Abstract
Rationale: Long-term survivors of cystic fibrosis (CF) (age > 40 yr) are a growing population comprising both patients diagnosed with classic manifestations in childhood, and nonclassic phenotypes typically diagnosed as adults. Little is known concerning disease progression and outcomes in these cohorts.
Objectives: Examine effects of age at diagnosis and gender on disease progression, setting of care, response to treatment, and mortality in long-term survivors of CF.
Methods: Retrospective analysis of the Colorado CF Database (1992–2008), CF Foundation Registry (1992–2007), and Multiple Cause of Death Index (1992–2005).
Measurements and Main Results: Patients with CF diagnosed in childhood and who survive to age 40 years have more severe CFTR genotypes and phenotypes compared with adult-diagnosed patients. However, past the age of 40 years the rate of FEV1 decline and death from respiratory complications were not different between these cohorts. Compared with males, childhood-diagnosed females were less likely to reach age 40 years, experienced faster FEV1 declines, and no survival advantage. Females comprised the majority of adult-diagnosed patients, and demonstrated equal FEV1 decline and longer survival than males, despite a later age at diagnosis. Most adult-diagnosed patients were not followed at CF centers, and with increasing age a smaller percentage of CF deaths appeared in the Cystic Fibrosis Foundation Registry. However, newly diagnosed adults demonstrated sustained FEV1 improvement in response to CF center care.
Conclusions: For patients with CF older than 40 years, the adult diagnosis correlates with delayed but equally severe pulmonary disease. A gender-associated disadvantage remains for females diagnosed in childhood, but is not present for adult-diagnosed females.
Keywords: cystic fibrosis, middle aged, aged, gender, outcome assessment
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Despite advances in cystic fibrosis (CF) survival and increasing awareness of adult-diagnosed patients, little is known concerning outcomes for long-term survivors of CF.
What This Study Adds to the Field
We describe here disease progression, setting of care, response to treatment, and patient survival of long-term survivors of CF in the context of gender and age at diagnosis.
Cystic fibrosis (CF) is the most common lethal autosomal recessive disease in the non-Hispanic white population, affecting 1 in 3,200 births (1). Over the past decades survival has dramatically improved, with a predicted median survival of 37.4 years for patients entered into the 2007 CF Foundation Registry (2). However, reaching middle age still remains relatively uncommon, as the current median age is 16.6 years, and nearly 95% of all patients with CF entered in the Cystic Fibrosis Foundation (CFF) Registry are currently under age 40 years (2). Patients with CF who are older than age 40 years are a little-studied population, but their long-term outcomes are of importance as these individuals provide us with our first glimpse at what CF care will look like in the coming decades (3).
The population of patients with CF who are more than 40 years of age is actually composed of two different cohorts that can generally be distinguished by the age at diagnosis. Nearly 90% of CF cases are diagnosed before age 10 years (2) with the classic clinical features of exocrine pancreatic function insufficiency (PI) and progressive pulmonary disease (4) combined with laboratory evidence of an abnormality in the CF transmembrane conductor regulator (CFTR) gene or protein. A second cohort is composed of patients diagnosed in adulthood, primarily due to the existence of CFTR mutations that afford partial function of the gene (5, 6), resulting in a variable phenotype with a later onset of clinically significant symptoms (7–10). Patients with an adult diagnosis (AD) of CF typically have milder lung disease than age-matched patients with a childhood diagnosis (CD) (11–13) and are predominantly pancreatic sufficient (PS) (11, 12, 14) or have recurrent pancreatitis (15, 16). In both cohorts obstructive azoospermia in males is common, and may be the clinical feature that leads to diagnosis as an adult (17, 18). As our understanding of CF has increased, diagnostic guidelines have been refined to better reflect the broad range of clinical features that may result from CFTR dysfunction (4). On occasion, laboratory testing may be inconclusive as sweat chloride testing is neither sensitive nor specific for the nonclassic phenotype (17, 19), and genetic testing may reveal rare CFTR mutations with variable or unknown consequences (4). But in most cases, the diagnosis can be made with certainty, and progressively larger and older cohorts of AD patients have been reported by CF centers worldwide (3, 11–14, 20–22).
In addition to age at diagnosis, gender is a second major influence on the diagnosis and progression of CF. In the setting of a childhood diagnosis, females are diagnosed later than males (23–25), possibly because of a gender-based bias by parents and physicians (25). When CF is diagnosed in adulthood, little attention has been paid to the effect of gender, although in several case series, the majority of AD patients were female (11, 12, 21, 26). Gender is implicated in modifying disease progression for patients with a childhood diagnosis, as females have been found to have a significantly decreased survival from age 1 to 20 years (27–31). However, this “gender gap” is reportedly not present after age 20 years (30), and in some younger cohorts (born after 1990) the historical gender gap is no longer observed (32, 33). The underlying cause of the CF gender gap likely represents the summation of genetic modifications imposed by sex, as well as the socioenvironmental effects of gender (34). Various biological mechanisms have been identified that can result in a disadvantage for women (25, 35–44), and from a psychosocial perspective, young females have been found to cope less effectively with the disease, and to be less adherent to therapy (45–53). However, these conclusions have been reached from analysis of children and adolescents; thus studies of long-term survivors of CF may help clarify the impact of sex and gender on disease progression.
We studied outcomes in a cohort of long-term survivors seen at the Colorado CF center from 1992 to 2008, as well as patients reported to the CFF Registry from 1992 to 2007, and the Mortality Multiple Cause-of-Death (MCOD) Public Use Record maintained by the Centers for Disease Control (54). In patients with CF older than 40 years, we compared CD and AD cohorts, and the impact of gender regarding progression of disease, patient survival, and cause of death. We also examined the setting in which these patients receive their care, the rate of clinic attendance, and the response to treatment of newly diagnosed adult patients with CF followed at accredited Care centers. These findings may help plan for the future in which survival past 40 years will be increasingly common for patients with CF. In addition, a better understanding of the progression of lung disease in patients who were not diagnosed until middle age may help shape lifelong treatment strategies for asymptomatic infants or young adults with the same CFTR mutations identified by newborn screening or genetic testing (55). Some of the results of these studies have previously been reported in abstract form (56).
METHODS
Cohorts Studied
A retrospective chart review was conducted to identify older patients with CF diagnosed, evaluated, or monitored at the Colorado CF center or at National Jewish Health (formerly National Jewish Medical and Research Center; Denver, CO). Clinical records of the Adult CF Program between 1992 and 2008 (475 patients) and the Infectious Disease Division (2,822 patients) were reviewed. A cohort of 156 individuals more than age 40 years was analyzed. All AD patients were referred because of chronic airway infection and/or bronchiectasis, which are characteristic clinical features of CF lung disease (57). All patients also had sweat chloride measurements diagnostic for CF or identification of two CFTR mutations or variants. Diagnostic and phenotypic features of this population can be found in Table E1 in the online supplement. The Colorado database includes many out-of-state referrals for specialized assessment, and thus does not represent a well-defined population base. However, this database provides detailed clinical data and outcomes measurements for a large cohort of long-term survivors independent of the U.S. Cystic Fibrosis Foundation (CFF) Registry. A parallel analysis was conducted on patients enrolled in the CFF Registry from 1992 to 2007 whose greatest age was more than 40 years (n = 2,964). The CFF Registry includes extensive demographic, diagnostic, and clinical data for all patients seen at U.S. CFF–accredited care centers. To more accurately characterize the classic and nonclassic phenotype utilizing age at diagnosis, CD was defined as diagnosis at age 10 years or younger, whereas AD was defined as diagnosis at age 18 years and older. Patients diagnosed between the ages of 10 and 18 years (n = 6 of the Colorado database and n = 350 of the CFF Registry) were not included for further analysis.
Assessment of Care for Older Patients with CF Independent of Accredited CF Centers
Many of the adult-diagnosed patients in the Colorado database were referred to National Jewish Health (NJH) from outside the region, or left the region during the 15-year study period. The CFF Registry was queried to identify patients in the Colorado database also entered into the CFF Registry, and the years that they were seen at CF centers. Entry into the CFF Registry serves as a marker that a patient has received care at a CF center. For each patient, the six most recent years of eligibility were analyzed, defined as the six most recent years of life. Patients who were diagnosed within the past 6 years were only eligible for entry into the CFF Registry in the year of diagnosis and subsequent years. For patients not currently enrolled in the CFF Registry, verification of survival was obtained by querying the Social Security Death Index. A second assessment of patients with CF receiving CF center care was conducted by comparing the number of deaths recorded in the CFF Registry to the number of deaths in which CF was recorded as the cause of death (or contributing condition) in the Mortality Multiple Cause-of-Death Public Use Record maintained by the Centers for Disease Control and Prevention (Atlanta, GA) (1992–2005) (54).
Response to CF Center Care
Within the Colorado database, 111 AD patients were identified, and 49 subsequently established care at the Colorado CF center. For patients with available medical records and more than 1 year of follow-up data (n = 32), the FEV1 (percent predicted) obtained at diagnosis was compared with the best available FEV1 for each subsequent year, for up to 4 years of follow-up. Response to treatment was determined by the change in percent-predicted FEV1, compared with the value obtained at the initiation of CF center care. A parallel analysis was performed with CFF Registry data, by comparing the first available FEV1 with the best available FEV1 for subsequent 12-month periods, with up to 4 years of follow-up.
Statistical Analysis
As age at diagnosis and greatest known age were not normally distributed (Figure 1 and Figures 4C and 4D), nonparametric Wilcoxon rank sum tests were used. A two-tailed Fisher's exact test was used to determine differences in gender frequency by diagnostic cohort (Figures 4A and 4B), prevalence of pancreatic insufficiency (Table 1), and high-risk CFTR mutation (Table 1). The significance of differences in sweat chloride (Table 1) was determined by Student t test. The average follow-up period between groups was analyzed by analysis of variance with a Tukey correction for multiple comparisons. Longitudinal mixed effects models were employed to characterize the age-related decline in FEV1, allowing for a random slope and intercept for each subject. Diagnostic and gender groups were included in one model for comparison, and linear contrasts were used to test differences between groups. Because of limited data for the CD group past the age of 60 years, observations were limited to those obtained between 40 and 60 years of age. Results are presented as estimated means and standard error. Differences in survival curves were analyzed by log-rank (Mantel-Cox) tests (Figures 3 and 6). Comparison of the rate of AD and CD attendance at CF centers over time (Figure 7) and response to CF center care over time (Figure 9) was performed by two-way analysis of variance. Comparison between numbers of recorded deaths in the CFF Registry and the MCOD for each age range, as well as the frequency of each cause of death between the diagnostic cohorts, was analyzed by Pearson chi-square test. For all tests, P < 0.05 was considered significant. Data analysis was conducted with StatView software and SAS (SAS Institute, Cary, NC). This study was approved by the NJH Institutional Review Board.
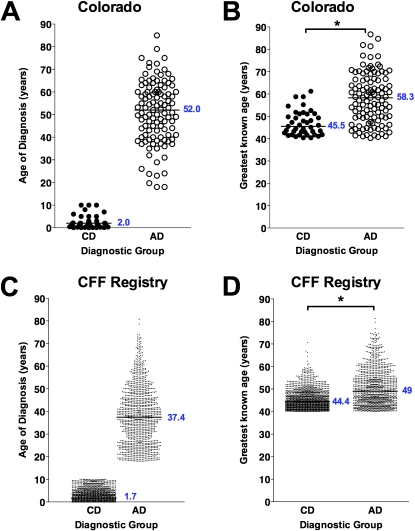
Figure 1.
Age at diagnosis and greatest known age of long-term survivors from the Colorado and Cystic Fibrosis Foundation (CFF) Registry databases. (A) Patients from the Colorado database diagnosed at 10 years or younger were designated as childhood diagnosis (solid circles, CD) and were compared with patients diagnosed at age 18 years or greater, designated as adult diagnosis (open circles, AD). (B) The greatest known age of patients shown in (A) from the Colorado database in the CD cohort was significantly less than that of the AD cohort (*P < 0.001). (C) Patients from the CFF Registry database diagnosed at 10 years or younger (solid circles, CD) were compared with patients diagnosed at age 18 years or greater (open circles, AD). (D) The greatest known age of patients shown in (C) from the CFF Registry database in the CD cohort was significantly less than that of the AD cohort (*P < 0.05).
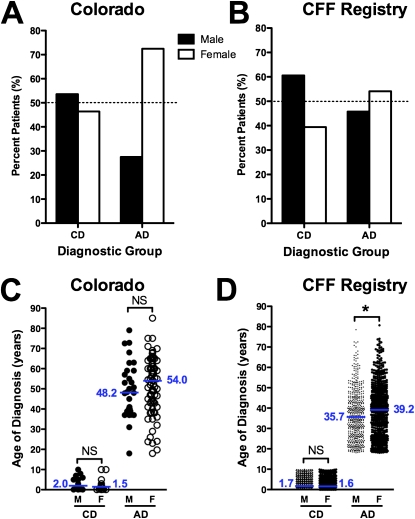
Figure 4.
The adult diagnosis of cystic fibrosis (CF) occurs with greater frequency and at an older age in women. (A) In the Colorado database, the majority (53.6%) of childhood-diagnosed (CD) patients over 40 years of age were men (solid column), whereas the majority (72.5%) of adult-diagnosed (AD) patients over 40 years of age were women (open column). (B) In the Cystic Fibrosis Foundation (CFF) Registry database, the same pattern was observed, with 60.6% of CD patients over 40 years of age being male (solid column), whereas 54.1% of AD patients over 40 years of age were women (open column). (C) In the Colorado database, the age at diagnosis was 5.8 years older in females (open circles) compared with males (solid circles) (p = NS). (D) In the CFF Registry, females (open circles) were 3.5 years older at the age at diagnosis (*P = 0.0014).
TABLE 1.
CLINICAL FEATURES AND OUTCOMES OF PATIENTS WITH CYSTIC FIBROSIS OVER AGE 40 YEARS
| Database Cohort | Subjects (n) | Dx* (yr) | Female (%) | PI† (%) | High-risk Genotype‡ (%) | Sweat Cl− (mmol/L) | Greatest Age (yr)§ | Transplant (%) | Deceased (%) |
|---|---|---|---|---|---|---|---|---|---|
| CFF (range) | 2,614 | 7.7 (0–80.7) | 46.0 | 66.1 | 68.5 | 96.8 ± 25.6 | 45.9 (40.1–81.3) | 2.4 | 24.2 |
| CD (range) | 1,436 | 1.7 (0–10) | 39.3 | 79.0 | 92.3 | 106.4 ± 20.2 | 44.4 (40.1–70.6) | 3.1 | 26.5 |
| AD (range) | 1,178 | 37.4‖ (18 –80.7) | 54.1‖ | 50.4‖ | 30.9‖ | 85.1 ± 26.8‖ | 49.0 (40.1–81.3) | 1.5¶ | 21.5** |
| Colorado (range) | 150 | 43.9 (0.1–85.0) | 65.3 | 32.0 | 26.7 | 82.8 ± 30.9 | 52.7 (40.3–86.7) | 8.0 | 25.3 |
| CD (range) | 41 | 2.0 (0.1–10.0) | 46.3 | 82.9 | 83.3 | 105.5 ± 19.1 | 45.5 (40.4–61.2) | 24.4 | 36.6 |
| AD (range) | 109 | 52.0‖ (18–85.0) | 72.5†† | 12.8‖ | 5.3‖ | 74.6 ± 30.4‖ | 58.3 (40.3–86.7) | 1.8‡‡ | 21.1§§ |
Definition of abbreviations: AD = adult diagnosis; CD = childhood diagnosis; CFF = Cystic Fibrosis Foundation.
Dx = median age at diagnosis in years.
PI = percentage of subjects with pancreatic insufficiency, based on reported use of pancreatic enzyme replacement therapy.
High-risk genotype defined by identification of two class I–III CFTR mutations.
Median greatest known age of patients in cohort, either alive or deceased, in years.
P < 0.0001 by Fisher's two-tail exact test compared with CD cohort.
P = 0.01 by Fisher's two-tail exact test compared with CD cohort.
** P = 0.003 by Fisher's two-tail exact test compared with CD cohort.
P = 0.038 by Fisher's two-tail exact test compared with CD cohort.
P < 0.001 by Fisher's two-tail exact test compared with CD cohort.
Not significantly different by Fisher's two-tail exact test compared with CD cohort.
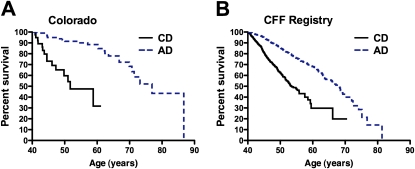
Figure 3.
Survival analysis of patients with cystic fibrosis (CF) more than 40 years of age. (A) For patients included in the Colorado database, adult-diagnosed (AD) patients (dashed line) demonstrated a median survival 25.2 years greater than that of childhood-diagnosed (CD) patients (solid line). (B) For patients included in the Cystic Fibrosis Foundation (CFF) Registry, AD patients (dashed line) demonstrated a median survival 14.7 years greater than that of CD patients (solid line).
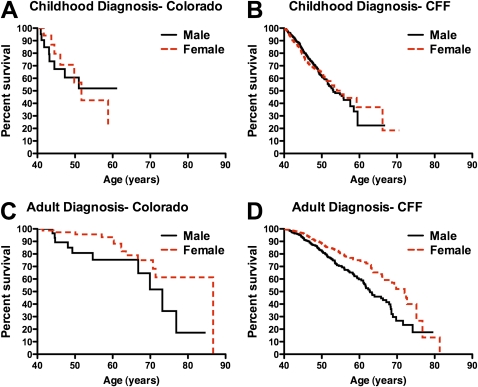
Figure 6.
Effect of gender on survival of patients with cystic fibrosis (CF) who are more than 40 years of age. (A) For childhood-diagnosed long-term survivors in the Colorado database no difference was appreciated between male (solid line) and female (dashed line) survival. (B) For childhood-diagnosed long-term survivors in the Cystic Fibrosis Foundation (CFF) Registry database, male (solid line) survival exceeded female (dashed line) survival by 2.4 years (not significant). (C) For adult-diagnosed long-term survivors in the Colorado database, female (dashed line) survival exceeded male (solid line) by 13.5 years. (D) For adult-diagnosed long-term survivors in the CFF Registry database, female (dashed line) survival exceeded male (solid line) survival by 9.2 years.
Figure 7.
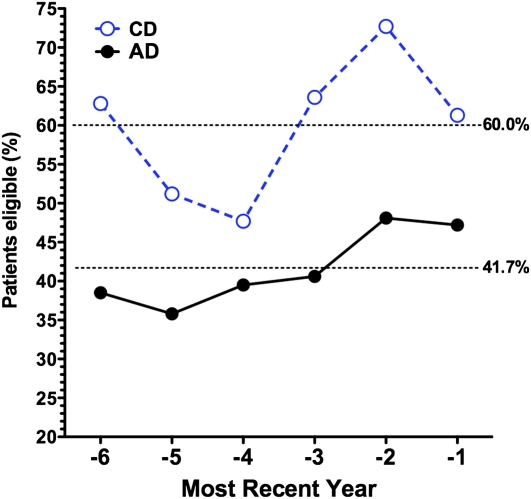
Adult-diagnosed (AD) patients are less likely to receive care at a cystic fibrosis (CF) care center. The percentage of patients in the Colorado database found to be entered in the Cystic Fibrosis Foundation (CFF) Registry was plotted against the six most recent years each subject was eligible for entry into the CFF Registry. During this time span, on average 60% of CD patients (open circles) were seen at a CF care center during a given year, compared with 41.7% of AD patients (closed circles) (P < 0.001 by two-way analysis of variance).
Figure 9.
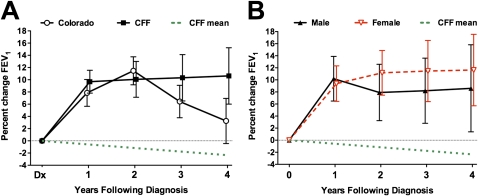
Response to cystic fibrosis (CF) center care by newly diagnosed adults. (A) Response to treatment was assessed in adult-diagnosed patients with CF who established CF center care in the Colorado database (open circles) or in the Cystic Fibrosis Foundation (CFF) Registry (solid squares) with a minimum of 1 year of follow-up visits. The best FEV1 for each subsequent 12-month period was compared with the FEV1 measured at the initiation of standard CF center care. The mean change in percent predicted FEV1 (±SEM) is depicted. For both cohorts, maximal improvement was observed between 1 and 2 years, but lung function remained improved over baseline for the entire 4-year period. The effect of the initial introduction of CF center care can be appreciated in comparison with the observed annual rate of decline for the adult-diagnosed CF population (more than 40 yr of age) as a whole in the CFF Registry (dotted line). (B) Responses of males (solid triangles) and females (open triangles) from the CFF Registry cohort were both significantly improved over baseline, but the difference between the genders was not significant.
RESULTS
Age at Diagnosis and Greatest Known Age in Long-term Survivors of CF
Clinical features of all patients with CF age 40 years or greater were reviewed from the Colorado (n = 156) and CFF Registry (n = 2,964) databases. The Colorado database was analyzed on the basis of age at diagnosis: a CD cohort (n = 41) with age at diagnosis ranging from birth to 10 years (median, 2.0 yr) and an AD cohort (n = 109; range, 18–84 yr; median, 52.0 yr) (Figure 1A). The greatest known age of the CD cohort (median, 45.5 yr) was significantly lower than that of the AD cohort (median, 58.3 yr; P < 0.001) (Figure 1B). Within the CFF Registry database, the median age at diagnosis for the CD cohort (n = 1,436) was 1.7 years, compared with 37.4 years for the AD cohort (n = 1,178), with age at diagnosis ranging from 18 to 80.7 years (median, 37.4 yr) (Figure 1C). For this cohort, the greatest known age of the CD cohort (median, 44.4 yr) was also significantly lower than that of the AD cohort (median, 49.0 yr; P < 0.001) (Figure 1D).
Adult Diagnosis Correlates with the Nonclassic CF Phenotype
Normal (PS) or absent (PI) exocrine pancreatic function is the clinical feature often used to distinguish between classic and nonclassic forms of CF. For long-term survivors in the Colorado database, only 12.8% of the AD cohort was PI, compared with 82.9% of the CD cohort (P < 0.0001) (Table 1). For the CFF Registry database, 50.4% of the AD cohort was PI, compared with 79% of the CD cohort (P < 0.0001) (Table 1). For most patients, data demonstrating formal testing of pancreatic function were lacking, and thus use of pancreatic enzyme replacement therapy was used as a marker of PI.
A “high-risk” genotype is defined by the presence of two mutations from class I to III, and correlates strongly with a more severe clinical phenotype (58). A “low-risk” genotype is defined by the presence of one (or more) class IV and V mutations, and is typically associated with later diagnosis, pancreatic sufficiency, and improved survival (58, 59). For subjects in the Colorado database with available mutational analysis (87.3%), only 5.3% of the AD cohort had a high-risk genotype, compared with 83.3% of the CD cohort (P < 0.0001) (Table 1). In the CFF Registry database, only 45% of subjects had sufficient data available from genetic analysis to determine CFTR mutation class. In the AD cohort, 30.9% had a high-risk genotype, compared with 92.3% of the CD cohort (Table 1).
In the patient cohorts studied and described in this article, the adult diagnosis correlated strongly with PS and with a low-risk genotype, which are the principal features of nonclassic CF. Thus, the age at diagnosis currently represents the most feasible criterion by which to designate classic and nonclassic forms of the disease for the purpose of reviewing databases of long-term survivors of CF.
Disease Severity and Decline in Lung Function of Patients with CF Older than 40 Years
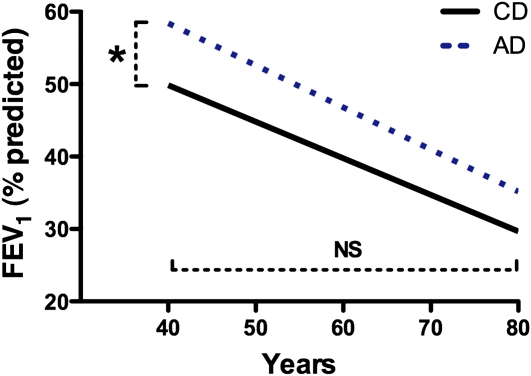
Progression of CF lung disease is most frequently approximated by the decline in FEV1 (percent predicted) over time. FEV1 values from all clinic visits between 1992 and 2007 (n = 35,128) captured in the CFF Registry for patients more than 40 years of age and less than 60 years of age were analyzed. The average length of follow-up in the AD group was longer (62.2 mo ± 1.4 [SEM], n = 1,169) than for the CD group (55.9 mo ± 1.3 [SEM], n = 1,180; P < 0.01). Although AD patients have a significantly higher FEV1 at age 40 years (57.9 ± 3.0%) compared with CD patients (50.0 ± 3.1%; P < 0.01), the annual age-related FEV1 decline from that age forward was not different between the two cohorts (CD group, −0.50 ± 0.07% vs. AD group, −0.36 ± 0.07%; P = 0.19) (Figure 2).
Figure 2.
Decline in lung function of childhood-diagnosed (CD) and adult-diagnosed (AD) patients older than 40 years was not different. FEV1 values from all clinic visits captured in the Cystic Fibrosis Foundation (CFF) Registry database for patients over 40 years of age were analyzed. Although AD patients (dotted line) have a significantly higher FEV1 at age 40 years compared with CD patients (solid line), the rate of FEV1 decline from age 40 to 60 years was not different between the two cohorts.
Survival of Patients with CF Older than 40 Years
Survival curves were calculated for patients with CF who survived past age 40 years. In both the Colorado and CFF Registry databases, median AD survival greatly exceeded CD survival (Figure 3). For patients in the Colorado database who survived past age 40 years, the median survival for the CD cohort was to age 51.7 years, versus 76.9 years for the AD cohort (P < 0.0001) (Figure 3A). For patients in the CFF Registry database, the median survival for the CD cohort was to age 53.5 years, versus 68.2 years for the AD cohort (P < 0.0001) (Figure 3B). In comparison, the average life span during the study period for the white population of the United States that survived to age 40 years was 79.2 years (60).
Gender and the Adult Diagnosis of CF
Previously, our group and others had noted a greater number of women in populations of late-diagnosed patients with CF (11, 12, 21, 26). In the Colorado database, 46.4% of CD patients more than 40 years of age were women (Figure 4A), likely due to decreased survival of young women present in CF birth cohorts before 1990 (27–31). In contrast, 72.5% of AD patients more than 40 years were women (P = 0.0038). In the CFF Registry database, 39.4% of CD patients more than 40 years of age were women (Figure 4B), whereas 54.1% of AD patients more than 40 years of age were women (P < 0.0001). For CD patients in the NJH and CFF cohorts, there was no difference between age at diagnosis between men and women (Figures 4C and 4D). However, for AD patients, the diagnosis was made later in women. For the Colorado database, the median age at diagnosis for males was 48.2 years compared with 54.0 years for women (P = 0.27) (Figure 4C). For the CFF Registry database, the median age at diagnosis for males was 35.7 years compared with 39.2 for women (P = 0.0014) (Figure 4D).
Effect of Gender on Lung Function Decline in Patients with CF Older than 40 Years
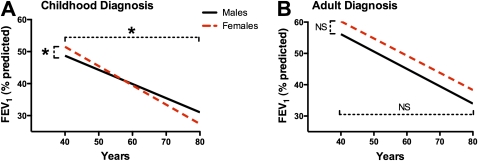
The average length of follow-up in CD females (51.2 mo ± 1.9 [SEM]) was shorter than for CD males (58.9 mo ± 1.7; P < 0.03), AD females (62.2 mo ± 1.9; P < 0.001), and AD males (58.9 ± 1.7; P < 0.001). No other differences in follow-up time were found. Women in the CD cohort had a small but significantly higher FEV1 at age 40 years (51.9 ± 5.0 vs. 48.8 ± 4.0), but a greater annual age-related decline than CD men (−0.58 ± 0.13 vs. −0.24 ± 0.10%; P = 0.034) (Figure 5A). There were no differences in FEV1 between women and men in the AD cohort (59.1 ± 4.4 vs. 56.6 ± 4.4; P = 0.64), and the annual age-related decline between the genders was not different (−0.43 ± 0.10 vs. −0.57 ± 0.10%; P = 0.34) (Figure 5B).
Figure 5.
Gender-dependent decline in lung function of patients with cystic fibrosis (CF) older than 40 years. (A) Women in the childhood diagnosis (CD) cohort (dashed line) had a higher FEV1 at age 40 years than did CD men (solid line) but demonstrated a greater rate of decline than men (*P ≤ 0.034). (B) Women in the adult diagnosis (AD) cohort (dashed line) did not have a significantly greater FEV1 and demonstrated a rate of decline in FEV1 not different from that of AD men (solid line).
Effect of Gender on Long-term Survival in CF
When the effect of gender on survival was analyzed, no difference was appreciated in survival between CD men and women. In the Colorado database, male median survival was undefined (due to low numbers) and female survival was 51.7 years (P = 0.89) (Figure 6A). In the CFF Registry, median survival for males was 53.1 years versus 55.5 years for females (P = 0.58) (Figure 6B). In contrast, a significant survival advantage was observed for AD women. In the Colorado database, median survival of AD women was 86.7 years compared with 73.2 years for their male counterparts (P = 0.038) (Figure 6C). For the CFF Registry database, female median survival was 72.0 years compared with 62.8 years for men (P < 0.0001) (Figure 6D). For reference, the average life span for white women in the U.S. population who survived past age 40 years was 81.3 years during the period of this study, compared with 77.0 years for men (60).
Together, Figures 5 and 6 support the conclusion that the effect of gender on long-term survivors of CF differs with the age at diagnosis. In the CD cohort, lung function declines more rapidly for females past age 40 years and the expected survival advantage for females who reach age 40 years is not present. However, in the AD cohort, the rate of FEV1 decline for women is equal to that of men, and women demonstrate a significantly longer life span than their male counterparts.
Phenotypic Consequence of 5T Splicing Variants in Long-term Survivors of CF
In populations of children and young adults, the presence of a 5T allele in trans with a known CF-causing mutation has been associated with a wide range of disease severity, but in the vast majority of cases appears to be clinically inconsequential (16, 18, 61–63). In the Colorado database, 20 individuals were identified with a 5T allele (Table E2). From the CFF Registry database, only 12 additional patients were identified, and were less completely characterized (data not shown). The most common accompanying mutation was ΔF508 (10 of 20). Each patient had phenotypic evidence of airway disease, predominantly bronchiectasis (17 of 20) or sinusitis (11 of 20), and all underwent extensive testing to eliminate a range of other immunodeficiency disorders. In addition, all had at least one respiratory tract pathogen, including characteristic CF infections such as mucoid or nonmucoid Pseudomonas aeruginosa (9 of 20) and Staphylococcus aureus (9 of 20), as well as nontuberculous mycobacteria (14 of 20). All of these patients were diagnosed as adults (median age, 54.5 yr). Of the 20 patients with CF with a 5T allele, more than 40 years of age, and in the Colorado database, 80% (16 of 20) were women, consistent with the overwhelming predominance of females in the AD population as a whole (Figure 4).
Setting of Care for Long-term Survivors of CF
Whereas patients diagnosed in childhood with classic CF have frequently received care at CF centers for their entire life, patients diagnosed as adults may not be willing or able to be monitored at a CF care center. The CFF Registry was queried to determine which patients in the Colorado database had ever been entered into the CFF Registry, and for which years CFF Registry data were available for each subject. Whereas 97.7% (43 of 44) of CD patients more than 40 years of age were found to be in the CFF Registry, only 63.6% (70 of 110) of AD patients had ever been entered in the CFF Registry (P < 0.001). Analysis of the six most recent years for which each subject was eligible for entry into the CFF Registry demonstrated that on average, 60% of CD patients were seen at a CF care center during any given year, compared with 41.7% of AD patients (P < 0.001) (Figure 7). There was no difference between the percentage of males and females attending CF centers in either the CD or AD cohorts (data not shown).
In young patients it has been reported that females are less adherent to treatment (45–47, 49, 64–66). In long-term survivors of CF who have been emancipated from their parents for decades, decreased adherence to recommended therapies would likely be reflected in less frequent attendance at CF centers. Current CFF care guidelines recommend four CF center visits each year, and the mean for all patients with CF in the CFF Registry is 5.0 per year (2). We queried the CFF Registry to determine the annual rate of clinic visits for males and females more than age 40 years from 1992 to 2007 (n = 66,371 visits). Female patients with the childhood diagnosis averaged 2.72 ± 1.1 (mean ± SD) annual visits compared with 2.54 ± 1.0 for male patients (P = 0.002). Female patients with an adult diagnosis averaged 2.82 ± 1.4 visits per year compared with 2.67 ± 1.3 for male patients (P = 0.03). When the number of annual visits for CD females was compared with AD females there was no difference (P = 0.86), and there was also no difference between CD males and AD males (P = 0.67).
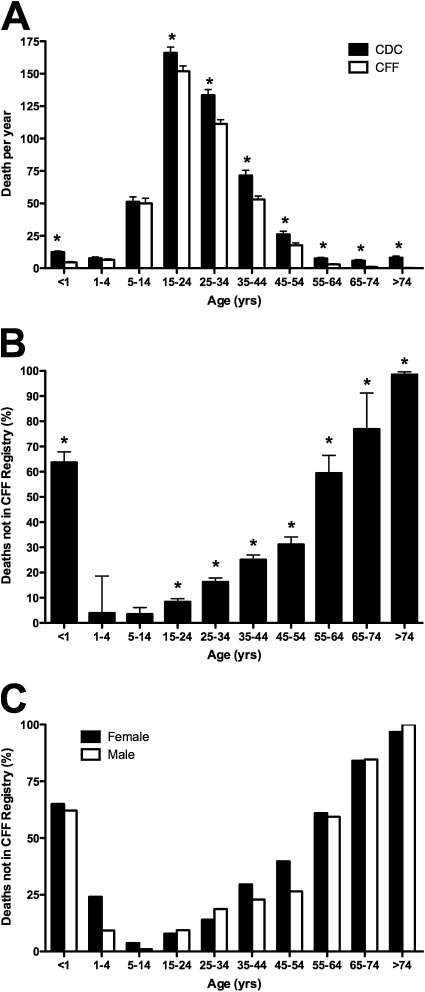
The majority of patients with CF ultimately die of respiratory complications as a result of progressive lung disease. As their condition worsens, patients monitored at a CF care center would typically be seen with greater frequency, and their death recorded in the CFF Registry database. The number of deaths captured in the CFF Registry from 1992 to 2005 (n = 5,589) was compared with the number of deaths in which CF was listed as the cause of death, or contributing condition, in the MCOD for the corresponding years (n = 6,866). CF deaths for age classifications ranging from less than 1 year to greater than 74 years were compared between the two databases. The number of deaths for patients aged 1–4 and 4–14 years recorded in the CFF Registry was nearly identical to the MCOD database; however, deaths of infants (age, <1 yr) and patients more than 14 years of age was significantly different (Figure 8A) between the two databases. For patients older than 45 years, which would encompass most of the AD cohort, only 45.9% of the deaths over the 14-year span were captured in the CFF Registry (Figure 8B). No gender-dependent difference was present in the percentage of deaths captured in the CFF Registry over all ages studied, or in the group of long-term survivors more than 45 years of age (Figure 8C).
Figure 8.
Long-term survivors are less likely to receive care at a cystic fibrosis (CF) center at the time of death. (A) Deaths recorded in the Cystic Fibrosis Foundation (CFF) Registry (open columns) were compared with the number of CF-related deaths in the CDC Mortality Multiple Cause-of-Death (MCOD) Public Use Record (CDC, solid columns). From 1 to 14 years of age, there was no significant difference between the databases. However, significantly fewer infants (age, <1 yr) and patients older than 14 years were entered into the CFF Registry (columns represent means ± SEM; *P < 0.05). (B) Percentage of CF-related deaths not captured in the CFF Registry. The inverse ratio of deaths recorded in the CFF Registry to CF-related deaths in the MCOD is plotted for each age interval (columns represent means ± SEM; P < 0.05). (C) Effect of gender on the percentage of CF-related deaths not captured in the CFF Registry. No significant difference was present between males and females over all ages, or in the subcohort greater than 45 years of age.
Together, results presented in Figures 7 and 8 support the conclusion that long-term survivors of CF are often not monitored at CF centers, and that patients with an adult diagnosis of CF are less likely to receive care at an accredited CF center, or to be represented in the CFF Registry, when compared with the CD cohort. Although females and males appear equally likely to establish care at a CF center, the females more than 40 years of age come closer to achieving the recommended number of annual visits, suggesting that poor adherence to treatment reported in young female cohorts is not present in long-term survivors.
Response to CF Center Care by Newly Diagnosed Adult Patients
With a different spectrum of infections, organ involvement, and CFTR mutations, the response of adult-diagnosed patients with CF to treatment strategies validated in classic childhood-diagnosed patients with CF is unknown. The current standard for response to treatment in CF is improvement in FEV1. Change in FEV1 of AD patients who established care at the Colorado CF center with a minimum of 1 year of follow-up (n = 32) was analyzed. The change in FEV1 (percent predicted) was determined by comparing the FEV1 measured at the initiation of standard CF center care (first CF clinic after a new diagnosis) with the subject's best FEV1 for each subsequent 12-month interval. After diagnosis, FEV1 improved 7.8 ± 2.2% within 12 months and 11.4 ± 2.3% within 24 months. Over a 4-year period, CF center care resulted in a sustained improvement in FEV1 over baseline (P = 0.0013) (Figure 9A). The identical analysis was performed on data from patients older than 40 years in the CFF Registry database and who were newly diagnosed between 1992 and 2007 (n = 308). As with the Colorado database, lung function improved significantly in newly diagnosed adults who received care at CF centers nationwide (Figure 9A). After diagnosis, FEV1 increased 9.7 ± 1.9% within 12 months and 10.1 ± 2.9% within 24 months, and was sustained for the 4-year period (P < 0.0001). This response can be compared with the adult CF population as a whole, which we found to have a lung function decline rate of 0.58% per year (Figure 2), or a historical rate of 0.77% annual decline previously reported in adult-diagnosed patients (12). The extent of FEV1 response from the CFF Registry database was not significantly different between men and women (Figure 9B).
Cause of Death in Long-term Survivors of CF
Little has been reported concerning the cause of death in long-term survivors of CF. In both the CD and AD cohorts, the vast majority of patients were still living, with a trend toward a lower percent mortality in the AD cohort in the Colorado database, and a significantly lower percent mortality in the AD CFF cohort (Table 1). In both databases, AD patients were less likely to have received a lung transplant compared with long-term CD survivors.
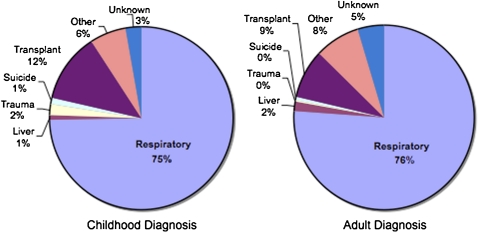
Historically, nearly all patients with CF died of respiratory failure, or of complications after transplantation (2). However, AD patients generally have more limited pancreatic involvement and less severe lung disease than CD patients of comparable ages (Figure 3). Cause of death data from the CFF Registry database (seven most common causes) were compared between deceased patients from the CD (n = 380) and AD (n = 252) cohorts. CD patients more than 40 years of age died predominantly of respiratory failure (75%) and transplantation-related complications (12%). Despite a significantly longer life span, AD patients also died predominantly of respiratory failure (76%) and transplantation-related complications (9%), at an almost identical frequency (Figure 10). The frequency of death from respiratory or transplant related complications was not different by gender for either the CD or AD cohorts (data not shown). These same trends were present in the Colorado database, although the number of deaths with known causes was insufficient to allow meaningful comparisons (data not shown).
Figure 10.
Cause of death analysis of patients in the Cystic Fibrosis Foundation (CFF) Registry whose greatest age exceeded 40 years. Deceased patients with CF in the childhood diagnosis (CD, left) and adult diagnosis (AD, right) cohorts were found to have frequencies for major causes of death that were not different. Specifically, 87% of CD patients died of respiratory or transplantation-related complications, compared with 85% of AD patients.
DISCUSSION
This report is the first to analyze disease progression, delivery of care, and cause of death for long-term survivors of CF in the context of gender and age at diagnosis. As previously reported in smaller patient populations, we found that patients with CF diagnosed in childhood (birth to age 10 yr) nearly always have PI and a high-risk CFTR genotype, whereas the adult diagnosis was strongly associated with PS and a low-risk CFTR genotype (Table 1). On average, AD patients have better lung function (FEV1) than age-matched CD patients at age 40 years. Unexpectedly, for patients older than age 40 years, the rate of FEV1 decline was not different between the AD and CD cohorts (Figure 2). More importantly, despite a significantly longer life span for AD patients, the frequency of death from respiratory failure or transplantation-related complications was nearly identical between the two cohorts (Figure 10). Thus, when the clinical consequence of CF diagnosed in adulthood is viewed in the context of the lifetime of the patient, instead of simply considering the features at the time of diagnosis, it is apparent that the pulmonary manifestations are not a mild form of the disease, but rather a delayed but equally serious form of the childhood presentation.
The effect of sex and gender on diagnosis, disease progression, and survival remains one of the most intensely studied aspects of CF pathophysiology and epidemiology. Differences in CF disease manifestations between males and females may result both from biological modifiers relating to sex, and from the cultural attribute of gender that determines norms for a variety of attitudes and behaviors that may affect outcome (34). The integrated effects of sex and gender are likely magnified in long-term survivors of CF. The earliest known impact of gender is the age at diagnosis in children. Reports from Ireland, the United Kingdom, and the United States have found that the CF diagnosis in female infants (in the absence of newborn screening) occurs later than in males (23–25). Despite equal disease severity, diagnosis of females in the United States lagged males by 4 months (25). As early diagnosis has been proven beneficial (67, 68), delayed diagnosis likely represents a disadvantage for female patients. In this report, we found that for patients who succeed in reaching 40 years, the age of childhood diagnosis was not different between genders, and females actually trended toward an earlier age at diagnosis (Figures 4C and 4D). This finding suggests that females diagnosed later in childhood were less likely to reach age 40 years, supporting the conclusion that delayed diagnosis is a disadvantage for survival. Accordingly, we found that women represent a significant minority of survivors who reach age 40 years in the CD cohorts, as only 35.7% of the CFF Registry and 44.5% of the Colorado database were female (Figures 4A and 4B). This finding is consistent with the well-described “gender gap” in survival, in which females were found to have a median life span 3 to 5 years shorter than that of males in cohorts of CD patients born before 1990 (27–31).
Unexpectedly, delayed diagnosis of females was present in the AD cohort, in which the median age at diagnosis for females was 3.5 years later than men in the CFF Registry, and 5.8 years later in the Colorado database (Figures 4C and 4D). In children, it has been suggested that the delay is due to reduced recognition of respiratory symptoms in female infants, or an unconscious gender-based bias by physicians against pursuing the CF diagnosis (25) in females. The greatest lag in diagnosis between genders has been reported to occur in the setting of patients presenting with predominantly respiratory symptoms (23–25). Within the Colorado database, virtually all women were referred because of unexplained bronchiectasis or chronic airway infection. Thus, it is possible that the same factors that contribute to delayed diagnosis in female infants with the classic phenotype are also responsible for delayed CF diagnosis of females in adulthood.
Despite a later age at diagnosis, we found that the prevalence of the adult CF diagnosis is significantly greater in females. In the CFF Registry, 54.1% of the AD cohort was female, and in the Colorado database there was nearly a three-to-one distribution of women to men (Figures 4A and 4B). A trend toward female predominance has also been observed in other AD cohorts (11, 12, 21, 26). A number of gender-related factors could contribute to an increased frequency of adult diagnosis in females, including greater persistence in seeking the diagnosis and overall greater use of the health care system. In addition, a bias may exist against referral of males presenting with congenital bilateral absence of the vas deferens (CBAVD) to CF centers, resulting in an underrepresentation of males in databases of adult-diagnosed patients with CF. If such a bias exists, it may be magnified within the Colorado database, in which virtually all referrals were based on respiratory complaints. However, it is also plausible that female predominance is based on molecular mechanisms and differences in phenotype. Clues to a biological mechanism may come from patients possessing a 5T-splicing variant as their most mild CFTR abnormality.
The effect of gender on 5T penetrance has been questioned from analysis of large populations undergoing CFTR screening, in which penetrance of the 5T allele in young females has been reported to be both higher (69) and lower (70, 71) than in males. A potential source of confusion is that the preponderance of genetic screening for CF mutations and phenotype analysis is performed in young adults of childbearing age or infants (69, 71). In the Colorado database of long-term survivors, 80% of patients with adult-diagnosed CF and a 5T allele were women (Table E2). This finding suggests that the 5T allele can have a clinically significant effect in an older cohort (median age, 58.0 yr), even when the second CFTR defect was another 5T allele (n = 1) or a low-risk class IV or V mutation (Table E2). It is possible that in certain individuals, pulmonary manifestations of the 5T allele are not clinically apparent until late middle age. In men, the 5T allele can result in CBAVD, but there is growing evidence that these men often have some degree of pulmonary or sinus disease (72–74). In a small cohort of men with CBAVD and no pulmonary symptoms (mean age, 39 yr), bronchoalveolar lavage demonstrated increased cytokines and subclinical infection (75). This finding supports the conclusion that CFTR mutations resulting in CBAVD can also induce pulmonary disease, but respiratory symptoms may not be apparent at the time of genetic testing. Likely, the true penetrance of the 5T allele in both males and females is underestimated in population screening of young adults. As newborn screening for CF is widely implemented, and most programs test for CFTR mutations, many infants are being identified with low-risk genotypes and are being designated as having CFTR-related metabolic syndrome (CRMS) (55). We believe that some infants diagnosed with CRMS today may in time have similar disease progression as AD patients described in this article.
In this study of long-term survivors of CF, we were especially interested in looking for evidence of the “gender gap” of decreased survival in females diagnosed in childhood (27–31). Mechanisms that contribute to worse outcomes in females include relatively poorer nutritional status, delayed age at diagnosis, differences in lung development, adverse effects of estrogen, increased frequency of pulmonary exacerbation, earlier onset of CF-related diabetes, and earlier first acquisition of P. aeruginosa (25, 35–44). Equally worrisome are reports of gender-related psychological and behavioral attributes of young female patients with CF that could place them at a disadvantage compared with their male peers. These include increased difficulty in coping with the disease, increased illness-related strains and worries, greater depression, decreased quality of life scores, greater treatment discouragement, and greater acceptance of being underweight (45–52), which may contribute to decreased treatment adherence and worse outcomes (45–47, 49, 64–66). The phenomenon of decreased survival in females appears to be confined to patients of age 1–20 years (30, 33, 76). In the current therapeutic environment, some have reported that the gender gap is no longer present in children (32, 33). However, in this study, the gender gap in the CD cohorts is still apparent. Despite successfully surviving to age 40 years, and having a small but significantly greater FEV1 than their male counterparts, the rate of FEV1 decline of CD women past age 40 years is greater (Figure 5A), and median survival is not significantly greater than that of men (Figures 6A and 6B). In the non-Hispanic white U.S. population, women who reach age 40 years are expected to have a 4.3-year survival advantage (60), and thus in these long-term female survivors, a relative gender gap is still present.
For long-term survivors of CF diagnosed as adults, gender appears to have a different effect on survival. Unlike the females in the CD cohorts, the AD females had an identical rate of FEV1 decline as males (Figure 5B), and enjoyed a distinct survival advantage of 13.5 years in the Colorado database and 9.2 years in the CFF Registry database (Figures 6C and 6D), far exceeding the expected survival advantage (60). Thus, a “reverse gender gap” is present within these databases, defined by increased survival for AD females. To our knowledge, this is the first report of any clinical parameter in which CF females have an advantage over males. However, further investigation is required to determine whether the preponderance of adult-diagnosed females (Figures 4A and 4B), and the survival advantage for females (Figures 6C and 6D), are due to underrepresentation of adult-diagnosed males with mild CF lung disease in the CFF Registry.
In this analysis, both age at diagnosis and age of the patient were major factors in determining setting of care for long-term survivors of CF. As most patients in the Colorado database live outside the region, we cross-referenced the CFF Registry to determine whether patients were being seen at accredited CF centers in their home states. Patients in the AD cohort were less likely than CD patients to ever appear in the CFF Registry or to have been seen at a CF care center over the six most recent years (Figure 7). Nearly one-third of CD patients greater than age 40 years were also not seen in a CF center over the same period, although virtually all had at one point been entered into the CFF Registry. A comparison of mortality data from the CFF Registry with the MCOD Index provided a second assessment of CF center attendance by long-term survivors. For the CF population as a whole, 81.4% of the deaths recorded in the MCOD Index were captured in the CFF Registry. However, for patients with CF greater than age 45 years, on average only 45.9% of deaths reported in the MCOD Index were included in the CFF Registry, and the percentage fell dramatically with advancing age (Figure 8B). Analysis of the 1990 CFF Registry, when the median age in the database was 12.5 years, demonstrated that 92% of CF deaths reported in the U.S. Vital Statistics were captured within the CFF Registry (77). Together, these findings support the conclusion that long-term survivors of CF as a whole are frequently not seen at CF centers, and this effect is greatest in the older AD cohort.
The lack of specific information concerning prognosis, and the absence of an evidence-based treatment approach, may contribute to poor attendance of AD patients at traditional CF care centers. It is not known whether current care recommendations will achieve demonstrable success in the AD cohort. Clinical trials of available CF therapeutics were conducted on patients with the classic phenotype, and AD patients typically have different CFTR mutations, different absorption of nutrition and medications, and different patterns of airway infection. However, in this study, we found that newly diagnosed adult patients with CF receiving CF center care achieved a significant and sustained improvement in FEV1 from baseline (time of diagnosis) over a period of 4 years (Figure 10A). This is particularly meaningful in comparison with the 0.58% annual rate of FEV1 (percent predicted) decline observed in the AD population as a whole (Figure 2), or a historical rate of 0.74% annual decline in FEV1 (percent predicted) reported in the mid-1990s for late-diagnosed patients with CF (12). Typically, CF center care represents a more aggressive approach to airway clearance, and pathogen-specific antibiotic treatment of airway infection, as compared with community standards. CF center care has previously been associated with improved outcomes for children and adolescents with a CD diagnosis (78, 79). This finding supports the idea that the CF center approach is of benefit to the AD cohort, even in the absence of specific, evidence-based therapies for this population.
There was no difference in CF center attendance between males and females, either in the Colorado database (Figure 7) or when the MCOD database was compared with the CFF Registry (Figure 8C). Current CFF Clinical Practice Guidelines recommend four center visits per year. Women more than 40 years of age from both the CD and AD cohorts had a small but significantly greater frequency of annual CF care center visits, compared with their male peers. In middle-aged or elderly patients, given the expense and effort required to seek care at a CF center, the number of annual visits likely serves as a marker of adherence to other treatment recommendations. No gender-specific difference was detected in the extent or duration of response to CF center care (Figure 9B). Thus, a gender-related difference in disease perception and adherence that may have contributed to a relative disadvantage for younger females was not evident in the CD cohort that survived to age 40 years, or in females diagnosed as adults. Further studies will be needed to determine to what degree better coping skills and greater adherence to therapy as adolescents and young adults predict long-term survival in patients with the childhood diagnosis.
Long-term survivors represent an important and growing segment of the CF population that deserves increased study. Although previously regarded as medical curiosities, these patients represent the vanguard of a rapidly aging CF population. However, meaningful conclusions can be reached only if gender and the classic and nonclassic phenotypes are considered independently. Particular focus needs to be placed on better characterizing the clinical course of patients with the nonclassic phenotype who have historically been diagnosed as adults. Although we have found that these patients generally benefit from CF center care, future studies of specific therapies are needed. Studies of adult-diagnosed patients are also critical, as only late in life can the true penetrance and complete phenotype associated with a vast number of rare CFTR mutations be conclusively defined. With tremendous acceleration in newborn screening and prereproductive genetic testing, infants and young adults are increasingly being identified with the genetic potential for CF in the absence of clinically significant disease. Identification of middle-aged or elderly patients with the identical CFTR genotype may assist physicians attempting to guide patients and families as to the implications of these genetic findings, as many of the adult-diagnosed patients were themselves asymptomatic as children and young adults. Analysis of a cohort of patients with the 5T allele further supports the conclusion that even the mildest CFTR mutations have the potential to induce severe CF lung disease in old age. Our data indicate that patients with the nonclassic form of the disease who are diagnosed early and treated aggressively may be expected to live a full life span. However, when diagnosis and treatment are delayed, the morbidity of nonclassic CF is severe. These findings support the conclusion that asymptomatic individuals identified with the genetic potential for CF should be monitored at CF centers, and monitored regularly for disease progression.
Supplementary Material
Acknowledgments
The authors thank Bruce Marshall, Monica Brooks, Ase Sewell, and the Clinical Research Committee of the Cystic Fibrosis Foundation for access to the database and support of this project.
Supported by the CF Foundation, Rebecca Runyon Bryan Chair for Cystic Fibrosis, Max and Yetta Karasik Foundation, and NIH 1R01HL090991.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201001-0092OC on May 6, 2010
Author Disclosure: J.A.N. served on the Board or Advisory Board for Pharmaxis ($5,001–$10,000), and received lecture fees from the France Foundation and MedLearning ($10,001–$50,000). He received grant support from Novartis, Genentech, Altus ($10,001–$50,000), Transave, Boehringer Ingelheim ($50,001–$100,000), the NIH, the CF Foundation, the Gates Foundation, the Karasik Foundation, and the Department of Defense (more than $100,001). C.S.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.J.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.C.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.M.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.G.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.L.Y. received grant support from the Cystic Fibrosis Foundation (more than $100,001). D.P.N. received grant support from Gilead Sciences Inc. ($10,001–$50,000), the Cystic Fibrosis Foundation (more than $100,001), and the Department of Defense ($10,001–$50,000). J.S.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.A.H. served on the Board or Advisory Board for Hill-Rom ($5,001–$10,000) and received lecture fees from Hill-Rom ($10,001–$50,000). She served as an expert witness for Lawson, Dugan, and Murray, Sutter Ins. Services Corp, and Sandberg, Phoenix & von Gontard, P.C. ($1,001–$5,000) and she received grant support from Bayer ($1,001–$5,000). M.D.I. served on the Board for OMNI-Bio and was an expert witness for Flynn, Gaskin, & Bennett, LLP ($5,001–$10,000). C.L.D. served on the Board or Advisory Board for Otsuka and Sanofi-Aventis ($1,001–$5,000). He received lecture fees from Cellestis ($1,001–$5,000) and served as an expert witness for Patterson Belknap Webb and Tyler LLP and May and Buege, Ltd ($1,001–$5,000). He received grant support from Oxford Immunotec, Designer Diagnostics, Gilead, Pharmaxis ($10,001–$50,000), the NIH, and the CDC (more than $100,001). He receives royalties from Bruckmeier Publishing (up to $1,000). J.L.T.-C. served as a consultant and was on the Advisory Board for Genentech ($1,001–$5,000). She received lecture fees from Novartis ($1,001–$5,000) and received grant support from Genentech ($5,001–$10,000), Vertex, Solvay, and Transave ($10,001–$50,000). She has received grant support from the NIH and the CFF ($50,001–$100,000). F.J.A. served on the Advisory Board for Inspire Pharmaceuticals and PTC Therapeutics (up to $1,000). He received lecture fees from Inspire Pharmaceuticals ($1,001–$5,000) and served as an expert witness for Gilead Sciences (up to $1,000). He received grant support from Digestive Care Inc. ($50,001–$100,000), PTC Therapeutics, Gilead Sciences, Vertex Pharmaceuticals (more than $100,001), Altus Pharmaceuticals ($50,001–$100,000), the NIH, and the Cystic Fibrosis Foundation (more than $100,001). He has other financial interests with KaloBios Pharma, Axcan Pharma, Yasoo Health, Inc. ($50,001–$100,000), INO Therapeutics, Inc., and MPEX Pharmaceuticals ($10,001–$50,000). M.T.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.K.S. has received grant support from Vertex Pharmaceutical ($5,001–$10,000) and the Cystic Fibrosis Foundation (more than $100,001). She served as a consultant and was on the Board or Advisory Board for the Cystic Fibrosis Foundation (up to $1,000).
References
- 1.Sontag MK, Hammond KB, Zielenski J, Wagener JS, Accurso FJ. Two-tiered immunoreactive trypsinogen-based newborn screening for cystic fibrosis in Colorado: screening efficacy and diagnostic outcomes. J Pediatr 2005;147(3, Suppl)S83–S88. [DOI] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2007 annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2008.
- 3.Hodson ME, Simmonds NJ, Warwick WJ, Tullis E, Castellani C, Assael B, Dodge JA, Corey M. International Study Of Aging In Cystic. An international/multicentre report on patients with cystic fibrosis (CF) over the age of 40 years. J Cyst Fibros 2008;7:537–542. [DOI] [PubMed] [Google Scholar]
- 4.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153:S4–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle MP. Nonclassic cystic fibrosis and CFTR-related diseases. Curr Opin Pulm Med 2003;9:498–503. [DOI] [PubMed] [Google Scholar]
- 6.Highsmith WE, Overbeek SE, Hilvering C. A novel mutation in the CF gene in patients with pulmonary disease but normal sweat chloride concentrations. N Engl J Med 1994;331:974–980. [DOI] [PubMed] [Google Scholar]
- 7.Sanders JS, Pryor TD, Wedel MK. Prolonged survival in an adult with cystic fibrosis. Chest 1980;77:226–227. [DOI] [PubMed] [Google Scholar]
- 8.Fiel S. Cystic fibrosis in an adult. Emerg Med 1988;20:109–121. [Google Scholar]
- 9.Hunt B, Geddes D. Newly diagnosed CF in middle and later life. Thorax 1984;40:23–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su C, Beanblossom B. Typical CF in an elderly woman. Am J Med 1989;86:701–703. [DOI] [PubMed] [Google Scholar]
- 11.Rodman DM, Polis JM, Heltshe SL, Sontag MK, Chacon C, Rodman RV, Brayshaw SJ, Huitt GA, Iseman MD, Saavedra MT, et al. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med 2005;171:621–626. [DOI] [PubMed] [Google Scholar]
- 12.Gan KH, Geus WP, Bakker W, Cornelis CBHW, Heijerman HGM. Genetic and clinical features of patients with cystic fibrosis diagnosed after the age of 16 years. Thorax 1995;50:1301–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating CL, Liu X, Dimango EA. Classic respiratory disease but atypical diagnostic testing distinguishes adult presentation of cystic fibrosis. Chest 2009;137:1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilljam M, Ellis L, Corey M, Zielenski J, Durie P, Tullis DE. Clinical manifestations of cystic fibrosis among patients with diagnosis in adulthood. Chest 2004;126:1215–1224. [DOI] [PubMed] [Google Scholar]
- 15.Sharer N, Schwarz M, Malone G, Howarth A, Painter J, Super M, Braganza J. Mutations of the cystic fibrosis gene in patients with chronic pancreatitis. N Engl J Med 1998;339:645–652. [DOI] [PubMed] [Google Scholar]
- 16.Cohn JA, Friedman KJ, Noone PG, Knowles MR, Silverman LM, Jowell PS. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 1998;339:653–658. [DOI] [PubMed] [Google Scholar]
- 17.Groman JD, Karczeski B, Sheridan M, Robinson TE, Fallin MD, Cutting GR. Phenotypic and genetic characterization of patients with features of “nonclassic” forms of cystic fibrosis. J Pediatr 2005;146:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chillon M, Casals T, Mercier B, Bassas L, Lissens W, Silber S, Romey MC, Ruiz-Romero J, Verlingue C, Claustres M, et al. Mutations in the cystic fibrosis gene in patients with congenital absence of the vas deferens. N Engl J Med 1995;332:1475–1480. [DOI] [PubMed] [Google Scholar]
- 19.Stewart B, Zabner J, Shuber AP, Welsh MJ, McCray PB Jr. Normal sweat chloride values do not exclude the diagnosis of cystic fibrosis. Am J Respir Crit Care Med 1995;151:899–903. [DOI] [PubMed] [Google Scholar]
- 20.McCloskey M, Redmond AO, Hill A, Elborn JS. Clinical features associated with a delayed diagnosis of cystic fibrosis. Respiration 2000;67:402–407. [DOI] [PubMed] [Google Scholar]
- 21.Widerman E, Millner L, Sexauer W, Fiel S. Health status and sociodemographic characteristics of adults receiving a CF diagnosis after age 18 years. Chest 2000;118:427–433. [DOI] [PubMed] [Google Scholar]
- 22.Simmonds NJ, Cullinan P, Hodson ME. Growing old with cystic fibrosis: the characteristics of long-term survivors of cystic fibrosis. Respir Med 2009;103:629–635. [DOI] [PubMed] [Google Scholar]
- 23.Farrell P, Joffe S, Foley L, Canny GJ, Mayne P, Rosenberg M. Diagnosis of cystic fibrosis in the Republic of Ireland: epidemiology and costs. Ir Med J 2007;100:557–560. [PubMed] [Google Scholar]
- 24.McCormick J, Sims EJ, Mehta A. Delayed diagnosis of females with respiratory presentation of cystic fibrosis did not segregate with poorer clinical outcome. J Clin Epidemiol 2006;59:315–322. [DOI] [PubMed] [Google Scholar]
- 25.Lai HC, Kosorok MR, Laxova A, Makholm LM, Farrell PM. Delayed diagnosis of US females with cystic fibrosis. Am J Epidemiol 2002;156:165–173. [DOI] [PubMed] [Google Scholar]
- 26.Modolell I, Alvarez A, Guarner L, De Gracia J, Malagelada JR. Gastrointestinal, liver, and pancreatic involvement in adult patients with cystic fibrosis. Pancreas 2001;22:395–399. [DOI] [PubMed] [Google Scholar]
- 27.Cystic Fibrosis Genotype–Phenotype_Consortium. Correlation between genotype and phenotype in patients with CF. N Engl J Med 1993;329:1308–1313. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor GT, Quinton HB, Kahn R, Robichaud P, Maddock J, Lever T, Detzer M, Brooks JG. Case-mix adjustment for evaluation of mortality in CF. Pediatr Pulmonol 2002;33:99–105. [DOI] [PubMed] [Google Scholar]
- 29.Fogarty A, Hubbard R, Britton J. International comparison of median age at death from cystic fibrosis. Chest 2000;117:1656–1660. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol 1997;145:794–803. [DOI] [PubMed] [Google Scholar]
- 31.Kerem E, Reisman J, Corey M, Canny GJ, Levison H. Prediction of mortality in patients with cystic fibrosis. N Engl J Med 1992;326:1187–1191. [DOI] [PubMed] [Google Scholar]
- 32.Verma N, Bush A, Buchdahl R. Is there still a gender gap in cystic fibrosis? Chest 2005;128:2824–2834. [DOI] [PubMed] [Google Scholar]
- 33.Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry: 2006 annual data report to the center directors. Bethesda, MD: Cystic Fibrosis Foundation; 2007.
- 34.Wolfenden LL, Schechter MS. Genetic and non-genetic determinants of outcomes in cystic fibrosis. Paediatr Respir Rev 2009;10:32–36. [DOI] [PubMed] [Google Scholar]
- 35.Milla CE, Billings J, Moran A. Diabetes is associated with dramatically decreased survival in female but not male subjects with cystic fibrosis. Diabetes Care 2005;28:2141–2144. [DOI] [PubMed] [Google Scholar]
- 36.van den Berg JM, Kouwenberg JM, Heijerman HG. Demographics of glucose metabolism in cystic fibrosis. J Cyst Fibros 2009;8:276–279. [DOI] [PubMed] [Google Scholar]
- 37.Levy H, Kalish LA, Cannon CL, Garcia KC, Gerard C, Goldmann D, Pier GB, Weiss ST, Colin AA. Predictors of mucoid Pseudomonas colonization in cystic fibrosis patients. Pediatr Pulmonol 2008;43:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ. Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol 2003;35:257–262. [DOI] [PubMed] [Google Scholar]
- 39.Demko CA, Byard PJ, Davis PB. Gender differences in CF: Pseudomonas aeruginosa infection. J Clin Epidemiol 1994;43:125–131. [DOI] [PubMed] [Google Scholar]
- 40.Grasemann H, Storm van's Gravesande K, Buscher R, Drazen JM, Ratjen F. Effects of sex and of gene variants in constitutive nitric oxide synthases on exhaled nitric oxide. Am J Respir Crit Care Med 2003;167:1113–1116. [DOI] [PubMed] [Google Scholar]
- 41.Block JK, Vandemheen KL, Tullis E, Fergusson D, Doucette S, Haase D, Berthiaume Y, Brown N, Wilcox P, Bye P, et al. Predictors of pulmonary exacerbations in patients with cystic fibrosis infected with multi-resistant bacteria. Thorax 2006;61:969–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zemel BS, Jawad AF, FitzSimmons S, Stallings VA. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr 2000;137:374–380. [DOI] [PubMed] [Google Scholar]
- 43.Pagtakhan RD, Bjelland JC, Landau LI, Loughlin G, Kaltenborn W, Seeley G, Taussig LM. Sex differences in growth patterns of the airways and lung parenchyma in children. J Appl Physiol 1984;56:1204–1210. [DOI] [PubMed] [Google Scholar]
- 44.Coakley RD, Sun H, Clunes LA, Rasmussen JE, Stackhouse JR, Okada SF, Fricks I, Young SL, Tarran R. 17β-Estradiol inhibits Ca2+-dependent homeostasis of airway surface liquid volume in human cystic fibrosis airway epithelia. J Clin Invest 2008;118:4025–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson JM, Wall M, Berge J, Milla C. Associations of psychosocial factors with health outcomes among youth with cystic fibrosis. Pediatr Pulmonol 2009;44:46–53. [DOI] [PubMed] [Google Scholar]
- 46.Czajkowski DR, Koocher GP. Medical compliance and coping with cystic fibrosis. J Child Psychol Psychiatry 1987;28:311–319. [DOI] [PubMed] [Google Scholar]
- 47.Patterson JM, Wall M, Berge J, Milla C. Gender differences in treatment adherence among youth with cystic fibrosis: development of a new questionnaire. J Cyst Fibros 2008;7:154–164. [DOI] [PubMed] [Google Scholar]
- 48.Besier T, Schmitz TG, Goldbeck L. Life satisfaction of adolescents and adults with cystic fibrosis: impact of partnership and gender. J Cyst Fibros 2009;8:104–109. [DOI] [PubMed] [Google Scholar]
- 49.Abbott J, Conway S, Etherington C, Fitzjohn J, Gee L, Morton A, Musson H, Webb AK. Perceived body image and eating behavior in young adults with cystic fibrosis and their healthy peers. J Behav Med 2000;23:501–517. [DOI] [PubMed] [Google Scholar]
- 50.Willis E, Miller R, Wyn J. Gendered embodiment and survival for young people with cystic fibrosis. Soc Sci Med 2001;53:1163–1174. [DOI] [PubMed] [Google Scholar]
- 51.Walters S. Sex differences in weight perception and nutritional behaviour in adults with cystic fibrosis. J Hum Nutr Diet 2001;14:83–91. [DOI] [PubMed] [Google Scholar]
- 52.Arrington-Sanders R, Yi MS, Tsevat J, Wilmott RW, Mrus JM, Britto MT. Gender differences in health-related quality of life of adolescents with cystic fibrosis. Health Qual Life Outcomes 2006;4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myers LB. An exploratory study investigating factors associated with adherence to chest physiotherapy and exercise in adults with cystic fibrosis. J Cyst Fibros 2009;8:425–427. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention (CDC). Mortality data, multiple cause-of-death public-use data files. Hyattsville, MD: National Center for Health Statistics, Publications and Information Products; 2010.
- 55.Borowitz D, Parad RB, Sharp JK, Sabadosa KA, Robinson KA, Rock MJ, Farrell PM, Sontag MK, Rosenfeld M, Davis SD, et al. Cystic fibrosis foundation practice guidelines for the management of infants with cystic fibrosis transmembrane conductance regulator–related metabolic syndrome during the first two years of life and beyond. J Pediatr 2009;155:S106–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nick J, Chacon C, Brayshaw S, Jones M, Barboa C, Young R, Janssen J, Gleeksman C, Huitt G, Iseman M, et al. Diagnosis, patterns of care, and long-term survival in nonclassic CF patients [abstract]. Pediatr Pulmonol 2008;S31:402. [Google Scholar]
- 57.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. J Pediatr 1998;132:589–595. [DOI] [PubMed] [Google Scholar]
- 58.McKone EF, Goss CH, Aitken ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest 2006;130:1441–1447. [DOI] [PubMed] [Google Scholar]
- 59.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet 2003;361:1671–1676. [DOI] [PubMed] [Google Scholar]
- 60.Arias E, Curtin LR, Wei R, Anderson R. US decennial life tables for 1999–2001, United States life tables. Natl Vital Stat Rep 2008;57:1–36. [PubMed] [Google Scholar]
- 61.Noone PG, Pue CA, Zhou Z, Friedman KJ, Wakeling EL, Ganeshananthan M, Simon RH, Silverman LM, Knowles MR. Lung disease associated with the IVS8 5T allele of the CFTR gene. Am J Respir Crit Care Med 2000;162:1919–1924. [DOI] [PubMed] [Google Scholar]
- 62.Weiss FU, Simon P, Bogdanova N, Mayerle J, Dworniczak B, Horst J, Lerch MM. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut 2005;54:1456–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dray X, Fajac I, Bienvenu T, Chryssostalis A, Sogni P, Hubert D. Association of pancreas divisum and recurrent acute pancreatitis with the IVS8-5T-12TG allele of the CFTR gene and CFTR dysfunction. Pancreas 2007;35:90–93. [DOI] [PubMed] [Google Scholar]
- 64.Anthony H, Paxton S, Bines J, Phelan P. Psychosocial predictors of adherence to nutritional recommendations and growth outcomes in children with cystic fibrosis. J Psychosom Res 1999;47:623–634. [DOI] [PubMed] [Google Scholar]
- 65.DeLambo KE, Ievers-Landis CE, Drotar D, Quittner AL. Association of observed family relationship quality and problem-solving skills with treatment adherence in older children and adolescents with cystic fibrosis. J Pediatr Psychol 2004;29:343–353. [DOI] [PubMed] [Google Scholar]
- 66.Collins CE, O'Loughlin EV, Henry R. Discrepancies between males and females with cystic fibrosis in dietary intake and pancreatic enzyme use. J Pediatr Gastroenterol Nutr 1998;26:258–262. [DOI] [PubMed] [Google Scholar]
- 67.Farrell PM, Kosorok MR, Rock MJ, Laxova A, Zeng L, Lai HC, Hoffman G, Laessig RH, Splaingard ML; Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Early diagnosis of cystic fibrosis through neonatal screening prevents severe malnutrition and improves long-term growth. Pediatrics 2001;107:1–13. [DOI] [PubMed] [Google Scholar]
- 68.Waters DL, Wilcken B, Irwing L, Van Asperen P, Mellis C, Simpson JM, Brown J, Gaskin KJ. Clinical outcomes of newborn screening for cystic fibrosis. Arch Dis Child Fetal Neonatal Ed 1999;80:F1–F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morea A, Cameran M, Rebuffi AG, Marzenta D, Marangon O, Picci L, Zacchello F, Scarpa M. Gender-sensitive association of CFTR gene mutations and 5T allele emerging from a large survey on infertility. Mol Hum Reprod 2005;11:607–614. [DOI] [PubMed] [Google Scholar]
- 70.Strom CM, Crossley B, Redman JB, Buller A, Quan F, Peng M, McGinnis M, Sun W. Cystic fibrosis screening: lessons learned from the first 320,000 patients. Genet Med 2004;6:136–140. [DOI] [PubMed] [Google Scholar]
- 71.Sun W, Anderson B, Redman J, Milunsky A, Buller A, McGinniss MJ, Quan F, Anguiano A, Huang S, Hantash F, et al. CFTR 5T variant has a low penetrance in females that is partially attributable to its haplotype. Genet Med 2006;8:339–345. [DOI] [PubMed] [Google Scholar]
- 72.Kerem E, Rave-Harel N, Augarten A, Madgar I, Nissim-Rafinia M, Yahav Y, Goshen R, Bentur L, Rivlin J, Aviram M, et al. A cystic fibrosis transmembrane conductance regulator splice variant with partial penetrance associated with variable cystic fibrosis presentations. Am J Respir Crit Care Med 1997;155:1914–1920. [DOI] [PubMed] [Google Scholar]
- 73.Culard JF, Desgeorges M, Romey MC, Malzac P, Demaille J, Claustres M. A novel splice site mutation in the first exon of the cystic fibrosis transmembrane regulator (CFTR) gene identified in a CBAVD patient. Hum Mol Genet 1994;3:369–370. [DOI] [PubMed] [Google Scholar]
- 74.Claustres M, Guittard C, Bozon D, Chevalier F, Verlingue C, Ferec C, Girodon E, Cazeneuve C, Bienvenu T, Lalau G, et al. Spectrum of CFTR mutations in cystic fibrosis and in congenital absence of the vas deferens in France. Hum Mutat 2000;16:143–156. [DOI] [PubMed] [Google Scholar]
- 75.Gilljam M, Moltyaner Y, Downey GP, Devlin R, Durie P, Cantin AM, Zielenski J, Tullis DE. Airway inflammation and infection in congenital bilateral absence of the vas deferens. Am J Respir Crit Care Med 2004;169:174–179. [DOI] [PubMed] [Google Scholar]
- 76.Kulich M, Rosenfeld M, Goss CH, Wilmott R. Improved survival among young patients with cystic fibrosis. J Pediatr 2003;142:631–636. [DOI] [PubMed] [Google Scholar]
- 77.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr 1993;122:1–9. [DOI] [PubMed] [Google Scholar]
- 78.Mahadeva R, Webb K, Westerbeek RC, Carroll NR, Dodd ME, Bilton D, Lomas DA. Clinical outcome in relation to care in centres specialising in cystic fibrosis: cross sectional study. BMJ 1998;316:1771–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collins CE, MacDonald-Wicks L, Rowe S, O'Loughlin EV, Henry RL. Normal growth in cystic fibrosis associated with a specialised centre. Arch Dis Child 1999;81:241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.