Abstract
Rationale: γδT lymphocytes are enriched within the epithelial microenvironment, where they are thought to maintain homeostasis and limit immunopathology. γδT cells are postulated to exert a regulatory influence during acute allergic airway disease, but the mechanism is unknown. Although regulation of allergic airway disease has been attributed to IL-17–producing T helper (Th) 17 cells, we have found that γδT cells represent the major source of IL-17 in the allergic lung.
Objectives: The aim of this study was to determine the contribution of these IL-17–producing γδT cells to regulation of allergic airway inflammation.
Methods: Flow cytometry revealed that IL-17–producing γδT cells are more prevalent than IL-17+αβT cells (Th17) in a murine model of ovalbumin-induced allergic inflammation.
Measurements and Main Results: Transfer of γδT cells at the peak of acute allergic responses ameliorated airway hyperresponsiveness with a corresponding acceleration in the resolution of eosinophilic and Th2-driven inflammation. Conversely, functional blockade of γδT cells led to exacerbation of injury. Neither treatment changed pulmonary Th17 cell numbers. Moreover, transfer of Th17 cells had no effect on disease outcome. Importantly, IL-17–deficient γδT cells were unable to promote resolution of injury. These data identify IL-17–producing γδT cells as key regulators of the allergic response in vivo.
Conclusions: This unfolds a new perspective for the understanding of γδT cell function with regard to innate regulation of the adaptive immune responses, emphasizing that resolution of responses are important in determining the outcome of acute inflammatory episodes as well as for maintenance of tissue integrity and homeostasis.
Keywords: airway hyperresponsiveness, airway inflammation, inflammatory resolution, γδT cells, IL-17
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
γδT cells have been found to be critical regulators of the allergic response; however, the underlying mechanism remains unclear. This study identifies IL-17 production as a key mechanism in γδT cell–dependent regulation of allergic inflammation.
What This Study Adds to the Field
Our results reveal a new perspective for understanding how appropriate resolution of acute inflammation is critical in determining the outcome of disease. Exploiting the properties of IL-17–producing γδT cells may hold therapeutic potential in the outcome of allergic inflammatory episodes.
The ability to mount a controlled inflammatory response is a fundamental component of host defense. However, should inflammation proceed unimpeded, unnecessary tissue damage may be incurred which can be detrimental to organ function. Thus, it is imperative that inflammatory responses be tightly regulated. The immunopathological consequences of sustained or dysregulated inflammation and/or ineffective resolution is exemplified in chronic inflammatory conditions, such as asthma. Airway hyperresponsiveness (AHR) to innocuous environmental allergen with predominant leukocytic infiltrates and self-amplifying mediator release represent the principal pathological characteristics of asthma. Studies of mouse models in conjunction with clinical studies have defined many of the proinflammatory pathogenic mechanisms that drive the asthmatic phenotype. Such studies suggest that infiltrating T helper (Th) 2 cells and eosinophils are responsible for acute inflammatory reactions characteristic of asthma (1, 2). The physiological consequence of such events represents a leading cause of asthmatic mortality, which remains difficult to prevent and reduce (1).
In comparison, the paradoxical necessity of appropriate termination and resolution of inflammation has only comparatively recently been recognized, but it is now clear that resolution of airway inflammation is an active process that functions to not only dampen inflammation, but also actively promote a return to homeostasis (3). Although this area of research has largely been neglected in favor of defining proinflammatory mechanisms, a number of endogenous proresolving and anti-inflammatory mechanisms have begun to be defined. Self-limitation of allergic inflammation has been attributed to a variety of processes, such as secretion of lipid mediators (4) and efferocytosis (5). In addition, regulatory cells, such CD25+ forked box (Fox) P3+ regulatory T cells (Tregs) (6, 7). In particular, γδT cells are thought to be important for the maintenance of normal airway tone (8).
γδT cells are a distinct T cell lineage prominent at mucosal surfaces, including the airway epithelium (9). Triggered by pattern recognition and endogenous stress signals, γδT cells increase at inflammatory sites, and act as sentinels of pulmonary homeostasis (10). Evidence suggests that the primary function of γδT cells is immunoregulation and protection of host tissue against the damaging side effects of the immune response (11). Certainly, γδT cells have been found to be essential for the resolution of inflammation after infection with Mycobacterium tuberculosis, Escherichia coli, Listeria, and Streptococcus pneumoniae (12). In murine models of ovalbumin (OVA)-induced acute allergic airway disease, absence of γδT cells dramatically elevates AHR, with a comparatively small effect on development of allergic sensitization (8). However, the mechanisms by which γδT cells down-regulate AHR remain poorly understood. Recently, Huang and colleagues (13) demonstrated regulation of acute allergic airway disease by γδT cells, highlighting the importance of the inflammatory context in determining lung γδT cell functionality. Considering their lack of antigen specificity and ability to recognize self-stress–associated antigen, it is now thought that γδT cells are environmentally responsive, requiring prior signals from the inflammatory environment to elicit their regulatory activity during an established response (14).
The pleiotropic cytokine, IL-17, plays an important role in mediating an appropriate immune response to various infectious agents (15), and is the signature cytokine of the differentiated (αβ)Th17 effector subset. IL-17+ T cells are elevated in the airways of subjects with asthma (16), and IL-17 has been identified as a critical regulator of allergic immunopathology (17). Although the majority of studies on IL-17 have focused on Th17 cells, at steady state, γδT cells from naive mice are the main IL-17–producing lymphocyte subset (18), and it is becoming increasingly acknowledged that this function represents an essential part of their protective role during infectious inflammation (19) and fibrotic lung injury (20). However, their role in allergic inflammation has not been determined.
Therefore, we sought to determine the particular contribution of IL-17 and γδT cells during resolution of allergic airway inflammation and AHR. Through the use of an in vivo model of acute allergic airway disease, we demonstrate, for the first time, that IL-17–producing γδT cells are an essential component of inflammatory resolution and restoration of airway function. We show that IL-17+γδT cells, rather than Th17 cells, are the principal producers of IL-17 during allergic airway disease; moreover, this property is critical for effective resolution of allergic airway inflammation. Some of the results of these studies have previously been reported in the form of an abstract.
METHODS
Animals
Female BALB/c or C57BL/6 mice, purchased from Harlan Olac Ltd. (Bicester, UK), and C57BL/6 Il17a−/− mice (gift from Professor F. Powrie, courtesy of Yoichiro Iwakura) were housed at the Imperial College animal facility, with food and water ad libitum. UK Home Office guidelines (Animals Scientific Procedures Act, 1986) were observed.
Sensitization and Airway Challenge
To induce acute allergic airway disease, BALB/c or C57BL/6 mice were sensitized with 0.1 mg per mouse OVA (grade V; Sigma, Poole, UK) in 0.2 ml Alum (aluminum hydroxide Alu-Gel-S; Serva Electrophoresis, Heidelberg, Germany) on Day 0. Control mice were sham-sensitized with an equivalent volume of phosphate-buffered saline (PBS) in Alum. All animals were challenged with 5% OVA for 20 minutes via the airways between Days 6 and 12. Peak allergic airway disease was assessed on Day 13 and, after 1 week without challenge, resolution was assessed on Day 20. Mice were killed by exsanguination under terminal anesthesia. Disease parameters were assessed in animals killed by exsanguination under terminal anesthesia (100 mg/kg ketamine; (Fort Dodge Animal Health, Southampton, UK) and 10 mg/kg domitor (Pfizer, Sandwich, UK) 24 hours after final OVA challenge (except where stated).
In Vivo Experimental Protocols
Protocol A.
OVA sensitization and challenge were achieved in BALB/c mice as described previously here. During the resolution phase, treated animals received anti–T cell receptor (TCR) δ monoclonal antibodies (mAbs) (100 μl of 200 μg/ml, intravenously, from the GL3 hybridoma, a kind gift from L. Lefrancois). Control mice received an equivalent volume of 100 μl PBS.
Protocol B.
OVA sensitization and challenge were achieved in BALB/c mice as described previously here. During the resolution phase, treated animals received treatment on Days 15 and 18 with either (1) γδT cells (500,000 intravenously) or (2) recombinant mouse IL-17 (40 μl of 2.5 μg/ml; R&D Systems, Abingdon, UK), intratracheally. Control mice received an equivalent volume of 100 μl PBS.
Protocol C.
OVA sensitization and challenge were achieved in C56BL/6 mice as described previously here. During the resolution phase after 48 hours without challenge, resolution was assessed on Day 15. During the resolution phase, treated animals received γδT cells (500,000 intravenously) from either (1) IL-17+/+ or (2) IL-17−/− animals. Control mice received an equivalent volume of 100 μl PBS.
Protocol D.
OVA sensitization and challenge of BALB/c mice were achieved as described previously here. During the resolution phase, treated animals received treatment on Days 15 and 18 with either (1) γδT cells (500,000 intravenously) or (2) CD4+CCR6+IL-17+Th17 cells (500,000 intravenously) from protocol S1. Control mice received an equivalent volume of 100 μl PBS.
Protocol E.
OVA sensitization and challenge of BALB/c mice were achieved as described previously here. During the resolution phase, treated animals received treatment on Days 15 and 18 with Vγ4+γδT cells (500,000 intravenously). Control mice received an equivalent volume of 100 μl PBS.
Protocol S1.
OVA sensitization and challenge of C57BL/6 mice were achieved as described previously here. Resolution was assessed on Days 15 and 16 without challenge. During the resolution phase, groups of mice were administered recombinant mouse IL-17 intratracheally (40 μl of 2.5 μg/ml; R&D Systems).
Protocol S2.
BALB/c mice were sensitized intraperitoneally with 100 μg OVA emulsified in 200 μl complete Freund's adjuvant (both from Sigma) on Days 0 and 5. On Day 9, lymphoid organs were isolated and Th17 cells isolated as described subsequently here.
Isolation of γδT Cells for Adoptive Transfer
Single-cell suspensions were recovered from lung tissue as previously described (6). γδT cells were purified with a γδT cell–specific cell isolation kit, according to the manufacturer's instructions (Miltenyi Biotec, Surrey, UK), with purity typically greater than 95%.
Isolation of CD4+CCR6+T Cells for Adoptive Transfer
Single-cell suspensions were recovered from spleen and lymph nodes. A CD4+T cell population was first obtained with a CD4+T cell isolation kit according to the manufacturer's instructions (Miltenyi Biotec), with purity typically greater than 95%. The isolated CD4+T cells were then labeled with an anti-mouse CCR6 mAb (R&D Systems), and CCR6+ cells selected with anti-rat IgG microbeads, according to the manufacturer's instructions (Miltenyi Biotec). IL-17 expression from positively selected cells was assessed by flow cytometry as described below. Recipient animals received 5 × 105 T cells intravenously (or equal volume of 100 μl PBS as a control).
Isolation of Vγ4+γδT Cells for Adoptive Transfer
Single-cell suspensions were recovered from lung tissue, as previously described (7). Cells were first labeled with anti-Vγ4 mAb (clone UC3-10A6; Antibodies Online, Aachen, Germany) and isolated with anti-hamster micro-beads according to the manufacturer's instructions (Miltenyi Biotec). Animals received Vγ4+γδT cells intravenously, or an equal volume of 100 μl PBS.
Delivery of Recombinant IL-17
A total of 40 μl of 2.5 μg/ml mouse recombinant IL (rIL)-17 (IL-17A, referred to throughout as IL-17; R&D Systems) was administered intratracheally. Control mice received an equivalent volume of 40 μl PBS, as previously described (17, 21, 22).
Measurement of AHR
Mice were ventilated with a small animal ventilator (Harvard Apparatus, Kent, UK). Direct measurements of lung resistance were collected from anesthetized and tracheotomized mice with an EMMS system (Electro-Medical Measurement Systems, Bordon, Hants, UK) in response to increasing concentrations of inhaled methacholine (Sigma), as previous described (6).
Cell Recovery
Bronchoalveolar lavage (BAL), lung tissue, spleen, and lymph node leukocyte suspensions were prepared as previously described (6).
Analysis of Cellular Composition from Cytospins
A total of 50,000 BAL and lung cells were added to glass slides by centrifugation. Eosinophils and neutrophils were quantified from differential counts of Wright-Gimsa (Sigma)–stained cells, performed as previously described (6).
Staining of BAL and Lung Cells for Flow Cytometric Analysis
BAL and lung tissue cells were stained with anti-mouse CD11c or anti-mouse CD27 (both from eBioscience, San Diego, CA), anti-mouse CD3, anti-mouse CD4, anti-mouse CD8, anti-mouse TCRδ, anti-mouse Vγ4 (all from BD Pharmingen, Oxford, UK; nomenclature from Ref. 23), anti-mouse CCR6, anti-mouse CD27 (R&D Systems), anti-mouse CD11b (BioLegend, Cambridge, UK), anti-mouse monocyte and macrophage antibody (MOMA)–2 (AbD Serotec, Oxford, UK) and anti-mouse T1/ST2 (Morwell Diagnostics, Zurich, CH), anti-mouse RAR-related orphan receptor (ROR)-γt (eBioscience), or relevant isotype controls. For intracellular cytokine staining, cells were stimulated with phorbol myristate acetate/ionomycin (Merck, Hertfordshire, UK) in the presence of brefeldin A (Sigma) for 6 hours before extracellular staining. Cells were permeabilized with 0.5% saponin (Sigma) and stained with anti-mouse IL-10, IFN-γ, IL-4, IL-5, IL-13, IL-17, or appropriate isotype control (BD Pharmingen). Macrophages (MOMA-2+) were further characterized as alveolar (CD11bloCD11chi) and tissue/inflammatory (CD11b+CD11c−).
Flow Cytometric Analysis
Flow cytometric analysis was performed with a FACSCalibar (Becton Dickenson, Oxford, UK) with CellQuest software. Dead cells were excluded on the basis of size (forward scatter of light) and granularity (side scatter of light). Cells contained within a low forward scatter/side scatter lymphocyte gate were plotted on a dot plot. Where representative plots are shown, axes represent log fluorescence intensity. The total number of double-positive cells in the BAL or lung was calculated as described previously (2).
Analysis of Lung Mediators
Paired antibodies for murine IL-4 (BD Pharmingen), eotaxin-1/CCL11, macrophage-derived chemokine (MDC)/CCL22, monocyte chemoattractant protein (MCP)-1/CCL2 and T cell directed CC chemokine (TARC)/CCL17, keratinocyte chemoattractant (KC)/CXCL1 (R&D Systems), and granulocyte-colony stimulating factor (G-CSF) (Insight Biotechnology, London, UK) were detected in lung homogenate supernatant by sandwich ELISA. IL-13, IL-17, and IL-23 were measured with an ELISA kit (R&D Systems), according to the manufacturer's instructions.
Statistical Analysis
Data are expressed as means (±SEM) unless otherwise stated. Statistical significance between groups was tested with a Mann-Whitney U test, with a P value less than 0.05 considered significant. Graph generation and statistical analysis were performed with GraphPad prism software (version 4.00; GraphPad, La Jolla, CA).
RESULTS
Functional Blocking of γδT Cells during Established Allergic AHR and Inflammation Leads to Impaired Resolution of Disease
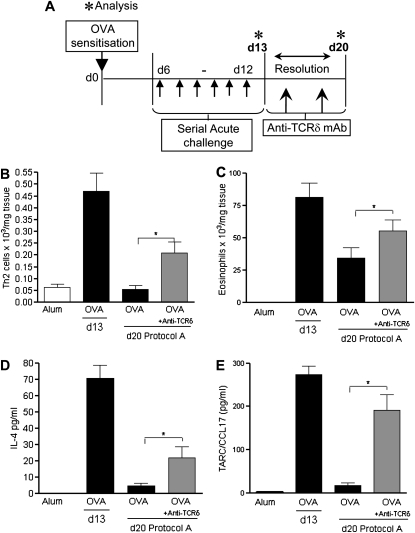
CD4+Th2 cells are the driving force behind parameters of allergic inflammation, such as airway eosinophilia and AHR in OVA-sensitized animals (2). To assess whether γδT cells play a key role in regulation of established allergic airway inflammation, we administered function-blocking anti-γδTCR mAb (8) or an Ig control during the resolution phase of OVA-induced allergic inflammation (Figure 1A, protocol A). Cellular lung inflammation was assessed on Days 13 and 20 after 1 week without further allergen exposure. OVA mice demonstrated a natural attenuation of cellular lung infiltration (Figures 1B and 1C). In contrast, mice receiving the anti–γδT cell mAb demonstrated delayed resolution, with significantly higher numbers of lung-infiltrating eosinophils (Figure 1B) and Th2 cells (Figure 1C) compared with control mice. Treatment was not associated with any effect on lung neutrophil numbers (see Figure E1A in the online supplement). Anti-TCRδ treatment (Figure 1A, protocol A) did not lead to a further elevation in AHR (data not shown). However, the mechanisms causing AHR are multifactorial. Moreover, AHR may already be maximal at this time point.
Figure 1.
Administration of anti–γδT cell function–blocking antibody negatively affects resolution of acute airway inflammation. Protocol A: resolution of acute allergic airway disease in BALB/c mice after anti–T cell receptor (TCR) δ treatment. BALB/c mice were sensitized with ovalbumin (OVA) (Day [d] 0) and challenged through the airways with aerosolized OVA (d6–d12). Peak allergic airway disease was assessed on d13, 24 hours after the final aerosol challenge. Additional groups of mice were left unchallenged for 1 week. Treated groups were given either 100 μl of 200 μg/ml anti-TCRδ monoclonal antibodies (mAbs) or Ig control on Days 15 and 18 during the resolution phase. Disease parameters were assessed on Day 20 (A). Resolution of Th2 inflammation was assessed by flow cytometric staining of lung CD4+T1/ST2+ Th2 lymphocytes (B). Airway eosinophils (C) were quantified by differential counting of Wright-Giemsa–stained lung cytospins on d13 and d20. Assessment of tissue mediators IL-4 (D) and T cell directed CC chemokine (TARC)/CCL17 (E) in homogenized lung supernatant by ELISA (n = 4–6 mice per group; *P < 0.05 in comparison to d20 OVA control mice).
IL-4 is the archetypal Th2 cytokine, and drives the immunopathology associated with OVA-driven asthma-like phenotypes. Peak levels of IL-4 and the chemoattractant, TARC/CCL17, an important mediator in inflammatory cell recruitment to the allergic lung, were detected on Day 13. After resolution, IL-4 and TARC/CCL17 levels were dramatically reduced in OVA animals (Figures 1D and 1E, Day 20). In comparison, mediator levels remained significantly elevated at Day 20 in OVA animals receiving anti–γδT cell treatment. No differences in pulmonary IFN-γ levels were detected (data not shown).
IL-17+γδT Cells Are Increased in the Allergic Lung
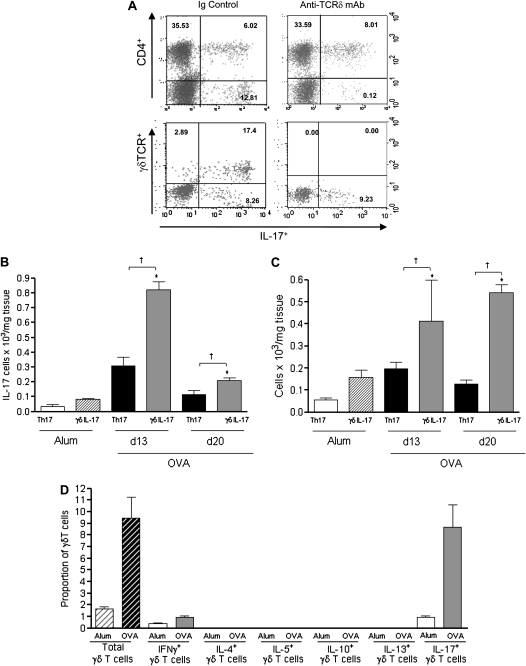
Flow cytometric assessment of IL-17–producing pulmonary CD3+ T lymphocytes revealed the presence of a CD4+IL-17+ (5.36%) and a larger CD4−IL-17+ (17.71%) population (Figure 2A, top left) within the lymphocyte gate. Administration of a γδT function-blocking antibody (24) (Figure 1A, protocol A) eradicated the CD4−IL-17+ T cell population (Figure 2A, top right plot) without any quantitative effect on CD4+IL-17+T lymphocytes. Given this striking change, the phenotype of the CD4−IL-17+ cells was examined further, revealing the presence of a γδTCR+ IL-17+ population (Figure 2A, bottom plots). Quantification of pulmonary IL-17+T lymphocytes revealed both populations to be significantly up-regulated in both the airway lumen (BAL) and lung tissue after acute airway challenge (Figures 2B and 2C, Day 13). Furthermore, significantly more IL-17+γδT cells than Th17 cells were present in both lung compartments after acute OVA challenge. Interestingly, IL-17+γδT cells remained significantly elevated 1 week after the cessation of allergen challenge. Previous studies have shown that γδT cells are able to produce a variety of mediators, including a host of inflammatory cytokines (12). However, there remains a paucity of knowledge regarding the cytokine profile of pulmonary γδT cells during in vivo allergic airway responses. Investigation of additional immune mediators revealed that, apart from IL-17, γδT cells produced very low levels of the archetypal Th1 cytokine (IFN-γ), and none of the asthma-associated Th2 cytokines (IL-4, IL-5, IL-10, or IL-13) (Figure 2D, Day 13). Interestingly, the majority of γδT cells from sham-immunized control animals also expressed IL-17.
Figure 2.
γδT cells produce IL-17 after induction of acute allergen-induced airway inflammation. Protocol A: resolution of acute allergic airway disease in BALB/c mice after anti–T cell receptor (TCR) δ treatment. (A) Representative plots from gated CD3+ cells stimulated ex vivo with phorbol myristate acetate/ionomycin in the presence of brefedin A to assess IL-17 production from CD4+T cells (top panels) and γδT cells (bottom panels) in lung cells from mice receiving an Ig control (left panels) or anti–TCRδ treatment (right panels). Axes represent log fluorescence intensity of gated lung lymphocytes from ovalbumin (OVA) mice (Day [d] 13) according to criteria outlined in the Methods section. Quantification of Th17 and IL-17–producing γδT cells in leukocyte suspensions from bronchoalveolar lavage (B) and lung tissue (C). Percentage of total lymphocytes expressing the γδTCR from alum (white hatched) and OVA (black hatched) mice and the percentage of γδT cells from alum (white bars) and OVA (gray bars) mice expressing different cytokines on d13 (D) (n = 4–6 mice per group; *P < 0.05 above alum controls and †P < 0.05 between IL-17–producing CD4+ and γδT cells. mAb = monoclonal antibody.
Both IL-17 and γδT Cells Promote Resolution of Allergic Lung Inflammation and AHR
IL-17 has been proposed as a negative regulator during established allergic inflammation, and, although Th17 cells were assumed to be the cellular source, this was not definitively determined (17). We found γδT cells to be the larger IL-17–expressing lymphocyte population in the airways, and that IL-17–producing γδT cells have been shown to exert a proresolving protective role during infectious inflammation (19) and fibrotic lung injury (20). As such, we sought to dissect the function of γδT cells during established allergic airway disease and specifically determine if IL-17 production by these cells contributed to resolution of allergic airway inflammation and AHR in vivo.
To directly assess the contribution of γδT cells and IL-17 to resolution of acute allergic airway inflammation, in vivo transfer of either γδT cells or instillation of rIL-17 was performed in OVA-allergic mice at the peak of inflammation. The effect on resolution of injury was assessed 1 week later (Figure 3A, protocol B). Given that OVA sensitization/challenge elicits an increase in IL-17 expressing γδT cells (Figure 2), γδT cells from the lungs of sensitized/challenged hosts were used to derive donor γδT cells, of which, on average, 75% of γδT cells expressed IL-17. Lung homing capacity of pulmonary γδT cells has previously been demonstrated (25).
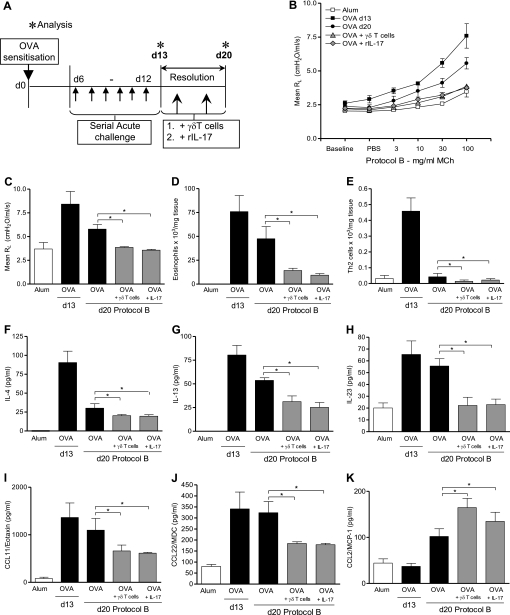
Figure 3.
IL-17 and γδT cells precipitate resolution of inflammation and re-establishment of normal lung function after acute allergic inflammation. Protocol B: resolution of acute allergic airway disease in BALB/c mice after addition of γδT cells or recombinant IL (rIL)-17. BALB/c mice were sensitized with ovalbumin (OVA) at Day (d) 0 and challenged through the airways with aerosolized OVA (d6–d12). Peak allergic airway disease was assessed on d13, 24 hours after the final aerosol challenge. Additional groups of mice were left unchallenged for 1 week without intervention. Treated groups given two doses of either (1) γδT cells (500,000 intravenously), or (2) rIL-17 (intratracheally) on d15 and d18 during the resolution phase (A). Invasive measurements of lung resistance (RL) were used to assess airway function on d13 and d20 against increasing concentrations of methacholine (MCh) (B). RL after 100 mg/ml MCh (C). Airway eosinophils were quantified by differential counting of Wright-Giemsa–stained lung cytospins on d13 and d20 (D). Resolution of Th2 inflammation was assessed by flow cytometric staining of lung CD4+T1/ST2+ Th2 lymphocytes (E). Assessment of IL-4 (F), IL-13 (G), IL-23 (H), eotaxin-1/CCL11 (I), macrophage-derived chemokine (MDC)/CL22 (J), and monocyte chemoattractant protein (MCP)/CCL2 (K) in homogenized lung supernatant by ELISA (n = 8–10 mice per group; *P < 0.05 in comparison to d20 OVA control mice from two independent experiments).
IL-17 and γδT cells promote resolution of inflammation after acute allergic airway disease.
The presence of Th2 cells in the allergic airway is strongly correlated with AHR, the hallmark of the asthma phenotype (2). Administration of γδT cells during the resolution phase significantly reduced AHR to levels comparable with alum control animals (Figures 3B and 3C). Strikingly, intratracheal instillation of rIL-17 mimicked this effect.
OVA sensitization and challenge was associated with eosinophilia (Figure 3D) and Th2 leukocytic infiltration (Figure 3E) on Day 13 after challenge. After 1 week without allergen challenge (Day 20), some degree of cellular resolution was evident, although significant Th2 and eosinophilic cellular infiltrates remained detectable in the airways. Treatment with γδT cells or IL-17 significantly enhanced the resolution of cellular infiltrates in comparison to untreated allergic mice at this time point.
γδT cells and IL-17 precipitate resolution of allergen-driven proinflammatory mediators of the lung.
Peak levels of the Th2 driving mediators, IL-4 (Figure 3F) and IL-13 (Figure 3G), were detected on Day 13, and remained significantly elevated in the absence of further allergen exposure (Day 20). In contrast, a further significant reduction in pulmonary levels of both IL-4 and IL-13 was observed in animals receiving either γδT cells or rIL-17 treatment (Figures 3F and 3G). However, pulmonary levels of the Th1-associated cytokine, IFN-γ, were not affected by either intervention (data not shown). Acute allergic inflammation (Day 13) was also associated with elevated pulmonary levels of IL-23, which were also sustained after cessation of allergen challenge (Figure 3H). Notably, we found that addition of either γδT cells or rIL-17 significantly reduced pulmonary IL-23 to baseline levels.
Chemokine responses are altered by addition of rIL-17 or γδT cells.
We determined if transfer of γδT cells or rIL-17 during acute allergic airway disease modulated production of chemokines known to direct leukocyte migration into the allergic lung. A significant decrease in eotaxin-1/CCL11 and MDC/CCD22 was observed after addition of γδT cells compared with OVA control animals (Figures 3I and 3J). This effect was mirrored by rIL-17 treatment, a finding in keeping with previous studies (17). In contrast, both γδT cell and rIL-17 treatment was associated with a concurrent increase in the macrophage recruitment factor MCP-1/CCL2 (Figure 3K).
IL-17–Deficient γδT Cells Cannot Regulate Pulmonary Allergic Responses
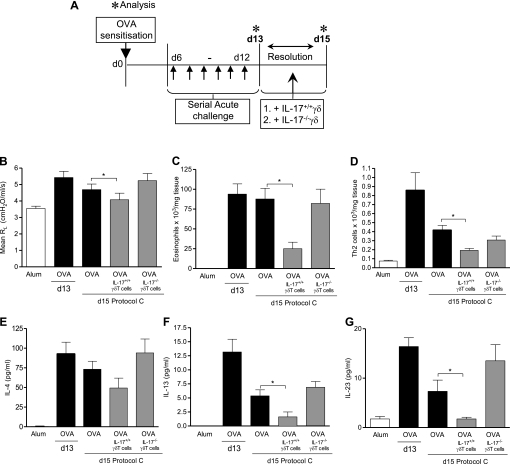
Several cell types have been associated with IL-17 production (15). To definitively demonstrate the importance of IL-17 production specifically from local pulmonary γδT cells, γδT cells were isolated from IL-17–sufficient (IL-17+/+) or IL-17–deficient (IL-17−/−) mice and transferred into lungs of allergic mice at the peak of inflammation to determine the effect on resolution. Because IL-17−/− mice are on a C57BL/6 background (26), we performed the whole experiment in C57BL/6 mice (Figure E2A, protocol S1). The resolution of inflammation was observed to be much quicker in this strain, and, by Day 16, inflammation had resolved spontaneously (Figures E2B–E2E). Importantly, delivery of rIL-17 did not induce lung neutrophilia (Figure 3A), or up-regulation of the neutrophil hematopoietic factor, granulocyte-colony stimulating factor (Figure E3B), or the neutrophil chemoattractant, KC/CXCL1 (Figure E3C). Therefore, γδT cells derived from IL-17+/+ or IL-17−/− mice were transferred at the peak of inflammation (Day 13), and analysis was performed on Day 15 (Figure 4A, protocol C). Critically, we found that only γδT cells from wild-type mice were able to effect resolution of key features of the allergic response. Similar to the results in BALB/c mice (Figure 3, protocol B), AHR and recruitment of eosinophils and Th2 cells was significantly reduced by transfer of IL-17+/+γδT cells (Figures 4B–4D). In direct contrast, lung function, airway eosinophilia, and Th2 cellular infiltrates remained elevated in animals receiving IL-17−/−γδT cells at comparable levels to OVA control mice. Addition of IL-17+/+γδT cells, but not IL-17–deficient γδT cells, was also associated with an accelerated decrease in the inflammatory Th2 cytokines, IL-4 and IL-13 (Figures 4E and 4F), as well as IL-23 (Figure 4G).
Figure 4.
Only IL-17–sufficient γδT cells aid resolution of allergic airway inflammation. Protocol C: resolution of acute allergic airway disease in C57BL/6 mice receiving IL-17+/+ or IL-17−/−γδT cells. C57BL/6 mice were sensitized with ovalbumin (OVA) (Day [d] 0) and challenged through the airways with aerosolized OVA (d6–d12). Peak allergic airway disease was assessed on d13, 24 hours after the final aerosol challenge. Additional groups of mice were left unchallenged for 1 week without intervention. During the resolution phase, treated groups received γδT cells from (1) IL-17–sufficient and (2) IL-17–deficient animals (A). Average resistance at 100 mg/ml dose of methacholine (MCh) (B). Lung parenchyma eosinophils quantified from differential counting of Wright-Giemsa–stained lung cytospins on d13 and d15 (C). Lung tissue Th2 cells quantified on d13 and d15 by flow cytometry according to the criteria outlined in the Methods section (D). Assessment of IL-4 (E), IL-13 (F), IL-23 (G) (n = 6–8 mice per group; *P < 0.05 in comparison to d20 OVA control mice from two independent experiments).
OVA-Induced CD4+CCR6+ Cells Cannot Regulate Pulmonary Allergic Responses
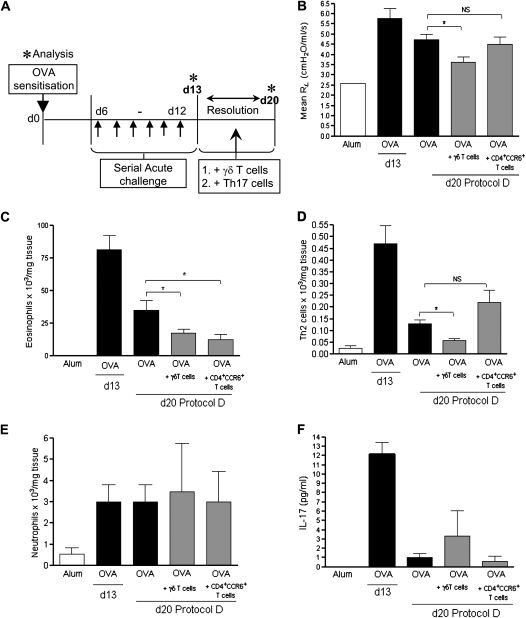
Although γδT cells were the predominant IL-17–producing lymphocyte population in the lung, CD4+17+T cells were also present in the airways during acute allergic airway inflammation (Figures 2B and 2C). IL-17 has previously been proposed to regulate acute allergic reactions (17, 27), whereas Th17 cells have been shown to induce pulmonary neutrophila (28). We found that Th17 cell numbers were not affected after intervention with either γδT cells or rIL-17 during the resolution phase (Figures E4A and E4B). Nevertheless, Th17 cells could conceivably be a potential source of IL-17 in the allergic lung, and thus contribute to resolution. To determine the contribution of this particular subset to resolution of inflammation, we generated OVA-specific CD4+CCR6+ in vivo (Figure E5A, protocol S2). CD4+T cells were isolated from the spleens of mice immunized with OVA in complete Freund's adjuvant, and selected on the basis of CD4 and CCR6 expression, as reported by Martin and colleagues (29). Isolated CD4+CCR6+T cells were checked for expression of OVA-specific IL-17 production, with 73% of the isolated CCR6+T cells expressing IL-17 (Figure E5B), and these cells also expressed the Th17-lineage transcription factor, RORγt (Figure E5C). Macrophage inflammatory protein3α/CCL20, the ligand for CCR6, was highly expressed in the airways after allergen challenge (Figure E5D), thus permitting CD4+CCR6+T cell trafficking as previously reported (77). These cells were transferred during the resolution phase (Figure 5A, protocol D).
Figure 5.
Th17 cells do not promote resolution of acute allergic airway disease. Protocol D: addition of either γδT cells or Th17 cells during resolution of acute allergic airway disease. Sensitized/challenged BALB/c mice were treated with two doses of either (1) γδT cells (500,000, intravenously), or (2) Th17 cells (500,000, intravenously) on Day (d) 15 and d18 during the resolution phase, and disease parameters were assessed on Day 20 (A). Average resistance at 100-mg/ml dose of methacholine (MCh) (B). Airway eosinophils were quantified by differential counting of Wright-Giemsa–stained lung cytospins on d13 and d20 (C). Resolution of Th2 inflammation was assessed by flow cytometric staining of lung CD4+T1/ST2+ Th2 lymphocytes (D). Airway neutrophils were quantified by differential counting of Wright-Giemsa–stained lung cytospins on d13 and d20 (E). Assessment of IL-17 (F) in homogenized lung supernatant by ELISA (n = 4–6 mice per group; *P < 0.05 in comparison to d20 ovalbumin [OVA] control mice).
Interestingly, animals receiving CD4+CCR6+ cells rather than γδT cells during resolution (Figure 5A, protocol D) did not demonstrate any restoration of lung function toward baseline (Figure 5B). Although transfer of CD4+CCR6+ cells did reduce eosinophil numbers (Figure 5C), there was no effect on Th2 cell numbers (Figure 5D), and, in contrast to published reports (28), there was no effect on lung neutrophils (Figure 5E). IL-17 was measured by ELISA at the peak of inflammation (Day 13) and after resolution on Day 20 (Figure 5F). OVA challenge of sensitized mice led to an increase in lung IL-17, as detected by ELISA. There were no significant differences between groups on Day 20, after resolution.
Vγ4+γδT Cells Promote Resolution of Allergic Airway Inflammation
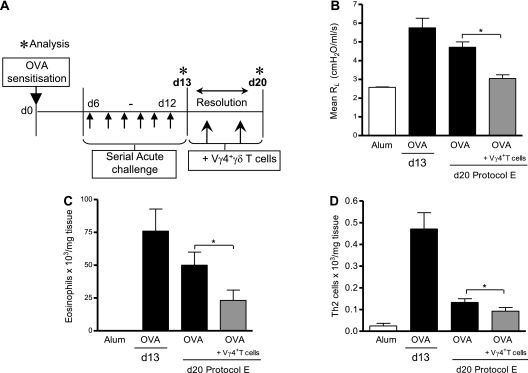
γδT cells are comprised of several functionally distinct subsets defined by surface receptor expression (30). In accordance with Ribot and colleagues (31), we found that IL-17–producing γδT cells were negative for CD27 (Figure E6A). Absence of this functional marker is indicative of a distinct, thymically established IL-17+IFN-γ−RORγt+γδT cell subset (31). In addition, there is a well established association between γδT cell function and surface phenotype, such as TCR chain expression (30). In particular, the Vγ4+γδT cell subset, based on the nomenclature by Heilig and Tonegawa (23), are thought to suppress AHR (13, 32–34). In keeping with recent reports (29, 31), we found that 64% of IL-17+γδT cells expressed the Vγ4− chain (Figure E3B). We next sought to determine if the Vγ4+γδT cell subpopulation was responsible for promoting resolution of established allergic airway disease in our model. Therefore, isolated Vγ4+γδT cells were transferred during the resolution period (Figure 6A, protocol E). After the inflammatory resolution period (Day 20), OVA mice receiving Vγ4+γδT cells demonstrated an improvement in lung function as compared with OVA control mice (Figure 6B). Similar to data in Figure 3 after transfer of total γδT cells, transfer of Vγ4+γδT cells also resulted in a reduction in cellular Th2 and eosinophilic infiltrates (Figures 6C and 6D).
Figure 6.
Addition of Vγ4+γδT cells during resolution of acute allergic airway disease. Protocol E: resolution of acute allergic airway disease in BALB/c mice after addition of Vγ4+γδT cells. Sensitized/challenged BALB/c mice received Vγ4+γδT cells on Day (d) 15 and d18 during the resolution phase, and disease parameters were assessed on Day 20 (A). Average resistance at 100-mg/ml dose of methacholine (MCh) (B). Airway eosinophils quantified by differential counting of Wright-Giemsa–stained lung cytospins (C). Resolution of Th2 inflammation was assessed by flow cytometric staining of lung CD4+T1/ST2+ Th2 lymphocytes (D) (n = 4–6 mice per group with *P < 0.05 in comparison to d20 ovalbumin [OVA] control mice).
Reduced Lung Tissue Macrophages Correlate with Inflammatory Resolution after Addition of γδT Cells
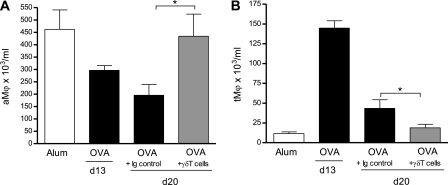
There is substantial evidence that macrophages are of major importance to the resolution of inflammation (35). In particular, alveolar macrophages (aMφ: MOMA2+CD11bloCD11chi), the predominant leukocyte in the normal airway, have a suppressive phenotype, and are critical to regulation of airway homeostasis (36). Airway inflammation is accompanied by an influx of inflammatory tissue macrophages into the airway spaces. To this end, both alveolar macrophage (aMφ) and tissue macrophage (tMφ: MOMA-2+CD11b+CD11c−) populations were quantified in the airway lumen (BAL) during peak airway inflammation (protocol A, Day 13) and after resolution (protocol A, Day 20) with the phenotypic criteria outlined in the Methods based on GeurtsvanKessel and Lambrecht (37). Peak allergic airway inflammation (Day 13) was accompanied by a reduction in luminal (BAL) aMφ and an increase in tMφ (Figures 7A and 7B). Interestingly, on Day 20 after resolution, mice receiving γδT cells had elevated luminal aMφ and reduced tMφ when compared with OVA control animals.
Figure 7.
Effect of γδT cells on pulmonary macrophage populations during resolution of allergic airway inflammation. Protocol B: resolution of acute allergic airway disease in BALB/c mice after addition of γδT cells. Alveolar macrophages (aMφ) (A) and tissue macrophages (tMφ) (B) were assessed by flow cytometry in bronchoalveolar lavage (n = 4–6 mice per group; *P < 0.05 in comparison to Day [d] 20 ovalbumin [OVA] control mice).
DISCUSSION
We have established that therapeutic blockade of γδT cells during established allergic airway disease impaired the resolution of pulmonary inflammation, demonstrating an important regulatory role for γδT cells in the allergic lung. Allergic airway inflammation elicited IL-17 production from local γδT cells and CD4+CCR6+ T cells in the airways. Interestingly, IL-17–producing γδT cells dominated this response. Delivery of γδT cells ameliorated key features of the allergic response. A parallel proresolving role for IL-17 was also revealed. Furthermore, we conclusively determined that the regulatory capacity of γδT cells is dependent upon their ability to secrete IL-17. Further investigation revealed the Vγ4+γδT cell subset to be the majority producers of IL-17 and responsible for the proresolving effect of γδT cell transfer during resolution.
Natural resolution of inflammation is a dynamic process that requires the removal of stimulus, down-regulation of mediators, and elimination of dead cells (3). Dysregulation or failure of this process prevents a return to homeostasis, and can contribute to the pathogenesis and progression of chronic inflammatory disorders, such as asthma (38). Our data provide evidence that delivery of either rIL-17 or IL-17+/+γδT cells promotes normalization of lung function and resolution of inflammation in the allergic lung. Bronchoconstriction, the cardinal feature of allergic asthma, is considered a primary contributor to asthma mortality (39). Migration of inflammatory cells—in particular, Th2 cells—into the allergic airways promotes changes to airway function (39, 40). IL-17+γδT cells or rIL-17 promoted resolution of pulmonary eosinophilic and Th2 cellular infiltrates, and decreased pulmonary levels of proasthmatic chemokines and IL-4 and IL-13 after addition of IL-17+γδT cells or rIL-17 treatment. Importantly, this was not the result of immune deviation to a Th1 phenotype. Attempts to replicate the eosinophilic airway inflammation of human asthma in mice have predominately focused on the genetically predisposed Th2 BALB/c strain (41). Importantly, by also using a “Th1”-associated C57BL/6 strain, our model also illustrates that the proresolving role of IL-17+γδT cells during allergic airway disease is not strain dependent. Conversely, we have shown that depletion of γδT cells has the opposite effect, delaying resolution of injury. This has ramifications for the maintenance and outcome of persistent inflammation, and implicates γδT cells as critical players in regulating chronic inflammatory diseases, such as asthma. Therapeutic blockade of γδT cells during established allergic airway disease impaired the resolution of pulmonary inflammation. Although the specific in vivo mechanism of the anti-TCRδ mAb, GL3, has not been established, it cannot be excluded that the effect seen is through cross-linking of the γδTCR, and this requires further exploration.
Maintenance of normal organ function is paramount in the airways, and previous studies have identified γδT cells as a vital component of airway homeostasis and key regulators of AHR (8). Although the effect was shown to be independent of the Th2 response, the mechanism was undefined (8). IL-17+T cells have previously been reported in the airways of subjects with asthma (16), and a pivotal role for IL-17 in regulation of established allergic airway inflammation by down-modulating proinflammatory mediators, such as CCL17/TARC and IL-13, and reduction of inflammatory cell recruitment and activation via the IL-4 receptor has been previously demonstrated (17). This protective role was further underscored by the demonstration that IL-17 receptor (IL-17R)–deficient mice demonstrate worse Th2 pathology in the airways (17). Although Th17 cells were assumed to be the source, this was not defined. Critically, we now show that only IL-17+/+γδT cells, but not Th17 cells, were able to down-regulate all of these key features of the allergic response, highlighting IL-17 production by γδT cells as a potential mechanism in this setting.
Our findings demonstrate a protective role for IL-17, which is paradoxically at odds with the literature describing the proinflammatory effects of this mediator in eradication of infectious pathogens (15). Nevertheless, our data are in agreement with emerging reports of a protective function for IL-17 during noninfectious inflammation (20, 42), and there is now evidence that IL-17+γδT cells represent a functionally distinct subset (29, 31). It is unclear why γδT cell–derived IL-17 plays such a decisive role in resolution when other readily available sources of IL-17 are present in the airways. Pulmonary Th17 cell numbers were not altered after intervention with either γδT cells or rIL-17 during the resolution phase. In addition, we did not observe a similar proresolving effect to after transfer of Th17 cells, further supporting the dominant proresolving function of IL-17–producing γδT cells shown in this study. However, induction of AHR in OVA-challenged mice after adoptive transfer of in vitro–polarized OVA-specific Th17 cells has been demonstrated (28). This may be explained by the use of in vitro–polarized cells in a cell transfer model that lacks contribution from the other layers of the immune system, such as mast cells and B cells.
Importantly, we demonstrate that the cellular source of IL-17 appears critical to functional outcome. Although IL-17+γδT cells and Th17 cells share many similar characteristics (29), functional contrasts in these populations have been noted, particularly with regard to their response to TCR, cytokine signals, and environment (29, 43, 44). In addition to being more numerous than Th17 cells in the allergic lung, IL-17+γδT cells have a spatial advantage in that they are normally locally resident in close proximity to the bronchial epithelium (9). It is therefore possible that location, in relation to airway structure, confers IL-17+γδT cells a functional immunoprotective advantage during inflammation, an idea that is consistent with the established pleiotropic and environment specific role played by γδT cells in the airways (11). Although the nature of γδT cell ligands is not well defined, recognition of endogenous stress–associated antigens has been reported (45, 46). Huang and colleagues (13) recently demonstrated that airway allergen exposure alters the outcome of the γδT cell response. Coupled with the recent demonstration of preferential expression of innate pattern recognition receptors on IL-17+γδT cells, but not Th17 cells (29), inflammation-induced liberation of γδT cell ligands may elicit a common proresolving IL-17–dependent mechanism that has been evolutionarily conserved to promote effective pathogen elimination and minimize collateral tissue damage. Interestingly, γδT cells are a rich source of IL-17 in the steady state (18), and are thought to be poised to produce IL-17 as a first-line defense. However, the data presented here show that γδT cells are intimately involved in the later stages of an immune response, and are crucial for effective resolution. The complex functions of IL-17 are likely to depend on the microenvironment and to be influenced by temporal and spatial considerations in addition to the cellular source.
The Vγ4+γδT cell subset is a key regulator of allergic airway immune responses (32, 34). Consistent with this, we found that the immunoregulatory IL-17+γδT cells were predominantly of the Vγ4+ subset. Furthermore, specific transfer of Vγ4+γδT cells promoted inflammatory resolution in a manner similar to pan-γδT cell transfer. Acquisition of antiinflammatory capacity by Vγ4+γδT cells is thought to be stage dependent, relating to the inflammatory status of the animal (13, 47), supporting the idea that environmental context differentially regulates the proresolving activities of γδT cells (12). Interestingly, Schnyder-Candrian and colleagues (17) proposed a dual role for IL-17 during allergic asthma, reporting this cytokine to be both necessary for allergic sensitization and protective during established disease. A similar context-specific bifunctionality has previously been reported for γδT cells (12). Together, these data strongly suggest that contextual influence, such as source of mediator, are important determinants of functional outcome, particularly in the lung, where immune homeostasis is vital for function. However, IL-17R has three ligands that have been described to date: IL-17A, IL-17F, and IL-25 (48). The dual role proposed by Schnyder-Candrian and colleagues (17) was interpreted from IL-17R–deficient mice, thus suggesting that the role of IL-17 during allergic sensitization may not be so clear cut, and commands further exploration.
The mechanism by which γδT cell–derived IL-17 exerts its protective function in the allergic airway requires further exploration. The heterodimeric IL-17R is ubiquitously expressed on lung parenchyma, epithelium, and leukocytes (48, 49, 49, 50). The activity of IL-17 is most classically defined by its ability to induce the expression of inflammatory cytokines, chemokines, and other mediators by the epithelium and stromal cells via its chief signaling component, actin-related gene-1, and activation of the proinflammatory nuclear factor–κB pathway (51). However, Act-1 has been reported to induce both pro- and anti-inflammatory effects during infection (52). Indeed, a protective role for IL-17R signaling on T cells during colitis was recently reported (42). There are also data to suggest that IL-17 signaling differs among resident cells (16, 53, 54). Thus, the functional outcome of the IL-17R–IL-17 interaction is likely dependent on the immunological context, cell type, and homo- and hetero-dimeric specificities of the ligand–receptor interaction (52, 55).
IL-23 is a proinflammatory cytokine with a pathological role in chronic inflammatory disease (56, 57). Notably, we found that addition of IL-17+/+γδT cells or rIL-17 significantly reduced pulmonary IL-23 levels. Eosinophils express the IL-23 receptor (IL-23R) (58), and IL-23R–ligand interactions induce proinflammatory mediator release (58). Neutralization of IL-23, therefore, has a beneficial effect on allergic airway inflammation by decreasing eosinophil recruitment (58). Interestingly, recent experiments demonstrate that the proresolving lipid molecule, resolvin E1, selectively regulated allergic mediators via inhibition of IL-23 from dendritic cells (DCs) (4). Indeed, IL-23 is produced by resident DCs and macrophages (59), both of which are known to interact with γδT cells (25). The accelerated resolution of inflammation observed after IL-17+γδT cell transfer may therefore be explained mechanistically by downstream suppression of IL-23 production. Furthermore, IL-23 has an important role in regulating the balance between effector and CD4+CD25+FoxP3+ Tregs in the gut (60), and IL-23R expression can suppress FoxP3 expression (61). Tregs have previously been illustrated to play a key role in regulation of allergic inflammation (6), and, thus, the decrease in IL-23 after γδT cell transfer might allow more effective regulation by CD4+CD25+Tregs.
Residency of γδT cells at epithelial borders fosters interaction with accessory cells, such as macrophages and DCs, which provide a means to amplify and elicit their broad-spectrum functions (9). Indeed, macrophages express multiple ligands for γδT cells (62), and pulmonary DCs and alveolar macrophages are regulated by γδT cells during resolution of inflammation after infection (25). In addition, γδT cell interactions with epithelial cells are thought to be important for their role in the maintenance of normal airway function (63). These interactions are likely to play an important role in returning airway homeostasis. Recruitment of inflammatory macrophages to the airway spaces is a central feature of allergic airway inflammation (64, 65). In addition, efferocytosis to clear inflammatory cell debris is fundamental to inflammatory resolution, and consequently releases antiinflammatory and proresolving mediators (5, 66). Recruitment of inflammatory macrophages through the macrophage recruiting MCP-1/CCL2 chemokine axis is a vital component of this process (67, 68). Induction of MCP-1/CCL2 by γδT cells has previously been reported (69), and, consistent with this, we observed elevated MCP-1/CCL2 levels after γδT cell transfer. Alveolar macrophages are residents in the airway spaces, and play a key role in maintaining homeostasis (70) and promoting resolution of inflammation (36). Inflammatory macrophages recruited to the alveoli spaces during inflammation can acquire the suppressive phenotype of alveolar macrophages (71), enabling them to actively suppress the antigen-presenting function of DCs (72) and the proliferative capacity of T cells (73, 74), and eventually restore homeostasis (75). Both γδT cells and IL-17 have independently been associated with resolution of inflammation via phagocyte infiltration (69, 76). We found that addition of γδT cells during resolution resulted in increased aMφ numbers in the airway lumen, with a concurrent decrease in tMφ numbers in this location, providing a potential mechanism by which resolution could be occurring.
To date, the focus has been on elucidating proinflammatory mechanisms associated with allergic inflammation, with little information on the time course of the natural resolution of allergic airway disease after discontinuation of allergen airway challenge. An understanding of the mechanisms leading to resolution of inflammation might reveal novel avenues for development of asthma therapy. Clearly, IL-17 is implicated in allergic asthma. We show here, for the first time, that γδT cells play a vital role in the resolution of allergic airway inflammation via an IL-17–dependent mechanism. This unfolds a new perspective for the understanding of γδT cell function with regard to the appropriate innate regulation of the adaptive immune system, emphasizing that resolution of such responses is equally important in determining the outcome of acute inflammatory episodes, and is critical for maintenance of tissue integrity and homeostasis. Exploiting the endogenous proresolving properties of IL-17–producing γδT cells may be of therapeutic benefit.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the generosity of Fiona Powrie and Sofia Buonocore (Sir William Dunn School of Pathology, University of Oxford) and Yoichiro Iwakura (Centre for Experimental Medicine, University of Tokyo) for the provision of the IL-17−/− mice. We would also like to thank Sara Mathie, Matthew Bell, and Simone Walker for technical assistance, in particular, with lung function measurements. The GL3 hybridoma was a kind gift courtesy of W. Born and L. LeFrancois, and was purified by Rebecca Beavil of the Medical Research Council and Asthma UK Centre in Allergic Mechanisms of Asthma.
Supported by Wellcome Trust grant 057,704. C.M.L. is a Wellcome Senior Fellow, and J.R.M. is supported by a Foundation Studentship from the National Heart and Lung Institute at Imperial College.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200911-1775OC on April 22, 2010
Author Disclosure: J.R.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; C.M.L. received $1,001–$5,000 from MedImmune in consultancy fees, $1,001–$5,000 from GlaxoSmithKline in lecture fees, and more than $100,001 from Leti in industry-sponsored grants.
References
- 1.Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw AJ, Pavord ID. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002;360:1715–1721. [DOI] [PubMed] [Google Scholar]
- 2.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2–IL-33 pathway. Am J Respir Crit Care Med 2009;179:772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol 2005;6:1191–1197. [DOI] [PubMed] [Google Scholar]
- 4.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol 2008;9:873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 2006;129:1673–1682. [DOI] [PubMed] [Google Scholar]
- 6.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med 2005;202:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewkowich IP, Herman NS, Schleifer KW, Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Kohl J, Belkaid Y, et al. CD4+CD25+ T cells protect against experimentally induced asthma and alter pulmonary dendritic cell phenotype and function 295. J Exp Med 2005;202:1549–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Kohler G, O'Brien R, Gelfand EW, Born W. Negative regulation of airway responsiveness that is dependent on gammadelta T Cells and independent of alphabeta T cells. Nat Med 1999;5:1150–1156. [DOI] [PubMed] [Google Scholar]
- 9.Wands JM, Roark CL, Aydintug MK, Jin N, Hahn YS, Cook L, Yin X, Dal PJ, Lahn M, Hyde DM, et al. Distribution and leukocyte contacts of gammadelta T cells in the lung. J Leukoc Biol 2005;78:1086–1096. [DOI] [PubMed] [Google Scholar]
- 10.Born W, Cady C, Jones-Carson J, Mukasa A, Lahn M, O'Brien R. Immunoregulatory functions of gamma delta T cells. Adv Immunol 1999;71:77–144. [PubMed] [Google Scholar]
- 11.Born WK, Lahn M, Takeda K, Kanehiro A, O'Brien RL, Gelfand EW. Role of gammadelta T cells in protecting normal airway function. Respir Res 2000;1:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carding SR, Egan PJ, Gammadelta T. Cells: functional plasticity and heterogeneity. Nat Rev Immunol 2002;2:336–345. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O'Brien RL, Born WK. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol 2009;183:849–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien R, Jin N, Huang Y, Aydintug MK, Roark C, Born W. Characteristics of IL-17–producing gammadelta T cells. Immunity 2010;32:1. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol 2007;51:1139–1147. [DOI] [PubMed] [Google Scholar]
- 16.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001;108:430–438. [DOI] [PubMed] [Google Scholar]
- 17.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med 2006;203:2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 2005;22:285–294. [DOI] [PubMed] [Google Scholar]
- 19.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL, Gammadelta T. Cells: an important source of IL-17. Curr Opin Immunol 2008;20:353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun RK, Ferrick C, Neubauer P, Sjoding M, Sterner-Kock A, Kock M, Putney L, Ferrick DA, Hyde DM, Love RB. IL-17 producing gammadelta T cells are required for a controlled inflammatory response after bleomycin-induced lung injury. Inflammation 2008;31:167–179. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 2009;31:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyamoto M, Prause O, Sjostrand M, Laan M, Lotvall J, Linden A. Endogenous IL-17 as a mediator of neutrophil recruitment caused by endotoxin exposure in mouse airways. J Immunol 2003;170:4665–4672. [DOI] [PubMed] [Google Scholar]
- 23.Heilig JS, Tonegawa S. Diversity of murine gamma genes and expression in fetal and adult T lymphocytes. Nature 1986;322:836–840. [DOI] [PubMed] [Google Scholar]
- 24.Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur J Immunol 2009;39:372–379. [DOI] [PubMed] [Google Scholar]
- 25.Kirby AC, Newton DJ, Carding SR, Kaye PM. Pulmonary dendritic cells and alveolar macrophages are regulated by gammadelta T cells during the resolution of S. pneumoniae–nduced inflammation. J Pathol 2007;212:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y, Antigen-Specific T. Cell sensitization is impaired in IL-17–deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002;17:375–387. [DOI] [PubMed] [Google Scholar]
- 27.Hellings PW, Kasran A, Liu Z, Vandekerckhove P, Wuyts A, Overbergh L, Mathieu C, Ceuppens JL. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am J Respir Cell Mol Biol 2003;28:42–50. [DOI] [PubMed] [Google Scholar]
- 28.McKinley L, Alcorn JF, Peterson A, Dupont RB, Kapadia S, Logar A, Henry A, Irvin CG, Piganelli JD, Ray A, et al. Th17 cells mediate steroid-resistant airway inflammation and airway hyperresponsiveness in mice. J Immunol 2008;181:4089–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17–producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 2009;31:321–330. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. Gammadelta T-cell receptors: functional correlations. Immunol Rev 2007;215:77–88. [DOI] [PubMed] [Google Scholar]
- 31.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17–producing gammadelta T cell subsets. Nat Immunol 2009;10:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahn YS, Taube C, Jin N, Takeda K, Park JW, Wands JM, Aydintug MK, Roark CL, Lahn M, O'Brien RL, et al. V gamma 4+ gamma delta T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol 2003;171:3170–3178. [DOI] [PubMed] [Google Scholar]
- 33.Lahn M, Kanehiro A, Takeda K, Terry J, Hahn YS, Aydintug MK, Konowal A, Ikuta K, O'Brien RL, Gelfand EW, et al. MHC class I–dependent Vgamma4+ pulmonary T cells regulate alpha beta T cell–independent airway responsiveness. Proc Natl Acad Sci USA 2002;99:8850–8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O'Brien RL, Gelfand EW, et al. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol 2004;172:2894–2902. [DOI] [PubMed] [Google Scholar]
- 35.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol 1995;6:385–393. [DOI] [PubMed] [Google Scholar]
- 36.Thepen T, Kraal G, Holt PG. The role of alveolar macrophages in regulation of lung inflammation. Ann N Y Acad Sci 1994;725:200–206. [DOI] [PubMed] [Google Scholar]
- 37.GeurtsvanKessel CH, Lambrecht BN. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol 2008;1:442–450. [DOI] [PubMed] [Google Scholar]
- 38.Haslett C. Introduction: the paradox of inflammation. Semin Cell Biol 1995;6:315–316. [DOI] [PubMed] [Google Scholar]
- 39.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 1999;17:255–281. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez-Ramos JC, Lloyd C, Kapsenberg ML, Gonzalo JA, Coyle AJ. Non-redundant functional groups of chemokines operate in a coordinate manner during the inflammatory response in the lung. Immunol Rev 2000;177:31–42. [DOI] [PubMed] [Google Scholar]
- 41.Wills-Karp M, Ewart SL. The genetics of allergen-induced airway hyperresponsiveness in mice. Am J Respir Crit Care Med 1997;156:S89–S96. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor W Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell–mediated intestinal inflammation. Nat Immunol 2009;10:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, Riethmacher D, Si-Tahar M, Di Santo JP, Eberl G. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma T+ T Cells. J Exp Med 2008;205:1381–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009;31:331–341. [DOI] [PubMed] [Google Scholar]
- 45.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science 1998;279:1737–1740. [DOI] [PubMed] [Google Scholar]
- 46.Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev 2007;215:46–58. [DOI] [PubMed] [Google Scholar]
- 47.Andrew EM, Carding SR. Murine gammadelta T cells in infections: beneficial or deleterious? Microbes Infect 2005;7:529–536. [DOI] [PubMed] [Google Scholar]
- 48.Toy D, Kugler D, Wolfson M, Vanden BT, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol 2006;177:36–39. [DOI] [PubMed] [Google Scholar]
- 49.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 1995;3:811–821. [DOI] [PubMed] [Google Scholar]
- 50.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol 2009;9:556–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol 2007;8:247–256. [DOI] [PubMed] [Google Scholar]
- 52.Aujla SJ, Dubin PJ, Kolls JK. Interleukin-17 in pulmonary host defense. Exp Lung Res 2007;33:507–518. [DOI] [PubMed] [Google Scholar]
- 53.Jones CE, Chan K. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am J Respir Cell Mol Biol 2002;26:748–753. [DOI] [PubMed] [Google Scholar]
- 54.Prause O, Laan M, Lotvall J, Linden A. Pharmacological modulation of interleukin-17–induced GCP-2-, GRO-alpha- and interleukin-8 release in human bronchial epithelial cells. Eur J Pharmacol 2003;462:193–198. [DOI] [PubMed] [Google Scholar]
- 55.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell–mediated model in mice. Gastroenterology 2007;132:2359–2370. [DOI] [PubMed] [Google Scholar]
- 56.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, et al. IL-23 is essential for T cell–mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest 2006;116:1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheung PF, Wong CK, Lam CW. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: implication for Th17 lymphocytes-mediated allergic inflammation. J Immunol 2008;180:5625–5635. [DOI] [PubMed] [Google Scholar]
- 59.Langrish CL, McKenzie BS, Wilson NJ, de Waal MR, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 2004;202:96–105. [DOI] [PubMed] [Google Scholar]
- 60.Izcue A, Hue S, Buonocore S, Rancibia-Carcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell–dependent colitis. Immunity 2008;28:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, et al. TGF-beta–induced Foxp3 inhibits T(h)17 cell differentiation by antagonizing RORgammaT function. Nature 2008;453:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aydintug MK, Roark CL, Chain JL, Born WK, O'Brien RL. Macrophages express multiple ligands for gammadelta TCRs. Mol Immunol 2008;45:3253–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Havran WL, Jameson JM, Witherden DA. Epithelial cells and their neighbors. III. Interactions between intraepithelial lymphocytes and neighboring epithelial cells. Am J Physiol Gastrointest Liver Physiol 2005;289:G627–G630. [DOI] [PubMed] [Google Scholar]
- 64.Moon KA, Kim SY, Kim TB, Yun ES, Park CS, Cho YS, Moon HB, Lee KY. Allergen-induced CD11b+ CD11c(Int) CCR3+ macrophages in the lung promote eosinophilic airway inflammation in a mouse asthma model. Int Immunol 2007;19:1371–1381. [DOI] [PubMed] [Google Scholar]
- 65.Warmington KS, Boring L, Ruth JH, Sonstein J, Hogaboam CM, Curtis JL, Kunkel SL, Charo IR, Chensue SW. Effect of C-C chemokine receptor 2 (CCR2) knockout on type-2 (schistosomal antigen-elicited) pulmonary granuloma formation: analysis of cellular recruitment and cytokine responses. Am J Pathol 1999;154:1407–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tsuyuki S, Bertrand C, Erard F, Trifilieff A, Tsuyuki J, Wesp M, Anderson GP, Coyle AJ. Activation of the Fas receptor on lung eosinophils leads to apoptosis and the resolution of eosinophilic inflammation of the airways. J Clin Invest 1995;96:2924–2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Welgus HG, Campbell EJ, Bar-Shavit Z, Senior RM, Teitelbaum SL. Human alveolar macrophages produce a fibroblast-like collagenase and collagenase inhibitor. J Clin Invest 1985;76:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol 2004;31:3–7. [DOI] [PubMed] [Google Scholar]
- 69.DiTirro J, Rhoades ER, Roberts AD, Burke JM, Mukasa A, Cooper AM, Frank AA, Born WK, Orme IM. Disruption of the cellular inflammatory response to listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun 1998;66:2284–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thepen T, Van RN, Kraal G. Alveolar macrophage elimination in vivo is associated with an increase in pulmonary immune response in mice. J Exp Med 1989;170:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bilyk N, Holt PG. Cytokine modulation of the immunosuppressive phenotype of pulmonary alveolar macrophage populations. Immunology 1995;86:231–237. [PMC free article] [PubMed] [Google Scholar]
- 72.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 1993;177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holt PG. Alveolar macrophages. II. Inhibition of lymphocyte proliferation by purified macrophages from rat lung. Immunology 1979;37:429–436. [PMC free article] [PubMed] [Google Scholar]
- 74.Down-regulation of immune responses in the lower respiratory tract: the role of alveolar macrophages. Clin Exp Immunol 1986;63:261–270. [PMC free article] [PubMed] [Google Scholar]
- 75.Lambrecht BN. Alveolar macrophage in the driver's seat. Immunity 2006;24:366–368. [DOI] [PubMed] [Google Scholar]
- 76.Sergejeva S, Ivanov S, Lotvall J, Linden A. Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol 2005;33:248–253. [DOI] [PubMed] [Google Scholar]
- 77.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F et al. Development, cytokine profile and function of human IL-17 interleukin 17-producing helper T cells. Nat Immunology 2007;8:950–957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.