Abstract
Heterologous viruses may transactivate or suppress human immunodeficiency virus (HIV)–1 replication. An adenovirus type 5 gene transfer vector (Ad5) HIV-1 vaccine was recently evaluated in a clinical trial, without efficacy. In this context, it is relevant to ask what effect Ad vectors have on HIV-1 replication, particularly in cells that are part of the innate immune system. Infection of HIV-1–infected human alveolar macrophages (AMs) obtained from HIV-1+ individuals with an Ad vector containing no transgene (AdNull) resulted in dose-responsive inhibition of endogenous HIV-1 replication. HIV-1 replication in normal AMs infected with HIV-1 in vitro was inhibited by AdNull with a similar dose response. Ad reduced AM HIV-1 replication up to 14 days after HIV-1 infection. Fully HIV-1–infected AMs were treated with 3′-azido-3′-deoxythymidine, after which Ad infection still inhibited HIV-1 replication, suggesting a postentry step was affected. Substantial HIV-1 DNA was still produced after Ad infection, as quantified by TaqMan real-time PCR, suggesting that the replication block occurred after reverse transcription. AdNull blocked HIV-1 long terminal repeat (LTR) transcription, as assessed by an vesicular stomatitis virus G protein pseudotyped HIV-1 LTR luciferase construct. The formation of HIV-1 DNA integrated into the host chromosome was not inhibited by Ad, as quantified by a two-step TaqMan real-time PCR assay, implying a postintegration block to HIV-1 replication. These data indicate that Ad vectors are inhibitory to HIV-1 replication in human AMs based, in part, on their ability to inhibit LTR-driven transcription.
Keywords: adenovirus, HIV-1 replication, human alveolar macrophage
CLINICAL RELEVANCE.
A recent adenovirus type 5 gene transfer vector (Ad5)-based human immunodeficiency virus (HIV)–1 vaccine trial was stopped due to a higher incidence of HIV-1 infection. It is timely to ask what effect Ad vectors have on HIV-1 replication in cells that support active replication of HIV-1 such as human alveolar macrophages.
Alveolar macrophages (AMs) are important reservoirs of human immunodeficiency virus (HIV)–1 infection in the lung (1, 2). In spite of highly active antiretroviral therapy (HAART) suppression of HIV-1 replication in T cells, various reservoirs, including AMs, dendritic cells, and other monocyte-derived tissue macrophages continue to harbor the virus (3, 4). We have previously shown that if AMs are infected with an E1−E3− adenovirus (Ad) gene transfer vector before in vitro HIV-1 infection, subsequent replication of HIV-1 by the AM is markedly suppressed independently of the transgene in the Ad vector (5). In the context that heterologous viruses have disparate effects on HIV-1 replication, and clinical trials are now underway with another HIV-1 vaccine based on a type 5 Ad gene transfer vector, the focus of this study is to further characterize the effect of Ad vectors on HIV-1 replication in human AMs with regard to the HIV-1 life cycle (6–18). Specifically, we assessed the effect of Ad vectors on HIV-1 replication in AMs obtained from HIV-1+ individuals, established the relevance of the model of HIV-1 infection of normal AMs in vitro with respect to the effects of Ad on HIV-1 replication, and characterized the step(s) in the HIV-1 life cycle during which AdNull suppresses HIV-1 replication. The data demonstrate that: (1) HIV-1 replication by endogenously HIV-1–infected AMs is blocked by Ad; (2) in vitro HIV-1 infection of normal AMs in vitro is a valid model with which to study the effects of Ad on HIV-1 replication; (3) the major effect of AdNull in causing inhibition of HIV-1 replication is at a step subsequent to reverse transcription and integration of the HIV-1 DNA into the human chromosome; (4) the effect of AdNull inhibiting HIV-1 replication is as a result of suppression of long terminal repeat (LTR)-dependent transcription; and (5) the suppression of LTR-dependent transcription by AdNull is due, in part, to activation of intracellular IFN-related signaling via phospho–signal transducer and activator of transcription (STAT)–1 and IFN regulatory factor (IRF)–8.
Some of the results of these studies have been previously reported in the form of an abstract (19–23).
MATERIALS AND METHODS
Cells
Human AMs were obtained by bronchoalveolar lavage (BAL) from healthy volunteers, as previously described, after obtaining written informed consent under a protocol approved by the Committee for Human Rights in Research of the Weill Medical College of Cornell University (24, 25). AMs were obtained from HIV-1+ individuals with lung infections who were not receiving highly active antiretroviral therapy because they were previously unaware of their HIV-1 status, noncompliant, or refused therapy. The AMs were purified from BAL fluid obtained from diagnostic bronchoscopies when the fluid recovered was in excess of that required for diagnostic studies under a separately approved protocol. AMs from HIV-1+ individuals were used for this study if spontaneous p24 elaboration was detectable. The lavage fluid was filtered through gauze to remove debris. The cells were pelleted, washed with PBS (pH 7.4), and resuspended in RPMI 1,640 medium containing 10% FBS, 2 mM glutamine, 100 U of penicillin/ml, and 10 μg/ml of streptomycin (GIBCO BRL, Gaithersburg, MD) at a final concentration of 2 × 105 AMs/ml. AMs were purified by adherence to plastic (2 h, 37°C) on a 24-well culture plate (COSTAR, Cambridge, MA). A 1-ml aliquot of the cell suspension (2 × 105 cells) was added per well. HeLa-CD4+/CCR5+ cells were infected with a strain of HIV-1 isolated from the frontal lobe of subject JR who died of AIDS dementia (JRFL-1) for 7 days and cultured in Dulbecco's modified Eagle medium with 10%FBS, 1% penicillin/streptomycin, and 0.1% Fungizone.
AdNull Vector
The AdNull vector is an E1a−, partial E1b−, partial E3− based on the Ad5 backbone (26, 27). The AdNull vector contains the cytomegalovirus immediate-early promoter/enhancer in the expression cassette in the E1 position, but no transgene. Empty Ad capsids were isolated as a separate band during ultracentrifugation by CsCl density gradient purification of the AdNull preparation.
Endogenous HIV-1 Replication by Human AMs In Vitro
AMs obtained from HIV-1+ individuals undergoing diagnostic bronchoscopy were purified by adherence, as described subsequently here. The following organisms were isolated from these bronchoscopies (n = 5 individuals): Pneumocystis jirovecii (n = 2); Mycobacterium avium intracellulare with methicillin-resistant Staphylococcus aureus (n = 1); Aspergillus fumigatus (n = 1); Rhizopus and Candida (n = 1). None of the individuals had viral pneumonia. After washing, the media were replaced with fresh media, and 50-μl aliquots were removed and replaced with 50 μl of fresh media to maintain constant volume in the well each time the media were assayed for viral replication (see below).
Ad Infection of Normal AMs
At 24 hours after plating AMs obtained from normal individuals, the cells were washed twice with medium (RPMI 1,640 medium containing 2% FBS, 2 mM glutamine, 100 U penicillin/ml, and 10 μg/ml streptomycin [GIBCO BRL]) to remove red blood cells and other nonadherent cells. Infections were based on particle units (pu) of Ad (28). AMs were infected with Ad at doses of 102 to 2.5 × 104 pu/cell or empty Ad capsids 2.5 × 104 pu/cell. Dilutions of thawed Ad stock were added to wells with a minimal volume of media (250 μl/well) to maximize contact of particles with AMs. Infections were initiated by placing the plates on a rocker for 1.5 hour at 37°C. The cells were then washed three times with culture medium to remove any residual Ad, and replaced in the incubator with 1 ml culture medium per well.
HIV-1 Infection of Normal AMs
At 2 or 72 hours after Ad infection, the normal AMs (2 × 105/well) were infected with the HIV-1 laboratory strain, JRFL, at 103 median tissue culture infectious doses (TCID50) per well, a dose known to infect cells of monocytic origin (29, 30). For some experiments, HIV-1 infection was performed before Ad infection, as indicated. A 10-μg/ml aliquot of 3′-azido-3′-deoxythymidine (AZT; Sigma, St. Louis, MO) was added daily to some wells as a control to inhibit HIV-1 replication. For this experiment, the media were left unchanged until 12 days after the Ad infection. After 12 days of incubation at 37°C, the cells were washed, and 1 ml fresh culture media was added.
Quantification of HIV-1 Replication
Productive HIV-1 infection for both JRFL and endogenous HIV-1 was determined by quantifying p24 antigen in the media supernatant by ELISA (Beckman Coulter, Miami, FL) at 0–28 days after HIV-1 JRFL infection. The effects of Ad infection of AMs on subsequent HIV-1 infection and replication were compared on Days 1–38.
Assessment of HIV-1 LTR Transcription
Vesicular stomatitis virus G protein (VSV-G)–1 is an HIV-1 VSV-G pseudotyped virus containing the firefly luciferase gene cloned downstream of the full-length HIV-1 LTR promoter (31, 32). AMs were plated at 2 × 105 and subsequently infected with AdNull at 2.5 × 104 pu/cell or left uninfected. After 72 hours, 150 μl of the VSV-G-1 preparation was added to the AM media. Alternatively, cells were infected with VSV-G-1 first, followed by subsequent AdNull infection after an additional 4 hours. After 72 hours, the media were removed, AMs were lysed with cell lysis buffer (Promega, Madison, WI), and luciferase activity was quantified by luminometry, and normalized to protein concentration, as determined by the bicinchoninic acid method (Pierce, Rockford, IL). Additional experiments were performed with HIV-1/LTR-Luc, a nonpseudotyped fully HIV-1 envelope virus containing the luciferase reporter downstream of the HIV-1 LTR promotor, derived from the pNL4–3 plasmid.
TaqMan Real-Time PCR Analysis of HIV-1 DNA Synthesis in AMs
The HIV-1 inoculum was pretreated with RNase-Free DNAase (Qiagen, Valencia, CA) for 1 hour at 37°C to degrade any HIV-1 DNA present in the viral preparation (33). AMs were infected with HIV-1 JRFL, as described previously here, and DNA was extracted from the AMs with the Dneasy Tissue Kit (Qiagen, Valencia, CA). Triplicate PCR reactions (50 μl each) were established with 5 μl of both 1:10 and 1:100 dilutions of the DNA in water. Primers and probes were developed to quantify the JRFL envelope gene. Amplification reactions were prepared with reagents from Perkin Elmer (Foster City, CA) and contained 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 10 μm ethylenediaminetetraacetate acid, 60 nM 5-carboxyrhodamine, 3 mM MgCl2, 200 nM each of dCTP, dGTP, and dATP, 400 nM dUTP, 0.5 U urical N-glycosylase, 0.25 U AmpliTaq Gold DNA polymerase, 200 nM forward primer (5′-TCCTTTGAGCCAATTCCCAT), 200 nM reverse primer (5′-TCCATTGAACGTCTTATCATTACACTT), and 100 nM TaqMan central probe (5′-ATTATTGTGCCCCGGCTGGTTTTGC). The TaqMan probe was labeled with the reporter group, FAM (6-carboxy fluorescein) on the 5′ end, and the quencher, 6-carboxy-N,N,N,N-tetramethylrhodamine on the 3′ end. The primer and probe combination was designed with the PrimerExpress software (Perkin Elmer). Samples (standard or unknown) were amplified for 40 cycles in a Perkin Elmer 7700 sequence detection system after 10 minutes at 50°C for urical N-glycosylase to degrade carryover contamination, and 10 minutes at 94°C to activate the AmpliTaq Gold. Cycling conditions were 15 seconds at 94°C, followed by 1 minute at 60°C with continuous monitoring of the fluorescence. Data were processed with SDS 1.6 software (Perkin Elmer) to generate standard curves and to determine the concentration of target in the unknowns by interpolation. A 25% difference in the vector amount adjusted for dilution in the 1:10 and 1:100 dilutions of the DNA was considered acceptable. Total DNA concentration was determined by measuring the A260 of dilutions of the DNA stocks.
TaqMan Real-Time PCR Analysis of Chromosomally Integrated HIV-1 DNA in AMs
The HIV-1 inoculum was pretreated with RNase-Free DNAase (Qiagen, Valencia, CA) for 1 hour at 37°C to degrade any HIV-1 DNA present in the viral preparation (33). For an integrated HIV-1 standard, HeLa-CD4+/CCR5+ cells were infected with JRFL. After 7 days of infection at 37°C, cells were trypsinized and DNA was purified with the Dneasy Tissue Kit (Qiagen, Valencia, CA). AMs were infected with HIV-1 JRFL, as described previously here, and DNA was extracted from the AMs after 72 hours with the Dneasy Tissue Kit (Qiagen). A two-step nested amplification was performed to initially amplify sequences exclusively containing only human 300–base pair repetitive DNA sequences (Alu) adjacent to HIV-1 envelope, based on a recently published method (34). Primers and probes were developed to quantify human Alu sequences and the JRFL envelope gene with the PrimerExpress software (Perkin Elmer). During the first-round PCR, integrated HIV-1 sequences in the samples (standard or unknown) were amplified with a JRFL-specific primer JRFL-R1: ctcgatgtcagcagtccttgtagtac) and Alu-targeting primers (Alu-f1 gcactttgggaggccgaggcg and Alu-r1 acagagcgagactccgtctcaaaaa) that annealed to conserved regions of the Alu repeat element. During the first-round PCR, Alu-JRFL sequences were amplified from 2.5 μl of purified cellular DNA in a 25-μl reaction mixture comprised of 1× TaqMan Universal PCR Master Mix (Applied Biosystems), 10 μM primers, and DEPC-Treated Water (Ambion). The first-round PCR cycle conditions were as follows: An initial denaturing of 95°C for 10 minutes, followed by 35 repetition cycles of: 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 3 minutes.
The second-round TaqMan PCR was performed with 2.5 μl of the first-round PCR product in a mixture comprised of: 1× TaqMan Universal PCR Master Mix (Applied Biosystems), 10 μM primers Inner JRFL-L2:gctacttccctgattggcagaa, Inner JRFL-R2:gaagcaccatccaaaggtcaa, and 10 μM probe and DEPC-Treated Water (Ambion). The second-round PCR cycle began with a DNA-denaturing and polymerase-activation step (95°C for 10 min), followed by 45 cycles of amplification (95°C for 15 s, 60°C for 60 s) with an ABI 7,700 sequence detector system (Applied Biosystems). The TaqMan probe, cacaccagggccaggaatcagatttc, internal to the sequence spanning the Inner JRFL-L2 and Inner JRFL-R2, was labeled with the reporter group, FAM (6-carboxy fluorescein), on the 5′ end and the quencher, 6-carboxy-N,N,N,N-tetramethylrhodamine, on the 3′ end. Data were processed by the SDS 1.6 software (Perkin Elmer) to generate standard curves based on serial 10-fold dilutions of DNA from JRFL-infected JC-48 cells, and to determine the concentration of target in the unknowns by interpolation.
Western Analysis
To assess intracellular levels of phosphorylated STAT-1 and IRF-1, NuPAGE 4–12% Bis-Tris Gel (Invitrogen, Carlsbad, CA) were used for electrophoresis, with equal amounts of AM cell lysate protein, 15 μg/lane added to each lane. Proteins were transferred to polyvinylidene flouride (PVDF) membrane (0.45-μm pore size; Invitrogen), washed, and blocked with 5% nonfat milk for 1 hour. The membrane was incubated with anti–phospho–STAT-1 rabbit immunoaffinity-purified IgG (Upstate, Lake Placid, NY) or anti–IRF-8 mouse monoclonal primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) 1:500–1:750 in 5% nonfat milk (16 h, 4°C). After washing and reblocking, the secondary horseradish peroxidase–labeled goat anti-mouse or anti-rabbit antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) 1:8,000–1:10,000 was applied (2 h, 23°C). Detection was performed with enhanced chemiluminescence Western analysis detection reagents (Amersham, Piscataway, NJ) and used BioMax Light Film (Kodak, Rochester, NY).
Statistical Analysis
Data are expressed as means (±SEM). Treatment group means were compared by ANOVA. A P value less than 0.05 was considered statistically significant. The Newman-Keuls post hoc test was performed when the ANOVA indicated significance. Statistics were compared with the Number Cruncher Statistical System software (NCSS, Kaysville, UT).
RESULTS
Effect of Ad on Endogenous HIV-1 Replication by Human AMs
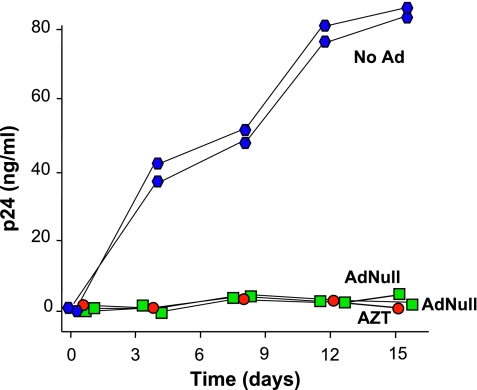
Although cultured AMs obtained from most HIV-1+ individuals exhibit no spontaneous HIV-1 replication (35), we were able to identify several individuals who, for various reasons, were not on HAART, whose CD4+ cell counts were less than 200 × 106/L, and whose AMs spontaneously replicated HIV-1 in the setting of acute lung infection. In the absence of Ad, spontaneous HIV-1 replication was observed as the level of p24 increased steadily over the first 12 days of culture in vitro (Figure 1). The HIV-1 replication was suppressed by 10 μg/ml AZT added daily to the culture media. Infection with AdNull (2.5 × 104 pu/cell) produced nearly complete inhibition of HIV-1 replication comparable to the effect of AZT (P < 0.05, ANOVA). Similar results were observed with AMs from four other HIV-1+ individuals (data not shown).
Figure 1.
Effect of an adenovirus vector with no transgene (AdNull) on endogenous human immunodeficiency virus (HIV)–1 replication by human alveolar macrophages (AMs). AMs were isolated from an HIV-1+ individual not on treatment with highly active antiretroviral therapy (HAART). The AMs (2 × 105/well) were infected with AdNull 2.5 × 104 particle units (pu)/cell, treated daily with 10 μg/ml 3′-azido-3′-deoxythymidine (AZT), or left untreated. Aliquots of media were collected every 4 days and assayed for HIV-1 p24 protein to quantify viral replication. Each symbol represents the measurement from a single well. Ordinate, p24 (ng/ml); abscissa, time (d). The results were similar in AMs obtained from five different individuals.
Dose–Response Effect of Ad Vectors on HIV-1 Replication in Endogenously Infected Human AMs
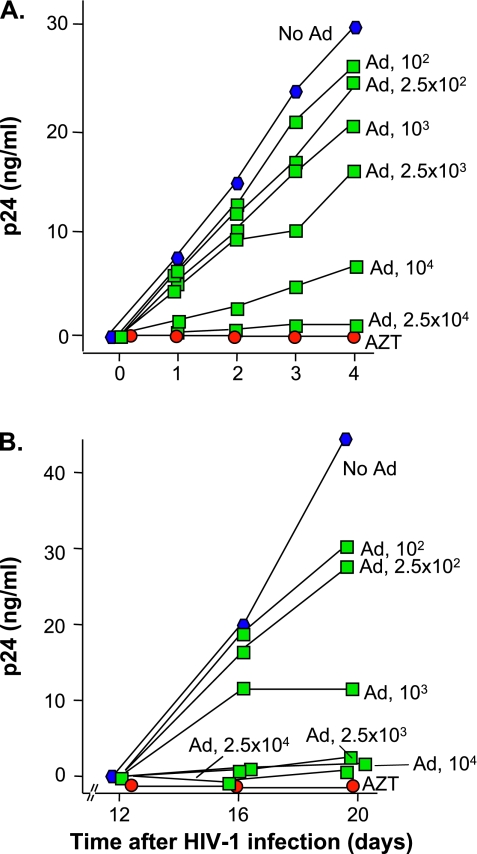
AMs were isolated from BAL fluid of an HIV-1+ individual (different from Figure 1) not on HAART in the setting of acute lung infection. In the absence of Ad, spontaneous HIV-1 replication was observed as the level of p24 increased linearly over the first 4 days of in vitro culture. The HIV-1 replication was suppressed by AZT added daily to the culture media (Figure 2A). Infection with AdNull (2.5 × 104 pu/cell) produced substantial inhibition of HIV-1 replication. This inhibition was dose dependent over a range of input Ad of 102–2.5 × 104 pu/cell. For Day 4, the correlation between percent inhibition versus log[AdNull] was significant (r2 = 0.96). These data indicate that infection with Ad vectors, in the absence of any transgene, block HIV-1 replication in endogenously infected AMs in a dose-responsive fashion.
Figure 2.
Dose- and time-dependent effect of AdNull on endogenous and laboratory-infected HIV-1 replication by human AMs. (A) Time dependency of HIV-1 replication in naive and AdNull-treated AMs. AMs were isolated from an HIV-1+ individual not on treatment with HAART. The AMs (2 × 105/well) were infected with AdNull 102 - 2.5 × 104 pu/cell or treated daily with AZT, 10 μg/ml or left untreated. Aliquots of media were collected and assayed for HIV-1 p24 protein to quantify viral replication. Each symbol represents the measurement from a single well. Ordinate, p24 (ng/ml); abscissa, time (d). The results were similar in AMs obtained from five different individuals. (B) Effect of time after HIV-1 infection on HIV-1 replication with laboratory infection of AMs with HIV-1. AMs were isolated from a normal volunteer. The AMs (2 × 105/well) were infected with 102 to 2.5 × 104 pu/cell AdNull, treated daily with 10 μg/ml AZT, or left untreated. After 2 hours, all of the wells were washed and infected with an HIV-1 strain isolated from the frontal lobe of subject JR who died of AIDS demetia (JRFL) 103 median tissue culture infectious dose (TCID50)/well. The media were replaced at Day 12. Aliquots of media were collected at Days 12, 16, and 20, and assayed for HIV-1 p24 protein to quantify viral replication. Ordinate, p24 (ng/ml); abscissa, time (d). The results were similar in AMs obtained from eight different individuals.
Effect of Ad Vectors on HIV-1 Replication in Normal Human AMs Infected with HIV-1 In Vitro
To model the effect of Ad on HIV-1 replication, normal AMs were infected with AdNull for 2 hours in vitro. After washing the AMs, they were infected with HIV-1 103 TCID50/well. The media were changed after 12 days, and p24 levels were monitored daily thereafter. To assess the fidelity of the in vitro HIV-1 infection of AMs as a model for endogenously infected AMs, the dose–response curves for Ad inhibition of endogenous HIV-1 replication in human AMs were compared with in vitro infection. The results indicate that, in the absence of Ad, robust HIV-1 replication was obtained in AMs under these conditions (Figure 2B). The HIV-1 replication was suppressed by AZT. Infection with AdNull (102–2.5 × 104 pu/cell) produced a dose-dependent inhibition of HIV-1 replication. These data demonstrate that the in vitro HIV-1 infection of normal AMs is a reasonable model for the effect of Ad on endogenously HIV-1–infected AMs.
Effect of Ad Vectors on HIV-1 Replication when AMs are First Infected with HIV-1
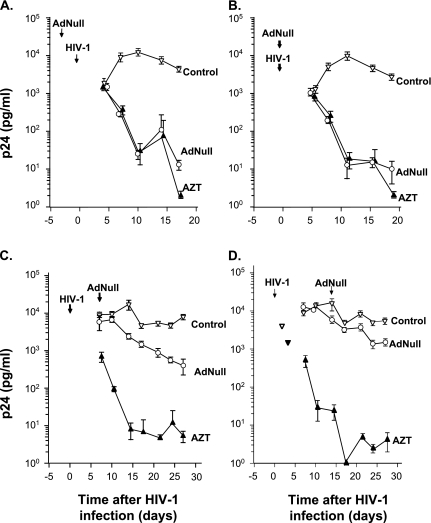
To begin to assess the steps in the HIV-1 life cycle where Ad exerts an inhibitory effect, we evaluated the ability of Ad vectors to inhibit HIV-1 replication after HIV-1 infection had already occurred. We compared four different scenarios for infection: (1) Ad infection followed by HIV-1 infection 3 days later; (2) Ad infection followed by HIV-1 infection 2 hours later; (3) HIV-1 infection followed by Ad infection 7 days later; and (4) HIV-1 infection followed by Ad infection 14 days later. Consistent with the previous results, Ad significantly reduced the levels of p24 when the AMs were infected 3 days or 2 hours before HIV-1 infections (Figures 3A and 3B). To a lesser degree, p24 production was reduced even when the AMs were infected with Ad 7 or 14 days after HIV-1 infection (Figures 3C and 3D). These data suggest that Ad could be affecting a step in the HIV-1 life cycle after HIV-1 cellular entry, but are not definitive.
Figure 3.
Effect of timing of Ad infection on HIV-1 replication by human AMs infected in vitro with HIV-1. AMs were isolated from a normal volunteer. The AMs (2 × 105/well) were infected with 102 to 2.5 × 104 pu/cell AdNull at Days −3 (before HIV-1 infection), 0, 7, or 14 after HIV-1 infection, treated daily 10 μg/ml with AZT, or left untreated. (A) Ad infection 3 days before HIV-1 infection. (B) Ad infection 2 hours before HIV-1 infection. (C) Ad infection 7 days after HIV-1 infection. (D) Ad infection 14 days after HIV-1 infection. Ordinate, p24 (pg/ml); abscissa, time (d). The results were similar in AMs obtained from three different individuals.
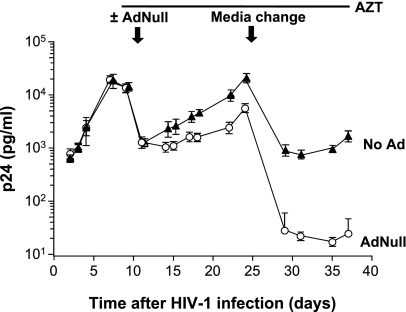
Ad Inhibits HIV-1 Replication by AMs under Conditions where Reverse Transcription Is Blocked
To establish that Ad is acting at a post–viral entry step, AMs were infected with HIV-1 in vitro and cultured for an additional 9 days to allow a near-maximal number of HIV-1–infected AMs. AZT was then added to all wells and maintained in the culture media to prevent any productive infection by blocking reverse transcription in previously uninfected cells. After an additional 2 days, some wells were infected with Ad (2.5 × 104 pu/cell). The media were changed at Day 14, and levels of p24 were measured to quantify HIV-1 replication. Under these conditions, all HIV-1 replication must occur from cells that had been previously infected, and the HIV-1 RNA reverse transcribed to DNA. In the absence of Ad, robust p24 production occurred (Figure 4). With Ad infection, the amount of p24 was reduced, even though HIV-1 was prevented from productively infecting new cells. The difference in p24 production between the Ad-infected and uninfected AMs continuously increased, up to nearly a 100-fold reduction by Day 45 (overall ANOVA, P < 0.01). These data indicate that Ad is acting at least in part at a post–reverse transcription step to inhibit HIV-1 replication.
Figure 4.
Effect of Ad on HIV-1 replication by human AMs under conditions where reverse transcription is blocked. AMs were isolated from a normal volunteer. The AMs (2 × 105/well) were infected with HIV-1 JRFL 103 TCID50/well. At Day 12, the cells were treated with 10 μg/ml AZT. After an additional 4 days, the cells were infected with 2.5 × 104 pu/cell AdNull or left uninfected. AZT was added daily. Aliquots of media were collected and assayed for p24 protein, but the media were not changed until 14 days later, as indicated. Ordinate, p24 (pg/ml); abscissa, time (d). The results were similar in AMs obtained from three different individuals.
Effect of Ad on the Formation of HIV-1–Specific DNA in AMs
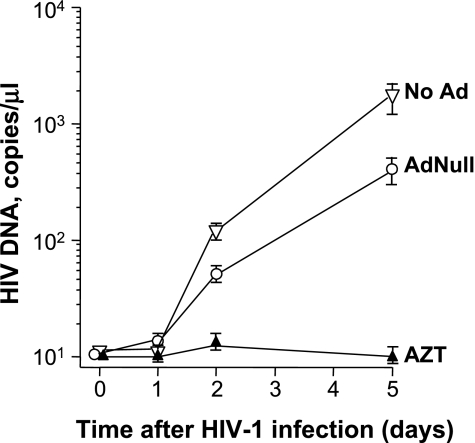
To confirm the effect of Ad on reverse transcription of HIV-1 RNA to DNA, AMs were infected with AdNull (2.5 × 104 pu/cell), treated with AZT, or left untreated. After 72 hours, the AMs were infected with HIV-1 JRFL (103 TCID50/well). DNA was then isolated at 0, 24, 48, and 120 hours, and HIV-1 DNA was quantified by TaqMan real-time PCR. On Days 2 and 5, untreated cells accumulated significant amounts of HIV-1 DNA, which was inhibited by AZT (P < 0.05, ANOVA; Figure 5). Although there was a numerical reduction in the number of HIV-1 DNA copies per cell, Ad-infected AMs still accumulated significant amounts of HIV-1 DNA. These data indicate that the block to HIV-1 replication by Ad is not at the level of reverse transcription from HIV-1 RNA to DNA, suggesting that the suppressive effect of AdNull occurs at a later stage of the HIV-1 life cycle.
Figure 5.
Effect of Ad on the transcription of HIV-1–specific DNA in AMs. To assess the effect of Ad on reverse transcription of HIV-1 RNA to DNA, AMs were infected with 2.5 × 104 pu/cell AdNull, treated with AZT, or left untreated. After 72 hours, the AMs were infected with HIV-1 JRFL, 103 TCID50/well. DNA was then isolated at 0, 24, 48, and 120 hours, and HIV-1 DNA was quantified by TaqMan real-time PCR. Ordinate, HIV-1 DNA copies/μl; abscissa, time (d). The results were similar in AMs obtained from three different individuals.
Effect of Ad on HIV-1 LTR Transcription in AMs
To determine whether AdNull suppresses HIV-1 replication via modulation of LTR-dependent transcription, a VSV-G pseudotyped HIV-1 LTR-luciferase construct (VSV-G-1) was used (31, 32). AMs were infected with AdNull 2.5 × 104 pu/cell for 2 hours, followed by washing and addition of fresh media, or were left uninfected. After an additional 3 days, the AMs were infected with VSV-G-1. In some wells, the cells were infected with VSV-G-1 first, followed by AdNull infection 4 hours later. At 72 hours later, the AMs were harvested, and luciferase expression was measured. The LTR expression, as quantified by luciferase expression, decreased from 3 × 104 relative light units (RLU)/mg to 3 × 103 RLU/mg (P < 0.05, ANOVA; Table 1). This represents a greater than 1 log inhibition of LTR transcription. Moreover, the inhibition was similar if the Ad infection occurred 4 hours after VSV-G-1 infection, at a time when all of the VSV-G-1 was inside the cell, eliminating the possibility that Ad had blocked VSV-G-1 entry (P < 0.05, ANOVA; Table 1; [36]). To further ascertain that the VSV-G pseudotyped virus was not merely interfering with HIV-1 entry into the cell by overwhelming the endocytotic pathway, the experiment was repeated with an HIV-1 LTR-luciferase constructed on a purely HIV-1 envelope, with similar results (P < 0.05, ANOVA; Table 1). These data indicate that Ad infection of AMs suppresses transcription from the HIV-1 LTR.
TABLE 1.
EFFECT OF AD ON HUMAN IMMUNODEFICIENCY VIRUS–1 LTR TRANSCRIPTION IN HUMAN ALVEOLAR MACROPHAGES
| Luciferase Activity (RLU/mg) | |
|---|---|
| VSV-G/HIV-1/LTR-Luc | 3,940 ± 1,640 |
| VSV-G/HIV-1/LTR-Luc + AdNull | 365 ± 164 |
| VSV-G/HIV-1/LTR-Luc + AdNull (4 h after HIV-1) | 231 ± 138 |
| Background | <10 |
| HIV-1/LTR-Luc | 1,307 ± 131 |
| HIV-1/LTR-Luc + AdNull | 76 ± 54 |
Definition of abbreviations: Ad, adenovirus vector; HIV, human immunodeficiency virus; LTR, long terminal repeat; Luc, luciferase; RLU, relative light units; VSV-G, vesicular stomatitis virus G protein.
AM were isolated from a normal volunteer. For the first two conditions, the alveolar macrophages (AMs) (2 × 105/well) were infected with AdNull 2.5 × 104 pu/cell or left uninfected. After 72 hours, the cells were infected with a VSV-G pseudotyped HIV-1 LTR-luciferase construct (VSV-G/HIV-1/LTR-Luc). As a third condition, the cells were infected with VSV-G/HIV-1/LTR-Luc first, followed by AdNull 4 hours later. After an additional 72 hours, cell lysates were collected and luciferase activity and protein were quantified. Data are presented as means (±SD) for triplicate detection. In a separate experiment, the AMs (2 × 105/well) were infected with AdNull 2.5 × 104 pu/cell or left uninfected. After 1 hour, the cells were infected with a nonpseudotyped HIV-1 LTR-luciferase construct (HIV-1/LTR-Luc). After an additional 72 hours, cell lysates were collected and luciferase activity and protein were quantified. This experiment was repeated with AMs from five separate volunteers with similar results.
Effect of Ad on the Formation of Integrated HIV-1 DNA in AMs
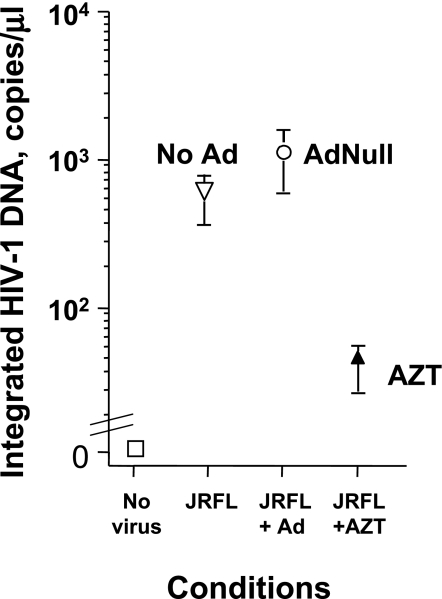
To quantify the effect of Ad on incorporation of HIV-1 DNA into the host chromosome, AMs were infected with AdNull (2.5 × 104 pu/cell), treated with AZT, or left untreated. After 1 hour, the AMs were infected with HIV-1 JRFL (103 TCID50/well). DNA was then isolated at 72 hours and HIV-1 DNA was quantified by a two-step nested TaqMan real-time PCR. In the first step, template DNA was amplified with primers designed to amplify HIV-1 DNA exclusively adjacent to human Alu sequences. In the second step, HIV-1 DNA was amplified and detected with primers, and a probe within the predicted sequence of the first HIV-1/human Alu primer sequence was quantified by TaqMan. JC-48, a HeLa cell line transfected with CD4 and CCR5, was infected with HIV-1 JRFL for 7 days, and the DNA was used as a standard. At 72 hours, untreated AMs infected with JRFL accumulated significant amounts of HIV-1 DNA specifically integrated into chromosomal DNA, which was inhibited by AZT (P < 0.05, ANOVA; Figure 6). Ad coinfected AMs still accumulated HIV-1 DNA specifically integrated into chromosomal DNA at levels comparable to AMs infected with JRFL in the absence of Ad. To show the specificity of this assay for integrated HIV-1 DNA, the samples were also run directly in the second-round TaqMan without the first-round Alu-HIV PCR. Although HIV-1 DNA was detectable, the level was 100-fold less in all samples, indicating that the detection in the two-step procedure was greater than 99% due to integrated HIV-1 DNA (data not shown). These data indicate that the block to HIV-1 replication by Ad is not before HIV-1 nuclear import and integration into chromosomal DNA, suggesting that the suppressive effect of AdNull occurs at a later stage of the HIV-1 life cycle. Taken together with the inhibition of LTR-driven transcription, the effect of Ad can be localized to the LTR-transcription step itself.
Figure 6.
Effect of Ad on integrated HIV-1–specific DNA in AMs. To assess the effect of Ad on integration of HIV-1 DNA into the chromosomal DNA, AMs were infected with 2.5 × 104 pu/cell AdNull, treated with AZT, or left untreated. After 1 hour, the AMs were infected with HIV-1 JRFL, 103 TCID50/well. DNA was isolated at 72 hours and integrated HIV-1 DNA was quantified by a two-step nested TaqMan real-time PCR. The initial PCR step specifically amplifies only sequences annealing to both human 300–base pair repetitive DNA sequences (Alu) primers and a JRFL env primer. In the second step, JRFL primers with an internal probe site quantify the Alu-JRFL amplification products with the first-round PCR products as a template. Ordinate, integrated HIV-1 DNA (copies/μl); abscissa, infection conditions (no virus, JRFL, JRFL + Ad, JRFL + AZT). The results were similar in AMs obtained from three different individuals.
Effect of Ad on IFN-Related Signal Transduction in AMs
To assess whether the Ad genome was necessary for the inhibition of HIV-1 replication in human AMs, we treated AMs in vitro with empty Ad capsids (2.5 × 104 pu/cell). At 5 and 10 days after infection with HIV-1, there was no inhibition of HIV-1 replication (Figure E1 in the online supplement). These results indicate that Ad DNA is absolutely required for the inhibitory effect of AdNull on HIV-1 replication. Because, previously, we were unable to identify a soluble factor(s) released by AMs that mediated the inhibitory effects of Ad on AMs HIV-1 replication, we focused on potential intracellular mediators of this effect. Because intracellular viral DNA is known to induce rapid activation of IFN-associated signaling pathways, we evaluated the effect of Ad infection of AMs on activation of STAT-1 by Western analysis. There was significant phosphorylation of STAT-1 induced by AdNull infection of AMs in the presence or absence of HIV-1 coinfection (Figure E2). These data indicate that the STAT-1 pathway is activated by AdNull infection of AMs. To determine if downstream IFN-inducible proteins could be playing a role in the suppression of LTR expression, we evaluated the effect of AdNull on the expression of IRF-8 by Western analysis. Up-regulation of IRF-8 is known to interfere with the induction of HIV-1 LTR transcription by the IRF-1/tat complex (37). AdNull infection of AMs significantly up-regulated IRF-8 expression in the presence and absence of HIV-1 coinfection (Figure E3). These data suggest a potential mechanism for suppression of LTR transcription via increased IRF-8 expression mediated by activation of STAT-1.
DISCUSSION
Individuals infected with HIV-1 are frequently coinfected with other viruses (38–43). Individual cells recovered from HIV-1+ individuals have been noted to be infected with HIV-1 and co-infected with a heterologous virus (44–47). These observations lead to the hypothesis that co-infections with heterologous viruses may accelerate the course of HIV-1 infection in vivo (48–50). For example, under some circumstances, HIV-1 replication is strongly stimulated in AMs by M. tuberculosis and by bacterial products (51, 52). HSV-1, CMV and HHV-8 gene products transactivate HIV-1 gene transcription in vitro (7, 8, 16). Depending upon the cell type and state of cellular activation and stage of HIV-1 infection, CMV and adeno-associated virus serotype 2 gene products inhibit HIV-1 transcription and replication (10, 11, 13, 14). This is consistent with the knowledge that viruses have evolved numerous methods for inhibiting replication of heterologous viruses within the same cell (12). These virally-induced blocks to HIV-1 replication are of interest since they may represent potential targets for therapeutic antiviral strategies.
The effect of type 5 Ad gene transfer vectors on HIV-1 replication is particularly relevant, since clinical trials are now underway with an HIV-1 vaccine based on type 5 Ad gene transfer vectors (17, 18). Wild-type adenovirus is known to block HIV-1 replication via the E1a region (9). We previously observed that replication deficient E1−, partial E3− type 5 Ad gene transfer vectors inhibit HIV-1 replication in human alveolar macrophages independently of the transgene (5). Ad infection had no significant effect in the viability of HIV-1 infected AMs (5). Suppression of HIV-1 replication by E1− Ad vectors was a function of time and dose, was independent of the transgene in the Ad vector, and was not associated with the E4 genes in the Ad backbone. Inhibition did not appear to be due to a soluble factor in the AMs supernatant, and was not significantly overcome by addition of the CCR5 or CXCR4 coding sequences to the AMs.
AMs are an important element of pulmonary host defense including during HIV-1 infection (1, 2). They are also known to contribute to HIV-1 related lung diseases, and serve as a reservoir for HIV-1 infection despite HAART (1–4, 49). Inhibition of HIV replication in AMs by other micro-organisms has been previously observed in some circumstances. While M. tuberculosis can activate HIV-1+ replication in AMs in some circumstances (51), in others it inhibits via an IFN-β–inducible inhibitory nuclear factor C/EBP-β which suppresses transcription from the HIV-1 LTR (53). A number of studies have examined the effect of co-infection of pathogens with HIV-1 in mononuclear phagocytes. Most in vitro studies reveal an upregulation of HIV-1 replication in AMs, mononuclear cell lines and peripheral blood mononuclear cells by co-pathogens, such as mycobacteria and cytomegalovirus (54, 55). M. tuberculosis and M. avium complex infection of blood monocytes in vitro both upregulate HIV-1 replication (56). In vitro studies of the effect of M. tuberculosis on HIV-1 replication in monocyte-derived macrophages have rendered conflicting results depending upon the experimental conditions (57). When inhibitory, the effect of M. tuberculosis is dependent on the state of differentiation of the monocytic cells (53).
Possible Mechanisms of Ad Vector–Mediated Suppression of LTR-Driven HIV-1 Replication in AMs
HIV-1 virion attachment onto the cell occurs by interaction with cell surface CD4 acting in concert with co-receptors CCR5 and CXCR4 (25, 58–60). HIV-1 strains predominantly utilizing CCR5 as the co-receptor readily infect AMs (25). It has been shown that upregulation of expression of CCR5 or CXCR4 via gene transfer did not significantly abrogate Ad vector-mediated inhibition of HIV-1 replication, suggesting that the effect of Ad vectors is unlikely to involve depletion or blockade of cell surface co-receptors (5).
After HIV-1 virion entry, the viral RNA genome is reverse transcribed into double stranded DNA. The viral dsDNA is translocated to the nucleus, followed by the initiation of HIV-1 transcription leading to HIV-1 protein synthesis. Suppression of HIV-1 post-entry events might be due to either the direct effect of an Ad vector gene product or to the indirect effect of an inducible cellular factor(s) on LTR transcription. HIV-1 LTR possesses multiple nuclear factor binding sites used to modulate transcription including Nef, SP-1, NF-κB and C/EBP β (61, 62).
Upregulation of the inhibitory isoform of C/EBPβ modulation of HIV-1 LTR has been implicated as the mechanism through which M. tuberculosis exerts its suppressive effect on HIV-1 replication in AMs. An example of post-entry block is IFN-β which acts via repression of HIV-1 LTR transcription (51). In the Janus kinase–STAT IFN signaling pathway, phosphorylation of STAT-1 to p-STAT-1 causes dimerization, nuclear transport, and the induction of transcription of numerous genes, including the IRF genes (IRF1–9), which stimulate or inhibit a variety of cellular genes related to the differentiated state of macrophages, immune response, et cetera. In lymphocytes and monocytic cells, IRF-1 also stimulates HIV-1 replication via an interaction with the HIV-1 tat, which, together, bind the HIV-1 LTR and potentiate transcription (37). IRF-8 opposes the effect of IRF-1 by preventing the binding of IRF-1/tat to the HIV-1 LTR, and inhibits HIV-1 replication in lymphocytes and monocytic cells (37). We observed striking phosphorylation of STAT-1 as well as up-regulation of IRF-8 protein in Ad-infected AMs, suggesting that activation of an IFN signaling pathway may mediate the effect of AdNull on HIV-1 LTR transcription. This is consistent with a recent report of up-regulation of IFN-related gene expression by Ad infection of mouse macrophages (63). Other soluble cytokines, such as IL-4 and -10, transforming growth factor–β, CD8+ T lymphocyte antiviral factor, and macrophage-derived chemokine, inhibit HIV-1 replication at one or more postentry levels (64–66). Influenza virus is known to inhibit subsequent HIV-1 replication via induction of release of soluble mediators (67). Previous studies have shown that passive transfer of media from Ad-infected AMs with conditioned media depleted of Ad vectors did not reveal a soluble inhibitor of HIV-1 replication (5).
Implications of Ad Vector–Mediated Suppression of LTR-Driven HIV-1 Replication in AMs
Monocyte-derived cells, such as alveolar macrophages remain a persistent source of transcriptionally activatable HIV-1, with the potential to initiate viral replication, even in the face of successful HAART where plasma levels of HIV-1 RNA become undetectable (3, 68). An Ad5 vector–based vaccine would be expected to transduce other types of cells of monocytic lineage when administered systemically. Our results do not directly address the question of whether a similar inhibitory effect of Ad on HIV-1 replication would be anticipated in other terminally differentiated tissue macrophages, such as Kupfer cells, as well as other cells of monocytic origin, such as dendritic cells, which may serve as reservoirs of HIV-1 and/or facilitate its transport in vivo. The inhibitory effect of Ad on HIV-1 replication observed here suggests that a suppressive effect in other monocytic cell types is plausible, but, until tested, remains a hypothesis. The observation that Ad mediates suppression of endogenous HIV-1 replication in AMs is reassuring in light of the ongoing clinical trial of an Ad-based HIV-1 vaccine (17, 18, 69; clinicaltrials.gov).
Supplementary Material
Acknowledgments
The authors thank Shawn Kuhman, Department of Microbiology, for the human immunodeficiency virus–1 vesicular stomatitis virus G protein–1 LTR-luciferase construct, B.-G. Harvey, Division of Pulmonary and Critical Care Medicine, Weill Cornell, for human alveolar macrophages, and N. Mohamed for help in preparing the manuscript.
This work was supported in part by National Institutes of Health grants R01 HL59861, AI45463, and the Will Rogers Memorial Fund (Los Angeles, CA).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2008-0063OC on October 5, 2009
Author Disclosure: R.J.K. has received compensation for serving on the Board of Eli Lilly ($1,001–$5,000) and Centocor($1,001–$5,000) and has served as an expert witness for the U.S. District Attorney, NY ($1,001–$5,000) and for a private attorney ($1,001–$5,000) for review of Medical Malpractice defense; received Royalties from the commercial entity Uptodate ($1,001–$5,000), and received sponsored grants from the National Institutes of Health ($100,001 or more). F.R. has received reimbursement for consultancies to Gielad ($1,001–$5,000), Actelion ($1,001–$5,000), United Therapeutics ($1,001–$5,000), CSL Behring ($1,001–$5,000), and Baxter ($5,001–$10,000); has served on the Board/Advisory Board for Gielad ($1,001–$5,000), Actelion ($5,001–$10,000), United Therapeutics ($1,001–$5,000), CSL Behring ($1,001–$5,000), and Baxter ($1,001–$5,000); has received reimbursement for Lectures from Gielad ($1,001–$5,000), Actelion ($5,001–$10,000), United Therapeutics ($1,001–$5,000), CSL Behring ($1,001–$5,000), Baxter ($1,001–$5,000) and Pfizer ($1,001–$5,000); and has an Industry Sponsored Grant from Baxter ($10,000–$50,000) and United Therapeutics ($1,001–$5,000). None of the other authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Agostini C, Trentin L, Zambello R, Semenzato G. HIV-1 and the lung. infectivity, pathogenic mechanisms, and cellular immune responses taking place in the lower respiratory tract. Am Rev Respir Dis 1993;147:1038–1049. [DOI] [PubMed] [Google Scholar]
- 2.Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection: report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med 2001;164:2120–2126. [DOI] [PubMed] [Google Scholar]
- 3.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A 1997;94:13193–13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White NC, Agostini C, Israel-Biet D, Semenzato G, Clarke JR. The growth and the control of human immunodeficiency virus in the lung: implications for highly active antiretroviral therapy. Eur J Clin Invest 1999;29:964–972. [DOI] [PubMed] [Google Scholar]
- 5.Rice J, Connor R, Worgall S, Moore JP, Leopold PL, Kaner RJ, Crystal RG. Inhibition of HIV-1 replication in alveolar macrophages by adenovirus gene transfer vectors. Am J Respir Cell Mol Biol 2002;27:214–219. [DOI] [PubMed] [Google Scholar]
- 6.Mosca JD, Bednarik DP, Raj NB, Rosen CA, Sodroski JG, Haseltine WA, Hayward GS, Pitha PM. Activation of human immunodeficiency virus by herpesvirus infection: identification of a region within the long terminal repeat that responds to a trans-acting factor encoded by herpes simplex virus 1. Proc Natl Acad Sci USA 1987;84:7408–7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrove JM, Leonard J, Weck KE, Rabson AB, Gendelman HE. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol 1987;61:3726–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan R, Bohan C, Shiao FC, Robinson R, Kaplan HJ, Srinivasan A. Activation of HIV LTR-directed expression: analysis with pseudorabies virus immediate early gene. Virology 1989;172:92–99. [DOI] [PubMed] [Google Scholar]
- 9.Ventura AM, Arens MQ, Srinivasan A, Chinnadurai G. Silencing of human immunodeficiency virus long terminal repeat expression by an adenovirus E1a mutant. Proc Natl Acad Sci USA 1990;87:1310–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antoni BA, Rabson AB, Miller IL, Trempe JP, Chejanovsky N, Carter BJ. Adeno-associated virus rep protein inhibits human immunodeficiency virus type 1 production in human cells. J Virol 1991;65:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koval V, Clark C, Vaishnav M, Spector SA, Spector DH. Human cytomegalovirus inhibits human immunodeficiency virus replication in cells productively infected by both viruses. J Virol 1991;65:6969–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rittner K, Heilbronn R, Kleinschmidt JA, Sczakiel G. Adeno-associated virus type 2-mediated inhibition of human immunodeficiency virus type 1 (HIV-1) replication: involvement of P78rep/P68rep and the HIV-1 long terminal repeat. J Gen Virol 1992;73:2977–2981. [DOI] [PubMed] [Google Scholar]
- 13.Jault FM, Spector SA, Spector DH. The effects of cytomegalovirus on human immunodeficiency virus replication in brain-derived cells correlate with permissiveness of the cells for each virus. J Virol 1994;68:959–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boccuni MC, Campanini F, Battista MC, Bergamini G, Dal MP, Ripalti A, Landini MP. Human cytomegalovirus product UL44 downregulates the transactivation of HIV-1 long terminal repeat. AIDS 1998;12:365–372. [DOI] [PubMed] [Google Scholar]
- 15.Moore JP, Stevenson M. New targets for inhibitors of HIV-1 replication. Nat Rev Mol Cell Biol 2000;1:40–49. [DOI] [PubMed] [Google Scholar]
- 16.Caselli E, Menegazzi P, Bracci A, Galvan M, Cassai E, Di LD. Human herpesvirus-8 (Kaposi's sarcoma-associated herpesvirus) ORF50 interacts synergistically with the Tat gene product in transactivating the human immunodeficiency virus type 1 LTR. J Gen Virol 2001;82:1965–1970. [DOI] [PubMed] [Google Scholar]
- 17.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 2002;415:331–335. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. HIV/AIDS. Hedged bet: an unusual AIDS vaccine trial. Science 2005;309:1003. [DOI] [PubMed] [Google Scholar]
- 19.Kaner RJ, Michael E, Cieciuch AA, Luettich K, Moore J, Crystal RG. Inhibition of HIV-1 replication in human alveolar macrophages by a modified adenovirus occurs after HIV-1 reverse transcription. Am J Respir Crit Care Med 2002;165:A367. [Google Scholar]
- 20.Kaner RJ, Michaels E, Rahaghi F, Cieciuch AA, Luettich K, Moore JP, Crystal RG. Adenovirus gene transfer vectors suppress HIV-1 replication in human alveolar macrophages due in part to inhibition of HIV-1 long terminal repeat transcription. Mol Ther 2002;5:S257. [Google Scholar]
- 21.Kaner R, Rahaghi F, Igonkin D, Kuhmann S, Moore J, Crystal R. Adenovirus gene transfer vectors inhibit HIV-1 replication in human alveolar macrophages at a post-reverse transcription step independent of Ad capsids. Am J Respir Crit Care Med 2003;167:A197. [Google Scholar]
- 22.Kaner RJ, Santiago F, Crystal RG. E1 adenovirus gene transfer vectors inhibit HIV-1 replication in human alveolar macrophages [abstract]. Mol Ther 2005;11:S384. [Google Scholar]
- 23.Kaner RJ, Santiago F, Crystal RG. Adenovirus vectors inhibit endogenous HIV-1 replication in human alveolar macrophages [abstract]. Proc Am Thorac Soc 2005;2:A454. [Google Scholar]
- 24.Russi TJ, Crystal RG. Bronchoalveolar lavage. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The lung: scientific foundations. Philadelphia: Lippencott-Raven, Inc.; 1997. pp. 371–382.
- 25.Worgall S, Connor R, Kaner RJ, Fenamore E, Sheridan K, Singh R, Crystal RG. Expression and use of human immunodeficiency virus type 1 coreceptors by human alveolar macrophages. J Virol 1999;73:5865–5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenfeld MA, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier LE, Paakko PK, Gilardi P, Stratford-Perricaudet LD, Perricaudet M, et al. Adenovirus-mediated transfer of a recombinant alpha 1-antitrypsin gene to the lung epithelium in vivo. Science 1991;252:431–434. [DOI] [PubMed] [Google Scholar]
- 27.Hersh J, Crystal RG, Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther 1995;2:124–131. [PubMed] [Google Scholar]
- 28.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 1996;70:7498–7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J Virol 1998;72:2855–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rich EA, Chen IS, Zack JA, Leonard ML, O'Brien WA. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest 1992;89:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 1995;206:935–944. [DOI] [PubMed] [Google Scholar]
- 32.Chang LJ, Urlacher V, Iwakuma T, Cui Y, Zucali J. Efficacy and Safety Analyses of a Recombinant Human Immunodeficiency Virus Type 1 Derived Vector System. Gene Ther 1999;6:715–728. [DOI] [PubMed] [Google Scholar]
- 33.Koh KB, Fujita M, Adachi A. Elimination of HIV-1 plasmid DNA from virus samples obtained from transfection by calcium-phosphate co-precipitation. J Virol Methods 2000;90:99–102. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, Tanaka C, Wu Y, Chang MO, Inagaki Y, Saito Y, Naito T, Ogasawara H, Sekigawa I, Hayashida Y. Analysis of human immunodeficiency virus type 1 integration by using a specific, sensitive and quantitative assay based on real-time polymerase chain reaction. Virus Genes 2006;32:105–113. [DOI] [PubMed] [Google Scholar]
- 35.Lebargy F, Branellec A, Deforges L, Bignon J, Bernaudin JF. HIV-1 in human alveolar macrophages from infected patients is latent in vivo but replicates after in vitro stimulation. Am J Respir Cell Mol Biol 1994;10:72–78. [DOI] [PubMed] [Google Scholar]
- 36.Saeed MF, Kolokoltsov AA, Davey RA. Novel, rapid assay for measuring entry of diverse enveloped viruses, including HIV and rabies. J Virol Methods 2006;135:143–150. [DOI] [PubMed] [Google Scholar]
- 37.Sgarbanti M, Borsetti A, Moscufo N, Bellocchi MC, Ridolfi B, Nappi F, Marsili G, Marziali G, Coccia EM, Ensoli B, et al. Modulation of human immunodeficiency virus 1 replication by interferon regulatory factors. J Exp Med 2002;195:1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drew WL. Cytomegalovirus infection in patients with AIDS. J Infect Dis 1988;158:449–456. [DOI] [PubMed] [Google Scholar]
- 39.Jacobson MA, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS): clinical findings, diagnosis, and treatment. Ann Intern Med 1988;108:585–594. [DOI] [PubMed] [Google Scholar]
- 40.Horvath J, Raffanti SP. Clinical aspects of the interactions between human immunodeficiency virus and the hepatotropic viruses. Clin Infect Dis 1994;18:339–347. [DOI] [PubMed] [Google Scholar]
- 41.Soriano V, Nedjar S, García-Samaniego J, Bravo R, Castro A, González J, Martínez-Odriozola P, Pedreira J, del Romero J, Suárez D, et al. High rate of co-infection with different hepatitis c virus subtypes in HIV-infected intravenous drug addicts in Spain: Hepatitis HIV Spanish Study Group. J Hepatol 1995;22:598–599. [DOI] [PubMed] [Google Scholar]
- 42.Soriano V, Garcia-Samaniego J, Bravo R, Gonzalez J, Castro A, Castilla J, Martinez-Odriozola P, Colmenero M, Carballo E, Suarez D, et al. Interferon alpha for the treatment of chronic hepatitis C in patients infected with human immunodeficiency virus: Hepatitis-HIV Spanish Study Group. Clin Infect Dis 1996;23:585–591. [DOI] [PubMed] [Google Scholar]
- 43.Yan Z, Nguyen S, Poles M, Melamed J, Scholes JV. Adenovirus colitis in human immunodeficiency virus infection: an underdiagnosed entity. Am J Surg Pathol 1998;22:1101–1106. [DOI] [PubMed] [Google Scholar]
- 44.Nelson JA, Reynolds-Kohler C, Oldstone MB, Wiley CA. HIV and HCMV coinfect brain cells in patients with AIDS. Virology 1988;165:286–290. [DOI] [PubMed] [Google Scholar]
- 45.Skolnik PR, Pomerantz RJ, de la Monte SM, Lee SF, Hsiung GD, Foos RY, Cowan GM, Kosloff BR, Hirsch MS, Pepose JS. Dual infection of retina with human immunodeficiency virus type 1 and cytomegalovirus. Am J Ophthalmol 1989;107:361–372. [DOI] [PubMed] [Google Scholar]
- 46.Finkle C, Tapper MA, Knox KK, Carrigan DR. Coinfection of cells with the human immunodeficiency virus and cytomegalovirus in lung tissues of patients with AIDS. J Acquir Immune Defic Syndr 1991;1991:735–737. [PubMed] [Google Scholar]
- 47.Heng MC, Heng SY, Allen SG. Co-infection and synergy of human immunodeficiency virus-1 and herpes simplex virus-1. Lancet 1994;343:255–258. [DOI] [PubMed] [Google Scholar]
- 48.Blanchard A, Montagnier L, Gougeon ML. Influence of microbial infections on the progression of HIV disease. Trends Microbiol 1997;5:326–331. [DOI] [PubMed] [Google Scholar]
- 49.Orenstein JM, Fox C, Wahl SM. Macrophages as a source of HIV during opportunistic infections. Science 1997;276:1857–1861. [DOI] [PubMed] [Google Scholar]
- 50.Wahl SM, Greenwell-Wild T, Peng G, Ma G, Orenstein JM, Vazquez N. Viral and host cofactors facilitate HIV-1 replication in macrophages. J Leukoc Biol 2003;74:726–735. [DOI] [PubMed] [Google Scholar]
- 51.Honda Y, Rogers L, Nakata K, Zhao BY, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M, Type I. Interferon induces inhibitory 16-KD CCAAT/enhancer binding protein (C/EBP)beta, repressing the HIV-1 long terminal repeat in macrophages: pulmonary tuberculosis alters C/EBP expression, enhancing HIV-1 replication. J Exp Med 1998;188:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Equils O, Faure E, Thomas L, Bulut Y, Trushin S, Arditi M. Bacterial lipopolysaccharide activates HIV long terminal repeat through Toll-like receptor 4. J Immunol 2001;166:2342–2347. [DOI] [PubMed] [Google Scholar]
- 53.Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. Differentiation of monocytes to macrophages switches the mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein beta expression. J Immunol 2000;165:2028–2039. [DOI] [PubMed] [Google Scholar]
- 54.Wahl SM, Greenwell-Wild T, Peng G, Hale-Donze H, Doherty TM, Mizel D, Orenstein JM. Mycobacterium avium complex augments macrophage HIV-1 production and increases CCR5 expression. Proc Natl Acad Sci USA 1998;95:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse DB, Shioda T, Rom WN, Weiden M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med 2002;195:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toossi Z, Xia L, Wu M, Salvekar A. Transcriptional activation of HIV by Mycobacterium tuberculosis in human monocytes. Clin Exp Immunol 1999;117:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goletti D, Carrara S, Vincenti D, Giacomini E, Fattorini L, Garbuglia AR, Capobianchi MR, Alonzi T, Fimia GM, Federico M, et al. Inhibition of HIV-1 replication in monocyte-derived macrophages by Mycobacterium tuberculosis. J Infect Dis 2004;189:624–633. [DOI] [PubMed] [Google Scholar]
- 58.Lewin SR, Sonza S, Irving LB, McDonald CF, Mills J, Crowe SM. Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res Hum Retroviruses 1996;12:877–883. [DOI] [PubMed] [Google Scholar]
- 59.Coffey MJ, Woffendin C, Phare SM, Strieter RM, Markovitz DM. RANTES inhibits HIV-1 replication in human peripheral blood monocytes and alveolar macrophages. Am J Physiol 1997;272:L1025–L1029. [DOI] [PubMed] [Google Scholar]
- 60.Singh A, Besson G, Mobasher A, Collman RG. Patterns of chemokine receptor fusion cofactor utilization by human immunodeficiency virus type 1 variants from the lungs and blood. J Virol 1999;73:6680–6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu YZ, Latchman DS. The octamer-binding proteins Oct-1 and Oct-2 repress the HIV long terminal repeat promoter and its transactivation by Tat. Biochem J 1997;322:155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohr O, Marban C, Aunis D, Schaeffer E. Regulation of HIV-1 gene transcription: from lymphocytes to microglial cells. J Leukoc Biol 2003;74:736–749. [DOI] [PubMed] [Google Scholar]
- 63.Nociari M, Ocheretina O, Schoggins JW, Falck-Pedersen E. Sensing infection by adenovirus: Toll-like receptor-independent viral DNA recognition signals activation of the interferon regulatory factor 3 master regulator. J Virol 2007;81:4145–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Naif HM, Li S, Ho-Shon M, Mathijs JM, Williamson P, Cunningham AL. The state of maturation of monocytes into macrophages determines the effects of IL-4 and IL-13 on HIV replication. J Immunol 1997;158:501–511. [PubMed] [Google Scholar]
- 65.Chang TL, Mosoian A, Pine R, Klotman ME, Moore JP. A Soluble factor(s) secreted from CD8(+) T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. J Virol 2002;76:569–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanaka N, Hoshino Y, Gold J, Hoshino S, Martiniuk F, Kurata T, Pine R, Levy D, Rom WN, Weiden M. Interleukin-10 induces inhibitory C/EBPbeta through STAT-3 and represses HIV-1 transcription in macrophages. Am J Respir Cell Mol Biol 2005;33:406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pinto LA, Blazevic V, Patterson BK, Mac TC, Dolan MJ, Shearer GM. Inhibition of human immunodeficiency virus type 1 replication prior to reverse transcription by influenza virus stimulation. J Virol 2000;74:4505–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aquaro S, Bagnarelli P, Guenci T, De LA, Clementi M, Balestra E, Calio R, Perno CF. Long-term survival and virus production in human primary macrophages infected by human immunodeficiency virus. J Med Virol 2002;68:479–488. [DOI] [PubMed] [Google Scholar]
- 69.Nanda A, Lynch DM, Goudsmit J, Lemckert AA, Ewald BA, Sumida SM, Truitt DM, Abbink P, Kishko MG, Gorgone DA, et al. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J Virol 2005;79:14161–14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.