Abstract
Objective
Evaluate intravitreal 0.5 mg ranibizumab or 4 mg triamcinolone combined with focal/grid laser compared with focal/grid laser alone for treatment of diabetic macular edema (DME).
Design
Multicenter, randomized clinical trial.
Participants
A total of 854 study eyes of 691 participants with visual acuity (approximate Snellen equivalent) of 20/32 to 20/320 and DME involving the fovea.
Methods
Eyes were randomized to sham injection + prompt laser (n=293), 0.5 mg ranibizumab + prompt laser (n=187), 0.5 mg ranibizumab + deferred (≥24 weeks) laser (n=188), or 4 mg triamcinolone + prompt laser (n=186). Retreatment followed an algorithm facilitated by a web-based, real-time data-entry system.
Main Outcome Measures
Best-corrected visual acuity and safety at 1 year.
Results
The 1-year mean change (±standard deviation) in the visual acuity letter score from baseline was significantly greater in the ranibizumab + prompt laser group (+9±11, P<0.001) and ranibizumab + deferred laser group (+9±12, P<0.001) but not in the triamcinolone + prompt laser group (+4±13, P=0.31) compared with the sham + prompt laser group (+3±13). Reduction in mean central subfield thickness in the triamcinolone + prompt laser group was similar to both ranibizumab groups and greater than in the sham + prompt laser group. In the subset of pseudophakic eyes at baseline (n=273), visual acuity improvement in the triamcinolone + prompt laser group appeared comparable to that in the ranibizumab groups. No systemic events attributable to study treatment were apparent. Three eyes (0.8%) had injection-related endophthalmitis in the ranibizumab groups, whereas elevated intraocular pressure and cataract surgery were more frequent in the triamcinolone + prompt laser group. Two-year visual acuity outcomes were similar to 1-year outcomes.
Conclusions
Intravitreal ranibizumab with prompt or deferred laser is more effective through at least 1 year compared with prompt laser alone for the treatment of DME involving the central macula. Ranibizumab as applied in this study, although uncommonly associated with endophthalmitis, should be considered for patients with DME and characteristics similar to those in this clinical trial. In pseudophakic eyes, intravitreal triamcinolone + prompt laser seems more effective than laser alone but frequently increases the risk of intraocular pressure elevation.
Introduction
Macular edema is a frequent manifestation of diabetic retinopathy and an important cause of impaired vision in individuals with diabetes.1-3 Focal/grid photocoagulation, the current standard care for diabetic macular edema (DME), has been the mainstay of treatment since its benefit was demonstrated in the Early Treatment Diabetic Retinopathy Study (ETDRS) in 1985.4 In a randomized, multicenter clinical trial, the Diabetic Retinopathy Clinical Research Network (DRCR.net) showed that focal/grid photocoagulation in eyes with center-involved DME and visual acuity ≤20/40 produces gradual visual acuity improvement of ≥2 lines in approximately one third of eyes after 2 years of follow-up, although approximately 20% of laser-treated eyes worsen by ≥2 lines.5 Thus, other treatment modalities, including anti-vascular endothelial growth factor (VEGF) therapy and steroids, alone or in combination with laser, are under investigation.
The rationale for anti-VEGF therapy for DME is based on the observation that VEGF levels are increased in the retina and vitreous of eyes with diabetic retinopathy.6 Vascular endothelial growth factor has been demonstrated to increase vessel permeability in vivo possibly by increasing the phosphorylation of tight junction proteins.7 Therefore, therapy that inhibits VEGF may represent a useful therapeutic modality that targets the underlying pathogenesis of DME. Pegaptanib (Macugen, Eyetech Pharmaceuticals, Palm Beach Gardens, FL) was the first anti-VEGF drug reported to have a favorable effect on macular edema,8 although more recently, the anti-VEGF drugs ranibizumab (Lucentis, Genentech, South San Francisco, CA) and bevacizumab (Avastin, Genentech), among others, also have been evaluated for DME. Prior studies, which were small with short-term follow-up, have reported promising results.9 Intravitreal triamcinolone also was evaluated previously as treatment for DME in a randomized trial conducted by the DRCR.net.5 Although the data suggest that triamcinolone treatment was superior to the expected untreated course in the ETDRS, it was not superior to focal/grid photocoagulation.5
The combination of intravitreal treatment (either triamcinolone or an anti-VEGF drug) with focal/grid photocoagulation, theoretically, could be more effective than either treatment alone. The intravitreal treatment might rapidly reduce macular edema and lead to more rapid visual acuity improvement, whereas slower benefit accrues over time as a result of laser treatment. In addition, combined treatment could enhance the effect of focal/grid photocoagulation because the retina would be less edematous if laser treatment was administered some time after the intravitreal treatment reduced macular edema. Also, laser treatment theoretically could reduce the number of repeat intravitreal injections required to optimize the outcome of DME treatment. In a study of 86 eyes randomized to 4 mg intravitreal triamcinolone alone or followed by macular laser photocoagulation, Kang et al10 reported that after 6 months visual acuity was better and more eyes had resolution of central edema with the combined treatment when compared with intravitreal triamcinolone without macular laser. Other studies have shown greater mean visual acuity improvements at 6 months using ranibizumab + laser, or ranibizumab alone, when compared with laser alone.9
To determine whether anti-VEGF therapy alone or in combination with focal/grid laser, or intravitreal triamcinolone combined with focal/grid laser, might result in improved outcomes compared with the standard treatment for DME of laser alone, the DRCR.net designed a clinical trial to evaluate 3 treatment modalities for DME in comparison with focal/grid photocoagulation: ranibizumab combined with prompt (within 1 week) focal/grid photocoagulation, intravitreal triamcinolone combined with prompt (within 1 week) focal/grid photocoagulation, and intravitreal ranibizumab with focal/grid photocoagulation deferred for at least 24 weeks. The study design also provided an opportunity to determine which regimen resulted in fewer treatments if safety and efficacy were comparable.
Materials and Methods
This phase 3 randomized, multicenter clinical trial was conducted by the DRCR.net at 52 clinical sites in the United States. The study adhered to the tenets of the Declaration of Helsinki. The protocol and informed consent forms were compliant with the Health Insurance Portability and Accountability Act and approved by multiple institutional review boards. Each study participant gave written informed consent before participation in the study. Study oversight was provided by an independent data and safety monitoring committee. The study was conducted under an Investigational New Drug Application from the Food and Drug Administration. The study is listed on www.clinicaltrials.gov under identifier NCT00445003 (website registration date 03-06-2007), and the protocol is available on the DRCR.net website (www.drcr.net, date accessed January 1, 2010). Key aspects of the protocol pertinent to this article are summarized next.
Study Population
Eligible patients were at least 18 years old with type 1 or 2 diabetes. The major eligibility criteria for a study eye included the following: (1) best-corrected Electronic-Early Treatment Diabetic Retinopathy Study (E-ETDRS Visual Acuity Test11) visual acuity letter score 78 to 24 (20/32–20/320), (2) definite retinal thickening due to DME on clinical examination involving the center of the macula assessed to be the main cause of visual loss, and (3) retinal thickness measured on time domain optical coherence tomography (OCT) ≥250 μm in the central subfield. Principal exclusion criteria included the following: (1) treatment for DME within the prior 4 months, (2) panretinal photocoagulation within the prior 4 months or anticipated need for panretinal photocoagulation within the next 6 months, (3) major ocular surgery within the prior 4 months, (4) history of open-angle glaucoma or steroid-induced intraocular pressure (IOP) elevation that required IOP-lowering treatment, and (5) IOP ≥25 mmHg. Patients were excluded if their systolic blood pressure was >180 mmHg or diastolic blood pressure was >110 mmHg, or if a myocardial infarction, other cardiac event requiring hospitalization, cerebrovascular accident, transient ischemic attack, or treatment for acute congestive heart failure occurred within 4 months before randomization. A patient could have 2 study eyes in the trial only if both were eligible at the time of study entry.
Synopsis of Study Design
After eligibility was determined and informed consent was obtained, study participants with 1 study eye were assigned randomly on the DRCR.net study website (using a permuted blocks design stratified by study eye visual acuity) with equal probability to 1 of 4 treatment groups: (1) sham injection plus prompt (within 3–10 days after injection) focal/grid photocoagulation (sham + prompt laser group), (2) 0.5 mg intravitreal ranibizumab plus prompt (within 3–10 days after injection) focal/grid photocoagulation (ranibizumab + prompt laser group), (3) 0.5 mg intravitreal ranibizumab with deferred (≥24 weeks) focal/grid photocoagulation (ranibizumab + deferred laser group), and (4) 4 mg intravitreal triamcinolone plus prompt (within 3–10 days after injection) focal/grid photocoagulation (triamcinolone + prompt laser group). For study participants with 2 study eyes, the right eye was assigned randomly with equal probability to 1 of the 4 groups as indicated above. If the right eye was assigned to a treatment group other than the sham + prompt laser group, then the left eye was assigned to the sham + prompt laser group. If the right eye was assigned to the sham + prompt laser group, then the left eye was assigned randomly to 1 of the other 3 groups. Thus, there were more eyes in the sham + prompt laser group than in the other 3 groups.
Follow-up was planned for 3 years, with the primary outcome at 1 year. During the first year, follow-up visits occurred every 4 weeks (±1 week). Study participants in the 3 groups receiving laser were masked to treatment assignment through the primary outcome visit, whereas the ranibizumab + deferred laser group was not masked. After the first year, visits occurred every 4 to 16 weeks depending on the treatment group, disease course, and treatment administered. After a study participant completed the primary outcome visual acuity examination at 1 year, the study participant was made aware of his or her treatment group assignment and sham injections were discontinued. Visual acuity examiners and OCT technicians were masked to treatment group assignment before and at the 1-year primary outcome visit.
Examination Procedures
At baseline and each follow-up visit, best-corrected visual acuity letter score was measured at 3 m by a certified examiner using an E-ETDRS Visual Acuity Test.11 The OCT images were obtained at baseline and each follow-up visit by a certified operator using the Zeiss Stratus OCT (OCT3) machine (Carl Zeiss Meditec, Inc., Dublin, CA). Scans were 6 mm in length and included the 6-radial line fast macular scan pattern for quantitative measures and the cross-hair pattern (6–12 o’clock and 9–3 o’clock) for qualitative assessment of retinal morphology. All baseline OCT scans, annual follow-up scans with a standard deviation of the center point ≥10.0%, and scans from any visits in which the investigator suspected erroneous measurements because of the algorithm placement of the lines created by the OCT software that delineate the inner and outer aspects of the retina were sent to the Fundus Photograph Reading Center (University of Wisconsin, Madison) for grading. If the automated thickness measurements were judged by the Reading Center to be inaccurate on any submitted image, center point thickness was measured manually, and this value was used to impute a value for the central subfield based on a correlation of the 2 measures of 0.98 as published previously12 (20% of 854 baseline scans were imputed and 1 scan was unable to be manually graded at baseline, and 2% of 10 849 follow-up scans were imputed and 22 [<1%] were unable to be manually graded during follow-up through 1 year). Manual grading of the baseline scans resulted in an imputed baseline central subfield value <250 μm for 60 eyes (7%), which does not necessarily mean that the true thickness measurement is <250 if measureable. Of note, 22 (37%) of the 60 scans with an imputed central subfield thickness <250 μm were from 1 clinical site and represented 85% of the 26 baseline scans from that site. All intent-to-treat results presented were similar when evaluated with exclusion of eyes from that clinical site (data not shown) and when evaluated with exclusion of eyes from any clinical site with a baseline central subfield thickness <250 μm. Baseline OCT images also were assessed by the Reading Center for cystoid abnormalities and subretinal fluid.
Additional testing at baseline and each follow-up visit included slit-lamp examination, measurement of IOP, and fundus examination after pupil dilation. Standard ETDRS 7-field color stereoscopic fundus photographs were obtained at baseline and 12 months by a certified photographer and graded at the reading center for level of diabetic retinopathy.13 Hemoglobin A1c was measured at baseline. Any untoward medical occurrence, regardless of whether the event was considered treatment related, was considered as an adverse event and recorded. Treatment of adverse events and proliferative diabetic retinopathy was at the discretion of the investigator.
Treatment Protocol
Overview
The treatment protocol (summarized in Appendix 1, available at http://aaojournal.org) included a baseline treatment followed by intravitreal study drug or sham injection retreatments every 4 weeks through the 12-week study visit. From the 16-week study visit and thereafter, a retreatment algorithm for study drug injections and sham injections (Appendices 2 and 3, available at http://aaojournal.org) was designed to require retreatments unless a study visit was deemed a ‘success’ (defined below and in Table 1, available at http://aaojournal.org) at which point retreatment was at investigator discretion. From the 24-week study visit and thereafter retreatment was at investigator discretion if the study visit was deemed ‘no improvement’ (defined in Table 1, available at http://aaojournal.org). If retreatment with a study drug or sham injection was not given, ‘alternative treatment’ (defined in Table 1, available at http://aaojournal.org) was permitted only if a study eye met criteria for ‘failure’ or ‘futility’ (defined in Table 1, available at http://aaojournal.org). When retreatment with a study drug or sham injection was indicated, eyes assigned to one of the ranibizumab groups could receive ranibizumab as often as every 4 weeks; eyes assigned to intravitreal triamcinolone could receive triamcinolone as often as every 16 weeks with sham injections as often as every 4 weeks in between triamcinolone injections; eyes assigned to sham + prompt laser could receive sham injections as often as every 4 weeks. A retreatment algorithm for focal/grid laser (Appendix 4, available at http://aaojournal.org) was designed to require retreatment if there was ‘edema involving the center of the macula’ or ‘edema threatening the center of the macula’ (defined in Table 1, available at http://aaojournal.org) and if ‘complete laser’ had not been given (defined in Table 1, available at http://aaojournal.org), provided that it had been at least 13 weeks since the last focal/grid laser application.
Table 1.
Diabetic Retinopathy Clinical Research Network Definitions for Laser-Ranibizumab-Triamcinolone Treatment for Diabetic Macular Edema
| Term | Definition |
|---|---|
| Sham + Prompt Laser group | Eyes assigned to receive sham injection plus prompt (within one week) focal/grid photocoagulation |
| Ranibizumab + Prompt Laser group | Eyes assigned to receive 0.5 mg intravitreal ranibizumab plus prompt (within one week) focal/grid photocoagulation |
| Ranibizumab + Deferred Laser group | Eyes assigned to receive 0.5 mg intravitreal ranibizumab with deferred (≥24 weeks) focal/grid photocoagulation |
| Triamcinolone + Prompt Laser group | Eyes assigned to receive 4 mg intravitreal triamcinolone plus prompt (within one week) focal/grid photocoagulation |
| Focal/grid laser | Focal/grid photocoagulation administered using modified ETDRS protocol |
| ‘Complete laser’ | Direct treatment to all microaneurysms within areas of macular edema and grid treatment to all other areas of macular edema |
| ‘Success’ criteria relative to retreatment decisions | Either visual acuity letter score ≥84 (20/20) or OCT central subfield thickness <250 microns since the last non-sham injection or since baseline for the sham+prompt laser group |
| ‘Improvement’ criteria relative to retreatment decisions | Either visual acuity improved by ≥5 letters or OCT central subfield thickness improved by ≥10% since the last non-sham injection or since baseline for the sham+prompt laser group |
| ‘No improvement’ criteria relative to retreatment decisions | Success and failure/futility criteria not met and visual acuity letter score improved by <5 letters (or worsened) and OCT central subfield thickness decreased by <10% (or increased) since the last non-sham injection or since baseline for the sham+prompt laser group |
| ‘Failure’ criteria relative to retreatment decisions | Visual acuity 10 or more letters worse than baseline, OCT central subfield thickness ≥250 um, DME judged to be the cause of visual acuity loss, and at least 13 weeks since ‘complete laser’ had been given with ‘no improvement’ since the last laser treatment |
| ‘Futility’ criteria relative to retreatment decisions | After 52 week visit: OCT central subfield ≥250 um, DME judged to be the cause of visual acuity loss, and at least 29 weeks since ‘complete laser’ had been given with ‘no improvement’ since the last laser treatment |
| ‘Extended follow-up’ | After 52 week visit: Follow-up visit in twice the time interval since the last visit, up to a maximum of 16 weeks between study visits (applies to eyes assigned to ranibizumab in which the injection was repeatedly deferred either due to ‘success’ or ‘no improvement’) |
| ‘Alternative treatment’ | Treatment for DME other than the randomization-assigned regimen |
| ‘Edema involving the center of the macula’ | OCT central subfield thickness ≥250 um |
| ‘Edema threatening the center of the macula’ | Edema on clinical exam within 500 microns of the foveal center or edema associated with lipid within 500 microns of the foveal center or 1 disc area of edema within 1 disc area of the foveal center |
DME = diabetic macular edema; ETDRS = Early Treatment Diabetic Retinopathy Study; OCT = optical coherence tomography.
Retreatment Algorithm System
Compliance with the details of the treatment protocol, which depended mainly on visual acuity and OCT measurements over time, was facilitated by a web-based, real-time data-entry system. At each follow-up visit, the system provided real-time feedback to the treating physician regarding whether treatment was required or at investigator discretion. If treatment was to be given, the system also provided feedback as to whether the treatment should be an intravitreal study drug or sham injection, whether focal/grid photocoagulation should be applied, and what the next follow-up interval should be.
Statistical Methods
Data are reported that were collected by the clinical sites from March 2007 to February 8, 2010. This includes at least 1-year follow-up for the entire study population and up to 2-year follow-up for participants enrolled early in the trial. Mean change in visual acuity from baseline to 1 year adjusted for baseline visual acuity was the primary outcome measure. The primary analysis consisted of 3 pairwise comparisons of the mean change in the sham + prompt laser group compared with each of the other 3 groups.
Sample size was estimated to be 842 eyes (~701 study participants assuming 20% of study participants would have 2 study eyes) on the basis of an expected population difference in the letter score of 6.0 and standard deviation of the visual acuity letter score of 18, a correlation between baseline and 1-year scores of 0.48, a type 1 error rate of 0.016 (adjusted for multiple comparisons and alpha spending for interim data reviews), and a power of approximately 90%.
The primary analysis included all randomized eyes and followed the intent-to-treat principle. Data were included in the 1-year analysis when an examination was performed between 308 and 420 days from randomization. When more than 1 visit occurred in this window, data from the visit closest to the 1-year target date were used. For eyes without 1-year data, the last-observation-carried forward method was used to impute data for the primary analysis. Similar results (data not shown) were produced when analyses (1) used Rubin’s method14 to impute for missing data; (2) included only eyes with a completed 1-year examination and used the last visual acuity before additional treatment for those who received a treatment other than the randomly assigned treatment before the 1-year examination (per-protocol analysis); (3) included adjustment for the following potential confounders in addition to baseline visual acuity: age, gender, race/ethnicity, baseline hemoglobin A1c, baseline OCT central subfield thickness, and prior panretinal scatter photocoagulation and prior DME treatment at baseline; (4) were performed with outlying values truncated to 3 standard deviations from the mean; and (5) used van der Waerden’s normal score transformation on the visual acuity scores. For analyses other than the primary analysis, only data from completed visits were used with no imputation for missing data. For some results, medians and interquartile ranges have been reported instead of, or in addition to, means and standard deviations to describe the distribution of the data. Analyses of the number of study treatments received before the 1- and 2-year visits included only the eyes of participants completing the 1- and 2-year visits.
Three pairwise comparisons were made for all analyses, except the ranibizumab groups were pooled for analysis of progression of diabetic retinopathy and all safety analyses. For all continuous outcomes, treatment group comparisons were made using analysis of covariance models with generalized estimating equations to account for correlated data from study participants with 2 study eyes. For binary outcomes, proportions similarly were compared between treatment groups using logistic regression models with generalized estimating equations. All analyses included adjustment for baseline visual acuity. In addition, models in which the central subfield thickness was the outcome included baseline central subfield thickness as a covariate, and models with retinal volume as the outcome included both baseline central subfield thickness and retinal volume as covariates. Similar analyses were performed on 2-year results. All P values are 2-sided. SAS version 9.1 (SAS Inc, Cary, NC) was used for all analyses.
Results
Between March of 2007 and December of 2008, 691 study participants (mean age 63±10 years; 44% women) were enrolled, 163 (24%) with 2 study eyes. The mean baseline visual acuity letter score in study eyes was 63±12 (~20/63±2.4 lines), and the mean OCT central subfield retinal thickness was 405±134 μm. The 854 study eyes were assigned to either sham + prompt laser (n=293), ranibizumab + prompt laser (n=187), ranibizumab + deferred laser (n=188), or triamcinolone + prompt laser (n=186). The baseline characteristics of the 4 groups were similar (Table 2, available at http://aaojournal.org).
Table 2.
Baseline Study Participant and Ocular Characteristics
| Sham + Prompt Laser N = 293 | Ranibizumab + Prompt Laser N = 187 | Ranibizumab + Deferred Laser N = 188 | Triamcinolone + Prompt Laser N = 186 | |
|---|---|---|---|---|
| Women, no. (%) | 123 (42%) | 85 (45%) | 78 (41%) | 86 (46%) |
| Age (yrs) Median (25th, 75th percentile) | 63 (57, 69) | 62 (56, 70) | 64 (58, 70) | 62 (55, 70) |
| Race, no. (%) | ||||
| White | 202 (69%) | 131 (70%) | 134 (71%) | 134 (72%) |
| African-American | 51 (17%) | 30 (16%) | 25 (13%) | 32 (17%) |
| Hispanic or Latino | 34 (12%) | 21 (11%) | 25 (13%) | 15 (8%) |
| Asian | 4 (1%) | 1 (1%) | 2 (1%) | 4 (2%) |
| Native Hawaiian/Other Pacific Islander | 0 | 1 (1%) | 0 | 0 |
| More than one race | 1 (<1%) | 1 (1%) | 1 (1%) | 0 |
| Unknown/ not reported | 1 (<1%) | 2 (1%) | 1 (1%) | 1 (1%) |
| Diabetes type, no. (%) | ||||
| Type 1 | 25 (9%) | 11 (6%) | 15 (8%) | 14 (8%) |
| Type 2 | 260 (89%) | 172 (92%) | 170 (90%) | 166 (89%) |
| Uncertain | 8 (3%) | 4 (2%) | 3 (2%) | 6 (3%) |
| Duration of diabetes (yrs) Median (25th, 75th percentile) | 16 (9, 22) | 18 (12, 24) | 17 (11, 22) | 17 (11, 24) |
| HbA1c Median (25th, 75th percentile)* | 7.3 (6.6, 8.3) | 7.3 (6.6, 8.4) | 7.5 (6.7, 8.4) | 7.4 (6.5, 8.6) |
| Prior cardiovascular event, no. (%)† | 93 (32%) | 66 (35%) | 61 (32%) | 61 (33%) |
| Hypertension, no. (%)† | 240 (82%) | 154 (82%) | 156 (83%) | 148 (80%) |
| Number of study eyes, no. (%) | ||||
| 1 study eye | 130 (44%) | 131 (70%) | 132 (70%) | 135 (73%) |
| 2 study eyes | 163 (56%) | 56 (30%) | 56 (30%) | 51 (27%) |
| Prior panretinal photocoagulation, no. (%) | 48 (16%) | 36 (19%) | 31 (16%) | 37 (20%) |
| No prior treatment for DME, no. (%) | 105 (36%) | 74 (40%) | 74 (39%) | 61 (33%) |
| Prior laser for DME, no. (%) | 173 (59%) | 101 (54%) | 101 (54%) | 114 (61%) |
| Prior IVT for DME, no. (%) | 39 (13%) | 22 (12%) | 36 (19%) | 31 (17%) |
| Prior vitrectomy for DME, no. (%) | 15 (5%) | 7 (4%) | 5 (3%) | 12 (6%) |
| Prior peribulbar triamcinolone for DME, no. (%) | 12 (4%) | 9 (5%) | 5 (3%) | 5 (3%) |
| Prior anti-VEGF for DME, no. (%) | 24 (8%) | 24 (13%) | 21 (11%) | 20 (11%) |
| Intraocular pressure (mmHg) Median (25th, 75th percentile) | 16 (14, 18) | 16 (14, 18) | 16 (14, 18) | 16 (14, 18) |
| Currently on IOP lowering medicine for glaucoma or ocular hypertension, no. (%) | 5 (2%) | 6 (3%) | 4 (2%) | 2 (1%) |
| Lens status (clinical exam), no. (%) | ||||
| Phakic | 192 (66%) | 131 (70%) | 134 (71%) | 124 (67%) |
| AC IOL | 3 (1%) | 1 (1%) | 1 (1%) | 0 |
| PC IOL | 98 (33%) | 55 (29%) | 53 (28%) | 62 (33%) |
| Classification of DME (clinical exam), no. (%) | ||||
| Predominantly focal | 78 (27%) | 60 (32%) | 68 (36%) | 53 (28%) |
| Neither predominantly focal or diffuse | 71 (24%) | 46 (25%) | 41 (22%) | 48 (26%) |
| Predominantly diffuse | 144 (49%) | 81 (43%) | 79 (42%) | 85 (46%) |
| Visual acuity letter score (approximate Snellen equivalent) by randomization strata | ||||
| Median (25th, 75th percentile) | 65 (56, 73) | 66 (55, 72) | 66 (58, 72) | 66 (57, 72) |
| ≥66 (better than 20/50) | 146 (50%) | 95 (51%) | 95 (51%) | 93 (50%) |
| ≤65 (20/50 or worse) | 147 (50%) | 92 (49%) | 93 (49%) | 93 (50%) |
| Visual acuity letter score (approximate Snellen equivalent) | ||||
| 78-74 (20/32) | 61 (21%) | 34 (18%) | 32 (17%) | 38 (20%) |
| 73-69 (20/40) | 57 (19%) | 36 (19%) | 37 (20%) | 36 (19%) |
| 68-64 (20/50) | 41 (14%) | 37 (20%) | 36 (19%) | 31 (17%) |
| 63-59 (20/63) | 47 (16%) | 22 (12%) | 33 (18%) | 24 (13%) |
| 58-54 (20/80) | 33 (11%) | 20 (11%) | 13 (7%) | 19 (10%) |
| 53-49 (20/100) | 18 (6%) | 16 (9%) | 12 (6%) | 16 (9%) |
| 48-44 (20/125) | 16 (5%) | 6 (3%) | 10 (5%) | 6 (3%) |
| 43-39 (20/160) | 10 (3%) | 5 (3%) | 11 (6%) | 6 (3%) |
| ≤ 38 (≤ 20/200) | 10 (3%) | 11 (6%) | 4 (2%) | 10 (5%) |
| Central subfield thickness (microns) on OCT Median (25th, 75th percentile)ठ| 407 (309, 505) | 371 (302, 464) | 382 (298, 488) | 374 (298, 463) |
| Retinal volume (mm3) on OCT§ Median (25th,75th percentile) | 8.7 (7.8, 10.0) | 8.4 (7.5, 9.6) | 8.4 (7.4, 9.8) | 8.5 (7.8, 9.7) |
| OCT cystoid abnormality (questionable or definite), no. (%) | 274 (93%) | 171 (91%) | 174 (92%) | 177 (95%) |
| OCT subretinal fluid present (questionable or definite), no. (%) | 70 (24%) | 36 (20%) | 45 (25%) | 38 (21%) |
| ETDRS Retinopathy severity level (ETDRS description), no. (%)§ | ||||
| Level 10, 12 (diabetic retinopathy absent) | 5 (2%) | 4 (2%) | 3 (2%) | 1 (1%) |
| Level 14, 15, 20 (minimal NPDR) | 2 (1%) | 2 (1%) | 3 (2%) | 3 (2%) |
| Level 35, 43, 47 (mild to moderately severe NPDR) | 171 (59%) | 103 (55%) | 107 (57%) | 95 (51%) |
| Level 53 (severe NPDR) | 22 (8%) | 16 (9%) | 11 (6%) | 15 (8%) |
| Level 60 (scars of full or partial PRP present; abnormalities of PDR absent) | 38 (13%) | 30 (16%) | 30 (16%) | 29 (16%) |
| Level 61, 65 (mild to moderate PDR) | 33 (11%) | 24 (13%) | 22 (12%) | 34 (18%) |
| Level 71, 75 (high risk PDR) | 7 (2%) | 4 (2%) | 1 (1%) | 3 (2%) |
DME = diabetic macular edema; E-ETDRS© = electronic Early Treatment Diabetic Retinopathy Study; ETDRS = Early Treatment Diabetic Retinopathy Study; HbA1c = hemoglobin A1c; IOP = intraocular pressure; IVT = intravitreal triamcinolone; NPDR = non-proliferative diabetic retinopathy; OCT = optical coherence tomography; PDR = proliferative diabetic retinopathy; PRP = panretinal photocoagulation; VEGF = vascular endothelial growth factor.
Missing HbA1c data for 17, 3,7 and 8 study participants in the sham+prompt laser, ranibizumab + prompt laser, ranibizumab+deferred laser, and triamcinolone + prompt laser groups, respectively.
Medical history of condition.
One OCT central subfield thickness (CST) had an ineligible site OCT value (<250) and 60 had an ineligible OCT CST from reading center grading. All are included in this table.
Missing (or ungradeable) OCT and fundus photograph data as follows for the sham+prompt laser, ranibizumab + prompt laser, ranibizumab+deferred laser, and triamcinolone+prompt laser groups, respectively: central subfield (1 in the ranibizumab+deferred laser), retinal volume (73, 49, 42, 48), and retinopathy severity (5, 3, 9, 2).
Follow-Up
The follow-up status for all study participants (eyes) is shown in Figure 1 (available at http://aaojournal.org). Thirteen study participants (2%) died before the 1-year primary outcome visit and 15 participants died subsequently of causes apparently unrelated to study treatment. For the remaining study participants, the 1-year primary outcome visit was completed for 94% to 96% of eyes in the 4 treatment groups. Those who completed the 1-year primary outcome visit completed 94% of the non-annual visits before 1 year. Baseline visual acuity was similar in the 55 study eyes of the 44 study participants who did not complete the 1-year primary outcome visit compared with the 799 eyes of the 647 study participants who completed the 1-year primary outcome visit (data not shown). The 2-year visit was completed for 484 eyes (57%), with 267 (31%) still pending, as of February 8, 2010.
Figure 1.
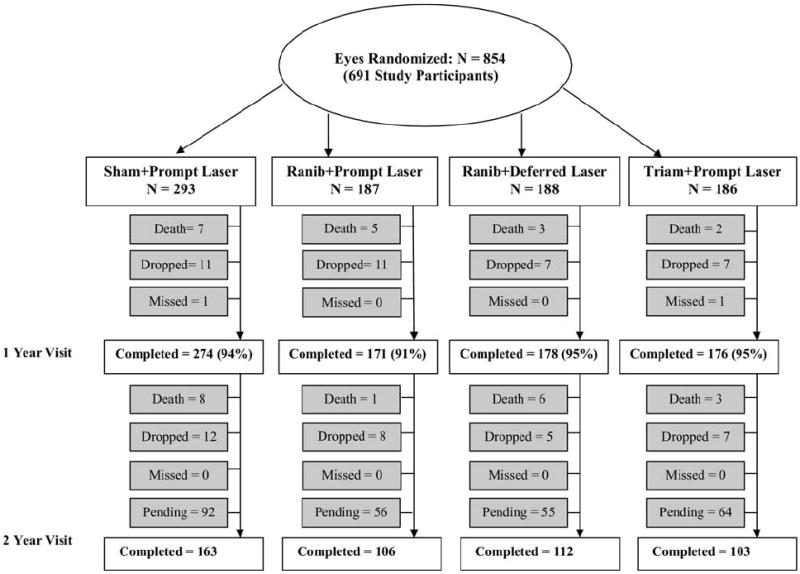
Completion of follow-up for study eyes. One-year completed visits include those that occurred between 308 and 420 days (between 44 and 60 weeks) from randomization. Two-year completed visits include those that occurred between 616 and 840 days (between 88 and 120 weeks) from randomization. Ranib = ranibizumab; Triam = triamcinolone.
Treatments
Sham Injections and Intravitreal Study Drug Injections
For each study participant, there were 13 possible sham or study drug injections during the first year of follow-up. The median (25th, 75th percentile) number of sham injections before the 1-year primary outcome visit was 11 (8, 13) in the sham + prompt laser group (of note, this excludes 56 eyes among 163 participants with 2 study eyes that were unmasked at baseline because the study participant’s other eye was in the ranibizumab + deferred laser group, precluding sham injections for the study eye assigned to sham + prompt laser). The median number of study drug injections before the 1-year primary outcome visit was 8 (6, 10) ranibizumab injections (of 13 maximally possible injections) in the ranibizumab + prompt laser group, 9 (6, 11) ranibizumab injections (of 13 maximally possible injections) in the ranibizumab + deferred laser group, and 5 (3, 7) sham injections (of 9 maximally possible sham injections) and 3 (2, 4) triamcinolone injections (of 4 maximally possible triamcinolone injections) for a total of 13 maximally possible sham plus triamcinolone injections in the triamcinolone + prompt laser group (Fig 2, available at http://aaojournal.org).
Figure 2.
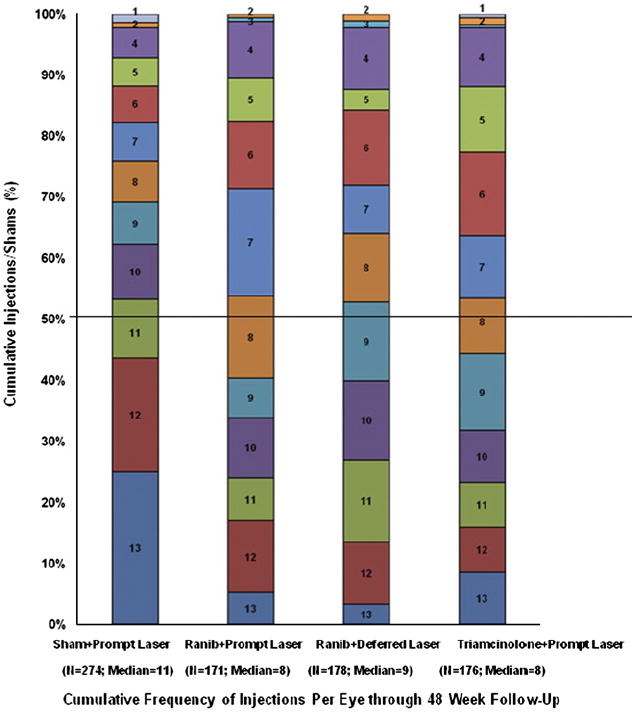
Cumulative distribution of injections/sham with randomized assigned treatment before the 52-week study visit. Includes eyes that completed the 52-week study visit; 56 eyes in sham group with other eye in the ranibizumab + deferred laser group are not included in figure because they were unmasked and a sham injection was not required per protocol. There were 13 possible sham or study drug injections. Study drug injections and sham injections included a baseline treatment and monthly retreatments through 12 weeks. After 16 weeks, eyes assigned to one of the ranibizumab groups could receive ranibizumab as often as every 4 weeks; eyes assigned to intravitreal triamcinolone could receive triamcinolone as often as every 16 weeks with sham injections as often as every 4 weeks in between triamcinolone injections; eyes assigned to sham + prompt laser could receive sham injections as often as every 4 weeks. Of 503 injections given in triamcinolone group before 1 year, 36% were triamcinolone injections. Ranib = ranibizumab.
Retreatments Relative to ‘Success’ and ‘Failure’ Criteria
At the 16-week study visit, 47 (25%) of the 187 eyes in the ranibizumab + prompt laser group and 41 (22%) of the 188 eyes in the ranibizumab + deferred laser group met ‘success’ criteria (visual acuity letter score ≥84 [~≥20/20] or OCT central subfield <250 μm) and did not receive an injection. A total of 17 eyes (9%) in the ranibizumab + prompt laser group and 15 eyes (8%) in the ranibizumab + deferred laser group met ‘success’ criteria at 16 weeks and did not receive an additional injection before the 1-year primary outcome visit. At the 1-year primary outcome visit, 89 (32%) of the eyes in the sham + prompt laser group, 109 (64%) of the eyes in the ranibizumab + prompt laser group, 92 (52%) of the eyes in the ranibizumab + deferred laser group, and 98 (56%) of the eyes in the triamcinolone + prompt laser group met the ‘success’ criteria, including 23 (8%), 23 (13%), 23 (13%), and 19 (11%), respectively, with a visual acuity letter score ≥84 (~≥20/20). ‘Failure’ criteria were met in 10 (4%), 3 (2%), 1 (1%), and 3 (2%) of the eyes in these 4 groups, respectively, during the first year of follow-up. Sham or study drug injections were not required for eyes meeting ‘success’ or ‘failure’ criteria.
Retreatments through Year 2
For the 218 study participants (58%) with 2 years of follow-up in the ranibizumab groups, there was a maximum of 25 possible ranibizumab injections. The median (25th, 75th percentile) number of ranibizumab injections between the 1-year visit, inclusive, and before the 2-year visit were 2 (0, 4) and 3 (1, 7) in the ranibizumab + prompt laser group and the ranibizumab + deferred laser group, respectively, for a total of 11 (7, 14) and 13 (8, 17) injections from baseline to the 2-year visit. Only 32% of participants in the ranibizumab + prompt laser group and 21% of participants in the ranibizumab + deferred laser group had no ranibizumab injections between the 1- and 2-year visits. The 103 study participants (55%) with 2 years of follow-up in the triamcinolone + prompt laser group received 1 (0, 2) triamcinolone injection between the 1-year visit, inclusive, and before the 2-year visit for a total of 4 (3, 5) from baseline to the 2-year visit of a total of 8 maximum possible injections.
Focal/Grid Laser Treatments
The distribution of laser treatments before the 1- and 2-year visits are shown in Table 3 (available at http://aaojournal.org). The median (25th, 75th percentile) number of focal/grid photocoagulation treatments before the 1-year primary outcome visit was 3 (2, 3) in the sham + prompt laser group, 2 (1, 3) in the ranibizumab + prompt laser group, and 2 (1, 3) in the triamcinolone + prompt laser group. In the ranibizumab + prompt laser group, after baseline and before the 1-year primary outcome visit, 53 (31%) study eyes received no additional focal/grid laser treatments, 54 (32%) received only 1 additional focal/grid laser treatment, 46 (27%) received only 2 additional focal/grid laser treatments, and 18 (11%) received 3 additional focal/grid laser treatments. Focal/grid laser treatment was not permitted in the ranibizumab + deferred laser group until the 24-week study visit; from the 24-week study visit and before the 1-year primary outcome visit, 128 (72%) of these study eyes received no focal/grid laser treatment, 35 (20%) received only 1 focal/grid laser treatment, and 15 (8%) received 2 focal/grid laser treatments. Forty-seven percent of the sham + prompt laser group, 57% of the ranibizumab + prompt laser group, 72% of the ranibizumab + deferred laser group, and 46% of the triamcinolone + prompt laser group received no focal/grid laser treatments between the 1- and 2-year visits.
Table 3.
Distribution of Focal/Grid Laser Treatments Received
| Sham + Prompt Laser | Ranibizumab + Prompt Laser | Ranibizumab + Deferred Laser∥ | Triamcinolone + Prompt Laser | |
|---|---|---|---|---|
| Number of laser treatments received prior to the 1 year visit, no. (%)* | N = 274 | N = 171 | N = 178 | N = 176 |
| 0 | 1 (<1%)† | 0 | 124 (70%) | 1 (1%)‡ |
| 1 | 35 (13%) | 53 (31%) | 36 (20%) | 46 (26%) |
| 2 | 75 (27%) | 54 (32%) | 17 (10%) | 53 (30%) |
| 3 | 107 (39%) | 46 (27%) | 1 (1%) | 49 (28%) |
| 4 | 56 (20%) | 18 (11%) | 0 | 27 (15%) |
| Proportion of eyes receiving laser at 48 week visit, no. (%)* | 242 (26%) | 155 (16%) | 160 (8%) | 154 (21%) |
| Number of laser treatments received prior to the 2 year visit, no. (%)§ | N = 163 | N = 106 | N = 112 | N = 103 |
| 0 | 1 (1%) | 0 | 65 (58%) | 0 |
| 1 | 14 (9%) | 21 (20%) | 21 (19%) | 16 (16%) |
| 2 | 28 (17%) | 24 (23%) | 7 (6%) | 23 (22%) |
| 3 | 38 (23%) | 23 (22%) | 11 (10%) | 28 (27%) |
| 4 | 29 (18%) | 22 (21%) | 8 (7%) | 13 (13%) |
| 5 | 25 (15%) | 9 (8%) | 0 | 11 (11%) |
| 6 | 13 (8%) | 5 (5%) | 0 | 7 (7%) |
| 7 | 15 (9%) | 2 (2%) | 0 | 5 (5%) |
Includes study participants completing the 1-year (52 week) visit.
One eye did not receive laser until post 1-year due to an adverse event unrelated to study treatment.
One eye did not receive laser until after 1-year due to missing 2 consecutive visits at the initial time of required laser treatment.
Includes study participants completing the 2-year visit.
Three eyes deviated from the protocol and received laser prior to 24 weeks (2 were given laser at the 1 week safety visit and 1 at the 20 week visit).
Alternative Treatments
Some eyes in the study were switched from the randomly assigned treatment to an alternative treatment during the first 2 years of follow-up because “failure” or “futility” criteria were met or the treating investigator determined deviating from the protocol would be in the best interest of the study participant as a patient. In the sham + prompt laser group, this occurred in 14 eyes during the first year and in 29 eyes during the second year. Of these eyes, 5 and 20, respectively, met the “failure” or “futility” criteria before receiving alternative treatment. In the ranibizumab + prompt laser group, 1 eye that met “failure” criteria received alternative treatment during the first year and 1 eye that met “failure” criteria received alternative treatment during the second year. There were no eyes in the ranibizumab + deferred laser group that received alternative treatment during the first or second year of follow-up. In the triamcinolone + prompt laser group, 1 and 3 eyes received alternative treatment during the first and second years, respectively. One of the 3 eyes in the second year of follow-up did not meet “failure” or “futility” criteria (Table 4, available at http://aaojournal.org, lists the alternative treatments received).
Table 4.
Alternative Treatments Received for Diabetic Macular Edema
| Sham + Prompt Laser N = 293 | Ranibizumab + Prompt Laser N = 187 | Ranibizumab + Deferred Laser N = 188 | Triamcinolone + Prompt Laser N = 186 | |
|---|---|---|---|---|
| Prior to the 1 year visit | ||||
| Eyes with alternative treatments (number of treatments applied) | 14 (25) | 1 (1) | 0 | 1 (1) |
| Per protocol, no.* | 5 | 1 | 0 | 1 |
| Deviations from protocol, no. | 9 | 0 | 0 | 0 |
| Alternative treatments, no.† | ||||
| Intravitreal Bevacizumab | 3 | 0 | 0 | 1 |
| Intravitreal Triamcinolone Acetonide | 5 | 1 | 0 | 0 |
| Vitrectomy | 2 | 0 | 0 | 0 |
| Intravitreal Bevacizumab + Intravitreal Triamcinolone Acetonide | 4 | 0 | 0 | 0 |
| 1 year through prior to the 2 year visit | ||||
| Eyes with alternative treatments (number of treatments) | 29 (55) | 1 (1) | 0 | 3 (4) |
| Per protocol, no.* | 20 | 1 | 0 | 2 |
| Deviations from protocol, no. | 9 | 0 | 0 | 1 |
| Alternative Treatments, no.† | ||||
| Intravitreal Bevacizumab | 9 | 0 | 0 | 0 |
| Intravitreal Ranibizumab | 2 | 0 | 0 | 0 |
| Intravitreal Triamcinolone Acetonide | 12 | 1 | 0 | 2‡ |
| Vitrectomy | 2 | 0 | 0 | 0 |
| Vitrectomy + Intravitreal Triamcinolone Acetonide | 0 | 0 | 0 | 1 |
| Intravitreal Bevacizumab + Intravitreal Ranibizumab | 1 | 0 | 0 | 0 |
| Intravitreal Bevacizumab + Intravitreal Triamcinolone Acetonide | 2 | 0 | 0 | 0 |
| Intravitreal Bevacizumab + Intravitreal Ranibizumab + Intravitreal Triamcinolone Acetonide | 1 | 0 | 0 | 0 |
Per protocol if met failure. Failure is defined as: Visual acuity 10 or more letters worse than baseline, optical coherence tomography central subfield thickness ≥250 microns, diabetic macular edema present on clinical exam that is the cause of the visual loss, complete laser given AND ≥13 weeks since last laser treatment with no improvement since the last laser treatment
Number of eyes, each combination of treatment only counted once
Non-study drug was given (intravitreal Kenalog)
Injection Treatment Compliance
Before the 1-year primary outcome visit, when a sham injection was required per protocol at each visit, the sham + prompt laser group was given 96% (1288) of the required sham injections. Required study drug injection rates in the 3 active treatment groups were 95% (462 injections), 97% (525 injections), and 97% (673 injections) in the ranibizumab + prompt laser group, ranibizumab + deferred laser group, and triamcinolone + prompt laser group, respectively.
Success with Masking of Sham Injections
At the 1-year primary outcome visit, study participants were asked to guess their treatment group assignment. Among the 430 study participants with 1 study eye who completed the masking questionnaire and had received only the randomized treatment, the correct assignment was stated by 10% of the sham + prompt laser group, 88% of the ranibizumab + prompt laser group, 90% of the ranibizumab + deferred laser group, and 44% of the triamcinolone + prompt laser group. Among the 117 study participants with 2 study eyes who completed the unmasking questionnaire and had received only the randomized treatment, the correct assignment was stated for both eyes by 28% in ranibizumab + prompt laser group, 23% of the ranibizumab + deferred laser group, and 3% of the triamcinolone + prompt laser group.
Effect of Treatment on Visual Acuity
As shown in Table 5, for the 1-year primary outcome, the mean change ± standard deviation in the visual acuity letter score from baseline was significantly greater in the ranibizumab + prompt laser group (+9±11, P<0.001) and ranibizumab + deferred laser group (+9±12, P<0.001) but not in the triamcinolone + prompt laser group (+4 ±13, P=0.31) compared with the sham + prompt laser group (+3±13). The results (Table 5) reflected both a greater proportion of eyes with a substantial improvement of ≥10 letters (50% and 47%) and ≥15 letters (30% and 28%) and a lower proportion of eyes with a substantial worsening of ≥10 letters (4% and 3%) and ≥15 letters (2% and 2%) in the 2 ranibizumab groups compared with the sham + prompt laser group (28% and 15% for ≥10 and ≥15 letter gain, respectively, and 13% and 8% for ≥10 and ≥15 letter loss, respectively). Outcomes at 2 years (Table 6, available at http://aaojournal.org) generally mirrored the 1-year primary outcome results. The distribution of the visual acuity letter score at the 1- and 2-year visits is shown in Table 7 (available at http://aaojournal.org).
Table 5.
Change in Visual Acuity (Last Observation Carried Forward) from Baseline to 1 Year (Primary Outcome)*
| Sham + Prompt Laser N=293 | Ranibizumab + Prompt Laser N=187 | Ranibizumab + Deferred Laser N=188 | Triamcinolone + Prompt Laser N=186 | |
|---|---|---|---|---|
| Change in visual acuity (letters) | ||||
| Mean ± SD | +3±13 | +9±11 | +9±12 | +4±13 |
| Median (25th, 75th percentile) | +5 (−2, +10) | +10 (+3, +16) | +9 (+5, +15) | +5 (−3, +12) |
| Difference in mean change from sham + prompt laser (95% CI) [P value]† | +5.8 (+3.2 to +8.5) [P <0.001] | +6.0 (+3.4 to +8.6) [P<0.001] | +1.1 (−1.5 to +3.7) [P=0.31] | |
| Distribution of change, No. (%) | ||||
| ≥15 letter improvement | 43 (15%) | 57 (30%) | 52 (28%) | 39 (21%) |
| 14–10 letter improvement | 38 (13%) | 38 (20%) | 36 (19%) | 22 (12%) |
| 9–5 letter improvement | 67 (23%) | 34 (18%) | 54 (29%) | 32 (17%) |
| Same ± 4 letters | 86 (29%) | 38 (20%) | 35 (19%) | 54 (29%) |
| 5–9 letters worse | 20 (7%) | 14 (7%) | 5 (3%) | 12 (6%) |
| 10–14 letters worse | 16 (5%) | 3 (2%) | 2 (1%) | 12 (6%) |
| ≥15 letters worse | 23 (8%) | 3 (2%) | 4 (2%) | 15 (8%) |
| Difference in proportion with ≥10 letter improvement from sham + prompt laser (95% CI)‡ | +23% (+13% to +34%) | +19% (+9% to +29%) | +6% (−4% to +16%) | |
| Relative risk (95% CI) [P value]§ for comparison with sham + prompt laser | 1.0 | 1.84 (1.40 to 2.42) [P<0.001] | 1.68 (1.27 to 2.21) [P<0.001] | 1.21 (0.88 to 1.66) [P=0.16] |
| Difference in proportion with ≥10 letter worsening from sham + prompt laser (95% CI)‡ | −10% (−16% to −5%) | −10% (−16% to −4%) | +1% (−7% to +9%) | |
| Relative risk (95% CI) [P value]‡ for comparison with sham + prompt laser | 1.0 | 0.24 (0.09 to 0.65) [P<0.001] | 0.24 (0.08 to 0.68) [P=0.001] | 1.08 (0.62 to 1.87) [P=0.75] |
| Difference in proportion with ≥15 letter improvement from sham + prompt laser (95% CI)‡ | +16% (+6% to +26%) | +13% (+4% to +22%) | +6% (−2% to +15%) | |
| Relative risk (95% CI) [P value]§ for comparison with sham + prompt laser | 1.0 | 2.09 (1.35 to 3.22) [P<0.001] | 1.89 (1.25 to 2.87) [P<0.001] | 1.43 (0.90 to 2.29) [P=0.07] |
| Difference in proportion with ≥15 letter worsening from sham + prompt laser (95% CI)‡ | −6% (−11% to −2%) | −6% (−10% to −1%) | 0 (−6% to +6%) | |
| Relative risk (95% CI) [P value]§ for comparison with sham + prompt laser | 1.0 | 0.21 (0.05 to 0.87) [P=0.009] | 0.28 (0.08 to 0.97) [P=0.01] | 1.02 (0.47 to 2.20) [P=0.95] |
CI = confidence interval; SD = standard deviation.
Visits occurring between 308 and 420 days (between 44 and 60 wks) from randomization were included as 1-yr visits. When > 1 visit occurred in this window, data from the visit closest to the 1-yr target date were used. For other eyes without any 1-yr data (19 eyes in the sham + prompt laser group, 16 eyes in the ranibizumab + prompt laser group, 10 eyes in the ranibizumab + deferred laser group, and 10 eyes in the triamcinolone + prompt laser group), the last observation carried forward method was used to impute data for the primary analysis.
Analysis of covariance adjusted for baseline visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Adjusted for correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logistic regression adjusted for correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Table 6.
Change in Visual Acuity from Baseline to 2 Years*
| Change in visual acuity (letters)† | Sham + Prompt Laser N = 163 | Ranibizumab + Prompt Laser N = 106 | Ranibizumab + Deferred Laser N = 112 | Triamcinolone + Prompt Laser N = 103 |
|---|---|---|---|---|
| Overall change | ||||
| Mean±SD | +2±16 | +7±13 | +10±15 | 0±21 |
| Median (25th, 75th percentile) | +5 (−2, +11) | +8 (+2, +15) | +10 (+4, +17) | +6 (−5, +13) |
| Difference in mean change from sham+prompt laser (95% CI) [P Value]‡ | +5.0 (0.1, +9.9) [P = 0.01] | +7.2 (+2.4, +12.0) [P < 0.001] | −1.6 (−6.6, +3.3) [P < 0.001] | |
| Distribution of change, no. (%) | ||||
| ≥15 letter improvement | 28 (17%) | 28 (26%) | 33 (29%) | 20 (19%) |
| 14-10 letter improvement | 22 (13%) | 18 (17%) | 24 (21%) | 21 (20%) |
| 9-5 letter improvement | 32 (20%) | 25 (24%) | 23 (21%) | 13 (13%) |
| Same ±4 letters | 46 (28%) | 25 (24%) | 25 (22%) | 22 (21%) |
| 5-9 letters worse | 13 (8%) | 3 (3%) | 3 (3%) | 5 (5%) |
| 10-14 letters worse | 3 (2%) | 4 (4%) | 1 (1%) | 6 (6%) |
| ≥15 letters worse | 19 (12%) | 3 (3%) | 3 (3%) | 16 (16%) |
| Difference in proportion with ≥10 letter improvement from sham+prompt laser (95% CI)§ | +13% (−2%, +27%) | +20% (+6%, +34%) | +9% (−5%, +23%) | |
| Relative risk (95% CI) [P Value]∥ for comparison with sham+laser | 1.0 | 1.41 (0.96, 2.07) [P = 0.03] | 1.65 (1.16, 2.36) [P<0.001] | 1.30 (0.88, 1.92) [P = 0.11] |
| Difference in proportion with ≥10 letter worsening from sham+prompt laser (95% CI)§ | −7% (−16%, +2%) | −10% (−18%, −2%) | +8% (−4%, +19%) | |
| Relative risk (95% CI) [P Value]∥ for comparison with sham+prompt laser | 1.0 | 0.49 (0.18, 1.33) [P = 0.09] | 0.26 (0.07, 0.95) [P = 0.01] | 1.58 (0.83, 3.02) [P = 0.09] |
CI = confidence interval; SD = standard deviation.
Visits occurring between 616 and 840 days (between 88 and 120 weeks) from randomization were included as 2-year visits. When more than 1 visit occurred in this window, data from the visit closest to the 2-year target date were used.
Among the 432 eyes with 2 year follow up, the 2-year results were similar to the 1-year results of the entire cohort of 854 eyes (data not shown)
Analysis of covariance adjusted for baseline visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Adjusted for correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logistic regression adjusted for correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Table 7.
Distribution of Visual Acuity at 1 and 2 Years
| Change in visual acuity letter score (approximate Snellen equivalent) | Sham + Prompt Laser N = 293 | Ranibizumab + Prompt Laser N = 187 | Ranibizumab + Deferred Laser N = 188 | Triamcinolone + Prompt Laser N = 186 |
|---|---|---|---|---|
| Baseline visual acuity letter score | ||||
| Median (25th, 75th percentile) | 65 (56, 73) | 66 (55, 72) | 66 (58, 72) | 66 (57, 72) |
| Visual acuity (LOCF) letter score (approximate Snellen equivalent) at the 1 year visit* | ||||
| Median (25th and 75th percentile) | 69 (59, 77) | 75 (66, 81) | 75 (66, 81) | 70 (58, 77) |
| ≥79 (≥20/25) | 59 (20%) | 64 (34%) | 65 (35%) | 40 (22%) |
| 78-69 (20/32 to 20/40) | 89 (30%) | 70 (37%) | 66 (35%) | 55 (30%) |
| 68-59 (20/50 to 20/63) | 73 (25%) | 27 (14%) | 34 (18%) | 43 (23%) |
| 58-49 (20/80 to 20/100) | 31 (11%) | 11 (6%) | 16 (9%) | 28 (15%) |
| 48-39 (20/125 to 20/160) | 24 (8%) | 8 (4%) | 4 (2%) | 14 (8%) |
| ≤38 (≤20/200) | 17 (6%) | 7 (4%) | 3 (2%) | 6 (3%) |
| Visual acuity letter score (approximate Snellen equivalent) at the 2 year visit† | N = 163 | N = 106 | N = 112 | N = 103 |
| Median (25th and 75th percentile) | 71 (59, 77) | 75 (62, 81) | 75 (65, 80) | 71 (54, 79) |
| ≥79 (≥20/25) | 34 (21%) | 39 (37%) | 36 (32%) | 30 (29%) |
| 78-69 (20/32 to 20/40) | 59 (36%) | 33 (31%) | 42 (38%) | 26 (25%) |
| 68-59 (20/50 to 20/63) | 30 (18%) | 12 (11%) | 22 (20%) | 13 (13%) |
| 58-49 (20/80 to 20/100) | 17 (10%) | 8 (8%) | 7 (6%) | 19 (18%) |
| 48-39 (20/125 to 20/160) | 7 (4%) | 11 (10%) | 1 (1%) | 5 (5%) |
| ≤38 (≤20/200) | 16 (10%) | 3 (3%) | 4 (4%) | 10 (10%) |
LOCF = last observation carried forward.
Visits occurring between 308 and 420 days (between 44 and 60 weeks) from randomization were included as 1-year visits. When more than 1 visit occurred in this window, data from the visit closest to the 1-year target date were used. For other eyes without any 1-year data (19 eyes in the sham+prompt laser group, 16 eyes in the ranibizumab+prompt laser group, 10 eyes in the ranibizumab+deferred laser group, and 10 eyes in the triamcinolone+prompt laser group) the last observation carried forward method was used to impute data for the primary analysis.
Visits occurring between 616 and 840 days (between 88 and 120 weeks) from randomization were included as 2-year visits. When more than 1 visit occurred in this window, data from the visit closest to the 2-year target date were used.
Most of the overall improvement in mean visual acuity (Fig 3) and proportion with ≥10 letter improvement from baseline (Fig 4A) within the ranibizumab-treated groups occurred by the 8-week study visit, with continued improvement through the 1-year primary outcome visit and stabilization thereafter. In contrast, the triamcinolone + prompt laser group showed a more complex picture with improvement in the change in mean visual acuity through the 24-week visit, with decline thereafter (Fig 3), whereas the proportion with ≥10 letter improvement gradually increased through 24 weeks, then decreased to 68 weeks and gradually increased again (Fig 4A). The sham + prompt laser group showed gradual improvement in these outcomes during the first year with stabilization thereafter. Few eyes deteriorated by ≥10 letters from baseline in the ranibizumab groups, whereas the proportion with this outcome in the triamcinolone + prompt laser group and sham + prompt laser group gradually increased throughout at least the first year (Fig 4B).
Figure 3.
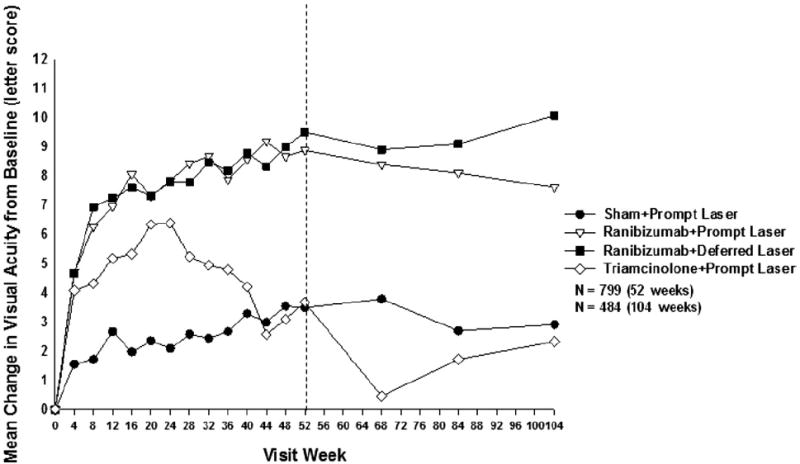
Mean change in visual acuity at follow-up visits. Values that were ±30 letters were assigned a value of 30. P values for difference in mean change in visual acuity from sham + prompt laser at 52 weeks: ranibizumab + prompt laser <0.001, ranibizumab + deferred laser <0.001, and triamcinolone + prompt laser groups = 0.31. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 44 and 60 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization.
Figure 4.
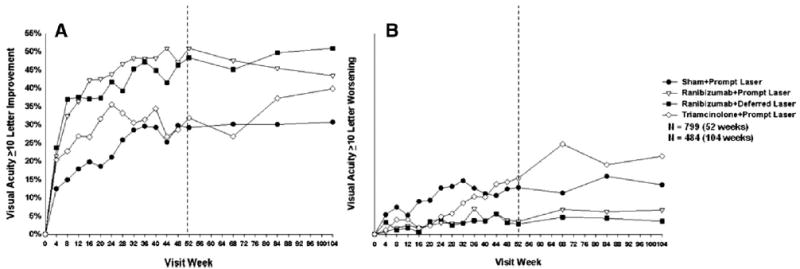
A, Ten letter or greater improvement in visual acuity at follow-up visits. P values for difference in proportion of ≥10 letter improvement in visual acuity from sham + prompt laser at the 52-week visit: ranibizumab + prompt laser <0.001, ranibizumab + deferred laser <0.001, and triamcinolone + prompt laser = 0.16. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 44 and 60 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization. B, Ten letter or greater loss in visual acuity at follow-up visits. P values for difference in proportion of 10 letter loss in visual acuity from sham + prompt laser at the 52-week visit: ranibizumab + prompt laser <0.001, ranibizumab + deferred laser <0.001, and triamcinolone + prompt laser = 0.75. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 44 and 60 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization.
By limiting the analysis to the 273 eyes that were pseudophakic at baseline, results appeared similar to the overall results for the sham + prompt laser and the 2 ranibizumab groups at 1 and 2 years. However, for the 62 pseudophakic eyes at baseline in the triamcinolone + prompt laser group, visual acuity results were substantially better than for phakic eyes such that the degree of improvement appeared comparable to that of the pseudophakic eyes in the ranibizumab groups and superior to that of the pseudophakic eyes in the sham + prompt laser group at 1 year (Table 8) and 2 years (Fig 5, available at http://aaojournal.org).
Table 8.
Change in Visual Acuity (Last Observation Carried Forward) from Baseline to 1 Year* among Baseline Subgroups
| Baseline Subgroup | Sham + Prompt Laser, Ranib + Prompt Laser, Ranib + Deferred Laser, Triam + Prompt Laser, N | Change in Visual Acuity Mean ± SD |
≥10 Letter Improvement |
≥10 Letter Worsening |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham + Prompt Laser | Ranib + Prompt Laser | Ranib + Deferred Laser | Triam + Prompt Laser | Sham + Prompt Laser | Ranib + Prompt Laser | Ranib + Deferred Laser | Triam + Prompt Laser | Sham + Prompt Laser | Ranib + Prompt Laser | Ranib + Deferred Laser | Triam + Prompt Laser | ||
| Pseudophakic at baseline | |||||||||||||
| No | 192, 131, 134, 124 | +2±13 | +9±10 | +10±14 | +2±14 | 27% | 54% | 54% | 26% | 15% | 2% | 3% | 20% |
| Yes | 101, 56, 54, 62 | +4±14 | +8±12 | +7±9 | +8±9 | 30% | 43% | 30% | 47% | 10% | 5% | 4% | 3% |
| Prior treatment for DME | |||||||||||||
| No | 105, 74, 74, 61 | +2±14 | +9±12 | +11±13 | +3±13 | 26% | 55% | 54% | 28% | 16% | 4% | 1% | 21% |
| Yes | 188, 113, 114, 125 | +3±13 | +9±10 | +8±12 | +5±13 | 29% | 48% | 42% | 35% | 12% | 3% | 4% | 11% |
| VA letter score (approximate Snellen equivalent) | |||||||||||||
| ≥66 (>20/50) | 146, 95, 95, 93 | +1±12 | +6±10 | +5±13 | +1±11 | 16% | 38% | 32% | 18% | 14% | 4% | 5% | 18% |
| ≤65 (≤20/50) | 147, 92, 93, 93 | +5±14 | +12±11 | +13±10 | +7±14 | 39% | 64% | 62% | 47% | 13% | 2% | 1% | 11% |
| OCT central subfield thickness | |||||||||||||
| <400 μm | 142, 111, 105, 114 | +3±11 | +7±11 | +7±12 | +3±12 | 23% | 43% | 41% | 25% | 13% | 4% | 4% | 14% |
| ≥400 μm | 151, 76, 82, 72 | +3±15 | +11±10 | +11±13 | +6±14 | 32% | 62% | 54% | 44% | 14% | 3% | 2% | 15% |
| Diabetic retinopathy severity | |||||||||||||
| Moderately severe NPDR or better | 178, 109, 113, 99 | 3±13 | 10±11 | 9±12 | 3±14 | 26% | 50% | 46% | 32% | 12% | 2% | 3% | 17% |
| Severe NPDR or worse | 100, 74, 64, 81 | 2±15 | 8±10 | 9±13 | 5±12 | 29% | 51% | 47% | 33% | 16% | 5% | 2% | 12% |
| Diffuse vs. focal edema as characterized by investigator† | |||||||||||||
| Typical/predominantly focal | 78, 60, 68, 53 | +3±13 | +8±11 | +8±13 | +3±11 | 27% | 53% | 43% | 26% | 10% | 2% | 4% | 15% |
| Neither predominantly focal nor diffuse | 71, 46, 41, 48 | +2±14 | +10±9 | +8±15 | +3±13 | 23% | 48% | 56% | 25% | 15% | 0 | 2% | 17% |
| Typical/predominantly diffuse | 144, 81, 79, 85 | +3±13 | +9±12 | +10±10 | +5±14 | 31% | 51% | 46% | 41% | 14% | 6% | 3% | 13% |
DME = diabetic macular edema; NPDR = non-proliferative diabetic retinopathy; OCT = optical coherence tomography; Ranib = ranibizumab; SD = standard deviation; Triam = triamcinolone; VA = visual acuity.
Visits occurring between 308 and 420 days (between 44 and 60 wks) from randomization were included as 1-yr visits. When > 1 visit occurred in this window, data from the visit closest to the 1-yr target date were used. For other eyes without any 1-yr data (19 eyes in the sham _ prompt laser group, 16 eyes in the ranibizumab + prompt laser group, 10 eyes in the ranibizumab + deferred laser group, and 10 eyes in the triamcinolone + prompt laser group), the last observation carried forward method was used to impute data for the primary analysis.
Question asked: If diabetic macular edema is present, indicate how you would characterize its type, focal vs. diffuse, in your own daily practice. You are free to use, or not use, OCT, fluorescein angiography, or fundus photographs in addition to your clinical examination.
Figure 5.
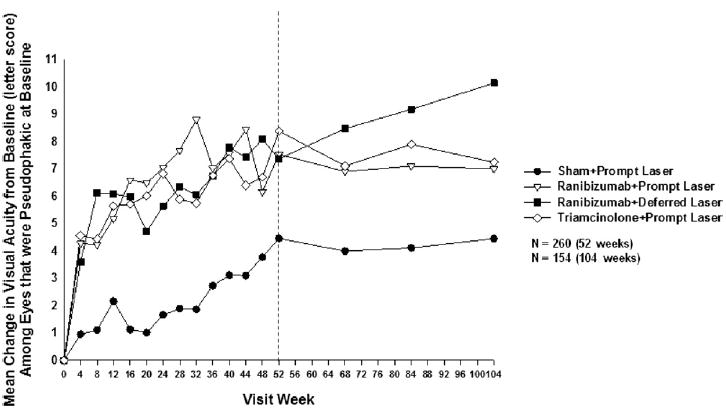
Mean change in visual acuity at follow-up visits among eyes that were pseudophakic at baseline. Values of ±30 or more letters were assigned a value of 30. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 44 and 60 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization.
There was no obvious clinically important difference in results at the 1-year primary outcome visit for any other of the following subgroups: prior treatment for DME, baseline visual acuity, baseline OCT-measured central subfield thickening, baseline level of diabetic retinopathy determined by grading of fundus photographs, or description of edema by the treating ophthalmologist as predominantly focal or predominantly diffuse (Table 8). One-year primary outcome results were similar to the overall results when limited to study participants with 2 study eyes (Table 9, available at http://aaojournal.org) and when excluding eyes from any clinical site with a baseline central subfield thickness <250 μm (Table 10, available at http://aaojournal.org).
Table 9.
Change in Visual Acuity (Last Observation Carried Forward) from Baseline to 1 Year* among Study Participants with 2 Study Eyes
| Change in visual acuity (letters) | Sham + Prompt Laser N = 163 | Ranibizumab + Prompt Laser N = 56 | Ranibizumab + Deferred Laser N = 56 | Triamcinolone + Prompt Laser N = 51 |
|---|---|---|---|---|
| Change from baseline | ||||
| Mean±SD | +2±13 | +11±8 | +7±17 | +4±14 |
| Difference in mean change from sham+prompt laser (95% CI)† | +7.1 (+3.4, +10.8) | +4.7 (−1.0, +10.3) | +2.8 (−1.9, +7.5) | |
| Median (25th, 75th percentile) | +4 (−3, +9) | +11 (+5, +16) | +7 (+3, +15) | +4 (−4, +12) |
| Distribution of change, no. (%) | ||||
| ≥15 letter improvement | 21 (13%) | 17 (30%) | 14 (25%) | 12 (24%) |
| 14-10 letter improvement | 19 (12%) | 16 (29%) | 7 (13%) | 3 (6%) |
| 9-5 letter improvement | 38 (23%) | 11 (20%) | 18 (32%) | 9 (18%) |
| Same ±4 letters | 47 (29%) | 9 (16%) | 14 (25%) | 15 (29%) |
| 5-9 letters worse | 12 (7%) | 2 (4%) | 0 | 6 (12%) |
| 10-14 letters worse | 9 (6%) | 1 (2%) | 1 (2%) | 1 (2%) |
| ≥15 letters worse | 17 (10%) | 0 | 2 (4%) | 5 (10%) |
CI = confidence interval; SD = standard deviation.
Visits occurring between 308 and 420 days (between 44 and 60 weeks) from randomization were included as 1-year visits. When more than 1 visit occurred in this window, data from the visit closest to the 1-year target date were used. For other eyes without any 1-year data (11 eyes in the sham+prompt laser group, 4 eyes in the ranibizumab+prompt laser group, 2 eyes in the ranibizumab+deferred laser group, and 5 eyes in the triamcinolne+prompt laser group) the last observation carried forward method was used to impute data for the primary analysis.
Analysis of covariance adjusted for baseline visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Table 10.
Change in Visual Acuity (Last Observation Carried Forward) from Baseline to 1 Year* Excluding Eyes with Baseline Optical Coherence Tomography Central Subfield Thickness <250 microns
| Sham + Prompt Laser N = 275 | Ranibizumab + Prompt Laser N = 175 | Ranibizumab + Deferred Laser N = 172 | Triamcinolone + Prompt Laser N = 171 | |
|---|---|---|---|---|
| Change in visual acuity (letters) | ||||
| Mean±SD | +3±13 | +9±11 | +9±11 | +4±13 |
| Median (25th, 75th percentile) | +4(−2, +10) | +10 (+4, +16) | +9(+5, +15) | +4(−3, +12) |
| Difference in mean change from sham+prompt laser (95% CI) [PValue]† | +6.3 (+3.6, +8.9) [P<0.001] | +6.8 (+4.1, +9.4) [P<0.001] | +1.1 (−1.6, +3.8) [P = 0.33] | |
| Distribution of change, no. (%) | ||||
| ≥15 letter improvement | 39 (14%) | 54 (31%) | 48 (28%) | 34 (20%) |
| 14-10 letter improvement | 36 (13%) | 37 (21%) | 33 (19%) | 19 (11%) |
| 9-5 letter improvement | 62 (23%) | 32 (18%) | 50 (29%) | 31 (18%) |
| Same ±4 letters | 81 (29%) | 34 (19%) | 32 (19%) | 51 (30%) |
| 5-9 letters worse | 19 (7%) | 13 (7%) | 5 (3%) | 10 (6%) |
| 10-14 letters worse | 15 (5%) | 3 (2%) | 2 (1%) | 12 (7%) |
| ≥15 letters worse | 23 (8%) | 2 (1%) | 2 (1%) | 14 (8%) |
| Difference in proportion with ≥10 letter improvement from sham+prompt laser (95% CI)‡ | +25% (+14%, +36%) | +20% (+9%, +30%) | +5% (−6%, +15%) | |
| Relative risk (95% CI) [P Value]§ for comparison with sham+prompt laser | 1.0 | 1.91 (1.44, 2.53) [P<0.001] | 1.73 (1.29, 2.30) [P<0.001] | 1.16 (0.83, 1.64) [P = 0.29] |
| Difference in proportion with ≥10 letter worsening from sham+prompt laser (95% CI)‡ | −11% (−17%, −5%) | −11% (−17%, −6%) | +1% (−7%, +9%) | |
| Relative risk (95% CI) [P Value]§ for comparison with sham+prompt laser | 1.0 | 0.20 (0.07, 0.61) [P<0.001] | 0.17 (0.05. 0.59) [P<0.001] | 1.08 (0.62, 1.91) [P = 0.74] |
| Difference in proportion with ≥15 letter improvement from sham+prompt laser (95% CI)‡ | +17% (+7%, +27%) | +14% (+5%, +23%) | +6% (−3%, +15%) | |
| Relative risk (95% CI) [P Value]§ for comparison with sham+prompt laser | 1.0 | 2.18 (1.39, 3.41) [P<0.001] | 1.97 (1.27, 3.07) [P<0.001] | 1.40 (0.85, 2.32) [P = 0.10] |
| Difference in proportion with ≥ 15 letter worsening from sham+prompt laser (95% CI)‡ | −7% (−12%, −3%) | −7% (−12%, −3%) | −0.2% (−7%, +6%) | |
| Relative risk (95% CI) [P Value]§ for comparison with sham+prompt laser | 1.0 | 0.14 (0.03, 0.77) [P = 0.006] | 0.14 (0.03, 0.78) [P = 0.006] | 0.97 (0.44, 2.13) [P = 0.93] |
CI = confidence interval; SD = standard deviation.
Adjusted for correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Visits occurring between 308 and 420 days (between 44 and 60 weeks) from randomization were included as 1-year visits. When more than 1 visit occurred in this window, data from the visit closest to the 1-year target date were used. For other eyes without any 1-year data (15 eyes in the sham+prompt laser group, 14 eyes in the ranibizumab+prompt laser group, 8 eyes in the ranibizumab+deferred laser group, and 8 eyes in the triamcinolone+prompt laser group) the last observation carried forward method was used to impute data for the primary analysis.
Analysis of covariance adjusted for baseline visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logistic regression adjusted for correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Effect of Treatment on Retinal Thickening
At the 1-year primary outcome visit, OCT results (Table 11; Fig 6; Figs 7 and 8, available at http://aaojournal.org) in the sham + prompt laser and the ranibizumab groups generally paralleled the overall visual acuity results, favoring the ranibizumab groups. In the triamcinolone + prompt laser group the reduction in mean central subfield thickness was greater than in the sham + prompt laser group and comparable with the ranibizumab groups. The pattern of OCT results were similar regardless of whether baseline central subfield thickness was <400 μm or ≥400 μm (Table 11).
Table 11.
Change in Retinal Thickness from Baseline to 1 Year*
| Change in OCT Central Subfield Thickness | Sham + Prompt Laser N=271 | Ranibizumab + Prompt Laser N=171 | Ranibizumab + Deferred Laser N=175 | Triamcinolone + Prompt Laser N=173 |
|---|---|---|---|---|
| Overall Change† | ||||
| Thickness (μm) Median (25th, 75th percentile) | 307 (234, 393) | 241 (209, 291) | 256 (206, 311) | 247 (206, 305) |
| Change from baseline (μm) Mean ±SD | −102±151 | −131±129 | −137±136 | −127±140 |
| Change from baseline (μm) Median (25th, 75th percentile) | −79 (−191, −7) | −112 (−210, −44) | −111 (−203, −35) | −90 (−219, −36) |
| Difference in mean change from sham + prompt laser (95% CI) [P value]‡ | −55 −78 to −32) [P<0.001] | −49 (−72 to −26) [P<0.001] | −52 (−75 to −29) [P<0.001] | |
| Thickness < 250 with at least a 25 μm decrease from baseline, No. (%) | 72 (27%) | 91 (53%) | 74 (42%) | 82 (47%) |
| Relative risk (95% CI) [P value]§ for comparison with sham + prompt laser | 1.0 | 2.00 (1.52 to 2.64) [P<0.001] | 1.55 (1.13 to 2.13) [P=0.001] | 1.76 (1.31 to 2.36) [P<0.001] |
| LogOCT, No. (%)∥ | ||||
| ≥2 step improvement | 81 (30%) | 72 (42%) | 71 (41%) | 65 (38%) |
| ≥2 step worsening | 6 (2%) | 1 (1%) | 0 | 4 (2%) |
| Baseline thickness < 400 μm | N=127 | N=100 | N=97 | N=104 |
| Thickness (μm) Median (25th, 75th percentile) | 286 (222, 353) | 235 (203, 266) | 241 (197, 285) | 246 (211, 287) |
| Change from baseline (μm) Mean ± SD | −21±88 | −65±78 | −64±73 | −53±85 |
| Change from baseline (μm) Median (25th, 75th percentile) | −27 (−79, +25) | −75 (−120, −8) | −54 (−112, −18) | −52 (−93, −9) |
| Thickness < 250 with at least a 25 μm decrease from baseline | 36 (28%) | 55 (55%) | 45 (47%) | 48 (46%) |
| LogOCT, No. (%)∥ | ||||
| ≥2 step improvement | 16 (13%) | 22 (22%) | 21 (22%) | 15 (14%) |
| ≥2 step worsening | 5 (4%) | 1 (1%) | 0 | 4 (4%) |
| Baseline thickness ≥ 400 μm | N=144 | N=71 | N=78 | N=69 |
| Thickness (μm) Median (25th, 75th percentile) | 333 (246, 423) | 249 (221, 320) | 279 (219, 356) | 253 (193, 337) |
| Change from baseline (μm) Mean ± SD | −174±158 | −225±128 | −226±142 | −239±134 |
| Change from baseline (μm) Median (25th, 75th percentile) | −175 (−263, −71) | −238 (−299, −158) | −208 (−306, −143) | −254 (−317, −172) |
| Thickness < 250 with at least a 25 μm decrease from baseline | 36 (25%) | 36 (51%) | 29 (37%) | 34 (49%) |
| LogOCT, No. (%)∥ | ||||
| ≥2 step improvement | 65 (45%) | 50 (70%) | 50 (64%) | 50 (72%) |
| ≥2 step worsening | 1 (1%) | 0 | 0 | 0 |
CI = confidence interval; logOCT = logarithmic transformation of optical coherence tomography; OCT = optical coherence tomography; SD = standard deviation.
Visits occurring between 308 and 420 days (between 44 and 60 wks) from randomization were included as 1-yr visits. When > 1 visit occurred in this window, data from the visit closest to the 1-yr target date were used.
Missing (or ungradeable) data as follows for the sham + prompt laser, ranibizumab + prompt laser, ranibizumab + deferred laser, and triamcinolone + prompt laser groups, respectively: 22, 16, 13, 13.
Analysis of covariance adjusted for baseline OCT retinal thickness and visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logistic regression adjusted for baseline OCT retinal thickness and visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logarithmic transformation of OCT central subfield thickness is calculated by taking the log base 10 of the ratio of the central subfield thickness divided by 200 and rounding to the nearest hundredth. The change is the change in the log values. (Ferris FL III, Miller KM, Glassman AR, Beck RW. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology. In Press.)
Figure 6.
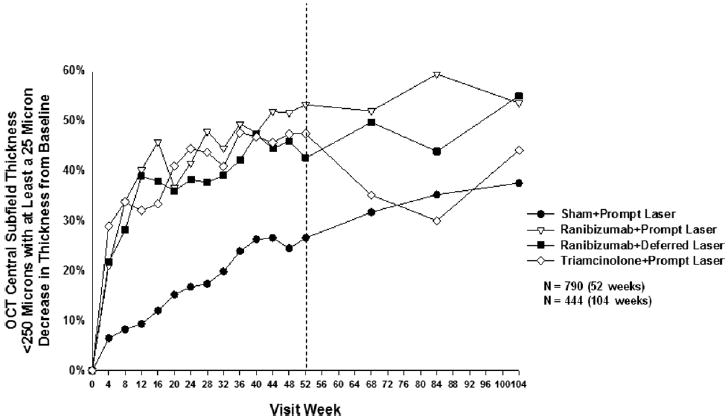
Optical coherence tomography central subfield thickness <250 μm with at least a 25 μm decrease in thickness from baseline at follow-up visits. P values for difference in proportion in OCT central subfield thickness <250 μm with at least a 25 μm decrease in thickness from sham + prompt laser at the 52-week visit: ranibizumab + prompt laser <0.001, ranibizumab + deferred laser = 0.001, and triamcinolone + prompt laser <0.001. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 44 and 60 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization. OCT = optical coherence tomography.
Figure 7.
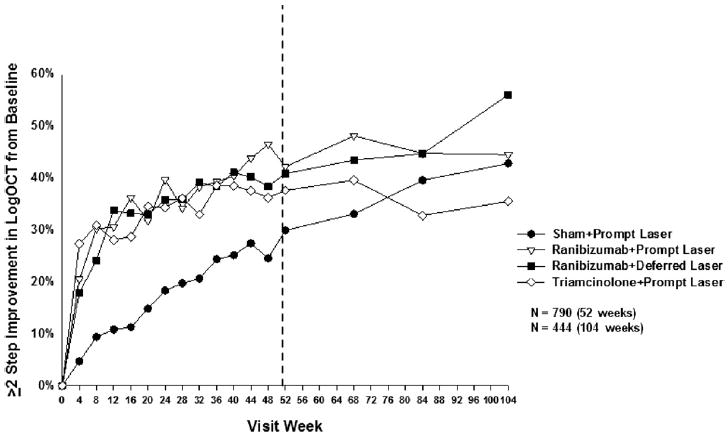
Two or more step improvement in the logarithmic transformation of OCT central subfield thickness from baseline. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 88 and 120 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization. logOCT = logarithmic transformation of optical coherence tomography calculated by taking the log base 10 of the ratio of the central subfield thickness divided by 200 and rounded to the nearest hundredth. (Ferris FL III, Miller KM, Glassman AR, Beck RW. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology. In Press.)
Figure 8.
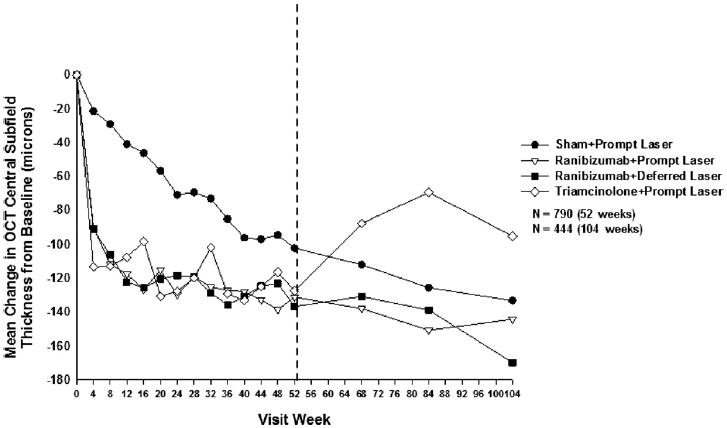
Mean change in OCT central subfield retinal thickening at follow-up visits. P values for difference in mean change in OCT central subfield retinal thickness from sham + prompt laser at the 52-week visit: ranibizumab + prompt laser <0.001, ranibizumab + deferred laser <0.001, and triamcinolone + prompt laser <0.001. Each visit week includes visits that are ±14 days, except the 52-week visit, which includes visits that occur between 308 and 420 days (between 44 and 60 weeks) from randomization, and the 104-week visit, which includes visits that occur between 616 and 840 days (between 88 and 120 weeks) from randomization. OCT = optical coherence tomography.
The change in OCT from the 1- to 2-year visit (Table 12, available at http://aaojournal.org; Fig 6; Figs 7 and 8, available at http://aaojournal.org) when contrasted with the change in visual acuity from the 1- to 2-year visit (Fig 3) differed among the treatments. For the ranibizumab groups, the OCT results remained relatively stable from the 1- to 2-year visit and paralleled the visual acuity results over this time. In the sham + prompt laser group, the OCT results from the 1- to 2-year visit did not parallel the visual acuity results because the mean change in visual acuity from baseline did not continue to increase from the 1- to 2-year visit, even though the mean central subfield thickness continued to decrease during this time. Unlike the ranibizumab groups and sham + prompt laser group, in the triamcinolone + prompt laser group, the mean central subfield thickness increased from the 1- to 2-year visit and paralleled the slight decline in mean visual acuity from the 1- to 2-year visit. The OCT retinal volume measurements (Table 13, available at http://aaojournal.org) at the 1-year primary outcome visit were similar to OCT central subfield thickness measurements (Table 11).
Table 12.
Change in Retinal Thickening from Baseline to 2 Years*
| Change in OCT Central Subfield Thickness† | Sham + Prompt Laser N = 152 | Ranibizumab + Prompt Laser N = 99 | Ranibizumab + Deferred Laser N = 100 | Triamcinolone + Prompt Laser N = 93 |
|---|---|---|---|---|
| Overall Change | ||||
| Thickness (microns) Median (25th, 75th percentile) | 267 (204, 350) | 240 (197, 289) | 231 (206, 288) | 258 (207, 330) |
| Change from baseline (microns) Mean±SD | −133±145 | −144±165 | −170±143 | −95±158 |
| Change from baseline (microns) Median (25th, 75th percentile) | −104 (−231, −25) | −107 (−255, −37) | −146 (−229, −81) | −78 (−176, −12) |
| Difference in mean change from sham+prompt laser (95% CI) [P value]‡ | −31 (−60, −0.9) [P = 0.01] | −36 (−66, −7) [P = 0.004] | −3 (−34, +28) [P = 0.81] | |
| Thickness <250 with at least a 25 micron decrease from baseline, no. (%) | 57 (38%) | 53 (54%) | 55 (55%) | 41 (44%) |
| Relative risk (95% CI) [P Value]§ for comparison with sham+prompt laser | 1.36 (1.01, 1.84) [P = 0.01] | 1.39 (1.01, 1.90) [P = 0.01] | 1.18 (0.85, 1.63) [P = 0.22] | |
| LogOCT, no. (%)∥ | ||||
| Two or more step improvement | 65 (43%) | 44 (44%) | 56 (56%) | 33 (35%) |
| Two or more step worsening | 2 (1%) | 1 (1%) | 0 | 4 (4%) |
CI = confidence interval; OCT = optical coherence tomography; SD = standard deviation.
Visits occurring between 616 and 840 days (between 88 and 120 weeks) from randomization were included as 2-year visits. When more than 1 visit occurred in this window, data from the visit closest to the 2-year target date were used.
Missing (or ungradeable) data as follows for the sham+prompt laser, ranibizumab+prompt laser, ranibizumab+deferred laser, and triamcinolone+prompt laser groups, respectively: 11, 7, 12, 10.
Analysis of covariance adjusted for baseline OCT retinal thickness and visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logistic regression adjusted for baseline OCT retinal thickness and visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Logarithmic transformation of OCT central subfield thickness (LogOCT) is calculated by taking the log base 10 of the ratio of the central subfield thickness divided by 200 and rounding to the nearest hundredth. The change is the change in the log values. (Ferris FL III, Miller KM, Glassman AR, Beck RW. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology. In Press.)
Table 13.
Change in Optical Coherence Tomography Retinal Volume from Baseline to 1 Year*
| Change in OCT Retinal Volume† | Sham + Prompt Laser N = 189 | Ranibizumab + Prompt Laser N = 117 | Ranibizumab + Deferred Laser N = 132 | Triamcinolone + Prompt Laser N = 121 |
|---|---|---|---|---|
| Total volume (mm3) at 1 year | ||||
| Mean±SD | 8.1±1.4 | 7.3±1.0 | 7.4±1.2 | 7.5±1.3 |
| Median (25th, 75th percentile) | 7.9 (7.2, 8.7) | 7.0 (6.6, 7.8) | 7.1 (6.6, 7.8) | 7.2 (6.7, 7.9) |
| Change in volume (mm3) from baseline | ||||
| Mean±SD | −1.0±1.4 | −1.4±1.4 | −1.5±1.5 | −1.4±1.6 |
| Median (25th, 75th percentile) | −0.6 (−1.7, −0.1) | −1.1 (−2.1, −0.6) | −1.1 (−2.1, −0.4) | −1.2 (−2.2, −0.6) |
| Difference in mean change from sham+prompt laser (95% CI) [P Value]‡ | −0.73 (−1.01, −0.44) [P<0.001] | −0.68 (−0.96, −0.41) [P<0.001] | −0.62 (−0.91, −0.34) [P<0.001] |
OCT = optical coherence tomography; SD = standard deviation; CI = confidence interval.
Visits occurring between 308 and 420 days (between 44 and 60 weeks) from randomization were included as 1 year visits. When more than 1 visit occurred in this window, data from the visit closest to the 1-year target date were used.
Missing (or ungradeable) data as follows for the sham+prompt laser, ranibizumab+prompt laser, ranibizumab+deferred laser, and triamcinolone+prompt laser groups, respectively: 85, 54, 46, 55.
Analysis of covariance adjusted for baseline OCT retinal volume, OCT retinal thickness and visual acuity and correlation between 2 study eyes. Confidence intervals are adjusted for multiple comparisons.
Effect of Treatment on Level of Diabetic Retinopathy
Eyes assigned to the ranibizumab-treated groups or the triamcinolone + prompt laser group were less likely to show progression of diabetic retinopathy from baseline to the 1-year primary outcome visit as graded on fundus photographs compared with the sham + prompt laser group (Table 14, available at http://aaojournal.org). Similarly, eyes assigned to the ranibizumab groups or the triamcinolone + prompt laser group appeared less likely to have a vitreous hemorrhage or receive panretinal photocoagulation than the sham + prompt laser group (3% [P=0.002] and 3% [P=0.02], respectively, vs. 8%) during the first year of follow-up.
Table 14.
Diabetic Retinopathy Progression from Baseline to 1 Year by Baseline Retinopathy Severity Group
| Change from Baseline to 1-Year Visit* | Sham N = 233 | Ranibizumab N = 303 | Triamcinolone N = 150 |
|---|---|---|---|
| Baseline severity: Moderately severe NPDR or better, No. (%) | N = 150 | N = 182 | N = 80 |
| Improved by 2 or more levels | 6 (4%) | 46 (25%) | 20 (25%) |
| Worsened by 2 or more levels | 11 (7%) | 5 (3%) | 2 (3%) |
| P value for comparison with Sham | P = 0.08 | P = 0.17 | |
| Baseline severity: Severe NPDR or worse, No. (%) | N = 83 | N = 121 | N = 70 |
| Improved by 2 or more levels† | 10 (19%) | 18 (28%) | 6 (13%) |
| Worsened by 2 or more levels | 7 (8%) | 1 (1%) | 2 (3%) |
| P value for comparison with Sham | P = 0.03 | P = 0.17 |
NPDR = non-proliferative diabetic retinopathy
N = 685; 113 eyes had missing or ungradeable photos at 1 year
Excludes 127 eyes with baseline Level 60 (scars of full or partial PRP present; abnormalities of PDR absent)
Safety
Ocular Adverse Events
Major ocular adverse events through 1 and 2 years are summarized in Tables 15 and 16 (available at http://aaojournal.org), respectively. There were 3 injection-related cases of infectious endophthalmitis (1 after a study injection at baseline, 1 after an injection at 4 weeks, and 1 after an injection at 56 weeks) following the 3973 ranibizumab injections (0.08%; 95% confidence interval [CI] 0.02% to 0.22%) among 375 study participants (0.8%; 95% CI, 0.2% to 2%). In these 3 cases, the maximum visual acuity letter score after the infectious endophthalmitis was unknown in the first case because of lack of follow-up after the 1-week study visit, 73 (~20/40) in the second case, and 58 (~20/80) in the third case. In addition, there was 1 case of inflammatory pseudoendophthalmitis after the 685 triamcinolone injections among 186 study participants (0.5%; 95% CI, 0.01% to 3%). There was 1 case of progression of traction retinal detachment that occurred in the ranibizumab + deferred laser group noted at an unscheduled visit 1 week before the 32-week study visit and after the eighth ranibizumab injection and 1 focal/grid photocoagulation. This one case had extramacular traction retinal detachment and prior panretinal photocoagulation at baseline that were considered stable before randomization and was among the 111 eyes in the ranibizumab groups with prior panretinal photocoagulation, evidence of proliferative diabetic retinopathy, or both, at baseline. Vitrectomy was uncommon among all 4 treatment groups, and there were 5 retinal vein occlusions (1 in the sham + prompt laser group, 1 in each of the ranibizumab groups, and 3 in the triamcinolone + prompt laser group).
Table 15.
Major Ocular Adverse Events during First Year of Follow-Up
| Sham + Prompt Laser N=293 | Ranibizumab + Prompt Laser N=187 No. of Injections = 1497 | Ranibizumab + Deferred Laser N=188 No. of Injections = 1613 | Triamcinolone + Prompt Laser N=186 No. of Injections = 541 | |
|---|---|---|---|---|
| Endophthalmitis, No. (%)* | 1 (<1%) | 1 (1%) | 1 (1%) | 0 |
| Pseudoendophthalmitis, No. (%)† | 1(<1%) | 0 | 0 | 1 (1%) |
| Ocular vascular event, No. (%)‡ | 1 (<1%) | 1 (1%) | 0 | 2 (1%) |
| Retinal detachment, No. (%) | 0 | 0 | 1 (1%)§ | 0 |
| Vitrectomy, No. (%) | 7 (2%) | 0 | 3 (2%) | 0 |
| Vitreous hemorrhage, No. (%) | 15 (5%) | 3 (2%) | 4 (2%) | 2 (1%) |
| Elevated intraocular pressure/glaucoma, No. (%) | ||||
| Increase ≥10 mmHg from baseline | 16 (5%) | 10 (5%) | 5 (3%) | 70 (38%) |
| IOP ≥30 mmHg | 3 (1%) | 2 (1%) | 4 (2%) | 46 (25%) |
| Initiation of IOP-lowering medication at any visit∥ | 9 (3%) | 5 (3%) | 4 (2%) | 41 (22%) |
| No. of eyes meeting ≥1 of the above | 23 (8%) | 12 (6%) | 7 (4%) | 79 (42%) |
| Glaucoma surgery | 0 | 0 | 0 | 0 |
| Cataract surgery | ||||
| Phakic at baseline | N=192 | N=131 | N=134 | N=124 |
| No. (%) with cataract surgery | 11 (6%) | 6 (5%) | 8 (6%) | 19 (15%) |
IOP = intraocular pressure
One case unrelated to study drug injection (after cataract extraction) in the sham + prompt laser group; 2 cases related to study drug injection in the ranibizumab groups (0.06% of ranibizumab injections given). One case occurred at baseline and 1 at the 4-wk visit. Endophthalmitis was defined as any patient having an intravitreal or anterior chamber tap for presumed endophthalmitits or treated for infectious endophthalmitis regardless of whether a tap was performed or whether a culture is positive.
One case was unrelated to the study drug injection (vitreous opacity with hypopyon), and 1 case was related to study drug injection in the triamcinolone group. Pseudoendophthalmitis was defined on the basis of investigator diagnosis and patient not tapped or treated for infectious endophthalmitis.
Includes 2 central retinal vein occlusions and 2 branch retinal vein occlusions.
Includes 1 progressive traction retinal detachment with proliferative diabetic retinopathy and prior panretinal photocoagulation at baseline. Visual acuity remained stable, within 5 letters of the baseline visual acuity letter score of 66 (20/50), while ranibizumab was given every 4 wks through the 24-wk visit when focal/grid laser also was applied. Ranibizumab was given again at the 28-wk visit and 5 wks later, sudden vision loss was reported, and a table-top detachment involving the central macula was noted at an unscheduled visit with a visual acuity letter score of 48 (20/125). Vitrectomy surgery was delayed for several weeks because of other medical problems; after surgery, the visual acuity letter score remained 0 (<20/800).
Excludes eyes with IOP-lowering medications at baseline.
Table 16.
Major Ocular Adverse Events During 2 Years of Follow Up
| Sham + Prompt Laser N = 293 | Ranibizumab + Prompt Laser N = 187 # injections = 1833 | Ranibizumab + Deferred Laser N = 188 # injections = 2140 | Triamcinolone + Prompt Laser N = 186 # injections = 685 | |
|---|---|---|---|---|
| Endophthalmitis, no. (%)* | 1 (<1%) | 2 (1%) | 2 (1%) | 0 |
| Pseudoendophthalmitis, no. (%)† | 1 (<1%) | 0 | 0 | 1 (1%) |
| Ocular vascular event, no. (%)‡ | 1 (<1%) | 1 (1%) | 1 (1%) | 3 (2%) |
| Retinal detachment, no. (%) | 0 | 0 | 1 (1%)§ | 0 |
| Vitrectomy, no. (%) | 15 (5%) | 4 (2%) | 7 (4%) | 2 (1%) |
| Vitreous hemorrhage, no. (%) | 27 (9%) | 6 (3%) | 8 (4%) | 7 (4%) |
| Elevated intraocular pressure/glaucoma, no. (%) | ||||
| Increase ≥10 mmHg from baseline | 22 (8%) | 16 (9%) | 11 (6%) | 78 (42%) |
| IOP ≥30 mmHg | 8 (3%) | 3 (2%) | 6 (3%) | 51 (27%) |
| Initiation of IOP-lowering medication at any visit∥ | 16 (5%) | 9 (5%) | 6 (3%) | 53 (28%) |
| Number of eyes meeting one or more of the above | 32 (11%) | 20 (11%) | 14 (7%) | 93 (50%) |
| Glaucoma surgerya | 1 (<1%) | 1 (1%) | 0 | 2 (1%) |
| Cataract surgery | ||||
| Phakic at baseline | N = 192 | N = 131 | N = 134 | N = 124 |
| No. (%) with cataract surgery | 23 (12%) | 16 (12%) | 17 (13%) | 68 (55%) |
IOP = intraocular pressure
One case unrelated to study drug injection (following cataract extraction) in the sham+prompt laser group; 1 case related to study drug injection and 1 case unrelated to injection (following cataract surgery) in the ranibizumab+prompt laser group; 2 cases related to study drug injection in the ranibizumab+deferred laser group. The 3 cases related to study drug injection in the ranibizumab groups are 0.08% of ranibizumab study drug injections given. Endophthalmitis was defined as any patient having an intravitreal or anterior chamber tap for presumed endophthalmitis or treated for infectious endophthalmitis regardless of whether a tap was performed or whether a culture is positive.
One case unrelated to the study drug injection (vitreous opacity with hypopyon) and one case related to study drug injection in the triamcinolone group. Pseudoendophthalmitis was defined based on investigator diagnosis and patient not tapped or treated for infectious endophthalmitis.
Includes 2 central retinal vein occlusions and 4 branch retinal vein occlusions.
Includes 1 traction retinal detachment with proliferative diabetic retinopathy and prior panretinal photocoagulation at baseline. Visual acuity had remained stable (within 5 letters) of the baseline visual acuity letter score of 66 (20/50) while ranibizumab was given every 4 weeks through the 24-week visit when focal/grid laser also was applied. Ranibizumab again was given at the 28-week visit and five weeks later, sudden vision loss was reported and a table top detachment involving the central macula was noted at an unscheduled visit with a visual acuity letter score of 48 (20/125). Vitrectomy surgery was delayed for several weeks because of other medical problems; following surgery, the visual acuity letter score remained 0 (<20/800).
Excludes eyes with IOP lowering medications at baseline.
Includes 2 filter and 2 cilliary body destruction.
The occurrence of IOP elevation >10 mmHg from baseline, IOP >30 mmHg, or initiation of IOP-lowering medications not in use at study entry at 1 or more visits during 2 years of follow-up was more frequent in eyes in the triamcinolone + prompt laser group than in the ranibizumab groups or the sham + prompt laser group (93 [50%] vs. 34 [9%] or 32 [11%], respectively; P<0.001 for both comparisons). Glaucoma surgery was performed in 4 eyes (1 eye in the sham + prompt laser group, 1 eye in the ranibizumab + prompt laser group, and 2 eyes in the triamcinolone + prompt laser group). Among the subgroup of 62 pseudophakic eyes at baseline in the triamcinolone + prompt laser group, 30 (48%) had ≥1 of the ocular hypertension events described above, compared with 10 (10%) and 15 (14%) among the 101 and 110 pseudophakic eyes at baseline in the sham + prompt laser and ranibizumab groups, respectively. The cumulative percentage of eyes in the triamcinolone + prompt laser group that underwent cataract surgery over the 2 years of follow-up was substantially greater compared with the sham + prompt laser group or the ranibizumab groups (59% vs. 14% and 14%, respectively; P<0.001 for both comparisons) (Fig 9).
Figure 9.
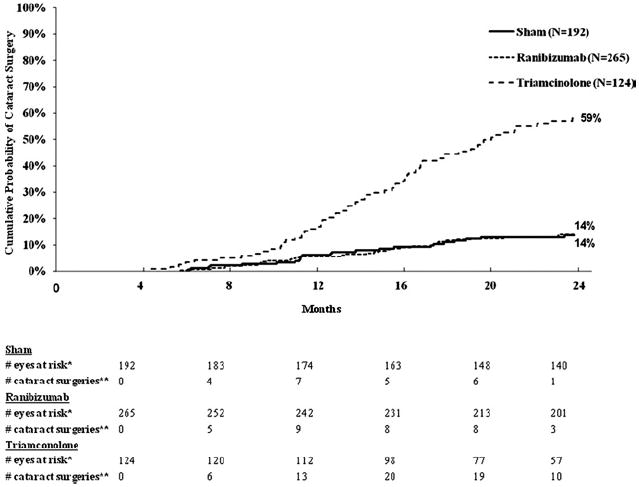
Cumulative probability of cataract surgery through 2 years of follow-up for all eyes phakic at baseline. Eyes pending a 2-year visit or that were lost to follow-up were censored at their last visit. N is the number of eyes phakic at baseline. *Number of eyes at the start of the interval without previous cataract surgery. **Number of eyes with cataract surgery during the subsequent 4-month period.
Systemic Adverse Events
There were no systemic adverse events with a difference in frequency among the 4 groups that could not be attributed to chance. In particular, there was no indication of an increase in the rate of cardiovascular or cerebrovascular events in the ranibizumab groups compared with the other groups (Table 17). The mean number of systemic adverse events reported per participant through 2 years with 1 study eye was 3±3 in the sham group, 3±3 in the 2 ranibizumab groups combined, and 3±4 in the triamcinolone group. All systemic adverse events and study eye ocular adverse events reported by the site are shown in Tables 18 and 19 (available at http://aaojournal.org).
Table 17.
Cardiovascular Events According to Antiplatelet Trialists’ Collaboration* through 1 and 2 Years
| Through 1 Yr |
Through 2 Yrs |
|||||
|---|---|---|---|---|---|---|
| Sham†N‡=130 | Ranibizumab N‡=375 | Triamcinolone N‡=186 | Sham†N‡=130 | Ranibizumab N‡=375 | Triamcinolone N‡=186 | |
| Nonfatal myocardial infarction, No. (%) | 3 (2%) | 1 (<1%) | 2 (1%) | 4 (3%) | 5 (1%) | 5 (3%) |
| Nonfatal cerebrovascular accident—ischemic or hemorrhagic (or unknown), No. (%) | 5 (4%) | 3 (1%) | 1 (1%) | 8 (6%) | 6 (2%) | 3 (2%) |
| Vascular death (from any potential vascular or unknown cause§), No. (%) | 4 (3%) | 7 (2%) | 2 (1%) | 6 (5%) | 8 (2%) | 4 (2%) |
| Any ATC cardiovascular event, No. (%) | 10 (8%) | 11 (3%) | 5 (3%) | 15 (12%) | 19 (5%) | 12 (6%) |
ATC = Antiplatelet Trialists’ Collaboration.
Collaborative overview of randomised trials of antiplatelet therapy—I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ 1994;308:81-106.
One participant had a nonfatal myocardial infarction and a nonfatal stroke and 1 participant had a nonfatal myocardial infarction and a subsequent vascular death through 1 year; an additional participant had a nonfatal stroke and a subsequent vascular death through 2 years. Multiple events are counted once in the any ATC cardiovascular event row.
N = number of study participants. Study participants with 2 study eyes are assigned to the non-sham group. Multiple events within a study participant are only counted once per event.
Four of the 6 vascular deaths in the sham group, 1 of the 8 vascular deaths in the ranibizumab group, and 1 of the 4 vascular deaths in the triamcinolone group were from an unknown cause.
Table 18.
Summary of all Systemic Adverse Events through 2 Years of Follow-up*
| Sham N = 319 | Ranibizumab N = 710 | Triamcinolone N=369 | Sham/ Ranibizumab N = 321 | Sham/ Triamcinolone N = 109 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse Event | ||||||||||
| Blood and lymphatic system disorders | ||||||||||
| Anaemia | 6 | 4 | 7 | 4 | 2 | |||||
| Anaemia of chronic disease | 0 | 1 | 1 | 0 | 0 | |||||
| Lymphadenopathy | 1 | 0 | 0 | 0 | 0 | |||||
| Lymphoedema | 0 | 2 | 0 | 0 | 0 | |||||
| Lymphoma | 0 | 0 | 1 | 0 | 0 | |||||
| Thrombocytopenia | 0 | 2 | 0 | 0 | 0 | |||||
| Cardiac disorders | ||||||||||
| Acute coronary syndrome | 0 | 0 | 1 | 0 | 0 | |||||
| Angina pectoris | 2 | 1 | 2 | 1 | 0 | |||||
| Arrhythmia | 1 | 2 | 1 | 1 | 0 | |||||
| Arteriosclerosis coronary artery | 3 | 1 | 3 | 1 | 0 | |||||
| Atrial fibrillation | 2 | 4 | 0 | 0 | 0 | |||||
| Bradycardia | 1 | 3 | 1 | 0 | 0 | |||||
| Cardiac failure | 0 | 1 | 2 | 1 | 0 | |||||
| Cardiac failure congestive | 6 | 14 | 7 | 8 | 2 | |||||
| Cardiomegaly | 0 | 2 | 0 | 2 | 0 | |||||
| Cardiomyopathy | 0 | 1 | 1 | 0 | 0 | |||||
| Chest discomfort | 1 | 0 | 0 | 0 | 0 | |||||
| Coronary artery disease | 1 | 3 | 1 | 2 | 0 | |||||
| Coronary artery occlusion | 0 | 0 | 1 | 0 | 0 | |||||
| Left ventricular hypertrophy | 0 | 0 | 0 | 1 | 0 | |||||
| Mitral valve incompetence | 0 | 1 | 0 | 0 | 0 | |||||
| Mitral valve prolapse | 0 | 0 | 1 | 0 | 0 | |||||
| Myocardial infarction | 6 | 3 | 5 | 4 | 1 | |||||
| Palpitations | 0 | 0 | 0 | 1 | 0 | |||||
| Supraventricular tachycardia | 0 | 1 | 0 | 0 | 0 | |||||
| Tachycardia | 0 | 0 | 0 | 2 | 0 | |||||
| Ear and labyrinth disorders | ||||||||||
| Deafness | 1 | 2 | 0 | 1 | 0 | |||||
| Ear pain | 0 | 1 | 0 | 1 | 0 | |||||
| Endocrine disorders | ||||||||||
| Diabetes mellitus inadequate control | 0 | 4 | 0 | 2 | 2 | |||||
| Diabetic gastroparesis | 0 | 0 | 1 | 0 | 0 | |||||
| Diabetic ketoacidosis | 0 | 0 | 2 | 3 | 0 | |||||
| Diabetic ulcer | 0 | 1 | 0 | 1 | 0 | |||||
| Goitre | 0 | 0 | 1 | 0 | 0 | |||||
| Hyperthyroidism | 1 | 0 | 0 | 1 | 0 | |||||
| Hypothyroidism | 2 | 4 | 2 | 1 | 0 | |||||
| Thyroid cancer | 0 | 0 | 1 | 0 | 0 | |||||
| Thyroid neoplasm | 1 | 0 | 0 | 0 | 0 | |||||
| Eye disorders | ||||||||||
| Conjunctival haemorrhage | 1 | 0 | 0 | 0 | 0 | |||||
| Punctate keratitis | 0 | 1 | 0 | 0 | 0 | |||||
| Visual impairment | 1 | 0 | 0 | 0 | 0 | |||||
| Gastrointestinal disorders | ||||||||||
| Abdominal pain | 3 | 1 | 2 | 2 | 0 | |||||
| Abdominal pain upper | 1 | 3 | 1 | 4 | 0 | |||||
| Constipation | 1 | 3 | 2 | 0 | 0 | |||||
| Diarrhoea | 4 | 4 | 6 | 6 | 0 | |||||
| Dyspepsia | 1 | 0 | 1 | 0 | 1 | |||||
| Gastric ulcer | 0 | 1 | 0 | 1 | 0 | |||||
| Gastritis | 1 | 1 | 0 | 1 | 1 | |||||
| Gastrointestinal haemorrhage | 1 | 1 | 0 | 0 | 0 | |||||
| Gastrooesophageal reflux disease | 3 | 4 | 5 | 0 | 0 | |||||
| Haematochezia | 1 | 0 | 0 | 0 | 0 | |||||
| Impaired gastric emptying | 0 | 3 | 0 | 0 | 0 | |||||
| Irritable bowel syndrome | 0 | 0 | 0 | 1 | 0 | |||||
| Nausea | 4 | 10 | 5 | 3 | 1 | |||||
| Oesophageal ulcer | 0 | 1 | 0 | 0 | 0 | |||||
| Oesophagitis | 0 | 1 | 0 | 1 | 0 | |||||
| Peptic ulcer | 1 | 1 | 0 | 0 | 0 | |||||
| Rectal haemorrhage | 0 | 0 | 0 | 1 | 0 | |||||
| Salivary gland disorder | 0 | 1 | 1 | 0 | 0 | |||||
| Tooth infection | 0 | 0 | 1 | 3 | 0 | |||||
| Vomiting | 2 | 3 | 4 | 1 | 2 | |||||
| General disorders and administration site conditions | ||||||||||
| Chest pain | 8 | 10 | 5 | 6 | 0 | |||||
| Chills | 2 | 1 | 0 | 0 | 0 | |||||
| Cyst | 1 | 3 | 2 | 1 | 0 | |||||
| Death | 5 | 5 | 2 | 3 | 0 | |||||
| Fatigue | 0 | 0 | 1 | 0 | 0 | |||||
| Gait disturbance | 0 | 1 | 0 | 0 | 0 | |||||
| Hernia | 1 | 0 | 1 | 2 | 1 | |||||
| Lethargy | 0 | 2 | 0 | 0 | 0 | |||||
| Oedema peripheral | 3 | 8 | 4 | 3 | 3 | |||||
| Pain | 0 | 1 | 1 | 0 | 0 | |||||
| Pyrexia | 3 | 2 | 0 | 4 | 0 | |||||
| Swelling | 4 | 3 | 1 | 2 | 0 | |||||
| Hepatobiliary disorders | ||||||||||
| Cholecystitis acute | 0 | 1 | 3 | 1 | 0 | |||||
| Cholelithiasis | 1 | 1 | 0 | 0 | 1 | |||||
| Hepatic failure | 0 | 1 | 1 | 0 | 0 | |||||
| Immune system disorders | ||||||||||
| Asthma | 0 | 1 | 1 | 2 | 0 | |||||
| Drug hypersensitivity | 1 | 0 | 0 | 1 | 0 | |||||
| Hypersensitivity | 0 | 0 | 2 | 0 | 0 | |||||
| Seasonal allergy | 4 | 7 | 1 | 4 | 3 | |||||
| Urticaria | 0 | 1 | 0 | 0 | 0 | |||||
| Infections and infestations | ||||||||||
| Bronchitis | 2 | 11 | 7 | 7 | 2 | |||||
| Cystitis | 3 | 3 | 0 | 2 | 4 | |||||
| Diverticulitis | 2 | 0 | 0 | 0 | 0 | |||||
| Ear infection | 2 | 7 | 5 | 2 | 3 | |||||
| Fungal infection | 1 | 2 | 0 | 1 | 1 | |||||
| Fungal skin infection | 0 | 0 | 1 | 0 | 0 | |||||
| Gangrene | 1 | 0 | 1 | 1 | 0 | |||||
| Gastroenteritis | 1 | 0 | 2 | 0 | 1 | |||||
| Gastroenteritis viral | 1 | 8 | 2 | 4 | 0 | |||||
| Gingival infection | 0 | 1 | 0 | 1 | 0 | |||||
| Herpes zoster | 2 | 1 | 4 | 2 | 0 | |||||
| Hordeolum | 0 | 0 | 0 | 1 | 0 | |||||
| Infection | 1 | 1 | 1 | 3 | 0 | |||||
| Influenza | 4 | 11 | 9 | 11 | 4 | |||||
| Kidney infection | 0 | 3 | 1 | 0 | 1 | |||||
| Localised infection | 1 | 8 | 7 | 3 | 1 | |||||
| Onychomycosis | 1 | 1 | 0 | 1 | 0 | |||||
| Oral herpes | 0 | 1 | 0 | 0 | 0 | |||||
| Osteomyelitis | 0 | 3 | 1 | 0 | 0 | |||||
| Pharyngitis streptococcal | 0 | 1 | 1 | 0 | 0 | |||||
| Pneumonia | 4 | 14 | 2 | 0 | 2 | |||||
| Respiratory tract infection | 0 | 2 | 1 | 1 | 1 | |||||
| Sepsis | 1 | 3 | 0 | 1 | 0 | |||||
| Sinusitis | 2 | 5 | 3 | 3 | 1 | |||||
| Skin bacterial infection | 0 | 0 | 1 | 0 | 0 | |||||
| Skin infection | 1 | 2 | 1 | 0 | 0 | |||||
| Staphylococcal infection | 0 | 0 | 0 | 1 | 2 | |||||
| Tooth abscess | 0 | 7 | 4 | 1 | 0 | |||||
| Upper respiratory tract infection | 2 | 22 | 7 | 7 | 1 | |||||
| Urinary tract infection | 5 | 16 | 5 | 4 | 3 | |||||
| Injury, poisoning and procedural complications | ||||||||||
| Accidental overdose | 0 | 0 | 1 | 0 | 0 | |||||
| Animal bite | 0 | 1 | 0 | 0 | 0 | |||||
| Animal scratch | 1 | 0 | 0 | 0 | 0 | |||||
| Arthropod bite | 1 | 0 | 1 | 0 | 1 | |||||
| Arthropod sting | 0 | 1 | 0 | 0 | 0 | |||||
| Asbestosis | 0 | 0 | 0 | 0 | 1 | |||||
| Caustic injury | 0 | 1 | 0 | 0 | 0 | |||||
| Fall | 4 | 14 | 4 | 3 | 2 | |||||
| Fibula fracture | 0 | 1 | 0 | 0 | 0 | |||||
| Food poisoning | 0 | 1 | 1 | 2 | 0 | |||||
| Foot fracture | 2 | 6 | 2 | 0 | 1 | |||||
| Head injury | 0 | 1 | 0 | 1 | 0 | |||||
| Hip fracture | 1 | 1 | 0 | 1 | 1 | |||||
| Joint injury | 2 | 6 | 4 | 0 | 0 | |||||
| Laceration | 2 | 2 | 1 | 0 | 0 | |||||
| Ligament rupture | 0 | 0 | 0 | 0 | 1 | |||||
| Ligament sprain | 0 | 1 | 0 | 0 | 1 | |||||
| Limb injury | 1 | 0 | 0 | 1 | 0 | |||||
| Nerve injury | 1 | l | 1 | 1 | 0 | |||||
| Post procedural complication | 0 | l | 1 | 0 | 0 | |||||
| Road traffic accident | 1 | 3 | 0 | 0 | 0 | |||||
| Spinal fracture | 0 | 0 | 0 | 1 | 0 | |||||
| Thermal burn | 1 | 0 | 0 | 0 | 0 | |||||
| Wound | 0 | 0 | 0 | 0 | 1 | |||||
| Wrist fracture | 0 | 0 | 2 | 0 | 0 | |||||
| Investigations | ||||||||||
| Angiogram | 1 | 0 | 0 | 0 | 0 | |||||
| Aortogram | 0 | 1 | 0 | 0 | 0 | |||||
| Arteriogram | 0 | 1 | 0 | 0 | 0 | |||||
| Biopsy thyroid gland | 0 | 1 | 1 | 0 | 0 | |||||
| Blood glucose decreased | 0 | 0 | 2 | 0 | 0 | |||||
| Blood potassium decreased | 1 | 0 | 0 | 0 | 0 | |||||
| Blood potassium increased | 2 | 3 | 4 | 1 | 0 | |||||
| Blood testosterone decreased | 0 | 1 | 0 | 0 | 0 | |||||
| Cardiac murmur | 1 | 0 | 0 | 0 | 0 | |||||
| Colonoscopy | 1 | 0 | 0 | 0 | 0 | |||||
| Heart rate increased | 0 | 1 | 1 | 0 | 0 | |||||
| Heart rate irregular | 0 | 5 | 1 | 0 | 0 | |||||
| Oesophagogastroduodenoscopy | 1 | 0 | 0 | 0 | 0 | |||||
| Weight decreased | 1 | 3 | 0 | 0 | 1 | |||||
| White blood cell count increased | 0 | 1 | 1 | 0 | 0 | |||||
| Metabolism and nutrition disorders | ||||||||||
| Abnormal weight gain | 1 | 1 | 0 | 0 | 0 | |||||
| Dehydration | 2 | 1 | 2 | 0 | 0 | |||||
| Diabetes mellitus | 0 | 2 | 2 | 2 | 0 | |||||
| Diabetes mellitus inadequate control | 0 | 2 | 3 | 0 | 1 | |||||
| Fluid overload | 0 | 0 | 1 | 0 | 1 | |||||
| Fluid retention | 0 | 0 | 0 | 1 | 0 | |||||
| Hypercholesterolaemia | 5 | 7 | 4 | 5 | 1 | |||||
| Hyperglycaemia | 5 | 6 | 6 | 3 | 1 | |||||
| Hyperkalaemia | 0 | 2 | 1 | 1 | 0 | |||||
| Hyperlipidaemia | 0 | 0 | 0 | 1 | 0 | |||||
| Hypertriglyceridaemia | 0 | 0 | 0 | 0 | 1 | |||||
| Hypogiycaemia | 3 | 11 | 3 | 5 | 5 | |||||
| Iron deficiency | 0 | 1 | 0 | 1 | 0 | |||||
| Oedema peripheral | 0 | 0 | 0 | 1 | 0 | |||||
| Osteoporosis | 0 | 1 | 2 | 0 | 0 | |||||
| Vitamin B12 deficiency | 0 | 1 | 0 | 0 | 0 | |||||
| Vitamin D deficiency | 1 | 1 | 1 | 0 | 1 | |||||
| Musculoskeletal and connective tissue disorders | ||||||||||
| Arthralgia | 5 | 7 | 2 | 4 | 1 | |||||
| Arthritis | 0 | 1 | 0 | 1 | 0 | |||||
| Back pain | 5 | 9 | 4 | 4 | 1 | |||||
| Bursitis | 1 | 0 | 0 | 1 | 0 | |||||
| Exostosis | 1 | 2 | 0 | 0 | 0 | |||||
| Facial bones fracture | 0 | 1 | 0 | 0 | 0 | |||||
| Fracture | 1 | 2 | 0 | 2 | 1 | |||||
| Gout | 1 | 3 | 0 | 4 | 2 | |||||
| Inclusion body myositis | 0 | 1 | 0 | 0 | 0 | |||||
| Intervertebral disc protrusion | 0 | 3 | 3 | 1 | 0 | |||||
| Joint sprain | 0 | 0 | 1 | 0 | 0 | |||||
| Lower limb fracture | 0 | 1 | 1 | 0 | 0 | |||||
| Multiple fractures | 1 | 0 | 0 | 1 | 0 | |||||
| Muscle spasms | 0 | 0 | 1 | 0 | 0 | |||||
| Muscular weakness | 1 | 3 | 0 | 0 | 0 | |||||
| Musculoskeletal pain | 6 | 4 | 2 | 1 | 0 | |||||
| Myalgia | 1 | 2 | 2 | 1 | 3 | |||||
| Neck pain | 3 | 6 | 1 | 1 | 0 | |||||
| Osteoarthritis | 1 | 2 | 2 | 2 | 1 | |||||
| Osteopenia | 0 | 1 | 0 | 0 | 0 | |||||
| Pain in extremity | 5 | 8 | 10 | 3 | 1 | |||||
| Rhabdomyolysis | 0 | 1 | 0 | 0 | 0 | |||||
| Rheumatoid arthritis | 0 | 3 | 0 | 0 | 0 | |||||
| Rib fracture | 1 | 1 | 1 | 1 | 0 | |||||
| Rotator cuff syndrome | 1 | 1 | 0 | 0 | 0 | |||||
| Spinal osteoarthritis | 1 | 0 | 0 | 0 | 0 | |||||
| Temporomandibular joint syndrome | 0 | 0 | 0 | 1 | 0 | |||||
| Tendonitis | 0 | 1 | 0 | 0 | 0 | |||||
| Trigger finger | 1 | 3 | 2 | 0 | 0 | |||||
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | ||||||||||
| Abdominal neoplasm | 1 | 0 | 0 | 0 | 0 | |||||
| Bladder cancer | 0 | 2 | 0 | 0 | 0 | |||||
| Bone neoplasm malignant | 0 | 1 | 0 | 0 | 0 | |||||
| Brain neoplasm | 0 | 0 | 0 | 1 | 0 | |||||
| Colon cancer | 0 | 2 | 0 | 0 | 0 | |||||
| Colonic polyp | 0 | 1 | 0 | 0 | 0 | |||||
| Lung cancer metastatic | 0 | 1 | 1 | 0 | 0 | |||||
| Multiple myeloma | 0 | 0 | 0 | 1 | 0 | |||||
| Polyp | 1 | 2 | 2 | 1 | 0 | |||||
| Skin cancer | 5 | 2 | 2 | 1 | 0 | |||||
| Nervous system disorders | ||||||||||
| Amnesia | 0 | 1 | 0 | 1 | 0 | |||||
| Carotid artery occlusion | 1 | 0 | 0 | 0 | 0 | |||||
| Carpal tunnel syndrome | 1 | 2 | 1 | 2 | 0 | |||||
| Cerebrovascular accident | 2 | 3 | 2 | 0 | 0 | |||||
| Concussion | 0 | 0 | 0 | 0 | 1 | |||||
| Convulsion | 0 | 0 | 1 | 1 | 0 | |||||
| Dementia Alzheimer’s type | 0 | 0 | 0 | 0 | 1 | |||||
| Dizziness | 6 | 8 | 2 | 2 | 0 | |||||
| Dysarthria | 0 | 0 | 0 | 0 | 1 | |||||
| Essential tremor | 0 | 0 | 0 | 0 | 1 | |||||
| Facial palsy | 1 | 0 | 1 | 0 | 0 | |||||
| Headache | 6 | 17 | 8 | 6 | 1 | |||||
| Hemiparesis | 2 | 0 | 0 | 0 | 0 | |||||
| Hypoaesthesia | 0 | 0 | 1 | 1 | 1 | |||||
| Insomnia | 2 | 3 | 1 | 0 | 0 | |||||
| Ischaemic stroke | 6 | 2 | 1 | 1 | 0 | |||||
| Migraine | 0 | 2 | 1 | 0 | 0 | |||||
| Neuropathy | 0 | 2 | 1 | 0 | 0 | |||||
| Neuropathy peripheral | 2 | 2 | 0 | 1 | 0 | |||||
| Post herpetic neuralgia | 0 | 0 | 1 | 0 | 0 | |||||
| Presyncope | 0 | 0 | 0 | 1 | 0 | |||||
| Restless legs syndrome | 0 | 1 | 0 | 0 | 0 | |||||
| Sciatica | 0 | 1 | 0 | 1 | 0 | |||||
| Syncope | 1 | 3 | 4 | 2 | 0 | |||||
| Tinnitus | 0 | 1 | 0 | 0 | 0 | |||||
| Tremor | 0 | 1 | 1 | 0 | 0 | |||||
| Vertigo | 3 | 1 | 2 | 3 | 0 | |||||
| Psychiatric disorders | ||||||||||
| Aggression | 0 | 0 | 1 | 0 | 0 | |||||
| Anxiety | 2 | 4 | 3 | 2 | 2 | |||||
| Bipolar disorder | 0 | 0 | 1 | 0 | 0 | |||||
| Depression | 3 | 8 | 0 | 4 | 0 | |||||
| Drug dependence | 0 | 0 | 1 | 0 | 0 | |||||
| Insomnia | 0 | 1 | 1 | 0 | 0 | |||||
| Mental disorder | 1 | 0 | 0 | 0 | 0 | |||||
| Renal and urinary disorders | ||||||||||
| Bladder spasm | 0 | 2 | 0 | 1 | 0 | |||||
| Haematuria | 0 | 1 | 0 | 0 | 0 | |||||
| Nephrolithiasis | 1 | 3 | 1 | 0 | 0 | |||||
| Proteinuria | 0 | 1 | 0 | 0 | 0 | |||||
| Renal disorder | 0 | 1 | 0 | 0 | 0 | |||||
| Renal failure | 3 | 11 | 4 | 6 | 2 | |||||
| Renal failure acute | 1 | 1 | 0 | 3 | 0 | |||||
| Renal failure chronic | 0 | 2 | 3 | 0 | 0 | |||||
| Renal impairment | 0 | 7 | 4 | 1 | 0 | |||||
| Urethral stenosis | 1 | 0 | 0 | 0 | 0 | |||||
| Urinary incontinence | 0 | 2 | 1 | 1 | 0 | |||||
| Urinary tract disorder | 0 | 1 | 0 | 0 | 0 | |||||
| Reproductive system and breast disorders | ||||||||||
| Breast cancer | 1 | 2 | 0 | 0 | 0 | |||||
| Erectile dysfunction | 1 | 0 | 2 | 1 | 0 | |||||
| Prostate cancer | 1 | 2 | 1 | 2 | 0 | |||||
| Prostatitis | 0 | 1 | 0 | 0 | 0 | |||||
| Prostatomegaly | 0 | 2 | 0 | 2 | 1 | |||||
| Respiratory, thoracic and mediastinal disorders | ||||||||||
| Asthma | 0 | 0 | 0 | 1 | 0 | |||||
| Cardio-respiratory arrest | 0 | 0 | 1 | 1 | 0 | |||||
| Chronic obstructive pulmonary disease | 1 | 1 | 0 | 0 | 1 | |||||
| Cough | 2 | 7 | 4 | 4 | 0 | |||||
| Dyspnoea | 3 | 5 | 2 | 5 | 1 | |||||
| Epistaxis | 0 | 3 | 0 | 1 | 0 | |||||
| Hypoxia | 0 | 1 | 0 | 0 | 0 | |||||
| Lung neoplasm | 0 | 1 | 0 | 0 | 0 | |||||
| Nasopharyngitis | 7 | 29 | 15 | 6 | 2 | |||||
| Oropharyngeal pain | 0 | 1 | 0 | 1 | 0 | |||||
| Pleural effusion | 0 | 0 | 1 | 0 | 0 | |||||
| Pulmonary hypertension | 0 | 1 | 0 | 0 | 0 | |||||
| Pulmonary oedema | 1 | 2 | 0 | 3 | 0 | |||||
| Respiratory distress | 0 | 0 | 1 | 0 | 0 | |||||
| Respiratory tract congestion | 1 | 3 | 1 | 1 | 1 | |||||
| Rhinitis allergic | 1 | 1 | 1 | 3 | 1 | |||||
| Rhinorrhoea | 0 | 0 | 0 | 1 | 0 | |||||
| Sinusitis | 4 | 13 | 4 | 6 | 2 | |||||
| Sleep apnoea syndrome | 3 | 3 | 3 | 2 | 1 | |||||
| Sneezing | 0 | 1 | 0 | 0 | 1 | |||||
| Throat cancer | 0 | 1 | 0 | 1 | 0 | |||||
| Wheezing | 0 | 0 | 0 | 0 | 1 | |||||
| Skin and subcutaneous tissue disorders | ||||||||||
| Alopecia | 0 | 1 | 0 | 0 | 0 | |||||
| Angioedema | 0 | 1 | 0 | 0 | 0 | |||||
| Blister | 0 | 1 | 0 | 0 | 0 | |||||
| Cellulitis | 0 | 4 | 3 | 1 | 0 | |||||
| Contusion | 2 | 3 | 0 | 2 | 0 | |||||
| Dermatitis contact | 0 | 2 | 0 | 1 | 1 | |||||
| Excoriation | 2 | 0 | 0 | 0 | 0 | |||||
| Furuncle | 1 | 1 | 0 | 0 | 0 | |||||
| Hyperhidrosis | 0 | 1 | 0 | 0 | 0 | |||||
| Hyperkeratosis | 0 | 1 | 0 | 0 | 0 | |||||
| Ingrowing nail | 3 | 1 | 0 | 0 | 0 | |||||
| Pruritus | 0 | 1 | 0 | 0 | 0 | |||||
| Psoriasis | 0 | 0 | 0 | 0 | 1 | |||||
| Rash | 0 | 7 | 1 | 1 | . | |||||
| Skin lesion | 2 | 0 | 0 | 1 | 1 | |||||
| Skin ulcer | 2 | 14 | 6 | 5 | 0 | |||||
| Subcutaneous abscess | 0 | 1 | 0 | 0 | 0 | |||||
| Swelling face | 1 | 0 | 0 | 0 | 0 | |||||
| Tinea capitis | 0 | 0 | 0 | 1 | 0 | |||||
| Surgical and medical procedures | ||||||||||
| Arterial bypass operation | 0 | 1 | 1 | 0 | 0 | |||||
| Benign tumour excision | 1 | 0 | 0 | 0 | 0 | |||||
| Cholecystectomy | 1 | 0 | 0 | 0 | 0 | |||||
| Coronary arterial stent insertion | 0 | 2 | 1 | 1 | 0 | |||||
| Gastric bypass | 0 | 0 | 1 | 2 | 0 | |||||
| Hysterectomy | 0 | 0 | 1 | 0 | 0 | |||||
| Knee operation | 0 | 0 | 1 | 0 | 1 | |||||
| Leg amputation | 1 | 0 | 0 | 0 | 0 | |||||
| Shoulder operation | 0 | 1 | 0 | 0 | 0 | |||||
| Skin lesion excision | 0 | 3 | 0 | 0 | 0 | |||||
| Stent placement | 1 | 1 | 1 | 2 | 0 | |||||
| Toe amputation | 0 | 0 | 1 | 0 | 0 | |||||
| Tooth extraction | 1 | 5 | 2 | 1 | 0 | |||||
| Vascular graft | 0 | 1 | 0 | 1 | 0 | |||||
| Wrist surgery | 0 | 1 | 0 | 0 | 1 | |||||
| Vascular disorders | ||||||||||
| Arterial occiusive disease | 0 | 2 | 0 | 0 | 0 | |||||
| Arteriosclerosis | 1 | 0 | 0 | 0 | 0 | |||||
| Arteriovenous fistula | 1 | 1 | 3 | 0 | 0 | |||||
| Carotid artery stenosis | 0 | 3 | 0 | 0 | 0 | |||||
| Cerebral haemorrhage | 0 | 0 | 0 | 0 | 1 | |||||
| Deep vein thrombosis | 1 | 1 | 0 | 0 | 0 | |||||
| Embolism | 1 | 0 | 0 | 0 | 0 | |||||
| Hypertension | 3 | 16 | 6 | 9 | 2 | |||||
| Hypotension | 2 | 4 | 1 | 1 | 0 | |||||
| Iliac artery occlusion | 1 | 0 | 0 | 0 | 0 | |||||
| Orthostatic hypotension | 0 | 1 | 1 | 0 | 0 | |||||
| Peripheral vascular disorder | 3 | 1 | 1 | 0 | 1 | |||||
| Poor peripheral circulation | 0 | 0 | 1 | 0 | 0 | |||||
| Pulmonary embolism | 0 | 1 | 1 | 0 | 0 | |||||
| Thrombosis | 0 | 0 | 1 | 0 | 0 | |||||
| Transient ischaemic attack | 2 | 1 | 0 | 0 | 0 | |||||
| Varicose vein | 0 | 1 | 0 | 0 | 0 | |||||
Comprehensive list of alt systemic adverse events as reported by the site using the Medical Dictionary for Regulatory Activities coding.
Table 19.
Summary of Study Eye Ocular Adverse Events
| Sham +Prompt Laser N = 488 | Ranibizumab+Prompt Laser N = 338 | Ranibizumab +Deferred Laser N = 384 | Triamcinolone +Prompt Laser N = 538 | |
|---|---|---|---|---|
| Anterior chamber | ||||
| Anterior chamber cell | 0 | 0 | 1 | 0 |
| Flat anterior chamber of eye | 1 | 0 | 1 | 0 |
| Hyphaema | 1 | 1 | 0 | 0 |
| Iris neovascularisation | 3 | 0 | 0 | 1 |
| Pigment dispersion syndrome | 0 | 0 | 1 | 0 |
| Cataract | ||||
| Cataract nuclear | 2 | 2 | 6 | 5 |
| Choroid | ||||
| Choroidal detachment | 1 | 0 | 0 | 1 |
| Choroidal neovascularisation | 4 | 1 | 0 | 0 |
| Conjunctiva | ||||
| Conjunctival haemorrhage | 7 | 31 | 42 | 25 |
| Conjunctival hyperaemia | 0 | 1 | 0 | 1 |
| Conjunctivitis | 3 | 5 | 1 | 4 |
| Conjunctivitis allergic | 3 | 3 | 2 | 1 |
| Conjunctivitis viral | 0 | 0 | 0 | 1 |
| Dry eye | 11 | 4 | 1 | 6 |
| Eye discharge | 6 | 3 | 1 | 1 |
| Eye inflammation | 0 | 0 | 0 | 1 |
| Keratoconjunctivitis sicca | 6 | 2 | 6 | 2 |
| Ocular hyperaemia | 4 | 6 | 3 | 1 |
| Pterygium | 1 | 0 | 0 | 0 |
| Cornea | ||||
| Arcus lipoides | 1 | 0 | 0 | 0 |
| Corneal abrasion | 5 | 2 | 6 | 4 |
| Comeal disorder | 0 | 3 | 0 | 0 |
| Corneal dystrophy | 0 | 0 | 1 | 0 |
| Corneal erosion | 1 | 0 | 0 | 0 |
| Corneal oedema | 0 | 1 | 0 | 1 |
| Corneal opacity | 0 | 1 | 0 | 0 |
| Comeal pigmentation | 0 | 1 | 0 | 0 |
| Corneal scar | 1 | 0 | 0 | 0 |
| Keratitis | 0 | 0 | 1 | 0 |
| Keratopathy | 1 | 1 | 0 | 0 |
| Punctate keratitis | 5 | 5 | 1 | 5 |
| Endocrine | ||||
| Retinal aneurysm | 0 | 0 | 1 | 0 |
| External | ||||
| Eye irritation | 13 | 10 | 14 | 11 |
| Eye swelling | 2 | 0 | 3 | 1 |
| Foreign body in eye | 1 | 0 | 2 | 4 |
| Hypersensitivity | 0 | 0 | 1 | 0 |
| Lacrimation increased | 12 | 8 | 23 | 5 |
| Seasonal allergy | 1 | 0 | 0 | 0 |
| Glaucoma-IOP | ||||
| Angle closure glaucoma | 0 | 1 | 0 | 0 |
| Borderline glaucoma | 2 | 2 | 0 | 4 |
| Glaucoma | 2 | 2 | 2 | 1 |
| Intraocular pressure decreased | 0 | 0 | 0 | 2 |
| Intraocular pressure increased | 16 | 8 | 11 | 79 |
| Ocular hypertension | 1 | 1 | 0 | 4 |
| Inflammation | ||||
| Iritis | 1 | 4 | 1 | 2 |
| Injection related | ||||
| Injection site extravasation | 0 | 0 | 0 | 1 |
| Lens | ||||
| Cataract | 23 | 19 | 20 | 50 |
| Cataract cortical | 8 | 4 | 4 | 1 |
| Cataract operation | 6 | 2 | 3 | 17 |
| Cataract operation complication | 1 | 0 | 0 | 0 |
| Cataract subcapsular | 8 | 6 | 7 | 28 |
| Posterior capsule opacification | 5 | 4 | 3 | 2 |
| Pseudoexfoliation of lens capsule | 0 | 1 | 0 | 0 |
| Lids | ||||
| Blepharitis | 4 | 2 | 6 | 2 |
| Chalazion | 1 | 0 | 1 | 0 |
| Entropion | 1 | 0 | 0 | 1 |
| Erythema of eyelid | 0 | 0 | 1 | 0 |
| Eyelid disorder | 0 | 0 | 0 | 1 |
| Eyelid oedema | 3 | 0 | 1 | 2 |
| Eyelid ptosis | 2 | 2 | 2 | 10 |
| Hordeolum | 1 | 0 | 1 | 0 |
| Periorbital haematoma | 0 | 1 | 0 | 0 |
| Pseudo-blepharoptosis | 0 | 0 | 0 | 1 |
| Miscellaneous | ||||
| Chemical eye injury | 0 | 1 | 0 | 0 |
| Eye discharge | 2 | 1 | 0 | 0 |
| Eye pain | 3 | 3 | 6 | 2 |
| Glaucoma | 2 | 1 | 0 | 3 |
| Ocular hyperaemia | 3 | 6 | 2 | 3 |
| Photopsia | 0 | 1 | 2 | 0 |
| Vision blurred | 1 | 2 | 1 | 1 |
| Visual impairment | 5 | 4 | 3 | 3 |
| Neurological | ||||
| Gliosis | 0 | 0 | 0 | 1 |
| Optic nerve | ||||
| Optic disc disorder | 1 | 0 | 0 | 0 |
| Retina | ||||
| Diabetic retinal oedema | 0 | 0 | 3 | 0 |
| Macular cyst | 1 | 1 | 0 | 0 |
| Macular degeneration | 0 | 1 | 0 | 2 |
| Macular hole | 0 | 1 | 2 | 0 |
| Macular ischaemia | 0 | 0 | 1 | 0 |
| Macular oedema | 1 | 1 | 1 | 0 |
| Maculopathy | 39 | 17 | 19 | 14 |
| Neovascularisation | 2 | 0 | 0 | 0 |
| Retinal aneurysm | 0 | 1 | 0 | 0 |
| Retinal degeneration | 0 | 1 | 0 | 1 |
| Retinal detachment | 0 | 1 | 1 | 0 |
| Retinal exudates | 2 | 3 | 1 | 1 |
| Retinal haemorrhage | 2 | 0 | 3 | 1 |
| Retinal neovascularisation | 6 | 2 | 1 | 0 |
| Retinal vein occlusion | 1 | 1 | 1 | 4 |
| Sensation-pain | ||||
| Abnormal sensation in eye | 0 | 0 | 1 | 1 |
| Asthenopia | 1 | 0 | 0 | 0 |
| Eye pain | 32 | 33 | 24 | 13 |
| Eye pruritus | 7 | 9 | 5 | 6 |
| Eyelid pain | 1 | 0 | 0 | 2 |
| Foreign body sensation in eyes | 8 | 3 | 6 | 12 |
| Skin | ||||
| Ecchymosis | 1 | 0 | 0 | 0 |
| Pruritus | 1 | 0 | 0 | 0 |
| Strabismus | ||||
| Extraocular muscle paresis | 0 | 1 | 0 | 0 |
| Visual field | ||||
| Visual field defect | 0 | 0 | 0 | 1 |
| Visual symptoms/abnormality | ||||
| Altered visual depth perception | 1 | 0 | 0 | 1 |
| Diplopia | 3 | 4 | 7 | 3 |
| Photophobia | 3 | 1 | 3 | 5 |
| Photopsia | 5 | 4 | 4 | 4 |
| Vision blurred | 55 | 23 | 29 | 42 |
| Visual acuity reduced | 19 | 9 | 15 | 21 |
| Visual disturbance | 16 | 7 | 6 | 14 |
| Visual field defect | 0 | 0 | 0 | 1 |
| Visual impairment | 4 | 2 | 6 | 1 |
| Vitreous | ||||
| Endophthalmitis | 2 | 2 | 2 | 0 |
| Hyalosis asteroid | 0 | 1 | 0 | 1 |
| Myodesopsia | 20 | 13 | 14 | 34 |
| Vitrectomy | 1 | 0 | 0 | 0 |
| Vitreous degeneration | 0 | 1 | 3 | 0 |
| Vitreous detachment | 9 | 5 | 7 | 8 |
| Vitreous disorder | 1 | 0 | 0 | 0 |
| Vitreous floaters | 9 | 14 | 14 | 28 |
| Vitreous haemorrhage | 30 | 6 | 9 | 9 |
| Vitreous opacities | 1 | 0 | 0 | 2 |
IOP = intraocular pressure
Discussion
In this randomized clinical trial, intravitreal ranibizumab, either with prompt or deferred (≥24 weeks) focal/grid laser, resulted in superior visual acuity and OCT outcomes compared with focal/grid laser treatment without ranibizumab at both 1 and 2 years of follow-up. Approximately half of the eyes treated with ranibizumab had substantial visual acuity improvement (≥10 letter gain from baseline), whereas approximately 30% gained ≥15 letters, equivalent to 3 lines on the eye chart, a reduction of the visual angle by half; substantial loss (≥10 letter loss from baseline) was uncommon. Among eyes treated with intravitreal ranibizumab, results were similar whether focal/grid laser was given starting with the first ranibizumab injection or it was deferred for at least 6 months. Overall, intravitreal triamcinolone combined with focal/grid laser did not result in superior visual acuity outcomes compared with laser without triamcinolone, although it did result in a greater reduction in retinal thickening at 1 year but not 2 years compared with laser alone. However, in an analysis limited to pseudophakic eyes, the triamcinolone + prompt laser group’s outcome for visual acuity was of similar magnitude to that of the 2 ranibizumab groups, suggesting that cataract formation, cataract surgery, or both, may have affected visual acuity outcomes adversely among phakic eyes in the triamcinolone + prompt laser group.
If ranibizumab is to be given as it was applied in this study, the 1- and 2-year data indicate a need to follow eyes continuously undergoing this treatment because the results indicate that additional ranibizumab or focal/grid laser, or both, are needed in most eyes through at least 2 years, even if ‘success’ criteria are met early in the course of treatment. According to the DRCR.net retreatment algorithm used in this study, eyes assigned to ranibizumab that met ‘success’ criteria at the 16-week study visit were not required to have continued injections unless visual acuity worsened or macular edema returned. Approximately two thirds of these early successes received additional ranibizumab at ≥1 visit after the 16-week visit. Furthermore, not all eyes avoided the need for focal/grid laser when following the protocol assigned to the ranibizumab + deferred laser group. Specifically, for eyes assigned to ranibizumab + deferred laser, approximately one third required focal/grid laser at least once between the 24-week and the 1-year study visits when the retreatment algorithm was followed.
These results are based on rigorous adherence to a detailed retreatment protocol facilitated by a web-based, real-time data-entry system that provided feedback to the treating physician regarding the treatment (intravitreal/sham injection or focal/grid photocoagulation) and subsequent follow-up interval to be prescribed at each follow-up visit. The retreatment algorithm followed in the study may appear detailed, but the underlying rationale is to continue anti-VEGF and focal/grid laser treatment, as needed, until stabilization or lack of further improvement is noted.
The details of the retreatment algorithm represent an attempt to have a rigorous protocol within a clinical trial that can address the many possible courses of the disease (variable improvements and deteriorations in visual acuity and retinal thickness). In addition, the retreatment algorithm attempts to minimize situations when retreatment might be recommended by the algorithm and yet not judged to be desired by the investigator, as might occur if monthly retreatments for 1 or 2 years were required. Once retreatment is withheld at a particular visit in lieu of monthly treatments for 1 or 2 years, the algorithm is designed to try to identify when the investigator might believe there is a need to reinitiate intravitreal anti-VEGF treatment, focal/grid laser treatment, or both, thereby avoiding substantial vision loss and a regimen that requires monthly treatments regardless of the clinical course. The relatively stable visual acuity outcomes between the 1- and 2-year visits in the ranibizumab groups suggest that this detailed retreatment algorithm accomplished these goals, although it is unknown whether treatment given every 4 weeks would have led to better outcomes. The impact of different retreatment approaches or use of other anti-VEGF drugs (e.g., bevacizumab) in clinical practice compared with this DRCR.net-specific protocol on visual acuity outcomes cannot be determined from this study.
We found no evidence that ranibizumab or triamcinolone is associated with an increased risk of systemic side effects or overall mortality, including cerebrovascular accidents and cardiovascular events. However, in view of the low number of observed events, a small increased risk cannot be ruled out. With respect to ocular adverse events, there was 1 case of progressive traction retinal detachment among the 375 eyes (0.3%) assigned to ranibizumab; this case had extramacular traction detachment before randomization, did not develop until 3 weeks after the 8 monthly consecutive intravitreal ranibizumab injections (when the concentration of the antibody in the vitreous should be low), and was among the 111 ranibizumab-treated eyes with prior panretinal photocoagulation, evidence of proliferative diabetic retinopathy, or both, at baseline. This complication has been suggested to be associated with intravitreal anti-VEGF injections in case series,15,16 although if ranibizumab truly is causative, the event seems to be uncommon, and the only case identified in this study did not have progressive detachment with vision loss until 8 monthly ranibizumab injections had been given. In fact, eyes in the ranibizumab groups were less likely to show progression of diabetic retinopathy, development of vitreous hemorrhage, or need for panretinal photocoagulation. More information regarding the possibility of this cause-and-effect relationship may be forthcoming in another DRCR.net protocol evaluating ranibizumab in the setting of proliferative diabetic retinopathy with DME.17
There were 3 cases of injection-related endophthalmitis, including 1 in which no antiseptic was applied to the injection site, which represented 1 of only 8 injections in which povidone iodine was not applied to the injection site. Fluorescein angiograms were not required in this study, so this study cannot determine whether there were cases of development or progression of macular capillary nonperfusion with anti-VEGF injections, as has been suggested in a case series.18 However, there were few eyes assigned to ranibizumab with a loss of ≥10 letters, suggesting that if this complication does occur in this setting, it is relatively uncommon and does not outweigh the benefits of treatment. As has been reported in prior studies,19-21 intravitreal triamcinolone was associated in this study with an increased risk of elevated IOP and cataract.
Subgroup analysis among pseudophakic eyes at baseline suggested that DRCR.net treatment using intravitreal triamcinolone combined with prompt focal/grid laser results in superior visual acuity outcomes compared with laser alone, although, as noted above, there is an elevated risk of increased IOP. These results are in contrast with a similar group of eyes treated with the same intravitreal triamcinolone formulation but without prompt focal/grid laser,5 in which outcomes for pseudophakic eyes at baseline were not superior using intravitreal triamcinolone compared with focal/grid laser. Although these differences in outcome could be due to differences in the characteristics of eyes between these 2 studies, it is logical to assume that the combination of 2 monotherapies (focal/grid laser and intravitreal triamcinolone) for DME in pseudophakic eyes, each of which seems to be superior to no treatment,5 is superior to focal/grid laser alone for pseudophakic eyes.
Some of the strengths of this study include its size, which provided relatively narrow CIs in the results presented, and good adherence to a strict protocol across multiple community- and institutional-based clinical sites throughout the United States. In addition, the data suggest that most study participants assigned to sham injection were successfully masked, because most believed they received actual injections. The data among study participants with 2 study eyes were similar to the overall results, suggesting there was little or no contralateral effect of ranibizumab on the fellow eye assigned to laser and no intravitreal injection, and providing a cohort in which all genetic, systemic, and environmental effects on DME should be well controlled. Although the 2-year data support the findings at 1 year, the 3-year data should help determine whether improvements noted to date are sustained and how often intravitreal ranibizumab or focal/grid laser is needed over time, whether starting with ranibizumab with prompt laser or ranibizumab with deferred laser. Some weaknesses of the study include the apparent complexity of the retreatment algorithm. Study participants assigned to the ranibizumab + deferred laser group or with 2 study eyes could not be masked if 1 eye was assigned to the ranibizumab + deferred laser group because prompt laser was not performed in that eye, thus unmasking the ranibizumab + deferred laser group to the treatment assignment. It also should be noted that neither intravitreal triamcinolone nor ranibizumab is currently approved for DME by the Food and Drug Administration; use of intravitreal ranibizumab or intravitreal triamcinolone for DME would be an off-label indication at this time.
The observed benefits of intravitreal anti-VEGF treatment for DME in this study are consistent with shorter-term improvements in visual acuity outcomes and resolution of DME on OCT noted with bevacizumab22 and ranibizumab.9 However, to our knowledge, no previous publications evaluating anti-VEGF drugs for DME have compared this treatment with concurrent controls receiving focal/grid laser with follow-up through at least 1 year. Other studies are under way that are comparing intravitreal ranibizumab alone, or in combination with laser, with laser alone over 1 year23 and comparing intravitreal ranibizumab alone with sham.24,25 Results from these and other studies should complement knowledge regarding the safety and efficacy of ranibizumab and other anti-VEGF drugs, alone, or in combination with laser, for the treatment of DME. The aggregate information from these studies also would be necessary to assess cost-effectiveness.
In conclusion, focal/grid laser has been the mainstay of treatment for DME during the past 25 years. On the basis of the data from this DRCR.net protocol, intravitreal ranibizumab with deferred (≥24 weeks) or prompt focal/grid laser is superior to focal/grid laser alone for the treatment of DME involving the center of the macula through at least 1 year of follow-up, with significantly more eyes gaining substantial vision and significantly fewer eyes losing substantial vision. Intravitreal ranibizumab as applied in this study, although uncommonly associated with endophthalmitis and theoretically associated with an increased risk of traction retinal detachments in eyes with proliferative diabetic retinopathy, should be considered for patients with DME and characteristics similar to those of the cohort in this clinical trial. In pseudophakic eyes, intravitreal triamcinolone with prompt focal/grid laser may be equally effective as ranibizumab at improving visual acuity and reducing retinal thickening but is associated with an increased risk of elevated IOP. Further follow-up is needed to determine even longer-term safety and efficacy of ranibizumab in the treatment of DME.
Acknowledgments
Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14229, and EY018817.
The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation of the manuscript. Genentech provided the ranibizumab for the study, and Allergan, Inc., provided the triamcinolone for the study. In addition, Genentech and Allergan, Inc., provided funds to the DRCR.net to defray the study’s clinical site costs. As described in the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, the ownership of the data, and all editorial content of presentations and publications related to the protocol. A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Neil M. Bressler:
Grants to investigators at The Johns Hopkins University are negotiated and administered by the institution (e.g., the School of Medicine) that receives the grants, typically through the Office of Research Administration. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the projects(s).
Dr. Neil M. Bressler is Principal Investigator of grants at The Johns Hopkins University sponsored by the following entities (not including the National Institutes of Health): Allergan, Bausch & Lomb, Carl Zeiss Meditec, EMMES Corporation, Genentech, Lumenis, Notal Vision Ltd.,* Novartis, QLT, Regeneron, Steba Biotech, Abbott Medical Optics, ForSight Labs, LLC, and Genzyme Corporation. Dr. Susan B. Bressler’s consulting arrangement with Notal Vision in connection with Dr. Neil M. Bressler’s role as principal investigator on a Notal Vision-sponsored research grant has been reviewed and managed by The Johns Hopkins University School of Medicine in accordance with its conflict of interest policy.
Appendix 1
Diabetic Retinopathy Clinical Research Network Laser-Ranibizumab-Triamcinlone Study Treatment
Baseline treatment
Injections of 0.5-mg ranibizumab and 4-mg preservative free triamcinolone (Trivaris, Allergan, Inc., Irvine, CA) were administrated with a standardized intravitreal injection technique which included a povidone iodine prep of the conjunctiva. Antibiotics in the pre-, peri-, and post-injection period were optional. The sham injection procedure consisted of placing the hub of a syringe against the conjunctival surface following the povidone–iodine prep. Focal/grid photocoagulation was administered using a technique modified from the original Early Treatment Diabetic Retinopathy Study (ETDRS) protocol as described previously and used in prior Diabetic Retinopathy Clinical Research Network (DRCR.net) protocols.26 The initial intravitreal (or sham) injection was given on the day of randomization. The 3 groups assigned to prompt laser received focal/grid photocoagulation 1 week (with a treatment window of 3 to 10 days) after the baseline intravitreal (or sham) injection.
Follow-up intravitreal study drug or sham retreatments through the 48-week study visit
The protocol required retreatment with intravitreal or sham injections (depending upon the randomized assignment at baseline) and focal/grid laser following guidelines outlined below, unless precluded by adverse events. The guidelines used to make retreatment decisions had different parameters for intravitreal or sham injections depending on which study visit was occurring. Prior to the 16-week study visit, treatment with sham or study drug was given every 4 weeks regardless of the visual acuity or optical coherence tomography (OCT) central subfield thickness. At the 16 and 20 week study visits, sham or study drug was required monthly unless ‘success’ criteria (defined as visual acuity letter score ≥84 (20/20) or OCT central subfield thickness <250; Table 1, available at http://aaojournal.org) was met, in which case sham or study drug injection was at investigator discretion. At each visit from 24 to 48 weeks, eyes were categorized as meeting either ‘success’ as defined above, ‘improvement’, ‘no improvement’, or ‘failure’ (defined below and Table 1, available at http://aaojournal.org). ‘Improvement’ required a sham or study drug injection and was defined as an eye that did not meet criteria for success but in which either visual acuity had improved by ≥5 letters or OCT central subfield thickness had improved by ≥10% since the last non-sham injection or since baseline for the sham+prompt laser group. If an eye was categorized as ‘no improvement’ because it met neither the criteria for ‘success’ or ‘improvement’, but had not yet met the criteria for ‘failure’ a sham or study drug injection could be given at investigator discretion. ‘Failure’ was defined as a visual acuity letter score 10 or more letters worse than the baseline score, OCT central subfield thickness ≥250 um, diabetic macular edema (DME) judged to be the cause of visual acuity loss and duration of at least 13 weeks since ‘complete laser’ (defined in Table 1, available at http://aaojournal.org) had been given with no improvement since the last laser treatment. Eyes that met ‘failure’ criteria could be treated at investigator discretion with a sham or randomized study drug injection or with an ‘alternative’ treatment regimen (defined in Table 1, available at http://aaojournal.org) other than that assigned at baseline, such as intravitreal bevacizumab or triamcinolone. Appendix 2 (available at http://aaojournal.org) provides a detailed flow chart of study drug or sham retreatments through the 48-week study visit.
Intravitreal study drug retreatments after the 48-week study visit
At and after the 1 year study visit (Appendix 3, available at http://aaojournal.org), sham injections were discontinued and follow-up study visits occurred at every 4 months instead of monthly for eyes in the sham+prompt laser group and the triamcinolone+prompt laser group. Eyes in the two ranibizumab groups that met failure criteria also had study visits every 4 months. For eyes assigned to receive injections, the same retreatment criteria were followed as at the 24 to 48 week visit with two additional considerations. First, the treatment regimen could be at investigator discretion, including treatments other than the randomly assigned treatment, not only for eyes that met ‘failure’ criteria but also for eyes that met ‘futility’ criteria (defined below and Table 1, available at http://aaojournal.org). ‘Futility’ criteria were defined similarly to failure criteria except that visual acuity was not required to be worse than baseline if it had been at least 29 weeks since ‘complete laser’ and all other criteria for ‘failure’ were met. Second, for eyes assigned to ranibizumab in which the injection was deferred at the current and previous two visits either due to ‘success’ or ‘no improvement’, as defined above, then the follow-up could be extended. ‘Extended follow-up’ (defined in Table 1, available at http://aaojournal.org) visits occurred at intervals that were twice the time since the previous visit, up to a maximum of 16 weeks between study visits.
Follow-up focal/grid laser treatment
Application of focal/grid laser treatment at and after the 16-week study visit (Appendix 4, available at http://aaojournal.org) for each group except the ranibizumab+deferred laser group occurred 3 to 10 days following each intravitreal (or sham) injection unless one of the following was present at the time of the injection: (1) laser was given in the previous 13 weeks; (2) the investigator considered that ‘complete laser’ (direct treatment to all microaneurysms within areas of edema and grid treatment to all other areas of macular edema) had already been applied; or (3) OCT central subfield thickness was <250 microns and there was ‘no edema threatening the center of the macula’, defined as no edema within 500 microns of the center of the macula, no edema associated with lipid within 500 microns of the center of the macula, and no edema ≥ 1 disc area within 1 disc area of the center of the macula (defined in Table 1, available at http://aaojournal.org). For the ranibizumab+deferred laser group, at the 24-week and any later visit, if there was ‘no improvement’ as defined above from the last two study injections and the investigator believed that macular edema was present for which focal/grid laser was indicated, the eye was to receive focal/grid laser until the edema resolved or ‘complete laser’ was given using the same criteria defined for eyes assigned to ‘prompt’ focal-grid photocoagulation.
Appendix 2
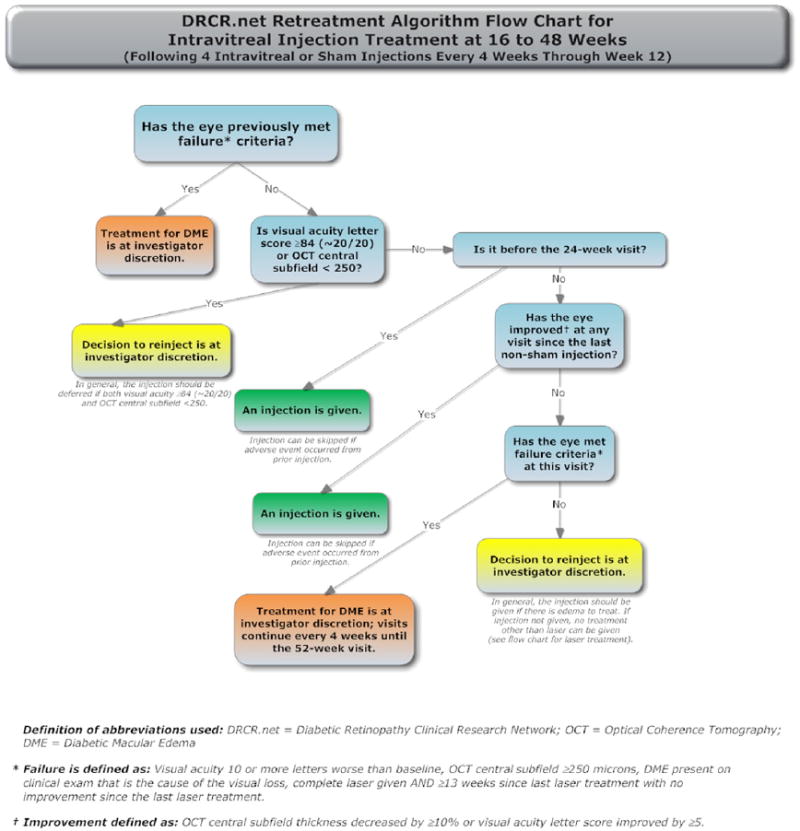
Appendix 3

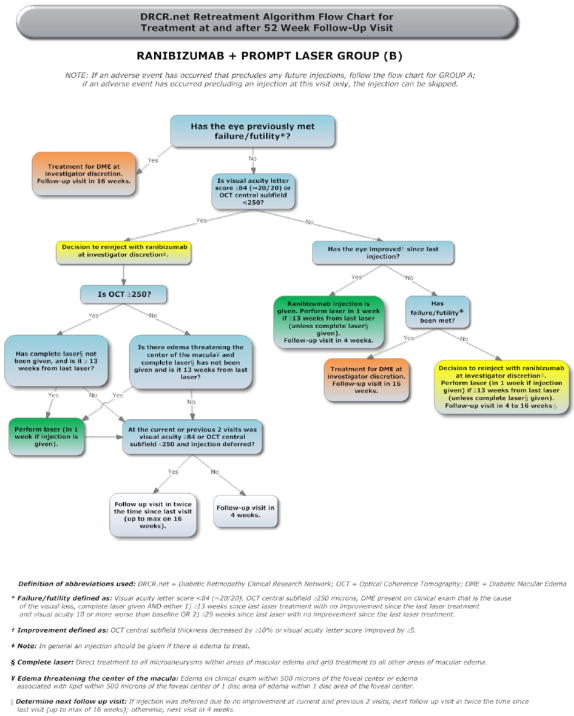
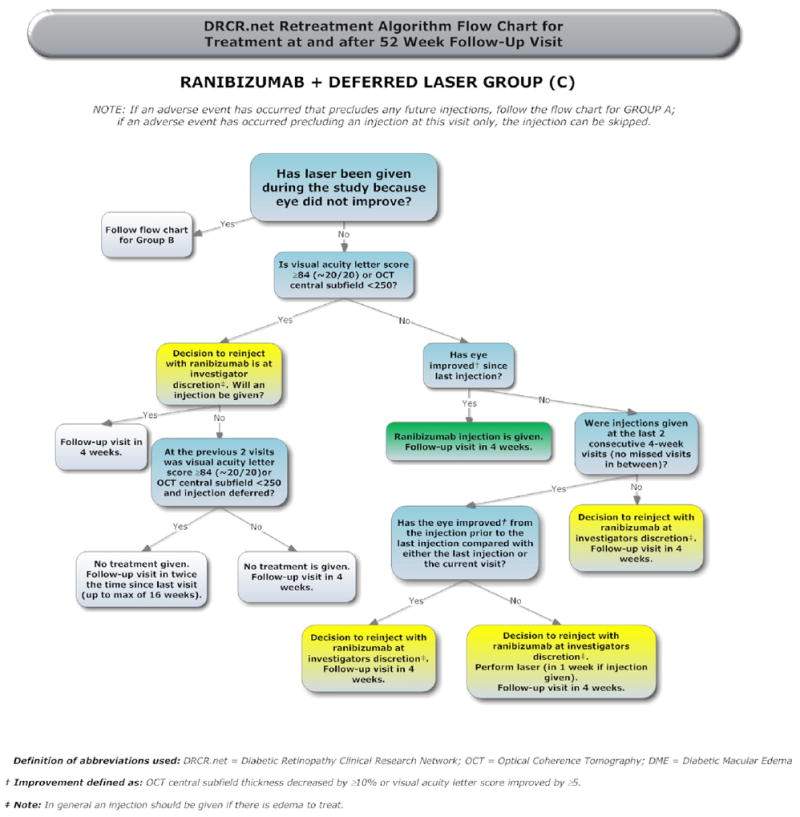

Appendix 4
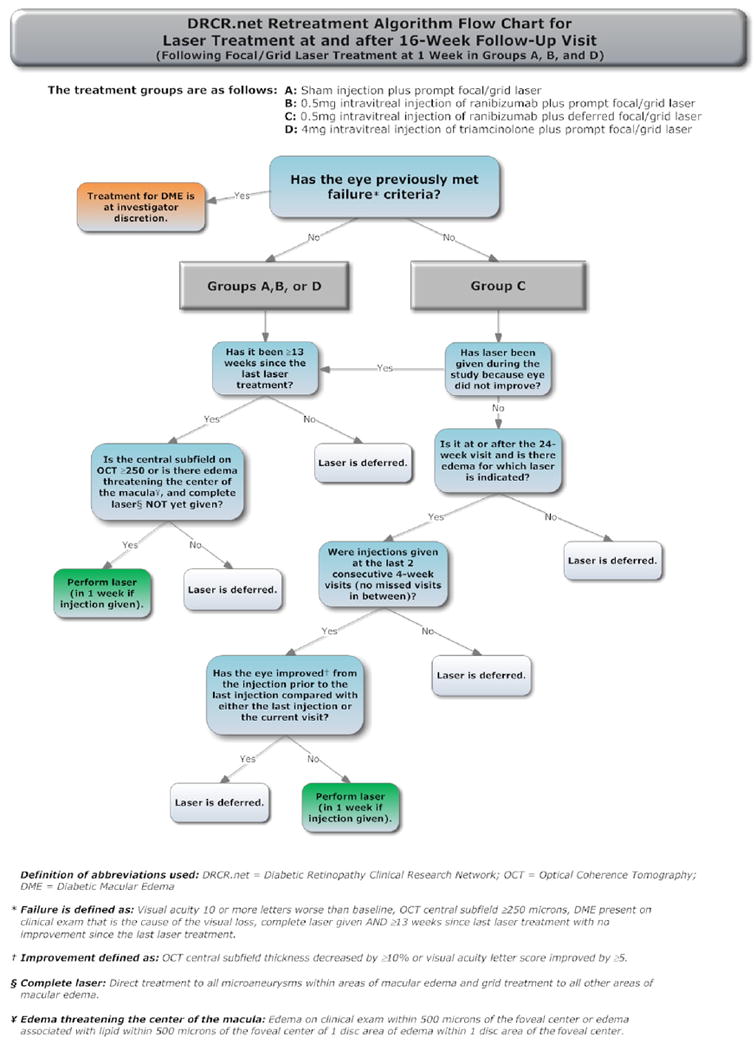
Appendix 5(website)
Diabetic Retinopathy Clinical Research Network Clinical Sites that participated on this protocol
Sites are listed in order by number of subjects enrolled into the study. The number of subjects enrolled is noted in parenthesis preceded by the site location and the site name. Personnel are listed as (I) for Study Investigator, (C) for Coordinator, (V) for Visual Acuity Tester, and (P) for Photographer.
Baltimore, MD Elman Retina Group, P.A. (90) Michael J. Elman (I); Michelle D. Sloan (C); Theresa M. Butcher (C); JoAnn Starr (C,V); Nancy Gore (V); Teresa Coffey (V); Pamela V. Singletary (V); Dena Y. Salfer-Firestone (V); Giorya Andreani (P); Daniel J. Ketner (P); Peter Sotirakos (P); Terri Cain (P) Jacksonville, FL University of Florida College of Med., Department of Ophthalmology, Jacksonville Health Science Cent (54) Kakarla V. Chalam (I); Sandeep Grover (I); Shailesh K. Gupta (I); Tamil M. Singh (C,P); Ravi Keshavamurthy (C,V); Swati Agarwal (C,P); William W. Phillips (C,P); Jason Sifrit (V); Manish C. Patel (V); Vikram S. Brar (P); John R. Carpentier (P) Indianapolis, IN Raj K. Maturi, M.D., P.C. (46) Raj K. Maturi (I); Thomas Ciulla (I); Nicholas F. Hrisomalos (I); Laura A. Bleau (C,P,V); Carolee K. Novak (V); Michelle Storie (V); Thomas Steele (P); Abby Maple (P); Jama L. Poston (P); Ashley Harless (P) Lakeland, FL Florida Retina Consultants (45) Scott M. Friedman (I); Oren Z. Plous (I); Kelly A. Blackmer (C); Jolleen S. Key (C,P,V); Karen Sjoblom (P,V); Jessica Maldonado (P); Sheila Walters-Treon (P); Allen McKinney (P,V); Katie Gostischa (P); Steve Carlton (P) Charlotte, NC Charlotte Eye, Ear, Nose and Throat Assoc., PA (31) David J. Browning (I); Justin C. Brown (I); Andrew N. Antoszyk (I); Danielle R. Brooks (C,V); Angela K. Price (C,V); Melissa K. Cowen (C,V); Jennifer V. Helms (C,V); Sarah A. Ennis (V); Rachel E. Pierce (V); Angella S. Karow (V); Wayne Lail (V); Michele E. Powers (P); Donna McClain (P); Richard J. George (P); Loraine M. Clark (P); Krystie A. Schlicker (P); Pearl A. Leotaud (P); Amanda R. Vittitow (P); Uma M. Balasubramaniam (P); Linda M. Davis (P); Michael D. McOwen (P); Jennifer A. Ballard (P) Portland, OR Casey Eye Institute (28) Andreas K. Lauer (I); Peter J. Francis (I); Steven T. Bailey (I); Thomas S. Hwang (I); Christina J. Flaxel (I); Susan I. Pope (C,V); Maureen D. Toomey (V); Susan K. Nolte (V); Shirley D. Ira (V); Teresa Liesegang (V); Ann D. Lundquist (V); Mitchell Schain (V); Debora R. Vahrenwald (V); Chris S. Howell (P); Joseph Cilio Rossi (P); Patrick R. Wallace (P); Kelly L. West (P); Peter N. Steinkamp (P); Patrick B. Rice (P); Scott R. Pickell (P) Lexington, KY Retina and Vitreous Associates of Kentucky (22) Thomas W. Stone (I); John W. Kitchens (I); William J. Wood (I); Rick D. Isernhagen (I); Diana M. Holcomb (C); Judith L. Cruz (V); Cathy A. Sears (V); Brenda VanHoose (V); Michelle Buck (V); Jenny L. Wolfe (V); Jeanne Van Arsdall (V); Wanda R. Heath (V); Edward A. Slade (P); Stephen T. Blevins (P); Terri Kidd (P) Knoxville, TN Southeastern Retina Associates, P.C. (19) Tod Alan McMillan (I); Stephen Lee Perkins (I); Nicholas Gray Anderson (I); Joseph M. Googe (I); Christina T. Higdon (C,V); Stephanie Evans (C); Charity D. Morris (C); Cecile Hunt (V); Misty Moore (V); Mary M. Johnson (V); Kristina Oliver (V); Vicky L. Seitz (V); Ann Arnold (V); Michael Jacobus (P); Jerry K. Whetstone (P); Paul A. Blais (P); Sarah M. Oelrich (P) West Columbia, SC Palmetto Retina Center (19) W. Lloyd Clark (I); John A. Wells (I); Mallie M. Taylor (C); Cassie P. Cahill (C,V); Marcia D. Gridine (C,V); Peggy D. McDougal (V); Kayla L. Henry (V); Robbin Spivey (P); Melissa L. Henderson (P); Pennie Tankersley (P); LaDetrick L. Oliver (P); Amy B. Hickman (P) Artesia, CA Sall Research Medical Center (18) Joseph B. Michelson (I); Laura Anne Teasley (I); Patricia Manjarrez (C); Anabelle Garcia (C,P); Cindy Lee (C); Gabriela Suderno (V); Jenny Keppler (V); Paul Yoo (V); Paul Paquette (P) Walnut Creek, CA Bay Area Retina Associates (18) Stewart A. Daniels (I); T. Daniel Ting (I); Subhransu K. Ray (I); Craig J. Leong (I); Maria Carmencita Aguilos (C); Kathleen J. Dowell (C); Grace M. Marudo (C,V); Cindy M. Moreci (C); Rouella J. Tejada (V); Tia H. Nguyen (V); Sean M. Teshima-McCormick (V); Ashley Schrock (V); William M. Combs (V); Nicole Hom (V); Matthew D. Hughes (P); Fred Hanamoto (P) Ft. Lauderdale, FL Retina Group of Florida (17) Mandeep Singh Dhalla (I); W. Scott Thompson (I); Scott Anagnoste (I); Jaclyn A. Brady-Lopez (C); Cindy V. Fernandez (C); Evelyn Quinchia (V); Jamie Mariano (V); Clifford M. Sherley (V); Patricia Aramayo (P); Melissa L. Ritchie (P); Karen L. McHugh (P); Brian M. Fernandez (P) Houston, TX Retina and Vitreous of Texas (16) H. Michael Lambert (I); Arthur W. Willis (I); Joseph A. Khawly (I); Roberto Diaz-Rohena (I); Pam S. Miller (C,V); Susan K. Busch (C,P,V); Debbie Fredrickson (V); Valerie N. Lazarte (V); Kevin L. Davis (V); Joseph A. Morales (P); Kristopher J. Chase (P); Donald K. Lowd (P); Jason E. Muniz (P); Allison W. Schmidt (P) Minneapolis, MN Retina Center, PA (16) Abdhish R. Bhavsar (I); Geoffrey G. Emerson (I); Michael Vaughn Emerson (I); Vu T. Huynh (C,P,V); Tanya M. Olson (C); DeAndra J. Boll (C); Miguelina Yafchak (C); Craig H. Hager (V); Samillya L. Pearson (V); Dwight L. Selders (V); Christopher M. Smith (P); Carmen Chan-Tram (P); William B. Carli (P); Jessica A. Kells (P); Laura Taylor-Reetz (P) Baltimore, MD Wilmer Eye Institute at Johns Hopkins (15) Sharon D. Solomon (I); Adrienne Williams Scott (I); Neil M. Bressler (I); Diana V. Do (I); Susan Bressler (I); Mary Frey (C,V); Sandra West (C,V); Deborah Donohue (V); Vanessa Kellner (V); Dennis Cain (P); Janis Graul (P); Jacquelyn Mc-Donald (P); David Emmert (P); Syed M. Shah (P); Judith Belt (P); Charles Herring (P) Loma Linda, CA Loma Linda University Health Care, Department of Ophthalmology (14) Joseph T. Fan (I); Mukesh Bhogilal Suthar (I); Michael E. Rauser (I); Cara L. Davidson (C,V); Gisela Santiago (C); Kara E. Rollins (C,P,V); Carrousel J. Corliss (C); Christy G. Quesada (C,V); William H. Kiernan (V); Rene G. Obispo (P); Jesse Knabb (P) Paducah, KY Paducah Retinal Center (14) Carl W. Baker (I); Tracey M. Caldwell (C); Tracey R. Martin (V); Mary J. Palmer (V); Lynnette F. Lambert (V); Tana R. Williams (P); Alecia B. Travis (P); Dawn D. Darden (P) Austin, TX Retina Research Center (12) Brian B. Berger (I); Eric Chen (I); Robert W. Wong (I); Kristen Davis (C); Julie R. Lummus (C); Ginger J. Manhart (C); Telisa L. Clevenger-Smith (C); Nicole Callen (V); Michael T. Gartner (V); Jamie L. Sun (V); Gilbert L. Abeyta (V); Ben Ostrander (P); Yong Ren (P) Columbia, SC Carolina Retina Center (11) Jeffrey G. Gross (I); Michael A. Magee (I); Amy M. Flowers (C,P,V); Kayla L. Henry (C,V); Angelique SA McDowell (V); Cori M. Fore (V); Heidi K. Lovit (V); Jason C. Rohrer (V); Kristin K. Bland (V); Ally M. Paul (P); Chris N. Mallet (P); Rick Christoff (P); Randall L. Price (P) Madison, WI University of Wisconsin-Madison, Dept of Ophthalmology/Retina Service (11) Justin L. Gottlieb (I); Barbara A. Blodi (I); Michael S. Ip (I); Kathryn F. Burke (C,V); Barbara H. Soderling (C,V); Shelly R. Olson (V); Angela M. Wealti (V); Guy F. Somers (V); Kristine A. Dietzman (V); Gene E. Knutson (P); Denise A. Krolnik (P); John C. Peterson (P) Beachwood, OH Retina Associates of Cleveland, Inc. (10) Michael A. Novak (I); Joseph M. Coney (I); David G. Miller (I); Lawrence J. Singerman (I); Larraine Stone (C); Elizabeth McNamara (C,P,V); Trina M. Nitzsche (V); Kimberly A. Dubois (V); Vivian Tanner (V); Tamara L. Cunningham (P); Sheila K. Smith-Brewer (P); John C. DuBois (P); Gregg A. Greanoff (P) Boston, MA Joslin Diabetes Center (10) Jennifer K. Sun (I); Lloyd Paul Aiello (I); Deborah K. Schlossman (I); Sabera T. Shah (I); Paul G. Arrigg (I); Paolo S. Silva (I); George S. Sharuk (I); Timothy J. Murtha (I); Margaret E. Stockman (C,V); Julie A. Barenholtz (C,V); Rita K. Kirby (V); Richard M. Calderon (P); Jerry D. Cavallerano (V); John C. BuAbbud (V); Elizabeth S. Weimann (P); Leila Bestourous (V); Robert W. Cavicchi (P); Ann Koplle (C) Lubbock, TX Texas Retina Associates (10) Michel Shami (I); Stephen R. Smith (I); Yolanda Saldivar (C); Phyllis Pusser (C); Ashaki Meeks (V); Natalie R. Garcia (V); Linda Squires (V); Carrie L. Tarter (V); Thom F. Wentlandt (P) Portland, OR Retina Northwest, PC (9) Mark A. Peters (I); Craig A. Lemley (I); Michael S. Lee (I); Irvin L. Handelman (I); Richard F. Dreyer (I); Stephen Hobbs (C,P,V); Dawn A. Brunelle (C,P,V); Marcia Kopfer (V); Wendy Raunig (V); Gina Durbin (V); Howard Daniel (P); Joe Logan (P); Christophe N. Mallet (P); Harry Wohlsein (P) Santa Barbara, CA California Retina Consultants (9) Dante J. Pieramici (I); Ma’an A. Nasir (I); Alessandro A. Castellarin (I); Melvin D. Rabena (C); Jerry Smith (C,V); Amy L. Sterling (V); Debbie Hernandez (V); Kelly Avery (V); Jessica C. Basefsky (V); Liz Tramel (V); Karen Boyer (P); Sarah M. Risard (P); Matthew Giust (P) Winston-Salem, NC Wake Forest University Eye Center (9) Craig Michael Greven (I); Madison M. Slusher (I); Joan Fish (C,V); Cara Everhart (C,V); Frances Marie Ledbetter (C,V); Lori N. Cooke (C,V); David T. Miller (P); Mark D. Clark (P); Marshall Tyler (P) Augusta, GA Southeast Retina Center, P.C. (8) Dennis M. Marcus (I); Harinderjit Singh (I); Graciela R. Zapata (C); Mari Carrie McAteer (C); Donyale Blair (C); Kasie A. Leverett (V); Catherine Powell (V); Carrie M. Hill (V); Kimbi Y. Overton (V); Julie C. Coxville (V); Ken Ivey (P); Victoria Lynne Oldag (P) Fort Myers, FL Retina Consultants of Southwest Florida (8) Thomas A. Ghuman (I); Glenn Wing (I); Joseph P. Walker (I); Paul A. Raskauskas (I); Ashish G. Sharma (I); Richard W. Grodin (I); Cheryl Kiesel (C); Jennifer L. Frederick (C); Eileen Knips (C,P); Cheryl Ryan (C); Crystal Y. Peters (C); Jennifer M. Banks (V); Danielle Dyshanowitz (V); Etienne C. Schoeman (P) Syracuse, NY Retina-Vitreous Surgeons of Central New York, PC (8) Robert G. Hampton (I); Paul F. Torrisi (I); Bryan K. Rutledge (I); Samuel C. Spalding (I); Cindy J. Grinnell (C); Michelle L. Manley (V); Lynn M. Kwasniewski (V); Peter B. Hay (P); Lynn A. Capone (P); Kelly M. Harrison (P) Beverly Hills, CA Retina-Vitreous Associates Medical Group (7) Roger L. Novack (I); David Boyer (I); Homayoun Tabandeh (I); Amanda Tam (C); Saba Mukarram (C); Tamara Gasparyan (C); Jaime K. Gilmour (V); Jackie Sanguinet (V); Julio Sierra (V); Sarah E. Pachman (V); Eric G. Protacio (P); Jeff Kessinger (P); Adam Smucker (P) Chapel Hill, NC University of North Carolina, Dept of Ophthalmology (7) Mary Elizabeth R. Hartnett (I); Travis A. Meredith (I); Seema Garg (I); Odette M. Houghton (I); Cassandra J. Barnhart (C,V); Fatoumatta N’Dure (C,V); Debbie Morck (V); Debra Cantrell (P); Rona Lyn Esquejo (P) Seattle, WA University of Washington Medical Center (7) James L. Kinyoun (I); Gurunadh Atmaram Vemulakonda (I); Susan A. Rath (C,V); Pendra Kay Burrows (V); Patricia K. Ernst (V); Juli A. Pettingill (V); Brad C. Clifton (P); James D. Leslie (P); Chuck Stephens (P) Boston, MA Ophthalmic Consultants of Boston (6) Trexler M. Topping (I); Tina A. Cleary (I); Lesley-Anne Freese (C,V); Lindsey Williams (C,V); Victoria M. Hurley (C); Paula P. Zand (C); Emily A. Corey (V); Jennifer L. Stone (V); Taneika N. Howard (V); Robin Ty (V); Sandy G. Chong (V); Katie L. Moses (V); Margie Graham (P); Steve A. Bennett (P); Michael Cullen Jones (P) Dubuque, IA Medical Associates Clinic, P.C. (6) Michael H. Scott (I); Philomina M. Wiegman (C); Maureen M. Runde (C); Thomas R. Dvorak (V); Marcia J. Humphrey (P); Brenda L. Tebon (P) Milwaukee, WI Medical College of Wisconsin (6) Judy E. Kim (I); Dennis P. Han (I); David V. Weinberg (I); Thomas B. Connor (I); William Wirostko (I); Kimberly E. Stepien (I); Vesper V. Williams (C); Jeanette Graf (C); Krissa L. Packard (C); Sharon Rekow (C); Dawn Alvarez (C,V); Judy Flanders (V); Vicki Barwick (V); Dennis B. Backes (P); Joseph R. Beringer (P); Kristy L. Keller (P); Kathy J. Selchert (P) Cleveland, OH Case Western Reserve University (5) Suber S. Huang (I); Shawn C. Wilker (I); Johnny Tang, MD (I); Anchal Malik (C); Kathy Carlton (V); Claudia Clow (V); Stephanie Burke (P); Geoffrey Pankhurst (P); Mark A. Harrod (P) Dallas, TX Texas Retina Associates (5) Gary E. Fish (I); Robert C. Wang (I); Jean Arnwine (C); Carrie L. Tarter (C); Brenda Sanchez (V); Sally Arceneaux (V); Hank Aguado (P); Kimberly Cummings (P); Keith Gray (P); Michael Mackens (P); Betsy L. Hendrix (P); Diana Jaramillo (P) Philadelphia, PA University of Pennsylvania Scheie Eye Institute (5) Alexander J. Brucker (I); Sheri Grand Drossner (C,V); Joan C. DuPont (C,V); Wei Xu (V); Cheryl Devine (P); William Nyberg (P); Laurel Weeney (P); Jim M. Berger (P) San Antonio, TX Retinal Consultants of San Antonio (5) Calvin E. Mein (I); Moises A. Chica (I); Lita Kirschbaum (C,V); Ercilia Riff (C); Marianne F. Tadros (C,P); Christopher Sean Weineke (P); Brenda Nakoski (P) Chicago, IL Illinois Retina Associates, S.C. (4) Mathew W. MacCumber (I); Katherine Lluen-Nunez (C); Chris Droira (V); Joanne Pleskovich (P) Hershey, PA Penn State College of Medicine (4) Kimberly A. Neely (I); Ingrid U. Scott (I); Thomas W. Gardner (I); Susan M. Chobanoff (C,V); Laura E. Walter (V); Mary Hershey (V); James D. Strong (P); Timothy J. Bennett (P) New Albany, IN John-Kenyon American Eye Institute (4) Howard S. Lazarus (I); Debra Paige Bunch (C,V); Angela D. Ridge (C,V); Kelly Booth (V); Jay Moore (P); Margaret Trimble (P) Irvine, CA University of California, Irvine (3) Baruch D. Kuppermann (I); Jeff Grijalva (C); Rosie Magallon (V); Bret Trump (P) Kingsport, TN Southeastern Retina Associates, PC (3) Howard L. Cummings (I); Deanna Jo Long (C,P); Jeni Jill Vermillion (C); Stacy Carpenter (V); Julie P. Berry (P) New York, NY The New York Eye and Ear Infirmary/Faculty Eye Practice (3) Ronald C. Gentile (I); Estuardo Alfonso Ponce (I); Anita Ou (C,V); Peggy Guerrero (C); Catiria Guerrero (V); Jenny M. Gallardo (V); Violete Perez (V); Katy W. Tai (V); Julie A. Paa (V); Dominique Jampol (P); Robert Masini (P); Paul Whitten (P); Wanda Carrasquillo-Boyd (P); Kenneth Boyd (P) Palm Desert, CA Southern California Desert Retina Consultants, MC (3) Clement K. Chan (I); David M. Salib (I); Steven G. Lin (I); Asha S.D. Nuthi (I); Kimberly S. Walther (C); Isela Aldana (C); Eric D. Dickerson (C); Lenise E. Myers (V); Sara Warren (V); Sandra U. Castillo (V); Kenneth M. Huff (P); Donna J. Chesbrough (P) Chicago, IL University of Illinois at Chicago Medical Center (2) Michael Blair (I); Jennifer I. Lim (I); Marcia Niec (C); Tametha Johnson (V); Yesenia Ovando (V); Mark Janowicz (P); Catherine Carroll (P) Denver, CO Denver Health Medical Center (2) Jon M. Braverman (I); Antonio P. Ciardella (I); Hugo Quiroz-Mercado (I); Leif S. Ryman (C); Rosemary C. Rhodes (V); Sasha I. Montalvo (V); Scott R. Harloff (P); Debbie M. Brown (P) Hermitage, PA Vitreo-Retinal Consultants, Inc. (2) Stephen R. Kaufman (I); Marc F.G. Estafanous (I); Kathleen A. Huff (C); Amy E. Lamancusa (C); Rhonda Fill (V); Meagan Peace (V); Denise M. Williams (P) Joliet, IL Illinois Retina Associates (2) John S. Pollack (I); Mathew W. MacCumber (I); Barbara J. Ciscato (C,V); Katherine Lluen-Nunez (C); Belinda M. Kosinski (V); Chris Droira (V); Daniel W. Muir (P); Joanne Pleskovich (P) Portsmouth, NH Eyesight Ophthalmic Services, PA (1) Richard Chace (I); Sunny Kallay (C); Nicole Dolbec (V); Kirsten Stevens (V); Ronda Baker-Hill (V); Janea Halbmaier (P) Providence, RI Retina Consultants (1) Caldwell W. Smith (I); Harold A. Woodcome (I); Edwina Rego (C); Collin L. DuCoty (C); Sylvia Varadian (C); Claudia Salinas (V); Erika Banalewicz (V); Alex L. Nagle (P); Mark Hamel (P)
DRCR.net Coordinating Center
Jaeb Center for Health Research, Tampa, FL (staff as of 02/10/2010): Adam R. Glassman (Director and Principal Investigator), Roy W. Beck (Executive Director) Talat Almukhtar, Bambi J. Arnold, Brian B. Dale, Alyssa Baptista, Lina Caicedo, Sharon R. Constantine, Simone S. Dupre, Allison R. Edwards, Lauren Huffman, Meagan L. Huggins, Paula A. Johnson, Lee Anne Lester, Brenda L. Loggins, Shannon L. McClellan, Michele Melia, Kellee M. Miller, Pamela S. Moke, Haijing Qin, Rosa Pritchard, Eureca Scott, Cynthia R. Stockdale, Emily Williams
Fundus Photograph Reading Center
University of Wisconsin-Madison, Madison, WI (staff as of 02/10/10): Matthew D. Davis (Director Emeritus), Ronald P. Danis (Director and Principal Investigator), Larry Hubbard (Associate Director), James Reimers (Lead Color Photography Evaluator), Pamela Vargo (Lead Photographer), Ericka Moeller (Digital Imaging Specialist), Dawn Myers (Lead OCT Evaluator), Kristjan Burmeister (Project Manager)
DRCR.net Operations Center
Johns Hopkins University School of Medicine, Baltimore, MD (staff as of February 10, 2010): Neil M. Bressler (Network Chair and Principal Investigator), Connie Lawson, Peggy R. Orr.
DRCR.net Vice Chairs
Susan B. Bressler (2009-current), Scott Friedman (2009-current), Ingrid U. Scott (2009-Current).
National Eye Institute
Eleanor Schron (2009-current), Donald F. Everett (2003–2006, 2007–2009), Päivi H. Miskala (2006 –2007)
Executive Committee
Raj K. Maturi (2009-present; Chair 2010) Neil M. Bressler (2006-Current; Chair 2006–2008), Lloyd Paul Aiello (2002-present; Chair 2002–2005), Carl Baker (2009-present), Roy W. Beck (2002-present), Susan B. Bressler (2009-Present), Alexander J. Brucker (2009–present), Kakarla V. Chalam (2009-present), Ronald P. Danis (2004-present), Matthew D. Davis (2002-present), Michael J. Elman (2006-present; Chair 2009), Frederick L. Ferris III (2002-present), Scott Friedman (2007-present), Adam R. Glassman (2005-present), Joseph Googe, Jr. (2009-present), Eleanor Schron (2009-present), Ingrid U. Scott (2009-Present), JoAnn Starr (2009-present), Jennifer K. Sun (2009-present). Prior Members: Andrew N. Antoszyk (2009), Abdhish Bhavsar (2007–2008), David M. Brown (2006–2007), David J. Browning (2005–2006), Donald F. Everett (2002–2009), Joan Fish (2008–2009), Andreas Lauer (2007–2008), Kim McLeod (2002–2006), Päivi H. Miskala (2005–2007), Cynthia J. Grinnell (2006–2007).
DRCR.net Data and Safety Monitoring Committee
John Connett, (Chair 2003-Current), Deborah Barnbaum (2006-Current), Harry W. Flynn, Jr. (2003-Current), Robert N. Frank (2003-Current), Saul Genuth (2003-Current), Lee Jampol (2003-Current), Stephen Wisniewski (2003- Current),
Prior Member: Jeanette Resnick (2003–2006)
Prior DRCR.net VEGF Steering Committee Members
Alexander J. Brucker (2007–2008), Michael J. Elman (2007–2008), Joseph Googe, Jr. (2008), Lloyd P. Aiello (2006–2008), Roy W. Beck (2006–2008), Neil M. Bressler (2006–2008), Kakarla V. Chalam (2008), Ronald P. Danis (2006–2008), Donald F. Everett (2006–2008), Frederick L. Ferris III (2006–2008), Adam Glassman (2006–2008), Tina Higdon (2008), Abdhish Bhavsar (2006–2007), David Browning (2006–2008), Peter Campochiaro (2006–2007), Joan Fish (2006–2007), Scott M. Friedman (2006–2008), Mary Elizabeth R. Hartnett (2006–2007), Raj Maturi (2008–2008), Päivi Miskala (2006–2007), Ingrid Scott (2006–2008).
Footnotes
Financial Disclosure(s): Proprietary or commercial disclosure may be found after the references.
The lead author(s) have made the following disclosure(s): Scott M. Friedman: Sirion Therapeutics (S), MacuSight (S), Pfizer (S), Vitreoretinal Technologies (S), Allergan (S), EMMES Corporation (S). Ingrid U. Scott: Genentech (C). Lloyd P. Aiello: Genentech (C). Susan B. Bressler: Glaxo- SmithKline (C). Frederick L. Ferris III; Bausch & Lomb (P).
This article contains online-only material. The following should appear online-only: Tables 1, 2, 3, 4, 6, 7, 9, 10, 12, 13, 14, 16, 18, and 19; Figures 1, 2, 5, 7, and 8; Appendices 1, 2, 3A-D, 4, and 5.
References
- 1.Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. IV Diabetic macular edema. Ophthalmology. 1984;91:1464–74. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 2.Moss SE, Klein R, Klein BE. The 14-year incidence of visual loss in a diabetic population. Ophthalmology. 1998;105:998–1003. doi: 10.1016/S0161-6420(98)96025-0. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Ten-year incidence of visual loss in a diabetic population. Ophthalmology. 1994;101:1061–70. doi: 10.1016/s0161-6420(94)31217-6. [DOI] [PubMed] [Google Scholar]
- 4.Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985;103:1796–806. [PubMed] [Google Scholar]
- 5.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–59. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 7.Antonetti DA, Barber AJ, Hollinger LA, et al. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1: a potential mechanism for vascular permeability in diabetic retinopaty and tumors. J Bio Chem. 1999;274:23463–7. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- 8.Macugen Diabetic Retinopathy Study Group. Changes in retinal neovascularization after pegaptanib (Macugen) therapy in diabetic individuals. Ophthalmology. 2006;113:23–8. doi: 10.1016/j.ophtha.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen QD, Shah SM, Heier JS, et al. READ-2 Study Group. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) Study. Ophthalmology. 2009;116:2175–81. doi: 10.1016/j.ophtha.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Kang SW, Sa HS, Cho HY, Kim JI. Macular grid photocoagulation after intravitreal triamcinolone acetonide for diffuse diabetic macular edema. Arch Ophthalmol. 2006;124:653–8. doi: 10.1001/archopht.124.5.653. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Moke PS, Turpin AH, et al. A computerized method of visual acuity testing: adaptation of the Early Treatment of Diabetic Retinopathy Study testing protocol. Am J Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- 12.Diabetic Retinopathy Clinical Research Network. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007;114:525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(suppl):823–33. [PubMed] [Google Scholar]
- 14.Little RJ, Rubin DB, Barnett V. Wiley Series in Probability and Mathematical Statistics. New York: Wiley; 1987. Statistical analysis with missing data; pp. 255–9. [Google Scholar]
- 15.Moradian S, Ahmadieh H, Malihi M, et al. Intravitreal bevacizumab in active progressive proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:1699–705. doi: 10.1007/s00417-008-0914-4. [DOI] [PubMed] [Google Scholar]
- 16.Arevalo JF, Maia M, Flynn HW, Jr, et al. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92:213–6. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 17.Laser-Ranibizumab-Triamcinolone for Proliferative Diabetic Retinopathy (LRTforDME+PRP) [January 5, 2010]; ClinicalTrials.gov. NCT00445003. Available at: http://public.drcr.net/Studies.aspx?ReclD5147.
- 18.Sabet-Peyman EJ, Heussen FM, Thorne JE, et al. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2009;40:316–8. doi: 10.3928/15428877-20090430-17. [DOI] [PubMed] [Google Scholar]
- Ockrim ZK, Sivaprasad S, Falk S, et al. Intravitreal triamcinolone versus laser photocoagulation for persistent diabetic macular oedema. Br J Ophthalmol. 2008;92:795–9. doi: 10.1136/bjo.2007.131771. [DOI] [PubMed] [Google Scholar]
- 20.Gillies MC, Sutter FK, Simpson JM, et al. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–8. doi: 10.1016/j.ophtha.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 21.Martidis A, Duker JS, Greenberg PB, et al. Intravitreal triamcinolone for refractory diabetic macular edema. Ophthalmology. 2002;109:920–7. doi: 10.1016/s0161-6420(02)00975-2. [DOI] [PubMed] [Google Scholar]
- 22.Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–7. doi: 10.1016/j.ophtha.2007.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Efficacy and safety of ranibizumab (intravitreal injections) in patients with visual impairment due to diabetic macular edema (RESTORE) [January 5, 2010]; ClinicalTrials.gov. NCT00687804. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00687804?term=nct00687804.
- 24.A study of ranibizumab injection in subjects with clinically significant macular edema with center involvement secondary to diabetes mellitus (RIDE) [January 5, 2010]; ClinicalTrials.gov. NCT00473382. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00473382?term=NCT00473382.
- 25.A study of ranibizumab injection in subjects with clinically significant macular edema with center involvement secondary to diabetes mellitus (RISE) [January 5, 2010]; ClinicalTrials.gov. NCT00473330. Available at: http://www.clinicaltrials.gov/ct2/show/NCT00473330.
- 26.Writing Committee for the Diabetic Retinopathy Clinical Research Network. Fong DS, Strauber SF, Aiello LP, et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80. doi: 10.1001/archopht.125.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]


