Abstract
The etiology of airway hyperresponsiveness associated with asthma requires an understanding of the regulatory mechanisms mediating human airway smooth muscle cell (SMC) contraction. The objective of this study was to determine how human airway SMC contraction (induced by histamine) and relaxation (induced by formoterol) are regulated by Ca2+ oscillations and Ca2+ sensitivity. The responses of human small airways and their associated SMCs were studied in human lung slices cut from agarose-inflated lungs. Airway contraction was measured with phase–contrast video microscopy. Ca2+ signaling and Ca2+ sensitivity of airway SMCs were measured with two-photon fluorescence microscopy and Ca2+-permeabilized lung slices. The agonist histamine induced contraction of human small airways by stimulating both an increase in intracellular Ca2+ concentration in the SMCs in the form of oscillatory Ca2+ waves and an increase in Ca2+ sensitivity. The frequency of the Ca2+ oscillations increased with histamine concentration, and correlated with increased contraction. Formoterol induced airway relaxation at low concentrations by initially decreasing SMC Ca2+ sensitivity. At higher concentrations, formoterol additionally slowed or inhibited the Ca2+ oscillations of the SMCs to relax the airways. The action of formoterol was only slowly reversed. Human lung slices provide a powerful experimental assay for the investigation of small airway physiology and pharmacology. Histamine induces contraction by simultaneously increasing SMC Ca2+ signaling and Ca2+ sensitivity. Formoterol induces long-lasting relaxation by initially reducing the Ca2+ sensitivity and, subsequently, the frequency of the Ca2+ oscillations of the airway SMCs.
Keywords: lung slice, smooth muscle cell, two-photon microscopy, hyperresponsiveness, histamine
CLINICAL RELEVANCE.
Increased airway contraction is a key event underlying airway hyperresponsiveness (AHR) in human asthma. The intracellular signaling mechanisms of the smooth muscle cells (SMCs) mediating this airway contraction are not fully understood, yet these mechanisms are a prerequisite to understanding the alterations that lead to AHR. This study uses the lung slice preparation to characterize the relationship between histamine-induced human airway contraction, Ca2+ oscillations, and increased Ca2+ sensitivity in airway SMCs. With this understanding of the mechanisms of human airway contraction, the mechanism of action of formoterol, a long-acting β2-agonist, was determined. Low concentrations of formoterol relax human airways by decreasing the Ca2+ sensitivity of the SMCs, whereas high concentrations of formoterol additionally reduce the frequency of, or stop, the Ca2+ oscillations.
Because increased airway smooth muscle cell (SMC) contraction is a major characteristic of airway hyperresponsiveness (AHR) associated with asthma (1), a prerequisite to address AHR is an understanding of the mechanisms that regulate airway SMC contraction. It is well established that airway SMC contraction is regulated by the phosphorylation of the regulatory myosin light chain (rMLC) (2, 3) to induce its interaction with adjacent actin filaments via the classical mechanism of cross-bridge cycling to generate force (4). This phosphorylation of rMLC is mediated by MLC kinase (MLCK), which itself is activated by calmodulin and an increase in intracellular Ca2+ concentration ([Ca2+]i). From studies with animals, it has been shown that agonist-induced increases in [Ca2+]i occur in the form of oscillatory Ca2+ waves rather than just a static increase in [Ca2+]i (5–8). Although the frequency of these Ca2+ oscillations has been found to increase with agonist concentration, and that this correlates with increased contraction, the frequency of the Ca2+ oscillations generated by similar agonist concentrations differs considerably between species (9).
To induce SMC relaxation, the increase in [Ca2+]i is generally reversed to inactivate MLCK, but the cessation of the cross-bridge cycle requires that the rMLC is dephosphorylated; this reaction is mediated by MLC phosphatase (MLCP). With the exception of mice (10), the activity of MLCP appears to be Ca2+ independent, but is regulated by the same agonists that stimulate increases in [Ca2+]i. Therefore, the extent of rMLC phosphorylation and SMC tone is the aggregate of the antagonistic activity of Ca2+-activated MLCK and agonist-regulated MLCP activity. The phenomenon by which MLCP activity modulates the effectiveness of MLCK is commonly referred to as Ca2+ sensitivity (3). Our previous studies with Ca2+-permeabilized mouse lung slices have shown that airway and vascular SMCs express different levels of Ca2+ sensitivity (10), and this has the result that the same stimulus generates different amounts of contraction. Similarly, airway SMCs from different species display different magnitudes of contraction in response to similar changes in [Ca2+]i (9).
The facts that airway SMC contraction results from the balance of MLCK activity regulated by Ca2+ oscillations and MLCP activity regulated by agonists, and that this regulation is highly variable between species and SMC types, makes the translation of results from one species to another very difficult. Consequently, to understand airway SMC contraction in human asthma, it is vital to investigate these SMC regulatory processes directly in human tissue.
The opportunity to study human airway SMC responses in an intact microenvironment is provided by the development of techniques employing living lung slices from human lungs. In lung slices, the relationships between the airway SMCs and the epithelial cells, extracellular matrix, and alveoli cells are retained and, as a result, airway contraction and relaxation occur under conditions resembling those in situ. Human lung slices have been used to study the early allergic response (11, 12) and the desensitization (13) and pharmacological characterization of β2-adrenergic receptors (14), but the signaling pathways mediating the contractile response in human small airway SMCs have not been fully investigated. Because β2-adrenergic agonists are widely used as bronchodilators by patients with asthma, it is also very important to understand how these β2-agonists counteract the contractile mechanisms in human airways. Consequently, in this study, we investigated, with human lung slices, how histamine, a putative inflammatory mediator important in asthma, stimulates Ca2+ oscillations and increases in Ca2+ sensitivity to induce airway contraction. Subsequently, we examined how formoterol, a long-acting β2-adrenergic agonist, affects these processes to induced airway relaxation.
MATERIALS AND METHODS
Reagents
Hanks' balanced salt solution (HBSS) was obtained from GIBCO-Invitrogen (Carlsbad, CA), supplemented with 20 mM Hepes buffer (sHBSS) and adjusted to pH 7.4. A zero Ca2+ solution (0 Ca2+) was prepared with HBSS without Ca2+ and Mg2+, supplemented with 20 mM Hepes, 0.407 mM MgSO4, 0.49 mM MgCl2, and 1 mM EGTA. Histamine, methacholine (MCh), serotonin (5HT), DMSO, sulfobromophthalein, caffeine, and (R,R)-formoterol were obtained from Sigma-Aldrich (St. Louis, MO). Other reagents were obtained from various sources as follows: Oregon-green 488 BAPTA-1-AM (Molecular Probes–Invitrogen Corp., Eugene, OR); Leukotriene D4 (LTD4; Cayman Chemicals, Ann Arbor, MI); Pluronic-F-127 (Calbiochem, La Jolla, CA); ultra pure agarose (low melting point, catalog no. 15517-022; Invitrogen, Carlsbad, CA); ryanodine (Asc-083; Ascent Scientific, Princeton, NJ); cell permeant, caged-IP3 (D-2,3-O-Isopropylidene-6-O-(2-nitro-4,5-dimethoxy) benzyl-myo-inositol 1,4,5-trisphosphate-hexakis(propionoxymethyl) ester, Cat # ALX-307-071; Enzo Life Sciences, Plymouth Meeting, PA).
Human Tissue
We obtained healthy human lung tissue from whole human lung lobes that were considered, but not used, for transplantation, or with informed consent from patients undergoing surgery (lobectomy) for lung cancer. Whole lung lobes were obtained from the National Disease Research Interchange in collaboration with Dr. R. A. Panettieri, Jr., University of Pennsylvania School of Medicine (Philadelphia, PA). Some normal lobectomy samples were obtained from the Division of Cardiothoracic Surgery, University of California San Diego Medical Center (San Diego, CA). Most lobectomy lung samples were obtained in collaboration with the Department of Surgery and the Department of Pathology at the University of Massachusetts Memorial Medical Center (Worcester, MA). The tumors were identified as non–small cell carcinoma (adenocarcinoma, squamous cell carcinoma). Tissues with diagnostic changes of emphysema were excluded from the study. The experimental protocols were approved by University of Massachusetts Medical School (Worcester, MA).
Human Lung Slices
Whole lung lobes were inflated with a warm agarose solution (2% in sHBSS, 37°C) via a main bronchus in accordance with previously published methods (11). In the case of lobectomy samples, only the distal part of the lobe, which was healthy and contained intact airways and acini, was inflated with warm agarose. This was generally achieved by cannulating multiple airways that were accessible on the cut surface of the lobe. To solidify the agarose, the tissue was cooled with ice for 15 to 35 minutes. After cooling, approximately 1-cm-thick tissue sections were cut perpendicular to the cross-section of the visible airways. From these sections, tissue cores containing airways were prepared, and these were cut into thin slices (∼250 μm thick) with a vibratome (VF-300; Precisionary Instruments, Greenville, NC) at room temperature (RT) in sHBSS. The slices were maintained in Dulbecco's modified Eagle's medium supplemented with antibiotics and antimycotics, but without serum, at 37°C and 10% CO2 for up to 4 days.
Measurement of Airway Contraction
Lung slices were placed in a custom-built perfusion chamber as previously described (5). Perfusion was also performed by a custom-built, gravity-fed perfusion system with a multitube manifold (8 or 16 inputs) with a single output, and regulated by electronic valves. Chamber volume was approximately 100 μl; perfusion rate was approximately 700 μl/minute. Lung slices were observed on an inverted microscope (Diaphot 200; Nikon, Melville, NY) with a 4× objective and phase–contrast optics. Images were recorded in time lapse (1 image/2 s) with a charge-coupled device camera (Pulnix, JAI Inc., San Jose, CA) and image acquisition software (Video Savant 3 or 4; IO Industries, London, ON, Canada). Image analysis was performed with custom-written scripts compatible with Video Savant 4. To calculate the airway lumen area with respect to time, the pixels within a selected grayscale range that distinguish the airway lumen from the surrounding alveolar tissue were summed. Airway areas were normalized to the initial area before stimulation; a decrease of airway area over time indicates airway contraction. Experiments were performed at RT (∼23–25°C).
Measurement of Ca2+ Signals in Airway SMCs
Human lung slices were loaded with the Ca2+ reporter dye, Oregon green, by incubation in 20 μM Oregon green BAPTA-1-AM, 0.1% Pluronic F-127, and 100 μM sulfobromophthalein for 90 minutes at 30°C in sHBSS. The lung slices were subsequently washed with sHBSS containing 100 μM sulfobromophthalein and kept for 30 minutes at RT to allow time for dye de-esterification before experimentation.
Fluorescence imaging of the lung slice was achieved with a custom-built two-photon laser scanning microscope. Details of the microscope design have been previously described (15). Video images were recorded at 15 frames per second, and changes in fluorescence intensity (F) were detected within a region of interest (∼8 × 8 pixels) located within a single airway SMC, and normalized to the initial F value (F0). Because airway SMCs can move as a result of airway contraction induced by agonists, it was necessary to track the region of interest; this was achieved by using custom-written scripts with Video Savant software. Line-scan images were performed by analyzing data frame-by-frame from a line selected along or across an airway SMC in Scion Image (Scion Corp./National Institutes of Health, Bethesda, MD).
Ca2+-Permeabilized Lung Slices
The cells within the lung slices were made permeable to Ca2+ by treatment with caffeine and ryanodine, as previously described (10). Briefly, to convert normal lung slices into slices permeable to Ca2+, the lung slices were simultaneously exposed to 20 mM caffeine and 50 μM ryanodine for 5 minutes. This treatment is believed to lock the ryanodine receptor (RyR) of the sarcoplasmic reticulum (SR) in an open state, and thereby depletes the SR of Ca2+. Emptied SR stores, in turn, induce Ca2+ influx from the extracellular space via activation of store-operated Ca2+ channels to refill the SR. Because the treatment with caffeine and ryanodine is irreversible, a constant leak of Ca2+ from the SR into the cytoplasm through the open RyR occurs, and this sustains a constant influx of extracellular Ca2+ into the cytoplasm. Under these conditions, the [Ca2+]i can be manipulated by changing the extracellular Ca2+ concentration ([Ca2+]o).
Flash Photolysis of Caged Inositol 1,4,5-Trisphosphate
An increase of the intracellular inositol 1,4,5-trisphosphate (IP3) concentration was achieved by flash photolysis of cell-permeant caged IP3, as previously described (15, 16). Lung slices were initially loaded with Oregon green 488 BAPTA-1 AM, as described previously here, and subsequently with 2 μM cell-permeant caged-IP3 in sHBSS containing 0.1% Pluronic F-127 and 100 μM sulfobromophthalein for 1 hour at RT. The lung slices were washed for 1 hour in sHBSS containing 100 μM sulfobromophthalein to allow de-esterification.
Protein Phosphatase and Kinase Activity Assay
Pieces of small human airways were dissected from the lung and incubated in sHBSS (control) or histamine (500 nM) for 10 minutes or histamine (500 nM, 10 min) followed by formoterol (50 nM, 10 min). Subsequently, the tissue was snap frozen in liquid nitrogen and stored at −80°C before protein extraction. To extract cell proteins, the tissue was homogenized in buffer containing 0.5 M NaCl, 50 mM Tris-HCl (pH 7.5), 0.5% Triton X-100, 2 mM EGTA, 30 mM MgCl2, and a protease inhibitor cocktail, followed by centrifugation at 14,000 × g for 5 minutes to separate membrane particles and cell debris from the supernatant. Enzyme activity was assayed with the supernatant as previously described (17). The buffer for the MLCK activity assay contained 0.1 M KCl, 5 mM MgCl2, 20 mM (3-(N-morpholino)propanesulfonic acid–potassium salt) (MOPS)-KOH (pH 7.5), 0.2 mM CaCl2, or 1 mM EGTA, 0.2 mM [γ-32P] ATP, 1 mM DTT, 0.1 μM microcystin-LR, 1.2 μM calmodulin, 0.5 μM turkey gizzard myosin, 0.2 mg/ml BSA, and 0.5 μl of supernatant. The assay was initiated by addition of myosin, and terminated by addition of 2 μl of 1 M perchloric acid and 0.35 M NaH2PO4; assay volume was 20 μl. The reaction mixture was filtered with a nitrocellulose membrane (0.45 μm), and the phosphorylated myosin was quantified by measuring the radioactivity emitted by 32P by Cerenkov counting (18). To ensure that the phosphorylation of the myosin by MLCK was specific for the rMLC, only whole myosin molecules containing the rMLC were used.
The buffer for the MLCP activity assay contained 0.1 M KCl, 20 mM MOPS-KOH (pH 7.5), 1 mM EDTA, 1 mM DTT, 0.1 μM 32P-labeled phosphorylated whole turkey gizzard myosin, 0.2 mg/ml BSA, and 0.5 μl of supernatant. The turkey gizzard myosin was initially phosphorylated by calmodulin-dependent MLCK, with [γ-32P] ATP as a substrate. MLCK specifically phosphorylates Ser19 of the rMLC. MLCK can also phosphorylate Thr18 of rMLC, but this occurs at a 1,000-fold lower rate. MLCK does not phosphorylate other sites of rMLC, the heavy chain, or the essential light chain (2, 19). Consequently, the rate of 32P release from this phosphorylated myosin only comes from the rMLC, and therefore represents the specific MLCP activity. The MLCP assay was initiated by the addition of supernatant, and terminated by addition of 5 μl of 50% trichloroacetic acid. Assay volume was 50 μl. The radioactivity of the liberated 32P was again determined by Cerenkov counting. Assays were performed at 25°C. Results were normalized to protein concentration.
Statistical Analysis
Student's t test was used to test for significant differences between means. Summary data are expressed as means (±SEM).
RESULTS
To characterize the contractile responses of human airways and their regulatory mechanisms, we examined the behavior of airways in lung slices obtained from a number of different individuals. The tissue samples were obtained from lungs that were considered, but not used, for lung transplantation, or from lung tissue resected from patients undergoing lung surgery. In both cases, the tissue used was macroscopically “healthy”; only lung slices with airways uniformly surrounded by alveoli tissue and displaying an intact epithelium with vigorous ciliary activity were used for experiments (Figure 1A). The lumens of all airways observed were free of agarose, and had a diameter smaller than 2 mm, with an airway area ranging from 0.01 to 0.5 mm2.
Figure 1.
Contractile responses of human airways within lung slices. (A) Phase-contrast image of a human airway in a lung slice under resting conditions. The airway is lined with epithelial cells (ECs; arrow), and is attached, via dense connective tissue, including the smooth muscle cells (SMCs), to the surrounding alveolar tissue (scale bar, 200 μm). (B) The contractile response of a human airway to the sequential application of histamine (His, 500 nM), methacholine (MCh; 500 nM), serotonin (5HT; 500 nM), KCl (50 mM), and leukotriene D4 (LTD4; 100 nM). The lung slice was perfused with sHBSS between each agonist to induce airway relaxation. Airway contraction is represented as the change in airway area (% of the initial area) with respect to time (min). Data are representative of six to eight slices from four individuals. (C) Representative data showing concentration-dependent airway contraction induced by His (1 nM to 10 μM). The lung slice was perfused with sHBSS between each concentration of His to relax the airway. (D) The mean concentration–airway contraction (%) response for His (filled squares; n = 6–16 slices from 6 individuals) and MCh (shaded circles; n = 3–19 slices from at least 3 individuals) measured 10 minutes after administration. Each point represents mean (±SEM). Data were fitted with a sigmoidal curve.
Airway Contraction in Response to Different Agonists
To confirm that airways in lung slices were viable and that the airway SMCs retained their functionality by maintaining their surface receptors, the airway responses to different contractile agonists were investigated (Figure 1). The perfusion of the lung slice with histamine (500 nM), MCh (500 nM), and LTD4 (100 nM) induced bronchoconstriction by reducing the airway lumen area to 57.67 (±3.47)%, 63.98 (±7.88)%, and 47.89 (±5.49)% (n = 8, 8, and 7 slices from 4 individuals), respectively, of the initial airway size (Figure 1B). After the removal of each agonist by perfusing the lung slice with sHBSS, the airway immediately relaxed, except in the case of LTD4, where the relaxation rate was slower. By contrast, 5HT (500 nM), a potent bronchoconstrictor of mouse and rat airways, did not induce airway contraction (Figure 1, n = 6 from 3 individuals) at any concentration tested. Membrane depolarization, induced by 50 mM KCl, also induced a substantial airway contraction to 60.98 (±5.06)% (n = 8 from 4 individuals). These contractile airway responses to each mediator were similar for 4 days after cutting the lung slices, indicating that the lung slices remain viable for a prolonged period.
To further quantify the contractile airway response of human airways to histamine and MCh, a concentration–contraction response curve was constructed for each agonist by exposing the airways to increasing concentrations of histamine or MCh (from 1 nM to 10 μM; Figures 1C and 1D). At low agonist concentrations, a small, slow bronchoconstriction, with twitching occurring in some airway SMCs, was observed. At higher agonist concentrations, the airways contracted more quickly and to a greater extent (Figure 1C). In comparison to MCh, histamine induced greater airway contraction at lower concentrations (10 and 50 nM; Figure 1D), but for both agonists, the extent of airway contraction was a function of agonist concentration. From these responses, it was also determined that a concentration of 500 nM histamine should be used for further contraction experiments, because this concentration gives a midrange airway contraction equal to a decrease in airway area of 36 ± 5.3% (n = 16 from 6 individuals).
Ca2+ Oscillations in Human Airway SMCs
The [Ca2+]i is an important parameter that determines airway contraction. Consequently, to investigate how the contraction induced by the different agonists is related to changes in [Ca2+]i, we studied the Ca2+ signaling of several single airway SMCs in lung slices loaded with the Ca2+ indicator, Oregon green. In response to histamine, MCh, and LTD4, human airway SMCs showed oscillatory increases of [Ca2+]i (Figure 2). Each Ca2+ oscillation occurred in the form of a Ca2+ wave, propagating along the length of the individual SMC (Figures 3C and 3D), but not to adjacent cells. As a result, the Ca2+ oscillations occurred asynchronously with respect to neighboring SMCs (Figure 2E). The Ca2+ oscillations in the airway SMCs were accompanied by contraction and shortening of the SMC, and this resulted in the contraction of the airway (movement of the SMCs toward the airway lumen [Figures 2A–2C, arrow]; upward displacement of the SMCs in the line-scan [Figure 2E]). No major differences in the form of Ca2+ oscillations in response to histamine, MCh, and LTD4 were observed. However, LTD4, at substantially lower concentrations (100 nM compared with 1 μM MCh or histamine), induced Ca2+ oscillations with a similar frequency compared with those induced by histamine or MCh (Figures 2D, 2F, and 2G).
Figure 2.
Ca2+ oscillations in human airway SMCs in response to different agonists. (A–C) Airway SMCs within the wall of a human airway in a lung slice loaded with the Ca2+ indicator dye, Oregon green, and observed with two-photon fluorescence microscopy before (A), or 1 minute (B) and 3 minutes (C) after exposure to His (500 nM). His induced an increase in the fluorescence intensity (F) to initial F (F0) ratio (F:F0) of the airway SMCs, indicating an increase of the intracellular Ca2+ concentration ([Ca2+]i). Along with this increase in the [Ca2+]i, the airway contracted (as indicated by a white arrow) toward the airway lumen. (D–I) In response to various agonists, airway SMCs showed an increase in [Ca2+]i in the form of repetitive Ca2+ oscillations, which occur in an asynchronous manner (E) with regard to adjacent airway SMCs, as shown in the line scan analysis (obtained from the pixel values analyzed over time, along a line drawn across several airway SMCs). Representative Ca2+ oscillations are shown in response to (D) 1 μM His, (F) 1 μM MCh, (G) 100 nM LTD4, and (H) 50 mM KCl. His, MCh, and LTD4 all induced Ca2+ oscillations with a frequency of approximately eight to nine peaks per minute. By contrast, KCl induced very slow Ca2+ oscillations (∼2–3 peaks/min) with a long duration of the elevated [Ca2+]i, as shown in the line scan analysis in I.
Figure 3.
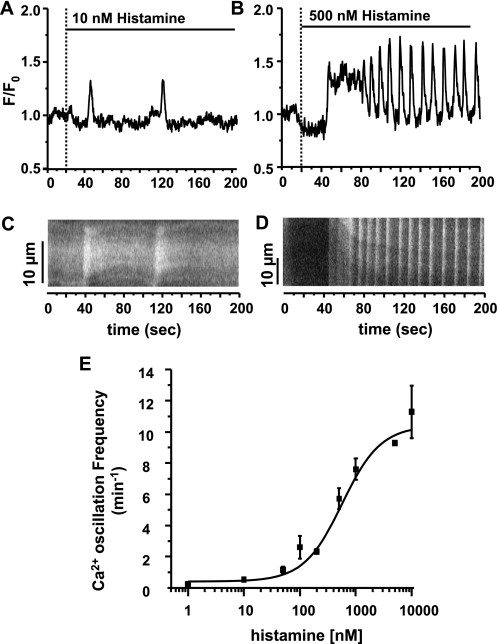
The frequency of agonist-induced Ca2+ oscillations in response to different concentrations of His. (A) Low concentrations of His (e.g., 10 nM) induced Ca2+ oscillations with a low frequency (0.53 ± 0.11 peaks/min), whereas higher concentrations (e.g., 500 nM) (B) induced Ca2+ oscillations with a greater frequency (5.73 ± 0.66 peaks/min). (C and D) Line scan analysis showing the Ca2+ wave propagation within a single airway SMC during the experiments shown in A and B. Scale bars indicates distance along the SMC (10 μm for each line scan analysis). (E) The Ca2+ oscillation frequency response for different concentrations of His (1 nM to 10 μM). Increasing concentrations of His induced Ca2+ oscillations with an increasing frequency. Data are means (±SEM); n = 8–27 (different cells in different slices) from 4–7 individuals for each concentration.
Ca2+ oscillations were also induced by KCl, but these Ca2+ oscillations had a much lower frequency, and a duration (peak width) that was substantially longer (Figures 2H and 2I). In contrast to the sustained contraction of the SMCs associated with agonist-induced Ca2+ oscillations, each KCl-induced Ca2+ oscillations was commonly observed to be associated with an SMC twitch. In keeping with the observation that 5HT did not induce airway contraction, 5HT also did not induce Ca2+ oscillations (data not shown).
To determine if the frequency of Ca2+ oscillations is dependent on agonist concentration, the effect of increasing concentrations of histamine on the frequency of the Ca2+ oscillations in airway SMCs was analyzed (Figures 3A–3E). Histamine at 10 nM induced Ca2+ oscillations with a frequency of 0.53 (±0.11) peaks min−1, whereas 500 nM histamine induced 5.73 (±0.66) peaks min−1 (Figures 3A–3D). At 1 and 10 μM histamine, the airway SMCs displayed Ca2+ oscillations with 7.61 (±0.69) and 11.28 (±1.69) peaks min−1 (Figure 3E). These results indicate that the frequency of histamine-induced Ca2+ oscillations is concentration dependent in human airway SMCs (Figure 3E).
Correlation of Airway Contraction with Ca2+ Oscillation Frequency
Increasing concentrations of histamine increased both airway contraction (Figure 1D) and the Ca2+ oscillation frequency (Figure 3E). By replotting the extent of airway contraction against the frequency of the Ca2+ oscillations in the SMCs at equivalent histamine concentrations, it can be seen in Figure 4 that an increase in the frequency of the Ca2+ oscillations correlates with an increase in airway contraction.
Figure 4.
The relationship between airway contraction and the frequency of Ca2+ oscillations in airway SMCs induced by His. Increasing concentrations of His induced increasing amounts of airway contraction (see Figure 1D) and increasing frequencies of Ca2+ oscillations in airway SMCs (see Figure 3E). By comparing and plotting these data, at equal His concentrations, an increasing airway contraction correlates with an increasing Ca2+ oscillation frequency; n = 7 to 20 lung slices from at least 4 individuals for each point. Data are means (±SEM).
Role of Ryanodine Receptor in Airway Contraction and Ca2+ Signaling
Because Ca2+ oscillations are important in driving airway contraction, we sought to determine whether the ryanodine receptor (RyR) was involved. Therefore, we examined the effect of ryanodine, an antagonist of the RyR, on airway contraction and Ca2+ oscillations. Ryanodine (50 μM) was found to have no major effect on airway contraction induced by histamine (500 nM) (Figure 5A) or the appearance and propagation of histamine-induced Ca2+ oscillations (Figure 5B). These results suggest that the RyR does not significantly contribute to histamine-induced Ca2+ oscillations. By contrast, the slow Ca2+ oscillations induced by KCl (50 mM) were quickly inhibited by ryanodine within 3 minutes (Figure 5C). This inhibition was accompanied by a sustained increase in [Ca2+]i. This result is consistent with an inhibition of the RyR in an open state and the emptying of Ca2+ from the SR.
Figure 5.
Effect of ryanodine on airway contraction and Ca2+ oscillations. (A) Representative response of the effect of ryanodine (50 μM) on His-induced (500 nM) airway contraction (% of the initial area with respect to time). Ryanodine had no significant effect on airway contraction during 15-minute exposure (n = 4 slices from 3 individuals). (B) Representative response of the effect of ryanodine on His-induced Ca2+ oscillations of airway SMCs (as indicated by the change in fluorescence intensity with respect to time). Ryanodine did not inhibit the Ca2+ oscillations or alter the propagation of the associated Ca2+ waves (n = 5 slices from 2 individuals). (C) A representative response indicating that KCl-induced (50 mM) Ca2+ oscillations were inhibited within 3 minutes of ryanodine exposure. In addition, the Ca2+ baseline was elevated as the Ca2+ oscillations were inhibited (n = 2 slice from 2 individual). (D) A representative line scan analysis across several airway SMCs (indicated by the white line in the left image) showing the immediate induction of Ca2+ oscillations when the lung slice loaded with caged IP3 was flashed with ultraviolet (UV) light to release IP3 from its caged compound. (E) Line scan plot showing that IP3 released by a UV light flash from caged IP3 induces Ca2+ oscillations in the presence of 50 μM ryanodine (n = 3 slices from 2 individuals).
The Role of IP3 Receptor in Ca2+ Oscillations
To investigate the role of the IP3 receptor (IP3R) in generating Ca2+ oscillations, we increased the intracellular IP3 concentration by photolysis of caged IP3 (15). Lung slices perfused with sHBSS alone did not display Ca2+ oscillations, but, upon the release of IP3 from caged IP3, Ca2+ oscillations were immediately observed (Figure 5D). The same result was obtained in the presence of ryanodine, indicating that IP3-induced Ca2+ oscillations are not influenced by the inhibition of the RyR and, therefore, most likely rely entirely on the IP3R (Figure 5E).
Ca2+ Sensitivity of Airway SMCs
To investigate the Ca2+ sensitivity of airway SMCs, human lung slices were permeabilized to Ca2+ by treatment with caffeine and ryanodine, as described in Materials and Methods here and in other studies (10). After this treatment, the [Ca2+]i can be clamped at a steady level, and the action of agonists at a constant [Ca2+]i can be examined. Before treatment with caffeine and ryanodine, the airways show a normal contraction (contraction to 51.76% of the initial area after 10 min [Figure 6A, left]) and Ca2+ oscillations (Figure 6B, left) in response to 500 nM histamine. Upon exposure to caffeine and ryanodine, the airways display an initial contraction (Figure 6A, middle) and a spike increase in [Ca2+]i (Figure 6B, middle). Subsequently, the airways relaxed due to the influence of caffeine and the [Ca2+]i settled to a sustained plateau. The failure of a second caffeine administration to induce a second contraction of the airway and to induce another Ca2+ spike indicates that the treatment with caffeine and ryanodine was complete, and that the SR was emptied of Ca2+.
Figure 6.
Ca2+sensitivity of human airway SMCs assayed with Ca2+-permeabilized lung slices. (A) A representative contractile response with respect to time of a human airway in response to His (500 nM) before (left) and after (right) Ca2+-permeabilization with caffeine and ryanodine (center). (B) A representative Ca2+ response of airway SMCs before and after Ca2+ permeabilization. The normal airway (A, left) displayed a standard airway contraction and repetitive Ca2+ oscillations in SMCs (B, left) in response to His (500 nM). The lung slice was permeabilized to Ca2+ by the simultaneous treatment with caffeine (Caff; 20 mM) and ryanodine (Rya; 50 μM). This Caff/Rya treatment induced a transient airway contraction (A, center), which was accompanied by an initial peak, and subsequently sustained increase in [Ca2+]i (B, center). Removal of Caff/Rya allowed the airway to partially recontract without influencing [Ca2+]i. A second caffeine exposure induced airway relaxation, but did not alter the [Ca2+]i (data not shown). This response was found to be characteristic of a fully Ca2+-permeabilized lung slice in which the [Ca2+]i can be manipulated by changing the extracellular Ca2+ concentration ([Ca2+]o). Perfusing the lung slice with a zero Ca2+ sHBSS (0 Ca2+) placed the airway in a relaxed state (A, right), and reduced the [Ca2+]i to below the original baseline (B, right). Subsequent addition of sHBSS containing 1.3 mM Ca2+ resulted in the contraction of the airway (A, right) and an elevation of the [Ca2+]i (B, right). Addition of His (500 nM) induced further airway contraction, but no change in [Ca2+]i. Upon removal of His, the airway relaxed to the contracted state induced with 1.3 mM Ca2+ alone. Removal of extracellular Ca2+ induced a full airway relaxation (A, right) and the decrease of [Ca2+]i (B, right) (n = 4 slices from 2 individuals).
By changing the [Ca2+]o to zero, the [Ca2+]i dropped below the original baseline (Figure 6B, right), and the airways became fully relaxed (Figure 6A, middle and right). When the [Ca2+]i was raised (Figure 6B, right) by perfusing the lung slice with sHBSS (containing 1.3 mM Ca2+), the airway contracted (to 66.13% of initial area after 10 min) (Figure 6A, right). When histamine (500 nM) was subsequently added, under these conditions of high [Ca2+]i, the airways showed an additional contraction (to 46.14% of the airway area after 10 min; Figure 6A, right) without a change in the [Ca2+]i (Figure 6B, right). These responses indicate that the Ca2+ sensitivity of the SMCs has been increased by histamine. Upon removal of histamine, the airway relaxed back to the Ca2+-induced contractile level (69.61% of initial airway area), and, upon the reduction of [Ca2+]i (Figure 6B, right) by perfusion with zero extracellular Ca2+, the airway almost completely relaxed (to 95.94% after 10 min; Figure 6A, right).
With these Ca2+-permeabilized slices, the amount of airway contraction induced by different levels of [Ca2+]o (0.1–10 mM) was investigated (Figures 7A and 7B). In response to 0.2 mM [Ca2+]o, the airway contracted to 90.82 (±2.09)% of the initial airway area, whereas 0.5 mM, 1.3 mM, and 10 mM [Ca2+]o constricted the airway lumen to 84.16 (±3.85)%, 74.99 (±2.34)%, and 58.50 (±3.83)% of the initial area, respectively. The contraction of the airway by exposure to increasing [Ca2+]o correlated with an increase in [Ca2+]i, as determined by the fluorescence ratio of Oregon Green (F:F0 [Figure 7B]). Exposure to a [Ca2+]o from 500 nM to 1.3 mM induced an increase in the fluorescence ratio from approximately 1.75 to 2.5; this range of steady-state ratio values is similar to the peak fluorescence ratio values induced by Ca2+ oscillations (Figure 6). Although, in these studies, it was not possible to quantify the fluorescence values in terms of absolute Ca2+ concentration, the similar relative change in the fluorescence ratio indicates that the peak [Ca2+]i associated with the Ca2+ oscillations is similar to the steady [Ca2+]i induced by high [Ca2+]o. The equivalence of these conditions appears to be confirmed by the fact that 500 nM histamine induced a similar airway contraction in either normal or Ca2+-permeabilzed lung slices with 1.3 mM external Ca2+ (Figures 6 and 7). Importantly, the peak fluorescence ratio of the Ca2+ oscillations does not substantially vary with agonist type, agonist concentration, or Ca2+ oscillation frequency (Figure 2).
Figure 7.
Airway contraction induced by different concentrations of Ca2+ and His in Ca2+-permeabilized lung slices. (A) Representative contractile response with respect to time of an airway within a Ca2+-permeabilized lung slice. While perfused with zero Ca2+ sHBSS (0 Ca2+), the airway is fully relaxed (100% of initial airway area). Upon addition of sHBSS containing increasing concentrations of Ca2+ (0.2–10 mM), the airway showed a stepwise increased contractile response, which was reversed by perfusing the lung slice with 0 Ca2+ sHBSS. (B) F value relative to the F0 of airway SMCs within Ca2+-permeabilized lung slices in response to increasing [Ca2+]o values (0.1–10 mM; n = 3 slices from one individual). (C) An airway within a Ca2+-permeabilized lung slice was contracted by administration of sHBSS containing 1.3 mM Ca2+. Subsequent addition of increasing concentrations of His (100 to 1,000 nM) induced a further stepwise increase in airway contraction, which was reversed by removal of His. A complete relaxation occurred in response to removal of extracellular Ca2+ (n = 2–4 from 2 individuals).
We next explored the process of agonist-induced Ca2+ sensitivity by examining the effect of histamine on airway contraction at the peak Ca2+ concentration induced by a constant [Ca2+]o of 1.3 mM. Increasing concentrations of histamine (100, 500, and 1,000 nM) induced increasing levels of airway contraction (to 59.70 ± 6.61%, 53.60 ± 2.72%, and 49.48 ± 3.35% of the initial airway area, respectively) (Figure 7C) without changing the [Ca2+]i (Figure 6). These results demonstrate that histamine increases the Ca2+ sensitivity of human airway SMCs in a concentration-dependent manner.
Effects of Formoterol on Airway Contraction Induced by Histamine
In the studies described previously here, we have identified two major mechanisms underlying human airway contraction; namely, agonist-induced Ca2+ oscillations and increased Ca2+ sensitivity. Consequently, we investigated how these two contractile mechanisms were influenced by formoterol, a long-acting β2-adrenergic agonist used for asthma therapy to induce airway relaxation.
Lung slices were perfused with histamine (500 nM) to induce a sustained airway contraction (to 55.75 ± 5.02% of initial area after 10 min [Figure 8A]). The subsequent exposure of the airway to formoterol (Figure 8A: 1 nM, gray line; 2.5 nM, black line) induced airway relaxation within 1 minute (1 and 2.5 nM formoterol induced 33.68 ± 10.37% and 76.86 ± 12.06% relaxation, respectively, after 10 min compared with the initial contraction; n = 4 slices from 3–4 individuals). Upon removal of formoterol, the airways slowly recontracted at a rate that was substantially lower than the initial contractile rate. After 20 minutes, the airway had recontracted to 95.42 (±4.91)% (1 nM) and 99.56 (±8.18)% (2.5 nM) of the initially contracted airway area. Higher concentrations of formoterol (Figure 8B: 10 nM, gray line; 100 nM, black line) induced relaxation of 84.53 (±5.55)% (n = 6 from 4 individuals) and 96.13% (n = 2 from 2 individuals), respectively. However, the recontraction observed 20 minutes after removal of formoterol (74.02 ± 9.45% [10 nM] and 17.20% [100 nM]) was significantly less than that observed after the removal of 1 or 2.5 nM formoterol. Summaries of airway relaxation and recontraction in response to the addition and removal of different concentrations of formoterol are shown in Figures 8C and 8D. A significant airway relaxation was induced by 1–2.5 nM formoterol, whereas a near full airway relaxation was induced by greater than 5 nM formoterol (Figure 8C). The longevity of formoterol-induced relaxation after washout significantly increased with formoterol concentration (Figure 8D).
Figure 8.
Effect of formoterol on His-induced airway contraction. (A and B) Representative airway responses showing the relaxant effect of different formoterol concentrations on airways contracted with 500 nM His. (A) Addition of low concentrations of formoterol (1 nM, gray line; 2.5 nM, black line) relaxed the airway. After the removal of formoterol, the airways slowly recontracted. (B) Higher concentrations of formoterol (10 nM, gray line; 100 nM, black line) fully relaxed the airway. However, after formoterol removal, airway recontraction was slow, and a return to the previous contracted state was not achieved within 30 minutes. (C) The mean airway relaxation (% normalized to the initial airway contraction) and (D) extent of airway relaxation (20 min after formoterol removal) in response to increasing concentrations of formoterol (50 pM to 100 nM; n = 2–6 slices from 2–4 individuals; [C] *P < 0.01; [D] *P < 0.05).
Effect of Formoterol on Ca2+ Oscillations
We next examined if formoterol affected the Ca2+ oscillation frequency of SMCs to induce airway relaxation. Before exposure to formoterol, Ca2+ oscillations with a stable frequency were induced by histamine (500 nM) (Figure 9). Formoterol at 1 nM had no effect on these Ca2+ oscillations (data not shown), but 2.5 nM formoterol slightly reduced the Ca2+ oscillation frequency by approximately 8% after 2 minutes (Figure 9A). Exposure to 10 nM formoterol reduced the Ca2+ oscillation frequency by approximately 35% (Figure 9B), whereas 100 nM formoterol completely stopped the Ca2+ oscillations (Figure 9C). In most cases, the Ca2+ oscillations began to increase their frequency after formoterol removal: 5, 10, and 20 minutes after the removal of 2.5 nM formoterol, the Ca2+ oscillation frequency was restored to 55, 63, and 93% of the initial rate, respectively. Similarly, the Ca2+ oscillation frequency was restored to 7% at 5 minutes, 14% at 10 minutes, and 37% at 20 minutes of the initial rate after removal of 10 nM formoterol. The Ca2+ oscillations remained stopped 5 minutes after the removal of 100 nM formoterol, but were reinitiated at 17% of the initial frequency at 10 minutes and 8.93% at 20 minutes. These results indicate that high concentrations of formoterol have a prolonged inhibitory effect on the frequency of Ca2+ oscillations.
Figure 9.
Effect of formoterol on His-induced Ca2+ oscillations. (A–C) Representative responses of airway SMCs showing the effects of increasing concentrations of formoterol on His-induced (500 nM) Ca2+ oscillations. (A) Formoterol (2.5 nM) induced a small decrease in the Ca2+ oscillation frequency within 2 minutes of exposure. This effect was completely reversed 20 minutes after the removal of formoterol (n = 5 slices from 3 individuals). (B) Formoterol (10 nM) slowed the Ca2+ oscillation frequency to a greater extent after 2 minutes of exposure. A reduction in the Ca2+ oscillation frequency continued even after formoterol had been removed (Minutes 11–13). The frequency of the Ca2+ oscillations began to increase again 20 minutes after the removal of formoterol (n = 4 slices from 2 individuals). (C) The Ca2+ oscillations were completely inhibited by 100 nM formoterol within 2 minutes of exposure. This effect was long lasting; Ca2+ oscillations only started to slowly reappear 20 minutes after the removal of formoterol (n = 4 slices from 3 individuals). (D) Line scan analysis of a single airway SMC showing Ca2+ oscillations induced by His (500 nM). Upon administration of 15 nM formoterol, the Ca2+ oscillations slowed down and stopped after 2 minutes of exposure. The release of IP3 from caged IP3 by UV flash photolysis reinitiated Ca2+ oscillations for approximately 30 seconds in the presence of formoterol (n = 5 from 2 individuals).
To investigate if formoterol affects the IP3 pathway to slow or stop the Ca2+ oscillations, we used flash photolysis of caged IP3. Again, a stable frequency of Ca2+ oscillations was induced with histamine (500 nM), and the subsequent addition of formoterol (15 nM) slowed and stopped the Ca2+ oscillation frequency. However, after the release of IP3 within the SMCs, the Ca2+ oscillations briefly reappeared (Figure 9D). These results suggest that formoterol does not fully inhibit the IP3R to stop Ca2+ oscillations, but may reduce the availability of IP3 to generate Ca2+ oscillations.
Effect of Formoterol on Ca2+ Sensitivity
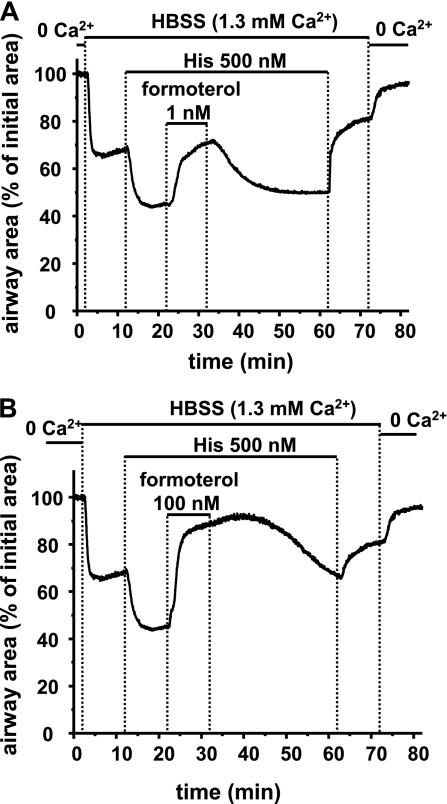
We also investigated the effect of formoterol on the Ca2+ sensitivity of airway SMCs. Airways in Ca2+-permeabilized slices were contracted (to 68.16% of the initial area) by a sustained increase in [Ca2+]i, and further contracted (to 44.69%) by an increase in Ca2+ sensitivity with histamine (500 nM) (Figures 6 and 10). Under these conditions, low concentrations of formoterol (1 nM) relaxed the contracted airway to 70.45% of the initial airway area within 1 minute, and fully reversed the histamine-induced airway contraction within 10 minutes. After 2 minutes of washout of formoterol (1 nM), the airway began to recontract, and after 20 minutes had recontracted to 49.87% (Figure 10A). Higher concentrations of formoterol (100 nM) immediately induced a near-full airway relaxation (to 88.25%; Figure 10B). After formoterol washout (100 nM), the airway recontracted slowly, and reached 81.65% of the initial airway area after 20 minutes.
Figure 10.
Effect of formoterol on His-induced Ca2+ sensitivity. Representative responses showing the effect of (A) 1 nM and (B) 100 nM formoterol on His-induced airway contraction in Ca2+-permeabilized lung slices. Perfusion with sHBSS containing 1.3 mM Ca2+ to increase [Ca2+]i induced airway contraction. The airway further contracted in response to 500 nM His. Addition of 1 nM formoterol reversed the His-induced contraction. The removal of formoterol allowed airway recontraction within 30 minutes. Addition of 100 nM formoterol relaxed the airway to a greater extent and for a longer duration. The subsequent sequential removal of His and extracellular Ca2+ relaxed the airway (n = 4 slices from 2 individuals).
Effect of Histamine and Formoterol on MLCK and MLCP Activity
To investigate if the additional contraction induced by histamine under high [Ca2+]i conditions in Ca2+-permeabilized slices results from MLCP inhibition, we assayed MLCP activity as a function of phosphate release (32P) from phosphorylated rMLC of whole myosin. The use of whole myosin (heavy and light chains) as the assay substrate imparts specificity to this assay, because the molecular structure of myosin determines which sites are phosphorylated. Whole myosin is specifically phosphorylated by MLCK at Ser19 of the rMLC. Although Thr18 of the rMLC can be phosphorylated, this occurs with 1,000-times less efficacy than phosphorylation of Ser19, and the heavy chains are not phosphorylated. Although phosphorylation of the regulatory myosin phosphatase target subunit (MYPT1) decreases MLCP activity, the phosphorylation level of MYPT1 is less reliable as an MLCP activity assay, because MLCP activity can also be regulated by other components, such as a 17-kD inhibitory phosphoprotein (CPI-17) and the RhoA-binding protein, p116Rip, without phosphorylating MYPT1. Consequently, we employed the approach of measuring the phosphate released from whole myosin to specifically reflect MLCP activity.
In comparison to the activity of MLCP activity in control conditions of high [Ca2+]i, MLCP activity (phosphate release) was significantly decreased by histamine (500 nM) (Figure 11A). Exposure of histamine-contracted airways to formoterol (50 nM) partially reversed the decreased MLCP activity (increased phosphate release), a response consistent with the observed relaxation induced by formoterol in Ca2+-permeabilized slices. The Ca2+-dependent MLCK activity, determined by a similar assay of phosphate uptake by the specific phosphorylation of rMLC, was not affected by histamine or formoterol (Figure 11B). This result implies that Ca2+ sensitivity was not mediated by changes in MLCK activity.
Figure 11.
Effects of His and formoterol on myosin light chain phosphatase (MLCP) and MLC kinase (MLCK) activity. Tissue extracts derived from human small airways were exposed to His (500 nM) for 10 minutes or His (10 min), followed by formoterol (50 nM) for 10 minutes before assaying for (A) MLCP and (B) MLCK activity. MLCP and MLCK activity was expressed in terms of radioactivity-labeled phosphate (32P) release or uptake (nmol/min/mg protein) by whole myosin, respectively. (A) MLCP activity was significantly reduced in airways exposed to His compared with untreated airways. The exposure of the airways to formoterol partially reversed the His-induced decrease in MLCP activity. (B) Ca2+-induced MLCK activity of the airways was not altered by either His alone or by His and formoterol combined (n = 4 assays from one individual; *P < 0.01).
DISCUSSION
Many factors, including repetitive airway inflammation and, over the longer term, airway remodeling, are believed to contribute to the development of AHR, but it is airway SMC contraction that is responsible for AHR. Therefore, it is surprising, especially because SMC hyperplasia and hypertrophy are also associated with AHR, that the physiological mechanisms regulating human airway SMC contraction are poorly understood.
The major reasons for this lack of understanding are that human airway SMCs within small airways are experimentally inaccessible and difficult to obtain, especially from patients with asthma. A common approach to this problem has been the use of cultured SMCs, muscle strips, or isolated tracheal rings from animals. However, SMC cultures often experience a change in phenotype with a loss of contractility, whereas muscle strips are usually only obtained from larger airways. Unfortunately, in these tissue preparations, other components of the lung, which may be important to airway contraction, such as epithelial cells or the extracellular matrix, are discarded.
Some of these problems associated with study of SMCs and airway contraction can be addressed by the use of lung slices in which the in situ organization of the lung is largely retained (7, 10–13, 20). The airways are lined with epithelial cells and, because the airways remain tethered to the surrounding alveoli tissue, contraction occurs against a variable load mediated by the elastic properties of the alveoli tissue and the inflation pressure of the alveoli mediated by the agarose. Surfactant forces present in air-filled alveoli are not present in the agarose-filled lung slice. Similarly, dynamic stretching of the airway, normally associated with breathing, is not yet simulated in these lung slices. However, the advantage of the static tethering forces present is that they limit contraction and bring about airway relaxation when the SMC contractile forces are terminated. As a result, the efficiency and mechanisms of action of bronchodilators can also be evaluated with lung slices. Most importantly, lungs slices are highly compatible with microscopy techniques, which enables the measurement of the dynamic changes in the size and SMC [Ca2+]i of small airways (21).
In view of theses advantages, we previously conducted studies with lung slices from mice and rats to characterize the contractile physiology of small airways (5–10, 15, 20). An important fact emphasized by these studies was that agonist-induced contraction is mediated by a dual process consisting of the initiation of Ca2+ oscillations and an increase in Ca2+ sensitivity mediated by a decrease in MLCP activity (22). Although a Ca2+ increase has been recognized as a fundamental contractile stimulus (23), the dynamic nature of this signal (i.e., Ca2+ oscillations and Ca2+ waves within the SMCs [6, 24–26]) had not been fully appreciated. Equally important is an increase in Ca2+ sensitivity. This response is commonly considered to only facilitate contraction, but our studies with mouse lung slices emphasize that without an increase in Ca2+ sensitivity, large elevations in [Ca2+]i were unable to sustain airway contraction (10).
Because Ca2+ oscillations and Ca2+ sensitivity make a major contribution to airway contraction, we compared these responses in mouse, rat, and human airway SMCs. It was clear that the specific responses of the SMCs from different species, as well as different airway locations, were different (9, 27). For example, human airways respond to histamine, whereas mouse airways only respond very weakly to histamine concentrations of 10 mM or greater. On the other hand, human airways do not contract in response to 5HT, which is a potent bronchoconstrictor in mice and rats (9, 12, 28). The frequency of the Ca2+ oscillations induced by the same concentration of agonist varies from 5 to 20 peaks per minute in these three species. Similarly, the Ca2+ sensitivity of mouse SMCs is Ca2+ dependent, whereas the Ca2+ sensitivity of rats or humans is not. The consequence of these species-specific differences is that it is difficult to extrapolate data from one species to another—in particular, to humans.
To overcome this difficulty, we have extended the lung slice technique for use with human tissue. Human lung tissue, available from transplantation services, is ideal for the production of lung slices, because the respiratory tract is intact and whole lung lobes can be easily inflated with agarose. However, human tissue from lobectomies is also useful. The only requirement for the use of this tissue is that the distal part of the lobe is obtained (free of disease) where the respiratory tract is intact and can be inflated.
Previous studies have indicated that human airways in lung slices were responsive to different agonists (11, 12). We also demonstrate here a consistent viability and functionality of the airways obtained from different individuals (varying in age, sex, and disease) over multiple cycles of contraction and relaxation for 4 days in response to a variety of contractile agonists, including histamine, MCh, and LTD4. These responses suggest that human SMCs in lung slices remain differentiated and retain their in situ phenotype related to receptors and internal structure. The longevity of the human lung slice makes them valuable for extended experimentation.
Although human lung slices are responsive to a variety of agonists, we focused on the contraction and associated regulatory mechanisms induced by histamine, an inflammatory mediator implicated in allergic asthma. Histamine, like MCh, induced airway contraction in a concentration-dependent manner that was fully reversible, and did not appear to accommodate and was accompanied by oscillatory increases of [Ca2+]i in single airway SMCs. Like the airway contractions of mice and rats (9), an increase in frequency of these Ca2+ oscillations correlated with an increased airway contraction. However, in humans, the extent of contraction for a similar rate of Ca2+ oscillations was greater than in mice, but less than in rats. We believe the reason for these differences in airway contraction is the result of differences in Ca2+ sensitivity (see subsequent discussion).
Because the extent of contraction correlates with the Ca2+ oscillation frequency, it is important to understand the mechanisms of these Ca2+ oscillations, because changes in these mechanisms may enhance AHR. In previous studies with isolated cell and tissue preparations from animals, SMC Ca2+ oscillations were proposed to be dependent on the RyR (29), and perhaps stimulated by cADP-ribose (30, 31). By contrast, our study of agonist-induced Ca2+ oscillations in mouse small airway SMCs indicated that MCh induced Ca2+ oscillations via an increase in IP3 and the IP3R (32), and that a role for the RyR was not supported.
We also found here that ryanodine, a RyR antagonist, had no effect on histamine-induced Ca2+ oscillations or IP3-induced Ca2+ oscillations of human airway SMCs. The possibility that ryanodine was impotent was ruled out by the fact that ryanodine did inhibit KCl-induced Ca2+ oscillations. KCl-induced Ca2+ oscillations differ from agonist-induced Ca2+ oscillations in that they are lower in frequency, longer in duration, dependent on Ca2+ influx, and result from an overload of intracellular stores as the cell compensates for excess Ca2+ influx initiated by KCl-induced membrane depolarization. This overfilling of the SR sensitizes and stimulates the periodic opening of RyR to generate Ca2+ waves by calcium-induced calcium release, and this explains why these Ca2+ oscillations are susceptible to inhibition by ryanodine. The failure of ryanodine to affect agonist- or IP3-induced Ca2+ oscillations also emphasizes that the Ca2+ transient associated with each Ca2+ oscillation does not activate RyR within the SR. The most likely reason for this is that the RyR remains insensitive to cytosolic Ca2+ when the Ca2+ content of the SR, which normally primes the RyR, is lowered by IP3-induced Ca2+ oscillations. Thus, the RyR has little role in histamine-induced Ca2+ oscillations in normal human airway SMCs.
Because Ca2+ sensitivity is a major determinate of airway contraction in mice and rat airways, we sought to characterize its role in human airway SMCs. By creating Ca2+-permeabilized SMCs by treatment with caffeine and ryanodine, a technique extensively used with mice and rats (9, 10), the contractile response can be separated into contraction induced by increasing [Ca2+]i and contraction induced by increases in Ca2+ sensitivity. As mentioned, caffeine activates the RyR, whereas ryanodine locks the RyR in the open state. The subsequent efflux of Ca2+ from the SR leads to activation of store-operated Ca2+ channels and a sustained Ca2+ influx from the extracellular space. This technique differs from previous methods with α toxin or β-escin in that the cell membrane is still intact. As a result, Ca2+ influx is limited, and it is likely that Ca2+ extrusion methods, such as the Ca2+ ATPase pump or Na+/Ca2+ exchanger, are still active. Consequently, the [Ca2+]i is not simply equal to [Ca2+]o. More accurately, the [Ca2+]i is stabilized when the opposite Ca2+ movements across the cell membrane reach a new equilibrium. This equilibrium is proportional to the [Ca2+]o, and was determined by measuring the F:F0 ratio. By progressively increasing the [Ca2+]o, the [Ca2+]i was increased (increased F:F0), and the human airway SMCs displayed increasing steps of sustained contraction. Most importantly, airway contraction was increased further by histamine, even though the [Ca2+]i was not altered. These data indicate that human airway SMCs also rely extensively on increases in Ca2+ sensitivity to mediate contraction. This has the important implication that inflammatory processes may lead to AHR by increasing the Ca2+ sensitivity of human SMCs.
With this ability to address Ca2+ signaling and Ca2+ sensitivity of human airway SMCs, we examined how the long-acting β2-agonist, formoterol (33), induced airway relaxation. The generally accepted mechanism of β2-agonist action has been a cAMP-induced reduction of the Ca2+ signaling driving MLCK activity (34). However, the specifics of this action vary with species and agonist. Isoproterenol appears to slow Ca2+ oscillations by inhibiting the open probability of the IP3R, whereas formoterol appears to act initially on Ca2+ sensitivity of mouse airways (35).
In human airways, low concentrations of formoterol induced a substantial relaxation of the airway, with relatively little effect on the frequency of the Ca2+ oscillations. Similar concentrations of formoterol relaxed Ca2+-permeablized human airways with sustained high [Ca2+]i. These results suggest this relaxation occurred by decreasing Ca2+ sensitivity, a response implying an increase in MLCP activity, and one supported by the increase of phosphate release from phosphorylated myosin of small airway extracts. Whether the effect of formoterol on MLCP activation is direct or indirect, possibly via activation of protein kinase A (PKA), remains to be determined.
At higher concentrations, formoterol additionally slowed or stopped Ca2+ oscillations. However, when the [IP3] was increased, the Ca2+ oscillations were briefly reinitiated. This response suggests that, although the IP3R remains functional, formoterol may have decreased the sensitivity of the IP3R to IP3 (15), the availability of IP3, or both. The transient induction of Ca2+ oscillations indicates that IP3 is rapidly metabolized. Similar results were found in mouse airways (35).
An important aspect of formoterol action was its longevity. Both the Ca2+ sensitivity and Ca2+ oscillations were affected for extended periods after formoterol removal. This implies either a high affinity of formoterol for the β2-receptor or, perhaps, a substantial partition of formoterol into the membrane of the airway SMCs (34). Interestingly, this longevity of action was not observed in mouse airways (35), implying that human β-receptors or membrane proteins are more susceptible to formoterol than are mouse receptors.
Although Ca2+ increases and Ca2+ sensitivity drive airway SMC contraction, these two processes have different sensitivities to Ca2+ dynamics of the SMC (i.e., Ca2+ oscillations versus steady-state Ca2+). Ca2+ sensitivity appears to be a Ca2+-independent process in human SMCs. Consequently, the dynamics of Ca2+ changes have little effect on Ca2+ sensitivity in human airways. The finding that step Ca2+ increases give rise to step increases in contraction implies an inherent level of Ca2+ sensitivity in the absence of agonist stimulation. This is not the case for mouse airway SMCs, where the Ca2+-sensitivity is down-regulated by increases in Ca2+ (9, 10). In mouse airway SMCs, a step increase in Ca2+ results in a transient contraction. This is consistent with a Ca2+-dependent increase in MLCK activity, followed by a slower Ca2+-dependent increase in MLCP activity that reverses the rMLC phosphorylation induced by MLCK. By contrast, MLCK activity is sensitive to the Ca2+ dynamics. Our mathematical modeling of mouse SMC responses (in which Ca2+ sensitivity can be clamped) predict that Ca2+ oscillations will induce a greater amount of contraction than the mean Ca2+ value of the oscillatory signal under steady-state conditions (36). The reason for this is that the peak Ca2+ of the oscillations has a disproportionate effect on activating the MLCK. In addition, the inactivation kinetics of MLCK are slow when Ca2+ is reduced. As a result, Ca2+ oscillations give rise to highly activated MLCK. Although an increase in the frequency of the Ca2+ oscillations experimentally correlates with increased contraction, our mathematical modeling indicates that a pure change in Ca2+ oscillation frequency, while retaining a constant average Ca2+, had little effect on contraction (36). The explanation for this appears to be that the shape of the Ca2+ oscillations also changes with frequency, thus average [Ca2+] can change, and this can contribute to the activation of the MLCK.
Although the range of responses of human airway SMCs reported here are qualitatively similar to rodent responses, many details of these responses are specific to human SMCs. The responses of human SMCs vary in a number of ways. At the agonist level, human airway SMCs respond to histamine, whereas mouse SMCs do not. The reverse is true for 5-HT. The fundamental signal of increased Ca2+ is represented by Ca2+ oscillations and, in mouse airways, these occur very quickly, whereas, in humans, the Ca2+ oscillations are slow and more similar to those observed in rat airways or mouse arterioles. With respect to the contractile mechanism of Ca2+ sensitivity, the human is again very different from mouse airways that have a Ca2+-dependent decrease in Ca2+ sensitivity. The sensitivity of rat airways is, like human airways, Ca2+ independent, but greater than that of human airways. With respect to formoterol-induced relaxation, humans are more sensitive than either rats or mice, and show substantially longer-lasting relaxation responses.
In summary, human lung slices are a valuable preparation with which to study airway SMC physiology. Human airway SMC contraction is dependent on both agonist-induced Ca2+ oscillations and increased Ca2+ sensitivity, and their interaction. Consequently, formoterol serves as a potent bronchodilator, because it reduces both these processes with a strong affinity in human airways. Finally, the differences in human airway responses, as compared with animal responses, emphasize the advantage of performing studies with human lung slices that mimic many in situ conditions.
Acknowledgments
The authors thank Prof. Reynold Panettieri, Dr. Philip Cooper, and William Jester from the University of Pennsylvania, Philadelphia, for help with obtaining human lung tissue.
This work was supported by National Institutes of Health grants HL071930 and HL087401 (M.J.S.) and HL073050 (M.I.).
Originally Published in Press as DOI: 10.1165/rcmb.2009-0222OC on September 18, 2009
Author Disclosure: M.J.S. received lecture fees from Sepracor Inc. of $1,001–$5,000, along with a basic research grant of more than $100,000, and a sponsored grant from National Institutes of Health (NIH) of more than $100,000. M.I. received a sponsored grant from NIH of more than $100,001. None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Martin JG, Duguet A, Eidelman DH. The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease. Eur Respir J 2000;16:349–354. [DOI] [PubMed] [Google Scholar]
- 2.Kamm KE, Stull JT. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 1985;25:593–620. [DOI] [PubMed] [Google Scholar]
- 3.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 2003;83:1325–1358. [DOI] [PubMed] [Google Scholar]
- 4.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 2000;15:600–616. [DOI] [PubMed] [Google Scholar]
- 5.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol 2005;125:535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergner A, Sanderson MJ. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am J Physiol Lung Cell Mol Physiol 2002;283:L1271–L1279. [DOI] [PubMed] [Google Scholar]
- 7.Bergner A, Sanderson MJ. Acetylcholine-induced calcium signaling and contraction of airway smooth muscle cells in lung slices. J Gen Physiol 2002;119:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Zoghbi JF, Sanderson MJ. Endothelin-induced contraction of bronchiole and pulmonary arteriole smooth muscle cells is regulated by intracellular Ca2+ oscillations and Ca2+ sensitization. Am J Physiol Lung Cell Mol Physiol 2007;293:L1000–L1011. [DOI] [PubMed] [Google Scholar]
- 9.Bai Y, Sanderson MJ. The contributions of Ca2+ signaling and Ca2+ sensitivity to the regulation of airway smooth muscle contraction is different in rats and mice. Am J Physiol Lung Cell Mol Physiol 2009;296:L947–L958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol 2006;291:L208–L221. [DOI] [PubMed] [Google Scholar]
- 11.Wohlsen A, Martin C, Vollmer E, Branscheid D, Magnussen H, Becker WM, Lepp U, Uhlig S. The early allergic response in small airways of human precision-cut lung slices. Eur Respir J 2003;21:1024–1032. [DOI] [PubMed] [Google Scholar]
- 12.Ressmeyer AR, Larsson AK, Vollmer E, Dahlen SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J 2006;28:603–611. [DOI] [PubMed] [Google Scholar]
- 13.Cooper PR, Panettieri RA Jr. Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol 2008;122:734–740. [DOI] [PubMed] [Google Scholar]
- 14.Sturton RG, Trifilieff A, Nicholson AG, Barnes PJ. Pharmacological characterization of indacaterol, a novel once daily inhaled 2 adrenoceptor agonist, on small airways in human and rat precision-cut lung slices. J Pharmacol Exp Ther 2008;324:270–275. [DOI] [PubMed] [Google Scholar]
- 15.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir Res 2006;7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leybaert L, Sanderson MJ. Intercellular calcium signaling and flash photolysis of caged compounds: a sensitive method to evaluate gap junctional coupling. Methods Mol Biol 2001;154:407–430. [DOI] [PubMed] [Google Scholar]
- 17.Koga Y, Ikebe M. p116Rip decreases myosin II phosphorylation by activating myosin light chain phosphatase and by inactivating RhoA. J Biol Chem 2005;280:4983–4991. [DOI] [PubMed] [Google Scholar]
- 18.Gould JM, Cather R, Winget GD. Advantages of the use of Cerenkov counting for determination of P32 in photophosphorylation research. Anal Biochem 1972;50:540–548. [DOI] [PubMed] [Google Scholar]
- 19.Pearson RB, Jakes R, John M, Kendrick-Jones J, Kemp BE. Phosphorylation site sequence of smooth muscle myosin light chain (Mr = 20 000). FEBS Lett 1984;168:108–112. [DOI] [PubMed] [Google Scholar]
- 20.Delmotte P, Sanderson MJ. Effects of albuterol isomers on the contraction and Ca2+ signaling of small airways in mouse lung slices. Am J Respir Cell Mol Biol 2008;38:524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper PR, McParland BE, Mitchell HW, Noble PB, Politi AZ, Ressmeyer AR, West AR. Airway mechanics and methods used to visualize smooth muscle dynamics in vitro. Pulm Pharmacol Ther 2009;22:398–406. [DOI] [PubMed] [Google Scholar]
- 22.Sanderson MJ, Delmotte P, Bai Y, Perez-Zogbhi JF. Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc Am Thorac Soc 2008;5:23–31. [DOI] [PubMed] [Google Scholar]
- 23.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 1994;372:231–236. [DOI] [PubMed] [Google Scholar]
- 24.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003;4:517–529. [DOI] [PubMed] [Google Scholar]
- 25.Prakash YS, Kannan MS, Sieck GC. Regulation of intracellular calcium oscillations in porcine tracheal smooth muscle cells. Am J Physiol 1997;272:C966–C975. [DOI] [PubMed] [Google Scholar]
- 26.Dai JM, Kuo KH, Leo JM, Pare PD, van Breemen C, Lee CH. Acetylcholine-induced asynchronous calcium waves in intact human bronchial muscle bundle. Am J Respir Cell Mol Biol 2007;36:600–608. [DOI] [PubMed] [Google Scholar]
- 27.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol 2007;36:122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol 1999;126:1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol 1997;272:L659–L664. [DOI] [PubMed] [Google Scholar]
- 30.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose–mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J 2003;17:452–454. [DOI] [PubMed] [Google Scholar]
- 31.Deshpande DA, White TA, Dogan S, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2005;288:L773–L788. [DOI] [PubMed] [Google Scholar]
- 32.Bai Y, Edelmann M, Sanderson MJ. The contribution of inositol 1,4,5-trisphosphate and ryanodine receptors to agonist-induced Ca2+ signaling of airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2009;297:L347–L361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derom EY, Pauwels RA. Time course of bronchodilating effect of inhaled formoterol, a potent and long acting sympathomimetic. Thorax 1992;47:30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kips JC, Pauwels RA. Long-acting inhaled beta(2)-agonist therapy in asthma. Am J Respir Crit Care Med 2001;164:923–932. [DOI] [PubMed] [Google Scholar]
- 35.Delmotte P, Sanderson MJ. Effects of formoterol on contraction and Ca2+ signaling of mouse airway smooth muscle cells. Am J Respir Cell Mol Biol 2010;42:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang I, Politi AZ, Tania N, Bai Y, Sanderson MJ, Sneyd J. A mathematical model of airway and pulmonary arteriole smooth muscle. Biophys J 2008;94:2053–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]