Abstract
The pathological hallmark lesions in idiopathic pulmonary fibrosis are the fibroblastic foci, in which fibroblasts are thought to be involved in the tissue remodeling, matrix deposition, and cross-talk with alveolar epithelium. Recent evidence indicates that some fibroblasts in fibrosis may be derived from bone marrow progenitors as well as from epithelial cells through epithelial–mesenchymal transition. To evaluate whether endothelial cells could represent an additional source for fibroblasts, bleomycin-induced lung fibrosis was established in Tie2-Cre/CAG-CAT-LacZ double-transgenic mice, in which LacZ was stably expressed in pan-endothelial cells. Combined X-gal staining and immunocytochemical staining for type I collagen and α-smooth muscle actin revealed the presence of X-gal–positive cells in lung fibroblast cultures from bleomycin-treated mice. To explore the underlying mechanisms, by which loss of endothelial-specific markers and gain of mesenchymal phenotypes could be involved in microvascular endothelial cells, the effects of activated Ras and TGF-β on the microvascular endothelial cell line MS1 were analyzed. Combined treatment with activated Ras and TGF-β caused a significant loss of endothelial-specific markers, while inducing de novo mesenchymal phenotypes. The altered expression of these markers in MS1 cells with activated Ras persisted after withdrawal of TGF-β in vitro and in vivo. These findings are the first to show that lung capillary endothelial cells could give rise to significant numbers of fibroblasts through an endothelial–mesenchymal transition in bleomycin-induced lung fibrosis model.
Keywords: fibroblasts, myofibroblasts, endothelial cells, LacZ, fibrosis
CLINICAL RELEVANCE.
This study is the first to show that lung endothelial cells could give rise to significant numbers of fibroblasts in lung fibrosis model. The underlying mechanism might be suggested by the findings that combined treatment with activated Ras and TGF-β could cause endothelial–mesenchymal transition in endothelial cells.
Idiopathic pulmonary fibrosis (IPF) is a devastating disease without effective therapy (1). The hallmark lesions are the fibroblastic foci representing focal areas of active fibrogenesis featuring vigorous fibroblast replication and exuberant extracellular matrix deposition, which may lead to obliteration of the distal air space. Fibroblasts represent the key source of interstitial collagens, but fibroblasts isolated from IPF lungs have heterogenous phenotypes and properties different from that of normal lung fibroblasts (2). While it has been assumed that they arise only from intrapulmonary mesenchymal cells, one potential explanation for this heterogeneity is that fibroblasts may be derived from multiple cell origins under pathological conditions such as IPF. Recent mounting evidence suggests that bone marrow (BM)-derived fibroblasts may be recruited to various injured tissue sites and play an important role in the establishment of fibrosis at those sites (3, 4). Another potential explanation for their heterogeneity is the possible emergence during pulmonary fibrosis of alveolar epithelial cells (AECs)-derived fibroblasts through epithelial–mesenchymal transition (epithelial-MT) (5, 6).
Epithelial-MT, in which persistent loss of epithelial markers and de novo expression of mesenchymal markers are involved, is assumed to have a critical role in not only tissue development during embryogenesis but also pathological condition (7, 8). One central feature of epithelial-MT is the co-operation of TGF-β signaling with receptor tyrosine kinase (RTK) signaling, which activates Ras/ERK/MAPK pathway, resulting in the establishment of epithelial-MT, not “scattering”, in which the phenotype changes are fully reversible after removal of scattering-inducing factor, such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), or TGF-β alone (7).
Endothelial cells in the lung vasculature represent one of main cellular components of structural cells in the lung (9). In animal models of various lung diseases, they have been shown to function not only as a mere barrier between the blood compartment and the interstitial and air spaces, but also be involved in new vessel formation (10). Although a few in vitro studies reported the possibility of endothelial cells as a source of α-smooth muscle actin (α-SMA)–expressing mesenchymal cells or that of type I collagen (Col I)–producing cells (11, 12), it has not fully been determined whether endothelial cells could give rise to another population of fibroblasts under certain pathological conditions such as lung fibrosis.
These previous findings led us to investigate whether lung capillary endothelial cells could represent an additional source of fibroblasts, possibly through endothelial–mesenchymal transition (endothelial-MT), in an experimental lung fibrosis model, and if so, to elucidate the underlying mechanism, that is, whether combined signaling with activated Ras and TGF-β could induce endothelial-MT.
We generated Tie2-Cre/CAG-CAT-LacZ double-transgenic mice (CAG mice), in which de novo LacZ expression will stably label the Tie2 promoter-activated cells after Cre-mediated recombination. In vivo studies using the bleomycin (BLM)-induced lung fibrosis model with these mice successfully demonstrated that lung capillary endothelial cells could represent another potential source of fibroblasts/myofibroblasts, possibly through an endothelial-MT. The results of the study in vitro showed that combined treatment with activated Ras and TGF-β could induce endothelial-MT in microvascular endothelial cells.
MATERIALS AND METHODS
Mice and Mouse Fibrosis Model
To obtain CAG mice, Tie2-Cre Tg mice were bred with CAG-CAT-LacZ Tg mice (13). Pulmonary fibrosis was induced by endotracheal BLM injection as before (4). All animal studies were reviewed and approved by the University Committee on Use and Care of Animals at Nagoya Graduate School of Medicine.
Collection of Lung Samples and Lung Fibroblasts from BLM-Treated Mice
At Day 28 after BLM injection, both lungs from treated mice were lavaged with PBS and then frozen in OCT compound (Miles, Elkhart, IN). Mouse lung fibroblasts were also isolated from lung tissues at Day 28 as before (14).
X-Gal Staining
For detection of β-galactosidase activity, cultured cells and lung tissues were incubated in an X-gal solution at 37°C overnight. Nuclei were stained with Hoechst 33342 for cultured fibroblasts or hematoxylin for lung tissues, respectively.
Cell Treatments and Analysis of Phenotype Changes In Vitro
The mouse microvascular endothelial cell line MS1 and activated Ras transduced MS1 (SVR) were maintained as before (15). After TGF-β treatment at 10 ng/ml for 24 hours, MS1 and SVR were harvested for assessment of targeted genes by real-time PCR, or endothelial-specific markers expression by FACS. For immunocytochemical determination of α-SMA, cultured cells in an 8-well Lab-Tek (Nalge Nunc International, Naperville, IL) Chamber Slide were also treated with 10 ng/ml TGF-β for 24 hours. To distinguish endothelial-MT in vitro from “scattering,” these cells were cultured for another 24 hours after TGF-β removal, followed by phenotype analysis. To evaluate whether the cells can retain the phenotype changes, SVR were also re-plated into the new culture dish after TGF-β treatment and then analyzed as above.
In Vitro Analysis of Phenotype Alteration in Endothelial Cell Line by FACS
After treatment, the harvested cells were stained with appropriate dilutions of biotin-conjugated rat anti-mouse CD31 antibody, PE-conjugated rat anti-mouse CD34 antibody, biotin-conjugated rat anti-mouse Tie2 antibody, purified rat anti-mouse VE-cadherin antibody, or the appropriate isotype-matched controls, and then detected by subsequent staining with SAv-Cy-chrome for CD 31 and Tie2, and SAv-488-conjugated anti rat antibody for VE-cadherin, respectively. Dead cells were excluded from flow cytometry analysis by appropriate gating (4), and a total of 2 × 104 living cells were collected for each analysis on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). The results are presented as overlaid histograms and the relative mean fluorescence intensity (MFI). The relative MFI was calculated by dividing the MFI units of CD31, CD34, Tie2, or VE-cadherin staining by the MFI units of isotype control staining in each sample (16).
Endothelial Cell Line Transplantation In Vivo and Isolation of Activated Ras-Transduced Endothelial Cells Ex Vivo
A million MS1 or SVR cells were inoculated subcutaneously into the flank of nude mice (17). At Day 28 after inoculation, ex vivo SVR cells from the resulting nodule were isolated as before (14). To eliminate host-derived cells such as fibroblasts, cultured cells were selected with neomycin. Isolated ex vivo SVR cells were treated with 10 ng/ml TGF-β and then harvested for the analysis as above. To evaluate lung metastasis in treated mice, H&E staining was performed for the lungs resected at Day 28.
Immunofluorescent Staining
Immunofluorescent stainings were performed for the collected samples as before (18). The slides were incubated with appropriate dilutions of primary antibodies, and then visualized by fluorescent reagents.
PCR Analysis for Expression of Targeted Genes
Real-time PCR was performed using a TaqMan ABI 7300 Sequence Detection System (PE Applied Biosystems, Foster City, CA). Fibronectin, Col I, Snail, and Twist mRNA were detected, using mixture reagents from TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA). The mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA signal using TaqMan rodent GAPDH control reagents from Applied Biosystems (19). RT-PCR analysis for mRNA of TGFβR I (GenBank accession no. NM_009370) and II (GenBank accession no. NM_009371 for variant1 and NM_029575 for variant2), and GAPDH was performed with platinum Taq.
Statistical Analysis
The results were analyzed using the Mann-Whitney test for comparison between any two groups, and by nonparametric equivalents of ANOVA for multiple comparisons. P < 0.05 was considered to indicate statistical significance.
RESULTS
Generation of CAG Mice and Endothelial-MT in BLM-Induced Pulmonary Fibrosis
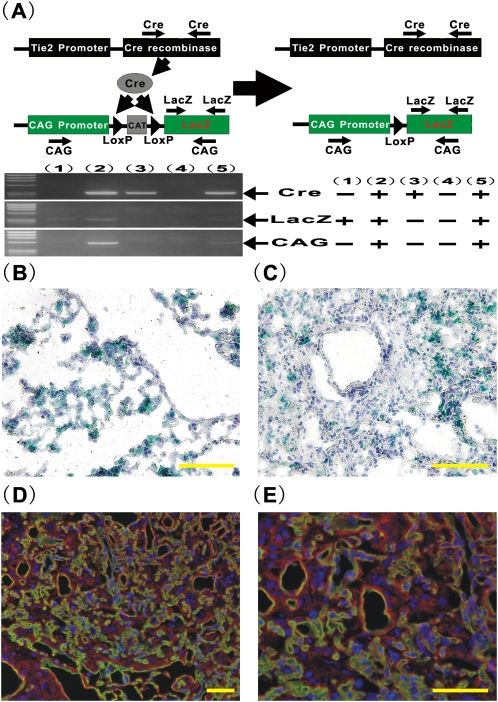
We investigated whether endogenous endothelial cells could represent a significant source of lung fibroblasts in an animal model of pulmonary fibrosis in vivo. To enable the tracking of fibroblasts derived from endothelial cell lineage in vivo, CAG mice were generated so that de novo irreversible LacZ expression by Tie2 promoter/enhancer–driven Cre-mediated recombination could be used as a marker for cells of endothelial cell origin (Figure 1A). As the primers used for amplification of CAG, designed on CAG promoter and the LacZ gene, can detect only the recombined allele of the CAG-CAT-LacZ target gene (13), the results of genotyping analysis revealed that the CAG mice carrying both Tie2-Cre gene and CAG-CAT-LacZ gene could express the de novo CAG promoter driven LacZ gene after Tie2 promoter/enhancer–driven Cre-mediated excision of the loxP-flanked chloramphenicol acetyltransferase (CAT) gene located between the CAG promoter and the LacZ gene, while littermates carrying either the Tie2-Cre or CAG-CAT-LacZ gene alone could not (Figure 1A). To evaluate the distribution of the cells with β-galactosidase activity in the lungs, X-gal staining was performed for the lung tissues from saline- or BLM-treated CAG mice at Day 28. Lungs from saline-treated CAG mice showed essentially normal lung architecture with expected blue staining of capillary endothelial cells (Figure 1B). Morphological evaluation for lungs from BLM-treated CAG mice revealed severe pulmonary fibrosis, characterized by loss of normal alveolar architecture, prominent disorganized thickening of the alveolar septa, and collapse of the alveolar space with organizing inflammatory infiltrate and fibroblasts (Figure 1C). These histological changes of lungs from BLM-treated CAG mice were comparable to those in BLM-treated wild type B6 mice (data not shown). In cellular fibrotic areas, large numbers of X-gal–positive cells were evident (Figure 1C), indicating that significant numbers of the cells in active fibrotic lesions were of endothelial origin. To further evaluate the distribution of these cells in fibrotic lungs of BLM-treated CAG mice, double immunofluorescent staining for CD31 (Figure 1D and 1E, green), a representative endothelial maker, and Col I (Figure 1D and 1E, red), a marker for fibroblasts, was performed (Figures 1D and 1E, and Figures E1E and E1F in the online supplement). Although the immunostaining for the lungs of saline-treated CAG mice exhibited that Col I expression was observed either in CD31-expressing endothelial cells or in accordance with alveolar architecture (Figures E1A–E1D), the findings for the lungs of BLM-treated CAG mice showed that Col I–expressing cells were observed in a significant number of the cells in fibrotic lesions, in which scattered CD31–co-expressing cells could be identified. These findings suggested that endothelial-MT could be mediating the emergence of these cells at an intermediate stage between complete acquisition of mesenchymal phenotype and loss of endothelial markers.
Figure 1.
Generation of Tie2-Cre/CAG-CAT-LacZ double-transgenic mice (CAG mice) and bleomycin (BLM)-induced lung fibrosis model. To obtain CAG mice, Tie2-Cre Tg mice were bred with CAG-CAT-LacZ Tg mice. (A) Top: Schematic description of gene constructs in CAG mice. Bottom: Genotyping analyses. Genotyping analyses were performed for off spring of CAG mice. The representative electropherogram of Cre (left top), LacZ (left middle), and CAG (left bottom) were shown for a set of five offsprings. The results were summarized (right panel). X-gal staining was performed for the lung tissues from (B) saline- or (C) BLM- treated CAG mice at Day 28 (×400 magnification). Double immunostaining for CD31 (green) and Col I (red) was performed for the lung tissues from BLM mice (D, ×200 magnification; E, ×400 magnification). Scale bars in B–D indicate 50 μm.
Characterization of Lung Fibroblasts Derived from BLM-Treated CAG Mice
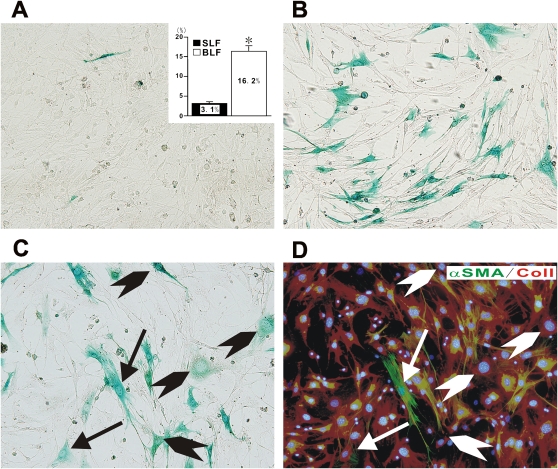
To directly prove the existence of endothelial-derived fibroblasts in BLM-induced lung fibrosis, lung fibroblasts from treated mice were evaluated for further studies. Lung fibroblasts from both saline- (SLF) and BLM-treated (BLF) mice were isolated, which in culture displayed the usual, primarily spindle-shaped fibroblast-like morphology. Upon X-gal staining, a few (3.1%) positive cells were identifiable in SLF cultures, while BLF cultures showed 16.2% to be positive (Figures 2A and 2B, respectively). To verify the fibroblastic nature or phenotype of the X-gal–positive fibroblast-like cells, BLFs were subjected to X-gal staining and sequentially followed by double immunocytochemical staining for Col I (Figure 2D, red) and α-SMA (Figure 2D, green). The results showed that the X-gal–positive BLFs consisted of two subpopulations: one that was both α-SMA– and Col I–positive (i.e., myofibroblastic), comprising 14.8% of X-gal–positive fibroblasts, and the other being α-SMA–negative but Col I–positive, comprising 85.2% of X-gal–positive fibroblasts (Figures 2C and 2D). It should be noted that X-gal–negative fibroblasts expressing α-SMA were also present, indicative of myofibroblasts not derived from endothelial cells. X-gal staining, followed by double immunostaining, for SLFs demonstrated that no or little α-SMA expression might be observed in X-gal–positive and –negative SLFs, although both SLFs exhibited Col I expression (Figures E2A and E2B). Both SLFs and BLFs were negative for CD31, indicating absence of contamination by endothelial cells in these cultured fibroblasts, or that all endothelial cell–derived fibroblasts had completely transitioned (data not shown). Thus significant numbers of fibroblasts from fibrotic lung were derived from endothelial cells, consistent with the occurrence of endothelial-MT in this model of pulmonary fibrosis.
Figure 2.
Characterization of BLM-induced lung fibroblasts derived from CAG mice. Saline-treated lung fibroblasts (SLF) or BLM-treated lung fibroblasts (BLF) derived from CAG mice were isolated and then stained with X-gal (SLF in A and BLF in B, respectively). The percentage of X-gal–positive cells among total counted cells, based on Hoechst 33342 nucleus staining, were shown in inset in A. Data shown in inset represent the means ± SEM from at least four samples (4 for SLF and 8 for BLF, respectively) in three independent experiments. Combined staining with X-gal and sequential immucytochemistry for Col I (red) and α-SMA (green) were performed for BLF derived from CAG mice (C, X-gal staining; D, immunocytochemistry for Col I and α-SMA, respectively). Arrows indicate X-gal (+)/Col I (+)/α-SMA (+) myofibroblasts. Arrowheads indicate X-gal (+)/Col I (+)/α-SMA (−) fibroblasts. A representative example of at least three independent experiments is shown. All images were photographed at ×200 magnification.
A recent study showed that tissue endothelial cells might be derived from endothelial progenitor cells (EPC) in bone marrow (BM) (20), thus suggesting that not all endothelial cell–derived fibroblasts necessarily arise from local lung capillary endothelial cells. To evaluate the contribution of Tie2-expressing BM cells (versus local lung capillary endothelial cells) to X-gal–positive BLFs, BM chimeras were prepared by injection of BM cells collected from CAG mice or B6 mice into lethally irradiated CAG or B6 recipient mice. After stable engraftment was established, BLM-induced lung fibrosis was induced in the chimera mice. BLFs from CAG mice with the BM cells from B6 mice (B6 to CAG mice) showed 15.3% to be X-gal–positive (% of X-gal–positive cells; 15.3 ± 0.92 in BLFs from B6 to CAG mice). On the other hands, BLFs from B6 mice with BM from CAG mice (CAG to B6 mice) showed only 0.8% of X-gal–positive cells (% of X-gal–positive cells; 0.82 ± 0.15 in BLFs from B6 to CAG mice). SLFs from B6 to CAG mice or CAG to B6 mice yielded no or little of X-gal–positive fibroblasts (data not shown). Together, these findings demonstrated that the contribution of EPC in BM to endothelial cell–derived BLFs was minor, and that the predominant source appeared to be endogenous lung capillary endothelial cells via endothelial-MT.
Altered Expression of Endothelial-Specific Markers on Endothelial Cells by Combined Treatment with Activated Ras and TGF-β
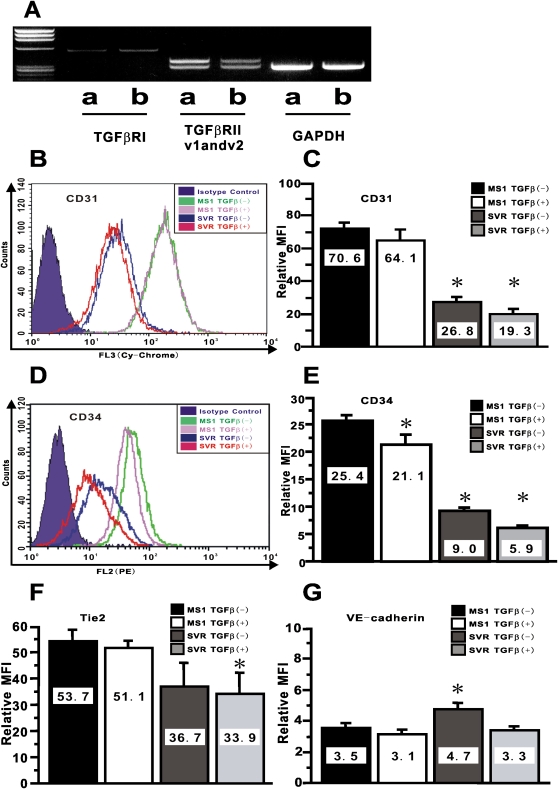
To illuminate the underlying mechanism by which microvascular endothelial cells can undergo endothelial-MT in vivo, microvascular endothelial MS1 cells and the cells transduced with activated Ras gene (SVR cells) were used, since a previous report indicates that the combination of activated Ras and TGF-β treatment induces repression of epithelial phenotype such as E-cadherin expression in epithelial cells (21). These cells also allow us to eliminate the possibility of contribution by contaminating fibroblasts to the observations from further experiments, since these were derived from a single cell (22). RT-PCR revealed that these endothelial cell lines expressed mRNAs for TGF-β receptor I and II (Figure 3A), thus suggesting that these cells would respond to TGF-β treatment. To evaluate the effect of these signals on endothelial cells, we examined the expression of endothelial-specific markers in response to combined treatment with activated Ras and TGF-β by FACS. Figures 3B and 3C showed that activated Ras signaling significantly repressed the expression of CD31, while TGF-β slightly inhibited expression, which was not statistically significant. However TGF-β caused significant repression of CD31 in the cells with activated Ras. On the other hand, Figures 3D and 3E showed that both activated Ras and TGF-β significantly repressed CD34 expression, although the effect of TGF-β was lower in magnitude relative to that of activated Ras alone. Nevertheless, combined treatment by these two agents significantly repressed the MFI for CD34 to 23% of that with vehicle treatment. Similar to the inhibitory pattern of CD31 expression, only the combination of activated Ras and TGF-β treatment caused significant repression of Tie2 expression (Figures 3F and E3A). TGF-β treatment of cells with activated Ras showed a significant repression of VE-cadherin expression, while activated Ras signaling slightly stimulated expression of VE-cadherin (Figures 3G and E3B). These data showed that although activated Ras alone caused significant repression of endothelial phenotype with the exception of VE-cadherin expression, combined treatment with TGF-β in vitro caused an additive steady repression of endothelial markers consistent with epithelial-MT (23).
Figure 3.
Altered expression of endothelial-specific markers by combined treatment with activated Ras and TGF-β. In A, the results of RT-PCR analysis for TGF-βRI, TGF-βRII variant1 (v1), variant2 (v2), and GAPDH are shown. Data shown are representative electropherograms of the indicated products using RNA samples from: MS1 (lane a) and SVR (lane b). These are representative of three independent experiments. CD31 in B and CD34 in D on endothelial MS1 and SVR treated with vehicle or TGF-β at 10 ng/ml for 24 hours were shown as overlaid histogram by FACS. The relative mean fluorescence intensity (MFI) for CD31 in C, for CD34 in E, for Tie2 in F, and for VE-cadherin in G were calculated as described in the online supplement. Data shown represent the means ± SEM from at least three independent experiments. Asterisks signify statistically significant difference (P < 0.05) in comparison with the relative MFI for each endothelial marker on MS1 with vehicle.
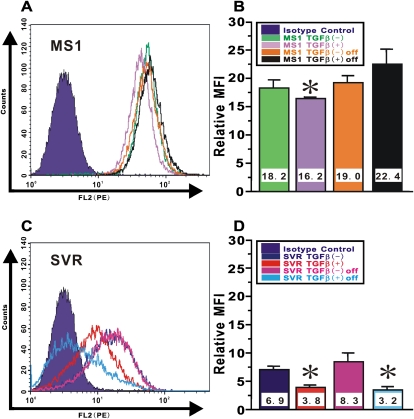
Persistence of Altered Endothelial Phenotype in Ras-Activated Endothelial Cells after Withdrawal of TGF-β In Vitro
Epithelial-MT can be distinguished from “scattering,” in which the phenotype changes are fully reversible after factor removal (21). In MS1 cells, the small but significant TGF-β–induced reduction in CD34 expression in the absence of activated Ras returned to normal levels upon TGF-β removal (Figures 4A and 4B). In contrast, the more robust TGF-β–induced suppression of CD34 expression in SVR cells with activated Ras, was persistent even up to 24 hours after TGF-β removal (Figures 4C and 4D). These findings would argue against the phenomena of scattering and instead would be consistent with endothelial-MT occurring in the cells exposed to activated Ras and TGF-β treatment.
Figure 4.
Persistence of altered endothelial phenotype in Ras-activated endothelial cells after withdrawal of TGF-β in vitro. Endothelial MS1 and SVR treated with vehicle or TGF-β at 10 ng/ml for 24 hours were cultured in complete medium after TGF-β removal for another 24 hours. CD34 expression on (A) MS1 cells and (C) SVR cells was shown as overlaid histogram by FACS. The relative MFI for CD34 in (B) MS1 cells and (D) SVR cells was calculated as described in the online supplement. Data shown represent the means ± SEM from at least three independent experiments. Asterisks signify statistically significant difference (P < 0.05) in comparison with the relative MFI for CD34 on MS1 or SVR with vehicle.
De Novo Expression of Mesenchymal Phenotypes in Endothelial Cells by Combined Treatment with Activated Ras and TGF-β
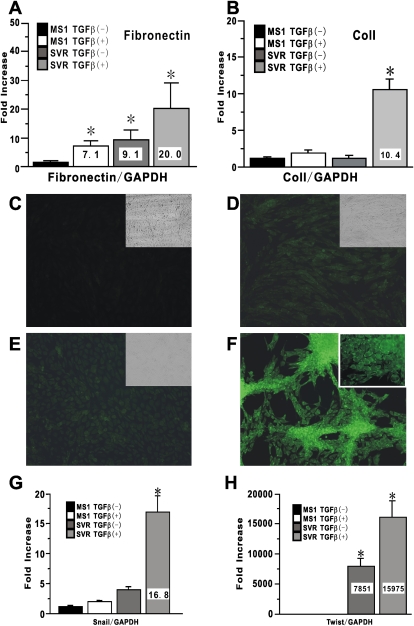
Next, we evaluated whether combined treatment with activated Ras and TGF-β could yield gain of mesenchymal markers in endothelial cells. Fibronectin mRNA expression in endothelial cells treated with either TGF-β or activated Ras alone was significantly increased relative to that in untreated cells by 7.1-fold and 9.1-fold, respectively (Figure 5A). Treatment with both TGF-β and activated Ras caused a more dramatic induction of up to 20-fold increase over that of untreated cells (Figure 5A). Although an induction of Col I expression was observed upon treatment with TGF-β, constitutive activated Ras alone failed to significantly induce Col I expression. Combined treatment with both TGF-β and activated Ras yielded a more dramatic induction of up to 10-fold increase over that of untreated cells (Figure 5B). Next, we evaluated α-SMA expression in treated endothelial cells as an indicator of myofibroblast transdifferentiation. As expected, untreated endothelial cells did not express α-SMA, and neither did cells treated with either TGF-β or activated Ras alone (Figures 5C–5E). In contrast, TGF-β treatment of cells with activated Ras induced significant morphological change and yielded cells exhibiting remarkable de novo α-SMA expression consistent with myofibroblast transdifferentiation (Figure 5F). Since the cells used in these experiments were derived from a single endothelial cell, a contribution to expression of these phenotypic markers from contaminating fibroblasts could be excluded. Interestingly, TGF-β treatment on the cells with activated Ras in vitro also caused significant induction of Snail and Twist, epithelial-MT–related transcription factors (Figures 5G and 5H).
Figure 5.
De novo induced expression of mesenchymal-specific markers in endothelial cells by combined treatment with activated Ras and TGF-β. Mesenchymal markers expression in MS1 and SVR treated with vehicle or TGF-β at 10 ng/ml for 24 hours were evaluated. Fibronectin mRNA in A and Col I mRNA in B were analyzed using real-time PCR. Data shown represent the means ± SEM from three independent experiments. Cultured cells in an 8-well Lab-Tek Chamber Slide were also treated with vehicle or 10 ng/ml TGF-β in medium without FCS for 24 hours for immunocytochemistry for α-SMA. (C–F) Treated cells were stained with FITC-conjugated mouse anti-α-SMA antibody. (C) MS1 with vehicle. (D) MS1 with TGF-β. (E) SVR with vehicle. (F) SVR with TGF-β. Magnification: ×200. The insets in C–E shows the light microscopic appearance of treated cells treated with each condition. Inset in F shows the cells stained with isotype-matched control IgG for α-SMA. A representative example of at least three independent experiments is shown. Snail mRNA in G and Twist mRNA in H were also analyzed using real-time PCR. Data shown represent the means ± SEM from three independent experiments. Asterisks signify statistically significant difference (P < 0.05) in comparison with the quantitative value of targeted mRNA in MS1 with vehicle.
Persistence of Endothelial-MT Phenotype in Ras-Activated Endothelial Cells after Withdrawal of TGF-β
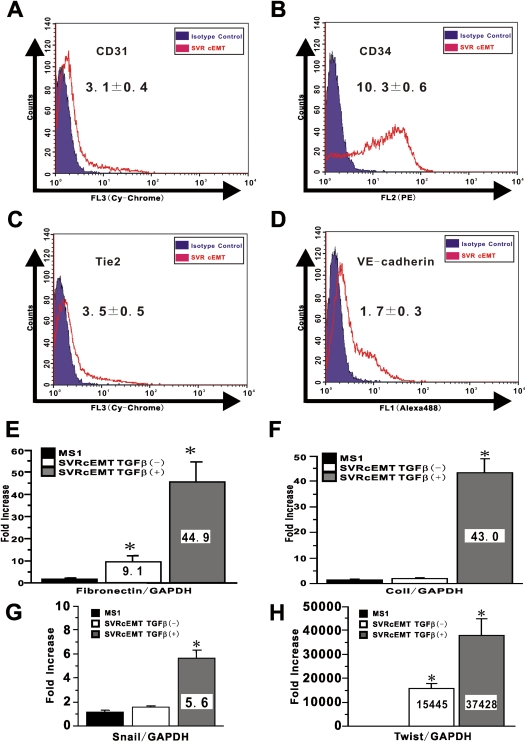
After treatment of SVR cells with TGF-β to induce endothelial-MT, they were detached and replated in new dishes for culture in the absence of TGF-β. After 24 hours without TGF-β, the cells appeared to maintain their morphology and organized in a monolayer with occasional compact circular aggregates. TGF-β treatment of cells with activated Ras did not induce apoptosis (data not shown). To confirm that these complete endothelial-MT (cEMT) cells could maintain this endothelial-MT phenotype even after withdrawal of TGF-β, they were assessed by FACS for expression of endothelial markers. Compared with the basal expression of endothelial cells markers (Figures 3B, 3D, E3A, and E3B), the results showed that culturing in the absence of TGF-β did not reverse the endothelial-MT–associated significant reduction in expression of the endothelial markers CD31, Tie2, and VE-cadherin, although CD34 expression on a few cEMT cells appeared to revert back to the basal level (Figures 6A–6D). However, re-treatment with TGF-β significantly and stably repressed CD34 expression on cEMT cells (MFI in cEMT cells; 10.3, MFI in cEMT cell with TGF-β; 6.3, respectively). Thus, once activated Ras-transduced endothelial cells were treated with TGF-β, loss of endothelial phenotype was persistent even after withdrawal of exogenous TGF-β stimulation.
Figure 6.
Persistence of endothelial-MT phenotype in Ras-activated endothelial cells after withdrawal of TGF-β. SVR treated with TGF-β were re-plated into the new culture dish and cultured with complete medium. The collected cells were called as cEMT cells. To evaluate whether cEMT cells can retain endothelial-MT phenotype or not, CD31 in A, CD34 in B, Tie-2 in C, and VE-cadherin expression in D on cEMT cells were shown as overlaid histogram by FACS. A representative example of at least three independent experiments is shown. Mesenchymal markers expressions in cEMT cells were also evaluated. Fibronectin mRNA in E, Col I mRNA in F, Snail mRNA in G, and Twist mRNA in H were analyzed using real-time PCR. Data shown represent the means ± SEM from three independent experiments. Asterisks signify statistically significant difference (P < 0.05) in comparison with the quantitative value of targeted mRNA in MS1.
In contrast analysis of mesenchymal cell markers revealed that the TGF-β induction of fibronectin and Col I expression in cEMT cells was reversible (Figures 6E and 6F). Thus, when cultured in TGF-β–free media, the fibronectin and Col I mRNA levels in cEMT cells reverted back to levels seen in SVR cells before TGF-β stimulation (Figures 5A and 5B). However, when these cEMT cells were re-stimulated with TGF-β, the mRNA levels of both fibronectin and Col I were markedly induced to levels (Figures 6E and 6F) that were more than 2-fold higher than the levels achieved by the initial treatment with TGF-β (Figures 5A and 5B). These data suggested that cEMT cells had stably acquired a potential to produce abundant extracellular matrix (ECM) constituents such as fibronectin and Col I in response to TGF-β stimulation, a property that would be consistent with a fibroblast phenotype. cEMT cells maintained high levels of Snail and Twist expression, which could be further stimulated by re-treatment with TGF-β (Figures 6G and 6H).
In Vivo Acquisition of Endothelial-MT Phenotype in Ras-Activated Endothelial Cells
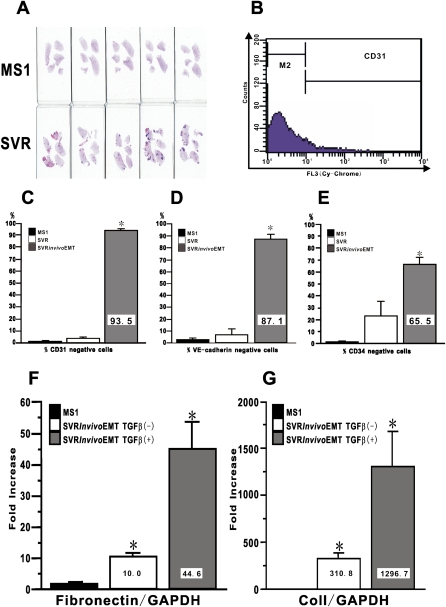
To evaluate whether endothelial cells could acquire endothelial-MT phenotype in vivo, MS1 or SVR cells were inoculated into the flank of nude mice with BALB/C background. At Day 28 after inoculation, primary tumors were established only in the group of SVR-treated nude mice, while only local hemangioma was detected in the group of MS1-treated nude mice even as long as 3 months after inoculation (data not shown). Gross morphological analysis of H&E-stained lung tissue sections showed that lung metastasis was observed only in SVR-treated nude mice (Figure 7A). The SVR cells from primary tumor tissue were isolated using selection with neomycin. In preliminary experiments, we confirmed that cultured naïve fibroblasts from skin and lung tissues were completely eliminated with the neomycin selection at the concentration of 400 μg/ml within the 14-day culture period. Immunocytochemistry confirmed that isolated cells were positive for H-2Kb, consistent with original derivation of the MS1 and SVR cells from C57BL/6 mice (data not shown). Evaluation of endothelial markers by FACS revealed that most of the isolated ex vivo SVR cells did not expresss CD31 (93.5%), VE-cadherin (87.1%), or CD34 (65.4%) (Figures 7B–7E). This is in contrast to the original MS1 and SVR cells, which were mostly positive for all three markers. Evaluation of mesenchymal phenotypes by real-time PCR revealed significant expression of both fibronectin and Col I by ex vivo SVR cells, which was significantly stimulated by more than 2-fold upon treatment with TGF-β (Figures 7F and 7G). Furthermore, ex vivo SVR cells also maintained high levels of Snail and Twist expression, which could be further stimulated by re-treatment with TGF-β (data not shown). These findings suggested that the implanted cells had undergone endothelial-MT in vivo that is consistent with the behavior of these cells in vitro.
Figure 7.
In vivo acquisition of endothelial-MT phenotype in Ras-activated endothelial cells. A quantity of 1 × 106 cells of MS1 or SVR were inoculated subcutaneously into the flank of nude mice. (A) The lungs from MS1-treated mice (upper row; n = 5) and SVR-treated mice (lower row; n = 5) were collected at Day 28 to evaluate lung metastasis. Collected primary tumors of SVR were digested and selected with neomycin. Selected ex vivo SVR cells, which we called in vivo EMT cells, were analyzed for the expression of endothelial-specific markers by FACS. (B) We defined the population of cells in M2 gate as marker-negative cells. The percentage of marker-negative cells for (C) CD31, (D) VE-cadherin, and (E) CD34 are shown. Data shown represent the means ± SEM from three independent experiments. Fibronectin mRNA in F and Col I mRNA in G were analyzed using real-time PCR. Data shown represent the means ± SEM from three independent experiments. Asterisks signify statistically significant difference (P < 0.05) in comparison with the quantitative value of targeted endothelial markers and mRNA in MS1.
DISCUSSION
Classification of idiopathic interstitial pneumonias (IIPs) based on histopathological patterns has had a significant impact on the prediction of clinical course of patients with the various IIPs, when supported by other parameters such as demographic, physiologic, and radiologic assessment (24). Nevertheless, the outcome of treatment for IIPs, especially for IPF, remains unsatisfactory. An explanation for poor response to anti-inflammatory therapies for IPF is that extensive accumulation and/or proliferation of heterogeneous fibroblast populations may be a more important pathogenetic factor than the much lower level of inflammation seen in affected lung tissue (25). We have previously described the presence of BM-derived fibroblast-like cells, but not myofibroblasts, in injured lungs undergoing pulmonary fibrosis (4). In this present study, the possibility that lung capillary endothelial cells could serve as another source for fibroblasts in BLM-induced pulmonary fibrosis was examined. While there is no completely satisfactory animal model of human IPF, the BLM-induced model is relatively well-characterized and does exhibit certain features found in the human disease (26). Elucidation of the cellular origin of fibroblast in the context of remodeling/fibrosis on the one hand, and the biology of the various fibroblast subpopulations on the other, has the potential to revolutionize the treatment of patients with lung disorders such as IPF, emphysema, and other fibrotic lung diseases (27). It is assumed that combined stimulations for epithelial-MT might induce the persistent repression of the promoter activity of epithelial markers, rather than its activation, resulting in persistent loss of epithelial markers (7, 8). Together with these reviews (7, 8), our present findings in vitro indicate that these stimulations for endothelial-MT might yield the repression of the promoter activity of endothelial markers and sequentially the vanishing endothelial markers expression in fibrosis model in vivo. Therefore, to confirm that endogenous endothelial cells in vivo could significantly contribute to the fibroblast population in lung fibrosis, we generated CAG mice, in which the activation of Cre recombinase under the control of the Tie2 promoter/enhancer induced the deletion of the CAT gene and consequently de novo irreversible LacZ gene expression in pan-endothelial cells, independent of the status of Tie2 promoter activity (28). X-gal staining of lungs from these double transgenic mice not only exhibited the expected distribution of Cre-mediated de novo LacZ expression in lung capillary endothelial cells under physiologic conditions, but also enable us to trace LacZ expressing endothelial-derived fibroblasts in the lungs of these mice under normal and/or pathological conditions. In control saline-treated mice, the distribution of LacZ expression in pulmonary capillary endothelial cells was clearly evident, similar to that seen in other studies of lung endothelial cells using Tie2-LacZ transgenic mice (29) as well as the distribution of Tie2 receptor in a study of idiopathic pulmonary hypertension (30). Thus our data indicated that Cre-mediated recombination in the lung occurred only in the intended cell type, namely the endothelial cell, with no detectable activity in other cell types, as other studies demonstrated using Tie2-Cre/ROSA26R mice (28, 31, 32). In contrast to the control lungs, those from BLM-injured CAG mice exhibited distorted morphology with extensive remodeling and vastly increased numbers of both X-gal–positive and –negative cells, virtually all densely clustered in areas undergoing active fibrosis. The extravascular and interstitial localization of some of these cells, and their morphology were consistent with interstitial mesenchymal cells, such as fibroblasts. As recent study with a surfactant protein C–driven Cre/loxP reporter system suggested that there were some epithelial cells with mesenchymal nature as early epithelial-MT stage in lung fibrosis (6), our double immunostaining for CD31 and Col I showed that fibrotic lesions involved substantial numbers of fibroblast-like cells positive for Col I, some of which expressed CD31, indicating to be under intermediate state of endothelial-MT. Since it was difficult, if not impossible, in lung tissue sections to distinguish X-gal–positive endothelial-derived fibroblasts from X-gal–positive endothelial cells amid the distorted architecture of the remodeling lung tissue with collapse of the alveolar space, lung fibroblasts were isolated for further studies. Cultured fibroblasts from the lungs of BLM-treated CAG mice revealed mostly typical spindle-shaped fibroblast morphology similar to previously cultured primary murine lung fibroblasts (14). X-gal staining of fibroblast cultures from BLM-treated mice revealed that 16.2% of cells were LacZ-positive, which also expressed Col I and have typical fibroblast morphology. Of this LacZ-positive population, less than 15% of them expressed α-SMA, indicating that only a small minority of endothelial cell–derived cells was able to differentiate to myofibroblasts. A much smaller (3.1%) proportion were LacZ-positive in cells isolated from saline-treated animals, probably due to basal contribution of endothelial-derived fibroblasts under physiological condition. The finding that both SLFs and BLFs were negative for CD31 in our fibroblasts culture system might be supported by the previous study (33) and also explained by the manufacturer's recommendations that many kinds of growth factors are usually needed to keep primary cultured endothelial cells in vitro (34). Recent lineage tracking analysis in combination with a Tie2-driven Cre/loxP reporter system suggested that only 1–5% of the endothelium in liver may be derived from BM-derived EPC (35). Furthermore, Zeisberg and coworkers also evaluated a lesser contribution by BM-derived cells in endothelial-derived fibroblasts in cardiac fibrosis using the Tie1-driven Cre/loxP reporter system (36). Our finding that BM-derived cells did not substantially contribute to the X-gal–positive BLFs in our lung fibrosis model was compatible with these previous studies (35, 36). These novel findings for the first time directly demonstrated that lung endothelial cells in BLM-induced fibrotic lungs could give rise to significant numbers of fibroblasts, while possible existence of nonendothelial resident lung Tie-2–positive progenitors could not be completely excluded.
To illuminate the underlying mechanism, by which “micro”-vascular endothelial cells can undergo endothelial-MT in vivo, we used the “micro”-vascular endothelial cell line MS1 cells and SVR cells, derived as a single cell clone using different drug selections, respectively (22). Although there are clear limitations to these “micro”-vascular endothelial cell lines in terms of tissue derivation from pancreatic islets, they remain useful for tracking endothelial-MT in vitro, since it allows us to eliminate the possibility of contribution by contaminating fibroblasts to the observations from experiments using these cells derived from a single clone. A recent review by Thiery and Sleeman suggested that the exact mechanisms remain unclear, although complex network of multiple signaling pathways organizes epithelial-MT process in epithelial lineages (8). In this study we showed that the combination of activated Ras and TGF-β treatment in vitro induced stable repression of endothelial markers reminiscent of endothelial-MT. Although the inability of TGF-β treatment alone to repress CD31 expression is also noted in aortic valve endothelial cells in vitro (37), a study using a hepatocyte cell line suggests that the expression of the junctional proteins remained unaffected or little changed during the first 24 hours, but progressively decreased after prolonged TGF-β treatment (38). Our finding that endothelial phenotypic changes in MS1 cells without an activated Ras gene were resistant to repressive effect of TGF-β treatment alone on endothelial markers (aside from CD34 expression), was compatible with these previous studies (37, 38). In our latter experiment, loss of endothelial phenotypes such as CD31, CD34, VE-Cadherin, and Tie2 was observed in endothelial cells after the completion of endothelial-MT (referred to as cEMT). These findings suggested that more rapid repression of CD34 expression than that of CD31 expression might depend on the differential response to combined signaling with activated Ras and TGF-β.
Growth factors such as FGF, HGF, or TGF-β alone are known to have scattering factor activity, which is reversible (i.e., upon their removal the growth factor-induced phenotype changes are fully reversible) (21). Our findings in endothelial cells are consistent with this report in that TGF-β treatment alone induced only the reversible “scattering” phenotype without any morphological change. In contrast, TGF-β treatment in combination with activated Ras gave rise to persistent repression of endothelial markers even after TGF-β removal.
Another essential characteristic of epithelial-MT is gain or de novo expression of mesenchymal markers (39). Fibronectin and Col I are the most critical ECM constituents in lung fibrosis. Fibronectin expression was increased 20-fold in SVR cells treated with TGF-β, compared with untreated endothelial (MS1) cells in our study. Although untreated MS1 cells exhibited no or little expression of Col I mRNA, the de novo expression of Col I was markedly induced in TGF-β–treated endothelial cells with activated Ras, up to 10-fold increase over that of untreated cells. A marker of myofibroblast differentiation that is often used is α-SMA expression (40). Our previous report showed the unexpected finding that BM-derived fibroblasts do not give rise to α-SMA–expressing myofibroblasts, even after in vitro TGF-β stimulation, indicating that the BM is not a significant source of progenitor cells for myofibroblasts in injured lung undergoing fibrosis (4). Previous reports indicate that de novo α-SMA expression could be induced in “macro”-vascular endothelial cells by prolonged (from 6–28 d) treatment with TGF-β alone (37, 41), but interestingly, not in “micro”-vascular endothelial cells (41). In our study, only the combination of activated Ras and TGF-β treatment could induce de novo α-SMA expression in “micro”-vascular endothelial cells, while neither activated Ras nor TGF-β alone was able to induce α-SMA expression. Triggering epithelial-MT is known to result in the activation of transcriptional regulators such as Snail and Twist, which regulate the changes in gene expression patterns that underlie epithelial-MT (8). In our study, substantial induction of Snail and Twist in endothelial cells treated with activated Ras and TGF-β seemed to be compatible with these findings, consistent with their importance during endothelial-MT as well.
Loss of endothelial phenotype after the completion of endothelial-MT (we called cEMT) appeared stable and irreversible since cessation of exogenous TGF-β treatment did not cause reversion to the endothelial phenotype. In addition, upon re-treatment with TGF-β, cEMT cells exhibited the potential for significantly higher levels of ECM expression compared with that in cells responding to initial treatment with TGF-β. These findings were supported with significantly increasing Snail and Twist expression in cEMT cells, compared with those observed in endothelial cells.
As previously reported (22), SVR with activated Ras, but not MS1 cells, caused tumors in the flank of nude mice. All nude mice with primary tumors of SVR endothelial cells also exhibited gross lung metastasis. There is accumulating evidence to indicate that epithelial-MT may be involved in tumor metastasis (8). In our study, neomycin-selected ex vivo SVR cells exhibited evidence of undergoing endothelial-MT in vivo, probably due to stimulation by endogenous TGF-β derived from tumor-associated cells or fibroblasts (42), and subsequently caused or promoted the distant metastasis observed in the lung. Although several studies suggest the possibility of myofibroblast-like cells being derived from primary “macro”-vascular endothelial cells (12, 43), our comprehensive evaluation for altered phenotypes in the endothelial cells derived from a single cell is the first to directly show that “micro”-vascular endothelial cells could undergo endothelial-MT in vitro by combination of activated Ras and TGF-β treatment. These findings using microvascular endothelial cell line in vitro allow us to speculate that the underlying mechanism by which capillary endothelial cell in BLM-treated lungs could yield another population of fibroblasts might be an endothelial-MT process, most likely under the influence of both TGF-β and growth factors capable of activating the Ras/MAPK pathway, which are known to be elaborated by both recruited and resident cells in fibrotic lesions (44).
Our previous study using GFP BM chimera mice demonstrated that α-SMA–expressing myofibroblasts appeared not to be of BM origin, and more likely originating from peribronchial and perivascular adventitial fibroblasts or other intrapulmonary precursor cells (4, 45). Our present finding suggested the endothelial cell as one of the candidate intrapulmonary precursor cell for the myofibroblast in lung fibrosis. The histopathology of IPF indicates increased capillary density around fibroblastic foci with evidence of vascular regression in the center of these foci (46). One interpretation for this vascular heterogeneity may be due to an inability to form new vessels in areas of established fibrosis and inhibition of angiogenesis (46, 47). Our evidence of transition of endothelial cells into fibroblasts through endothelial-MT in BLM-induced lung fibrosis provides an alternative explanation by suggesting that the basis for the vascular regression may be the loss of endothelial cells via endothelial-MT to contribute to the fibroblastic elements in these foci. Pulmonary vascular remodeling in IPF-associated pulmonary hypertension (PH), as one of the most critical complications, represents not only pathological and biological endothelial loss but also the neointima formation with α-SMA–expressing mesenchymal cells and ECM beneath a dysfunctional endothelial layer, both of which are assumed to be common features in idiopathic pulmonary hypertension and atherosclerosis (48, 49). Futhermore, the question of their potential myofibroblastic nature has been suggested from comprehensive analysis for α-SMA–expressing mesenchymal cells in atherosclerosis, although smooth muscle cells (SMCs) originating from media have been assumed to contribute to α-SMA–expressing mesenchymal cells in neointima (40, 48). Sumioka and coworkers as well as Zeisberg and colleagues suggested the possibility of endothelial-derived fibroblasts in cardiac, kidney, and eye fibrosis (36, 50, 51) and carcinoma as well (52). Further investigation into endothelial-derived fibroblasts/myofibroblasts in the pathogenesis of not only pulmonary vascular remodeling in IPF-associated PH, but also neointima formation in atherosclerosis, is warranted.
In summary, we confirmed that endogenous lung endothelial cells in intact animals could also give rise to significant numbers of fibroblasts in a murine model of bleomycin-induced lung injury and fibrosis. The underlying mechanism was suggested by in vitro studies showing that endothelial-MT could occur in endothelial cell lines when treated with TGF-β in combination with activation of Ras signaling. Furthermore, this same cell line could undergo a similar transition to fibroblasts in vivo when implanted in nude mice.
Supplementary Material
This work was supported by Nagao Memorial Fund, Grant-in-Aid for Young Scientists (B) (17790530), Grant-in-Aid for Scientific Research (C) (19590890), and the 21st Century COE Program “Integrated Molecular Medicine for Neuronal and Neoplastic Disorders” of the Ministry of Education, Culture, Sports, Science and Technology. Support from National Institutes of Health grants HL28737 and HL52285 (to S.H.P.) is also acknowledged.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0031OC on September 18, 2009
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517–525. [DOI] [PubMed] [Google Scholar]
- 2.Thannickal VJ, Toews GB, White ES, Lynch JP III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004;55:395–417. [DOI] [PubMed] [Google Scholar]
- 3.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 2002;110:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 2006;103:13180–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol 2003;4:657–665. [DOI] [PubMed] [Google Scholar]
- 8.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006;7:131–142. [DOI] [PubMed] [Google Scholar]
- 9.Franks TJ, Colby TV, Travis WD, Tuder RM, Reynolds HY, Brody AR, Cardoso WV, Crystal RG, Drake CJ, Engelhardt J, et al. Resident cellular components of the human lung: current knowledge and goals for research on cell phenotyping and function. Proc Am Thorac Soc 2008;5:763–766. [DOI] [PubMed] [Google Scholar]
- 10.Dutly AE, Andrade CF, Verkaik R, Kugathasan L, Trogadis J, Liu M, Waddell TK, Stewart DJ, Keshavjee S. A novel model for post-transplant obliterative airway disease reveals angiogenesis from the pulmonary circulation. Am J Transplant 2005;5:248–254. [DOI] [PubMed] [Google Scholar]
- 11.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res 2002;90:1189–1196. [DOI] [PubMed] [Google Scholar]
- 12.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2007;293:L1–L8. [DOI] [PubMed] [Google Scholar]
- 13.Sakai K, Miyazaki J. A transgenic mouse line that retains Cre recombinase activity in mature oocytes irrespective of the cre transgene transmission. Biochem Biophys Res Commun 1997;237:318–324. [DOI] [PubMed] [Google Scholar]
- 14.Huaux F, Liu T, McGarry B, Ullenbruch M, Phan SH. Dual roles of IL-4 in lung injury and fibrosis. J Immunol 2003;170:2083–2092. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto N, Kawabe T, Hara T, Imaizumi K, Wakayama H, Saito H, Shimokata K, Hasegawa Y. Effect of erythromycin on matrix metalloproteinase-9 and cell migration. J Lab Clin Med 2001;137:176–183. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Q, Austen KF, Friend DS, Heidtman M, Boyce JA. Human peripheral blood eosinophils express a functional c-kit receptor for stem cell factor that stimulates very late antigen 4 (VLA-4)-mediated cell adhesion to fibronectin and vascular cell adhesion molecule 1 (VCAM-1). J Exp Med 1997;186:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi M, Imaizumi K, Kawabe T, Wakayama H, Horio Y, Sekido Y, Hara T, Hashimoto N, Takahashi M, Shimokata K, et al. Induction of antitumor immunity by transduction of CD40 ligand gene and interferon-gamma gene into lung cancer. Cancer Gene Ther 2001;8:421–429. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto N, Kawabe T, Imaizumi K, Hara T, Okamoto M, KojimaK, Shimokata K, Hasegawa Y. CD40 plays a crucial role in lipopolysaccharide-induced acute lung injury. Am J Respir Cell Mol Biol 2004;30:808–815. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 20.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997;275:964–967. [DOI] [PubMed] [Google Scholar]
- 21.Janda E, Lehmann K, Killisch I, Jechlinger M, Herzig M, Downward J, Beug H, Grunert S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 2002;156:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA 1997;94:861–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotzmann J, Huber H, Thallinger C, Wolschek M, Jansen B, Schulte-Hermann R, Beug H, Mikulits W. Hepatocytes convert to a fibroblastoid phenotype through the cooperation of TGF-beta1 and Ha-Ras: steps towards invasiveness. J Cell Sci 2002;115:1189–1202. [DOI] [PubMed] [Google Scholar]
- 24.Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, Lama V, Kazerooni EA, Gross BH, Toews GB, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute-walk test. Am J Respir Crit Care Med 2006;174:803–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheppard D. Pulmonary fibrosis: a cellular overreaction or a failure of communication? J Clin Invest 2001;107:1501–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu T, Jin H, Ullenbruch M, Hu B, Hashimoto N, Moore B, McKenzie A, Lukacs NW, Phan SH. Regulation of found in inflammatory zone 1 expression in bleomycin-induced lung fibrosis: role of IL-4/IL-13 and mediation via STAT-6. J Immunol 2004;173:3425–3431. [DOI] [PubMed] [Google Scholar]
- 27.Gharaee-Kermani M, Gyetko MR, Hu B, Phan SH. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: a potential role for stem cells in the lung parenchyma and implications for therapy. Pharm Res 2007;24:819–841. [DOI] [PubMed] [Google Scholar]
- 28.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol 2001;230:230–242. [DOI] [PubMed] [Google Scholar]
- 29.Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, Sato TN. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc Natl Acad Sci USA 1997;94:3058–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dewachter L, Adnot S, Fadel E, Humbert M, Maitre B, Barlier-Mur AM, Simonneau G, Hamon M, Naeije R, Eddahibi S. Angiopoietin/Tie2 pathway influences smooth muscle hyperplasia in idiopathic pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1025–1033. [DOI] [PubMed] [Google Scholar]
- 31.Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: impaired lymphocyte migration to bone marrow. J Exp Med 2001;193:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limbourg FP, Takeshita K, Radtke F, Bronson RT, Chin MT, Liao JK. Essential role of endothelial Notch1 in angiogenesis. Circulation 2005;111:1826–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiting CV, Tarlton JF, Bailey M, Morgan CL, Bland PW. Abnormal mucosal extracellular matrix deposition is associated with increased TGF-beta receptor-expressing mesenchymal cells in a mouse model of colitis. J Histochem Cytochem 2003;51:1177–1189. [DOI] [PubMed] [Google Scholar]
- 34.Takagi Y, Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Hashimoto I, Hayashi Y, Kawabe T, Shimokata K, et al. Erythromycin-induced CXCR4 expression on microvascular endothelial cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L420–L431. [DOI] [PubMed] [Google Scholar]
- 35.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci USA 2006;103:13156–13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 2007;13:952–961. [DOI] [PubMed] [Google Scholar]
- 37.Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol 2001;159:1335–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gotzmann J, Fischer AN, Zojer M, Mikula M, Proell V, Huber H, Jechlinger M, Waerner T, Weith A, Beug H, et al. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene 2006;25:3170–3185. [DOI] [PubMed] [Google Scholar]
- 39.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003;112:1776–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res 2006;99:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121:335–348. [DOI] [PubMed] [Google Scholar]
- 43.Arciniegas E, Neves CY, Carrillo LM, Zambrano EA, Ramirez R. Endothelial-mesenchymal transition occurs during embryonic pulmonary artery development. Endothelium 2005;12:193–200. [DOI] [PubMed] [Google Scholar]
- 44.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest 2004;113:148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. Am J Pathol 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 46.Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, Sasano H, Kondo T, Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2004;169:1203–1208. [DOI] [PubMed] [Google Scholar]
- 47.Renzoni EA. Neovascularization in idiopathic pulmonary fibrosis: too much or too little? Am J Respir Crit Care Med 2004;169:1179–1180. [DOI] [PubMed] [Google Scholar]
- 48.Hao H, Gabbiani G, Camenzind E, Bacchetta M, Virmani R, Bochaton-Piallat ML. Phenotypic modulation of intima and media smooth muscle cells in fatal cases of coronary artery lesion. Arterioscler Thromb Vasc Biol 2006;26:326–332. [DOI] [PubMed] [Google Scholar]
- 49.Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 2007;175:875–880. [DOI] [PubMed] [Google Scholar]
- 50.Sumioka T, Ikeda K, Okada Y, Yamanaka O, Kitano A, Saika S. Inhibitory effect of blocking TGF-beta/Smad signal on injury-induced fibrosis of corneal endothelium. Mol Vis 2008;14:2272–2281. [PMC free article] [PubMed] [Google Scholar]
- 51.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 2008;19:2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res 2007;67:10123–10128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.