Abstract
Rationale: Many lines of evidence point toward the gastrointestinal (GI) tract in the pathophysiology of organ dysfunction in sepsis. Splanchnic hypoperfusion during sepsis leads to enterocyte apoptosis, diminished barrier function, and release of bacterial products. Sepsis lowers levels of insulin-like growth factor (IGF)–1, a known antiapoptotic factor. We recently demonstrated that treatment with IGF-1 is protective in murine sepsis.
Objectives: We hypothesize that decreased IGF-1 levels in sepsis contributes to the development of bacterial translocation.
Methods: Sepsis was induced in C57BL/6 mice via intratracheal instillation of Pseudomonas aeruginosa. Human subjects with sepsis were enrolled if they had a documented positive blood culture with a nonenteric organism. Bacterial translocation was measured in serum by quantitative real-time polymerase chain reaction with primers specific for enteric bacteria. Serum IGF-1 was measured by ELISA. Apoptosis of the GI epithelium was assessed via immunohistochemistry.
Measurements and Main Results: We found that mice with severe sepsis had evidence of bacterial translocation by 24 hours. Enteric bacterial load correlated inversely with levels of serum IGF-1. If we treated mice with IGF-1, bacterial translocation was significantly decreased. In addition, we found increased GI epithelial cell apoptosis after sepsis, which was significantly decreased after IGF-1 treatment. Human subjects with nonenteric sepsis developed progressive enteric bacteremia over 3 days. The degree of enteric bacteremia correlated inversely with serum IGF-1 levels.
Conclusions: These data support the hypothesis that sepsis-induced reductions in IGF-1 levels contribute to the development of bacterial translocation in both a murine model and human subjects.
Keywords: sepsis, bacteria, insulin-like growth factor–1, bacterial translocation
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Patients with sepsis have low levels of insulin-like growth factor (IGF)–1. Translocation of bacteria across the gastrointestinal (GI) epithelium is believed to occur in the setting of inflammation.
What This Study Adds to the Field
The degree of bacterial translocation across the GI epithelium is inversely correlated to the decline in IGF-1 levels during sepsis in both human subjects and a murine model. Treatment with IGF-1 decreases GI epithelial cell apoptosis and bacterial translocation in a murine model of severe sepsis.
Sepsis is a systemic response to infection that results in organ dysfunction. It affects 750,000 Americans annually with an overall mortality of nearly 30% (1). Many lines of evidence point toward the gastrointestinal (GI) tract in the pathophysiology of organ dysfunction in sepsis (2). Gastric tonometry studies suggest that mucosal ischemia may be an important determinant of survival in critical illness (3, 4). Splanchnic hypoperfusion during sepsis leads to mucosal injury, which causes diminished barrier function, release of bacterial products, and production of inflammatory cytokines (5). Inflammation within the GI tract can lead to loss of barrier function of the gut. Tumor necrosis factor–α, probably through increased production of nitric oxide, causes enterocyte death and, therefore, increases mucosal permeability in animals (6). Similar findings were shown in animals after exposure to endotoxin (6).
Studies have shown that GI permeability is increased in sepsis (7). In an animal model of pneumonia and sepsis, there was evidence of increased intestinal permeability and bacterial translocation (8). The increased intestinal permeability preceded the onset of bacterial translocation, lending credence to the hypothesis that altered barrier function predisposes to bacterial translocation. Apoptosis of epithelial cells may be important in the pathophysiology of altered intestinal permeability and bacterial translocation, because the inhibition of intestinal epithelial apoptosis by overexpression of B-cell lymphoma 2 led to improved survival in a murine model of sepsis (9). Furthermore, in a study of intensive care unit patients, increased intestinal permeability was associated with the development of Multiple Organ Dysfunction Syndrome, and the increase in permeability preceded the onset of Multiple Organ Dysfunction Syndrome (10). Although there is evidence of bacterial translocation in animal models of sepsis, convincing evidence for whole-bacteria translocation to the systemic bloodstream in patients with sepsis is lacking (11). Studies in trauma, thermal injury, and cirrhosis have used quantitative real-time polymerase chain reaction (PCR) to evaluate for bacterial translocation (12–15). However, no prior study has used this highly sensitive technique to detect bacterial translocation in sepsis.
Insulin like growth factor (IGF)–1 is known to have antiapoptotic properties. We have previously demonstrated that IGF-1 levels are reduced in both human sepsis and murine models of sepsis (16). In addition, we demonstrated that treatment with IGF-1 protected mice against organ injury and mortality after a sepsis insult (16). This was true even when the IGF-1 was given 12 hours after the onset of sepsis. We focused our initial work on the potential for IGF-1 to impact survival of immune cells, including Kupffer cells. However, it is likely that the mechanism of improved outcome after IGF-1 is multifactorial. In this study, we hypothesized that low levels of IGF-1 in sepsis would correlate with GI epithelial cell apoptosis and resultant bacterial translocation. Furthermore, we hypothesized that treatment with IGF-1 would decrease GI epithelial cell apoptosis and translocation of enteric bacteria. Some of the results of these studies have been reported previously in the form of an abstract (17).
METHODS
Human Subjects
Subjects were enrolled from the Inpatient Medical Units, including the Medical Intensive Care Unit and the Cardiovascular Intensive Care Unit, at the University of Iowa Hospitals and Clinics. Subjects were included if they had systemic inflammation, as defined by consensus statement and at least one positive blood culture growing a nonenteric organism (18). Briefly, systemic inflammation was defined as two or more of the following: temperature greater than 38°C or less than 36°C; heart rate greater than 90 beats per minute; respiratory rate greater than 20 or PaCO2 less than 22 mm Hg; and white blood cell count greater than 12,000 per mm3 or less than 4,000 per mm3, or greater than 10% immature forms. Blood cultures were considered positive for a nonenteric organism if they grew a gram-positive organism, Pseudomonas aeruginosa, or yeast. If the culture was positive for gram-positive cocci in clusters, then two positive blood cultures were required for enrollment. Subjects were excluded if they had multiple organisms growing in culture, a recent history of abdominal surgery or trauma, pancreatitis or other abdominal pathology, a GI source of infection, or were on immunosuppressive therapy. Subjects were enrolled within 24 hours of the culture turning positive. After informed consent by either the subject or legally authorized representative, blood was obtained daily for 3 days for measurement of enteric bacteremia by quantitative real-time PCR. Serum was obtained for measurement of IGF-1. This study was approved by the University of Iowa Institutional Review Board.
ELISA
ELISA kits for human and murine IGF-1 were obtained from R&D Systems (Minneapolis, MN) and were used according to the manufacturer's instructions.
Pneumonia and Bacteremia Model
Sepsis was induced in C57BL/6 mice via intratracheal intubation and inoculation, as previously described (16, 19, 20). Briefly, after anesthesia with ketamine/xylazine, C57BL/6 mice (female, age 6–8 wk; Harlan Laboratories, Indianapolis, IN) underwent intratracheal intubation with a 24-gauge angiocath and infection with Pseudomonas aeruginosa strain PA103 (5 × 104 cfu in a 50-μl volume). Sham animals received 50 μl of intratracheal saline. A subset of animals was treated with 24 mg/kg IGF-1 subcutaneously 12 hours after the onset of infection. Animals were killed at predetermined time points and blood was obtained from the portal vein and right ventricle as previously described (19). Livers were harvested for assessment of hepatic bacterial load. Animal studies were approved by the University of Iowa Institutional Animal Care and Use Committee.
DNA Isolation and Quantitative Real-Time PCR
Bacterial DNA was isolated from murine and human whole-blood and murine liver homogenates with the Bugs N' Beads kit (GenPoint, Oslo, Norway), per the manufacturer's instructions. For real-time PCR analysis, 2 ng of experimental sample DNA was added to 48 μl of reaction mixture containing 1× iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and 0.2 mM of each sense and anti-sense primers (IDT, Coralville, IA). For amplification of the wec F gene of the enterobacterial (EB) common antigen, we used a modified protocol previously described by Bayardelle and Zafarullah (21). Primers used to amplify the wec F gene are as follows (5′ to 3′): forward, GGTAAGCGTCGGCATCTTCTT; reverse, AAACAGCCACGCTTTGCTGT. These primers are specific for enteric bacterial species (21). Amplification was then performed in an iCycler iQ Fluorescence Thermocyler (Bio-Rad) as follows: 5 minutes at 95°C, followed by 25 cycles of 2 minutes at 94°C, 1 minute at 60°C, and 2 minutes at 72°C. Fluorescence data were captured during the 10-second dwell to ensure that primer-dimers were not contributing to the fluorescence signal generated with SYBR Green I DNA dye. Specificity of the amplification was confirmed by melting curve analysis. Data were collected and recorded by iCycler iQ software (Bio-Rad), and expressed as a function of threshold cycle, the cycle at which the fluorescence intensity in a given reaction tube rises above background (calculated as 10 times the mean SD of fluorescence in all wells over the baseline cycles). The experimental threshold cycle was compared with an Escherichia coli bacterial DNA standard curve for quantification.
Apoptosis Quantification
Apoptosis was detected by immunohistochemistry for activated caspase-3 (22) in formalin-fixed, paraffin-embedded cecal tissues. Briefly, tissue blocks were sectioned (4 μm) and rehydrated in graded alcohols. Antigen unmasking was performed by incubation with Tris/ethylenediaminetetraacetic acid buffer (pH 8.0) and steam. Endogenous peroxidase activity was quenched with 3% hydrogen peroxide, and nonspecific background blocked by Background Buster reagent (Innovex Biosciences, Richmond, CA). Primary rabbit monoclonal antibody to activated caspase-3 (no. 9664L; Cell Signaling Technologies, Danvers, MA) was applied and, after a series of rinses, secondary antibody (Rabbit Envision HRP System; DAKO, Carpinteria, CA) and chromogen (Rabbit Envision HRP System reagents, DAB Plus, and DAB Enhancer; DAKO) kits were applied according to manufacturers' instructions. Slides were dehydrated through graded alcohol and xylene baths, counterstained (Surgipath hematoxylin), and coverslipped. Apoptosis was further detected by a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay with a commercial kit (ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit, no. 7101; Millipore, Billerica, MA), as previously described (23), and stained cells were identified as apoptotic. Morphometric analysis of apoptosis was performed by a two-member pathologist team blinded to the study groups. Examiners selected random high-power fields (400× magnification with BX41 microscope; Olympus, Center Valley, PA) of the cecum mucosal wall to count cellular staining within a standardized length of mucosa per viewing field. Data from each examiner were compiled (total of 11 samples per tissue) and data presented as apoptotic cells per standardized length (1 mm) of cecal wall.
Statistical Analyses
Statistical analyses were performed with GraphPad Prism (GraphPad, San Diego, CA), and are specifically described in each figure legend.
RESULTS
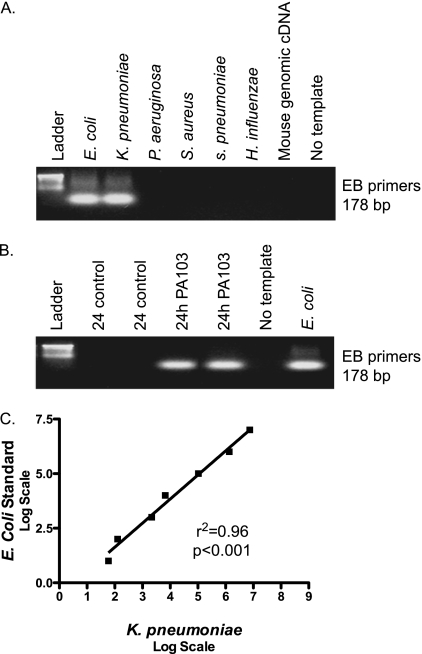
The Utility of Enteric Bacterial Primers in the Quantitative Assessment of Bacterial Translocation
Using primers that detect DNA from enteric bacteria, we sought to develop a protocol whereby we could effectively quantify enteric bacterial load in the bloodstream of subjects with sepsis. We used a modified version of a protocol described by Bayardelle and Zafarullah (21). This protocol involves primers that amplify a region of wec F, a gene that encodes a transferase important in creating the repetitive trisaccharide unit of the EB common antigen. These EB primers amplify DNA from enteric bacterial species E. coli, K. pneumoniae, Salmonella spp., Enterobacter spp., Serratia spp., and Shigella spp (21). In our study, we confirmed that EB primers amplify DNA from E. coli and K. pneumoniae, but not P. aeruginosa or gram-positive organisms (Figure 1A). Next, we examined whether there was qualitative evidence of enteric bacterial DNA in the blood stream of mice infected with P. aeruginosa. Murine sepsis was induced in C57BL/6 mice via intratracheal instillation of P. aeruginosa (strain PA103). We have previously demonstrated that this model of sepsis is associated with organ injury and 40% mortality (19). At 24 hours after the onset of sepsis, we found evidence of enteric bacteria in the blood, whereas control animals had no enteric bacteremia (Figure 1B). To determine whether these primers could be used to measure enteric bacterial load quantitatively, we serially spiked human blood samples with increasing amounts of K. pneumoniae. After DNA isolation, we compared the amount of enteric bacterial DNA to a known E. coli standard by quantitative real-time PCR with the EB primers. The load of bacterial DNA in spiked blood correlated significantly with the E. coli standard (Figure 1C), suggesting that these primers can be used to quantitatively measure the presence of enteric bacteria.
Figure 1.
Enterobacterial (EB) primers are useful in the quantitative measurement of enteric bacteria. (A) Standard polymerase chain reaction (PCR) was performed with EB primers, which amplify the wec F gene of the EB Common Antigen. We found evidence of the 178 base pair amplicon in enteric bacterial DNA (Escherichia coli, Klebsiella pneumoniae), but not in nonenteric bacteria (Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae) or mouse genomic DNA. (B) Sepsis was induced in C57BL/6 mice via intratracheal inoculation with Pseudomonas aeruginosa (strain PA103). Control mice received intratracheal saline. After 24 hours, mice were killed and DNA was isolated from whole blood. Standard PCR with EB primers demonstrated evidence of enteric bacteria in the blood of septic mice, but not that of control mice. (C) Human blood was spiked with serially increasing inocula of K. pneumoniae. Subsequently, DNA was isolated, and real-time PCR was performed with EB primers to compare amounts of amplification with that of an E. coli DNA standard. We found a significant correlation between the E. coli standard and the DNA isolated from spiked blood (r2 = 0.96; P < 0.001).
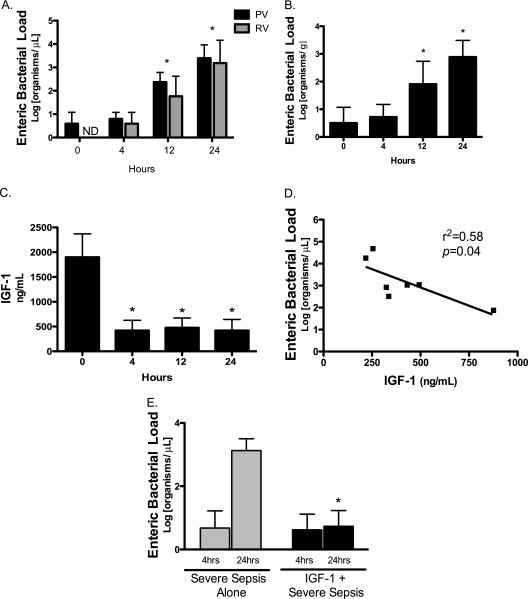
Enteric Bacteremia Is Inversely Correlated with Serum IGF-1 in Murine Sepsis
Murine sepsis was induced in C57BL/6 mice via intratracheal instillation of P. aeruginosa strain PA103. At serial time points after induction of sepsis, we evaluated for enteric bacteremia by quantitative real-time PCR of DNA harvested from whole blood obtained simultaneously from the portal vein and right ventricle. We found that there was a significant increase in enteric bacterial load in the blood at 12 and 24 hours after onset of sepsis (Figure 2A), indicating an increase in bacterial translocation over time. In addition, we found that enteric bacteria were present in the portal vein at baseline, providing evidence that the origin of these bacteria is indeed the GI tract. To further evaluate this, we measured levels of enteric bacteria in liver homogenates. We found that the enteric bacterial load in the liver increase significantly over time (Figure 2B). Similar to the portal vein bacterial load, there was evidence of enteric bacteria in the liver at baseline, suggesting the GI tract as the source of these bacteria. We have previously demonstrated that IGF-1 levels are reduced during sepsis (16). Figure 2C demonstrates confirmation that serum IGF-1 levels, as measured by ELISA, are reduced in our murine model of Pseudomonas bacteremia and sepsis.
Figure 2.
Enteric bacterial load inversely correlates with insulin-like growth factor (IGF)–1 in murine sepsis. (A) C57BL/6 mice received intratracheal inoculation with 5 × 104 organisms of Pseudomonas aeruginosa (PA103) to generate severe sepsis, and were killed at 4, 12, and 24 hours (each group represents the mean and SD of n > 6 mice). Enteric bacterial load was measured in portal vein (PV) and right ventricular (RV) blood by real-time polymerase chain reaction (PCR) with primers specific for enteric bacteria. A log transformation was performed to correct for unequal variances. Analysis of variance (ANOVA) followed by Bonferroni's test for multiple comparisons demonstrates a significant increase in enteric bacterial load in both the PV and RV blood at 12 and 24 hours (*P < 0.001). ND = none detected. (B) Enteric bacterial load was measured in liver homogenates at 4, 12, and 24 hours after onset of sepsis. Results are normalized per gram of liver tissue and are represented as a log transformation to correct for unequal variances. ANOVA, followed by Bonferroni's test for multiple comparisons, demonstrates a significant increase in enteric bacterial load in the liver at 12 and 24 hours (*P < 0.01). (C) Serum IGF-1 was measured by ELISA at baseline and 4, 12, and 24 hours after onset of murine sepsis (each group represents the mean and SD of n > 6 mice). We found that serum IGF-1 levels decrease significantly during sepsis, and remain decreased at 24 hours after the septic insult (*P < 0.001). (D) Results of linear regression analysis indicate a significant inverse correlation between the log transformation of enteric bacterial load and serum IGF-1 levels 24 hours after the onset of sepsis (r2 = 0.58; P = 0.04). (E) A subset of mice was treated with IGF-1 (24 mg/kg) 12 hours after infection (each group represents the mean and SD of n > 6 mice). ANOVA followed by Bonferroni's test for multiple comparisons demonstrates a significant decrease in enteric bacterial load at 24 hours in the mice treated with IGF-1 12 hours after infection compared with the septic mice (*P < 0.001).
We evaluated whether there was a correlation between elevated enteric bacterial load and decreased IGF-1 levels at 24 hours after the onset of sepsis. Through linear regression analysis, we demonstrated a significant inverse correlation between elevated enteric bacterial load and reduced IGF-1 levels (Figure 2D). To determine whether supplementation of IGF-1 would impact the degree of bacterial translocation, we treated mice with IGF-1 12 hours after the onset of sepsis. We found that treatment with IGF-1 12 hours after the onset of infection prevented the increase in enteric bacteremia seen in the septic mice at 24 hours (Figure 2E). This suggests that decreased IGF-1 levels during sepsis likely contribute to the subsequent development of bacterial translocation. Our previous studies showed that IGF-1 treatment also results in decreased organ injury and improved outcome in murine sepsis (16). As a group, these data suggest that one potential mechanism for improved outcome after IGF-1 therapy is decreased bacterial translocation.
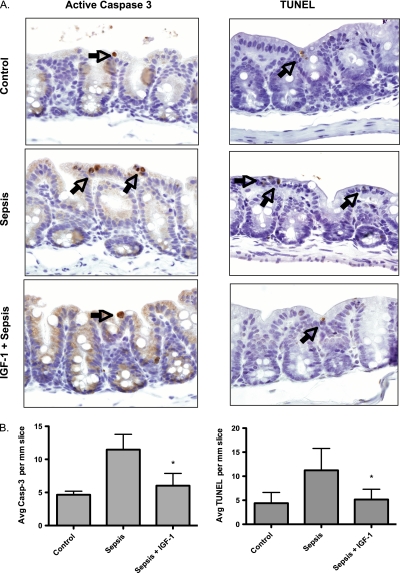
IGF-1 Treatment Decreases GI Epithelial Cell Apoptosis
To evaluate whether bacterial translocation during sepsis was associated with apoptosis of the GI epithelium, we evaluated the cecum of septic and control mice for evidence of apoptosis. Animal studies suggest that mesenteric perfusion to the distal ileum and cecum is reduced during endotoxemia (24). Therefore, the cecum is considered most vulnerable to ischemic injury in the setting of hypoperfusion. Interestingly, we found no gross disruption of the GI epithelium after sepsis on histologic evaluation. In fact, measurements of epithelial cell height were not different in the septic animals compared with control animals. However, when we probed further by staining with an antibody for activated caspase-3, a marker of apoptosis, we found that there was an increase in apoptotic cells in the cecum after sepsis (Figure 3A). This was decreased in the mice treated with IGF-1. In addition, we performed TUNEL staining as further evidence of apoptosis. We found that there was an increase in the number of TUNEL-positive cells in the cecum after sepsis, and this was decreased in the mice treated with IGF-1 (Figure 3A). To characterize further the degree of apoptosis, we quantified apoptotic cells (activated caspase-3 positive and TUNEL positive), and found a significant increase in the number of apoptotic cells in the septic epithelium compared with both control and IGF-1–treated mice (Figure 3B).
Figure 3.
Insulin-like growth factor (IGF)–1 treatment decreases gastrointestinal (GI) epithelial cell apoptosis in murine sepsis. (A) Sepsis was induced as previously described. A subset of mice received 24 mg/kg IGF-1 12 hours after the onset of sepsis. At 24 hours, the cecum was harvested, fixed, sectioned, and stained with an antibody for active caspase-3 (Casp-3) or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay to evaluate for apoptotic cells. Through both TUNEL assay and staining for active caspase-3, we found increased apoptotic cells in the cecum of septic mice, and this was reduced in the mice treated with IGF-1. The arrows indicate apoptotic GI epithelial cells. (B) To quantify the degree of apoptosis, we analyzed random high-power fields (n = 11) of cecal mucosa. Results for each mouse (six mice per group) and then each group were reported as average apoptotic (i.e., stained for activated caspase-3 or positive TUNEL staining) cells per 1 mm cecal wall length. The analysis was blinded. Analysis of variance (ANOVA), followed by Bonferroni's test for multiple comparisons, demonstrates a significant increase in apoptotic cells in septic mice compared with control animals. Importantly, there was a significant decrease in apoptotic epithelial cells at 24 hours in the mice treated with IGF-1 12 hours after infection compared with septic mice (*P < 0.05).
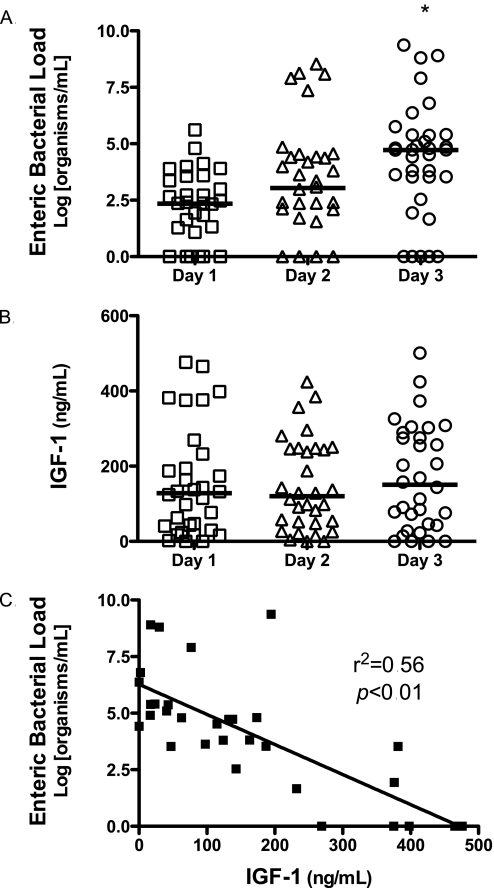
Bacterial Translocation Inversely Correlates with IGF-1 in Human Sepsis
We studied human subjects to see if a putative IGF-1 mechanism might also be implicated in human sepsis. Human subjects were enrolled if they had evidence of systemic inflammation and bacteremia from a nonenteric source. Patient characteristics, including source of infection, are shown in Table 1. All but one of the subjects had sepsis with a gram-positive organism; therefore, our cohort represents gram-positive sepsis. The majority of subjects had pneumonia or catheter-related bloodstream infections. Of note, all subjects had negative urine cultures, making it less likely that the urinary tract is the source of the enteric bacterial load seen. After informed consent, blood was drawn daily for 3 days. We evaluated for bacterial translocation by measuring bacterial load of enteric organisms daily. We found that subjects had an increase in enteric bacterial load over 3 days (Figure 4A). We have previously shown that human subjects with sepsis have low serum IGF-1 levels compared with healthy control subjects. In our prior study, the median IGF-1 level in healthy control subjects was 231.3 ng/ml, with an interquartile range of 132.5 ng/ml to 514.2 ng/ml (16). Consistent with this prior study, we found that subjects with sepsis had low IGF-1 levels at time of enrollment and over the subsequent 2 days; however, we did not find a significant difference between IGF-1 levels on Days 1–3 (Figure 4B). Using linear regression, we compared enteric bacterial load on Day 3 with IGF-1 levels measured at time of enrollment. We found a statistically significant inverse correlation between elevated enteric bacterial load and reduced IGF-1 levels (Figure 4C). This suggests that reduced IGF-1 levels during sepsis in humans may be an important factor in the development of bacterial translocation.
TABLE 1.
PATIENT CHARACTERISTICS
| Characteristics | n (%) |
|---|---|
| Male sex | 17 (53) |
| Bacterial species | |
| Staphylococcus species | 19 (59) |
| Streptococcus species | 12 (38) |
| Pseudomonas species | 1 (3) |
| Source of infection | |
| Pneumonia/respiratory tract | 10 (31) |
| Meningitis | 2 (6) |
| Central venous catheter | 6 (19) |
| Cellulitis | 5 (16) |
| Endocarditis | 3 (9) |
| Unknown | 6 (19) |
| Urinary tract | 0 (0) |
| 28-d mortality | 6 (19) |
Median age (interquartile range) = 61 (47–72) years.
Figure 4.
Enteric bacterial load inversely correlates with insulin-like growth factor (IGF)–1 in human sepsis. (A) Human subjects with nonenteric sepsis were enrolled within 24 hours of having a positive blood culture. Blood was drawn daily for 3 days, and enteric bacterial load was measured by real-time polymerase chain reaction (PCR). A log transformation was performed to correct for unequal variances. We found that subjects with nonenteric sepsis had demonstrable levels of enteric bacteria in the blood stream, and that the degree of enteric bacteremia increased over time. Analysis of variance (ANOVA), followed by Bonferroni's test, demonstrates a significant increase in enteric bacterial load on Day 3 compared with Day 1 (*P < 0.05). (B) Serum IGF-1 from human subjects was measured by ELISA daily for 3 days. There was no significant change I IGF-1 level over the course of 3 days. (C) Through linear regression analysis, we found a significant inverse correlation between Day 1 IGF-1 levels and Day 3 enteric bacterial load (r2 = 0.56; P < 0.01).
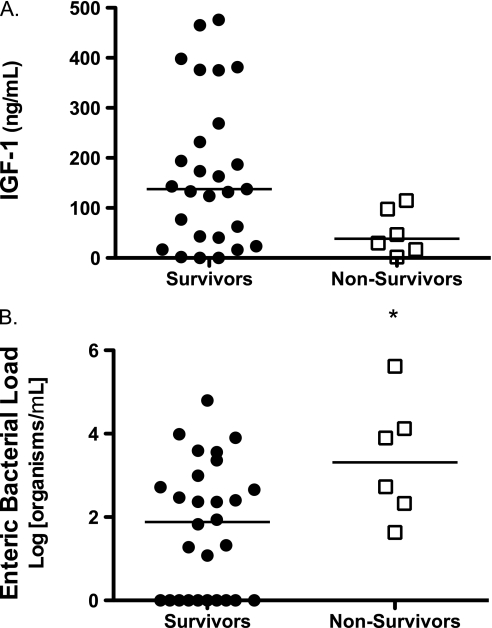
IGF-1 Levels are Lower and Enteric Bacterial Load Is Higher among Nonsurvivors
Although this was a small study designed to evaluate the relationship between IGF-1 and bacterial translocation in sepsis, we investigated whether there were differences in IGF-1 level and enteric bacterial load among subjects who did not survive. In our group of 32 subjects with sepsis, there were 6 deaths, giving an overall mortality rate of 19%. This mortality rate is lower than the murine model of severe sepsis, because the human subjects did not need to meet criteria for severe sepsis for enrollment. This mortality rate for bacteremic sepsis is consistent with prior studies (25). We compared IGF-1 levels on day of enrollment between survivors and nonsurvivors, and found a trend toward lower IGF-1 levels in the nonsurvivors, although this did not reach statistical significance (Figure 5A). We subsequently compared levels of enteric bacteria on day of enrollment between survivors and nonsurvivors, and found that there were significantly higher levels of enteric bacteria among the nonsurvivors (Figure 5B). Although these findings suggest that the degree of bacteria translocation may play a role in predicting outcome in human subjects with sepsis, a larger study is needed to evaluate further the role of bacterial translocation in human sepsis.
Figure 5.
Insulin-like growth factor (IGF)–1 is lower and enteric bacterial load is higher in nonsurvivors. (A) IGF-1 levels on day of enrollment of survivors and nonsurvivors were compared. Although Mann-Whitney test did not show a statistically significant difference (P = 0.06), there was a trend toward lower IGF-1 levels in the nonsurvivors. (B) Enteric bacterial loads on day of enrollment of survivors and nonsurvivors were compared. Mann-Whitney test demonstrates significantly higher enteric bacterial load among the nonsurvivors (P = 0.04).
DISCUSSION
In this study, we evaluated the relationship between reduced IGF-1 levels and the development of bacterial translocation during sepsis. Using a murine model of Pseudomonas bacteremia and sepsis, we found that levels of IGF-1 correlate inversely with enteric bacterial load. The increase in enteric bacterial load occurred concomitant with an increase in GI epithelial cell apoptosis. Furthermore, we demonstrated that treatment with IGF-1 prevents the development of GI epithelial cell apoptosis and bacterial translocation. To understand further how this relationship impacts human subjects with sepsis, we measured serum IGF-1 levels and enteric bacterial load in human subjects with systemic inflammation and nonenteric bacteremia. We found that enteric bacterial load increases over time. In addition, we demonstrated an inverse correlation between low levels of IGF-1 and increased enteric bacterial load, suggesting that reduced IGF-1 levels may play a role in the development of bacterial translocation in human sepsis. Finally, we found that increased enteric bacterial load was present among nonsurvivors, suggesting that degree of bacterial translocation may be a predictor of outcome in sepsis.
Bacterial translocation is defined as the passage of viable bacteria, or their products, from the GI tract to the mesenteric lymph nodes, liver, and bloodstream (11). There are three mechanisms that have been postulated to lead to bacterial translocation: increased intestinal permeability; small bowel overgrowth of bacteria; and local and systemic immune deficiency states. Increased intestinal permeability is thought to be the most important mechanism leading to bacterial translocation. Normally, the intestinal mucosa prevents transepithelial movement of water-soluble compounds with a radius greater than 0.4 nm (26). Many studies have evaluated the role of increased intestinal permeability and bacterial translocation after trauma and thermal injury. The systemic inflammatory response to traumatic injury is thought to cause altered intestinal permeability. In addition, low-flow states, such as hemorrhagic and cardiogenic shock, produce a decrease in splanchnic perfusion that is out of proportion to the drop in total cardiac output (26). This can lead to intestinal ischemia, with subsequent increased intestinal permeability to enteric organisms.
The evidence for altered gastrointestinal permeability after cutaneous burn injury is compelling. The increased intestinal permeability in patients with burns correlates with the extent of burn injury (27). In addition, infection in patients with burns has been shown to be associated with increased intestinal permeability (28). A retrospective review of patients with burns showed that 80% of the patients who died were septic with enteric organisms (29). The pathophysiology of increased intestinal permeability after thermal injury may be related to neutrophilic infiltration of the gastrointestinal tract, which occurs as a result of ischemic injury. In a rat model of burn injury, depletion of neutrophils resulted in decreased intestinal permeability and decreased bacterial translocation of enteric organisms (15, 30). In an animal model of pneumonia and sepsis, there was evidence of increased intestinal permeability and bacterial translocation (8). The increased intestinal permeability preceded the onset of bacterial translocation, lending credence to the hypothesis that altered barrier function predisposes to bacterial translocation.
Previous studies of human subjects with traumatic injuries, thermal injury, and cirrhosis have used quantitative real-time PCR to evaluate for bacterial translocation (12–15). To our knowledge, this is the first study to use this highly sensitive technique to detect bacterial translocation in sepsis. PCR is more sensitive than standard plating techniques in that it can quantify exact numbers of bacteria and is able to detect low-level bacteremia (31, 32). In addition, sepsis is associated with an active cellular and humoral response, resulting in bacterial killing. Standard culture techniques, which rely on bacterial viability, may not represent the true bacterial load in the setting of a brisk bactericidal response (33). Using primers specific for enteric bacteria allowed us to differentiate between the bacteria responsible for the primary infection and the secondary bacteremia from translocation of enteric bacteria. PCR also allowed us to detect and quantify low levels of enteric bacteria in human subjects, which may have been missed with standard culture techniques.
Our data clearly demonstrate the presence of increasing enteric bacterial loads during sepsis in both a murine model of P. aeruginosa sepsis and human subjects with sepsis. In the murine model, we have established that the source of this enteric bacterial load is the GI tract by demonstrating the increasing bacterial load in the portal vein and the liver. In human subjects with sepsis, the certainty of the origin of the enteric bacteria was more difficult to establish. Although we believe that the GI tract is the most likely source of these bacteria, particularly given the negative urinary culture in all subjects, we cannot state with 100% certainty that these bacteria did not arise from another source. Another limitation of this study is that nearly all of the human subjects enrolled had gram-positive sepsis. Therefore, the results of this study cannot be extrapolated to subjects with gram-negative sepsis. In this study, we found that enteric bacterial load was higher in subjects who ultimately did not survive. Given the small size of this study, this was an unexpected finding, and we believe that a larger study is needed to evaluate further the role of enteric bacterial load in predicting outcome in human subjects with sepsis.
Sepsis is associated with low levels of serum IGF-1 (34–36), although the exact mechanism for this remains unclear. IGF-1 is a potent inhibitor of apoptosis. The antiapoptotic effects of IGF-1 have been demonstrated in numerous cell types, and are not limited to the GI tract (37–39). Activation of the IGF-1 receptor leads to stimulation of several phosphorylation cascades that regulate a variety of biological effects (40). There is one major pathway of IGF-1 signaling that leads to cell survival. This pathway involves activation of phosphatidylinositol 3-kinase and Akt, and has been linked to cell metabolism, growth, and antiapoptotic responses (41). We have demonstrated previously that IGF-1 treatment improves outcome in murine sepsis (16). We found that one mechanism of improved outcome from sepsis with IGF-1 treatment involved improved hepatic bacterial clearance, likely due to decreased Kupffer cell apoptosis. However, it is more likely that the mechanism of improved outcome with IGF-1 is multifactorial. Prior animal studies have demonstrated a decrease in translocation of bacteria across the gut after IGF-1 therapy (42–44) in models of liver injury and thermal injury. However, ours are the first studies to demonstrate that IGF-1 treatment protects the GI epithelium from apoptosis in the setting of a septic insult and, thus, decreases bacterial translocation. In addition, this is the first study to report a significant correlation between low levels of IGF-1 and bacterial translocation in human sepsis.
In the last decade, intensive insulin therapy has been proposed to treat the critically ill. Putative benefits include: anabolic effects via the phosphatidylinositol 3-kinase signaling pathway; suppressed inflammation by modulating nuclear factor–κB–regulated cascades; prevention of endothelial and mitochondrial dysfunction (45, 46); and limiting apoptosis. An initial trial in surgical intensive care unit patients was positive (47), but four subsequent trials have failed to confirm a benefit, and all were dogged by a serious risk of hypoglycemia (48–51). Because activation at the IGF-1 receptor has similar downstream effects as signaling from the insulin receptor, but decoupled from insulin's impact on blood glucose levels, IGF-1 might have a role in critical illness. Future studies are needed to determine whether treatment with IGF-1 will be beneficial in human sepsis.
Supported by a Veterans' Administration Merit Review Grant, National Institutes of Health (NIH) grants HL-073967-02, HL-077431-01 (G.W.H.), and K08DK073519 (A.A.), and by National Center for Research Resources/NIH grant UL1RR024979.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the Clinical and Translational Science Award or the National Institutes of Health.
Originally Published in Press as DOI: 10.1164/rccm.200911-1757OC on April 22, 2010
Author Disclosure: G.W.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; K.C.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.B.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; G.A.S. received $10,000–$49,999 from ABS Corp. and more than $100,000 from Pneuma Partners, LLC, in current institutional grants, more than $100,000 from Agennix, Inc., and $50,000–$99,999 from Chiron Corp. in past institutional grants, and $1,000–$4,999 from Agennix Inc. in consultant or advisory board fees; D.K.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the united states: analysis of incidence, outcome, and associated costs of care. Crit Care Med 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 2.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 1996;20:411–417. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez G, Palizas F, Doglio G, Wainsztein N, Gallesio A, Pacin J, Dubin A, Schiavi E, Jorge M, Pusajo J, et al. Gastric intramucosal pH as a therapeutic index of tissue oxygenation in critically ill patients. Lancet 1992;339:195–199. [DOI] [PubMed] [Google Scholar]
- 4.Maynard N, Bihari D, Beale R, Smithies M, Baldock G, Mason R, McColl I. Assessment of splanchnic oxygenation by gastric tonometry in patients with acute circulatory failure. JAMA 1993;270:1203–1210. [PubMed] [Google Scholar]
- 5.Karimova A, Pinsky DJ. The endothelial response to oxygen deprivation: biology and clinical implications. Intensive Care Med 2001;27:19–31. [DOI] [PubMed] [Google Scholar]
- 6.Xu DZ, Lu Q, Deitch EA. Nitric oxide directly impairs intestinal barrier function. Shock 2002;17:139–145. [DOI] [PubMed] [Google Scholar]
- 7.Johnston JD, Harvey CJ, Menzies IS, Treacher DF. Gastrointestinal permeability and absorptive capacity in sepsis. Crit Care Med 1996;24:1144–1149. [DOI] [PubMed] [Google Scholar]
- 8.Yu P, Martin CM. Increased gut permeability and bacterial translocation in Pseudomonas pneumonia–induced sepsis. Crit Care Med 2000;28:2573–2577. [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM II, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA 2002;287:1716–1721. [DOI] [PubMed] [Google Scholar]
- 10.Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med 1998;158:444–451. [DOI] [PubMed] [Google Scholar]
- 11.Macintire DK, Bellhorn TL. Bacterial translocation: clinical implications and prevention. Vet Clin North Am Small Anim Pract 2002;32:1165–1178. [DOI] [PubMed] [Google Scholar]
- 12.Frances R, Benlloch S, Zapater P, Gonzalez JM, Lozano B, Munoz C, Pascual S, Casellas JA, Uceda F, Palazon JM, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology 2004;39:484–491. [DOI] [PubMed] [Google Scholar]
- 13.Kane TD, Alexander JW, Johannigman JA. The detection of microbial DNA in the blood: a sensitive method for diagnosing bacteremia and/or bacterial translocation in surgical patients. Ann Surg 1998;227:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang WZ, Han TQ, Tang YQ, Zhang SD. Rapid detection of sepsis complicating acute necrotizing pancreatitis using polymerase chain reaction. World J Gastroenterol 2001;7:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fazal N, Shamim M, Khan SS, Gamelli RL, Sayeed MM. Neutrophil depletion in rats reduces burn-injury induced intestinal bacterial translocation. Crit Care Med 2000;28:1550–1555. [DOI] [PubMed] [Google Scholar]
- 16.Ashare A, Nymon AB, Doerschug KC, Morrison JM, Monick MM, Hunninghake GW. Insulin-like growth factor–1 improves survival in sepsis via enhanced hepatic bacterial clearance. Am J Respir Crit Care Med 2008;178:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashare A, Doerschug KC, Nymon AB, Monick MM, Schmidt GA, Hunninghake GW. Low levels of IGF-1 correlate with the development of bacterial translocation in sepsis [abstract]. Am J Respir Crit Care Med 2009;179:A1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: the ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 19.Ashare A, Monick MM, Powers LS, Yarovinsky T, Hunninghake GW. Severe bacteremia results in a loss of hepatic bacterial clearance. Am J Respir Crit Care Med 2006;173:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Humlicek AL, Pang L, Look DC. Modulation of airway inflammation and bacterial clearance by epithelial cell ICAM-1. Am J Physiol Lung Cell Mol Physiol 2004;287:L598–L607. [DOI] [PubMed] [Google Scholar]
- 21.Bayardelle P, Zafarullah M. Development of oligonucleotide primers for the specific pcr-based detection of the most frequent Enterobacteriaceae species DNA using wec gene templates. Can J Microbiol 2002;48:113–122. [DOI] [PubMed] [Google Scholar]
- 22.Meyerholz DK, Samuel I. Morphologic characterization of early ligation-induced acute pancreatitis in rats. Am J Surg 2007;194:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK, McCaffrey AP, McNamara JO II, Giangrande PH. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat Biotechnol 2009;27:839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D, Qi L, Guillory D, Cruz N, Berg R, Deitch EA. Mechanisms of endotoxin-induced intestinal injury in a hyperdynamic model of sepsis. J Trauma 1993;34:676–682; discussion 682–673. [DOI] [PubMed] [Google Scholar]
- 25.Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA 1995;273:117–123. [PubMed] [Google Scholar]
- 26.Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med 1991;19:627–641. [DOI] [PubMed] [Google Scholar]
- 27.Ryan CM, Yarmush ML, Burke JF, Tompkins RG. Increased gut permeability early after burns correlates with the extent of burn injury. Crit Care Med 1992;20:1508–1512. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler TR, Smith RJ, O'Dwyer ST, Demling RH, Wilmore DW. Increased intestinal permeability associated with infection in burn patients. Arch Surg 1988;123:1313–1319. [DOI] [PubMed] [Google Scholar]
- 29.Desai MH, Herndon DN, Rutan RL, Abston S, Linares HA. Ischemic intestinal complications in patients with burns. Surg Gynecol Obstet 1991;172:257–261. [PubMed] [Google Scholar]
- 30.Sir O, Fazal N, Choudhry MA, Gamelli RL, Sayeed MM. Neutrophil depletion prevents intestinal mucosal permeability alterations in burn-injured rats. Am J Physiol Regul Integr Comp Physiol 2000;278:R1224–R1231. [DOI] [PubMed] [Google Scholar]
- 31.Cursons RT, Jeyerajah E, Sleigh JW. The use of polymerase chain reaction to detect septicemia in critically ill patients. Crit Care Med 1999;27:937–940. [DOI] [PubMed] [Google Scholar]
- 32.Ashare A, Powers LS, Butler NS, Doerschug KC, Monick MM, Hunninghake GW. Anti-inflammatory response is associated with mortality and severity of infection in sepsis. Am J Physiol Lung Cell Mol Physiol 2005;288:L633–L640. [DOI] [PubMed] [Google Scholar]
- 33.Aronson MD, Bor DH. Blood cultures. Ann Intern Med 1987;106:246–253. [DOI] [PubMed] [Google Scholar]
- 34.Heemskerk VH, Daemen MA, Buurman WA. Insulin-like growth factor-1 (IGF-1) and growth hormone (GH) in immunity and inflammation. Cytokine Growth Factor Rev 1999;10:5–14. [DOI] [PubMed] [Google Scholar]
- 35.Karinch AM, Pan M, Lin CM, Strange R, Souba WW. Glutamine metabolism in sepsis and infection. J Nutr 2001;131:2535S–2538S; discussion 2550S–2551S. [DOI] [PubMed] [Google Scholar]
- 36.Timmins AC, Cotterill AM, Hughes SC, Holly JM, Ross RJ, Blum W, Hinds CJ. Critical illness is associated with low circulating concentrations of insulin-like growth factors–I and –II, alterations in insulin-like growth factor binding proteins, and induction of an insulin-like growth factor binding protein 3 protease. Crit Care Med 1996;24:1460–1466. [DOI] [PubMed] [Google Scholar]
- 37.Muta K, Krantz SB. Apoptosis of human erythroid colony-forming cells is decreased by stem cell factor and insulin-like growth factor I as well as erythropoietin. J Cell Physiol 1993;156:264–271. [DOI] [PubMed] [Google Scholar]
- 38.Jung Y, Miura M, Yuan J. Suppression of interleukin-1 beta–converting enzyme-mediated cell death by insulin-like growth factor. J Biol Chem 1996;271:5112–5117. [DOI] [PubMed] [Google Scholar]
- 39.Matthews CC, Feldman EL. Insulin-like growth factor I rescues SH-SY5Y human neuroblastoma cells from hyperosmotic induced programmed cell death. J Cell Physiol 1996;166:323–331. [DOI] [PubMed] [Google Scholar]
- 40.Petley T, Graff K, Jiang W, Yang H, Florini J. Variation among cell types in the signaling pathways by which IGF-I stimulates specific cellular responses. Horm Metab Res 1999;31:70–76. [DOI] [PubMed] [Google Scholar]
- 41.Dolcet X, Egea J, Soler RM, Martin-Zanca D, Comella JX. Activation of phosphatidylinositol 3-kinase, but not extracellular-regulated kinases, is necessary to mediate brain-derived neurotrophic factor–induced motoneuron survival. J Neurochem 1999;73:521–531. [DOI] [PubMed] [Google Scholar]
- 42.Fukushima R, Saito H, Inoue T, Fukatsu K, Inaba T, Han I, Furukawa S, Lin MT, Muto T. Prophylactic treatment with growth hormone and insulin-like growth factor i improve systemic bacterial clearance and survival in a murine model of burn-induced gut-derived sepsis. Burns 1999;25:425–430. [DOI] [PubMed] [Google Scholar]
- 43.Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, Vagianos CE. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg 2000;190:423–431. [DOI] [PubMed] [Google Scholar]
- 44.Jeschke MG, Bolder U, Chung DH, Przkora R, Mueller U, Thompson JC, Wolf SE, Herndon DN. Gut mucosal homeostasis and cellular mediators after severe thermal trauma and the effect of insulin-like growth factor-I in combination with insulin-like growth factor binding protein–3. Endocrinology 2007;148:354–362. [DOI] [PubMed] [Google Scholar]
- 45.Langouche L, Vanhorebeek I, Vlasselaers D, Vander Perre S, Wouters PJ, Skogstrand K, Hansen TK, Van den Berghe G. Intensive insulin therapy protects the endothelium of critically ill patients. J Clin Invest 2005;115:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet 2005;365:53–59. [DOI] [PubMed] [Google Scholar]
- 47.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med 2001;345:1359–1367. [DOI] [PubMed] [Google Scholar]
- 48.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med 2008;358:125–139. [DOI] [PubMed] [Google Scholar]
- 49.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297. [DOI] [PubMed] [Google Scholar]
- 50.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the glucontrol study. Intensive Care Med 2009;35:1738–1748. [DOI] [PubMed] [Google Scholar]
- 51.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med 2006;354:449–461. [DOI] [PubMed] [Google Scholar]