Abstract
Background
Physician adherence to National Cholesterol Education Program clinical practice guidelines has been poor.
Methods
We recruited 68 primary care family and internal medicine practices; 66 were randomly allocated to a study arm; 5 practices withdrew, resulting in 29 receiving the Third Adult Treatment Panel (ATP III) intervention and 32 receiving an alternative intervention focused on the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7). The ATP III providers received a personal digital assistant providing the Framingham risk scores and ATP III–recommended treatment. All practices received copies of each clinical practice guideline, an introductory lecture, 1 performance feedback report, and 4 visits for intervention-specific academic detailing. Data were abstracted at 61 practices from random samples of medical records of patients treated from June 1, 2001, through May 31, 2003 (baseline), and from May 1, 2004, through April 30, 2006 (follow-up). The proportion screened with subsequent appropriate decision making (primary outcome) was calculated. Generalized estimating equations were used to compare results by arm, accounting for clustering of patients within practices.
Results
We examined 5057 baseline and 3821 follow-up medical records. The screening rate for lipid levels increased from 43.6% to 49.0% (ATP III practices) and from 40.1% to 50.8% (control practices) (net difference, −5.3% [P=.22]). Appropriate management of lipid levels decreased slightly (73.4% to 72.3%) in ATP III practices and more markedly (79.7% to 68.9%) in control practices. The net change in appropriate management favored the intervention (+9.7%; 95% confidence interval [CI], 2.8%-16.6% [P<.01]). Appropriate drug prescription within 4 months decreased in both arms (38.8% to 24.8% in ATP III practices and 45.3% to 24.1% in control practices; net change, +7.2% [P=.37]) Overtreat-ment declined from 6.6% to 3.9% in ATP III and rose from 4.2% to 6.4% in control practices (net change, −4.9% [P=.01]).
Conclusions
A multifactor intervention including personal digital assistant–based decision support may improve primary care physician adherence to the ATP III guidelines.
Lowering High Blood Cholesterol levels is effective in reducing the risk of cardiovascular disease (CVD), the leading cause of death in the United States.1,2 The National Cholesterol Education Program Adult Treatment Panel (ATP) guidelines have been disseminated to promote the appropriate management of dyslipidemia; the ATP III guidelines were released in 2001.3 Use of the previous ATP guidelines was suboptimal because many eligible patients were not prescribed therapy to lower lipid levels (LLT).4-7 There is ample evidence that physician adherence to many clinical practice guidelines (CPGs) has been poor.8 Simple CPG dissemination alone is typically ineffective in changing medical practice; rather, multifaceted interventions targeting different barriers to change are likely to be necessary.9 One strategy to bolster CPG adherence is academic detailing (AD), in which experts and/or opinion leaders visit clinicians to discuss medical topics; AD studies have documented improvements in care.10-12 We considered the ATP III recommendation that the 10-year risk of coronary heart disease (CHD) be estimated via the Framingham risk score (FRS) and used to determine optimal cholesterol level management to be a potentially important barrier to physician adherence. A computerized decision support system (CDSS) that calculates the FRS and delivers recommendations was developed for a personal digital assistant (PDA) (Palm Inc, Milpitas, California) and included on the National Heart, Lung, and Blood Institute ATP III Web site. A CDSS may improve clinical decision making13; however, to our knowledge, this specific tool has not been evaluated previously with respect to enhancing adherence to ATP III guidelines. We thus designed Guideline Adherence for Heart Health (GLAD HEART), a practice-based trial to test the hypothesis that a multifaceted intervention consisting of guideline dissemination enhanced by a CDSS would improve the appropriateness of primary care physicians' management of cholesterol levels. This study reports this trial's effect on screening of lipid levels and appropriate management of lipid level test results.
Methods
The GLAD HEART Trial was aimed at enhancing physician adherence to the ATP III guidelines. Practices were randomized, rather than physicians or patients. Our inclusion criteria were self-described primary care practices, staffed by internal medicine or family medicine providers who were willing to be randomized and to have patient medical record abstraction performed. To avoid limited commitment to the study, we required at least half of the providers (physicians, physician assistants, and nurse practitioners) at nonsolo provider practices to agree to participate. Finally, all practices were within a 3-hour driving radius of Winston-Salem. We excluded practices with a direct affiliation to a medical school or a residency program, practices that had been open less than 1 year after the publication of the ATP III guidelines, practices that provided subspecialty care exclusively, and sites outside North Carolina. Details regarding recruitment procedures have been published.14 Briefly, we recruited practices from June 1, 2003, through April 30, 2004, from several sources. We solicited practices via advertising (ie, newsletter inserts, conference presentation/flyers, mass faxes, and direct mail), which yielded 53 inquiries; of these, 18 met study criteria and agreed to participate. We identified 357 practices for direct communication (using cold calls, previous relationships, or in-office presentations), which yielded 31 practices. Finally, we surveyed 176 practices that had established relationships with the Northwest Area Health Education Center; of these, 90 returned a survey and 19 met criteria and agreed to participate. The Wake Forest University institutional review board reviewed and approved this study. Because this project involved quality improvement, individual patient-level consent for medical record review was not deemed to be required by the institutional review board. We complied with Health Insurance Portability and Accountability Act privacy directives.
Practice characteristics were obtained via a standard survey and included practice size and provider sex, ethnicity, and training. Practices were considered to consist of predominantly female or minority providers if more than 50% of the providers were women or nonwhite. Practices were considered to be urban or rural according to their location's census designation.
Intervention
The intervention was not blinded; practices received an intervention focused on the ATP III guidelines or an alternative intervention focused on the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7).15 Randomization was stratified by practice type and size and blocked (block size, 8). Providers in both arms participated in CPG dissemination activities, which included distribution of paper copies of the ATP III and JNC-7 guidelines, an initial didactic session (focused on both CPGs) for which physicians could obtain continuing medical education (CME) credits, and 1-hour intervention-specific AD visits to practices every 6 months. The 4 AD sessions were open to the providers and the clinic staff and were designed to reinforce ATP III or JNC-7 content (depending on their practice's intervention assignment). Each visit was conducted by a GLAD HEART physician-investigator and a study staff member. The second AD visit provided practice-specific feedback regarding their screening rate for lipid levels and appropriate management of lipid levels using data from the baseline medical record abstraction.
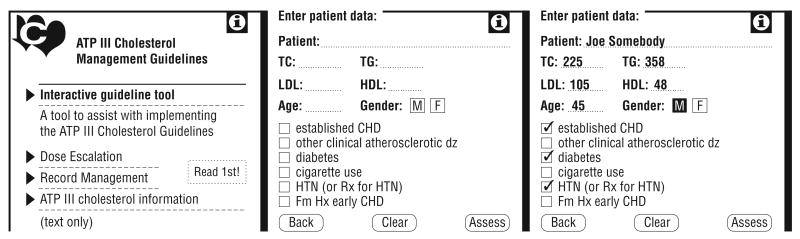
We furnished providers in the ATP III practices with a PDA programmed with a CDSS based on the National Heart, Lung and Blood Institute program but modified to include information about LLT dosing, management of triglyceride levels, and a report-printing function (Figure 1). A 1-page report that summarized the patient-specific data that had been entered, the patient-specific ATP III goals for low-density lipoprotein cholesterol (LDL-C) levels, and treatment recommendations could be printed via the PDA infrared port to a compatible printer. Practices that did not have such a printer were provided with an adaptor. A tracking program on each device recorded the number of times the CDSS program was used; this information was collected at AD visits to identify infrequent users, who were then encouraged to increase their use. At each AD session, providers were given cases for which to use the CDSS program and encouraged to do so for patients who had undergone testing of lipid levels. The CDSS was reprogrammed to reflect the modifications to the ATP III guidelines, published in 2004, which made more persons, especially high-risk patients, eligible for drug treatment and advocated a goal of less than 70 mg/dL for LDL-C levels in a subset of high-risk patients (to convert cholesterol levels to millimoles per liter, multiply by 0.0259).16 The modified CDSS was distributed to all ATP III providers. The JNC-7 practices received automatic blood pressure measurement devices (HEM907; Omron Healthcare, Bannockburn, Illinois) at the rate of 1 for every 3 practice providers for use in their clinic.
Figure 1.
Computerized decision support tool screen views from the Guideline Adherence for Heart Health Trial. ATP III indicates Third Adult Treatment Panel; CHD, coronary heart disease; dz, disease; Fm Hx, family history; HDL, high-density lipoprotein cholesterol level; HTN, hypertension; LDL, low-density lipoprotein cholesterol level; Rx, prescription; TC, total cholesterol level; and TG, triglyceride level.
Data Collection
We have previously published details regarding data collection, which entailed medical record abstraction at each practice at baseline and follow-up.17 Briefly, from December 1, 2003, through October 31, 2004 (baseline), and again from December 1, 2006, through September 30, 2007 (follow-up), nurse abstractors from the Carolinas Center for Medical Excellence used a standardized data collection tool and visited practices for medical record abstraction. Abstractors were not informed regarding the practice's intervention arm; they were aware that ATP III and JNC-7 guideline adherence was being assessed. The baseline 2-year data collection window (June 1, 2001, through May 31, 2003) was after publication of the ATP III guidelines but before any research contact with practices. Eligible patients included adults aged 21 to 84 years who visited the practices during the baseline period because the ATP III guidelines recommend screening for all adults 20 years or older every 5 years. The medical record abstraction period extended to September 30, 2003, to allow for follow-up and management decisions to be documented for the patients screened at the end of the window. During the follow-up data abstraction window (May 1, 2004, through April 30, 2006), we used the same eligibility criteria; similarly, the follow-up period extended to August 31, 2006.
We a priori expected the following 3 categories of patients: (1) those receiving LLT before the data abstraction window (for whom testing of lipid levels was to monitor therapy rather than to screen, and no further data abstraction was performed); (2) those not receiving prior LLT and without lipid data during the data collection period; and (3) those not receiving therapy before the window, with testing of lipid levels during the abstraction period (ie, those screened). Data elements collected for categories 2 and 3 included demographics (age, sex, and race/ethnicity) and major comorbidities (CVD and diabetes mellitus). For patients screened, additional variables abstracted included the initial lipid profile values, stroke, peripheral vascular disease, risk factors (ie, smoking, blood pressure, hypertension, prescription of antihypertensive medicine, and family history of heart disease), date of the follow-up lipid profile and lipid values (if measured), and date of prescription of LLT. In addition, documentation of the provider giving advice regarding therapeutic lifestyle changes was recorded. For race/ethnicity and sex, abstractors could record “unknown”; for all other data elements, lack of documentation was recorded as not present (comorbidities), not performed (measurement of lipid levels and blood pressure), or not prescribed (medications). We planned to select independent samples of 140 patients randomly at each practice to yield 30 full abstractions (ie, among patients screened). This sample represented the entire practice; we did not take independent samples for each provider within a practice. This was accomplished using lists of active patients generated by each practice; at practices unable to provide a list, medical records were systematically retrieved using a random number table to determine starting location and sampling frequency. When 30 medical records with full lipid profiles were abstracted, no further medical records were abstracted or retrieved. At some practices, additional samples of 140 patient medical records were prepared to reach those practices' abstraction goals. Selected records were reabstracted for intraobserver and interobserver reliability, which were 95.2% and 89.9%, respectively, at baseline and 93.0% and 88.2%, respectively, at follow-up.
Definitions
The ATP III guidelines recommend that all adults undergo screening of lipid levels once every 5 years; for certain high-risk persons (eg, those with diabetes mellitus or known CVD), annual screening is appropriate.3 We therefore considered all patients not already receiving LLT as eligible to be screened. In low-risk groups, approximately 40% should be screened in a 2-year period. We considered a person to have been screened if (1) they did not previously receive LLT and (2) a lipid-profile (including measurement of total cholesterol, triglyceride, high-density lipoprotein cholesterol, and LDL-C levels) report (with values) was obtained during the data collection window and recorded in the medical record. The screening rate consists of the number of patients screened divided by the total number of patients sampled who were eligible for screening. All screened persons were categorized into ATP risk categories on the basis of documented history and, if required, the 10-year risk of CHD calculated using the FRS.18 Patients were assigned to 1 of the following 4 ATP III risk categories: (1) low risk (defined as 0-1 risk factor for CHD), (2) intermediate low risk (≥2 risk factors and a 10-year risk of <10%), (3) intermediate high risk (≥2 risk factors and a 10-year risk of 10%-20%), and (4) high risk (CHD risk equivalent [diabetes, CHD, stroke, or peripheral vascular disease] and/or ≥2 risk factors with a 10-year risk of >20%). At baseline, patients were classified as having dyslipidemia if their LDL-C level exceeded the risk group–specific goal recommended in the ATP III guidelines (160, 130, 130, or 100 mg/dL, respectively). Patients were considered eligible for consideration of drug therapy if their LDL-C level exceeded the therapy-initiation thresholds specific to their risk category (190, 160, 130, or 130 mg/dL, respectively). We considered statins, fibrates, cholesterol absorption inhibitors, niacin, and bile acid sequestrants to be acceptable LLT. At follow-up, we used the modified recommendations, which made drug therapy optional for intermediate high-risk patients with LDL-C levels from 100 to 130 mg/dL, lowered the treatment threshold from 130 to 100 mg/dL for high-risk patients, and made drug therapy optional for high-risk patients with LDL-C levels of greater than 70 mg/dL. Patients were classified as being treated appropriately with respect to their LDL-C level if any of the following criteria were met:
The LDL-C level was below the risk stratum–specific LDL-C level goal, and drug therapy was not initiated during the 4 months after initial testing.
The LDL-C level was greater than the risk stratum–specific drug therapy initiation cutoff point, and drug therapy was prescribed within 120 days.
The LDL-C level was greater than the drug therapy–initiation cutoff point, results of a follow-up lipid profile confirmed this finding within 120 days, and, only if the lipid profile was remeasured, drug therapy was prescribed within 150 days of the original lipid profile.
The LDL-C level was greater than the drug treatment initiation threshold, was documented during the 120 days after initial testing to have decreased below the LDL-C goal, and drug therapy was not initiated.
The LDL-C level was greater than the drug treatment initiation threshold, was documented during the 120 days after initial testing to have decreased below the drug therapy–initiation point (but still above the goal), drug therapy was not initiated, and advice regarding therapeutic lifestyle changes was documented.
The LDL-C level was greater than the LDL-C goal but below the risk stratum–specific drug treatment initiation threshold, and advice regarding therapeutic lifestyle changes was documented.
The LDL-C level was in a “gray zone” (where the ATP III guidelines indicated that drug therapy was optional) and drug therapy was initiated within 120 days or, if a drug was not prescribed, advice regarding therapeutic lifestyle changes was documented.
Otherwise, patients were classified as being treated inappropriately with respect to their dyslipidemia. Patients were at risk for being inappropriately prescribed LLT if the initial LDL-C level was less than the drug therapy–initiation or drug-optional threshold. Such patients who were prescribed LLT within 4 months were considered to be inappropriately treated. Patients were at risk of being undertreated if their initial LDL-C level was greater than the drug therapy–initiation threshold; patients not treated within 4 months were considered to be undertreated. Patients for whom the FRS was required but could not be calculated owing to missing data were not included in analyses of appropriate management, as were those with elevated triglyceride levels resulting in missing LDL-C levels. We did not collect data on direct measurement of LDL-C levels because the ATP III guidelines recommend addressing elevated triglyceride levels first, then repeating the lipid profile.
Statistical Analysis
The primary outcome for this trial was the proportion of patients treated appropriately with respect to LLT within 4 months after testing. The analyses for this study take into account the nested design in which randomization and intervention were performed at the level of the practices, but the actual outcomes data were collected on individual patients within practices. Patients within a practice are more likely to be similar to one another than to patients in other practices, resulting in a positive, nonzero intraclass correlation coefficient (ICC). This correlation results from within-practice similarities in patient characteristics including age, ethnicity, and socioeconomic status and from similarities in provider behavior. We did not assess the additional potential correlation attributable to clustering at the provider level for 2 reasons. First, we had fiscal constraints regarding the number of medical records we could afford to abstract within each practice, making collection of a sufficiently large sample at the provider level infeasible. Second, we recognized the collaborative nature of practice, especially with midlevel providers involved; hence, we judged that practice level correlation was more important and more feasible to address.
Practice and patient level statistics were compared by intervention arm using descriptive statistics. The primary and secondary outcomes assessed represent dichotomous variables that are usually reported in terms of the proportion or the odds of success or failure. These include appropriate medication treatment decisions (primary) and the components of appropriate decision making, including appropriate prescribing, overprescribing, and appropriate screening. We performed a stratified analysis of the primary outcome according to ATP III categories, combining the 2 intermediate-risk categories into 1 (thus low, intermediate, and high risk). We analyzed the data using a generalized estimating equation approach with a logit link.19 Regression models took into account the within-practice correlation, and we used robust variance estimates.
To determine sample size, we estimated that appropriate treatment at baseline would be 70% in both arms. Recruiting 32 practices per group (64 total) yielded 80% power to detect a minimum difference of 9% in treatment rates between control and intervention practices at follow-up, with a 5% 2-sided significance level, assuming 30 patients would undergo assessment per practice, a between-practice variance of 0.21, and an ICC of 0.01.
Results
Recruitment and Practice Characteristic
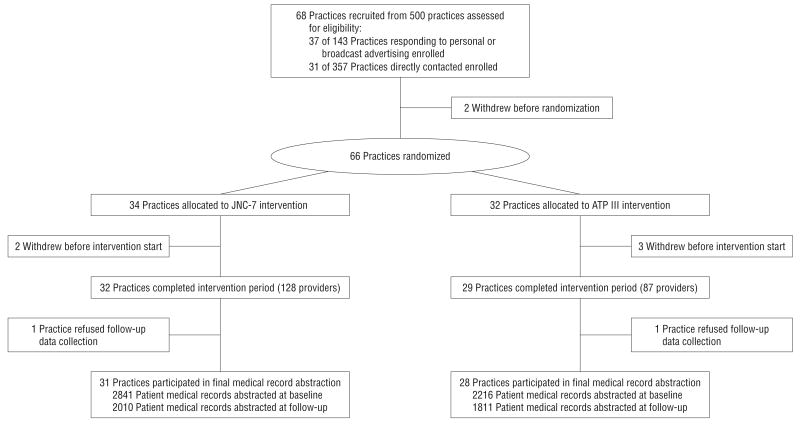
Our recruitment efforts yielded 68 practices (Figure 2). Two practices withdrew before randomization and an additional 5 practices withdrew after randomization but before the start of the intervention. This resulted in 61 practices entering the intervention period with 29 in the ATP III arm and 32 in the control intervention. One practice was discovered after randomization to have not met 1 inclusion criterion (it had been open for <1 year); we did not drop this practice and it is included in the results. Characteristics of practices recruited are listed in Table 1. The average number of health care providers per practice in the intervention arm was 3 (range, 1-11) in the ATP III arm and 4 (range, 1-14) in the control arm. There were no substantive differences in practice characteristics by arm. At the final AD visit, we ascertained the number of practices that had electronic medical record systems; the proportions were similar across arms (8 [28%] for ATP III vs 11 [34%] for JNC-7 practices [P=.60]).
Figure 2.
Consort diagram for the Guideline Adherence for Heart Health (GLAD HEART) Trial. ATP III indicates Third Adult Treatment Panel; JNC-7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.
Table 1. Characteristics of Enrolled Practices, GLAD HEART Trial 2003-2007.
| No. (%) of Practices | ||||
|---|---|---|---|---|
| Characteristic | ATP III Arm (n=29) |
Control Arm (n=32) |
P Valuea | |
| Practice type | ||||
| Family medicine | 22 (76) | 24 (75) | ||
| Internal medicine | 7 (24) | 8 (25) | .94 | |
| Practice size, No. of providers | ||||
| 1 | 8 (28) | 5 (16) | ||
| 2-4 | 16 (55) | 17 (53) | .33 | |
| ≥5 | 5 (17) | 10 (31) | ||
| Rural practice location | 7 (24) | 6 (19) | .61 | |
| Female provider predominant | 8 (28) | 8 (25) | .82 | |
| Minority provider predominant | 3 (10) | 7 (22) | .31b | |
Abbreviations: ATP III, Third Adult Treatment Panel; GLAD HEART, Guideline Adherence for Heart Health.
Unless otherwise indicated, P values for comparison are based on the χ2 test.
Indicates that the P value is based on the Fisher exact test owing to small expected cell sizes.
Intervention Fidelity
All 4 AD sessions were completed at all ATP III and JNC-7 practices. Average attendance of the providers (physicians and midlevel staff) in the ATP III arm ranged from 77% to 89% and in the JNC-7 arm from 76% to 83%. Overall attendance was 86% in the ATP III practices and 80% in JNC-7 practices (P=.15). In ATP III practices, at AD visit 2, 83% of the providers' PDAs indicated use of the CDSS. At AD visit 3, 95% of the providers' PDAs indicated some use of the CDSS; however, 46% of the PDAs indicated that the provider had discontinued use between visits 2 and 3. The most frequent reasons given for discontinued use were related to having learned the ATP III guidelines because of prior use and finding it inconvenient to input data during the clinical encounter. Several providers reported that, once they adopted electronic medical record systems, they were less inclined to enter data into the PDA (to avoid having to interface with 2 different computers).
Patient Characteristics and Screening Rates
We could not obtain complete follow-up data from 2 practices owing to withdrawal from the study after completion of the intervention but before the completion of follow-up medical record abstraction (1 practice in each arm). The characteristics of the 5057 patients at baseline and the 3821 patients at follow-up who were not receiving LLT are presented in Table 2. There were no differences by arm in the average age, the proportion who were women, the racial/ethnic composition of the sample, or the proportion with CVD and/or diabetes mellitus. Race/ethnicity was not available from medical records for about one-third of the overall sample. The patient characteristics were also similar between the baseline and follow-up samples.
Table 2. Characteristics of Patients With Medical Records Abstracted at Baseline and Follow-up in the GLAD HEART Triala.
| Baseline | Follow-up | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | ATP III Arm (n=2216) |
Control Arm (n=2841) |
P Value | ATP III Arm (n=1811) |
Control Arm (n=2010) |
P Value | ||
| Female, % | 59 | 56 | .18 | 60 | 58 | .42 | ||
| Mean age, y | 47.6 | 45.0 | .11 | 46.9 | 45.5 | .33 | ||
| Race/ethnicity, % | ||||||||
| Non-Hispanic white | 59 | 63 | 59 | 50 | ||||
| African American | 7 | 11 | 5 | 11 | ||||
| Hispanic | 2 | 2 | .59 | 3 | 1 | .19 | ||
| Other | 1 | 1 | 1 | 1 | ||||
| Unknown | 31 | 23 | 31 | 37 | ||||
| Medical history, % | ||||||||
| CVD | 9 | 6 | .11 | 6 | 5 | .38 | ||
| Diabetes mellitus | 10 | 9 | .42 | 9 | 10 | .45 | ||
Abbreviations: ATP III, Third Adult Treatment Panel; CVD, cardiovascular disease; GLAD HEART, Guideline Adherence for Heart Health.
Based on clustered analysis with patients nested within practices.
At baseline, a similar proportion of patients had been screened for dyslipidemia in both arms; screening rates increased (Table 3) such that about half were screened in each study arm. The net increase in screening was higher in the JNC-7 arm, but this difference was not statistically significant. The ICC for the screening rate was 0.22.
Table 3. Lipid Screening and Management Among 61 Primary Care Practices in North Carolina.
| Quality Indicator | ATP III Practice | JNC-7 Practice | ATP III–JNC-7 Comparison | P Value |
|---|---|---|---|---|
| Screening | ||||
| Sample size before/after intervention | 2216/1811 | 2841/2010 | ||
| Patients screened, % | ||||
| Baseline | 43.6 | 40.1 | +3.5 | .41 |
| Follow-up | 49.0 | 50.8 | −1.8 | .72 |
| Change from follow-up to baseline | +6.6 | +10.7 | −5.3 | .22 |
| Appropriate management | ||||
| Sample size before/after intervention | 842/709 | 855/771 | ||
| Patients appropriately managed, % | ||||
| Baseline | 73.4 | 79.7 | −6.3 | .02 |
| Follow-up | 72.3 | 68.9 | +3.4 | .18 |
| Change from follow-up to baseline | −1.1 | −0.8 | +9.7 | <.01 |
| Inappropriate prescription of LLT | ||||
| Sample size before/after intervention | 626/519 | 650/571 | ||
| Patients who received inappropriate prescription, % | ||||
| Baseline | 6.6 | 4.2 | +2.4 | .15 |
| Follow-up | 3.9 | 6.4 | −2.5 | .07 |
| Change from follow-up to baseline | −2.7 | +2.2 | −4.9 | .01 |
| Appropriate prescription of LLT | ||||
| Sample size before/after intervention | 216/190 | 205/200 | ||
| Patients who received appropriate prescription, % | ||||
| Baseline | 38.8 | 45.3 | −6.5 | .27 |
| Follow-up | 24.8 | 24.1 | +0.7 | .88 |
| Change from follow-up to baseline | −14.0 | −21.2 | +7.2 | .37 |
Abbreviations: ATP III, Third Adult Treatment Panel; JNC-7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LLT, therapy to lower lipid levels.
Appropriate Management
Of the 1776 patients screened at baseline and 1658 at follow-up, risk level and appropriate management could be determined for 1697 at baseline and 1480 at follow-up. In the JNC-7 arm, 41.8% of patients at baseline were low risk per the ATP III definition compared with 43.6% at follow-up. The CHD equivalent status was 22.6% prior to and 20.0% during the intervention. Only 24.0% (before) and 25.9% (after) of those screened required LLT. In the ATP III arm, 35.2% were low risk at baseline compared with 43.6% at follow-up. The CHD equivalent status was 22.8% and 16.5%. At baseline, 25.7% of those screened required LLT compared with 26.8% at follow-up. Appropriate management of cholesterol levels was higher in the control arm practices at baseline (Table 3). Appropriate management decreased slightly in the ATP III practices, and more markedly in the control practices. The net difference in appropriate management was 9.7% in favor of the intervention group (P<.01); the ICC was 0.01. We observed a reduction in inappropriate prescription of LLT in the ATP III arm, whereas inappropriate prescription increased in the control arm. There was a decline in appropriate prescribing of LLT in both arms of the study (Table 3), with no significant intervention effect on this measure. A sensitivity analysis that includes data from only 58 practices with both baseline and follow-up data yielded similar results (a net difference in appropriate management favoring the ATP III intervention of 9.2% [P=.02]).
The impact of the intervention on appropriate management by level of risk is presented in Table 4. The appropriate decision was made for approximately 90% of the low-risk patients in both arms during both periods, with no intervention effect. In moderate-risk patients, appropriate management declined in the JNC-7 arm and remained essentially unchanged in the ATP III arm, resulting in a net difference favoring the intervention. Among high-risk patients, appropriate decision making declined substantially in both arms, although to a slightly lesser extent in the ATP III arm. The ICC was similar in each risk category (0.01 for the low-, intermediate-, and high-risk categories).
Table 4. Appropriate Lipid Management by ATP III Risk Category Among 61 Primary Care Practices in North Carolina.
| Quality Indicator | ATP III Practice | JNC-7 Practice | ATP III–JNC-7 Comparison | P Value |
|---|---|---|---|---|
| Low risk | ||||
| Sample size before/after intervention | 296/309 | 357/336 | ||
| Patients appropriately managed, % | ||||
| Baseline | 91.4 | 94.1 | −2.7 | .29 |
| Follow-up | 90.9 | 89.2 | +1.7 | .49 |
| Change from follow-up to baseline | −0.5 | −4.9 | +4.4 | .21 |
| Moderate risk | ||||
| Sample size before/after intervention | 315/253 | 281/254 | ||
| Patients appropriately managed, % | ||||
| Baseline | 69.4 | 73.9 | −4.5 | .60 |
| Follow-up | 70.3 | 62.6 | +7.7 | .07 |
| Change from follow-up to baseline | +0.9 | −7.3 | +8.2 | .03 |
| High risk | ||||
| Sample size before/after intervention | 231/147 | 217/181 | ||
| Patients who received appropriate prescription of LLT, % | ||||
| Baseline | 47.5 | 55.6 | −8.1 | .14 |
| Follow-up | 24.4 | 28.7 | −4.3 | .41 |
| Change from follow-up to baseline | −23.1 | −26.9 | +3.8 | .65 |
Abbreviations: ATP III, Third Adult Treatment Panel; JNC-7, Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; LLT, therapy to lower lipid levels.
Comment
These results demonstrate that a multifaceted intervention that included guideline dissemination, AD audit and feedback, and provision of a CDSS resulted in better adherence to the ATP III guidelines than was observed in the control intervention. Stable adherence was observed in the ATP III intervention group, whereas a decline in guideline adherence was observed in the control group. The modest relative improvement in adherence was related to a decrease in overtreatment (prescribing LLT to patients who did not require it, as recommended by the ATP III) and to a smaller increase in undertreatment. Screening rates increased in both arms to a similar extent. Appropriate drug treatment was not improved by this intervention.
The ATP III recommends that all adults without CVD or diabetes be screened once every 5 years with a lipid profile; this translates into an expected screening rate of 40% during a 2-year window. Practices were meeting this expectation at baseline. As such, the observed screening rates of approximately 50% at follow-up may represent modest overscreening. It is possible that this screening rate reflects an increased awareness of the need to obtain lipid profiles to ascertain a patient's status with respect to the ATP III guidelines. Also, providers in both arms were aware that the study focused on cholesterol level management. On the other hand, we did not attempt to limit our medical record review to only those patients who had not been screened in the preceding 5 years, and it was not feasible to determine whether patients had been screened elsewhere; thus, our inferences regarding an appropriate screening rate are limited.
Many patients screened in these primary care practices were at low risk according to the ATP III guidelines. For these patients, the appropriate decision is not to prescribe a drug. A minority of patients were eligible for LLT. Thus, the average patient undergoing examination and screening in primary care had a greater risk of overtreatment than of undertreatment. The proportion of screened patients who were at lower risk increased during follow-up in both arms of the study. The fact that appropriate management decreased in the control arm suggests that screening without concomitant reinforcement of the ATP III recommendations, via AD or a CDSS, may lead to overtreatment.
We observed a decrease in appropriate LLT prescribing in both arms of this study. The analysis of appropriate decision making by risk category suggests one plausible explanation. Among high-risk patients, appropriate management declined markedly between baseline and follow-up in both arms. The criteria for appropriate management at follow-up were the revised ATP III guidelines, which made more high-risk patients eligible for LLT by lowering the drug therapy–initiation threshold from 130 to 100 mg/dL. The change in lipid level management recommendations was made early in the follow-up abstraction window, and we disseminated the revised recommendations to practices; therefore, we held practices to the revised standards at follow-up.
Systematic reviews have noted that many studies, particularly those evaluating single interventions, have failed to demonstrate improved quality.9 A multifaceted, cluster-randomized trial conducted in 20 community-based primary care practices (a design similar to that of the GLAD HEART Trial) aimed at improving a range of CVD prevention–related quality indicators demonstrated improved quality in both arms (mean indicators at or above the targets in the areas of hypertension, hyperlipidemia, CHD, heart failure, atrial fibrillation, and diabetes mellitus, 11.3%-33.7% in the intervention and 6.3%-22.7% in the control practices) but failed to show a statistically significant intervention effect.20 In that trial, cholesterol level screening did not appreciably change (50.2%-53.5% in the intervention arm and 45.3%-43.1% in the control arm). A CDSS failed to improve adherence of British primary care providers to recommendations for patients with angina overall, including specifically for statin prescription, compared with no CDSS.21 A recent German trial22 randomized primary care physicians to an intervention based on CME, a text with guideline-based recommendations, a paper-based CVD risk calculator, and a patient-physician shared decision-making tool on the patient's CVD risk compared with a control group featuring CME on non-CVD topics. That trial assessed change in the FRS as the outcome; in both arms the FRS decreased, but there was no difference across study arms. In a Canadian study23 that randomized patients rather than providers or practices, statin prescription within a 6-month window for patients with ischemic heart disease did not improve as a result of an intervention using an opinion leader–generated, patient-specific report faxed to primary care providers compared with no report (17% in each arm). However, a recent primary practice–based intervention24 aimed to improve physician adherence to Dutch national lipid level management guidelines suggests that the timing of decision support is critical and also suggests that a CDSS may be useful in the right setting. All practices in that study used an electronic medical record system; patient-level data were used by the CDSS to determine which patients required cholesterol level screening and which met criteria for LLT. Practices were randomized to receive the CDSS-generated recommendations automatically when patients were seen in the office (alerting function), when physicians requested the CDSS-generated advice, or when no CDSS was available (control). Both CDSS conditions increased screening rates, but only alerts improved LLT prescribing compared with a control condition.24
The major strengths of our study are the use of a randomized, controlled design; a substantial sample size; and data collection in a random sample of patients from practices. Our study has several limitations. Our practice sample was limited to practices that agreed to participate in a quality improvement trial; thus, our results may not be generalizable to unselected primary care practices. We experienced withdrawals from the trial. It is not clear why practices did so before the start of the intervention; however, we suspect that we may not have emphasized sufficiently the differences between our study methods and those of more typical quality improvement studies, specifically that they needed to be willing to accept the random allocation to the intervention. In traditional clinical trials, blinding may facilitate participant adherence, but it was not feasible to mask providers as to which arm they were in. We could not mandate or monitor use of the CDSS for every patient who was screened and could not limit control practice providers from obtaining the more limited National Heart, Lung, and Blood Institute version of the CDSS. We did not determine whether the recommendations were being printed or placed in the medical records. By assessing CDSS use at AD visits, we determined that many providers discontinued use of the CDSS during the trial. It is possible that the CDSS was not a significant factor in the observed intervention effect. Both arms received AD visits; those for the ATP III arm reinforced using the PDA tool at each visit and provided updates regarding the ATP III. At least 1 trial25 suggests that online CME presentations increased knowledge about the ATP III and increased pharmacotherapy for dyslipidemia for high-risk patients of primary care providers, although traditional in-person CME presentations did not. Furthermore, in our study, initial dissemination presentations and the performance feedback report were not tailored by arm; thus, it is unlikely that these aspects of the intervention are related to the differences observed. We also did not address potential patient-level barriers to LLT, including contraindications (although these are rare), prescription drug coverage, and patient preferences. We did not assess differences in control of dyslipidemia because this was not a primary aim of our study. By design we were interested in decision making with respect to screening lipid profiles, not longer-term results. Another limitation is the possibility that the selection of an active comparator (JNC-7 guidelines) had a deleterious effect on practices in that arm with respect to lipid level management. A separate report fully describes the results of the JNC-7 intervention; in brief, we found no difference between the 2 groups in any of the JNC-7 adherence measures, including the percentage of patients who achieved their blood pressure goals (intervention group, 49.2%; control group, 50.6%) or the use of a thiazide-type diuretic as first-line therapy (32.0% vs 29.5%).26 Therefore, we do not suspect that the JNC-7 intervention would affect cholesterol level management.
An alternative view of our results is that the intervention prevented significant deterioration of cholesterol level management in the ATP III arm, whereas in the JNC-7 arm, less exposure to the modified ATP III guidelines without ongoing education or decision support led to worse performance. We had expected our intervention to lead to increased use of LLT in appropriate patients. Our results, however, still translate into better quality because fewer patients in the ATP-III arm were offered LLT that they did not need and because the provision of needed therapy (as recommended by the guideline update) deteriorated less in the ATP III intervention group. The overall appropriateness of management indicates substantial room for improvement. Future efforts focused on enhancing the management of lipid levels using a CDSS likely need to incorporate features suggested in other trials to enhance success, including provision of automatic decision support as part of the clinic flow, especially when and where decisions are made.27 Our results, placed in the context of several other quality improvement studies focused on CVD, suggest that requiring providers to take the initiative to activate a CDSS (whether on a PDA or within an electronic medical record system) is unlikely to substantially improve medical decision making.
Acknowledgments
Obtained funding: Goff. Administrative, technical, and material support: Crago, Rosenberger, Barham, and Clinch.
Funding/Support: This study was supported by grant R01 HL70742 from the National Heart, Lung, and Blood Institute.
Footnotes
Author Contributions: Study concept and design: Bertoni and Goff. Acquisition of data: Bertoni, Bonds, Crago, Rosenberger, Clinch, and Goff. Analysis and interpretation of data: Bertoni, Chen, Hogan, Barham, and Goff. Drafting of the manuscript: Bertoni and Hogan. Critical revision of the manuscript for important intellectual content: Bonds, Chen, Crago, Rosenberger, Barham, Clinch, and Goff. Statistical analysis: Chen and Hogan.
Additional Contributions: The physicians and staff of the participating medical practices, the data collection team at the Carolinas Center for Medical Excellence, and Michael Lischke, EdD, MPH, of the Northwest Area Health Education Center made valuable contributions to this project.
Financial Disclosure: None reported.
Role of the Sponsor: The sponsor was not involved in the trial's design or conduct, data analysis, manuscript writing, or approval.
References
- 1.LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282(24):2340–2346. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Friday G, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Nieto FJ, Alonso J, Chambless LE, et al. Population awareness and control of hypertension and hypercholesterolemia: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1995;155(7):677–684. [PubMed] [Google Scholar]
- 5.Hoerger TJ, Bala MV, Bray JW, Wilcosky TC, LaRosa J. Treatment patterns and distribution of low-density lipoprotein cholesterol levels in treatment-eligible United States adults. Am J Cardiol. 1998;82(1):61–65. doi: 10.1016/s0002-9149(98)00227-6. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC., Jr Clinical treatment of dyslipidemia: practice patterns and missed opportunities. Am J Cardiol. 2000;86(12A):62L–65L. doi: 10.1016/s0002-9149(00)01473-9. [DOI] [PubMed] [Google Scholar]
- 7.Straka RJ, Taheri R, Cooper SL, Tan AW, Smith AC. Assessment of hypercholesterolemia control in a managed care organization. Pharmacotherapy. 2001;21(7):818–827. doi: 10.1592/phco.21.9.818.34563. [DOI] [PubMed] [Google Scholar]
- 8.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? a framework for improvement. JAMA. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 9.Grimshaw JM, Shirran L, Thomas R, et al. Changing provider behavior: an overview of systematic reviews of interventions. Med Care. 2001;39((8)(suppl 2)):II2–II45. [PubMed] [Google Scholar]
- 10.Avorn J, Soumerai SB. Improving drug-therapy decisions through educational outreach: a randomized controlled trial of academically based “detailing.”. N Engl J Med. 1983;308(24):1457–1463. doi: 10.1056/NEJM198306163082406. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales R, Steiner JF, Lum A, Barrett PH., Jr Decreasing antibiotic use in ambulatory practice: impact of a multidimensional intervention on the treatment of uncomplicated acute bronchitis in adults. JAMA. 1999;281(16):1512–1519. doi: 10.1001/jama.281.16.1512. [DOI] [PubMed] [Google Scholar]
- 12.Soumerai SB, McLaughlin TJ, Gurwitz JH, et al. Effect of local medical opinion leaders on quality of care for acute myocardial infarction: a randomized controlled trial. JAMA. 1998;279(17):1358–1363. doi: 10.1001/jama.279.17.1358. [DOI] [PubMed] [Google Scholar]
- 13.Hunt DL, Haynes RB, Hanna SE, Smith K. Effects of computer-based clinical decision support systems on physician performance and patient outcomes: a systematic review. JAMA. 1998;280(15):1339–1346. doi: 10.1001/jama.280.15.1339. [DOI] [PubMed] [Google Scholar]
- 14.Ellis SD, Bertoni AG, Bonds DE, et al. Value of recruitment strategies used in a primary care practice-based trial. Contemp Clin Trials. 2007;28(3):258–267. doi: 10.1016/j.cct.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289(19):2560–2571. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 16.Grundy SM, Cleeman JI, Merz CN, et al. National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 17.Bertoni AG, Bonds DE, Steffes S, et al. Quality of cholesterol screening and management with respect to the National Cholesterol Education's Third Adult Treatment Panel (ATPIII) guideline in primary care practices in North Carolina. Am Heart J. 2006;152(4):785–792. doi: 10.1016/j.ahj.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 19.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44(4):1049–1060. [PubMed] [Google Scholar]
- 20.Ornstein S, Jenkins RG, Nietert PJ, et al. A multimethod quality improvement intervention to improve preventive cardiovascular care: a cluster randomized trial. Ann Intern Med. 2004;141(7):523–532. doi: 10.7326/0003-4819-141-7-200410050-00008. [DOI] [PubMed] [Google Scholar]
- 21.Eccles M, McColl E, Steen N, et al. Effect of computerised evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomised controlled trial. BMJ. 2002;325(7370):941. doi: 10.1136/bmj.325.7370.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krones T, Keller H, Sonnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Ann Fam Med. 2008;6(3):218–227. doi: 10.1370/afm.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumdar SR, Tsuyuki RT, McAlister FA. Impact of opinion leader–endorsed evidence summaries on the quality of prescribing for patients with cardiovascular disease: a randomized controlled trial. Am Heart J. 2007;153(1):22e1–22e8. doi: 10.1016/j.ahj.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 24.van Wyk JT, van Wijk MA, Sturkenboom MC, Mosseveld M, Moorman PW, van der Lei J. Electronic alerts versus on-demand decision support to improve dyslipidemia treatment: a cluster randomized controlled trial. Circulation. 2008;117(3):371–378. doi: 10.1161/CIRCULATIONAHA.107.697201. [DOI] [PubMed] [Google Scholar]
- 25.Fordis M, King JE, Ballantyne CM, et al. Comparison of the instructional efficacy of Internet-based CME with live interactive CME workshops: a randomized controlled trial. JAMA. 2005;294(9):1043–1051. doi: 10.1001/jama.294.9.1043. [DOI] [PubMed] [Google Scholar]
- 26.Bonds DE, Hogan PE, Bertoni AG, et al. A multifaceted intervention to improve blood pressure control: the Guideline Adherence for Heart Health (GLAD) study. Am Heart J. 2009;157(2):278–284. doi: 10.1016/j.ahj.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ. 2005;330(7494):765. doi: 10.1136/bmj.38398.500764.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]