Abstract
Ligands of CCR5, the major coreceptor of HIV-1, costimulate T lymphocyte activation. However, the full impact of CCR5 expression on T cell responses remains unknown. Here, we show that compared with CCR5+/+, T cells from CCR5−/− mice secrete lower amounts of IL-2, and a similar phenotype is observed in humans who lack CCR5 expression (CCR5-Δ32/Δ32 homozygotes) as well as after Ab-mediated blockade of CCR5 in human T cells genetically intact for CCR5 expression. Conversely, overexpression of CCR5 in human T cells results in enhanced IL-2 production. CCR5 surface levels correlate positively with IL-2 protein and mRNA abundance, suggesting that CCR5 affects IL-2 gene regulation. Signaling via CCR5 resulted in NFAT transactivation in T cells that was blocked by Abs against CCR5 agonists, suggesting a link between CCR5 and downstream pathways that influence IL-2 expression. Furthermore, murine T cells lacking CCR5 had reduced levels of intranuclear NFAT following activation. Accordingly, CCR5 expression also promoted IL-2-dependent events, including CD25 expression, STAT5 phosphorylation, and T cell proliferation. We therefore suggest that by influencing a NFAT-mediated pathway that regulates IL-2 production and IL-2-dependent events, CCR5 may play a critical role in T cell responses. In accord with our prior inferences from genetic-epidemiologic studies, such CCR5-dependent responses might constitute a viral entry-independent mechanism by which CCR5 may influence HIV-AIDS pathogenesis.
During adaptive immune responses, T lymphocytes encounter chemokine gradients as they migrate into lymph nodes and inflamed tissues. Consequently, T lymphocytes are exposed to chemokines before they encounter APCs (1). Binding of CC chemokines to their cognate CCRs on the surface of T cells influences the type of lymphocyte response that occurs during subsequent antigenic stimulation (2). In this respect, previous reports indicate that in addition to their well-described effects on leukocyte migration (3–5), CC chemokines have the ability to costimulate activation of T lymphocytes (2, 6–9). However, the specific set of CCRs through which this effect is mediated by has not been completely elucidated. CCR5, the major coreceptor for the cell entry of HIV-1, constitutes a potential candidate, as it is known to be sequestered at the immunological synapse, and signals mediated via CCR5 might enhance T cell activation by stabilizing T cell-APC interactions (9). Furthermore, in addition to its role in immunological synapse formation (9), there is substantial evidence suggesting that the CCR5-CCR5 ligand axis might be involved in modulating other facets of T cell responses (6 –16), including T lymphocyte differentiation (11), survival (10), and polarization (12–16). Additionally, CCR5 expression is closely tied to the type of effector function acquired during T cell activation, that is, Th1 response (17).
Previous reports have also highlighted a link between CCR5 and the IL-2 system. For example, IL-2 up-regulates CCR5 expression both in vitro and in vivo (18 –20). Accordingly, rapamycin, a drug that disrupts IL-2 receptor signaling, reduces CCR5 surface expression on T cells (21). These observations are also consistent with the prominent influence of IL-2 on the responsiveness of T lymphocytes to CCR5 ligands, and in turn the recruitment of Ag-activated T cells into inflammatory tissues where immune responses occur (22). However, the converse is unknown, that is, whether CCR5 expression affects adaptive immunity by influencing IL-2 production. An improved understanding of this possibility is of importance, as novel therapeutic agents to block CCR5 are currently being used for the treatment of HIV infection (23) and are also being evaluated for their therapeutic efficacy in other diseases (e.g., rheumatoid arthritis). The impetus to develop CCR5 antagonists was based on the observation that individuals homozygous for the gene inactivating 32-bp deletion in the coding region of CCR5 (CCR5-Δ32) are profoundly resistant to HIV-1 infection (24 –26). Although it has been traditionally assumed that CCR5 deficiency in humans is harmless (24 –26), the frequency of individuals with the CCR5-Δ32/Δ32 genotype is very low and restricted to individuals of European descent (24 –26), precluding a rigorous evaluation of this assumption. The immunologic consequences of natural genetic-based deficiency of CCR5 as well as blockade of CCR5 remain unknown but are important to evaluate, as we found recently that healthy individuals with the CCR5-Δ32/Δ32 genotype have reduced cell-mediated immune responses (27), and others have shown that this genotype might be associated with increased susceptibility to viral infections other than HIV (28). Furthermore, the CCR5 antagonist maraviroc (29) is associated with a higher incidence of viral infections (www.pfizerpro.com/product_info/selzentry_pi_warnings.jsp).
To address the aforementioned gaps in knowledge, we used complementary analytical models (e.g., cell lines, knockout mice, and primary human T cells) to investigate the role of CCR5 in T lymphocyte responses. We demonstrate that functional expression of CCR5 but not CCR1 or CCR2 regulates IL-2 production as well as IL-2-dependent events such as expression of the α-chain of the IL-2 receptor (IL-2Rα; also known as CD25), transactivation of the NFAT, phosphorylation of STAT5, and cell proliferation during T cell activation. These findings indicate that there is an intimate cross-talk between CCR5 and IL-2, which in turn constitutes a biological network that might have a significant impact on adaptive immune responses.
Materials and Methods
Study subjects
Peripheral blood samples were obtained from adult healthy subjects who provided written consent. All studies in humans were approved by the Institutional Review Board at the University of Texas Health Science Center at San Antonio (UTHSCSA).
Mice
Ccr5−/− mice in C57BL/6J background were generated as described previously (30, 31). Ccr1−/− and Ccr2−/− were purchased from Taconic Farms. Wild type (WT)4 mice were purchased from The Jackson Laboratory. At time of examination, mice were between 8 and 12 wk old and on the C57BL/6J background unless stated otherwise. All animal studies were approved by the UTHSCSA Institutional Animal Care and Use Committee.
Cell preparation
Human PBMC were isolated by Histopaque gradient separation. For experiments that used murine leukocytes, mice were sacrificed and the spleen was dissected along with the lymph nodes (cervical, axillary, brachial, and inguinal). In some experiments the mouse intestines were carefully removed by excising connective and fat tissues, and the Peyer’s patches (PP) were collected from the terminal ileum. Single-cell suspensions from either splenocytes and lymph nodes or PP were prepared by mechanically homogenizing the tissues using the frosted ends of glass slides. Cells were filtered with a 70-µm nylon cell strainer (BD Falcon). Cell count and viability were determined by trypan blue stain (Invitrogen). T cells from human PBMC or mice splenocytes and lymph node cell suspensions were isolated using a magnetic separation method (autoMACS) following instructions of the manufacturer (Miltenyi Biotec). Cell purity was consistently >95% as estimated by FACS in all experiments. Jurkat cell line was obtained from the American Type Culture Collection. MOLT-4 clone 8 (from Dr. Ronald Desrosiers) and MOLT-4/CCR5 (32) (from Drs. Masanori Baba, Hiroshi Miyake, and Yuji Iizawa) cell lines (Fig. 1) were obtained through the AIDS Research and Reference Reagent Program (National Institute of Allergy and Infectious Diseases, National Institutes of Health). Overexpression of full-length CCR5 (Fig. 1) in human primary T cells (designated as CCR5high) as well as Jurkat cells (designated Jurkat/CCR5) was achieved by using a lentiviral vector system described previously (33). CC chemokine-induced down-regulation of surface CCR5 demonstrated that CCR5 in these overexpressing cells was functional.
FIGURE 1.
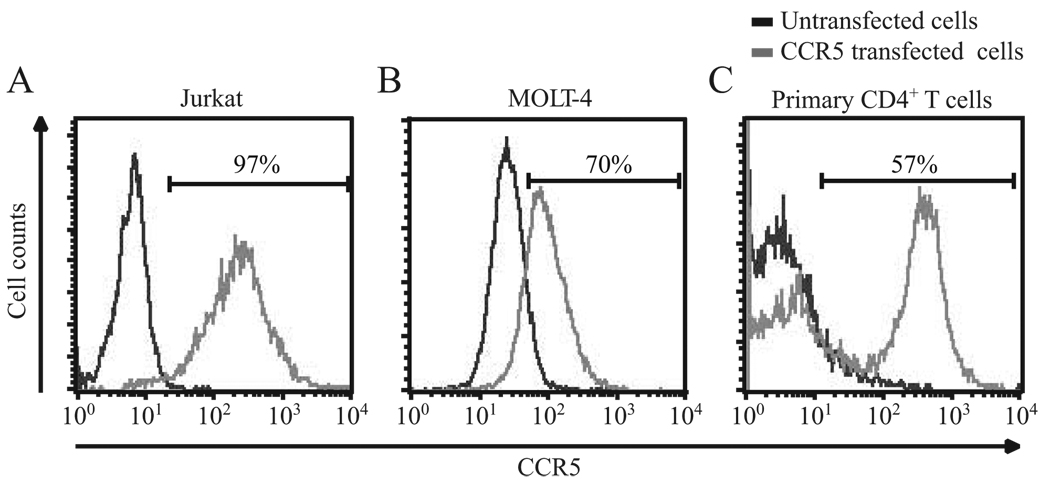
CCR5 surface expression in human T cells. Plots showing the CCR5 surface expression in (A) Jurkat, (B) MOLT-4, and (C) primary human CD4+ T cells before and after engineering cells to overexpress CCR5. In untransfected cells (black lines), dim levels of CCR5 were detected in less than 1%, 3%, and 5% of Jurkat, MOLT-4, and primary CD4+ T cells, respectively. In contrast, very high surface levels of CCR5 were detected in 97%, 70%, and 57% of the cells engineered to overexpress CCR5 (gray lines) in Jurkat CCR5, MOLT-4, and primary CD4+ T cells, respectively. These cells were designated herein as Jurkat/CCR5, MOLT-4/CCR5 and CCR5high cells, respectively. CCR5 mean fluorescence intensity values were also significantly different between untransfected vs CCR5 transfected cells in Jurkat, MOLT-4, and primary human CD4+ T cells, and in these cell types the values were 43 vs 209, 74 vs 112, and 35 vs 271, respectively.
Cell culture and T cell stimulation
Primary cells from humans or mice were resuspended in RPMI 1640 supplemented with heat-inactivated FBS (10%), penicillin/streptomycin, and l-glutamine and cultured at 37°C in a 5% CO2 incubator. Cell lines were cultured in the same media without antibiotics. Culture media for MOLT-4/CCR5 cells also contained G418 to maintain stable surface expression of CCR5. Mice T cells and human PBMC (1 × 106 cells/ml) were stimulated with soluble anti-CD3 (2.5 µg/ml) and anti-CD28 (1.25 µg/ml; BD Pharmingen). Human T cells were stimulated by using CD3-coated beads (bead-to-cell ratio 1:2; Miltenyi Biotec) or plate-bound anti-CD3 (1 µg/ml) and soluble anti-CD28 (0.5–1 µg/ml) Abs. CCR5 blockade was achieved by preincubating cells with anti-CCR5 Ab clone 2D7 (50 µg/ml; BD Pharmingen) or maraviroc (0.001 nM to 100 µM; Selzentry; Pfizer) for 1 h at room temperature or 37°C, respectively. In some experiments, 25 µg/ml (unless stated otherwise) of neutralizing Ab against CCL3/CCL3L1 (clone MAB670) and/or CCL5 (clone MAB2781; R&D Systems) were added to the culture. Prevention of CC chemokine-induced down-regulation of surface CCR5 by anti-CCR5 Ab or maraviroc was used as a functional readout in experiments conducted to demonstrate that blockade of CCR5 was optimal. Blockade of CCL3 and CCL3L1-induced down-regulation of surface CCR5 by MAB670 Ab was also used to confirm the specificity of this clone for both CCL3 and CCL3L1. Lymphoid cell lines were stimulated with PMA (50 ng/ml; Sigma-Aldrich) and ionomycin (1 µg/ml; Fisher Scientific).
FACS
Expression of surface markers was assessed using standard protocols (34). For intracellular cytokine staining, cells were incubated with GolgiStop containing monensin (0.7 µl/ml; BD Pharmingen) and brefeldin A (1 µl/ml; Sigma-Aldrich) for 5–6 h in the presence of anti-CD3/CD28 Abs at 37°C, 5% CO2. Cells were fixed with BD Cytofix/Cytoperm (BD Pharmingen) for 20 min at 4°C and stained with anti-IL-2 FITC for 30 min at 4°C. In some experiments, anti-CCR5-allophycocyanin was also added at this point. Cells were washed with 0.2% saponin between incubations. Phosho-protein staining was performed using standard protocols (35, 36). rIL-2 (0.1 µg/ml; R&D Systems) for 15 min was used to induce STAT5 phosphorylation. Cells were stained with anti-CD3-PE or anti-CD25-PE and anti-phosphoSTAT5 Alexa-647 Abs for 30 min at room temperature, washed, and resuspended before FACS analysis. Fc blocking reagent and appropriate isotype controls were included in all assays. Samples were analyzed with a FACSCalibur flow cytometer using CellQuest Pro software (BD Pharmingen). Dead cells were excluded based on forward and side scatter properties. At least 10,000 gated events were collected for each sample. All Abs were obtained from BD Pharmingen. Cell proliferation was measured by using CFSE dilution assay (CFDA SE Cell Tracer kit; Molecular Probes).
Quantitative RT-PCR
After 4–6 h of stimulation, total mRNA was isolated (Ambion) and cDNA synthesized (Invitrogen) using standard protocols. IL2 gene expression was measured by using TaqMan gene expression assays from Applied Biosystems.
Transient transfections and luciferase assays
A NFAT luciferase vector that contains a multimerized NFAT binding site cloned upstream of the firefly luciferase gene was kindly provided by Dr. Anjana Rao (Harvard Medical School, Boston, MA). Human T cell lines were transfected with a NFAT reporter construct and a Renilla luciferase plasmid as described previously (34). One hour posttransfection, cells were stimulated with PMA and ionomycin or left untreated. Luciferase activity was measured 24 h posttransfection with the Dual-Luciferase assay kit (Promega). The relative luciferase activity was calculated as described previously (37, 38). Data were normalized for transfection efficiency by dividing firefly luciferase activity with that of corresponding Renilla luciferase, and expressed as fold increase over the promoterless vector. Assays were conducted in triplicate and at least three separate experiments were performed. Nucleofection of mouse T cells with NFAT reporter vector and a Renilla luciferase plasmid was performed using the mouse T cell Nucleofector kit following instructions of the manufacturer (Amaxa Biosystems). After an overnight incubation with the plasmids, mouse T cells were stimulated with CCL5 (200 ng/ml; PeproTech) for 4 h. At this time, luciferase activity was determined as above.
ELISA
IL-2 levels were measured in cell culture supernatants following instructions of the manufacturer (eBioscience and BD Pharmingen for mouse (sensitivity of 2 pg/ml) and human (sensitivity of 7 pg/ml) IL-2, respectively).
NFAT2 detection
T cells were separated from WT and Ccr5−/− mice using the Pan T cell isolation kit (Miltenyi Biotec) following the manufacturer’s instructions using the autoMACS (Miltenyi Biotec). By FACS, >80% of the cells stained positive for CD3. T cell stimulation was performed as described previously by Srinivasan and Frauwirth (39). In brief, T cells were incubated with murine anti-CD3 and anti-CD28 Abs (10 µg/ml; BD Biosciences) in complete RPMI 1640 on ice for 30 min. Cells were then stimulated with goat anti-hamster secondary cross-linking Ab (10 µg/ml; Pierce Biotechnology) for 10 min at 37°C. Stimulation was stopped by the addition of ice-cold PBS. Nuclear protein extraction and NFAT2 detection were performed according to the manufacturer’s protocol (TransAM NFATc1 kit; Active Motif).
Multianalyte profile
Supernatants from purified mouse T cells from WT and Ccr5−/− mice after 48 h of stimulation with anti-CD3/CD28 Abs were analyzed by a rodent multianalyte profile (Rules-Based Medicine, Austin, TX). The sensitivity of this bioassay is comparable to that from ELISA. Notably, we and others have used this approach previously to establish critical murine phenotypes at the protein level (30, 40–42). A total of three mice per group were analyzed. The complete list of parameters assessed by this assay is shown in supplemental Table I.5
Genotyping
CCR5-Δ32 mutation was genotyped as previously described (43, 44). Wild type (CCR5-WT) refers to subjects who do not have this mutation.
Statistical analysis
Statistical analyses were performed using Stata 8.2 program. Mann-Whitney two-sample rank sum test and two-tailed paired Student’s t tests were used when appropriate. A p value of <0.05 was considered as statistically significant.
Results
CCR5 expression influences IL-2 production
IL-2 is produced soon after T cell activation and regulates the expansion of recently activated T cell clones (45– 47). For the initial studies that focused on whether CCR5 expression influences IL-2 production by T lymphocytes, we used mice genetically inactivated for Ccr5 (Ccr5−/−). To determine the specificity of the data, we also investigated Ccr1 (Ccr1−/−) and Ccr2 (Ccr2−/−) knockout mice. IL-2 levels were measured by ELISA in the supernatants of purified T cells from these and WT C57BL/6 mice after 48 h of stimulation with anti-CD3 and anti-CD28 Abs. IL-2 levels in T cell supernatants from Ccr1−/− or Ccr2−/− mice did not differ significantly from those detected in WT mice (data not shown). In contrast, compared with WT (i.e., Ccr5+/+) mice, IL-2 levels were significantly lower in supernatants of Ccr5−/− T cells (Fig. 2A). Similar findings were observed when comparing WT and Ccr5−/− T cells obtained from mice generated on a DBA/1J background (data not shown), suggesting that this association is independent of the strain of mice used. Thus, although CCR5 and CCR1 share common ligands (e.g., CCL3 and CCL5), only inactivation of CCR5 was associated with a significant reduction in IL-2 production. These findings suggested that only specific chemokine receptors might influence IL-2 production.
FIGURE 2.
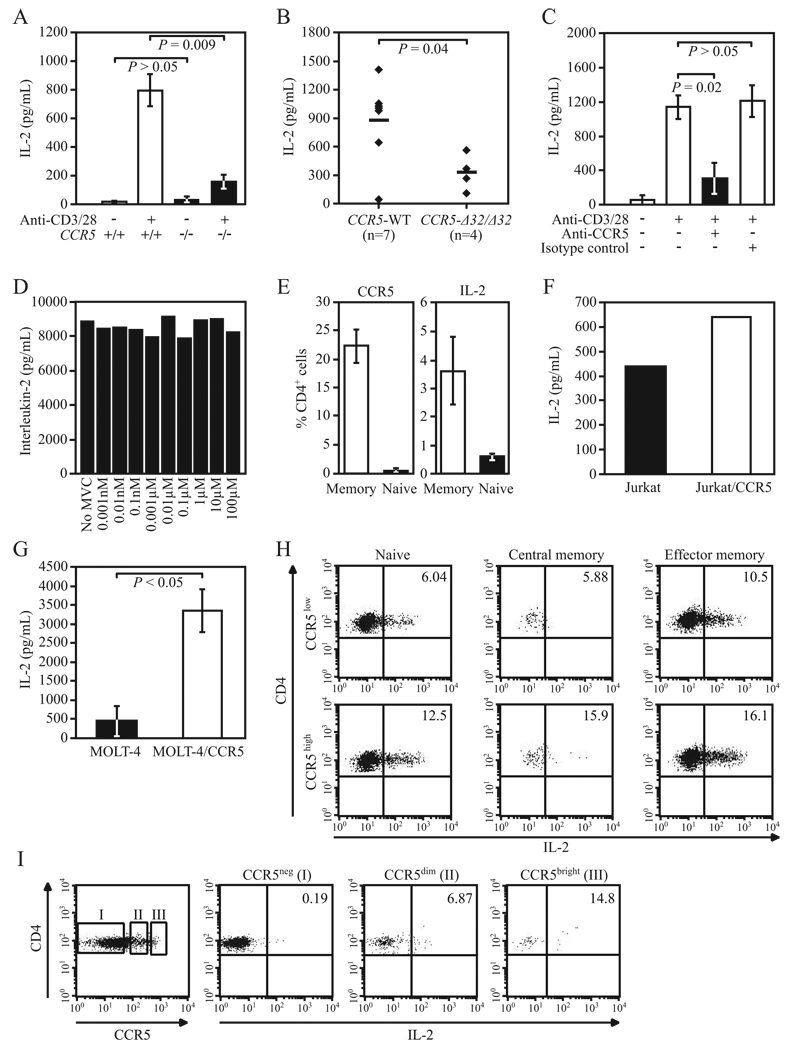
CCR5 expression levels influence IL-2 levels in T cells. A–C, IL-2 levels were measured by ELISA in culture supernatants obtained after 48 h of cell stimulation with anti-CD3 and anti-CD28 Abs; the sources of the cells were (A) purified T cells from C57BL/6J CCR5−/− and CCR5+/+ mice (data from three independent experiments), (B) PBMC from CCR5 WT (i.e., those lacking the CCR5-Δ32 allele) and CCR5-Δ32/Δ32 healthy individuals (n = no. of individuals/group), and (C) PBMC from three CCR5 WT healthy donors preincubated with anti-CCR5 or nonspecific isotype control Abs. Data in A and C are means ± SEM, and in B, horizontal lines reflect mean values. D, Purified human T cells were treated with different concentrations of maraviroc (0.001 nM to 100 µM; Selzentry; Pfizer) for 1 h at 37°C followed by stimulation with plate-bound anti-CD3 Ab (1 µg/ml; BD Pharmingen) and soluble anti-CD28 Ab (0.5 µg/ml; BD Pharmingen). IL-2 levels were measured by ELISA in culture supernatants after 48 h of stimulation. Results are representative of four experiments. E, Surface levels of CCR5 and intracellular levels of IL-2 were measured at the single cell level by FACS in PBMC from five healthy donors. Histograms correspond to percentage (mean ± SEM) of memory (CD45RO+) and naive (CD45RO−) CD4+ cells expressing CCR5 (left) and IL-2 (right). F, Representative data of IL-2 levels measured by ELISA in culture supernatants of CCR5 overexpressing Jurkat T cells (Jurkat/CCR5) and native Jurkat T cells after 24 h of stimulation with PMA/ionomycin. Data are representative of two independent experiments. G, Experimental approach as in E, but using native MOLT-4 and MOLT-4/CCR5 T cells. Data (mean ± SEM) are from three independent experiments. H, FACS plots showing CD3/CD28-induced IL-2 production (see x-axis; numbers in upper right box indicate percentage of IL-2-producing cells in naive (CD45RO−CCR7+), central memory (CD45RO+CCR7+), and effector memory (CD45RO+CCR7−) primary human CD4+ T cells before (top panels; CCR5low: CCR5 surface expression <5%) and after overexpression of CCR5 using a lentiviral system (bottom panels; CCR5high: CCR5 surface expression ~60%). I, IL-2 expression correlates with CCR5 expression. Leftmost panel shows the extent of intracellular expression of CCR5 in primary human CD4+ cells. I, II, and III indicate, respectively, CCR5-negative (CCR5neg), CCR5-positive dim (CCR5dim), and CCR5-positive bright (CCR5bright) CD4+ cells. The next three panels show the extent of intracellular expression of IL-2 in these three groups of CD4+ cells. Results are representative of three independent experiments.
To assess whether the reduced IL-2 production associated with deficiency of CCR5 in mice was tracking a broader perturbation in cytokine production, we quantified the levels of additional cytokines, chemokines, and other biomarkers in T cell supernatants from Ccr5−/− and WT mice (supplemental Table I). Results of a multianalyte bioassay profile not only confirmed the differential production of IL-2 between WT and CCR5−/− mice but also revealed differences in the levels of other cytokines and chemokines involved in inflammation, cell migration, hematopoiesis, and tissue regeneration (Table I). For example, the multianalyte bioassay profile confirmed our previous observation of reduced IL-6 production in Ccr5−/− mice (Table I) (30). However, among the parameters assessed, the differences between WT and Ccr5−/− mice in IL-2 levels were among the most striking, and the median levels of IL-2 in Ccr5−/− mice were one-fifth of those found in WT mice (Table I). Notably, there were no differences in the levels of CCR5 ligands between WT and CCR5−/− mice (supplemental Table I; highlighted in yellow).
Table I.
Differential T cell cytokine profile between CCR5+/+-- and CCR5−/−-activated T cellsa
| CCR5+/+ | CCR5−/− | |||
|---|---|---|---|---|
| Parameter | Median | Range | Median | Range |
| FGF-basic (fibroblast growth factor–basic), ng/ml | 0.75 | 0.70–0.82 | 0.47 | 0.40–0.62 |
| IL-11, pg/ml | 5.32 | 4.1–6.5 | Und.b | Und.–0.3 |
| IL-2, pg/ml | 116.00 | 73–162 | 25.90 | 12–30 |
| IL-3, pg/ml | 7.02 | 4.8–12 | 1.98 | 1.3–4.0 |
| IL-6, pg/ml | 6.81 | 1.5–17 | 1.97 | Und.–4.2 |
| MCP-1, pg/ml | 0.78 | 0.62–2.9 | Und. | Und.–0.53 |
| MCP-3, pg/ml | 1.53 | 1.2–2.2 | 0.70 | 0.57–1.1 |
| M-CSF, ng/ml | 0.0092 | 0.0071–0.01 | 0.0038 | 0.0028–0.0069 |
| MIP-1γ, ng/ml | 0.027 | 0.021–0.097 | 0.013 | 0.0072–0.014 |
| MPO (myeloperoxidase), ng/ml | 0.56 | 0.38–1.7 | 0.12 | 0.066–0.19 |
| OSM (oncostatin M), ng/ml | 0.03 | 0.024–0.03 | 0.02 | 0.022–0.023 |
| SCF (stem cell factor), pg/ml | 11.50 | 9.7–15 | 6.17 | 4.5–8.8 |
| TIMP-1 (tissue inhibitor of metalloproteinase type 1), ng/ml |
0.051 | 0.051–0.064 | 0.047 | 0.037–0.045 |
Chemokine-cytokine and other biomarker concentrations were measured in culture supernatants of purified mouse T cells after 48 h of stimulation with anti-CD3/CD28 Abs. Three mice per group were analyzed. Only those cytokines are listed for which significant differences in their levels were detected between CCR5+/+ and CCR5−/− T cell culture supernatants (p < 0.05). The complete list of biomarkers assessed and their corresponding levels detected in CCR5+/+ and CCR5−/− mice are shown in supplemental Table 1. Data were obtained by Rules-Based Medicine (www.rulesbasedmedicine.com), a multianalyte profile bioassay (31, 41–43).
Und. indicates undetectable.
To further extend the phenotype of reduced IL-2 production observed in CCR5-deficient T lymphocytes, we measured IL-2 production in anti-CD3/CD28 stimulated PBMC from healthy donors homozygous for the CCR5-Δ32 mutation. Although there was considerable inter-donor variability in IL-2 production, CCR5-Δ32/Δ32 donors produced significantly less IL-2 than did noncarriers of the CCR5-Δ32 mutation (Fig. 2B). These results further suggested the association of “low CCR5–low IL-2 expression.”
An argument could be made that the observed reduction in IL-2 levels in humans deficient in CCR5 expression was secondary to germline absence of CCR5. To address this possible confounding factor, we used an analytical approach in which we abrogated CCR5 function in T cells that normally express this receptor. This approach also allowed us to assess the effects of blocking CCR5 function in the context of the same genetic background, thus minimizing inter-donor variability. Blockade of CCR5 was performed using either maraviroc, a small molecule CCR5 inhibitor (29), or 2D7, a mAb that recognizes the second extracellular loop of CCR5, a critical region involved in binding of CCR5 ligands as well as docking of HIV to CCR5 (48). In contrast to the latter, inhibitor-bound CCR5 has a con- formation that is different than that of native (inhibitor-free) CCR5, and this allosteric change in structure is thought to mediate CCR5 antagonism (29, 49). Supporting our observations in CCR5-null human cells, preincubation of human PBMC with the neutralizing Ab against CCR5 was associated with significantly lower levels of IL-2 (Fig. 2C). However, incubation of human T cells (both primary T cells and MOLT-4 cells) with maraviroc at concentrations between 0.001 nM and 100 µM was not associated with reduced IL-2 production (Fig. 2D and data not shown). These findings suggested that the functional consequences of inhibitor-bound CCR5 and Ab-mediated blockade of CCR5 on IL-2 were distinct, a possibility that is consistent with the contrasting mechanisms by which the small molecule CCR5 inhibitor and the anti-CCR5 mAb mediate their effects (29, 49).
We surmised that if there was a strong relationship between CCR5 expression and IL-2 production, then the converse of the relationship low CCR5–low IL-2 expression must also exist, that is, the relationship of “high CCR5–high IL-2 expression.” To test for this relationship, we determined whether specific T cell subsets that express higher amounts of CCR5 also express high levels of IL-2. To this end, PBMCs obtained from healthy donors were stimulated with anti-CD3/CD28 Abs and IL-2 production was assessed at the intracellular level by FACS. Memory T lymphocytes are known to have significantly higher CCR5 surface levels than do naive lymphocytes (Fig. 2E, left), and analyses of IL-2 production at the single cell level revealed that memory T cells also produced significantly higher levels of IL-2 (Fig. 2E, right). In a complementary approach, we next determined whether overexpression of CCR5 in human cell types that do not normally express CCR5 will result in increased IL-2 production. To test this, we conducted proof-of-principle experiments using human T cell lines that are known to express minimal amounts of surface CCR5 (50), and in which high levels of CCR5 expression were induced (Fig. 1, A and B) as described previously (32, 33). Notably, CCR5-overexpressing T cells (Jurkat/CCR5 and MOLT-4/CCR5) produced significantly higher levels of IL-2 after in vitro stimulation than did native T cells (Jurkat and MOLT-4) that do not express high levels of surface CCR5 (Fig. 2, F and G, respectively).
To further confirm the high CCR5–high IL-2 relationship, we next extended the aforementioned approach to primary human lymphocytes and determined IL-2 production in CD4+ T cells before (CCR5low; Fig. 1C) and after transfection of a CCR5-containing lentiviral vector that results in higher CCR5 expression (CCR5high; Fig. 1C). A small percentage of CCR5low cells had dim expression of CCR5, whereas ~60% of the CD4+ T CCR5high cells expressed high surface levels of CCR5. Notably, as compared with CCR5low cells, a higher proportion of IL-2-producing cells were detected in naive and memory CCR5highCD4+ T cells (Fig. 2H).
Finally, we surmised that establishment of a dose-dependent relationship between CCR5 levels and IL-2 expression in CD4+ cells could provide a more definitive means to confirm that IL-2 levels correlate with CCR5 expression. To accomplish this, and to minimize confounding, fresh PBMC were stimulated with anti-CD3/CD28 Abs and IL-2 and CCR5 levels were determined at the intracellular level. This approach allowed us to assess IL-2 production in CD4+ cells that exhibited low, intermediate, and high CCR5 expression levels, and they were denoted as CD4+CCR5−, CD4+CCR5dim, and CD4+CCR5bright, respectively (Fig. 2I, leftmost panel). We detected a stepwise increase in the proportion of IL-2-producing cells in CD4+CCR5−, CD4+CCR5dim, and CD4+CCR5bright cells, respectively (Fig. 2I). Similar results were obtained with purified T cells (data not shown). Taken together, the results obtained using different analytical approaches provided strong evidence in support of the notion that CCR5 expression might be a major determinant of IL-2 production in activated T cells.
CCR5 expression influences IL-2 transcript levels
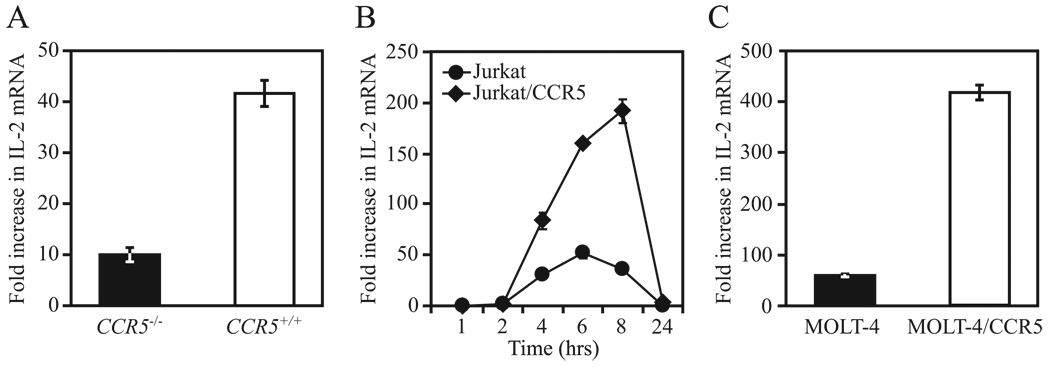
We surmised that low or high CCR5 surface expression would result in reduced or increased de novo production of IL-2, respectively. Consistent with this possibility, IL-2 mRNA expression levels in purified murine CCR5+/+ T lymphocytes that had been stimulated with anti-CD3/CD28 Abs were almost 4-fold higher than in similarly treated cells obtained from CCR5−/− mice (Fig. 3A). Accordingly, IL-2 mRNA expression was higher in Jurkat and MOLT-4 cells engineered to express high levels of surface CCR5 (Fig. 3, B and C). Taken together, these findings indicated that CCR5 surface expression levels impact positively the abundance of IL-2 mRNA transcripts during T cell activation.
FIGURE 3.
CCR5 surface expression levels influence IL-2 transcript levels. A, IL-2 mRNA levels (mean ± SEM) were determined by quantitative RT-PCR in purified T cells from C57BL/6J CCR5−/− and CCR5+/+ mice after 4 h of stimulation with anti-CD3/28 Abs. Data are representative of three independent experiments. B, IL-2 mRNA levels determined by quantitative RT-PCR in Jurkat T cells overexpressing CCR5 (Jurkat/CCR5) and native Jurkat at the indicated time points after stimulation with PMA/ionomycin. Results are representative of three independent experiments. C, Experimental protocol is as in B except using MOLT-4 and MOLT-4/CCR5 cells. IL-2 mRNA levels (mean ± SEM) were assessed after 6 h of stimulation. Results are representative of three independent experiments.
CCR5 expression favors NFAT transactivation
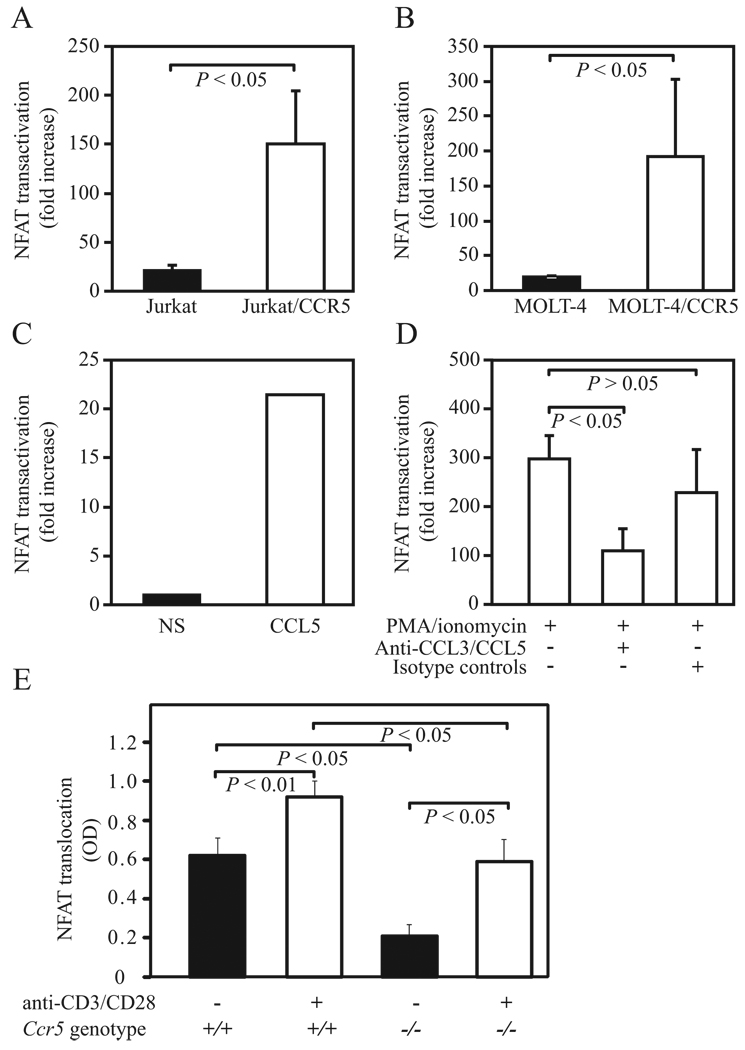
Both TCR activation and PMA/ionomycin treatment are strong inducers of IL-2 transcription and predominantly mediate their effects through calcium mobilization, calcineurin-dependent dephosphorylation of NFAT, and NFAT translocation and binding to IL-2 cis regulatory regions (45, 51, 52). As signaling through CCR5 also leads to intracellular calcium mobilization (53), we tested the hypothesis that this might serve as a costimulatory signaling pathway through which CCR5 levels influence IL-2 expression. To test this possibility, CCR5-overexpressing human T cells were transfected with a luciferase expression construct that contained consensus cis sequences for binding of NFAT proteins, and transactivation of NFAT was assessed after in vitro polyclonal stimulation. In accord with our hypothesis, NFAT transactivation was greater in T cell lines that expressed higher levels of surface CCR5 (Fig. 4, A and B).
FIGURE 4.
CCR5 surface expression influences NFAT transactivation and levels. A and B, NFAT transactivation (represented as mean fold increase ± SEM) was measured by luciferase reporter assays in (A) native and Jurkat/CCR5 T cells and (B) native MOLT-4 and MOLT-4/CCR5 T cells 24 h after stimulation with PMA/ionomycin. Data are representative of three independent experiments. C, NFAT transactivation was measured by luciferase reporter assay in purified T cells from C57BL/6J WT mice after 4 h of stimulation with CCL5. NS, nonstimulated cells. Data are representative of three independent experiments. D, NFAT transactivation in MOLT-4/CCR5 cells stimulated as described in B in the presence or absence of neutralizing Abs against CCL3/CCL3L1 and CCL5 or nonspecific isotype controls (10 µg/ml). Data are from three independent experiments. E, Levels of NFAT2 in the nuclear extracts obtained from unstimulated and TCR-stimulated T cells from WT (Ccr5+/+) and Ccr5−/− mice. Each bar represents the mean (±SD) OD measured from three independent samples. ANOVA with Bonferroni correction was used to determine statistical significance between the groups. Differences were considered statistically significant at p ≤ 0.05.
These observations suggested the possibility that the interaction between CCR5 ligands and its receptor trigger NFAT-mediated transcriptional activity. To determine whether there was a direct link between CCR5 signaling and NFAT, we measured NFAT transactivation in mouse primary T cells before and after stimulation with the CCR5 ligand CCL5 (RANTES). In vitro stimulation of mouse primary T cells with CCL5 induced NFAT transactivation (Fig. 4C). To determine whether this association between CCR5 signaling and NFAT transactivation was also present in human cells, we next measured NFAT transactivation in CCR5 overexpressing MOLT-4 cells in the presence or absence of neutralizing Abs against the major CCR5 ligands, that is, CCL3/CCL3L1 and CCL5. Treatment with anti-CCL3/CCL3L1 and anti-CCL5 neutralizing Abs but not nonspecific isotype controls resulted in a dramatic reduction (ranging from 39% to 78%) in levels of NFAT transactivation induced by PMA and ionomycin in MOLT-4/CCR5 cells (Fig. 4D). Notably, anti-CCL3/CCL3L1 and anti-CCL5 neutralizing Abs had no impact on NFAT transactivation in native MOLT-4 cells that did not express CCR5 (data not shown).
CCR5 surface expression modulates NFAT nuclear translocation
Of the five known isoforms of NFAT (54), NFAT1 (also known as NFATc2 or NFATp) and NFAT2 (NFATc1/NFATc) are thought to be critical regulators of IL-2 gene expression (55). The trans-activation experiments suggested that absence of CCR5 may lead to decreased nuclear translocation of the NFAT. To directly test this hypothesis, we measured the intranuclear levels of NFAT2 following activation of Ccr5−/− and WT T cells with anti-CD3/anti-CD28 Abs. In support of our hypothesis, we found that there were substantially higher intranuclear levels of NFAT2 under basal conditions and following T cell activation in WT mice than in Ccr5−/− mice (Fig. 4E). Of note, the intranuclear NFAT2 levels in the stimulated T cells from Ccr5−/− mice were comparable to the basal NFAT2 expression levels in WT T cells (Fig. 4E). These results further reinforced the notion that expression levels of CCR5 strongly influence the intranuclear translocation of dephosphorylated NFAT2 that plays an important role in IL-2 expression.
CCR5 expression influences CD25 expression
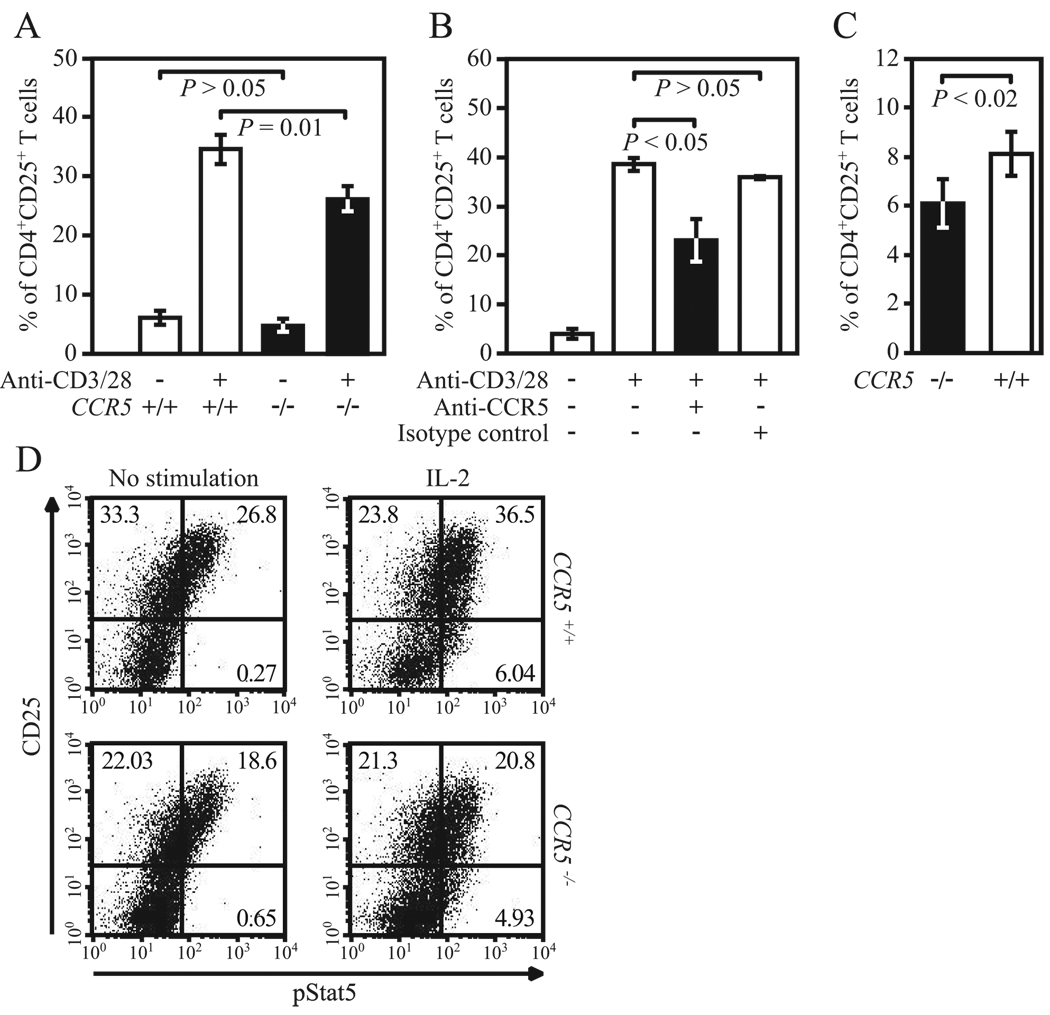
The aforementioned data suggested that signaling via CCR5 affects intranuclear NFAT levels and NFAT trans-activation (Fig. 4) and, consequently, IL-2 gene expression (Figs. 2 and 3). However, if this were true, then CCR5 expression should have broader effects, as NFAT has been shown to control not only IL-2 but also CD25 expression in activated T lymphocytes (45, 55). Further underscoring this possibility was the fact that expression of CD25 in activated T cells is to a large extent dependent on IL-2, which acts as a positive feedback regulator of the expression of CD25, its high-affinity receptor (46). Our studies affirmed this possibility. After stimulation in vitro, the percentage of CD4+CD25+ but not CD4+CD69+ T cells was significantly lower in T cells from the C57BL/6 CCR5−/− than CCR5+/+ mice (Fig. 5A). Similar results were obtained when using cells from DBA/1J CCR5−/− and CCR5+/+ mice (data not shown).
FIGURE 5.
CCR5 level influences expression of CD25. A and B, CD25 expression in CD4+ T cells was measured by FACS after 48 h of stimulation with anti-CD3/28 Abs in (A) purified T cells from C57BL/6J CCR5−/− and CCR5+/+ mice (data from three independent experiments) and (B) purified T cells from WT healthy donors (n = 3) preincubated with anti-CCR5 or nonspecific isotype control Abs. C, CD25 expression in CD4+ T cells was measured by FACS in PP from C57BL/6J CCR5−/− and CCR5+/+ mice (data from three independent experiments). D, Plots show relationship between CCR5 and STAT5 phosphorylation assessed by FACS 15 min after stimulation with rIL-2 (100 ng/ml) in primary T blasts (i.e., after 48 h of stimulation with anti-CD3/CD28 Abs followed by 24 h of serum starvation) from C57BL/6J CCR5−/− and CCR5+/+ mice. Numbers in quadrants correspond to percentage of cells. Data are representative of three independent experiments.
We replicated these findings in human cells. Expression levels of activation markers were assessed in anti-CD3/CD28-treated human T lymphocytes before and after Ab-mediated blockade of CCR5. Preincubation of human T lymphocytes with an anti-CCR5-neutralizing Ab resulted in significantly reduced expression of CD25, but not other activation markers such as CD69, CD38, or HLA-DR in CD4+ T cells (Fig. 5B and data not shown). Consistent with the notion that Ab and small molecule blockade are associated with contrasting functional effects, preincubation of human T cells with maraviroc was not associated with reduced expression of activation markers (data not shown). These observations also suggested that the reduced expression of CD25 observed in murine CD4+ T lymphocytes deficient in CCR5 may be due to impaired CCR5-CCR5 ligand interactions rather than to an intrinsic defect of CCR5-null T cells in their responsiveness to TCR-mediated activation.
To demonstrate further the role of CCR5-CCR5 ligand interactions in T cell activation, in addition to blocking CCR5, we also neutralized the activity of its main ligands by using mAbs. Blockade of either CCL3/CCL3L1 or CCL5 was associated with lower expression levels of CD25 in human CD4+ T cells, and blockade of these two ligands concurrently had additive effects with a reduction in CD25 levels comparable to that observed after Ab-mediated blockade of CCR5 (data not shown). Thus, the abrogation of CCR5-CCR5 ligand interactions by genetic- or Ab-mediated means resulted in a reduced expression of CD25 in CD4+ T cells after activation in vitro.
We next sought a means to assess whether the phenotype of “absence of CCR5–low CD25 levels” is also present in vivo. For this, we focused on GALT, which is the largest lymphoid organ in the body (56). A hallmark of T cells found in the GALT is their constitutively activated phenotype as compared with T cells that are present in blood or other peripheral lymphoid organs (57–59). We therefore investigated whether CCR5 expression influences the number of activated T cells in PP, the prototype of organized lymphoid tissues in GALT (58). Notably, we observed that as compared with WT mice, CCR5−/− mice had a slight but statistically significant reduction in the numbers of CD4+CD25+ cells in the PP but not in lymph nodes or spleen (Fig. 5C and data not shown). These findings are consistent with results of a previous report where an in vitro correlation between CD25 and CCR5 was detected in Ag-specific human PP T cells, but not blood T cells (60). Together with these previous observations, the data presented in Fig. 5, A–C suggested that CCR5 influences the expression of CD25 on CD4+ T cells both in vitro and in vivo.
CCR5 expression influences STAT5 phosphorylation
Having observed that CCR5 influenced CD25 expression, it was important to investigate whether the reduced expression of CD25 detected in the absence or following blockade of CCR5 had a functional impact, that is, whether it also translated to a perturbation in IL-2-IL-2R-dependent signaling pathways. We used STAT5 phosphorylation as a functional readout, as it occurs rapidly following IL-2-IL-2R interactions (46). For these analyses, T cell blasts from healthy individuals homozygous for the CCR5-Δ32 mutation and WT controls were stimulated in vitro with rIL-2, and the abundance of the phosphorylated isoform of STAT5 following IL-2 stimulation was determined at the single cell level by using a previously described FACS-based method (35, 36). Two of the three CCR5-Δ32/Δ32 individuals we studied had reduced IL-2-induced STAT5 phosphorylation as compared with age-, gender-, and race-matched WT controls (data not shown).
As the CCR5-Δ32/Δ32 genotype is rare and restricted to individuals of European descent (24–26), we were unable to expand the sample size to further confirm this association between the CCR5-null state and reduced IL-2-induced STAT5 phosphorylation in humans. Therefore, we used a similar experimental approach in T lymphocytes derived from CCR5+/+ and CCR5−/− mice. Consistent with our observation in human CCR5-null cells, the proportion of CCR5−/− mouse T cells that expressed the phosphorylated isoform of STAT5 following in vitro stimulation with IL-2 was lower than that observed in CCR5+/+ T cells (Fig. 5D). Additionally, we observed that compared with T cell blasts from CCR5+/+ mice, those from CCR5−/− mice also exhibited reduced CD25 expression at the intracellular level before and after stimulation with IL-2 (Fig. 5D), further confirming the impact of CCR5 levels on CD25 expression (Fig. 5, A–C). Thus, because STAT5 phosphorylation is detected predominantly in CD25+ T cells (Fig. 5D), these data suggest that impaired CD25 expression is a characteristic of the CCR5-null state that may result in a reduced number of target T cells available for mediating IL-2-dependent signaling pathways.
CCR5 expression influences T cell proliferation
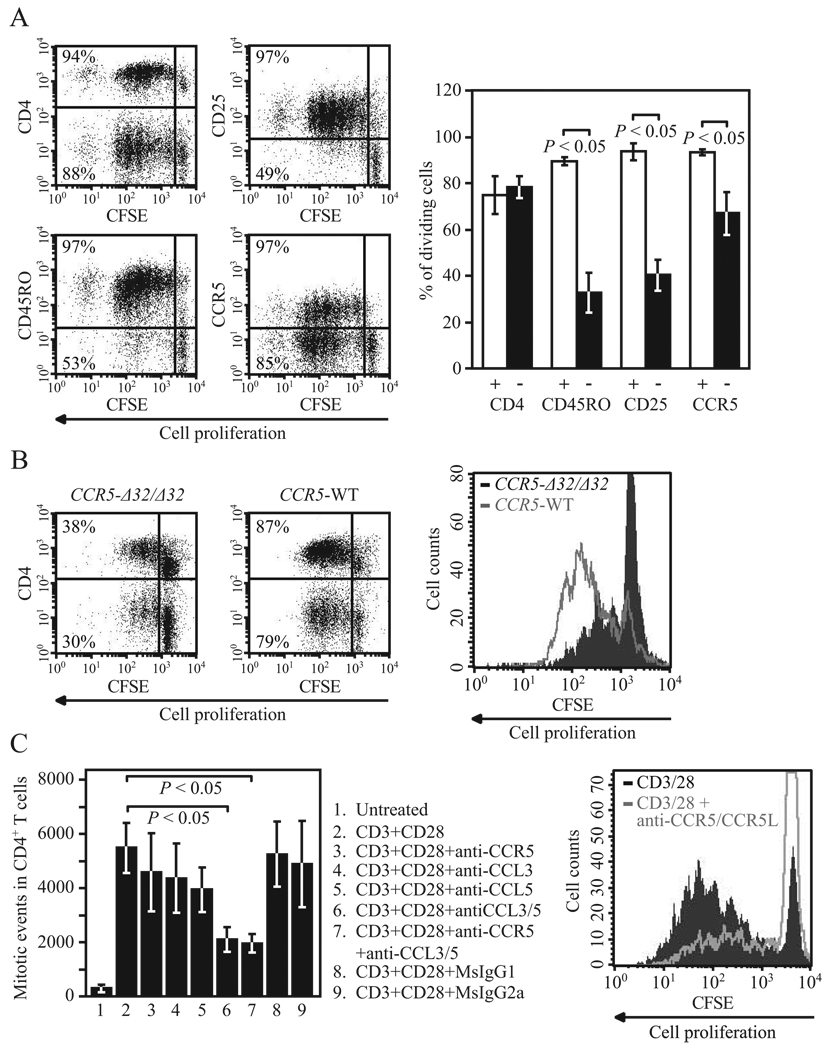
As T cell proliferation is highly dependent on IL-2-IL-2R interactions (45–47), we next determined whether by impacting IL-2 and CD25 expression, CCR5 also influences T cell proliferation. To address this, we stimulated human PMBC with anti-CD3/CD28 Abs and compared the extent of cell proliferation (assessed by CFSE dilution assay) in different T cell subsets including CCR5+ and CCR5− populations. Not surprisingly, T cell proliferation was almost exclusive to CD25+ and CD45RO+ cells (Fig. 6A). Although cell proliferation in CCR5+ and CCR5− populations did not follow the same binary pattern, the proportion of cells undergoing cell division was significantly higher in CCR5-expressing cells (Fig. 6A). Highlighting the specificity of these results, differences in the proportion of dividing cells were not discriminated by the presence of other surface markers such as CD4 (Fig. 6A). These findings are consistent with the observed correlation between CCR5 and CD25 expression (Fig. 5) as well as with the preferential expression of CCR5 on effector and memory (CD45RO+) T cells (Fig. 2D).
FIGURE 6.
CCR5 surface expression levels influence T cell proliferation. A, T cell proliferation was determined by CFSE dilution assay in PBMC from healthy donors after 5 days in culture. The experimental protocol used to induce CCR5 expression was as described previously (35) and included 72 h of stimulation with anti-CD3/CD28 Abs followed by 48 h of cell culture without Abs. Plots on the left show representative data of CFSE dilution assay in different cell populations defined by expression of CD4, CD25, CD45RO, and CCR5. Numbers in the quadrants correspond to the proportion of dividing cells within each specific cell population. In the panel on the right, histograms (mean ± SEM) correspond to percentage of dividing cells from five healthy individuals. Symbols in the bottom represent the presence (+) or absence (−) of the indicated surface marker. B, CFSE dilution assay after 96 h of stimulation in PBMC from an individual homozygous for the CCR5-Δ32 mutation (left) and an age- and race-matched WT control (middle). Numbers in the quadrants correspond to percentage of dividing cells within CD4+ and CD4− cell populations. Panel on the right shows overlay of the plots depicting cell proliferation in CD4+ cells from these two donors. C, T cell proliferation was determined by CFSE dilution assay in purified T cells from healthy donors (n = 3) after 6 days of stimulation with anti-CD3/CD28 Abs in the presence or absence of neutralizing Abs against the CCR5 ligands CCL3/CCL3L1 and CCL5 or nonspecific isotype controls. In some experiments, cells were preincubated with anti-CCR5 or nonspecific isotype control Abs. Histograms (mean ± SEM) correspond to number of mitotic events calculated as described previously (90). Numbers at the bottom correspond to the experimental conditions depicted on the side. On the right, the overlay of CFSE plots from experimental condition nos. 2 (CD3/CD28 stimulation only) and 7 (CD3/CD28 stimulation following blockade of CCR5 and the CCR5 ligands) are shown; CCR5L represents CCL3/CCL3L1 and CCL5.
However, the aforementioned approach did not allow us to address a possible cause-effect relationship between CCR5 expression and T cell proliferation. To test this possibility, we measured T cell proliferation in PBMC from a CCR5-Δ32/Δ32 donor and an age- and race-matched WT control. Consistent with our hypothesis, there was a higher proportion of CD4+ T cells going through cell division in control PBMC than in those from the CCR5-Δ32/Δ32 donor (Fig. 6B). Impaired proliferation of CCR5-null T cells was only evident after stimulation, and there were no differences in basal proliferation or cell death (data not shown). These findings suggested that CCR5 expression promotes cell proliferation after T cell activation.
To assess whether the reduced T cell proliferation seen in CCR5-deficient T cells could be due to impaired CCR5-CCR5 ligand interactions, we determined anti-CD3/CD28-induced cell proliferation of purified human T cells after blockade of CCR5 and/or neutralization of the main CCR5 ligands. This approach also allowed us to determine whether optimal T cell proliferation requires CCR5 expression on the surface membrane of T cells rather than on the surface of accessory cells (i.e., APCs) present in bulk PBMC. Compared with untreated or isotype control treated cells, blockade of CCR5, CCL3/CCL3L1, or CCL5 resulted in a reduced CD4+ T cell proliferation in vitro (Fig. 6C). This effect, however, was only significant when blocking both ligands simultaneously (Fig. 6C); the observed negative impact of blockade of CCR5 ligands on T cell proliferation is consistent with a previous report (8).
Discussion
CC chemokines impart crucial chemotactic and nonchemotactic effects on T cells that are thought to play critical roles in immune responses (2–16). Thus, given the costimulatory functions of CCR5 ligands (7, 8) and the recruitment of CCR5 at the immunological synapse (9), we hypothesized that reduced CCR5 expression might result in impaired T cell activation. Consistent with this thesis, here we report that CCR5 is a determinant of IL-2 and CD25 expression in activated T lymphocytes and provide evidence that these effects are mediated via NFAT and affect IL-2-dependent biological processes such as STAT5 phosphorylation and T cell proliferation.
Taub et al. demonstrated that CC chemokines costimulate T cell proliferation through the release of IL-2 and the increased expression of CD25 (8). The results of our study extend this observation by indicating that CCR5 is one of the receptors through which these effects might be mediated. Given the redundant nature of the chemokine system, it is possible that other CCRs are involved in T cell responses. However, our results obtained in three knockout models (i.e., CCR1−/−, CCR2−/−, and CCR5−/−) mice suggest that among these three CCRs, CCR5 might have a greater impact on IL-2 production and IL-2-dependent events than CCR1 or CCR2. There are abundant data demonstrating that IL-2 positively regulates the expression of CCR5 (18–21). Here we report the converse: CCR5 favors IL-2 production and CD25 expression in activated T cells. This inference is supported not only by our findings in primary T cells from CCR5-deficient mice, but also from in vitro experimental approaches such as Ab-mediated blockade of CCR5. That maraviroc did not affect IL-2 production in vitro may be due to differences in the receptor configuration and the resulting functionality of Ab-bound vs inhibitor-bound forms of CCR5 (29, 49). Taken together, these observations support the existence of a positive bi-directional feedback between CCR5 expression and IL-2 production during T cell activation. This might ensure survival, expansion, and migration of Ag-specific memory as well as Th1 cells (i.e., CCR5-bearing cells) during the effector phase of adaptive immune responses.
An important but often underappreciated phenotypic difference between naive and memory T cells is the differential expression of CCR5 on these cell types, which is significantly greater in memory cells. We observed that memory T cells (high CCR5 expression) produce higher levels of IL-2 in response to TCR stimulation than naive T cells (low CCR5 expression) and that signaling via CCR5 results in NFAT transactivation during T cell activation. In this regard, our findings might account for the expanded functional capacity of memory cells in terms of IL-2 secretion and cell proliferation (61), and they are also consistent with a recent report showing that accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells (62).
As compared with naive cells, memory T cells have less stringent activation requirements and are thus more permissive to activation (63). Therefore, based on our findings, here we propose that CCR5 might serve as a costimulatory receptor for memory T cells during secondary immune responses. We acknowledge fully that there are many other differences between naive and memory T cells and, consequently, IL-2 production might be influenced by factors independent of CCR5 expression. Despite this caveat, our findings strongly suggest that in addition to conferring inflamed tissue homing capacity, the acquisition of memory/effector function (characterized by a shift from CCR7 to CCR5 surface expression) might confer a preferential advantage for enhanced secretion of IL-2 and proliferation. Similarly, our findings might help explain why Th1 cells, which akin to memory cells exhibit a phenotype characterized by high surface expression of CCR5, produce more IL-2 than do Th2 cells, which do not express high levels of this receptor (64).
The activation of the NFAT pathway following stimulation of CCR5 is not unprecedented but is controversial. For example, Paya and colleagues showed that CCR5 stimulation alone is sufficient to induce expression of FasL by a NFAT-dependent pathway in primary CD4+ T cells (65). In contrast, Cicala et al. (66) found that treatment of PBMC with the CCR5 ligand CCL4 does not induce NFAT. However, in the latter study the authors also found that specific inhibitors of CCR5 prevented R5 gp120-mediated NFAT activation. The results of the present study provide additional support for the presence of NFAT-dependent pathways that are mediated via CCR5 in T lymphocytes.
The enhanced NFAT transactivation observed in the context of high CCR5 expression appears to be the result of a quantitative increase in NFAT levels. Specifically, we observed a significant increase in the levels of NFAT intranuclear translocation in CCR5-expressing T cells that also exhibited increased IL-2 production. This observation is consistent with a recent report suggesting that the translocation of NFAT into the nucleus in activated T cells serves as a switch of the cellular phenotype from an IL-2-nonproducing to an IL-2-producing cell (67). Thus, the link between CCR5 and NFAT expression has biological relevance, as it suggests that the NFAT-influencing effects of CCR5 might regulate the number of Th cells actively producing IL-2 during an immune response (67).
IL-2R is a heterotrimer composed of three protein subunits (α, β, γ) (45, 46). The β-chain exists in a complex with the γ-chain and binds to IL-2 with intermediate affinity (45, 46). This complex is converted into a high-affinity receptor by binding to the α-chain (CD25), whose expression is highly induced by T cell activation (45, 46). Here we demonstrate that CCR5 expression influences the up-regulation of CD25 during T cell activation, and that it may underlie the previously reported correlation between CCR5 and CD25 expression on activated T cells (60, 68). Although IL-2 may signal through its intermediate affinity receptor, the influence of CCR5 on CD25 expression is likely to be biologically relevant, as evidenced by the fact that activated CD25-null T cells are resistant to Fas-mediated activation-induced cell death, a mechanism of peripheral tolerance that is dependent on IL-2 expression (69, 70).
The observation that CCR5-CCR5 ligand interactions influence IL-2 production are consistent with a previous report showing that mice genetically inactivated for CCL5 exhibit not only impaired IL-2 production and T cell proliferation in vitro but also reduced delayed-type hypersensitivity responses in vivo (71). Similarly, we previously found that both humans and mice deficient in CCR5 expression have impaired cell-mediated immunity, as evidenced by reduced delayed-type hypersensitivity skin test responses (27). Since IL-2 is a critical component of anti-viral cell-mediated immunity, the findings reported herein provide additional support to our premise that by influencing cell-mediated immunity, CCR5 may affect anti-HIV responses (27). Notably, the phenotype of low CCR5 expression–low IL-2 levels may provide a basis for the recent reports demonstrating increased susceptibility to infection with West Nile virus (28) in individuals homozygous for the CCR5-Δ32 mutation, and with the higher incidence of herpesvirus and influenza infections observed during clinical trials in individuals who received the CCR5 inhibitor maraviroc as compared with individuals from the placebo arm of the study (www.pfizerpro.com/product_info/selzentry_pi_warnings.jsp). The observation of reduced T cell activation in the context of deficient CCR5 expression might also help explain the association between CCR5-Δ32 mutation and lower susceptibility to inflammatory disorders, including rheumatoid arthritis, Kawasaki disease, and coronary artery disease (72–75). On the other hand, our findings might account for the prolonged graft survival observed after blockade of CCR5 in vivo as well as in CCR5-deficient recipients (76–79). Thus, the perturbations in IL-2 production and T cell proliferation resulting from deficient CCR5 expression might be therapeutically relevant in graft rejection and inflammatory disorders where down-regulation of T cell responses is highly desirable.
Our results have implications for the treatment of HIV infection with CCR5 antagonists. For example, administration of maraviroc has been shown to have beneficial effects on CD4+ T cell recovery in HIV+ individuals even in clinical settings where CCR5 may not play a major role as a coreceptor (80). This observation suggests that in addition to their effects on viral entry, absence or blockade of CCR5 might provide an immunological benefit by regulating the degree of host immune activation. However, in our study we did not find that maraviroc influences IL-2 and CD25 levels, whereas a mAb that specifically binds to the second extracellular loop of CCR5 did. This is a surprising observation considering that both 2D7 Ab as well as maraviroc inhibit ligand-induced calcium flux. It is possible that 2D7 and maraviroc have differential effects on other secondary pathways downstream of CCR5 that may be required for optimal IL-2 expression. Such a scenario is plausible, as it has been previously suggested that distinct biological responses of CCR5 may be mediated through different sets of receptor conformations (81, 82). However, additional studies are required to confirm that maraviroc does not influence immune responses.
The results of our studies with 2D7 have translational value as in addition to small molecule CCR5 antagonists, CCR5 mAbs are also being developed for human use. The results of a phase I clinical trial with HGS004, a fully human IgG4 mAb, was recently reported (83). HGS004 inhibits HIV envelope-dependent cell fusion and viral entry as well as chemokine binding to CCR5 in a manner that is akin to 2D7. Intriguingly, administration of low doses of this Ab was associated with a striking increase in circulating CD4+ and CD8+ T cell counts as early as day 1 after Ab administration without having any effects on HIV viral loads. Based on the studies reported herein, it will be important to evaluate whether these prominent effects on CD4 recovery yet minimal effects on viral replication that are associated with administration of low doses of CCR5 mAb are in part because this therapeutic agent affects the immune activation state as observed for 2D7 herein.
At least three additional lines of evidence further support a link between CCR5 and T cell activation in the context of HIV infection. The first is the fact that natural hosts of SIV/HIV infection are characterized by both low CCR5 expression (84) as well as low levels of activation (85–87), despite having high levels of viral replication. In light of these reports, our findings indicate that low CCR5 expression may be one of the driving forces that underlie the low T cell activation status observed in these animals. Supporting this hypothesis and consistent with the synchronized expression of CD25 and CCR5 in CD4+ PP T cells activated in vitro (60), here we found a significantly reduced number of CD4+CD25+ T cells in PP from CCR5-null mice. This circumstantial evidence suggests that the level of T cell activation in the GALT might be influenced by the level of CCR5 expression. Second, we have found that in HIV+ individuals the level of CCR5 expression is highly correlated with the degree of T cell activation (our unpublished data), which is consistent with the preferential expression of CCR5 in activated CD4+ T cells from these patients (88). Finally, the fact that HIV R5 but not X4 strains induce increased T cell activation favoring HIV survival and spread (89) further supports a role for CCR5-mediated T cell costimulation in HIV pathogenesis.
In summary, we herein demonstrate that CCR5 expression is a regulator of IL-2 and CD25 expression during T lymphocyte activation, an effect that might be mediated through NFAT transactivation/translocation. Furthermore, these effects of CCR5 levels have biological consequences, as they impact IL-2-dependent processes such as STAT5 phosphorylation and T cell proliferation. These findings may have important clinical implications, as they emphasize careful immunological evaluation of HIV+ patients receiving CCR5 antagonists such as mAbs and suggest that blockade of CCR5 might be a beneficial intervention in T cell-driven conditions such as autoimmunity, graft rejection, and possibly HIV.
Supplementary Material
Acknowledgments
We thank to Birju Patel and Delma Thompson for superb technical assistance, and Dr. John Moore for advice on experiments with CCR5 blockers. We dedicate this paper to the memory of David King, who over the years provided expert flow cytometry support; L. Song for outstanding dedication to the graphic work; and to the other members of Dr. Sunil Ahuja’s laboratory for support and helpful discussions. Because of space constraints, we regret our inability to cite additional excellent work.
Footnotes
This work was supported by the Veterans Administration Center on AIDS and HIV infection of the South Texas Veterans Health Care System, a MERIT (R37046326) and other awards (AI043279 and MH069270) from the National Institutes of Health to S.K.A., and separate Merit Review grants from the Department of Veterans Affairs to S.K.A., S.S.A., and S.M. This work was also supported by a National Institutes of Health grant to D.U. (RO1-AI49131). S.K.A. is a recipient of the Elizabeth Glaser Scientist Award and the Burroughs Wellcome Clinical Scientist Award in Translational Research. M.P.Q. acknowledges support by the Stanley Research Foundation and a Young Investigator NARSAD award.
Abbreviations used in this paper: WT, wild type; PP, Peyer’s patches.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu. Rev. Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 2.Friedman RS, Jacobelli J, Krummel MF. Surface-bound chemokines capture and prime T cells for synapse formation. Nat. Immunol. 2006;7:1101–1108. doi: 10.1038/ni1384. [DOI] [PubMed] [Google Scholar]
- 3.Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- 4.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 5.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat. Immunol. 2001;2:123–128. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 6.Bacon KB, Premack BA, Gardner P, Schall TJ. Activation of dual T cell signaling pathways by the chemokine RANTES. Science. 1995;269:1727–1730. doi: 10.1126/science.7569902. [DOI] [PubMed] [Google Scholar]
- 7.Taub DD, Ortaldo JR, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Beta chemokines costimulate lymphocyte cytolysis, proliferation, and lymphokine production. J. Leukocyte Biol. 1996;59:81–89. doi: 10.1002/jlb.59.1.81. [DOI] [PubMed] [Google Scholar]
- 8.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation, I: Beta chemokines costimulate human T lymphocyte activation in vitro. J. Immunol. 1996;156:2095–2103. [PubMed] [Google Scholar]
- 9.Molon B, Gri G, Bettella M, Gomez-Mouton C, Lanzavecchia A, Martinez AC, Manes S, Viola A. T cell costimulation by chemokine receptors. Nat. Immunol. 2005;6:465–471. doi: 10.1038/ni1191. [DOI] [PubMed] [Google Scholar]
- 10.Pinto LA, Williams MS, Dolan MJ, Henkart PA, Shearer GM. β-chemokines inhibit activation-induced death of lymphocytes from HIV-infected individuals. Eur. J. Immunol. 2000;30:2048–2055. doi: 10.1002/1521-4141(200007)30:7<2048::AID-IMMU2048>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 11.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat. Immunol. 2001;2:102–107. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 12.Olszewski MA, Huffnagle GB, McDonald RA, Lindell DM, Moore BB, Cook DN, Toews GB. The role of macrophage inflammatory protein-1α/CCL3 in regulation of T cell-mediated immunity to Cryptococcus neoformans infection. J. Immunol. 2000;165:6429–6436. doi: 10.4049/jimmunol.165.11.6429. [DOI] [PubMed] [Google Scholar]
- 13.Zou W, Borvak J, Marches F, Wei S, Galanaud P, Emilie D, Curiel TJ. Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by β-chemokines rather than IL-12. J. Immunol. 2000;165:4388–4396. doi: 10.4049/jimmunol.165.8.4388. [DOI] [PubMed] [Google Scholar]
- 14.Lillard JW, Jr, Singh UP, Boyaka PN, Singh S, Taub DD, McGhee JR. MIP-1α and MIP-1β differentially mediate mucosal and systemic adaptive immunity. Blood. 2003;101:807–814. doi: 10.1182/blood-2002-07-2305. [DOI] [PubMed] [Google Scholar]
- 15.Sato N, Kuziel WA, Melby PC, Reddick RL, Kostecki V, Zhao W, Maeda N, Ahuja SK, Ahuja SS. Defects in the generation of IFN-γ are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1α-, or CCR2-deficient mice. J. Immunol. 1999;163:5519–5525. [PubMed] [Google Scholar]
- 16.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J. Immunol. 1997;158:4129–4136. [PubMed] [Google Scholar]
- 17.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YF, Tomura M, Iwasaki M, Mukai T, Gao P, Ono S, Zou JP, Shearer GM, Fujiwara H, Hamaoka T. IL-12 as well as IL-2 up-regulates CCR5 expression on T cell receptor-triggered human CD4+ and CD8+ T cells. J. Clin. Immunol. 2001;21:116–125. doi: 10.1023/a:1011059906777. [DOI] [PubMed] [Google Scholar]
- 19.Zou W, Foussat A, Houhou S, Durand-Gasselin I, Dulioust A, Bouchet L, Galanaud P, Levy Y, Emilie D. Acute upregulation of CCR-5 expression by CD4+ T lymphocytes in HIV-infected patients treated with interleukin-2: ANRS 048 IL-2 Study Group. AIDS. 1999;13:455–463. doi: 10.1097/00002030-199903110-00003. [DOI] [PubMed] [Google Scholar]
- 20.Weissman D, Dybul M, Daucher MB, Davey RT, Jr, Walker RE, Kovacs JA. Interleukin-2 up-regulates expression of the human immunodeficiency virus fusion coreceptor CCR5 by CD4+ lymphocytes in vivo. J. Infect. Dis. 2000;181:933–938. doi: 10.1086/315303. [DOI] [PubMed] [Google Scholar]
- 21.Heredia A, Amoroso A, Davis C, Le N, Reardon E, Dominique JK, Klingebiel E, Gallo RC, Redfield RR. Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV β-chemokines: an approach to suppress R5 strains of HIV-1. Proc. Natl. Acad. Sci. USA. 2003;100:10411–10416. doi: 10.1073/pnas.1834278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fatkenheuer G, Pozniak AL, Johnson MA, Plettenberg A, Staszewski S, Hoepelman AI, Saag MS, Goebel FD, Rockstroh JK, Dezube BJ, et al. Efficacy of short-term monotherapy with maraviroc, a new CCR5 antagonist, in patients infected with HIV-1. Nat. Med. 2005;11:1170–1172. doi: 10.1038/nm1319. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 25.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman PA, Buckler-White A, Alkhatib G, Spalding T, Kubofcik J, Combadiere C, Weissman D, Cohen O, Rubbert A, Lam G, et al. Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol. Med. 1997;3:23–36. [PMC free article] [PubMed] [Google Scholar]
- 27.Dolan MJ, Kulkarni H, Camargo JF, He W, Smith A, Anaya JM, Miura T, Hecht FM, Mamtani M, Pereyra F, et al. CCL3L1 and CCR5 influence cell-mediated immunity and affect HIV-AIDS pathogenesis via viral entry-independent mechanisms. Nat. Immunol. 2007;8:1324–1336. doi: 10.1038/ni1521. [DOI] [PubMed] [Google Scholar]
- 28.Glass WG, McDermott DH, Lim JK, Lekhong S, Yu SF, Frank WA, Pape J, Cheshier RC, Murphy PM. CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 2006;203:35–40. doi: 10.1084/jem.20051970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005Q;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinones MP, Martinez HG, Jimenez F, Estrada CA, Dudley M, Willmon O, Kulkarni H, Reddick RL, Fernandes G, Kuziel WA, et al. CC chemokine receptor 5 influences late-stage atherosclerosis. Atherosclerosis. 2007;195:e92–e103. doi: 10.1016/j.atherosclerosis.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A, Chenaux G, Reddick RL, Kuziel WA, Ahuja SS. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J. Clin. Invest. 2004;113:856–866. doi: 10.1172/JCI20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba M, Miyake H, Okamoto M, Iizawa Y, Okonogi K. Establishment of a CCR5-expressing T-lymphoblastoid cell line highly susceptible to R5 HIV type 1. AIDS Res. Hum. Retroviruses. 2000;16:935–941. doi: 10.1089/08892220050058344. [DOI] [PubMed] [Google Scholar]
- 33.Unutmaz D, KewalRamani VN, Marmon S, Littman DR. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 1999;189:1735–1746. doi: 10.1084/jem.189.11.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mummidi S, Adams LM, VanCompernolle SE, Kalkonde M, Camargo JF, Kulkarni H, Bellinger AS, Bonello G, Tagoh H, Ahuja SS, et al. Production of specific mRNA transcripts, usage of an alternate promoter, and octamer-binding transcription factors influence the surface expression levels of the HIV coreceptor CCR5 on primary T cells. J. Immunol. 2007;178:5668–5681. doi: 10.4049/jimmunol.178.9.5668. [DOI] [PubMed] [Google Scholar]
- 35.Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J. Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- 36.Perez OD, Krutzik PO, Nolan GP. Flow cytometric analysis of kinase signaling cascades. Methods Mol. Biol. 2004;263:67–94. doi: 10.1385/1-59259-773-4:067. [DOI] [PubMed] [Google Scholar]
- 37.Mummidi S, Bamshad M, Ahuja SS, Gonzalez E, Feuillet PM, Begum K, Galvis MC, Kostecki V, Valente AJ, Murthy KK, et al. Evolution of human and non-human primate CC chemokine receptor 5 gene and mRNA: potential roles for haplotype and mRNA diversity, differential haplotype-specific transcriptional activity, and altered transcription factor binding to polymorphic nucleotides in the pathogenesis of HIV-1 and simian immunodeficiency virus. J. Biol. Chem. 2000;275:18946–18961. doi: 10.1074/jbc.M000169200. [DOI] [PubMed] [Google Scholar]
- 38.Mummidi S, Ahuja SS, McDaniel BL, Ahuja SK. The human CC chemokine receptor 5 (CCR5) gene: multiple transcripts with 5-end heterogeneity, dual promoter usage, and evidence for polymorphisms within the regulatory regions and noncoding exons. J. Biol. Chem. 1997;272:30662–30671. doi: 10.1074/jbc.272.49.30662. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan M, Frauwirth KA. Reciprocal NFAT1 and NFAT2 nuclear localization in CD8+ anergic T cells is regulated by suboptimal calcium signaling. J. Immunol. 2007;179:3734–3741. doi: 10.4049/jimmunol.179.6.3734. [DOI] [PubMed] [Google Scholar]
- 40.Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 41.Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J. Immunol. 2005;174:7141–7146. doi: 10.4049/jimmunol.174.11.7141. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, Kanaly ST, Towne JE, Willis CR, Kuechle MK, et al. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J. Exp. Med. 2007;204:2603–2614. doi: 10.1084/jem.20070157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez E, Bamshad M, Sato N, Mummidi S, Dhanda R, Catano G, Cabrera S, McBride M, Cao XH, Merrill G, et al. Race-specific HIV-1 disease-modifying effects associated with CCR5 haplotypes. Proc. Natl. Acad. Sci. USA. 1999;96:12004–12009. doi: 10.1073/pnas.96.21.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mummidi S, Ahuja SS, Gonzalez E, Anderson SA, Santiago EN, Stephan KT, Craig FE, O’Connell P, Tryon V, Clark RA, et al. Genealogy of the CCR5 locus and chemokine system gene variants associated with altered rates of HIV-1 disease progression. Nat. Med. 1998;4:786–793. doi: 10.1038/nm0798-786. [DOI] [PubMed] [Google Scholar]
- 45.Kim HP, Imbert J, Leonard WJ. Both integrated and differential regulation of components of the IL-2/IL-2 receptor system. Cytokine Growth Factor Rev. 2006;17:349–366. doi: 10.1016/j.cytogfr.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Lin JX, Leonard WJ. Signaling from the IL-2 receptor to the nucleus. Cytokine Growth Factor Rev. 1997;8:313–332. doi: 10.1016/s1359-6101(97)00021-x. [DOI] [PubMed] [Google Scholar]
- 47.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu. Rev. Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 48.Wu L, LaRosa G, Kassam N, Gordon CJ, Heath H, Ruffing N, Chen H, Humblias J, Samson M, Parmentier M, et al. Interaction of chemokine receptor CCR5 with its ligands: multiple domains for HIV-1 gp120 binding and a single domain for chemokine binding. J. Exp. Med. 1997;186:1373–1381. doi: 10.1084/jem.186.8.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milligan G, Smith NJ. Allosteric modulation of heterodimeric G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28:615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Wu L, Paxton WA, Kassam N, Ruffing N, Rottman JB, Sullivan N, Choe H, Sodroski J, Newman W, Koup RA, Mackay CR. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J. Exp. Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 52.Jain J, Loh C, Rao A. Transcriptional regulation of the IL-2 gene. Curr. Opin. Immunol. 1995;7:333–342. doi: 10.1016/0952-7915(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 53.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell. Signal. 2004;16:1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol. Cells. 2004;18:1–9. [PubMed] [Google Scholar]
- 55.Kim HP, Leonard WJ. The basis for TCR-mediated regulation of the IL-2 receptor α chain gene: role of widely separated regulatory elements. EMBO J. 2002;21:3051–3059. doi: 10.1093/emboj/cdf321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mowat AM, Viney JL. The anatomical basis of intestinal immunity. Immunol. Rev. 1997;156:145–166. doi: 10.1111/j.1600-065x.1997.tb00966.x. [DOI] [PubMed] [Google Scholar]
- 57.Veazey RS, Rosenzweig M, Shvetz DE, Pauley DR, DeMaria M, Chalifoux LV, Johnson RP, Lackner AA. Characterization of gut-associated lymphoid tissue (GALT) of normal rhesus macaques. Clin. Immunol. Immunopathol. 1997;82:230–242. doi: 10.1006/clin.1996.4318. [DOI] [PubMed] [Google Scholar]
- 58.MacDonald TT, Spencer J, Viney JL, Williams CB, Walker-Smith JA. Selective biopsy of human Peyer’s patches during ileal endoscopy. Gastroenterology. 1987;93:1356–1362. doi: 10.1016/0016-5085(87)90266-6. [DOI] [PubMed] [Google Scholar]
- 59.Zeitz M, Greene WC, Peffer NJ, James SP. Lymphocytes isolated from the intestinal lamina propria of normal nonhuman primates have increased expression of genes associated with T-cell activation. Gastroenterology. 1988;94:647–655. doi: 10.1016/0016-5085(88)90235-1. [DOI] [PubMed] [Google Scholar]
- 60.Nagata S, McKenzie C, Pender SL, Bajaj-Elliott M, Fairclough PD, Walker-Smith JA, Monteleone G, MacDonald TT. Human Peyer’s patch T cells are sensitized to dietary antigen and display a Th cell type 1 cytokine profile. J. Immunol. 2000;165:5315–5321. doi: 10.4049/jimmunol.165.9.5315. [DOI] [PubMed] [Google Scholar]
- 61.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 2000;164:2338–2346. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 62.Dienz O, Eaton SM, Krahl TJ, Diehl S, Charland C, Dodge J, Swain SL, Budd RC, Haynes L, Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc. Natl. Acad. Sci. USA. 2007;104:7175–7180. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen: memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J. Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 64.Asselin S, Conjeaud H, Minty A, Fradelizi D, Breban M. Stable polarization of peripheral blood T cells towards type 1 or type 2 phenotype after polyclonal activation. Eur. J. Immunol. 1998;28:532–539. doi: 10.1002/(SICI)1521-4141(199802)28:02<532::AID-IMMU532>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 65.Algeciras-Schimnich A, Vlahakis SR, Villasis-Keever A, Gomez T, Heppelmann CJ, Bou G, Paya CV. CCR5 mediates Fas- and caspase-8 dependent apoptosis of both uninfected and HIV infected primary human CD4 T cells. AIDS. 2002;16:1467–1478. doi: 10.1097/00002030-200207260-00003. [DOI] [PubMed] [Google Scholar]
- 66.Cicala C, Arthos J, Censoplano N, Cruz C, Chung E, Martinelli E, Lempicki RA, Natarajan V, VanRyk D, Daucher M, Fauci AS. HIV-1 gp120 induces NFAT nuclear translocation in resting CD4+ T-cells. Virology. 2006;345:105–114. doi: 10.1016/j.virol.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 67.Podtschaske M, Benary U, Zwinger S, Hofer T, Radbruch A, Baumgrass R. Digital NFATc2 activation per cell transforms graded T cell receptor activation into an all-or-none IL-2 expression. PLoS ONE. 2007;2:e935. doi: 10.1371/journal.pone.0000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ebert LM, McColl SR. Up-regulation of CCR5 and CCR6 on distinct subpopulations of antigen-activated CD4+ T lymphocytes. J. Immunol. 2002;168:65–72. doi: 10.4049/jimmunol.168.1.65. [DOI] [PubMed] [Google Scholar]
- 69.Zheng L, Trageser CL, Willerford DM, Lenardo MJ. T cell growth cytokines cause the superinduction of molecules mediating antigen-induced T lymphocyte death. J. Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
- 70.Van Parijs L, Biuckians A, Ibragimov A, Alt FW, Willerford DM, Abbas AK. Functional responses and apoptosis of CD25 (IL-2Rα)-deficient T cells expressing a transgenic antigen receptor. J. Immunol. 1997;158:3738–3745. [PubMed] [Google Scholar]
- 71.Makino Y, Cook DN, Smithies O, Hwang OY, Neilson EG, Turka LA, Sato H, Wells AD, Danoff TM. Impaired T cell function in RANTES-deficient mice. Clin. Immunol. 2002;102:302–309. doi: 10.1006/clim.2001.5178. [DOI] [PubMed] [Google Scholar]
- 72.Prahalad S. Negative association between the chemokine receptor CCR5-δ32 polymorphism and rheumatoid arthritis: a meta-analysis. Genes Immun. 2006;7:264–268. doi: 10.1038/sj.gene.6364298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burns JC, Shimizu C, Gonzalez E, Kulkarni H, Patel S, Shike H, Sundel RS, Newburger JW, Ahuja SK. Genetic variations in the receptor-ligand pair CCR5 and CCL3L1 are important determinants of susceptibility to Kawasaki disease. J. Infect. Dis. 2005;192:344–349. doi: 10.1086/430953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breunis WB, Biezeveld MH, Geissler J, Kuipers IM, Lam J, Ottenkamp J, Hutchinson A, Welch R, Chanock SJ, Kuijpers TW. Polymorphisms in chemokine receptor genes and susceptibility to Kawasaki disease. Clin. Exp. Immunol. 2007;150:83–90. doi: 10.1111/j.1365-2249.2007.03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gonzalez P, Alvarez R, Batalla A, Reguero JR, Alvarez V, Astudillo A, Cubero GI, Cortina A, Coto E. Genetic variation at the chemokine receptors CCR5/CCR2 in myocardial infarction. Genes Immun. 2001;2:191–195. doi: 10.1038/sj.gene.6363760. [DOI] [PubMed] [Google Scholar]
- 76.Fischereder M, Luckow B, Hocher B, Wuthrich RP, Rothenpieler U, Schneeberger H, Panzer U, Stahl RA, Hauser IA, Budde K, et al. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357:1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- 77.Abdi R, Smith RN, Makhlouf L, Najafian N, Luster AD, Auchincloss H, Jr, Sayegh MH. The role of CC chemokine receptor 5 (CCR5) in islet allograft rejection. Diabetes. 2002;51:2489–2495. doi: 10.2337/diabetes.51.8.2489. [DOI] [PubMed] [Google Scholar]
- 78.Akashi S, Sho M, Kashizuka H, Hamada K, Ikeda N, Kuzumoto Y, Tsurui Y, Nomi T, Mizuno T, Kanehiro H, et al. A novel small-molecule compound targeting CCR5 and CXCR3 prevents acute and chronic allograft rejection. Transplantation. 2005;80:378–384. doi: 10.1097/01.tp.0000166338.99933.e1. [DOI] [PubMed] [Google Scholar]
- 79.Schroder C, Pierson RN, III, Nguyen BN, Kawka DW, Peterson LB, Wu G, Zhang T, Springer MS, Siciliano SJ, Iliff S, et al. CCR5 blockade modulates inflammation and alloimmunity in primates. J. Immunol. 2007;179:2289–2299. doi: 10.4049/jimmunol.179.4.2289. [DOI] [PubMed] [Google Scholar]
- 80.Mayer H, Van der Ryst E, Saag M, Clotet B, Fatkenheuer G, Clumeck N, Turner K, Goodrich JM. Safety and efficacy of maraviroc (MVC), a novel CCR5 antagonist, when used in combination with optimized background therapy (OBT) for the treatment of antiretroviral-experienced subjects infected with dual/mixed-tropic HIV-1: 24-week results of a phase 2b exploratory trial. Programs and Abstracts of the XVI International AIDS Conference; 2006; Toronto. 2006. Abstract THLB0215. [Google Scholar]
- 81.Blanpain C, Vanderwinden JM, Cihak J, Wittamer V, Le Poul E, Issafras H, Stangassinger M, Vassart G, Marullo S, Schlndorff D, et al. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol. Biol. Cell. 2002;13:723–737. doi: 10.1091/mbc.01-03-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lagane B, Ballet S, Planchenault T, Balabanian K, Le Poul E, Blanpain C, Percherancier Y, Staropoli I, Vassart G, Oppermann M, et al. Mutation of the DRY motif reveals different structural requirements for the CC chemokine receptor 5-mediated signaling and receptor endocytosis. Mol. Pharmacol. 2005;67:1966–1976. doi: 10.1124/mol.104.009779. [DOI] [PubMed] [Google Scholar]
- 83.Lalezari J, Yadavalli GK, Para M, Richmond G, Dejesus E, Brown SJ, Cai W, Chen C, Zhong J, Novello LA, et al. Safety, pharmacokinetics, and antiviral activity of HGS004, a novel fully human IgG4 monoclonal antibody against CCR5, in HIV-1-infected patients. J. Infect. Dis. 2008;197:721–727. doi: 10.1086/527327. [DOI] [PubMed] [Google Scholar]
- 84.Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, Sumpter B, Roques P, Marx PA, Hirsch VM, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood. 2007;109:1069–1076. doi: 10.1182/blood-2006-05-024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, Staprans SI, Feinberg MB. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity. 2003;18:441–452. doi: 10.1016/s1074-7613(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 86.Sumpter B, Dunham R, Gordon S, Engram J, Hennessy M, Kinter A, Paiardini M, Cervasi B, Klatt N, McClure H, et al. Correlates of preserved CD4+ T cell homeostasis during natural, nonpathogenic simian immunodeficiency virus infection of sooty mangabeys: implications for AIDS pathogenesis. J. Immunol. 2007;178:1680–1691. doi: 10.4049/jimmunol.178.3.1680. [DOI] [PubMed] [Google Scholar]
- 87.Kornfeld C, Ploquin MJ, Pandrea I, Faye A, Onanga R, Apetrei C, Poaty-Mavoungou V, Rouquet P, Estaquier J, Mortara L, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J. Clin. Invest. 2005;115:1082–1091. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reynes J, Portales P, Segondy M, Baillat V, Andre P, Reant B, Avinens O, Couderc G, Benkirane M, Clot J, et al. CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J. Infect. Dis. 2000;181:927–932. doi: 10.1086/315315. [DOI] [PubMed] [Google Scholar]
- 89.Locher CP, Witt SA, Kassel R, Dowell NL, Fujimura S, Levy JA. Differential effects of R5 and X4 human immunodeficiency virus type 1 infection on CD4+ cell proliferation and activation. J. Gen. Virol. 2005;86:1171–1179. doi: 10.1099/vir.0.80674-0. [DOI] [PubMed] [Google Scholar]
- 90.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion: signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J. Clin. Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.