prime neutrophils and cause PMN-mediated damage to human pulmonary microvascular endothelial cells in a two-event in-vitro model. Blood. 2007;109:1752–1755. doi: 10.1182/blood-2006-05-025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kopko PM, Holland PV. Transfusion-related acute lung injury. British Journal of Haematology. 1999;105:322–329. doi: 10.1111/j.1365-2141.1999.01357.x. [DOI] [PubMed] [Google Scholar]
- 63.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: Definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 64.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 65.Silliman CC, Dickey WO, Paterson AJ, et al. Analysis of the priming activity of lipids generated during routine storage of platelet concentrates. Transfusion. 1996;36:133–139. doi: 10.1046/j.1537-2995.1996.36296181925.x. [DOI] [PubMed] [Google Scholar]
- 66.de Jong M, Ray M, Crawford S, et al. Platelet and leukocyte activation in salvaged blood and the effect of its reinfusion on the circulating blood. Clin Orthop Relat Res. 2007;456:238–242. doi: 10.1097/BLO.0b013e31802dc4ba. [DOI] [PubMed] [Google Scholar]
- 67.Khan SY, Kelher MR, Heal, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silliman CC, Bjornsen AJ, Tuder R, et al. Pre-storage leukoreduction of packed red blood cells inhibits tranfusion-related acute lung injury (TRALI) in an animal model. Blood. 2000;96:655a. (abstr) [Google Scholar]
- 69.Silliman CC, Paterson AJ, Dickey WO, et al. The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion. 1997;37:719–726. doi: 10.1046/j.1537-2995.1997.37797369448.x. [DOI] [PubMed] [Google Scholar]
- 70.Medeiros BC, Kogela KE, Kaneab MA. Transfusion-related acute lung injury (TRALI) following platelet transfusion in a patient receiving high-dose interleukin-2 for treatment of metastatic renal cell carcinoma. Transfus Apheresis Sci. 2003;29:25–27. doi: 10.1016/S1473-0502(03)00094-6. [DOI] [PubMed] [Google Scholar]
- 71.Silliman CC, Boshkov LK, Mehdizadehkashi Z, et al. Transfusion-related acute lung injury: epidemiology and a prospective analysis of etiologic factors. Blood. 2003;101:454–462. doi: 10.1182/blood-2002-03-0958. [DOI] [PubMed] [Google Scholar]
- 72.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imoto S, Araki N, Shimada E, et al. Comparison of acute non-haemolytic transfusion reactions in female and male patients receiving female or male blood components. Transfus Med. 2007;17:455–465. doi: 10.1111/j.1365-3148.2007.00802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kelher MR, Masuno T, Moore EE, et al. Plasma from Stored PRBCs and MHC Class I Antibodies Cause ALI in an Two-Event in-vivo Model. Blood. 2008;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bux J, Sachs UJ. The pathogenesis of transfusion-related acute lung injury (TRALI) Br J Haematol. 2007;136:788–799. doi: 10.1111/j.1365-2141.2007.06492.x. [DOI] [PubMed] [Google Scholar]
- 76.Kopko PM, Marshall CS, MacKenzie MR, Holland PV, Popovsky MA. Transfusion-related acute lung injury: Report of a clinical look-back investigation. JAMA. 2002;287:1968–1971. doi: 10.1001/jama.287.15.1968. [DOI] [PubMed] [Google Scholar]
- 77.Lalezari P, Bernard GE. Identification of a Specific Leukocyte Antigen: Another presumed example of 5b. Transfusion. 1965;45:135–142. doi: 10.1111/j.1537-2995.1965.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 78.Nordhagen R, Conradi M, Drömtorp SM. Pulmonary Reaction Associated with Transfusion of Plasma Containing Anti-5b. Vox Sanguinis. 1986;51:102–107. doi: 10.1111/j.1423-0410.1986.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 79.Davoren A, Curtis BR, Shulman IA, et al. TRALI due to granulocyte-agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma: A report of 2 fatalities. Transfusion. 2003;43(5):641–645. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 80.Muniz M, Sheldon S, Schuller RM, et al. Patient-specific transfusion-related acute lung injury. Vox Sang. 2008;94:70–73. doi: 10.1111/j.1423-0410.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 81.Wallis JP, Haynes S, Stark G, et al. Transfusion-related alloimmune neutropenia: An undescribed complication of blood transfusion. Lancet. 2002;360:1073–1074. doi: 10.1016/S0140-6736(02)11132-9. [DOI] [PubMed] [Google Scholar]
- 82.Leach M, Vora AJ, Jones DA, Lucas G. Transfusion-related acute lung injury (TRALI) following autologous stem cell transplant for relapsed acute myloid leukemia: A case report and review of the litereture. Transfusion Medicine. 1998;8:333–337. doi: 10.1046/j.1365-3148.1998.00165.x. [DOI] [PubMed] [Google Scholar]
- 83.Sachs UJ, Bux J. TRALI after the transfusion of cross-match-positive granulocytes. Transfusion. 2003;43:1683–1686. doi: 10.1111/j.0041-1132.2003.00568.x. [DOI] [PubMed] [Google Scholar]
- 84.Wallis JP, Lubenko A, Wells AW, et al. Single hospital experience of TRALI. Transfusion. 2003;43:1053–1059. doi: 10.1046/j.1537-2995.2003.00466.x. [DOI] [PubMed] [Google Scholar]
- 85.Daveron A, Curtis B, Shulman IA, et al. TRALI due to granulocyte agglutinating human neutrophil antigen-3a (5b) alloantibodies in donor plasma; A report of 2 fatalities. Transfusion. 2003;43:641–645. doi: 10.1046/j.1537-2995.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 86.Leger R, Palm S, Wulf H, et al. Transfusion-related lung injury with leukopenic reaction caused by fresh frozen plasma containing anti-NB1. Anesthesiology. 1999;91:1529–1532. doi: 10.1097/00000542-199911000-00048. [DOI] [PubMed] [Google Scholar]
- 87.Nishimura M, Takanashi M, Okazaki H, Satake M. Detection of anti-CD32 alloantibody in donor plasma implicated in development of transfusion-related acute lung injury. Cell Biochem Funct. 2007;25:179–183. doi: 10.1002/cbf.1298. [DOI] [PubMed] [Google Scholar]
- 88.Fadeyi EA, Los Angeles MM, Wayne AS, et al. The transfusion of neutrophil-specific antibodies causes leukopenia and a broad spectrum of pulmonary reactions. Transfusion. 2007;47:545–550. doi: 10.1111/j.1537-2995.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 89.Sachs UJ, Chavakis T, Fung L, et al. Human alloantibody anti-Mart interferes with Mac-1-dependent leukocyte adhesion. Blood. 2004;104:727–734. doi: 10.1182/blood-2003-11-3809. [DOI] [PubMed] [Google Scholar]
- 90.Bux J, Stroncek D. Human neutrophil antigens. Transfusion. 2002;42:1523. doi: 10.1046/j.1537-2995.2002.00265.x. (letter) [DOI] [PubMed] [Google Scholar]
- 91.Fung L. The recipient side of TRALI - the role of patient neutrophils. Vox Sang. 2007;2:135–140. ISBT Science Series. [Google Scholar]
- 92.Bierling P, Bux J, Curtis B, et al. Recommendations of the ISBT Working Party on Granulocyte Immunobiology for leukocyte antibody screening in the investigation and prevention of antibody-mediated TRALI. Vox Sang. 2008;96:266–269. doi: 10.1111/j.1423-0410.2008.01144.x. [DOI] [PubMed] [Google Scholar]
- 93.Bux J, Chapman J. Report on the Second International Granulocyte Serology Workshop. Transfusion. 1997;37:977–983. doi: 10.1046/j.1537-2995.1997.37997454028.x. [DOI] [PubMed] [Google Scholar]
- 94.Lucas G, Rogers S, De Haas M, et al. Report on the Fourth International Granulocyte Immunology Workshop: progress toward quality assessment. Transfusion. 2002;42:462–468. doi: 10.1046/j.1525-1438.2002.00053.x. [DOI] [PubMed] [Google Scholar]
- 95.McCulloch J, Clay M, Press C, Kline W. Granulocyte Serology - A Clinical and Laboratory Guide. Chicago: ASCP; 1988. Methods for detection of granulocyte antibodies; pp. 5–32. [Google Scholar]
- 96.Fung YL, Naidu A, Minchinton RM. Is it time to re-evaluate TRALI serology? Transfusion. 2005;45[s3]:114A. (absrt) [Google Scholar]
- 97.Lalezari P, Radel E. Neutrophil-specific antigens: Immunology and clinical significance. Seminars in Hematology. 1974;11:281–290. [PubMed] [Google Scholar]
- 98.Verheugt FW, dem Borne AE, Noord-Bokhorst JC, et al. Serological, immunochemical and immuoncytological properties of granulocyte antibodies. Vox Sang. 1978;35:394–303. doi: 10.1111/j.1423-0410.1978.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 99.Zeevi A, Girnita A, Duquesnoy R. HLA antibody analysis: Sensitivity, specificity, and clinical significance in solid organ transplantation. Immunol Res. 2006;36:255–264. doi: 10.1385/IR:36:1:255. [DOI] [PubMed] [Google Scholar]
- 100.Fuggle SV, Martin S. Tools for human leukocyte antigen antibody detection and their application to transplanting sensitized patients. Transplantation. 2008;86:384–390. doi: 10.1097/TP.0b013e31817c90f5. [DOI] [PubMed] [Google Scholar]
- 101.Cooling L. Transfusion-related acute lung injury. JAMA. 2002;288:315–316. doi: 10.1001/jama.288.3.315. [DOI] [PubMed] [Google Scholar]
- 102.Toy P, Hollis-Perry KM, Jun J, Nakagawa M. Recipients of blood from a donor with multiple HLA antibodies: A lookback study of transfusion-related acute lung injury. Transfusion. 2004;44:1683–1688. doi: 10.1111/j.0041-1132.2004.04193.x. [DOI] [PubMed] [Google Scholar]
- 103.Triulzi DJ, Kakaiya R, Schreiber G. Donor risk factors for white blood cell antibodies associated with transfusion-associated acute lung injury: REDS-II leukocyte antibody prevalence study (LAPS) Transfusion. 2007;47:563–564. doi: 10.1111/j.1537-2995.2007.01184.x. [DOI] [PubMed] [Google Scholar]
- 104.Fadeyi E, Adams S, Peterson B, et al. Analysis of a high-throughput HLA antibody screening assay for use with platelet donors. Transfusion. 2008;48:1174–1179. doi: 10.1111/j.1537-2995.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.O'Brien S, Lin Y, Bonacossa I, et al. HLA Class I and II antibodies in deferred blood donors at Canadian Blood Service. Transfusion. 2008;48[s2]:42A. (abstr) [Google Scholar]
- 106.Rhamy J, Yarnell V, Susskind B. Evaluation of HLA screening methods for TRALI in random donors. Transfusion. 2008;48[s2]:41A. (abstr) [Google Scholar]
- 107.Kao KJ, Scornik JC, Riley WJ, McQueen CF. Association between HLA phenotype and HLA concentration in plasma or platelets. Hum Immunol. 1988;21:115–124. doi: 10.1016/0198-8859(88)90086-9. [DOI] [PubMed] [Google Scholar]
- 108.Jensen S, Jersild C, Grunnet N. Severe transfusion reaction to donor plasma containing HLA antibody. Vox Sang. 1990;59:62. doi: 10.1111/j.1423-0410.1990.tb02119.x. [DOI] [PubMed] [Google Scholar]
- 109.Kelher MR, Masuno T, Moore EE, et al. Plasma from stored packed red blood cells and MHC class I antibodies cause acute lung injury in a two-event in-vivo rat model. Blood. 2009;113:2079–2083. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Triulzi DJ, Kleinman S, Busch MP, et al. Relationship of HLA antibodies in blood donors to pregnancy and transfusion history. Transfusion. 2007;47[Suppl]:2A. (abstr) [Google Scholar]
- 111.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: Implications for a transfusion related acute lung injury (TRALI) risk reduction strategy. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02206.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kopko PM, Popovsky MA, MacKenzie MR, et al. HLA class II antibodies in transfusion-related acute lung injury. Transfusion. 2001;41:1244–1248. doi: 10.1046/j.1537-2995.2001.41101244.x. [DOI] [PubMed] [Google Scholar]
- 113.Kao GS, Wood IG, Dorfman DM, et al. Investigations into the role of anti-HLA class II antibodies in TRALI. Transfusion. 2003;43:185–191. doi: 10.1046/j.1537-2995.2003.00285.x. [DOI] [PubMed] [Google Scholar]
- 114.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–1490. [PubMed] [Google Scholar]
- 115.Nicolle AL, Chapman CE, Carter V, Wallis JP. Transfusion-related acute lung injury caused by two donors with anti-human leucocyte antigen class II antibodies: A look-back investigation. Transfus Med. 2004;14:225–230. doi: 10.1111/j.0958-7578.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 116.Stroncek DF, Fadeyi E, Adams S. Leukocyte antigen and antibody detection assays: Tools for assessing and preventing pulmonary transfusion reactions. Transfus Med Rev. 2007;21:273–286. doi: 10.1016/j.tmrv.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McCullough J, Press C, Clay M, Kline W. Granulocyte serology – a clinical and laboratory guide. ASCP Press; 1988. Implementation of Granulocyte Serology Testing; pp. 145–154. [Google Scholar]
- 118.Wolter LS. An improved method for polymerase chain reaction typing of granulocyte antigens NA1 and NA2. Transfusion. 1995;35:885. doi: 10.1046/j.1537-2995.1995.351096026375.x. (letter) [DOI] [PubMed] [Google Scholar]
- 119.Bux J, Stein EL, Bierling P, Fromont P, et al. Characterization of a new alloantigen (SH) on the human neutrophil Fc gamma receptor IIIb. Blood. 1997;89:1027–1034. [PubMed] [Google Scholar]
- 120.Clague HD, Fung YL, Minchinton RM. HNA-4a gene frequencies in an Australian population, determined by a new PCR method using sequence specific primers. Transfusion Medicine. 2003;13:149–152. doi: 10.1046/j.1365-3148.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- 121.Sachs UJ, Reil A, Bauer C, et al. Genotyping of human neutrophil antigen-5a (Ond) Transfus Med. 2005;15:115–117. doi: 10.1111/j.0958-7578.2005.00560.x. [DOI] [PubMed] [Google Scholar]
- 122.Bux J, Becker F, Seeger W, et al. Transfusion-related acute lung injury due to HLA-A2-specific antibodies in recipient and NB1-specific antibodies in donor blood. Br J Haematol. 1996;93:707–713. doi: 10.1046/j.1365-2141.1996.d01-1703.x. [DOI] [PubMed] [Google Scholar]
- 123.Fung YL, Fraser JF, Wood P, et al. The systemic inflammatory response syndrome induces functional changes and relative hypo-responsiveness in neutrophils. Journal of Critical Care. 2008;23:542–549. doi: 10.1016/j.jcrc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 124.Bohmer RH, Trinkle LS, Staneck JL. Dose effects of LPS on neutrophils in a whole blood flow cytometric assay of phagocytosis and oxidative burst. Cytometry. 1992;13:525–531. doi: 10.1002/cyto.990130512. [DOI] [PubMed] [Google Scholar]
- 125.Botha AJ, Moore FA, Moore EE, et al. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery. 1995;118:358–364. doi: 10.1016/s0039-6060(05)80345-9. [DOI] [PubMed] [Google Scholar]
- 126.Partrick DA, Moore EE, Fullerton DA, et al. Cardiopulmonary bypass renders patients at risk for multiple organ failure via early neutrophil priming and late neutrophil disability. J Surg Res. 1999;86:42–49. doi: 10.1006/jsre.1999.5702. [DOI] [PubMed] [Google Scholar]
- 127.Fung YL, Silliman CC, Minchinton RM, et al. Cardiopulmonary bypass induces enduring alterations to host neutrophil physiology: A single-centre longitudinal observational study. Shock. 2008;30:642–648. doi: 10.1097/SHK.0b013e318173e717. [DOI] [PubMed] [Google Scholar]
- 128.Chapman CE, Williamson LM. National blood service TRALI reduction policies: Implementation and effect. Transfus Med Hemother. 2008;35:93–96. doi: 10.1159/000119116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2008;49:440–452. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 130.Hume HA. TRALI: moving toward prevention. Transfusion. 2009;49:402–405. doi: 10.1111/j.1537-2995.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- 131.Maslanka K, Michur H, Zupanska B, et al. Leucocyte antibodies in blood donors and a look back on recipients of their blood components. Vox Sang. 2007;92:247–249. doi: 10.1111/j.1423-0410.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 132.Sachs UJ, Link E, Hofmann C, et al. Screening of multiparous women to avoid transfusion-related acute lung injury: A single centre experience. Transfus Med. 2008;18:348–354. doi: 10.1111/j.1365-3148.2008.00889.x. [DOI] [PubMed] [Google Scholar]