Abstract
Rationale: Current tools for the rapid diagnosis of tuberculous meningitis (TBM) are suboptimal. We evaluated the clinical utility of a quantitative RD-1 IFN-γ T-cell enzyme-linked immunospot (ELISPOT) assay (T-SPOT.TB), using cerebrospinal fluid cells for the rapid immunodiagnosis of TBM.
Objectives: To evaluate the diagnostic utility of the RD1 antigen- specific ELISPOT assay for the diagnosis of tuberculous meningitis.
Methods: The ELISPOT assay was evaluated in 150 patients with suspected TBM who were categorized as definite-TBM, probable-TBM, and non-TBM. Culture or polymerase chain reaction positivity for Mycobacerium tuberculosis served as the reference standard. To determine the diagnostic value of the ELISPOT assay, a clinical prediction rule was derived from baseline clinical and laboratory parameters using a multivariable regression model.
Measurements and Main Results: A total of 140 patients (81% HIV-infected; median CD4 count, 160 cells/mm3) were included in the final analysis. When comparing the definite-TBM (n = 38) and non-TBM groups (n = 48), the ELISPOT assay (cut point of ≥228 spot-forming cells per 1 million mononuclear cells) was a useful rule-in test: sensitivity 58% (95% confidence interval [CI], 41–74); specificity 94% (95% CI, 83–99). However, ELISPOT outcomes improved when other rapid tests were concurrently used to exclude bacterial (Gram stain) and cryptococcal meningitis (latex-agglutination test) within the non-TBM group. Using this approach, the ELISPOT assay (cut point of ≥46 spot-forming cells) was an excellent rule-in test: sensitivity 82% (95% CI, 66–92); specificity 100% (95% CI, 78–100); positive predictive value, 100% (95% CI, 89–100); negative predictive value, 68% (95% CI, 45–86); area under the curve, 0.90. The ELISPOT assay had incremental diagnostic value compared with the clinical prediction rule.
Conclusions: The RD-1 ELISPOT assay, using cerebrospinal fluid mononuclear cells and in conjunction with other rapid confirmatory tests (Gram stain and cryptococcal latex-agglutination test), is an accurate rapid rule-in test for TBM in a TB and HIV endemic setting.
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Tuberculous meningitis requires prompt initiation of treatment. However, current tools for the rapid diagnosis of tuberculous meningitis (smear microscopy and polymerase chain reaction) have poor sensitivity. Diagnostic delay may translate into increased morbidity and mortality, and empiric treatment may result in inappropriate exposure to toxic drugs.
What This Study Adds to the Field
Our findings indicate that a tuberculosis-specific quantitative T-cell ELISPOT assay, using cerebrospinal fluid mononuclear cells and in conjunction with other rapid confirmatory tests (Gram stain and cryptococcal latex-agglutination test), is an accurate and rapid rule-in test for tuberculous meningitis.
In 2007 there were approximately 10 million new cases of pulmonary tuberculosis diagnosed globally (1). In many parts of Africa, fueled by human immunodeficiency virus (HIV) coinfection, tuberculosis (TB) is out of control. HIV infection is also associated with higher rates of extrapulmonary tuberculosis (EPTB), including TB meningitis (TBM), which has a prevalence of between 1 and 6.3% in populations with pulmonary TB (2–4) and 5 to 18% in populations with EPTB (5–7). Although an accurate figure for the prevalence of TBM is not available in the South African population, in 2007 there were approximately 46,000 (14.5%) recorded cases of EPTB among approximately 315,000 patients with new and recurrent TB (1).
Delaying therapy in patients with TBM, which frequently occurs because the diagnosis is often difficult to confirm, increases mortality (∼30% despite treatment) (8–11). Smear microscopy under high-burden programmatic conditions has a dismal yield (0–20%) (8, 9, 12, 13). Culture results are delayed for several weeks, and the yields are variable (5–83%) (10, 14). The currently applied laboratory parameters are frequently unhelpful because cerebrospinal fluid (CSF) changes may vary from acellular with normal glucose (9) to predominant lymphocytosis or neutrophilia (8, 9). Thus, therapy is often empirical after rapid exclusion of other appropriate locally prevalent causes. In many settings this comprises rapidly excluding bacterial infection by Gram stain and cryptococcal meningitis by the latex agglutination test.
More recently, rapid immunodiagnostic tests, using peripheral whole blood or mononuclear cells, for the diagnosis of latent TB infection (LTBI) have become commercially available (15). These newer tests enumerate the frequency of peripheral blood effector T cells driven by RD-1 TB-specific antigens (early secreted antigenic target 6 [ESAT-6] and culture filtrate protein 10 [CFP-10]) (15, 16). However, this blood-based immunologic approach is unsuitable for the diagnosis of active TB because the test cannot distinguish effector cells in the context of LTBI from cells present during active disease (17–21). An alternative approach is to quantify the frequency of antigen-specific effector cells at the site of disease rather than in whole blood. This approach has recently been shown to be highly accurate when using bronchial alveolar lavage lymphocytes to diagnose pulmonary TB (22–24) but not when using site-specific lymphocytes to diagnose pleural TB (25). Case reports and small series suggest that this approach may also be useful for the diagnosis of TBM (26–29). We hypothesized that the frequency of TB antigen-specific lymphocytes would be increased in TBM but not in other causes of meningitis and could thus serve as a useful rapid diagnostic tool. We therefore evaluated the performance outcome of this technology in a prospective study that included 150 subjects with suspected TBM in a high-TB and high-HIV prevalence setting. To meaningfully assess the diagnostic value of the enzyme-linked immunospot (ELISPOT) assay, we compared its performance with a clinical prediction rule derived from pretreatment clinical and laboratory parameters.
METHODS
Study Participants
A total of 150 consecutive patients with suspected meningitis were prospectively recruited between January 2008 and April 2009. The study protocol was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal. Patients who were referred from regional hospitals with a meningitic illness and those investigated at Inkosi Albert Luthuli Central Hospital, a tertiary hospital, with arachnoiditis and neck stiffness were included in the study.
Patients were clinically assessed, had a computerized tomography scan done to exclude contraindications to a lumbar puncture, and provided written informed consent. In patients with an abnormal mental state, consent was obtained from a first-degree relative or from the Head of Department when a lumbar puncture was clinically justified (30). All patients had routine blood tests (see online supplement for details), including HIV and CD4 counts. CSF was obtained by lumbar puncture, and the following tests were performed: microscopy (Gram stain and for acid-fast bacilli), bacterial culture, Mycobacterium tuberculosis culture, fungal culture, TB polymerase chain reaction (PCR) (Roche Amplicor), routine chemistry (protein, glucose, chloride), viral PCR (Roche Amplicor) for (cytomegalovirus, herpes simplex virus type 1, and varicella zoster virus), fluorescent treponemal antibody test and venereal disease research laboratory for neurosyphilis if the fluorescent treponemal antibody was positive, cysticercal ELISA, and a cryptococcal antigen latex agglutination test (CLAT), which has a high specificity and sensitivity (31). Matched cerebrospinal fluid (10 ml) and blood samples (10 ml) were collected for IFN-γ ELISPOT testing (T-SPOT.TB; Oxford Immunotec, Oxford, Abingdon, UK). The clinical information recorded included demographic information, duration of symptoms and antituberculous therapy, HIV status, duration of steroid therapy, past history of TB, and history of TB contact.
Categorization of Patients
Patients were categorized as definite TBM (CSF culture or PCR positive for M. tuberculosis), probable TBM (see online supplement for details), or nonTBM (an alternate definite cause for meningitis identified and response to appropriate non-TB therapy) (32, 33). Outcomes were recorded as improved, deteriorated, or demised.
Throughout the study, the clinician was blinded to the ELISPOT results, and the laboratory technician was blinded to the clinical diagnosis. Thus, laboratory interpretation and clinical diagnosis were independent of the test result.
CSF Lymphocyte Antigen-specific IFN-γ Responses
CSF lymphocyte IFN-γ responses to the following antigens were evaluated: (1) RD-1 ELISPOT IFN-γ responses to ESAT-6/CFP-10 peptide pools were performed in duplicate according to the manufacturer's instructions, using 3 × 104 to 2.5 × 105 cells per well (cell numbers used per well were determined using titration experiments in seven patients; see Table E1 in the online supplement). (2) IFN-γ ELISPOT responses to 5 μg/ml purified protein derivative (PPD) (Staten Serum Institute, Copenhagen, Denmark) were performed in duplicate using IFN-γ precoated ELISPOT plates (Mabtech, Nacka Strand, Sweden) seeded with 3 × 104 to 2.5 × 105 lymphocytes derived from the CSF.
Results were deemed to be inconclusive (1) if the positive control failed (indeterminate), (2) if there was a high spot count (>10 spot-forming cells [SFCs]) in the negative control well, or (3) if there was high background discoloration of the ELISPOT wells precluding meaningful evaluation of the plate. A count of 20 or higher SFCs per million cells in the ESAT-6 or CFP-10 wells was deemed a positive result (see online supplement for further details). Given the use of different cut points in specific body compartments (22, 25) when compared with peripheral blood and that none has been defined for CSF, which has unique physiologic and anatomic characteristics, we derived new cut-offs using receiver operating characteristic (ROC) curves analysis. The manufacturer's cut-off and cut-offs generated from the study were used for reporting results.
Peripheral Blood-derived, Antigen-specific IFN-γ Responses
The following responses were evaluated in peripheral blood: (1) RD-1 ELISPOT responses using 2.5 × 105 peripheral blood mononuclear cells (PBMC) per well (T-SPOT.TB) and (2) PPD ELISPOT responses using 2.5 × 105 PBMC per well.
Statistical Analysis
Detailed statistical methods are outlined in the online supplement. Correlation between the CD4 count and the SFCs was sought by ROC curve comparisons of ESAT-6 and CFP-10 summated values (square root transformed), which were adjusted for CD4 (log transformed) count using separate regression models for patients with definite TBM and for patients without TBM (34) (Figure E1). Diagnostic accuracy was assessed using the ROC curve. Youden's Index (35) was used to select the optimum cut points on the ROC curve (optimal balance between sensitivity and specificity). This was compared with the manufacturer suggested cut points for PBMCs and ROC-derived cut points defining utility as a rule-in test. Univariate and multivariate analysis was used to generate a clinical prediction rule, which was used to define whether the T-SPOT.TB added incremental value over easily derived clinical and CSF laboratory parameters (details are provided in the online supplement). Study reporting and analysis were consistent with the STARD criteria (36). Results were analyzed using STATA V10 software (Stata Corp., College Station, TX).
RESULTS
Demographic and Clinical Results
Of the 150 recruited patients, there were 140 with evaluable RD-1 ELISPOT results (Figure 1). The study included 145 patients with clinical meningitis and 5 patients with an archnoiditis. Two patients were excluded (no CSF obtained from one patient, no definite diagnosis made in one patient). There were 1, 1, and 6 inconclusive RD-1 ELISPOT results in the definite, probable, and non-TBM groups, respectively, all due to high background discoloration within the wells, which precluded spot visualization. Sociodemographic data comparing definite and non-TBM groups are presented in Table 1 (a comparison of definite + probable TBM groups with non-TBM is presented in Table E2). Routine smear microscopy for acid fast bacilli, processed in the hospital service laboratory, had a 0% yield in this cohort.
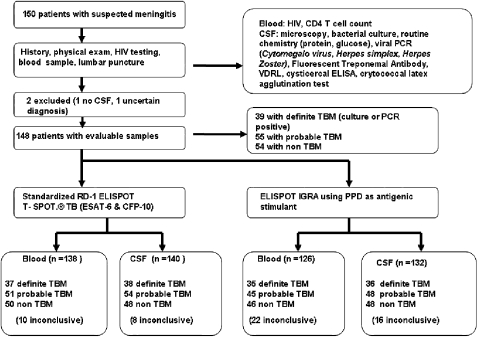
Figure 1.
Summary flow chart of patients recruited, investigations performed, and patient categorization. PCR = polymerase chain reaction; VDRL = venereal disease research laboratory.
TABLE 1.
COMPARISON OF THE CLINICAL AND LABORATORY PARAMETERS IN THE DEFINITE TUBERCULOSIS MENINGITIS (CULTURE OR POLYMERASE CHAIN REACTION POSITIVE; N = 38) AND NONTUBERCULOSIS MENINGITIS (N = 48) GROUPS
| Characteristic | Definite TBM, n (%) | Non-TBM, n (%) | P Value |
|---|---|---|---|
| Mean age (± SD) | 33.5 (9.5) | 32.9 (9.7) | 0.8 |
| Age, yr | |||
| <36 | 24 (61.5) | 35 (64.8) | 0.7 |
| ≥36 | 15 (38.5) | 19 (35.2) | |
| Sex | |||
| Male | 18 (46.1) | 16 (29.6) | 0.1 |
| Female | 21 (53.9) | 38 (70.4) | |
| Ethnic group | |||
| Black African | 38 (97.4) | 53 (98.2) | 0.3 |
| Mixed race | 1 (2.6) | 0 (0.0) | |
| European descent | 0 (0.0) | 0 (0.0) | |
| Indian | 0 (0.0) | 1 (1.8) | |
| HIV status | |||
| Positive | 34 (87.2) | 47 (87.0) | 0.9 |
| Negative | 4 (10.3) | 6 (11.3) | |
| Unknown/refused | 1 (2.6) | 1 (1.9) | |
| Previous TB infection | |||
| Yes | 8 (20.5) | 24 (44.4) | 0.007 |
| No | 27 (69.2) | 30 (55.6) | |
| Unknown | 4 (10.3) | 0 (0.0) | |
| TB contact (within 2 yr) | |||
| Yes | 9 (23.1) | 14 (25.9) | 0.6 |
| No | 26 (66.7) | 40 (74.1) | |
| Unknown | 4 (10.3) | 0 (0.0) | |
| Duration of illness, d† | |||
| <6 | 6 (16.2) | 9 (16.7) | 0.9 |
| ≥6 | 31 (83.8) | 45 (83.3) | |
| Steroid treatment at diagnosis | |||
| Yes | 12 (30.8) | 8 (14.8) | 0.07 |
| No | 27 (69.2) | 46 (85.2) | |
| CLAT | |||
| Positive | 4 (10.3) | 27 (50.0) | <0.001 |
| Negative | 35 (89.7) | 27 (50.0) | |
| Median CD4 (IQR) per μl | 84 (53–173) | 161 (54–261) | 0.05 |
| Hydrocephalus on CT or MRI | |||
| Yes | 17 (56.7) | 10 (43.5) | 0.3 |
| No | 13 (43.3) | 13 (56.5) |
Definition of abbreviations: CLAT = cryptococcal antigen latex agglutination test; CT = computed tomography; IQR = interquartile ratio; MRI = magnetic resonance imaging; TB = tuberculosis; TBM = tuberculous meningitis.
The 6-d cut point to discriminate between definite TBM and non-TBM patients was chosen based on Thwaites criteria (44).
The laboratory CSF changes in the definite-TBM and non-TBM groups are presented in Table 2. The alternate diagnoses in the non-TBM group (n = 48) included cryptococcal meningitis (n = 28), viral meningitis (n = 10), mucormycosis (n = 1), acute bacterial meningitis (n = 5), neoplastic meningitis (n = 2), neurosyphilis (n = 1), and venous sinus thrombosis (n = 1). Seven patients in the definite-TBM group had dual infections (TB culture or PCR positive and CLAT positive). Within the definite-TBM group, 23% of the patients died, compared with 7% in the probable-TBM and 7% in the non-TBM groups. A median of 1 × 105 (interquartile ratio, 4.8 × 104 to 1.4 × 105) cells were used per well; there was no correlation between the number of SFCs and cell count per well (Spearman coefficient = 0.05; P = 0.5).
TABLE 2.
CEREBROSPINAL FLUID CHANGES COMPARING THE DEFINITE TUBERCULOUS MENINGITIS (N = 38) WITH THE NONTUBERCULOUS MENINGITIS (N = 48) GROUP
| Characteristic | Definite TBM | Non-TBM | P Value |
|---|---|---|---|
| Lymphocytes, cells × 103/ml | 99 (16–242) | 28 (10–82) | 0.01 |
| Neutrophils, cells × 103/ml) | 67 (20–134) | 9 (0–66) | 0.002 |
| Protein, g/L | 1.77 (1.14–2.7) | 1.0 (0.8–1.9) | 0.005 |
| CSF, lucose mmol/L | 1 (1.0–1.7) | 2.1 (1.5–2.7) | <0.001 |
| Ratio of CSF to serum glucose | 0.2 (0.16–0.3) | 0.38 (0.2–0.5) | <0.001 |
| Ratio of CSF lymphocytes to total cells | 0.59 (0.2–0.8) | 0.81 (0.2–1.0) | 0.2 |
Definition of abbreviations: CSF = cerebrospinal fluid; TBM = tuberculous meningitis.
Values are medians with interquartile ranges in parentheses.
RD1 Antigen-driven ELISPOT Assays Using CSF Mononuclear Cells
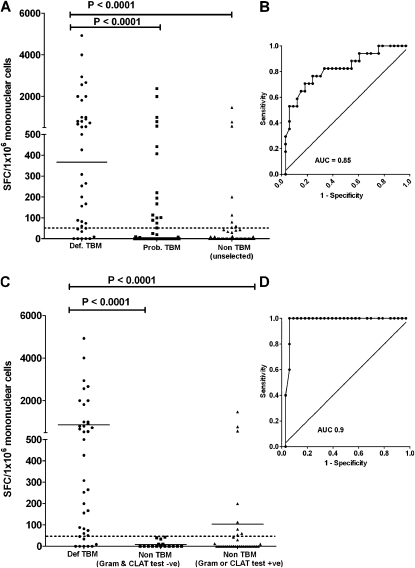
Given that the optimal combined sensitivity and specificity of approximately 80%, using Youden's index, lacks clinical utility as a rule-in or rule-out test, we determined a clinically more useful ROC-derived cut point with high specificity while sacrificing sensitivity (selection of a rule-in test). Performance outcomes when using the manufacturer's blood-specific cut point, an optimal cut-point using Youden's Index, and the AUC-derived cut point selecting for high specificity at the expense of sensitivity are shown in Table 3. Outcomes are shown when the definite TBM group (n = 38) was compared with (1) unselected non-TBM patients (n = 48; Table 3A), (2) non-TBM patients who were gram positive or CLAT positive (n = 33; Table 3B), and (3) non-TBM patients who were gram positive or CLAT negative (n = 15; Table 3C). Thus, each of the categories specified results for three different cut points. Figure 2 illustrates the comparison between the definite-TBM and the unselected non-TBM groups (Figure 2A) with the corresponding ROC curve (Figure 2B). It also illustrates (Figure 2C) the comparison between definite-TBM and the non-TBM groups when split into CLAT and gram-positive (n = 33) and CLAT and gram-negative patients (n = 15) and the corresponding ROC curve comparing the definite TBM with the non-TBM group that had negative Gram stain and CLAT tests.
TABLE 3.
PERFORMANCE OUTCOMES OF EARLY SECRETED ANTIGENIC TARGET 6 AND CULTURE FILTRATE PROTEIN 10 IFN-γ RELEASE ASSAY ELISPOT RESPONSES USING CEREBROSPINAL FLUID MONONUCLEAR CELLS AT DIFFERENT CUT POINTS IN THE DEFINITE TUBERCULOUS MENINGITIS AND NONTUBERCULOUS MENINGITIS GROUPS
| A. Definite-TBM (n = 38) compared with unselected non-TBM (n = 48) (AUC, 0.85) | |||||
|---|---|---|---|---|---|
| Cut point* | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
| ≥20 SFC† | 84% (69–94) | 73% (58–85) | 71% (56–84) | 85% (71–94) | 78% (68–86) |
| ≥46 SFC† | 82% (66–92) | 83% (70–93) | 80% (64–91) | 85% (72–94) | 83% (73–90) |
| ≥228 SFC§ | 58% (41–74) | 94% (83–99) | 88% (69–97) | 74% (61–84) | 78% (68–86) |
| B. Definite-TBM (n = 38) compared with non-TBM (n = 33) that were gram positive CLAT positive (i.e. alternative rapid tests that were concurrently positive) (AUC, 0.83) | |||||
| Cut point |
Sensitivity (CI) |
Specificity (CI) |
PPV (CI) |
NPV (CI) |
Accuracy (CI) |
| ≥20 SFC† | 84% (69–94) | 70% (51–84) | 76% (61–88) | 79% (60–92) | 77% (66–87) |
| ≥59 SFC‡ | 79% (63–90) | 79% (61–91) | 81% (65–92) | 76% (59–89) | 79% (66–88) |
| ≥800 SFC§ | 34% (20–51) | 97% (84–100) | 93% (66–100) | 56% (42–69) | 63% (51–74) |
| C. Definite-TBM (n = 38) compared with non-TBM (n = 15) patients who were gram positive or CLAT negative (i.e., all causes of meningitis other than cryptococcal and acute bacterial meningitides) (AUC, 0.90) | |||||
| Cut point |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
| ≥20 SFC† | 84% (69–94) | 80% (52–96) | 91% (77–98) | 67% (41–87) | 83% (70–92) |
| ≥46 SFC‡,§ | 82% (66–92) | 100% (78–100) | 100% (89–100) | 68% (45–86) | 87% (75–95) |
Definition of abbreviations: AUC = area under the curve; CI = confidence interval; CLAT = cryptococcal antigen latex agglutination test; NPV = negative predictive value; PPV = positive predictive value; SFC = spot-forming cells; TBM = tuberculous meningitis.
Cut point defined as SFCs per million cells.
The manufacturer-recommended cut point for cerebrospinal fluid is ≥ 20 SFC per million mononuclear cells in the early secreted antigenic target 6– or culture filtrate protein 10–containing wells.
Area under the curve–derived cut point by the Youden index, which allows for the selection of an optimal best fit specificity and sensitivity cut point from the receiver operating characteristic curve.
Area under the curve–derived cut-point defining utility as a rule-in test (i.e. high specificity at the expense of suboptimal sensitivity). In Table 3C) the Youdens index and area under the curve are identical).
Figure 2.
RD1 antigen–specific responses to early secreted antigenic target 6 and culture filtrate protein 10 using cerebrospinal fluid mononuclear cells comparing definite-, probable-, and non-tuberculous meningitis (TBM) groups. (A) Definite-TBM compared with the unselected non-TBM group and (B) the corresponding receiver operating characteristic (ROC) curve. (C) Responses when the non-TBM group was stratified by rapid test results (gram positive or cryptococcal antigen latex agglutination test [CLAT] positive vs. gram negative and CLAT negative) and (D) the corresponding ROC curve. (C) For clarity, the probable-TBM group is not shown.
In summary, when unselected non-TBM patients were used in the analysis (Table 3A), the ELISPOT assay was a good rule-in test (sensitivity, 58%; specificity, 94%). These outcomes improved further (sensitivity, 82%; specificity, 100%) when the ELISPOT was used in conjunction with other rapid tests (Gram stain and CLAT) to rapidly exclude bacterial and fungal infections in the non-TBM group. Comparison for all groups when the definite-TBM and probable-TBM groups were combined is shown in Table E3.
There were three strongly positive IFN-γ release assay (IGRA) results in the non-TBM group and in five patients in whom the test failed to elicit a T-cell response in the definite TBM group (Table E4). The likelihood of a positive ELISPOT result in the definite-TBM group increased with increasing CD4 T-cell counts (P = 0.003; Figure E1).
Derivation of a Clinical Prediction Rule Suited to High HIV Prevalence Settings
Out of several parameters identified in the univariate analysis (Table 4), only three variables were included in the multivariable regression model: CLAT (P = 0.002), CSF glucose to serum glucose ratio (P = 0.08), and CD4 count (P = 0.002). A clinical prediction rule, appropriately weighted according to the model, was developed (Table 5), and its sensitivity and specificity were calculated (Table 6). When the CLAT was excluded from the model, CSF glucose (P = 0.08) had borderline significance, and only CD4 count (P = 0.004) was a useful discriminatory parameter.
TABLE 4.
UNIVARIATE AND MULTIVARIATE ANALYSES FOR THE PREDICTION OF DEFINITE TUBERCULOUS MENINGITIS
| Univariate Analysis |
||||
|---|---|---|---|---|
| Laboratory Characteristic (cut point) | OR (95% CI) | P Value | β-coefficient | Score |
| Lymphocyte count, cells × 103/ml (≥200 cells] | 6.5 (1.9–22.0) | 0.003 | n/a | n/a |
| Neutrophil count, cells × 103/ml (≥36 cells] | 5 (2.0–12.3) | <0.001 | n/a | n/a |
| Protein level, g/L (≥2.5 g/L) | 3.6 (1.2–10.5) | 0.02 | n/a | n/a |
| CSF glucose mmol/l (≤1.0 mmol/L) | 8.4 (2.9–24.2) | <0.001 | n/a | n/a |
| Ratio of CSF/serum glucose (≤0.2 mmol/L) | 9.3 (3.1–28) | <0.001 | n/a | n/a |
| CD4 count, cells/mm3 (≤200) | 2.9 (1.1–7.4) | 0.03 | n/a | n/a |
| CLAT negative | 8.8 (2.7–28.0) | <0.001 | n/a | n/a |
| Previous TB | 2.7 (1.0–7.0) | 0.04 | n/a | n/a |
| Multivariate analysis* | ||||
| Ratio of CSF/serum glucose | 7.8 (2.1–28.7) | 0.002 | 2.06 | |
| ≤0.2 | 2 | |||
| >0.2 | 0 | |||
| CD4 count, cells/mm3 | 6.2 (1.9–20.6) | 0.002 | 1.83 | |
| ≤200 | 2 | |||
| >200 | 0 | |||
| CLAT test | 11.6 (3.1–43.3) | <0.001 | 2.45 | |
| Positive | 0 | |||
| Negative | 2 | |||
Definition of abbreviations: CLAT = cryptococcal latex agglutination test; CSF = cerebrospinal fluid; OR = odds ratio; TB = tuberculosis.
Shown in the multivariate analysis are the parameter-specific β-coefficients, which determined the weighting of the scores used in the clinical prediction rule. None of the clinical features (symptoms and signs) was significant in the univariate analysis.
TABLE 5.
PATIENT DISTRIBUTION OF THE CLINICAL PREDICTION RULE COMPARED WITH THE T-SPOT.TB ASSAY
| Clinical Score* | Patients with TBM, n (%) | Patients with non-TBM, n (%) | Total |
|---|---|---|---|
| 0 | 0 (0) | 5 (100) | 5 |
| 2 | 8 (19) | 35 (81) | 43 |
| 4 | 16 (53) | 14 (47) | 30 |
| 6 | 15 (100) | 0 (0) | 15 |
Definition of abbreviations: TBM = tuberculous meningitis.
A score of ≥4 favors TBM.
TABLE 6.
PERFORMANCE OUTCOMES OF THE CLINICAL PREDICTION RULE COMPARED WITH THE T-CELL ENZYME-LINKED IMMUNOSPOT TUBERCULOSIS ASSAY
| Restricting to patients with both clinical index and T-SPOT.TB results (n = 86) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|
| Clinical prediction rule§ (cut-point ≥4) | 79%*,† (64–91) | 74% (6–85) | 69% (53–82) | 83% (70–93) | 76% (66–85) |
| T-SPOT.TB (T) ≥228 | 58% (41–74) | 94% (83–99) | 88% (69–97) | 74% (61–84) | 78% (68–86) |
| T-SPOT.TB ≥46‡ | 82% (66–92) | 100% (78–100) | 100% (89–100) | 68% (45–86) | 87% (75–95) |
| Clinical prediction rule + T-SPOT.TB (≥228) | 95%† (82-97) | 75% (60-86) | 75% (60-86) | 94.7% (82-97) | 84% (74-91) |
| Clinical prediction rule + T-SPOT.TB (≥46) | 97%* (86–100) | 75% (60–86) | 75.5% (61–87) | 97.3% (86–100) | 85% (76–92) |
Definition of abbreviations: NPV = negative predictive value; PPV = positive predictive value; T-SPOT.TB = T-cell enzyme-linked immunospot tuberculosis assay.
The P value was 0.003 comparing clinical prediction rule to a combination of the clinical prediction and the spot-forming cells at cut point of ≥46.
The P value was 0.03 comparing clinical prediction rule to a combination of the clinical prediction and the spot-forming cells at cut point of ≥228.
Negative cryptococcal latex agglutination test and gram-negative result in the non-TBM group.
A score of ≥4 favors TBM.
Incremental Value of RD1 Antigen-driven ELISPOT Assay over Clinical and Laboratory Parameters
A clinical score of 4 or higher had a similar sensitivity to the ELISPOT assay (∼80%). However, the specificity of the ELISPOT assay was significantly better (the prediction rule erroneously misclassified 1 in 4 non-TBM patients as having definite TBM). A strategy combining the clinical prediction rule and ELISPOT was also evaluated (a clinical score higher than 4 necessitated TB treatment, whereas a score of less than 4 necessitated use of the ELISPOT test in conjunction with Gram staining). A combination strategy (clinical score plus ELISPOT) had a higher sensitivity than ELISPOT alone and was thus a useful rule-out test (NPV ≥ 95%).
In summary, these data collectively indicate that the ELISPOT assay has incremental clinical value over and above baseline clinical and laboratory parameters when used in a high-burden setting.
PPD-driven CSF ELISPOT Responses
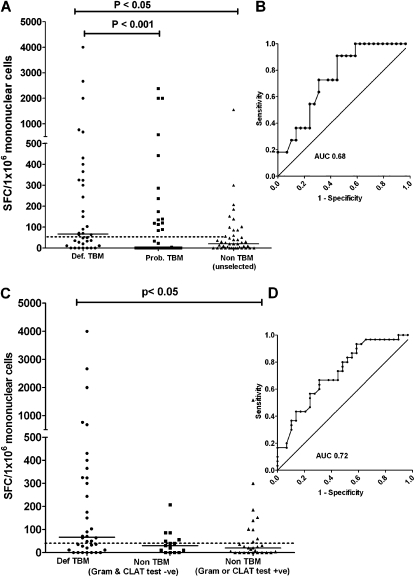
PPD is a less expensive and more widely available substitute antigen for the ELISPOT assay. Therefore, we compared its performance with the RD1-specific antigens. PPD-driven CSF ELISPOT responses are shown in Table E5 and in Figure 3. In summary, PPD ELISPOT responses discriminated poorly between the definite TBM and non-TBM groups.
Figure 3.
IFN-γ responses to purified protein derivative, using cerebrospinal fluid (CSF) mononuclear cells, comparing the definite-, probable-, and unselected non-tuberculous meningitis (TBM) groups. (A) Definite-TBM compared with the unselected non-TBM group (cut-point 46 spot-forming cells per 106 CSF mononuclear cells) and (B) the corresponding receiver operating characteristic (ROC) curve. (C) Responses when the non-TBM group was stratified by rapid test results (gram positive or cryptococcal antigen latex agglutination test [CLAT] positive versus gram negative and CLAT negative) and (D) the corresponding ROC curve.
RD-1 ELISPOT Responses Using Peripheral Blood Mononuclear Cells
RD-1 PBMC ELISPOT responses, in contrast to CSF responses, discriminated poorly between the TBM and non-TBM groups (Table E6). The frequencies of effector T cells were similar in blood and CSF (P = 0.8).
DISCUSSION
This study included a cohort of patients, routinely seen by clinicians in a high-burden setting, who had a meningitic illness and nondiagnostic CSF. The RD-1 ELISPOT IGRA using CSF cells, in contrast to the PPD IGRA, was a rapid and accurate rule-in test for TBM (sensitivity, 58%; specificity, 94%). This performance was further improved when used in conjunction with other inexpensive and routinely used rapid diagnostic tests (Gram stain and CLAT). Moreover, the RD-1 ELISPOT assay had a low rate of inconclusive results and had incremental value over a clinical prediction rule using simple and widely available clinical and laboratory parameters.
This is the first large prospective study using consecutively recruited unselected patients to evaluate the utility of a standardized quantitative T cell ELISPOT assay. The excellent performance outcomes of this assay likely represent a major advance in the rapid diagnosis of TBM, where diagnostic uncertainty often delays treatment, thus increasing mortality (8–11). Preliminary reports suggest that that the ELISPOT assay may be a useful diagnostic tool (26–29). The literature only cites two pilot studies and two case reports. The pilot studies had only 1 and 7 patients with definite TBM each, respectively (26, 29). Both studies, however, confirmed the possible utility of this test in CSF (26, 29). By contrast, the present study contained 38 culture or PCR-positive patients, recruited unselected TBM suspects, used clinical and technical operators blinded to the diagnostic categories and simultaneously evaluated PPD-specific responses.
There were three patients with cryptoccoccal meningitis who had strongly positive ELISPOT assays. However, given that they were all lost to follow-up or died, it is impossible to determine if these were true false positives or due to dual infection (TB and cryptoccocal meningitis). Indeed, our study had seven patients with both cryptococcal and TB meningitis, and dual infection is well described (37, 38). There were also five patients, most with very low CD4 counts, with definite TBM who had negative RD-1 ELISOPT assays. We found that negative ELISPOT results were more likely with very low CD4 counts, reflecting an attenuated antigen-specific response in those with advanced HIV (21, 39–41) Nevertheless, most patients with TBM with advanced HIV had positive results.
The RD-1 ELISPOT assay had good discriminatory value and a high specificity when using CSF mononuclear cells. This is similar to findings when using alveolar lavage cells in high- (22) and low-burden settings (23). In contrast, the specificity of the assay is poor in the pleural compartment (25) where patients with non-TB diagnoses (e.g., malignancy may have a high frequency of antigen-specific T cells). This may reflect the cellular translocation dynamics of different compartments, and in patients with LTBI there may be a compartment-specific leak of reactive T cells down a chemokine gradient (42).
Clinical diagnosis is a dynamic decision-making process assimilating an accumulating series of pre-test probabilities. Thus, the true clinical value of a test lies in its ability to improve pre-test probability (43) over and above that of readily available clinical and laboratory variables. We therefore evaluated the performance outcomes of a clinical prediction rule and showed that the ELISPOT assay has incremental value over basic clinical and laboratory parameters. We have, in the process, designed a clinical prediction rule suited to high HIV prevalence settings. An alternative prediction rule, developed by Thwaites and colleagues (44), was designed to distinguish TBM from bacterial meningitis, but it is less suited to high HIV prevalence settings. In a resource-poor setting, our clinical prediction rule misses 1 in 5 patients. A sensible approach in a resource-poor setting (i.e., to minimize test usage) might be to rule out TBM using the combination of the clinical prediction rule and the ELISPOT assay (i.e., score < 4 in combination with a negative ELISPOT assay has a high NPV). However, the ELISPOT assay, as recently shown in bronchoalveolar lavage fluid (45), cannot be used as a rule-out test for TBM. Thus, better diagnostic tools are required to rule out TBM in a HIV and TB endemic environment. The cost-effectiveness and clinical validity of such an approach remains to be evaluated.
Despite reports of up to a 60% yield in a research setting (44), smear-microscopy is less promising in a clinical setting and is routinely unhelpful in the local programmatic setting (8, 9, 12, 46–49). This is due to the high workload precluding accurate reading of microscopy slides (one laboratory technician reviews, approximately 300 patient slides for acid fast bacilli per day). It is recommended that large CSF volume (6 ml) and a slide examination for 30 minutes improves the yield of CSF microscopy (50). However, this is not feasible in our setting. For the sake of external validity, we processed CSF in the routine clinical, rather than research, laboratory setting.
Successful clinical implementation of this technology will require the harvesting of appropriate volumes of CSF. Given the requirement of 30,000 to 100,000 cells per well (Table E1), we recommend that for this test to be successfully used in clinical practice approximately 10 ml of CSF should be tapped, with approximately 3–4 ml used for routine tests and the remainder used for the ELISPOT assay. This volume of CSF (10 ml) can safely be taken (12).
Our study has several limitations. The confidence intervals of our test outcomes could have been narrower if we had recruited a larger cohort. However, we were limited by resource constraints and funding. Nevertheless, this is the largest TBM diagnostic study for the utility of IFN-γ release assays undertaken thus far. The findings presented here apply only to a high HIV prevalence setting. Thus, similar and larger studies are required in low TB and HIV burden settings. However, given the good outcomes in this high HIV prevalence setting, it is likely that outcomes will be better in immunocompetent HIV-negative individuals. The number of patients recruited for this study was too small to be examined for the influence of steroids and duration of therapy. Given the ethical implications of withholding therapy during patient transfer over large distances within our multitiered referral system, we were obliged to include patients who were empirically on treatment for up to 14 days before recruitment. This may have affected our M. tuberculosis culture yields and reduced the power of our study. It is likely that using the definite-TBM group in the analysis may overestimate sensitivity while using the definite plus the probable group may underestimate sensitivity. The true performance probably lies somewhere in between (39).
In conclusion, the RD-1 IFN-γ T cell ELISPOT assay is a good rule-in test for TBM, particularly when combined with other rapid and inexpensive tests, such as Gram stain and CLAT, and has incremental value over clinical judgment based on easily available clinical and laboratory parameters. These findings, if confirmed in other geographical and clinical settings, may help in the diagnosis of TBM in high- and low-burden environments and add may confidence to our often empirical decision to commit patients to approximately 12 months of potentially toxic antituberculous therapy.
Supplementary Material
Acknowledgments
The authors thank the patients and registrars in the department of neurology and nurses for facilitating this study; the Province of Kwa-Zulu Natal and the TB program for facilitating the study; and Madhukar Pai and Karel Moons for their Advanced Diagnostic Course (Montreal, Canada) notes, which were indispensable for formulating the clinical index.
Supported by the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP) funded by the Fogarty International Center, National Institutes of Health (grant D43TW00231, V.P.), a South African MRC grant, the EU FP7 programme (V.P.), and the South African NRF Research Chairs Initiative (SARChI; T.N. and K.D.). K.D. is also supported by a MRC Career Development Award (MRC), the EDCTP, and the EU FP7 program (TBsusgent).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200912-1931OC on May 4, 2010
Author Disclosure: V.B.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.K.C.P. received $1,001 to $5,000 from Jansen Pharmaceutical in advisory board fees and up to $1,000 from Jansen Pharmaceutical in lecture fees. P.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.N. received more than $100,001 from the NIH for several NIH HIV research grants, more than $100,001 from the Gates Foundation as an HIV research grant, and $50,001 to $100,000 from HHMI as an HIV research grant. K.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.WHO. Global tuberculosis control: surveillance, planning, financing. WHO report, 2009 [accessed October 20, 2009]. Available from: http://www.WHO/HTM/TB/2008.393
- 2.CDC. Extrapulmonary tuberculosis cases and percentages by site of disease: reporting areas. Centers for disease control and prevention, Atlanta, GA [accessed October 30, 2000]. Available from: www.cdc.gov/tb/surv/surv2005/PDF/table27.pdf
- 3.Phypers M, Harris T, Power C. CNS tuberculosis: a longitudinal analysis of epidemiological and clinical features. Int J Tuberc Lung Dis 2006;10:99–103. [PubMed] [Google Scholar]
- 4.Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary tuberculosis in the United States, 1993–2006. Clin Infect Dis 2009;49:1350–1357. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E, Fauchi AS, Kasper DL, Hauser SL, Longo DL, Jameson JL. Harrisons's principles of internal medicine. New York: McGraw-Hill; 2001.
- 6.Elder NC. Extrapulmonary tuberculosis: a review. Arch Fam Med 1992;1:91–98. [DOI] [PubMed] [Google Scholar]
- 7.Ozbay B, Uzun K. Extrapulmonary tuberculosis in high prevalence of tuberculosis and low prevalence of HIV. Clin Chest Med 2002;23:351–354. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Monco JC. Central nervous system tuberculosis. Neurol Clin 1999;17:737–759. [DOI] [PubMed] [Google Scholar]
- 9.Karsteadt AS, Valtatchanova S, Barriere R, Crewe-Brown HH. Tuberculous meningitis in South African urban adults. Q J Med 1998;91:743–747. [DOI] [PubMed] [Google Scholar]
- 10.Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev 2008;21:243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinnard C, Macgregor RR. Tuberculous meningitis in HIV-infected individuals. Curr HIV/AIDS Rep 2009;6:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhigjee AI, Padayachee R, Paruk H, Hallwirth-Pillay KD, Marais S, Connoly C. Diagnosis of tuberculous meningitis: clinical and laboratory parameters. Int J Infect Dis 2007;11:348–354. [DOI] [PubMed] [Google Scholar]
- 13.Kashyap RS, Ramteke SS, Morey SH, Purohit HJ, Taori GM, Daginawala HF. Diagnostic value of early secreted antigenic target-6 for the diagnosis of tuberculous meningitis patients. Infection 2009;37:508–513. [DOI] [PubMed] [Google Scholar]
- 14.Patel VB, Padayatchi N, Bhigjee AI, Allen J, Bhagwan B, Moodley AA, Mthiyane T. Multidrug-resistant tuberculous meningitis in Kwazulu-Natal, South Africa. Clin Infect Dis 2004;38:851–856. [DOI] [PubMed] [Google Scholar]
- 15.Dheda K, Smit RZ, Badri M, Pai M. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr Opin Pulm Med 2009;15:188–200. [DOI] [PubMed] [Google Scholar]
- 16.Pai M, O'Brien R. New diagnostics for latent and active tuberculosis: state of the art and future prospects. Semin Respir Crit Care Med 2008;29:560–568. [DOI] [PubMed] [Google Scholar]
- 17.Dewan PK, Grinsdale J, Kawamura LM. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin Infect Dis 2007;44:69–73. [DOI] [PubMed] [Google Scholar]
- 18.Hinks TS, Dosanjh DP, Innes JA, Pasvol G, Hackforth S, Varia H, Millington KA, Liu XQ, Bakir M, Soysal A, et al. Frequencies of region of difference 1 antigen-specific but not purified protein derivative-specific gamma interferon-secreting t cells correlate with the presence of tuberculosis disease but do not distinguish recent from remote latent infections. Infect Immun 2009;77:5486–5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, Ewer K, Hill AV, Mehta A, Rodrigues C. Enumeration of T cells specific for rd1-encoded antigens suggests a high prevalence of latent mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis 2001;183:469–477. [DOI] [PubMed] [Google Scholar]
- 20.Mazurek GH, Weis SE, Moonan PK, Daley CL, Bernardo J, Lardizabal AA, Reves RR, Toney SR, Daniels LJ, LoBue PA. Prospective comparison of the tuberculin skin test and 2 whole-blood interferon-gamma release assays in persons with suspected tuberculosis. Clin Infect Dis 2007;45:837–845. [DOI] [PubMed] [Google Scholar]
- 21.Rangaka MX, Diwakar L, Seldon R, van Cutsem G, Meintjes GA, Morroni C, Mouton P, Shey MS, Maartens G, Wilkinson KA, et al. Clinical, immunological, and epidemiological importance of antituberculosis T cell responses in HIV-infected Africans. Clin Infect Dis 2007;44:1639–1646. [DOI] [PubMed] [Google Scholar]
- 22.Dheda K, van Zyl-Smit RN, Meldau R, Meldau S, Symons G, Khalfey H, Govender N, Rosu V, Sechi LA, Maredza A, et al. Quantitative lung T cell responses aid the rapid diagnosis of pulmonary tuberculosis. Thorax 2009;64:847–853. [DOI] [PubMed] [Google Scholar]
- 23.Jafari C, Ernst M, Strassburg A, Greinert U, Kalsdorf B, Kirsten D, Lange C. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur Respir J 2008;31:261–265. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson KA, Wilkinson RJ, Pathan A, Ewer K, Prakash M, Klenerman P, Maskell N, Davies R, Pasvol G, Lalvani A. Ex vivo characterization of early secretory antigenic target 6-specific T cells at sites of active disease in pleural tuberculosis. Clin Infect Dis 2005;40:184–187. [DOI] [PubMed] [Google Scholar]
- 25.Dheda K, van Zyl-Smit RN, Sechi LA, Badri M, Meldau R, Meldau S, Symons G, Semple L, Maredza A, Dawson R, et al. Utility of quantitative T cell responses versus unstimulated interferon-{gamma} for the diagnosis of pleural tuberculosis. Eur Respir J 2009;34:1118–1126. [DOI] [PubMed] [Google Scholar]
- 26.Kim SH, Chu K, Choi SJ, Song KH, Kim HB, Kim NJ, Park SH, Yoon BW, Oh MD, Choe KW. Diagnosis of central nervous system tuberculosis by t-cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Vaccine Immunol 2008;15:1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosters K, Nau R, Bossink A, Greiffendorf I, Jentsch M, Ernst M, Thijsen S, Hinks T, Lalvani A, Lange C. Rapid diagnosis of CNS tuberculosis by a T-cell interferon-gamma release assay on cerebrospinal fluid mononuclear cells. Infection 2008;36:597–600. [DOI] [PubMed] [Google Scholar]
- 28.Murakami S, Takeno M, Oka H, Ueda A, Kurokawa T, Kuroiwa Y, Ishigatsubo Y. Diagnosis of tuberculous meningitis due to detection of esat-6-specific gamma interferon production in cerebrospinal fluid enzyme-linked immunospot assay. Clin Vaccine Immunol 2008;15:897–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas MM, Hinks TS, Raghuraman S, Ramalingam N, Ernst M, Nau R, Lange C, Kosters K, Gnanamuthu C, John GT, et al. Rapid diagnosis of mycobacterium tuberculosis meningitis by enumeration of cerebrospinal fluid antigen-specific T-cells. Int J Tuberc Lung Dis 2008;12:651–657. [PMC free article] [PubMed] [Google Scholar]
- 30.Simmons CP, Thwaites GE, Quyen NT, Torok E, Hoang DM, Chau TT, Mai PP, Lan NT, Dung NH, Quy HT, et al. Pretreatment intracerebral and peripheral blood immune responses in Vietnamese adults with tuberculous meningitis: diagnostic value and relationship to disease severity and outcome. J Immunol 2006;176:2007–2014. [DOI] [PubMed] [Google Scholar]
- 31.Tanner DC, Weinstein MP, Fedorciw B, Joho KL, Thorpe JJ, Reller L. Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol 1994;32:1680–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thwaites G, Chau TT, Mai NT, Drobniewski F, McAdam K, Farrar J. Tuberculous meningitis. J Neurol Neurosurg Psychiatry 2000;68:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thwaites G, Fisher M, Hemingway C, Scott G, Solomon T, Innes J. British Infection Society guidelines for the diagnosis and treatment of tuberculosis of the central nervous system in adults and children. J Infect 2009;59:167–187. [DOI] [PubMed] [Google Scholar]
- 34.Faraggi D. Adjusting receiver operating characteristic curves and related indices for covariates. Statistician 2003;52:179–192. [Google Scholar]
- 35.Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding Youden index to discriminate individuals using pooled blood samples. Epidemiology 2005;16:73–81. [DOI] [PubMed] [Google Scholar]
- 36.Bruns DE. The stard initiative and the reporting of studies of diagnostic accuracy. Clin Chem 2003;49:19–20. [DOI] [PubMed] [Google Scholar]
- 37.Lanjewar DN, Jain PP, Shetty CR. Profile of central nervous system pathology in patients with aids: an autopsy study from India. AIDS 1998;12:309–313. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg A, Bloch KC, Li S, Tang YW, Palmer M, Tyler KL. Dual infections of the central nervous system with Epstein-Barr virus. J Infect Dis 2005;191:234–237. [DOI] [PubMed] [Google Scholar]
- 39.Raby E, Moyo M, Devendra A, Banda J, De Haas P, Ayles H, Godfrey-Faussett P. The effects of HIV on the sensitivity of a whole blood IFN-gamma release assay in Zambian adults with active tuberculosis. PLoS One 2008;3:e2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aabye MG, Ravn P, PrayGod G, Jeremiah K, Mugomela A, Jepsen M, Faurholt D, Range N, Friis H, Changalucha J, et al. The impact of HIV infection and cd4 cell count on the performance of an interferon gamma release assay in patients with pulmonary tuberculosis. PLoS One 2009;4:e4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dheda K, Lalvani A, Miller RF, Scott G, Booth H, Johnson MA, Zumla A, Rook GA. Performance of a T-cell-based diagnostic test for tuberculosis infection in HIV-infected individuals is independent of cd4 cell count. AIDS 2005;19:2038–2041. [DOI] [PubMed] [Google Scholar]
- 42.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 2006;354:610–621. [DOI] [PubMed] [Google Scholar]
- 43.Moons KG, Harrell FE, Steyerberg EW. Should scoring rules be based on odds ratios or regression coefficients? J Clin Epidemiol 2002;55:1054–1055. [DOI] [PubMed] [Google Scholar]
- 44.Thwaites GE, Chau TT, Stepniewska K, Phu NH, Chuong LV, Sinh DX, White NJ, Parry CM, Farrar JJ. Diagnosis of adult tuberculous meningitis by use of clinical and laboratory features. Lancet 2002;360:1287–1292. [DOI] [PubMed] [Google Scholar]
- 45.van Zyl-Smit R, Meldau R, Dheda K. Quantitative pulmonary T-cell responses for the diagnosis of active tuberculosis [letter]. Am J Respir Crit Care Med 2010;181:289, author reply 289–290. [DOI] [PubMed] [Google Scholar]
- 46.Verdon R, Chevret S, Laissy JP, Wolff M. Tuberculous meningitis in adults: review of 48 cases. Clin Infect Dis 1996;22:982–988. [DOI] [PubMed] [Google Scholar]
- 47.Nagami PH, Yoshikawa TT. Tuberculosis in the geriatric patient. J Am Geriatr Soc 1983;31:356–363. [DOI] [PubMed] [Google Scholar]
- 48.Davis LE, Rastogi KR, Lambert LC, Skipper BJ. Tuberculous meningitis in the southwest United States: a community-based study. Neurology 1993;43:1775–1778. [DOI] [PubMed] [Google Scholar]
- 49.Patel VB, Bhigjee AI, Paruk HF, Singh R, Meldau R, Connolly C, Ndung'u T, Dheda K. Utility of a novel lipoarabinomannan assay for the diagnosis of tuberculous meningitis in a resource-poor high-HIV prevalence setting. Cerebrospinal Fluid Res 2009;6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thwaites GE, Chau TT, Farrar JJ. Improving the bacteriological diagnosis of tuberculous meningitis. J Clin Microbiol 2004;42:378–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.