Abstract
Rationale: Endothelin-1 (ET1) is dysregulated in pulmonary hypertension (PH). It may be important in the pathobiology of congenital diaphragmatic hernia (CDH).
Objectives: We hypothesized that ET1 levels in the first month would be higher in infants with CDH who subsequently expired or were discharged on oxygen (poor outcome). We further hypothesized that ET1 levels would be associated with concurrent severity of PH.
Methods: We sampled plasma at 24 to 48 hours, and 1, 2, and 4 weeks of age in 40 prospectively enrolled newborns with CDH. We performed echocardiograms to estimate pulmonary artery pressure at less than 48 hours of age and weekly to 4 weeks. PH was classified in relationship to systemic blood pressure (SBP): less than 2/3 SBP, 2/3 SBP-systemic is related to pressure, or systemic-to-suprasystemic pressure.
Measurements and Main Results: ET1 levels at 1 and 2 weeks were higher in infants with poor outcome compared with infants discharged on room air (median and interquartile range: 27.2 [22.6, 33.7] vs. 19.1 [16.1, 29.5] pg/ml, P = 0.03; and 24.9 [17.6, 39.5] vs. 17.4 [13.7, 21.8] pg/ml, P = 0.01 at 1 and 2 weeks, respectively). Severity of PH was significantly associated with increasing ET1 levels at 2 weeks (16.1 [13.7, 21.8], 21.0 [17.4, 31.1], and 23.6 [21.9, 39.5] pg/ml for increasing PH class, P = 0.03). Increasing severity of PH was also associated with poor outcome at that time (P = 0.001).
Conclusions: Infants with CDH and poor outcome have higher plasma ET1 levels and severity of PH than infants discharged on room air. Severity of PH is associated with ET1 levels.
Keywords: bronchopulmonary dysplasia, biological markers, echocardiography
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Vascular structure and reactivity are abnormal in congenital diaphragmatic hernia (CDH) and there remains a subset of severely affected children who are refractory to conventional therapies.
What This Study Adds to the Field
Endothelin-1 (ET1) has a potential role in the pathobiology of infants with severe CDH. Therapies that modulate ET1 or its effectors may be effective treatment for severely affected infants.
Congenital diaphragmatic hernia (CDH) occurs in 1 of 3,000 live births (1–3). This developmental defect is characterized by varying degrees of lung and vascular hypoplasia (4–6). Mortality for live-born infants remains high (30–50%) in population-based studies, despite treatment strategies focused on limitation of ventilator- and hyperoxia-induced lung injury (1–3). Chronic respiratory morbidity, growth failure, and neurodevelopmental delay are prevalent among survivors (7–9). Infants initially discharged requiring supplemental oxygen are at greatest risk for these morbidities (7, 8).
Pulmonary hypertension (PH) is common in infants with CDH (10). In one series, there were no survivors among eight infants with persistent systemic or suprasystemic echocardiographic estimates of pulmonary artery pressure (Ppa) at 6 weeks of age (11). Chronic lesser elevations in pulmonary vascular resistance (PVR) or Ppa are also an important indicator of the status of an infant with CDH (11, 12). The mechanism of prolonged elevation in PVR is unknown, but may be multifactorial, with varying contributions related to both size and function of the vascular bed. The hypoplastic vascular bed (4) is subject to lung and vascular injury due to ongoing respiratory support measures (13) and abnormal blood pressures and flow patterns (14, 15). Abnormal endothelial function results in delayed perinatal transition with an imbalance of vasoactive mediators (15–17). Together these insults may result in a postnatal arrest of alveolar and microvascular growth with worsening PH (18–22).
Endothelin-1 (ET1) is a candidate marker or mediator of this physiologic abnormality as a potent vasoconstrictor and smooth muscle cell mitogen (23). Prior studies demonstrate that elevated plasma ET1 levels correlate with severity of various diseases (24–27) and decreased lung clearance (26, 28, 29) in PH from diverse etiologies. In newborns with PH (PPHN) plasma ET1 levels are highest in the most severely-affected infants requiring extracorporeal membrane oxygenation support and decrease with resolution of the illness (17, 30). ET1 is elevated at admission in patients with CDH compared with healthy control subjects (31), and pulmonary expression of both endothelin A and B (ETA and ETB) receptors are increased in infants who died with PH at less than 3 days of age (32). Experimental models have yielded limited data, suggesting a possible developmental role of ET1 in CDH-associated lung hypoplasia (33) and abnormal ET1 signaling in fetal CDH (34–36). However, there are no longitudinal data in either humans or animal models to lend insight into the potential pathophysiological role of ET1 in vascular remodeling and growth in infants with CDH.
The primary aim of this prospective cohort study was to evaluate the relationship between plasma ET1 levels in the first month of life in infants with CDH and clinical outcome based on discharge status (discharged on room air versus death or discharged on supplemental oxygen). We also sought to evaluate the relationship of serial estimates of Ppa by echocardiogram to the clinical outcome of these infants and their plasma ET1 levels. A subset of these results was previously presented in abstract form (37).
METHODS
Study Enrollment
The study was approved by the University of California San Francisco Institutional Review Board. Written informed consent was obtained. Infants with CDH were eligible if they were admitted to the neonatal intensive care unit. Exclusion criteria were Morgagni hernia, known syndrome or anomalies that affect prognosis, or unable to enroll by 48 hours of age. Patients were recruited over 40 months (9/2002–12/2005); 54 infants were admitted, 9 were excluded, 5 declined participation, and 40 were enrolled.
Neonatal Clinical Management
All infants were intubated with assisted ventilation from birth. Infants were managed with gentle ventilation, using permissive hypercapnia, pressure limitation, and permissive oxygenation (38). Details of neonatal respiratory support are available in the online data supplement. Inhaled nitric oxide (iNO) was administered for impaired oxygen delivery due to PH. Infants with imminent inadequate oxygen delivery were placed on extracorporeal membrane oxygenation support. Surgical repair was performed after clinical stabilization. Supplemental oxygen was administered to maintain oxygen saturation by pulse oximetry of 95% or greater when breathing without assistance. Infants who failed weaning were discharged on supplemental oxygen.
Plasma ET1 Levels
We collected 1 ml of blood from an indwelling arterial catheter or puncture into a chilled ethylenediaminetetraacetic acid tube at 24 to 48 hours and 1, 2, and 4 weeks of age. Samples were immediately centrifuged at 2,000 × g for 15 minutes at 4°C. Plasma was stored (−70°C) and processed as previously described (39). Immunoreactive ET1 was detected in duplicate by antibodies purchased from Peninsula Labs (Belmont, CA). The cross-reactivity for human ET2 and ET3 is 7%. The coefficient of variability was 2.1%.
Echocardiogram Protocol
Echocardiograms were obtained with the Acuson Sequoia C256 or C512 ultrasound system (Mountain View, CA) at less than 48 hours, and 1, 2, 3, and 4 weeks of age. Standard echocardiographic views were obtained to classify estimated Ppa in relationship to systemic systolic blood pressure: less than 2/3 systemic pressure, greater than or equal to 2/3 systemic-to-systemic pressure, or systemic-to-suprasystemic pressure. Echocardiograms were interpreted by a single reader (T.A.T.) blinded to patient outcome. Three measures (in descending order of importance) were used to classify pulmonary-to-systemic pressure relationship by echocardiogram: (1) pressure differential by direction and velocity of ductus arteriosus (DA) flow (Bernoulli equation) (40); (2) two-dimensional interventricular septum position (parasternal short axis) graded as normal, flat, or D-shaped, with a substantially flattened septum indicating right ventricular pressure greater than or equal to 2/3 systemic pressure, and D-shaped considered to be systemic-to-suprasystemic (41, 42); and (3) peak tricuspid regurgitant (TR) jet velocity measured by Doppler with right ventricular systolic pressure estimated by the modified Bernoulli equation with no allowance for right atrial pressure (43). There were no structural cardiac abnormalities that could confound the classification.
Statistical Analysis
We used χ2, Fisher exact, nonparametric rank sum and trend tests, and we analyzed receiver-operator characteristic (ROC) curves to evaluate the association of ET1 levels with clinical outcomes (Stata 9.0; College Station, TX). Longitudinal changes in ET1 levels were evaluated with mixed-effects linear models. ET1 was used as the dependent variable after natural log-transformation due to its skewed distribution. We explored quadratic terms for time and used the likelihood ratio test to determine the best models for the relationships. P values less than 0.05 were considered significant.
RESULTS
Characteristics of the study population are shown (Table 1). These infants represent a spectrum of disease severity for infants with CDH diagnosed by prenatal ultrasound (75%) or presenting with respiratory distress from birth. From an anatomic perspective, 80% of infants with right-sided defects had liver herniated into the chest and 51% of infants with left-sided defects had liver herniated into the chest. All infants with herniated liver required prosthetic patch repair of the diaphragm. Three infants did not undergo repair. One expired at 1 hour of age from respiratory failure and two had life support withdrawn (one was diagnosed with Fryn syndrome postenrollment and another with additional anomalies complicated by prematurity). Overall, 22 infants had a poor outcome, as determined by their status at hospital discharge; 11 infants expired and 11 infants were discharged on supplemental oxygen. Infants discharged on supplemental oxygen had higher Pco2 levels at discharge than those discharged on room air (57 ± 9 mm Hg vs. 48 ± 6 mm Hg; P = 0.008), although PH was well compensated in both groups (7.38 ± 0.06 vs. 7.39 ± 0.03; P = 0.89).
TABLE 1.
SELECTED CHARACTERISTICS OF STUDY COHORT (N = 40)
| Gestational age, wk | 37.2 ± 2.7 (30–41) |
|---|---|
| Birth weight, kg | 3.0 ± 0.7 (1.3–4.83) |
| Male sex | 27 (67.5%) |
| Left-sided hernia | 35 (87.5%) |
| Liver herniated into hemithorax | 22 (55%) |
| Inhaled nitric oxide | 24 (60%) |
| Extracorporeal support | 4 (10%) |
| Prosthetic patch repair* | 25 (68%) |
| Age at CDH repair, d | 4 (1–26) |
| Age at extubation, d | 15.5 (2–41) |
| Age at death, d | 25 (0–87) |
| Survival | 29 (72.5%) |
| Discharge on supplemental oxygen† | 11 (38%) |
Definition of abbreviation: CDH = congenital diaphragmatic hernia.
Data are mean ± SD, median (range), or number (%).
N = 37 infants that underwent repair.
Survivors only.
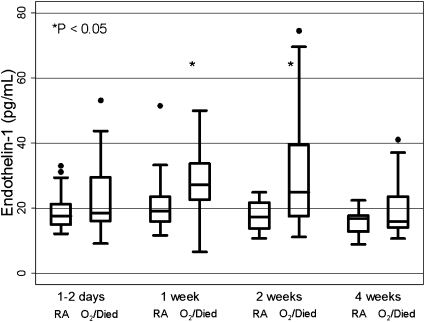
ET1 levels were higher in infants with a poor outcome at all study time points (1–2 d, 1, 2, and 4 wk). However, these levels were only significantly different at 1 and 2 weeks of age (Table 2, Figure 1). The area under the ROC curve for plasma ET1 levels at 2 weeks was 0.77 (95% CI, 0.58–0.89). A cut-off of 25.8 pg/ml at 2 weeks of age yielded 47% sensitivity for a poor outcome and 100% specificity, correctly classifying 70% of the cohort. In a longitudinal model, infants with a poor outcome were predicted to have higher ET1 levels throughout the first month of life. There was a quadratic relationship with patient age at sample time, with little change over time among infants discharged on room air, and an ET1 peak at approximately 2 weeks of age among infants who were discharged on oxygen or expired. In a sensitivity analysis, results were similar when the two infants for whom life support was withdrawn before 2 weeks of age were excluded. Discharge status maintained an independent association with ET1 level, without significant alteration in the relationship, after adjusting for concurrent use of iNO therapy, assisted ventilation, or the degree of elevation of Ppa.
TABLE 2.
PLASMA ENDOTHELIN-1 LEVELS (pg/ml) AT VARIOUS TIME POINTS BY STATUS AT HOSPITAL DISCHARGE (DISCHARGE ON ROOM AIR vs. EXPIRED OR DISCHARGE ON SUPPLEMENTAL OXYGEN)
| Values by Outcome Group, pg/ml | 1–2 d | 1 wk | 2 wk | 4 wk |
|---|---|---|---|---|
| Discharge on room air, median (interquartile range) | 17.7 (14.9, 21.3) (n = 18) | 19.1 (16.0, 23.5) (n = 18) | 17.4 (13.7, 21.8) (n = 14) | 17.0 (12.8, 17.8) (n = 8) |
| Discharge on oxygen/expired, median (interquartile range) | 18.5 (16.1, 29.5) (n = 21) | 27.2 (22.6, 33.7) (n = 20) | 24.9 (17.6, 39.5) (n = 19) | 15.9 (14.0, 23.6) (n = 17) |
| P value* | 0.37 | 0.03 | 0.01 | 0.47 |
| Discharged to home/other hospital, n | 0 | 0 | 4 | 10 |
| Expired, n | 1 | 2 | 3 | 5 |
Rank sum test for comparison by discharge status (room air vs. supplemental oxygen/expired).
Figure 1.
Box-and-whisker plots of plasma endothelin-1 (ET1) levels in the first month of life in infants with congenital diaphragmatic hernia. At each time point, data from infants discharged on room air (RA) are on the left, and data from those discharged on oxygen or expired (O2/Died) are on the right. ET1 levels are significantly higher in infants discharged on oxygen or expired at 1 and 2 weeks of age.
iNO therapy at the time of plasma ET1 measurement had a significant interaction with sample time (P = 0.01) and a quadratic relationship with patient age at sample time. This modeled interaction predicted that ET1 levels after 9 days of age were higher with iNO therapy than without iNO. Although iNO was independently associated with ET1 levels in these models, there was not a statistically significant difference at any single time point in univariate analyses (data not shown). Concurrent assisted ventilation was not independently associated with ET1 levels at any time (data not shown).
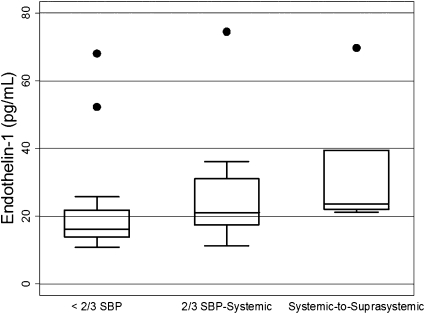
Higher ET1 levels were generally associated with higher estimates of Ppa at all time points, in analyses restricted to ET1 samples (n = 118) obtained within 3 days of the echocardiogram (Table 3). This pattern was statistically significant only at 2 weeks of age (Figure 2). Longitudinal linear models predicting log-transformed ET1 levels demonstrated a relationship between Ppa classification and ET1 levels. Using less than 2/3 systemic Ppa as the reference group, there was a statistically significant elevation in ET1 levels for the comparison of systemic-to-suprasystemic Ppa versus less than 2/3 systemic Ppa, but not for the comparison of greater than or equal to 2/3 systemic-to-systemic Ppa versus less than 2/3 systemic Ppa. These relationships were maintained after controlling for concurrent inhaled nitric oxide therapy and patient discharge status.
TABLE 3.
RELATIONSHIP OF PLASMA ENDOTHELIN-1 LEVELS (pg/ml) TO ESTIMATED PULMONARY ARTERY PRESSURE FOR SAMPLES OBTAINED WITHIN 3 DAYS OF ECHOCARDIOGRAM
| Estimated Pulmonary Pressure by Echocardiogram |
||||
|---|---|---|---|---|
| Values (pg/ml) at Time Points | <2/3 Systemic Blood Pressure | 2/3 Systemic – Systemic | Systemic – Suprasystemic | P Value* |
| 1–2 d | 21.1 (n = 1) | 17.3 (14.5, 21.6) (n = 16) | 18.7 (15.8, 31.2) (n = 22) | 0.37 |
| 1 wk | 18.2 (14.5, 26.0) (n = 8) | 22.9 (13.9, 26.0) (n = 14) | 23.4 (22.4, 36.6) (n = 9) | 0.08 |
| 2 wk | 16.1 (13.7, 21.8) (n = 14) | 21.0 (17.4, 31.1) (n = 8) | 23.6 (21.9, 39.5) (n = 6) | 0.03 |
| 4 wk | 16.0 (14.2, 20.6) (n = 12) | 13.8 (13.1, 25.6) (n = 4) | 19.9 (17.9, 30.5) (n = 4) | 0.24 |
Data are median (interquartile range).
Nonparametric test for trends.
Figure 2.
Box-and-whisker plots of plasma endothelin-1 (ET1) levels by echocardiographic classification of pulmonary artery pressure (Ppa) at 2 weeks of age. P = 0.03 for relationship of ET1 to Ppa classification by nonparametric test for trends. SBP = systemic blood pressure.
Four of 144 echocardiograms (3%) had inadequate data to classify Ppa. The ductus arteriosus was patent in 74% of the echocardiograms obtained at or before 2 days of age. In contrast, at 1 week of age or older, the ductus arteriosus was not patent in 72 to 79% of studies. The proportion of studies with a measureable TR jet ranged from 43 to 69% at the different study time points. Studies with a higher Ppa estimate were more likely to have a measureable TR jet (69 vs. 62 vs. 39% for systemic-to-suprasystemic Ppa, greater than or equal to 2/3 systemic-to-systemic Ppa, and less than 2/3 systemic Ppa, respectively; P = 0.008).
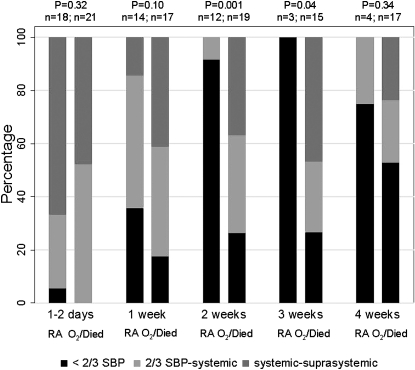
All infants except one had elevated Ppa (≥2/3 systemic blood pressure) at 1 to 2 days of age. Persistent elevations in Ppa were demonstrated in infants with poor outcome (discharged on oxygen or expired), compared with infants discharged on room air, with statistically significant differences at 2 and 3 weeks of age (Figure 3). Only 2 of 12 infants discharged on room air had an estimated Ppa greater than or equal to 2/3 systemic blood pressure at 2 weeks of age or older; one estimate was based on interventricular septum position only and the other on the ductus arteriosus shunt. The area under the ROC curve for classification of estimated Ppa at 2 weeks was 0.84 (95% CI, 0.72–0.96). The addition of concurrent ET1 levels resulted in significant improvement in the model (by likelihood ratio test) and an increase in the area under the curve to 0.90 (95% CI, 0.74–0.98). The combination of echocardiographic findings and ET1 levels improved correct classification to 90% at the previously described threshold ET1 level of 25.8 pg/ml, due to an increase in sensitivity for poor outcome to 89%. A subanalysis comparing infants discharged on oxygen to those who expired (Table 4) demonstrated that infants who expired generally had higher estimates of Ppa (P = 0.03) and higher ET1 levels, although this relationship did not reach statistical significance (P = 0.07).
Figure 3.
Pulmonary artery pressure estimate in infants discharged on room air (RA) and those discharged on oxygen or expired (O2/Died). Proportion of infants in each classification group is shown (estimated pressure < 2/3 systemic blood pressure [SBP] in black, estimated pressure 2/3 systemic-systemic in light gray, and estimated pressure systemic-to-suprasystemic in dark gray). P value is for difference between groups by nonparametric test for trends.
TABLE 4.
COMPARISON OF PLASMA ENDOTHELIN-1 LEVELS (pg/ml) AND ESTIMATED PULMONARY ARTERY PRESSURE FOR INFANTS WITH POOR OUTCOME AT 2 WEEKS OF AGE
| Discharge on Oxygen (n = 11) | Expired (n = 8) | P Value | |
|---|---|---|---|
| Plasma ET1 (pg/ml) | 19.2 (16.7, 26.1) | 34.1 (23.4, 53.8) | 0.07 |
| Estimated pulmonary pressure by echocardiogram | |||
| <2/3 systemic blood pressure | 4 (36) | 1 (12.5) | 0.03 |
| 2/3 systemic – systemic | 6 (55) | 1 (12.5) | |
| Systemic – suprasystemic | 1 (9) | 6 (75) |
Definition of abbreviation: ET1 = endothelin-1
Data are median (interquartile range) or n (%).
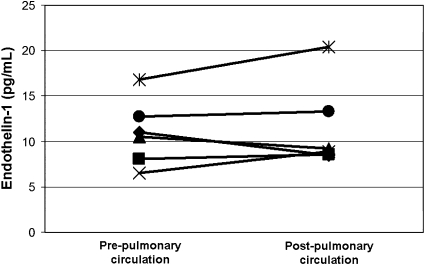
We performed six cardiac catheterization procedures for clinical indications in five infants enrolled in this study (all with poor outcome). The age at catheterization ranged from 44 to 121 days (median 62 d). There was no significant difference (−0.5 pg/ml, P = 0.60) in ET1 levels from samples collected proximal (right ventricle or pulmonary artery) and those collected distal (pulmonary vein or aorta) to the pulmonary vascular bed, with modest (>1 pg/ml) increases in ET1 levels across the pulmonary circulation in two studies and modest decreases in two studies (Figure 4).
Figure 4.
Plasma endothelin-1 levels obtained pre- and post–pulmonary circulation during six cardiac catheterization procedures in infants with congenital diaphragmatic hernia. There was not a significant difference in endothelin-1 levels across the pulmonary circulation.
DISCUSSION
In this longitudinal cohort of infants with CDH, which is the largest prospective study in this patient population to date, we found that ET1 levels at 2 weeks of age are significantly associated with patient outcome at hospital discharge. In addition, ET1 levels are associated with the degree of elevation of Ppa in infants with CDH, by echocardiographic estimates. Thus, our data suggest that plasma ET1 levels are an indicator of disease severity in CDH, although the pathobiology of ET1 in CDH remains undetermined.
In our cohort, almost all infants who are ultimately discharged on room air have transitioned to a lower estimate of Ppa by 2 weeks of age. Our findings at 2 weeks of age suggest that lung and vascular injury attenuate or delay the expected decrease in Ppa seen in almost all infants who are discharged on room air. Thus, the first 2 weeks of life in these infants may be a critical time for changes in the lung and pulmonary vascular bed, which affect later growth and function of the lung. These findings are consistent with data from animal models, which demonstrate a critical period of lung growth during which more severe pulmonary vascular injury will result in irrecoverable arrest of growth and development (20, 22).
In infants with CDH, vascular injury likely occurs secondary to abnormal pulmonary blood flow patterns. Prolonged elevations in PVR, a hypoplastic pulmonary vascular bed, and chronic increased blood flow to the lung contralateral to the hernia are all likely factors in this process, along with biochemical abnormalities in NO signaling demonstrated with vascular remodeling (4, 5, 10–12, 15, 44, 45). Thus, elevations in ET1 at 2 weeks of age in those infants who have a poor outcome (expired or discharged on oxygen) may reflect differences that are both biochemical (affecting endothelial and vascular smooth muscle cell function) and structural.
We found that ET1 levels in patients with poor outcome tended to increase at 1 to 2 weeks of age and then decrease by 4 weeks among patients who were alive at that point. We also found that ET1 levels were associated with the severity of PH, after adjusting for the timing of the sample. Prior studies using a similar ET1 assay have shown that newborns with PPHN have elevated ET1 levels compared with unaffected control subjects (31 ± 4.7 vs. 15.1 ± 4.1 pg/ml) (30). Findings in other patient populations may help explain the elevations in ET1 seen in our patients with poor outcome. Increased pulmonary ET1 production (46, 47) and plasma ET1 levels are demonstrated in adults with PH (with the degree of elevation correlated with the degree of hypertension) (48, 49). In healthy adults, prior studies have demonstrated either no transpulmonary gradient or a decrease in ET1 levels across the pulmonary circulation, which is the site of clearance of almost half of circulating ET1 (48, 50). In PH, ET1 concentration may increase across the pulmonary circulation, implicating increased lung ET1 production or decreased clearance in the disorder (26, 29, 47, 48). Normalization of the ET1 transpulmonary gradient after chronic therapy suggests that these changes in clearance or production can be reversible (51). One mechanism to explain changes in lung ET1 clearance could be alterations in ETB receptor expression. ETB receptors localized to the endothelium are primarily responsible for lung ET1 clearance (52). Aberrant expression of the ETB receptor has been demonstrated in the vascular smooth muscle layer in adults with PH (53) and infants with CDH and PH (32). Lambs with PH and abnormal reactivity have similar findings (54), but rats exposed to chronic exogenous ET1 resolve aberrant ETB receptor expression while abnormal vascular reactivity persists (55). Thus, in our patients, changes in pulmonary clearance or production of ET1 could explain the fall in plasma ET1 levels we observed. Our later results from cardiac catheterization, at 1.5 to 4 months of age, demonstrate no definitive pattern for transpulmonary handling of ET1 and generally lower ET1 levels than we found at 4 weeks.
Increased local or circulating ET1 may be a direct effector of the lung and vascular abnormalities seen in CDH (decreased and dysplastic growth of the lung and its vasculature) rather than just a marker of abnormal endothelial or vascular smooth muscle function. ET1 could be acting as a profibrotic factor, a smooth muscle cell mitogen, or via interactions with the NO-cGMP signaling system. Structural changes are seen in a transgenic mouse model with pulmonary overexpression of ET1, resulting in peribronchial and perivascular fibrosis, with no evidence of PH and normal alveoli (56). ET1 acts as a vascular smooth muscle cell mitogen via production of reactive oxygen species (ROS) (57), and ROS production may also result in decreased pulmonary NO bioavailability. Excessive production of ROS is implicated in the pathophysiology of vascular remodeling demonstrated in ovine models of PH that are associated with impairment of NO signaling (58, 59). Further, the addition of N-acetyl cysteine improves the impaired endothelium-dependent and -independent vasodilation seen in adult rats receiving a chronic infusion of ET1 (55). We have previously demonstrated abnormal vascular reactivity in infants and children with CDH (60). Thus, there are potential implications of these findings for treatment in CDH. Therapies focused on enhancing NO bioavailability or modulating the ET1 system may be effective in preventing these biochemical changes or in reversing them once they have occurred. By this mechanism, therapy could result in enhanced lung and vascular growth, which is seen in newborn animal models of PH and lung injury (13, 14, 19, 61).
Chronic iNO resulted in increased plasma ET1 levels in these patients only after 9 days of age (with most patients treated continuously from Day 1). Increased lung and circulating ET1 has been described with NO inhalation in animals with normal pulmonary vasculature and in children and adults with cardiopulmonary disease (62–64). In human newborns with PPHN, treatment with iNO resulted in lower ET1 levels compared with placebo-treated infants after weaning off therapy, with no differences noted at 1 day of treatment (17). Interestingly, although plasma ET1 levels are elevated in ARDS (47, 48), increased respiratory insufficiency as indicated by the need for ongoing assisted ventilation was not independently associated with ET1 levels in our cohort.
The strength of this study is that the children had a spectrum of acute outcomes for newborns presenting at birth with CDH. The combined outcome of death or discharge on oxygen identifies the most severely affected group of infants, and more than half of the children in this study had this poor outcome. Our study is limited by the difficulty in obtaining accurate Ppa estimates by echocardiography, despite the prospective collection of these data. Due to the lack of ductus arteriosus shunt or measureable TR jet, we confined our classification to the relationship of estimated Ppa to systemic pressure, which may have limited our ability to document significant associations. Regardless, previous studies have shown that TR jet in infants with chronic lung disease does not accurately estimate the systolic Ppa obtained at cardiac catheterization, and echocardiography has limited sensitivity and specificity for the severity of PH in this population (43). Also, we did not control conditions at the time of each echocardiogram, although these patients were treated under a consistent clinical protocol, adding additional variability to our estimates.
In conclusion, this study demonstrates that plasma ET1 levels are significantly associated with disease severity in infants with CDH. Although permissive ventilation strategies have improved survival in these patients, there is still significant mortality and morbidity in this population, with ongoing symptomatic lung dysplasia in survivors. Animal models have demonstrated the importance of dysregulation of the ET1 system and its interaction with the NO signaling cascade in the pathobiology of PH in the developing lung. Thus, therapies that modulate the ET1 system or its effectors (decreased NO signaling or excessive oxidative stress) in infants with severe CDH may impact lung and vascular growth and remodeling in this patient population and improve outcomes.
Supplementary Material
Supported by the National Center for Research Resources (NCRR), a component of the National Institutes of Health and NIH Roadmap for Medical Research, grant UL RR024131, the NHLBI, grants K23 HL-079922 (R.L.K.) and HL-61284 (J.R.F), and the Pulmonary Hypertension Association (R.L.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200907-1126OC on April 22, 2010
Author Disclosure: R.L.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.A.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.H.-M. received lecture fees from the March of Dimes (up to $1,000). J.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.J.M-G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.M. received lecture fees from W.L. Gore ($1,001–$5,000) and received grant support from AGA Medical ($5,001–$10,000). K.K.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.F. served as an expert witness for Norman, Hanson & DeTroy, LLC ($1,001–$5,000).
References
- 1.Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics 2003;112:532–535. [DOI] [PubMed] [Google Scholar]
- 2.Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics 2005;116:e356–e363. [DOI] [PubMed] [Google Scholar]
- 3.Gallot D, Boda C, Ughetto S, Perthus I, Robert-Gnansia E, Francannet C, Laurichesse-Delmas H, Jani J, Coste K, Deprest J, et al. Prenatal detection and outcome of congenital diaphragmatic hernia: a French registry-based study. Ultrasound Obstet Gynecol 2007;29:276–283. [DOI] [PubMed] [Google Scholar]
- 4.Kitigawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. Br J Surg 1971;58:342–346. [DOI] [PubMed] [Google Scholar]
- 5.Naeye RL, Shochat SJ, Whitman V, Maisels MJ. Unsuspected pulmonary vascular abnormalities associated with diaphragmatic hernia. Pediatrics 1976;58:902–906. [PubMed] [Google Scholar]
- 6.Geggel RL, Murphy JD, Langleban D, Crone RK, Vacanti JP, Reid LM. Congenital diaphragmatic hernia: arterial structural changes and persistent pulmonary hypertension after surgical repair. J Pediatr 1985;107:457–464. [DOI] [PubMed] [Google Scholar]
- 7.Muratore C, Utter S, Jaksic T, Lund D, Wilson J. Nutritional morbidity in survivors of congenital diaphragmatic hernia. J Pediatr Surg 2001;36:1171–1176. [DOI] [PubMed] [Google Scholar]
- 8.Cortes RA, Keller RL, Townsend T, Harrison MR, Farmer DL, Lee H, Piecuch RE, Leonard CH, Hetherton M, Bisgaard R, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. J Pediatr Surg 2005;I40:36–46. [DOI] [PubMed] [Google Scholar]
- 9.Chiu PPL, Sauer C, Mihailovic A, Adatia I, Bohn D, Coates AL, Langer JC. The price of success in the management of congenital diaphragmatic hernia: is improved survival accompanied by an increase in long-term morbidity? J Pediatr Surg 2006;41:888–892. [DOI] [PubMed] [Google Scholar]
- 10.Bos AP, Tibboel D, Koot VCM, Hazebroek FWJ, Molenaar JC. Persistent pulmonary hypertension in high-risk congenital diaphragmatic hernia patients: incidence and vasodilator therapy. J Pediatr Surg 1993;28:1463–1465. [DOI] [PubMed] [Google Scholar]
- 11.Dillon PW, Cilley RE, Mauger D, Zachary C, Meier A. The relationship of pulmonary artery pressure and survival in congenital diaphragmatic hernia. J Pediatr Surg 2004;39:307–312. [DOI] [PubMed] [Google Scholar]
- 12.Thibeault DW, Haney B. Lung volume, pulmonary vasculature, and factors affecting survival in congenital diaphragmatic hernia. Pediatrics 1998;101:289–295. [DOI] [PubMed] [Google Scholar]
- 13.Bland RD, Albertine KH, Carlton DP, MacRitchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med 2005;172:899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fratz S, Meyrick B, Ovadia B, Johengen MJ, Reinhartz O, Azakie A, Ross G, Fitzgerald R, Oishi P, Hess J, et al. Chronic endothelin A receptor blockade in lambs with increased pulmonary blood flow and pressure. Am J Physiol Lung Cell Mol Physiol 2004;287:L592–L597. [DOI] [PubMed] [Google Scholar]
- 15.Storme L, Parker TA, Kinsella JP, Rairigh RL, Abman SH. Chronic hypertension impairs flow-induced vasodilation and augments the myogenic response in fetal lung. Am J Physiol Lung Cell Mol Physiol 2002;282:L56–L66. [DOI] [PubMed] [Google Scholar]
- 16.Fineman JR, Wong J, Morin FC, Wild LM, Soifer SJ. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J Clin Invest 1994;93:2675–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christou H, Adatia I, Van Marter LJ, Kane JW, Thompson JE, Stark AR, Wessel DL, Kourembanas S. Effect of inhaled nitric oxide on endothelin-1 and cyclic guanosine 5′-monophosphate plasma concentrations in newborn infants with persistent pulmonary hypertension. J Pediatr 1997;130:603–611. [DOI] [PubMed] [Google Scholar]
- 18.Ladha F, Bonnet S, Michelakis ED, Eaton F, Hashimoto K, Archer SL, Thebaud B. Sildenafil decreases pulmonary hypertension and improves alveolarization in experimental hyperoxia-induced BPD in newborn rats [abstract]. Pediatr Res 2004;55:436A. [Google Scholar]
- 19.Lin YJ, Markham NE, Balasubramaniam V, Tang JR, Maxey A, Kinsella JP, Abman SH. Inhaled nitric oxide enhances distal lung growth after exposure to hyperoxia in neonatal rats. Pediatr Res 2005;58:22–29. [DOI] [PubMed] [Google Scholar]
- 20.McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM. Vascular endothelial growth factor receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol 2005;32:420–427. [DOI] [PubMed] [Google Scholar]
- 21.Tang J, Markham N, Lin Y, McMurty I, Maxey A, Kinsella J, Abman S. Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 2004;287:L344–L351. [DOI] [PubMed] [Google Scholar]
- 22.Todd L, Mullen M, Olley PM, Rabinovitch M. Pulmonary toxicity of monocrotaline differs at critical periods of lung development. Pediatr Res 1985;19:731–737. [DOI] [PubMed] [Google Scholar]
- 23.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- 24.Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, Temma S, Shirakami G, Imura H, Mikawa H. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects: evidence for increased production of endothelin in pulmonary circulation. Circulation 1991;84:2280–2285. [DOI] [PubMed] [Google Scholar]
- 25.Tutar HE, Imamoglu A, Atalay S, Gumus H, Akar N. Plasma endothelin-1 levels in patients with left-to-right shunt with or without pulmonary hypertension. Int J Cardiol 1999;70:57–62. [DOI] [PubMed] [Google Scholar]
- 26.Dupuis J, Cernacek P, Tardif JC, Stewart DJ, Gosselin G, Dyrda I, Bonan R, Crepeau J. Reduced pulmonary clearance of endothelin-1 in pulmonary hypertension. Am Heart J 1998;135:614–620. [DOI] [PubMed] [Google Scholar]
- 27.Rubens C, Ewert R, Halank M, Wensel R, Orzechowski HD, Schultheiss HP, Hoeffken G. Big endothelin-1 and endothelin-1 plasma levels are correlated with the severity of primary pulmonary hypertension. Chest 2001;120:1562–1569. [DOI] [PubMed] [Google Scholar]
- 28.Staniloae C, Dupuis J, White M, Gosselin G, Dyrda I, Bois M, Crepeau J, Bonan R, Caron A, Lavoie J. Reduced pulmonary clearance of endothelin in congestive heart failure: a marker of secondary pulmonary hypertension. J Card Fail 2004;10:427–432. [DOI] [PubMed] [Google Scholar]
- 29.Langleban D, Dupuis J, Hirsch A, Giovinazzo M, Langleban I, Khoury J, Ruel N, Caron A. Pulmonary endothelin-1 clearance in human pulmonary arterial hypertension. Chest 2005;128:622S. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfiels B, Abman SH. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr 1993;123:109–114. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi H, Puri P. Plasma endothelin levels in congenital diaphragmatic hernia. J Pediatr Surg 1994;29:1258–1261. [DOI] [PubMed] [Google Scholar]
- 32.de Lagausie P, de Buys-Roessingh A, Ferdadji L, Saada J, Aisenfisz S, Martinez-Vinson C, Fund X, Cayuela JM, Peuchmaur M, Mercier JC, et al. Endothelin receptor expression in human lungs of newborns with congenital diaphragmatic hernia. J Pathol 2005;205:112–118. [DOI] [PubMed] [Google Scholar]
- 33.Kavanagh M, Battistini B, Kluth D, Jean S, Fournier L, Yeng AY, Major D, Cloutier R. Effect of CGS 26303, an endothelin-converting enzyme-neutral endopeptidase inhibitor, on nitrofen-induced congenital diaphragmatic hernia in the rat. J Pediatr Surg 2000;35:780–784. [DOI] [PubMed] [Google Scholar]
- 34.Thebaud B, de Lagausie P, Forgues D, Aigrain Y, Mercier JC, Dinh-Xuan AT. ETA-receptor blockade and ETB-receptor stimulation in experimental congenital diaphragmatic hernia. Am J Physiol Lung Cell Mol Physiol 2000;278:L923–L932. [DOI] [PubMed] [Google Scholar]
- 35.Kavanagh M, Battistini B, Jean S, Crochetiere J, Fournier L, Wessale J, Opgenorth TJ, Cloutier R, Major D. Effect of ABT-627 (A-147627), a potent selective ETA receptor antagonist, on the cardiopulmonary profile of newborn lambs with surgically-induced diaphragmatic hernia. Br J Pharmacol 2001;134:1679–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kavanagh M, Seaborn T, Crochetiere J, Fournier L, Battistini B, Piedboeuf B, Major D. Modulating effect of a selective endothelin A receptor antagonist on pulmonary endothelin system protein expression in experimental diaphragmatic hernia. J Pediatr Surg 2005;40:1382–1389. [DOI] [PubMed] [Google Scholar]
- 37.Keller RL, Tacy T, Xu J, Hendricks-Munoz K, Hawgood S, Fineman J. Elevated plasma endothelin-1 (ET1) levels are associated with death in newborns with congenital diaphragmatic hernia (CDH). E-PAS 2006;59:5560.349.
- 38.Keller RL, Hawgood S, Neuhaus JM, Farmer DL, Lee H, Albanese CT, Harrison MR, Kitterman JA. Infant pulmonary function in a randomized trial of fetal tracheal occlusion for severe congenital diaphragmatic hernia. Pediatr Res 2004;56:818–825. [DOI] [PubMed] [Google Scholar]
- 39.Wong J, Reddy VM, Hendricks-Munoz K, Liddicoat JR, Gerrets R, Fineman JR. Endothelin-1 vasoactive responses in lambs with pulmonary hypertension and increased pulmonary blood flow. Am J Physiol 1995;269:H1965–H1972. [DOI] [PubMed] [Google Scholar]
- 40.Musewe NN, Smallhorn JF, Benson LN, Burrows PE, Freedom RM. Validation of Doppler-derived pulmonary arterial pressure on patients with ductus arteriosus under different hemodynamic states. Circulation 1987;76:1081–1091. [DOI] [PubMed] [Google Scholar]
- 41.Reisner SA, Azzam Z, Halmann M, Rinkevich D, Siderman S, Markiewicz W, Beyar R. Septal/free wall curvature ratio: a noninvasive index of pulmonary arterial pressure. J Am Soc Echocardiogr 1994;7:27–35. [DOI] [PubMed] [Google Scholar]
- 42.Dong SJ, Smith ER, Tyberg JV. Changes in the radius of curvature of the ventricular septum at end diastole during pulmonary arterial and aortic constrictions in the dog. Circulation 1992;86:1280–1290. [DOI] [PubMed] [Google Scholar]
- 43.Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008;121:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okuyama H, Kubota A, Kawahara H, Oue T, Kitayama Y, Yagi M. Correlation between lung scintigraphy and long-term outcome in survivors of congenital diaphragmatic hernia. Pediatr Pulmonol 2006;41:882–886. [DOI] [PubMed] [Google Scholar]
- 45.Nagaya M, Akatsuka H, Kato J, Niimi N, Ishiguro Y. Development in lung function of the affected side after repair of congenital diaphragmatic hernia. J Pediatr Surg 1996;31:349–356. [DOI] [PubMed] [Google Scholar]
- 46.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993;328:1732–1739. [DOI] [PubMed] [Google Scholar]
- 47.Druml W, Steltzer H, Waldhausl W, Lenz K, Hammerle A, Vierhapper H, Gasic S, Wagner OF. Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis 1993;148:1169–1173. [DOI] [PubMed] [Google Scholar]
- 48.Langleban D, DeMarchi M, Laporta D, Spanier AH, Schlesinger RD, Stewart DJ. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis 1993;148:1646–1650. [DOI] [PubMed] [Google Scholar]
- 49.Reesink HJ, Meijer RC, Lutter R, Boomsma F, Jansen HM, Kloek JJ, Bresser P. Hemodynamic and clinical correlates of endothelin-1 in chronic thromboembolic pulmonary hypertension. Circ J 2006;70:1058–1063. [DOI] [PubMed] [Google Scholar]
- 50.Dupuis J, Stewart DJ, Cernacek P, Gosselin G. Human pulmonary circulation is an important site for both clearance and production of endothelin-1. Circulation 1996;94:1578–1584. [DOI] [PubMed] [Google Scholar]
- 51.Langleban D, Barst RJ, Badesch D, Groves BM, Tapson VF, Murali S, Bourge RC, Ettinger N, Shalit E, Clayton LM, et al. Continuous infusion of epoprostenol improves the net balance between pulmonary endothelin-1 clearance and release in primary pulmonary hypertension. Circulation 1999;99:3266–3271. [DOI] [PubMed] [Google Scholar]
- 52.Burkhardt M, Barton M, Shaw SG. Receptor- and non-receptor-mediated clearance of big-endothelin and endothelin-1: differential effects of acute and chronic ETA receptor blockade. J Hypertens 2000;18:273–279. [DOI] [PubMed] [Google Scholar]
- 53.Bauer M, Wilkens H, Langer F, Schneider SO, Lausberg H, Schafers HJ. Selective upregulation of endothelin B receptor gene expression in severe pulmonary hypertension. Circulation 2002;105:1034–1036. [DOI] [PubMed] [Google Scholar]
- 54.Black SM, Mata-Greenwood E, Dettman RW, Ovadia B, Fitzgerald RK, Reinhartz O, Thelitz S, Steinhorn RH, Gerrets R, Hendricks-Munoz K, et al. Emergence of smooth muscle cell endothelin B-mediated vasoconstriction in lambs with experimental congenital heart disease and increased pulmonary blood flow. Circulation 2003;108:1646–1654. [DOI] [PubMed] [Google Scholar]
- 55.Migneault A, Sauvageau S, Villeneuve L, Thorin E, Fournier A, Leblanc N, Dupuis J. Chronically elevated endothelin levels reduce pulmonary vascular reactivity to nitric oxide. Am J Respir Crit Care Med 2005;171:506–513. [DOI] [PubMed] [Google Scholar]
- 56.Hocher B, Schwarz A, Fagan KA, Thone-Reineke C, El-Hag K, Kusserow H, Elitok S, Bauer C, Neumayer HH, Rodman DM, et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol 2000;23:19–26. [DOI] [PubMed] [Google Scholar]
- 57.Wedgewood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 2001;281:L1058–L1067. [DOI] [PubMed] [Google Scholar]
- 58.Wedgewood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, Black SM. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol 2005;289:L660–L666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol 2006;290:L1069–L1077. [DOI] [PubMed] [Google Scholar]
- 60.Keller RL, Moore P, Teitel D, Hawgood S, McQuitty J, Fineman JR. Abnormal vascular tone in infants and children with lung hypoplasia: findings from cardiac catheterization and the response to therapy. Pediatr Crit Care Med 2006;7:589–594. [DOI] [PubMed] [Google Scholar]
- 61.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thebaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 2005;172:750–756. [DOI] [PubMed] [Google Scholar]
- 62.McMullan DM, Bekker JM, Johengen MJ, Hendricks-Munoz K, Gerrets R, Black SM, Fineman JR. Inhaled nitric oxide-induced rebound pulmonary hypertension: role for endothelin-1. Am J Physiol Heart Circ Physiol 2001;280:H777–H785. [DOI] [PubMed] [Google Scholar]
- 63.Chen L, He H, Mondejar EF, Freden F, Wiklund P, Alving K, Hedenstierna G. Endothelin-1 and nitric oxide synthase in short rebound reaction to short exposure to inhaled nitric oxide. Am J Physiol Heart Circ Physiol 2001;281:H124–H131. [DOI] [PubMed] [Google Scholar]
- 64.Pearl JM, Nelson DP, Raake JL, Manning PB, Schwartz SM, Koons L, Shanley TP, Wong HR, Duffy JY. Inhaled nitric oxide increases endothelin-1 levels: a potential cause of rebound pulmonary hypertension. Crit Care Med 2002;30:89–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.