Abstract
Mutations in the sister of P-glycoprotein (Spgp) or bile salt export pump (BSEP) are associated with Progressive Familial Intrahepatic Cholestasis (PFIC2). Spgp is predominantly expressed in the canalicular membranes of liver. Consistent with in vitro evidence demonstrating the involvement of Spgp in bile salt transport, PFIC2 patients secrete less than 1% of biliary bile salts compared with normal infants. The disease rapidly progresses to hepatic failure requiring liver transplantation before adolescence. In this study, we show that the knockout of spgp gene in mice results in intrahepatic cholestasis, but with significantly less severity than PFIC2 in humans. Some unexpected characteristics are observed. Notably, although the secretion of cholic acid in mutant mice is greatly reduced (6% of wild-type), total bile salt output in mutant mice is about 30% of wild-type. Also, secretion of an unexpectedly large amount of tetra-hydroxylated bile acids (not detected in wild-type) is observed. These results suggest that hydroxylation and an alternative canalicular transport mechanism for bile acids compensate for the absence of Spgp function and protect the mutant mice from severe cholestatic damage. In addition, the spgp−/− mice display a significant increase in the secretion of cholesterol and phospholipids into the bile. This latter observation in spgp−/− mice suggests that intrahepatic, rather than intracanalicular, bile salts are the major driving force for the biliary lipid secretion. The spgp−/− mice thus provide a unique model for gaining new insights into therapeutic intervention for intrahepatic cholestasis and understanding mechanisms associated with lipid homeostasis.
Bile acids are critical as carriers for elimination of cholesterol from the body through biliary secretion and as a detergent for the ingestion of fatty acids and fat-soluble vitamins (1). They also play important roles in regulating cell apoptosis/survival (2–6) and in regulating gene expression through the farnesoid X-activated receptor (7–12) in hepatocytes. Bile acids are synthesized in hepatocytes from cholesterol, secreted into the bile after being conjugated with glycine or taurine, reabsorbed in the small intestine, and recirculated back to hepatocytes through the portal vein. Canalicular secretion of bile acids from liver into the bile is a key process in the enterohepatic circulation of bile acids and its malfunction results in different hepatic diseases (1). Canalicular bile salt transport appears to be mediated by the sister of P-glycoprotein (Spgp) (13) also known as the bile salt export pump (BSEP) (14)
Spgp is a canalicular-specific ATP binding cassette (ABC) transporter and a close relative of the multidrug-resistant P-glycoprotein (mdr) (15, 16). Expression of the rat spgp gene in Xenopus laevis oocytes results in a 1.5-fold higher [3H]taurocholate efflux and the reconstituted membrane vesicles from Spgp-transfected sf9 insect cells exhibits 5-fold stimulation of ATP-dependent taurocholate transport, with a Km of 5.3 μM, consistent with Spgp being a bile acid transporter (14). Mutations in the spgp gene have been associated with a severe human genetic disease, type 2 Progressive Familial Intrahepatic Cholestasis (PFIC2) (17). Total bile acid secretion in PFIC2 patients is only 1% of normal, and Spgp was not detected in these patients by immunostaining (18). In mice, spgp or a nearby locus may be associated with cholesterol gallstone susceptibility (19). All of the above evidence agrees with Spgp being a canalicular bile acid transporter.
To further investigate the role Spgp plays in biliary secretion of bile acids and to understand how spgp deficiency results in PFIC2 and affects bile formation and cholesterol homeostasis, we have generated a knockout mice model by using homologous recombination. This study provides insights into the role Spgp plays in regulating bile salts and lipid transport in these mutant mice.
Materials and Methods
Generation of spgp−/− Mutant Mice.
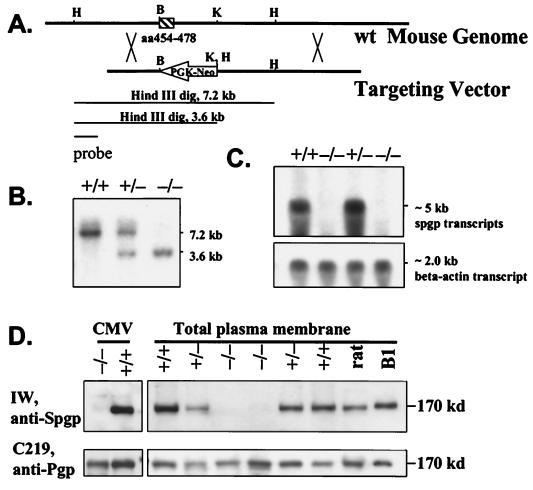
Two different ES cell lines were used. TL-1 embryonic stem cells and E14K embryonic stem cells from 129Sv/Ev mice were generous gifts from Dr. Patricia Labosky (University of Pennsylvania, Philadelphia) and Dr. Tak Mak (Amgen Institute, Toronto), respectively. Genomic DNA of murine spgp gene was isolated from a λ phage library of 129/J genomic DNA by using rat spgp cDNA as probe (15). One of the obtained genomic clones, 129J-9, and an additional fragment of 1.6 kb on the 5′ end of 129J-9, which was cloned by PCR amplification, were used to generate the targeting vector (Fig. 1A). The targeting vector was linearized with SstI and electroporated into TL-1 and E14k embryonic stem cells at 340 V and 250 μF of capacitance. ES cell clones surviving G418 selection were screened by Southern blot analysis. Four of the 11 targeted ES cell lines were subsequently used to produce chimeric mice and chimeric mice from three lines with germline transmission were used in this study. The heterozygous mutant mice from each generation were crossed into C57BL/6J. Homozygous mutant mice were produced by intercross of the heterozygous mice. Genotypes of the ES cells and mice were determined by Southern blot analysis using a 1-kb probe upstream of the 5′ end of the short arm of the targeting vector (Fig. 1 A and B). The expression level of the spgp gene was determined by Northern and Western blot analysis using standard methods (16). Antiserum IW is specific for Spgp, whereas monoclonal antibody C219 detects predominantly Pgp, but will also detect Spgp (15, 16). Both Pgp and Spgp migrate with a similar molecular size. Adult mice from generation three to four (2 to 6 months of age) were used in this study.
Figure 1.
Generation of spgp−/− mice. (A) Structure of targeting vector. A 1.4-kb BamHI (B) and KpnI (K) fragment, containing the coding region of Walker A of the N-terminal ATP-binding domain of Spgp (aa454–478), was deleted and replaced by a neomycin-resistant cassette in an antisense orientation of the spgp gene. The construct also introduced three missense mutations and a premature stop codon in the spgp gene starting at aa 454. A restriction site of HindIII (H) was introduced next to the KpnI site. (B) Southern blot analysis of spgp−/− mice. Genomic DNA obtained from wild-type, heterozygous, and homozygous mice, and digested with HindIII. The 7.2-kb and 3.6-kb bands correspond to wild-type and spgp−/− alleles, respectively. (C) Northern blot analysis. Total RNA was extracted from the livers of wild-type, heterozygous, and homozygous mice and 30 μg/lane was loaded. The mouse β-actin probe was used as control. (D) Western blot analysis of membrane proteins by using antibodies against Spgp and Pgp; 45 μg/well of total proteins was loaded. Spgp and Pgp have similar molecular weight. Antiserum IW is specific for Spgp. Monoclonal antibody C219 detects predominantly Pgp, but will also detect Spgp (15, 16). CMV, isolated canalicular membrane vesicles; rat, isolated plasma membrane from the rat liver; B1, isolated plasma membrane from SKOV3 cells expressing rat spgp cDNA (15).
Bile Duct Cannulation and Collection of Bile.
Animal surgery was performed by using the approved protocols of the Committee on Animal Care, University of British Columbia, according to the guidelines of the Canadian Council on Animal Care. Mice were killed by i.p. injection of Ketamine (112.5 mg/kg) and Xylazine (11.3 mg/kg) after 2 hours of fasting. The abdomen was opened, and the gall bladder was cannulated after distal common bile duct ligation (20). Bile was collected in 15 min intervals for a total of 30 min, followed by injection of 3.5 μmol/100 g body weight of [14C]taurocholate as a bolus into the jugular vein. Bile was then further collected through the cannula for another 30 min.
Transmission Electron Microscopy.
Mice were killed and their livers perfusion fixed in situ by using 2.5% glutaraldehyde and postfixed in 1% OsO4. Dehydration and embedding were performed as described in ref. 21. Sections were stained with uranyl acetate and examined by using a Philips EM400T transmission electron microscope (Eindhoven, The Netherlands).
Bile Acid Determinations.
Liquid chromatography–electrospray tandem mass spectrometry (LC/MS/MS) and gas chromatography–mass spectrometry (GC/MS) were used for the identification and determination of bile acids in bile, plasma, and liver tissues. Briefly, bile acids were extracted with a C18 (octadecyl) reversed-phase column. For LC/MS/MS, bile acids were analyzed by simultaneous monitoring of parent and daughter ions for the identification of glycine and taurine conjugates (22). Identification and quantification of conjugated bile acids was achieved in 5 min. The detection limit was 1 ng and the determination was linear up to 100 ng. For GC/MS, conjugated bile acids were hydrolyzed in 2.5 M NaOH at 160°C overnight. Bile acids were then extracted, methylated, and acetylated. Identification and quantification of bile acids were achieved by GC/MS using a Hewlett–Packard 5896 gas chromatograph equipped with a Hewlett–Packard 5971A mass selective detector (MSD) in selected ion-monitoring mode (SIM). Quantification was carried out by using a correction factor obtained by using 5β-cholanic acid as internal standard.
Biliary Phospholipid and Cholesterol Determinations.
Biliary lipids were extracted as described by Folch et al. (23). Phospholipids were determined enzymatically with phospholipase D and choline oxidase by using a kit supplied by Wako Chemicals (Richmond, VA). Cholesterol was determined by GC/MS as described above.
Results
Inactivation of the spgp Gene in Mice.
A vector for inactivation of the spgp gene was constructed by deleting a 1.4-kb fragment containing the coding region of Walker A of the N-terminal ATP-binding domain of Spgp (Fig. 1A, aa454–478). After electroporation with the targeting vector and G418 selection, 11 ES colonies were isolated by Southern blot screening. Four of the 11 targeted ES cell lines were subsequently introduced into pseudopregnant foster mother to produce chimeric mice. Germline transmission of the targeted mutation on spgp gene was achieved in chimeric mice from three of the four injected lines. The heterozygous mutant mice were crossed into C57Black/6J for each generation to eliminate genetic diversity, and the homozygotes were obtained by back-crossing of heterozygotes. Inactivation of the spgp gene resulting in spgp−/− mice (Fig. 1A) was confirmed by the absence of detectable Spgp mRNA and protein product (Fig. 1 C and D).
Analysis of spgp−/− Mice.
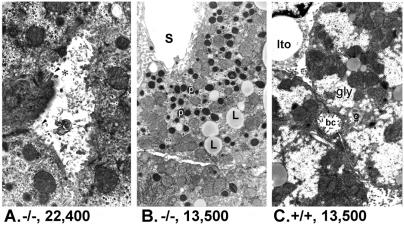
The spgp−/− mice are viable and fertile, but displayed growth retardation. The body weight of spgp−/− mice is about 20% lower than that of the wild-type littermates at weaning (21 days after birth). They tended to have a lower body weight throughout their life, but no advanced cholestasis was observed up to 1 year of age. Indicators of liver function such as gamma-glutamyl transferase, alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, 5′-nucleotidase, plasma albumin, and bilirubin showed no significant change in spgp−/− mice compared with wild-type mice (data not shown). Ultrastructural changes of the canaliculi were observed, including dilation of canalicular lumens, partial or complete loss of microvilli, and retained biliary material in the form of lamellar whorls or more membranous appearing “fingerprints” in canalicular lumens (Fig. 2A). There were also hepatocyte cytoplasmic changes, including increases in peroxisomes, lysosomes, and lipid droplets, and a decrease in glycogen content (Fig. 2B). The liver of spgp−/− mice was enlarged to 10.1% of the body weight (vs. 4.7% in wild-type; Table 1). Overall, however, the spgp−/− mice did not exhibit signs of overt cholestasis (1, 24–27).
Figure 2.
Ultrastructual changes in hepatocytes of spgp−/− mice. (A) Canalicular changes in hepatocytes of spgp−/− mice at 6 weeks. Bile canaliculus (✻) in center of micrograph shows dilated lumen, partial loss of microvilli (arrowhead), and retained biliary material in the form of lamellar and more membranous appearing “fingerprints.” (B) Cytoplasmic changes in hepatocytes of spgp−/− mice at 6 weeks. Note abundance of peroxisomes (p) and frequent small lipid droplets (L). Peroxisomes are thought to be the site of bile acid metabolism and modification (51). Mitochondria and other organelles are normal as is the hepatic sinusoid(s). (C) Hepatocytes of wild-type mice at 6 weeks. Part of two cells is shown with bile canaliculus (bc). Occasional lipid droplets and an abundance of glycogen (gly) are seen. Part of a normal stellate (Ito) cell is also present. g, Golgi.
Table 1.
Comparison of the spgp−/− and wild-type mice
| Wild type | spgp−/− | Significance† | |
|---|---|---|---|
| Body weight at weaning, g | 10.40 ± 0.67 (5) | 8.16 ± 0.72 (6) | P < 0.01 |
| Liver weight, g/100g BW | 4.72 ± 0.47 (4) | 10.14 ± 0.5 (3) | P < 0.01 |
| Bile flow rate μl/min/100g BW | 8.33 ± 2.71 (3) | 6.85 ± 2.58 (3) | P = 0.53 |
| Bile salt output, nmol/min/100g BW | 249.92 ± 80.90 (3) | 84.62 ± 30.41 (5) | P < 0.01 |
| Bile acids concentration in bile, mM | 32.33 ± 7.66 (3) | 7.60 ± 4.58 (3) | P < 0.01 |
| Deoxycholate | 1.16 ± 0.49 | ND | Significant |
| Chenodeoxycholate | 0.49 ± 0.12 | 0.11 ± 0.12 | P < 0.02 |
| Cholate | 21.56 ± 6.31 | 1.26 ± 1.05 | P < 0.01 |
| Ursodeoxycholate | 0.51 ± 0.54 | 0.06 ± 0.08 | P = 0.23 |
| Muricholate (alfa) | 0.96 ± 0.37 | 0.10 ± 0.04 | P ≤ 0.02 |
| Muricholate (omega) | 2.50 ± 0.48 | 1.57 ± 1.27 | P = 0.30 |
| Muricholate (beta) | 5.15 ± 4.18 | 3.16 ± 2.62 | P = 0.52 |
| Tetrahydroxy | ND | 1.37 ± 0.84 | Significant |
| Bile acids concentration in liver, μM | 40.07 ± 10.30 (3) | 232.06 ± 105.07 (3) | P < 0.04 |
| Bile acids concentration in plasma, μM | 7.22 ± 1.93 (4) | 29.43 ± 24.06 (5) | P = 0.11 |
| Biliary phospholipids, μM | 6.17 ± 3.60 (5) | 15.26 ± 7.06 (3) | P < 0.05 |
| nmole/min/100g BW | 46.27 ± 26.98 (5) | 128.16 ± 59.30 (3) | P < 0.03 |
| Biliary cholesterol, μM | 0.49 ± 0.02 (5) | 3.45 ± 1.71 (3) | P < 0.01 |
| nmole/min/100g BW | 3.69 ± 1.98 (5) | 28.97 ± 14.4 (3) | P < 0.01 |
| Bile acids/phospholipids ratio* | 8.58 ± 3.72 (3) | 1.48 ± 0.15 (3) | P < 0.03 |
| Bile acids/cholesterol ratio* | 99.96 ± 36.02 (3) | 6.62 ± 2.01 (3) | P < 0.01 |
Body weights were obtained from mice at weaning. All other numbers are from male adult mice. All numbers are expressed as mean ± SD (n). ND, not detectable; BW, body weight.
The bile acids:lipids ratio is the average of the ratio of bile salt output to lipids output for three animals.
The statistical significance of differences between spgp−/− and wild-type mice was assessed using Student's two-tailed t test.
Secretion of Bile Acids.
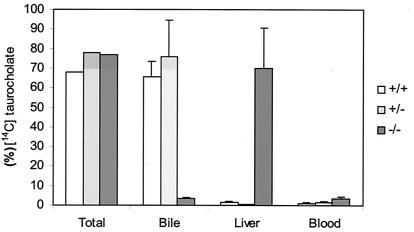
Because cholestasis is defined as a reduction in bile flow, we measured bile flow in the spgp−/− mice to assess the degree of cholestasis. We observed that the average bile flow is reduced only slightly in spgp−/− mice compared with wild-type controls (Table 1). This lack of significant reduction in bile flow in spgp−/− mice is surprising because bile salt secretion is a major driving force of bile flow and Spgp is considered the major canalicular bile salt transporter (14, 17, 18). Therefore, we examined the mutant mice for secretion of specific bile salts by infusing [14C]-labeled taurocholate, a major form of bile salt, into the jugular vein. Thirty minutes after infusion, radioactivity was distributed in spgp−/− vs. wild-type mice as follows: 4.6% vs. 65.5% secreted into the bile, 69.7% vs. 1.6% accumulated in the liver, and 3.5% vs. 1.0% remained in the plasma (Fig. 3). Injection of labeled glycocholic acid also yielded similar results (data not shown). The impairment of the bile salt secretion in spgp−/− mice was further confirmed by feeding experiments. The total bile acid in plasma of spgp−/− mice, after being fed with diet containing 0.02% (wt/wt) sodium taurocholate or glycocholate for 8 weeks, was elevated approximately 20–30 times (unpublished results). These data indicate a dramatic impairment in biliary secretion of conjugated cholic acid in spgp−/− mice.
Figure 3.
Distribution of infused radiolabeled bile acids in the bile, liver, and blood. Mice were cannulated and bile was collected in 15 min intervals for 30 min after ligation. [14C]taurocholate (3.5 μmol/100 g body weight) was infused. Accumulation of radioactivity in the bile, liver, and blood was determined. Results are expressed as percent of total radiolabeled material infused and represent means ± SD from three animals of each genotype. Similar results were obtained with labeled glycocholate.
To investigate further whether impaired or altered bile acid metabolism exist in the mutant mice, we identified and quantitated the individual bile acids by GC/MS. The total concentration of biliary bile salts in the spgp−/− mice was reduced by more than 4-fold. Correspondingly, the total bile salt concentrations in the liver and plasma were elevated 4- and 5.8-fold, respectively (Table 1). Individually, the amount of biliary cholic acid in spgp−/− mice was reduced by 17-fold, confirming the radiolabeled data in Fig. 3, but total biliary muricholic acids were not significantly reduced (Table 1). Taken together, the above data indicate that Spgp is the main transporter of the major hydrophobic bile salts, such as taurine and glycine-conjugated cholic acid. However, secretion of the more hydrophilic muricholic acids appeared not to be significantly impaired in the spgp−/− mice (Table 1).
Tetra-Hydroxylated Bile Acids.
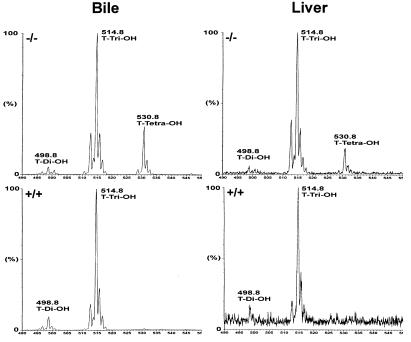
We detected an unusually large amount of tetra-hydroxylated bile acids (18% of total biliary bile acids) in the spgp−/− mice that were not detected in wild-type controls. This was confirmed with tandem mass spectrometry (LC/MS/MS), which showed that the tetra-hydroxylated bile salt was present mainly as a taurine conjugate with a fragment ion at m/z 530 (Fig. 4). Several investigators have reported the presence of tetra-hydroxylated bile salts in human cholestatic diseases (28–34). Furthermore, it was demonstrated that chenodeoxycholic, cholic, and deoxycholic acids can be hydroxylated and transformed into tri- or tetra-hydroxylated bile acids in cholestasis patients (30). Increased hydroxylation in rodents has also been observed following administration of toxic mono-hydroxylated bile salts (34) and after bile duct ligation (35, 36). Thus, the hydroxylation of bile salts in spgp−/− mice is consistent with a cholestatic phenotype. However, the amount of tetra-hydroxylated bile salts secreted into the bile was substantial, and this may help to modulate the severity of cholestasis and may explain why the bile flow rate was only slightly reduced in spgp−/− mice.
Figure 4.
Taurine-conjugated bile acids from the bile and liver of spgp−/− and wild-type mice detected by LC/MS/MS. The bile and the liver used were from the same mouse for each genotype. The molecular weights of the different species detected representing di-, tri-, and tetra-hydroxylated bile acids are indicated.
Because of a lack of standards for tetrahydroxylated bile acids, the structure of the tetrahydroxylated bile acid species observed in spgp−/− mice can only be postulated. However, analysis using ionization GC/MS confirms the presence of four hydroxyl groups and suggests that the structure of the tetrahydroxylated bile acid is predominantly 3α,6β,7α,12α-tetrahydroxy-5β-cholanic acid (unpublished observation).
Lipid Composition of Bile in spgp−/− Mice.
The secretion of cholesterol and phospholipids into the bile is mediated by a system(s) different from that for bile salts (14, 20, 37). However it is well established that bile salts, especially hydrophobic bile salts, promote secretion of biliary phospholipids and cholesterol (1, 37, 38). The mechanisms by which the bile salts induce biliary secretion of phospholipids and cholesterol is not understood (39). We analyzed biliary lipids in the spgp−/− mice to examine whether the biliary secretion of cholesterol and phospholipids was affected. The spgp−/− mice displayed a marked increase in biliary cholesterol (7-fold) and phospholipids (2.5-fold), possessed a more hydrophilic bile salt pool, expressed a 4-fold reduced biliary secretion of bile salts, and expressed a 6-fold hepatic accumulation of bile salts (Table 1, Fig. 4). Furthermore, the biliary cholesterol:phospholipid ratio was dramatically increased in the spgp−/− mice (0.23 vs. 0.08 in the wild-type mice; Table 1). Thus, the disruption of the spgp gene in mice appears to greatly affect the homeostasis of cholesterol and phospholipids.
Discussion
Spgp is considered to be the major bile salt transporter in the canaliculus (14, 40); and genetic defect of spgp gene in humans results in very severe progressive cholestasis (17, 18). This study shows that canalicular secretion of bile salts, especially conjugated cholic acid in spgp−/− mice, is severely impaired. The surprising finding in this study is that the spgp−/− mice display only mild nonprogressive cholestasis. A less severe phenotype in spgp−/− mice compared with human PFIC2 is likely because of another canalicular bile salt transport system (as yet unidentified) that allows for the secretion of significant amounts of bile salts. The spgp−/− mice thus provide a unique model system to investigate the pathogenesis of intrahepatic cholestasis and other related liver diseases; moreover, it may be a useful system for exploring potential approaches for therapeutic intervention for these diseases.
In the spgp−/− mice, biliary secretion of conjugated cholic acid and some di-hydroxyl bile salts was greatly reduced, but secretion of the more hydrophilic bile salts such as the muricholates were affected. Also, a significant amount of another hydrophilic bile acid, tetrahydroxylated bile acid, appeared (Table 1 and Fig. 4). These observations imply that (i) Spgp mediates the secretion of conjugated cholic acids and other hydrophobic bile acids. This is in line with previous publications demonstrating that Spgp is a transporter of taurocholates and other bile salts in transfected systems (14, 40); and (ii) there is an alternative bile salt transport system in the canaliculus for the more hydrophilic bile salts. Rodents have a more efficient hydroxylation/detoxification mechanism in the liver (36), and the bile acid pool in mice is more hydroxylated, less hydrophobic, and less toxic (32, 41, 42). Thus in mice, elevated hydroxylation of bile salts may serve not only as a detoxification mechanism of hydrophobic bile salts, but may also allow for their clearance via the predicted alternative transport system. It is yet to be discovered whether a similar system for the transport of hydrophilic bile salts exists in humans. In addition to Spgp, other proteins, such as FIC1 (43, 44), MRP3 (45–47), and ecto-ATPase (48, 49), have also been proposed as potential bile acid transporters in the canaliculus. Some of these may be responsible for this alternative transport of hydrophilic bile acids. The spgp−/− mice should be a useful system for investigating this possibility.
Whereas biliary secretion of bile salts was greatly reduced in the spgp−/− mice, the secretion of phospholipids and cholesterol into the bile was significantly elevated (Table 1). Secretion of phospholipids and cholesterol usually correlates positively with changes in the amount of bile salts secreted in vivo (37, 39), and it is commonly accepted that bile salts drive the amount of phospholipids and cholesterol secreted into the bile. Whether or not this is accomplished at the intracanalicular level or the intrahepatic level is not well defined (37–39). The spgp−/− mice allow us to differentiate between these two possibilities. In these mice, the increased biliary lipid secretion is observed under conditions where bile salt is reduced but intrahepatic bile salt is increased. We therefore propose that the intrahepatic site is the location for the bile salt stimulation of biliary lipid secretion.
An increased accumulation of intrahepatic bile salts could also explain the greatly increased cholesterol:phopholipid ratio in the bile of spgp−/− mice (Table 1). Bile acid synthesis from cholesterol accounts for about 50% of cholesterol eliminated from the body (50). Accumulation of hepatic bile acids resulting from an absence of Spgp would very likely inhibit bile acid biosynthesis (8, 9) and could lead to the accumulation of cholesterol in hepatocytes. This, in turn, may stimulate elevated biliary secretion of cholesterol. Hence, the observed higher cholesterol:phospholipid ratio in the bile of spgp−/− mice may reflect the need for cholesterol clearance. It is noteworthy that the Lith1 locus of cholesterol gallstone susceptibility in mice has been colocalized with the spgp locus (19). A higher cholesterol secretion in the spgp−/− mice would be consistent with the suggestion that the spgp gene is the Lith1 site. The spgp−/− mice are therefore an excellent model for investigating lipid secretion and understanding lipid homeostasis.
Acknowledgments
We thank Annick Itie, Dr. Tak Mak, and Dr. Keith Humphres for helpful advice regarding the generation of spgp−/− knockout mice; Diane Mignault for assistance in MS analysis; Dr. Wenchao Song for advice and support in ES cell culture and screening; and Rewa Grewal and Robert Schamborzki for technical support. This work was supported by funding from the National Cancer Institute of Canada and the Canadian Institutes of Health Research (to V.L.) and Medical Research Council of Canada (to I.M.Y. and B.T.).
Abbreviations
- Spgp
sister of P-glycoprotein
- PFIC2
type 2 progressive familial intrahepatic cholestasis
- GC/MS
gas chromatography–mass spectrometry
- LC/MS/MS
liquid chromatography–electrospray tandem mass spectrometry
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031465498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031465498
References
- 1.Hofmann A F. Arch Intern Med. 1999;159:2647–2658. doi: 10.1001/archinte.159.22.2647. [DOI] [PubMed] [Google Scholar]
- 2.Rust C, Karnitz L M, Paya C V, Moscat J, Simari R D, Gores G J. J Biol Chem. 2000;275:20210–20216. doi: 10.1074/jbc.M909992199. [DOI] [PubMed] [Google Scholar]
- 3.Sodeman T, Bronk S F, Roberts P J, Miyoshi H, Gores G J. Am J Physiol Gastrointest Liver Physiol. 2000;278:G992–G999. doi: 10.1152/ajpgi.2000.278.6.G992. [DOI] [PubMed] [Google Scholar]
- 4.Jones B A, Rao Y P, Stravitz R T, Gores G J. Am J Physiol. 1997;272:G1109–G1115. doi: 10.1152/ajpgi.1997.272.5.G1109. [DOI] [PubMed] [Google Scholar]
- 5.Kwo P, Patel T, Bronk S F, Gores G J. Am J Physiol. 1995;268:G613–G621. doi: 10.1152/ajpgi.1995.268.4.G613. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues C M, Fan G, Ma X, Kren B T, Steer C J. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Chen J, Hollister K, Sowers L C, Forman B M. Mol Cell. 1999;3:543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 8.Makishima M, Okamoto A Y, Repa J J, Tu H, Learned R M, Luk A, Hull M V, Lustig K D, Mangelsdorf D J, Shan B. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 9.Chiang J Y, Kimmel R, Weinberger C, Stroup D. J Biol Chem. 2000;275:10918–10924. doi: 10.1074/jbc.275.15.10918. [DOI] [PubMed] [Google Scholar]
- 10.Parks D J, Blanchard S G, Bledsoe R K, Chandra G, Consler T G, Kliewer S A, Stimmel J B, Willson T M, Zavacki A M, Moore D D, et al. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin B, Jones S A, Price R R, Watson M A, McKee D D, Moore L B, Galardi C, Wilson J G, Lewis M C, Roth M E, et al. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 12.Sinal C J, Tohkin M, Miyata M, Ward J M, Lambert G, Gonzalez F J. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 13.Childs S, Ling V. Important Adv. Oncol. 1994. 21–36. [PubMed] [Google Scholar]
- 14.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, Roth J, Hofmann A F, Meier P J. J Biol Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 15.Childs S, Yeh R L, Hui D, Ling V. Cancer Res. 1998;58:4160–4167. [PubMed] [Google Scholar]
- 16.Childs S, Yeh R L, Georges E, Ling V. Cancer Res. 1995;55:2029–2034. [PubMed] [Google Scholar]
- 17.Strautnieks S S, Bull L N, Knisely A S, Kocoshis S A, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V, et al. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 18.Jansen P L, Strautnieks S S, Jacquemin E, Hadchouel M, Sokal E M, Hooiveld G J, Koning J H, De Jager-Krikken A, Kuipers F, Stellaard F, et al. Gastroenterology. 1999;117:1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 19.Bouchard G, Nelson H M, Lammert F, Rowe L B, Carey M C, Paigen B. Mamm Genome. 1999;10:1070–1074. doi: 10.1007/s003359901163. [DOI] [PubMed] [Google Scholar]
- 20.Smit J J, Schinkel A H, Oude Elferink R P, Groen A K, Wagenaar E, van Deemter L, Mol C A, Ottenhoff R, van der Lugt N M, van Roon M A, et al. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 21.Tsukada N, Azuma T, Phillips M J. Proc Natl Acad Sci USA. 1994;91:6919–6923. doi: 10.1073/pnas.91.15.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Libert R, Hermans D, Draye J P, Van Hoof F, Sokal E, de Hoffmann E. Clin Chem. 1991;37:2102–2110. [PubMed] [Google Scholar]
- 23.Folch J, Lees M, Sloane Stanley G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 24.Steiner J W, Phillips M J, Miyai K. Int Rev Exp Pathol. 1964;3:165–167. [PubMed] [Google Scholar]
- 25.Philips M J, Poucell S, Patterson J, Valencia P. The Liver: A Textbook and Atlas of Ultrastructural Pathology. New York: Raven; 1987. [Google Scholar]
- 26.Trauner M, Meier P J, Boyer J L. N Engl J Med. 1998;339:1217–1227. doi: 10.1056/NEJM199810223391707. [DOI] [PubMed] [Google Scholar]
- 27.Jansen P L, Muller M M. Gut. 1998;42:766–767. doi: 10.1136/gut.42.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bremmelgaard A, Sjovall J. Eur J Clin Invest. 1979;9:341–348. doi: 10.1111/j.1365-2362.1979.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 29.Thomassen P A. Eur J Clin Invest. 1979;9:425–432. doi: 10.1111/j.1365-2362.1979.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 30.Bremmelgaard A, Sjovall J. J Lipid Res. 1980;21:1072–1081. [PubMed] [Google Scholar]
- 31.Radominska-Pyrek A, Zimniak P, Irshaid Y M, Lester R, Tephly T R, St. Pyrek J. J Clin Invest. 1987;80:234–241. doi: 10.1172/JCI113053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setchell K D, Dumaswala R, Colombo C, Ronchi M. J Biol Chem. 1988;263:16637–16644. [PubMed] [Google Scholar]
- 33.Nakagawa M, Setchell K D. J Lipid Res. 1990;31:1089–1098. [PubMed] [Google Scholar]
- 34.Yousef I M, Bouchard G, Tuchweber B, Plaa G L. Drug Metab Rev. 1997;29:167–181. doi: 10.3109/03602539709037579. [DOI] [PubMed] [Google Scholar]
- 35.Greim H, Trulzsch D, Roboz J, Dressler K, Czygan P, Hutterer F, Schaffner F, Popper H. Gastroenterology. 1972;63:837–845. [PubMed] [Google Scholar]
- 36.Coleman R. Biochem J. 1987;244:249–261. doi: 10.1042/bj2440249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coleman R. Biochem Soc Trans. 1987;15:68S–80S. [PubMed] [Google Scholar]
- 38.Coleman R, Rahman K. Biochim Biophys Acta. 1992;1125:113–133. doi: 10.1016/0005-2760(92)90036-u. [DOI] [PubMed] [Google Scholar]
- 39.Verkade H J, Vonk R J, Kuipers F. Hepatology. 1995;21:1174–1189. [PubMed] [Google Scholar]
- 40.Green R M, Hoda F, Ward K L. Gene. 2000;241:117–123. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- 41.Oude Elferink R P, Ottenhoff R, van Wijland M, Frijters C M, van Nieuwkerk C, Groen A K. J Lipid Res. 1996;37:1065–1075. [PubMed] [Google Scholar]
- 42.Rossi S S, Converse J L, Hofmann A F. J Lipid Res. 1987;28:589–595. [PubMed] [Google Scholar]
- 43.Bull L N, van Eijk M J, Pawlikowska L, DeYoung J A, Juijn J A, Liao M, Klomp L W, Lomri N, Berger R, Scharschmidt B F, et al. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 44.Strautnieks S S, Kagalwalla A F, Tanner M S, Knisely A S, Bull L, Freimer N, Kocoshis S A, Gardiner R M, Thompson R J. Am J Hum Genet. 1997;61:630–633. doi: 10.1086/515501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ortiz D F, St. Pierre M V, Abdulmessih A, Arias I M. J Biol Chem. 1997;272:15358–15365. doi: 10.1074/jbc.272.24.15358. [DOI] [PubMed] [Google Scholar]
- 46.Ortiz D F, Li S, Iyer R, Zhang X, Novikoff P, Arias I M. Am J Physiol. 1999;276:G1493–G1500. doi: 10.1152/ajpgi.1999.276.6.G1493. [DOI] [PubMed] [Google Scholar]
- 47.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. J Biol Chem. 2000;275:2905–2910. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 48.Sippel C J, Suchy F J, Ananthanarayanan M, Perlmutter D H. J Biol Chem. 1993;268:2083–2091. [PubMed] [Google Scholar]
- 49.Sippel C J, Dawson P A, Shen T, Perlmutter D H. J Biol Chem. 1997;272:18290–18297. doi: 10.1074/jbc.272.29.18290. [DOI] [PubMed] [Google Scholar]
- 50.Vlahcevic Z R, Pandak W M, Stravitz R T. Gastroenterol Clin North Am. 1999;28:1–25. doi: 10.1016/s0889-8553(05)70041-8. [DOI] [PubMed] [Google Scholar]
- 51.Lazarow P B. In: The Liver: Biology and Pathobiology. Arias I M, Boyer J L, Fausto N, Jakoby W B, Schacter D, Shafritz D A, editors. New York: Raven; 1994. pp. 293–307. [Google Scholar]