INTRODUCTION
Although environmental insults such as smoking have been implicated in the initiation of rheumatoid arthritis (RA) in patients who express the shared epitope, our understanding of the role of innate immunity in the pathogenesis of this disease is also expanding. The clinical picture of pain, stiffness, swelling, and joint destruction seen in RA is a result of chronic inflammation of the synovium, characterized by interactions of fibroblast-like synoviocytes with cells of the innate immune system, including macrophages, dendritic cells, mast cells and NK cells, as well as cells of the adaptive immune system, B and T lymphocytes (1). Also present are immune complexes, proteins of the complement system, autocrine and paracrine-acting cytokines as well as chemokines that have inflammatory, homeostatic, and even anti-inflammatory properties (2). As knowledge of the complexities of RA grows, gaps in the understanding of its pathogenesis are filled, and new potential therapeutic targets are uncovered.
The best known function of the innate immune system is the initial recognition of microbial pathogens. Upon encounter with non-self, primarily by macrophages and dendritic cells via membrane-bound or intracellular pattern recognition receptors (PRRs), cells of the innate system become activated leading to the production of inflammatory cytokines and chemokines. Effector cells and molecules of the innate system are recruited locally, and if unable to overcome the pathogen alone, macrophages and dendritic cells travel to local lymphoid tissues. There, processed antigens are presented by MHC molecules to naïve T-cells, thus initiating an adaptive response complete with lasting immunological memory. Upon clearance of the organism, with the help of opposing anti-inflammatory mediators, the inflammatory response is terminated (3). In RA however, “self” is either the primary target, or an innocent bystander that then becomes the focus of attack. In RA there is abundant evidence that the innate immune system is persistently activated, as evidenced by the continual expression of macrophage derived cytokines such as TNFα, IL-1 and IL-6. As our understanding of the innate immune system in RA continues to expand, enticing targets for new therapeutic interventions continue to be identified. This review will focus on cells of myelomonocytic origin, their receptors and factors that interact with them.
MONOCYTES AND MACROPHGES
Background and Role in RA
Macrophages, together with osteoclasts and myeloid dendritic cells are derived from myelomonocytic origins and are key cellular components of the innate immune system. Macrophages differentiate from circulating monocytes and have primary roles in tissues as phagocytes of invading pathogens, and as scavengers of apoptotic debris. In addition, macrophage activation results in the expression of chemokines and cytokines, such as TNFα and IL-1β, that help to attract other cells and proteins to the sites of inflammation (3). The central role of macrophages in RA pathogenesis is supported by the fact that conventional therapies, including methotrexate and cytokine inhibitors, act to decrease the production of cytokines produced primarily by macrophages (4). Indeed, a correlation has been found between synovial macrophage infiltration and subsequent radiographic joint destruction (5). A remarkable fact is that a reduction of sublining macrophages in RA synovial tissue has been shown to strongly correlate to the degree of clinical improvement regardless of the type of therapy chosen (6). In addition to local effects of macrophages in the synovial tissue, systemic consequences of macrophage-mediated inflammation in RA may be manifested by damage to other areas such as the subendothelial space where macrophages become foam cells contributing to atherosclerotic plaques (7).
Mechanisms of Increased Macrophage Number in RA Tissue
Possible mechanisms for the increased number of macrophages in diseased tissue include increased chemotaxis (8, 9) and reduced emigration (10). Some studies also suggest local proliferation of macrophages in areas of inflammation (11–14). Decreased apoptosis may also contribute to the accumulation of macrophages in the RA joint. Several studies have shown that induction of synoviocyte apoptosis in animal models of inflammatory arthritis ameliorates both joint inflammation and joint destruction (15). In both experimental arthritis and RA patient synovial tissue, reduced expression of the proapoptotic Bcl-2 family member, Bim, was seen in macrophages and corresponded to the increased expression of IL-1β by macrophages. Furthermore, administration of a Bim mimetic dramatically reduced the incidence of arthritis and successfully ameliorated established arthritis in mice (16). This result suggests that therapies that restore the homeostasis between survival and cell death of RA macrophages may be successful in ameliorating arthritis in patients.
Heterogeneity of Monocyte and Macrophage Populations
Within monocyte and macrophage populations there is a great deal of heterogeneity. For example, two human monocyte populations have been defined based on their surface marker expression, that is the CD14+CD16− and the CD14lowCD16+ subsets (17). CD16 is a receptor for IgG, FcγIIIA, which binds to IgG containing immune complexes, and will be discussed later in this review. The number of CD14lowCD16+ monocytes is elevated in RA peripheral blood and CD14lowCD16+ macrophages are enriched in RA synovial tissue (18). CD14lowCD16+ monocytes produce more TNF-α in response to the microbial TLR4 ligand LPS compared with the CD14+CD16− subset (19, 20). These observations suggest that the proinflammatory CD14lowCD16+ monocytes migrate to the RA joint and become highly responsive macrophages. However, CD14+CD16− monocytes also express the chemokine receptor CCR2, which binds Monocyte Chemotactic Protein-1, and thereby promotes monocyte migration to the site of inflammation. Since the RA joint is rich in this chemokine, it is possible that CD14+CD16−, CCR2+ monocytes are recruited to the joint, where CD16 expression is then induced. Nonetheless, since monocytes migrate from the peripheral blood into RA synovial tissue, identification of circulating monocyte subpopulations may be an extremely useful clinical tool in tracking disease activity and for identifying additional therapeutic targets (20–22).
In addition, diversity in activation states of macrophages has been found (23). In general, macrophages exhibiting a more inflammatory phenotype have been named M1, or classically activated macrophages, while those that trend toward a more anti-inflammatory and repair role are known as M2, or alternatively activated macrophages (23). Most macrophages in the RA joint express proinflammatory cytokines and are thus most consistent with classically activated macrophages. Therapies that promote the balance in favor of an M2 phenotype may be useful in RA (8).
Therapies Targeting Macrophages
Conventional therapies such as prednisone, methotrexate, leflunomide, sulfasalazine, and TNFα inhibitors have been shown to decrease the number of CD68+ macrophages in the synovial sublining (24). Another study of synovial tissue response to rituximab found a significant reduction of sublining macrophages at 16 weeks, providing evidence for synovial tissue sublining macrophage reduction following B-cell depletion therapy in RA as well (25). Furthermore, a reduction in the number of synovial sublining macrophages correlated clinically with improvement of DAS 28 scores, suggesting an association between sublining CD68+ macrophages and therapeutic efficacy (24). The positive correlation between the change in RA clinical activity and CD68 expression in the synovial sublining has been independently confirmed (26).
Specifically targeting activated macrophages at sites of inflammation would be a way of circumventing the potential untoward effects of systemic macrophage depletion. The bisphosphonate clodronate, encapsulated within liposomes, has been used to specifically deplete macrophages. After injecting rats intraperitoneally with streptococcal cell wall fragments (SCW) to induce arthritis, IV liposomal clodronate suppressed the development of chronic arthritis for up to 26 days after treatment. Treatment was also associated with depletion of synovial and hepatic, but not splenic, macrophages, as well as a reduction in articular IL-1β, IL-6, TNFα, and MMP-9 (27). Similarly, in the K/B × N serum transfer model of arthritis, where spontaneously produced anti-GPI antibodies from a K/B × N mouse are injected into a naïve host, treatment with liposomal clodronate prior to serum transfer caused depletion of macrophages in the bone marrow and liver, and the treated mice were completely resistant to arthritis. Resistance to arthritis was reversed when the macrophage-depleted mice were reconstituted with macrophages from naïve animals and immediately injected with K/B × N serum (28). In rabbits with established antigen-induced-arthritis (AIA), repeated intraarticular administrations of low, noncytotoxic doses of liposomal clodronate led to an early reduction of joint swelling, delay in radiographic progression, and decrease in synovial lining macrophage number. However, no difference was seen in pannus formation or radiographic erosions at 8 weeks (29). In patients with RA undergoing knee replacement surgery, a single intraarticular dose of clodronate liposomes significantly reduced the number of CD68+ cells as well as the expression of adhesion molecules in the synovial lining. In contrast, no immunohistologic difference was observed in the control group (30). These observation suggest that depletion of synovial tissue macrophages may be an important therapeutic goal in RA.
Systemic depletion of all macrophages could have serious consequences in patients, and this may be avoided by specifically targeting receptors present on activated macrophages. Folate receptor β (FRβ) has been described on both activated macrophages from RA synovial fluid and animal models of arthritis, but not on resting/quiescent macrophages or normal cells of the body except for the proximal tubule cells of the kidneys (31–33). The FRβ has been used to deliver folate-conjugated imaging agents to inflamed joints in patients with RA (34), and is a target for novel therapeutic agents. Several new generation folate antagonists are selectively taken up by the FRβ and show growth inhibition capabilities against FRβ-expressing cells, thus circumventing the systemic effects (35). In addition, antibodies or fragments of antibodies against FRβ linked with immunotoxins have been developed and have been shown to reduce the number of macrophages and levels of IL-6, and to increase the number of apoptotic cells in RA synovial tissue engrafted into severe-combined-immunodeficient mice (36, 37). Another approach involved the conjugation of folate to superoxide dismutase and catalase, two enzymes that scavenge the damaging reactive oxygen species secreted by activated macrophages. Folate conjugation dramatically enhanced the ability of catalase and superoxide dismutase to scavenge reactive oxygen species produced by activated macrophages in cell culture experiments and enhanced uptake of the enzyme catalase into activated macrophages (38). Folate has also been conjugated to small molecules, or haptens such as fluorescein, and given to rodents previously immunized to the hapten after the onset of experimental arthritis. The folate-hapten conjugate selectively “decorated” and promoted immune-mediated elimination of activated macrophages, as well as decreased paw swelling, spleen size, systemic inflammation, arthritis score, and bone erosion (39). Thus, the presence of select folate receptors on activated macrophages offers exciting potential to target activated macrophages in the RA joint.
DENDRITIC CELLS
Background
Dendritic cells (DCs), along with macrophages and B cells, have the ability to present antigen to T cells, and therefore play a central role in the development of innate and adaptive immune responses. In the periphery, immature DCs are stimulated to undergo differentiation by an array of pathogens, mainly via the activation of TLRs by exogenous or endogenous stimuli, but also in response to cytokines or immune complexes produced during the inflammatory response. TLR signaling results in a significant change in chemokine receptors expressed by DCs, allowing for maturation of DCs and migration to the lymphoid tissue. There, mature DCs display antigen on MHC molecules to naïve T cells. DCs also express the critical co-stimulatory molecules, CD80 and CD86, which interact with CD28 on T cells, completing the necessary signal for antigen-specific effector T cell maturation to occur. In addition to stimulation of naïve T cells, DCs can process and display antigen in local tissues, and contribute to the inflammatory response by the production of cytokines such as TNFα, IL-1β, and IL-6. Furthermore, DCs can direct the formation of distinct T-helper (Th) cells by producing key cytokines, such as IL-12 and IL-18 for Th1 cells, and IL-23 for Th17 cells (3). Finally, DCs are important in the development of both central and peripheral tolerance, and their depletion in animal models is associated with the onset of fatal autoimmune-type disease (40). In the thymus, DCs present endogenous self-antigens to T cells and delete those that are strongly reactive, while in the periphery, interaction between autoreactive T cells and immature DCs bearing self-antigen may result in anergy, apoptosis, or differentiation into T regulatory cells (3). Deviations in this pathway, either failed clearance of dead cells or exposure of DCs bearing self-antigens to maturation signals, can abrogate their tolerogenic ability and are implicated in the development of autoimmunity (41).
DCs may be categorized into subtypes based on the expression of various cell surface markers (42, 43). Functionally, however, DCs may be separated into 2 main classes: classical or conventional DCs (cDCs), which are resident in lymphoid tissues or migratory in non-lymphoid tissues and plasmacytoid DCs (pDCs). Both types may be activated by particular TLRs which induce the molecules necessary to promote antigen presentation, T cell stimulation, and cytokine production. cDCs express CD11c:CD18, also known as complement receptor 4 (CR4), and express all known TLRs except for TLR9. cDCs are the main participants in antigen presentation and activation of naïve T cells as well mediators of peripheral tolerance. pDCs, on the other hand, are particularly important in modifying the immune response toward viruses. They do not express high levels of CD11c, and have been identified by the expression of specific markers, such as blood dendritic cell antigen 2 (BDCA-2). In addition, pDCs express TLRs 1, 7, and 9, and other TLRs to a lesser degree (3). In response to stimuli such as viruses, pDCs are able to generate abundant amounts of type-I interferons (IFN-α and IFN-β) as well as other cytokines such as TNF-α and IL-12. These cytokines increase the production of inflammatory mediators by macrophages, and, in the case of IL-12, can direct a potent Th1 response (44).
Dendritic Cells and RA
The vast majority of studies that have examined the role of DCs in RA have relied on immunohistochemical techniques or have isolated DCs from peripheral blood and characterized their phenotype and function. In RA synovial tissue, the number of pDCs which are localized to perivascular lymphocytic infiltrates (45) correlates with anti-CCP antibodies (46). These pDCs produced BAFF, IL-18, and INFα/β, while the cDCs secreted IL-12 and IL-23 (46). The total numbers of cDCs or pDCs, or the number of mature DCs in RA synovial tissue were not significantly different from patients with OA or PsA (46, 47), although there was a statistical increase in the ratio of pDC/cDC in RA synovial tissue (46). These data suggest that the numbers of DCs in synovial tissue may not reflect their true contribution to RA pathogenesis.
Few cDCs or pDCs are detected in peripheral blood of RA patients and the numbers are lower compared to healthy controls. The expression of the inhibitory FcγRIIB on DCs derived from peripheral blood monocytes of patients with RA correlated with disease activity. Additionally, DCs expressing higher levels of FcγRIIB inhibited T-cell proliferation and promoted the T-regulatory phenotype following TLR and FcR stimulation in co-culture studies (48). Treatment with methotrexate or infliximab dramatically affects the number, maturation, and function of the DC. DCs derived from monocytes of patients treated infliximab displayed an anti-inflammatory phenotype (49) and increased numbers of cDCs in the circulation (50). Additionally, the numbers of pDCs were increased in the peripheral blood in patients in clinical remission induced by either methotrexate or infliximab. Further, isolation of these pDCs and co-culture with naïve T-cells led to an induction of the T-regulatory phenotype, which was capable of inhibiting autologous T-cell proliferation (51). In contrast, there was a reduction of both circulating cDCs and pDCs in anti-IL-6 receptor treated RA patients, which was not observed in anti-TNF or CTLA4-Ig treated RA patients (52). These data suggest that clinically relevant information may be gleaned from examining circulating DCs in RA patients and that there are differences related to the mode of therapy.
Dendritic Cells and Murine Models of RA
The utilization of murine models of inflammatory arthritis has supported the human studies on the roles of DCs in RA. Follicular DCs are required for development of the K/BxN mouse model of arthritis (53). In contrast, selective depletion of pDCs enhanced the severity and pathology of collagen-induced arthritis (CIA) (54). These data suggest that pDCs may prevent the break of tolerance and that the follicular DC or cDC may be the central culprit that leads to the activation of autoreactive lymphocytes. Adoptive transfer of DCs from CTLA4-Ig-treated mice was sufficient to inhibit arthritis in CIA recipient mice (55). Additionally, adoptive transfer of TLR-stimulated DCs following immunization reduced CIA (56). Thus, similar to RA patients, modification of the DC function by biologic therapies may lead to a skewing of T-cell development towards a T-regulatory phenotype mediated by DCs.
PATTERN RECOGNITION RECEPTORS
Background
There are several mechanisms by which macrophages and other innate immune cells become activated. One way is via pattern recognition receptors (PRRs) that are designed to recognize simple and regular non-self patterns of molecular structure, conserved during evolution, called pathogen-associated molecular patterns (PAMPs). Furthermore, when cells are under duress, such as in chronic inflammation, they may express danger-associated molecular patterns (DAMPs), such as uric acid, ATP, heat shock proteins (HSPs) or glycoprotein 96 (gp96), that may also be recognized by PRRs (57–60). PRRs may be membrane bound, or soluble plasma proteins. Examples include mannose-binding lectin (MBL) that is important in the lectin pathway of complement activation, the transmembrane PRRs composed of ten known human toll-like receptors (TLRs) that may be activated on cell-surfaces or within endosomal compartments, and the cytosolic PRRs which include the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and the retinoic acid-inducible gene I (RIG-1)-like receptors.
Toll-like Receptors
The cell surface TLRs include TLR1, 2, 4, 5 and 6, with the recognition motifs outside the cell, while TLR 3,7, 8 and 9 are on the endosomal membrane with the PRR recognition motifs within the endosomal compartment. The TLR system recognizes PAMPs, including lipopolysaccharide (LPS) (TLR4), peptidoglycans (TLR2 together with TLR1 or 6), and unmethylated CpG DNA motifs (TLR9) from bacteria, ssRNA (TLR7) and dsRNA (TLR3) from viruses. The cytoplasmic domain of the TLR receptor is called the Toll-IL1 receptor (TIR) motif because it is also present in the IL-1 receptor. TLR signals are mediated through the TIR, which interacts with adaptor molecules. All TLRs except TLR3 signal through the adaptor molecule myeloid differentiation factor 88 (MyD88), while TLR3 signals only through the adaptor molecule toll-interleukin-1-receptor-domain-containing adaptor-inducing interferon β (TRIF), and TLR4 signals through both MyD88 and TRIF. Signaling through the MyD88 leads to the activation of NF-κB and the MAP kinases: c-Jun N-terminal kinase (JNK) and p38. Activation of NF-κB and the MAP kinases leads to the transcription of genes involved in inflammation, proliferation and protection against apoptosis (3, 61, 62), whereas activation through TRIF results in the expression of type I interferons (IFN), IFN α and β (63). Clinically, further elucidation of TLR signaling cascades is important because they offer attractive targets for intervention.
TLR Expression in RA
TLRs are expressed in the RA joint. In RA synovial tissue, CD16+ synovial lining macrophages also expressed TLR2 (64), and the expression of both TLR-2 and TLR-4 was significantly higher in RA tissue than in samples from patients with OA (65). Interestingly, RA synovial tissues have a pronounced expression of TLR2 mRNA in the synovial lining and at sites of attachment and invasion into cartilage or bone tissue (66). In addition, TLR3 and TLR7 were also found to be highly expressed in RA synovium (67), and samples of tissue from patients with either early or longstanding RA showed similar levels of TLR3 and TLR4, both of which were significantly higher than in patients with OA (68).
In RA synovial fibroblasts, levels of baseline mRNA for TLR2 and TLR4, did not differ compared to OA tissue fibroblasts. However, compared with OA fibroblasts, RA synovial fibroblasts demonstrated a significantly increased expression of TLR2 after treatment with IL-1β, TNFα, LPS, or synthetic bacterial lipopeptide (sBLP), leading to a strong increase of NF-κB translocation into the nucleus (66). In contrast, another study found that levels of both TLR2 and TLR4 mRNA were significantly greater in RA synovial fibroblasts compared with those from patients with OA and normal skin fibroblasts (69). In contrast to whole synovial tissue, TLR7 mRNA expression by synovial fibroblasts was not seen, suggesting that previous TLR7 staining may have been reflecting expression by macrophages or dendritic cells (68).
Peripheral blood monocytes also express TLRs. Both TLR2 and TLR4 were increased on CD16+ and CD16− peripheral blood monocytes from RA patients versus healthy controls, and the TLR2 expression on the CD16+ subset was higher than the CD16− subset (64). Furthermore, IFN-γ increased the expression of TLR2 and TLR4 on RA peripheral blood monocytes (65). In RA synovial fluid, CD14+ macrophages demonstrated increased expression of TLR2 and TLR4 expression compared to peripheral blood monocytes or control macrophages differentiated in vitro from normal monocytes (70).
Activation of Cells From the RA Joint by Microbial TLR Ligands
Isolated RA synovial fibroblasts treated with microbial TLR2 and TLR4 ligands demonstrated a marked increase in the osteoclast activator RANKL, at both the mRNA and protein levels (69). Inflammatory cytokines IL-6 and IL-8 as well as matrix metalloproteases (MMP) 1 and 3 were induced by stimulation of RA synovial fibroblasts with bacterial peptidoglycan (PGN) (71). Furthermore, RASFs demonstrated increased expression of vascular endothelial growth factor (VEGF) after stimulation with bacterial PGN, and increased IL-15 after stimulation by both TLR2 and TLR4 ligands (72). Stimulation of RA synovial fibroblasts with the synthetic TLR3 ligand, poly I:C, led to the production of IL-6 and TNFα, which was significantly enhanced when the cells were pre-incubated with IFN-α compared to cells stimulated with poly I:C alone (73). These observations suggest that in RA synovial fibroblasts may be activated through the TLR pathway.
RA synovial fluid macrophages demonstrate an increased response to microbial TLR2 and TLR4 ligands compared to control macrophages differentiated in vitro from normal monocytes, macrophages from the joints of patients with other forms of inflammatory arthritis, or RA peripheral blood monocytes (70). In addition, treatment of RA synovial fluid macrophages with a microbial TLR2 ligand significantly increased the levels of both IFN-γ and IL-23 mRNA compared to in vitro differentiated control macrophages (74). It is possible that alterations of intracellular signaling pathways, such as those regulated by IFNγ or IL-10 might be responsible (75).
Endogenous TLR Ligands in RA
Since microbial ligands are not likely the cause of TLR signaling in the RA joint, studies have examined RA synovial fluids and tissues for the presence of potential endogenous TLR ligands. RA synovial fluid activated HEK293 cells only when these cells expressed TLR4, suggesting the presence of endogenous TLR4 ligands in RA synovial fluid (76). Additionally, we have demonstrated that RA synovial fluid is capable of activating normal macrophages, and that this activation was mediated through TLR2 and TLR4 (unpublished observations). Together, these observations suggest that endogenous TLR ligands or DAMPs may be released in response to inflammation in early RA and result in continuous persistence of inflammatory mediators through activation of cells of the innate immune system.
A number of potentially relevant endogenous TLR ligands or DAMPs have been identified in the RA joint. Ligands such as HSP22, tenascin-C, high-mobility group box chromosomal protein-1 (HMGB1), serum amyloid A, and fragments of hyaluronic acid are highly expressed in the RA joint and are capable of activating monocytic cells through TLR2 or TLR4, or both. Another DAMP, gp96, was also highly expressed in RA synovial fluid and synovial tissue. The addition of gp96 in vitro to RA synovial fluid macrophages induced significantly greater levels of TNF-α, IL-8, and TLR2 compared with control macrophages (60). While gp96 bound to both TLR2 and TLR4, macrophage activation was mediated primarily through TLR2. The quantity of TLR2 expression on synovial fluid macrophages strongly correlated with the level of gp96 in the same synovial fluids. Further studies, employing a murine model of arthritis mediated by immune complexes, demonstrated that in the normal joint an extracellular matrix glycoprotein, tenascin-C was not expressed. However during the early phase of the arthritis, tenascin-C was induced and it contributed to the progression of the arthritis, mediated through TLR4 activation of macrophages and synovial fibroblasts (77). These observations support a potentially important role of endogenous TLR ligands in the persistent activation of macrophages and synovial fibroblasts that is observed in the joints of patients with RA.
TLR Signaling as a Target in RA
Understanding the potential role of TLRs in inflammation has led to its therapeutic exploitation. In mice with CIA, in which intradermal injections of type II collagen leads to both the priming and the effector phases of inflammatory arthritis, treatment with a TLR4 antagonist both before the onset of disease and during established arthritis led to a significant reduction of arthritis (78). Furthermore, there was decreased histologic destruction of the cartilage matrix and infiltration of inflammatory cells into the joint space. Additionally, IL-1 receptor antagonist deficient mice develop a spontaneous chronic arthritis which was ameliorated when treated with a TLR4 antagonist (78). The results of these animal models support the potential benefits of suppressing TLR signaling as a therapeutic approach in RA.
In ex vivo synovial tissue cultures from RA patients, the addition of a TLR4 antagonist suppressed the spontaneous secretion of IL-1β and TNF-α, supporting the role of TLR4 in the production of inflammatory cytokines (76). Currently available anti-rheumatic therapies that are known to have a suppressive effect on the TLR signaling pathway including hydroxychloroquine (TLR7, 8 and 9) and auranofin (TLR4). Antagonists of TLR4 are being studied for possible use in sepsis and endotoxemia. Lipid A, the innermost of the three regions of LPS, was created in a stable, synthetic form called E5564 (Eritoran) (79). It is currently undergoing clinical trials and may become a viable agent in RA. Another TLR4 receptor antagonist, chaperonin 10 (HSP 10), has been studied in a randomized, double-blind, multicenter study of 23 patients with moderate to severe active RA who received twice weekly IV therapy at different concentrations for 12 weeks. All 3 treatment groups tolerated the therapy well, and had significant improvement in the primary endpoint of clinical improvement as measured by the DAS28 score. The effect appeared to be dose-dependent, with 4 out of 7 patients in the highest group reachinging an ACR 50 response, and 2 out of 7 achieving an ACR 70. The highest treatment group also had significant improvement in all secondary endpoint measures, including swollen and tender joint count, patient’s assessment of pain on the visual analog scale, disability index on the health assessment questionnaire, and morning stiffness (80). In summary, the TLR signaling pathway, which may be activated by endogenous TLR ligands or DAMPs, is a novel target for therapeutic intervention in patients with RA.
Nucleotide-binding Oligomerization Domain (NOD)-like receptors (NLRs)
Similar to TLRs, the NLRs are intracellular, cytosolic receptors that sense PAMPs or DAMPS and mediate an inflammatory response. Thus far, there are 22 proteins in the NLR family, including the NOD and NALP subfamilies, with the 14 NALPs characterizing the largest subfamily. Common features of the NLRs include a central nucleotide binding domain, a C-terminal leucine rich-repeat domain, and N-terminal caspase-recruitment and pyrin domains (81). The NOD proteins recognize fragments of bacterial cell-wall proteoglycans and activate the transcription factor NF-κB. Although NOD proteins are expressed in phagocytes along with TLRs, they are especially important activators of the innate response in epithelial cells, where expression of TLRs is weak or absent (3). Members of the NALP family, including NALP1, 3, and 12, are capable of forming functional caspase-1 activation complexes, or inflammasomes, that are important in the processing and release of IL-1β and IL-18 in response to PAMPs or DAMPs.
Recently, it has been shown that induction of SCW-driven arthritis in NOD-2 gene-deficient mice results in reduced joint swelling and decreased levels of TNF-α and IL-1β, whereas the opposite effect was seen in NOD-1 deficient mice, suggesting an anti-inflammatory role for NOD-1. Moreover, the microbial ligand for NOD-2, muramyl dipeptide (MDP), has been detected in RA synovium, whereas none was found in synovium from OA patients (82). NOD-2 was expressed by both macrophages and synovial fibroblasts, as detected by immunohistochemistry, but not by lymphocytes or blood vessels. Transcription of NOD-2 mRNA by RA synovial fibroblasts was induced by TNF-α, poly I:C, and LPS. Adding MDP to other inflammatory stimuli augmented RA synovial fibroblast production of inflammatory cytokines and matrix-degrading enzymes compared to the stimuli alone. These observations suggest that TLR activation may induce NOD-2 expression, and that TLRs and NOD-2 may act synergistically in promoting inflammation and matrix destruction in the RA joint (83). Further studies will be required to determine whether NODs may potentially become effective therapeutic targets in RA.
The Inflammasome and RA
In macrophages, the essential link between pro-IL1β and pro-IL18, and their bioactive counterparts is the protease caspase 1, which cleaves these molecules and generates the active cytokines that are then released from the cell (84). Activation of caspase 1 depends on the formation of inflammasomes, which are multiprotein complexes consisting of a NLR protein such as a NALP, the adaptor molecule ASC and caspase 1, and which are assembled in response to cellular recognition of DAMPs or PAMPs (85). Inflammasomes are implicated in diseases such as systemic-onset juvenile idiopathic arthritis (JIA) and familial Mediterranean fever (86, 87). Additionally, mutations in NALP3 are responsible for cryopyrin-associated periodic syndromes (88), and NALP3-containing inflammasomes are activated by monosodium urate (MSU) and calcium pyrophosphate dihydrate (CPPD) crystals in gout and pseudogout, respectively (89). In addition to MSU and CPPD crystals, other ligands of the NALP3 inflammasome include Alzheimer’s disease-associated amyloid deposits (amyloid-β), and the extracellular matrix components, biglycan and hyaluronan (90).
NALP3 is expressed in the RA joint. Employing real-time PCR, it has been shown that NALP3 mRNA levels were increased in RA synovium compared with OA, and that monocyte-derived macrophages from healthy donors differentiated in vitro increased NALP3 expression when stimulated by TNFα (91). Another study did not find any differences between RA and OA synovial expression of NALP1, NALP3, NALP12, or ASC using densitometric analysis of Western blots. However, using ELISA, caspase-1 levels were significantly enhanced in RA synovial tissues, even though there was no difference in concentrations of IL-1β (92). Thus, further studies are needed to clarify the role of the inflammasome in RA pathogenesis.
Interestingly, caspase 1 is not the only IL-1β converting enzyme (ICE) involved in IL-1β processing, which is likely the reason why inhibition of caspase 1 only partially inhibits experimental models of RA, whereas deficiency of IL-1β completely ameliorates the arthritis (85). Alternative ICEs, such as elastase or proteinase 3 in neutrophils, or chymase in mast cells, may be involved in the early stages of inflammatory arthritis by contributing to the processing of IL-1, whereas caspase 1 may play more of a role in the chronic stage of the arthritis (93, 94).
Retinoic Acid-inducible Gene I (RIG-I)-like Receptors (RLRs)
Three genes encode RIG-I-like receptors (RLRs) in human and mouse genomes (95). One of these, retinoic acid-inducible gene-I (RIG-I), encodes an RNA helicase protein whose expression is induced by IFN γ, and which is found in cells such as endothelial cells, bronchial epithelial cells, smooth muscle cells, and macrophages (96–98). It is a sensor of viral RNAs and activates cells of the innate immune system (99). It is also associated with various chronic inflammatory diseases including lupus nephritis, and is considered important in mediating reactions induced by IFN-γ (100). Recently, high levels of RIG-I expression have been found in RA synovial tissues compared to OA controls. Treatment of RA synovial fbroblasts with IFN-γ significantly induced expression of RIG-I, and knockdown of RIG-I in RA synovial fibroblasts with small interfering RNA (siRNA) resulted in the inhibition of the expression of the chemokine CXCL10 (101). In addition, RIG-I was expressed in RA synovial fibroblasts stimulated with TNFα and knockdown of RIG-I with siRNA suppressed TNF-α-induced RANTES (CCL5) expression, suggesting a possible role for the TNFα/RIG-I/CCL5 pathway in RA pathogenesis (102). Further studies are needed to show whether RIG-I is a plausible target for therapy in RA.
Complement Pathways
Background
The complement system is a key mediator of inflammation in the effector phase of RA. It is composed of a family of plasma proteins that act alone or in concert with antibodies to opsonize pathogens and dying cells of the host, enhance phagocytosis, and recruit effector cells to areas of inflammation. In addition, the coating of antigens with complement facilitates uptake by antigen presenting cells, and thus enhances the presentation of antigen to the adaptive immune system. Several complement proteins, including C3 and C5, are present in inactive states called zymogens. A cleaved zymogen yields 2 fragments. The larger fragment is a serine protease that remains covalently bound to the immune complex or pathogen surface (e.g. C3b), while the smaller peptide fragment acts locally as a mediator of inflammation (e.g. C3a). Upon activation, the proteases cleave other complement proteins into their active forms, amplifying the response.
There are 3 pathways of complement activation. The classical pathway is initiated by the binding of C1 to antigen: antibody complexes, either circulating or tissue bound or on pathogen surfaces, or directly to the surface components of some bacteria. Once activated, C1 cleaves C2 and C4, which together form C4b2a, the C3 convertase of the classical pathway. The lectin pathway is initiated by the binding of carbohydrate-binding proteins, such as mannose-binding lectin (MBL) to arrays of carbohydrates on the surface of pathogens. This pathway also leads to the creation of the C3 convertase formed by C4b2a. The alternative pathway primarily amplifies activation that is initiated by the other two pathways. C3b generated by the cleavage of C3 by the classical or lectin pathways is able to bind factor B which is cleaved by factor D, ultimately forming the alternative pathway C3 convertase, C3bBb. The most important activity of the C3 convertase is to cleave large numbers of C3 molecules into C3b fragments that opsonize pathogens, dying cells, and immune complexes, and amplify the complement cascade. Surfaces opsonized by C3b and its derivatives, are recognized by effector cells bearing complement receptors (CRs), and can stimulate phagocytosis as well as augment inflammatory signals in both innate and adapative cells.
Formation of the C3 convertases is the merging point of the three complement pathways. The next step involves the formation of the C5 convertase, capable of generating the most potent inflammatory peptide, C5a. Finally, the terminal complement proteins, C5b-C9, form the membrane-attack complex, which constructs pores in the cell membranes of some pathogens, causing death. The enzymatic complement cascade is balanced by membrane and plasma proteins, such as decay-accelerating factor (DAF) or factor I, that inhibit formation of the C3 convertase or promote its dissociation, thus preventing complement activation on normal host cells. Deficiencies in these proteins may lead to excessive complement activation and inflammation, as well as complement depletion and susceptibility to recurrent bacterial infections (3).
Evidence of Complement Activation in Murine Models of RA
Findings from experimental models suggest roles for both the alternative and classical pathways in the development of arthritis. In the CIA model, mice deficient for C3 demonstrate a significantly lower arthritis score than controls, while the arthritis was intermediate in mice deficient in the factor B, suggesting that C3 activation occurs via both classical and alternative C3 pathways in CIA (103). In contrast, another study found no resistance to CIA in C4 deficient mice, suggesting a more important role for the alternative pathway in this model (104). Intriguingly, however, both CIA and a passive serum-transfer model showed that injection of C4 binding protein (C4BP) was effective at delaying the onset of arthritis and reducing the severity of already established disease by inhibiting activity of both the classical and alternative pathways (105). Mice with C5 deficiency were highly resistant to CIA despite evidence of normal cellular and humoral immune responses to type II collagen and intra-articular deposition of IgG antibodies (106). In the K/B × N serum-transfer model, anti-GPI antibodies were effective at causing arthritis in mice deficient in C4, whereas no arthritis was seen in C3 or C5 deficient mice, suggesting that the pathogenesis of K/B × N serum-induced arthritis relies on activation of the alternative pathway (107, 108). Together these observations support a major role for the alternative pathway in CIA and anti-GPI-mediated arthritis.
Evidence of Complement Activation in RA
Complement-activating immune complexes are abundant in the joints of RA patients and appear to be crucial mediators of the effector phase of inflammation in the pathogenesis of RA (109–111). In RA synovial fluid, a decrease in C3 and C4 along with increased cleavage products of C3, suggests increased complement consumption within the joint (112, 113).
The concentration of C5a in plasma and synovial fluid of RA patients is significantly higher than in patients with OA (114), and levels of C5a in RA synovial fluid are sufficient to induce both neutrophil chemotaxis and microvascular plasma protein leakage, two important features of inflammation in RA (115). Neutrophils that migrate into inflamed joints demonstrate upregulated expression of complement receptors that enhance phagocytosis of material opsonized by C3b (116). The terminal complement proteins, C5b-C9 have also been implicated in RA. Plasma levels of C5b-C9 are significantly higher in RA than controls (117). Compared to crystal-induced and OA, RA synovial fluid levels of the C5b-9 complex were significantly higher and positively correlated with synovial fluid C3a and Bb levels (118).
Further supporting the importance of the classical pathway, microparticles from RA SF were found to contain abundant quantities of bound C1q, C4, and C3 as well as IgM and IgG antibodies (119). Components of the extracellular matrix (ECM) exposed during cartilage damage can also activate the classical pathway. ECM stabilizing proteins, fibromodulin and osteoadherin, are able to bind to and activate C1q by its globular head domain, resulting in a significant deposition of C3b and C4b (120). In addition, new activation products have recently been described in the classical complement pathway. One such molecule is a covalent complex between C1q and activated C4. The plasma levels of C1q-C4 complex were found to be significantly higher in patients with active RA as compared to patients with RA in clinical remission (121). Finally, it has recently been shown that human anti-cyclic citrullinated peptide (anti-CCP) antibodies activate both the classical and alternative pathways of complement in vitro (122).
In addition to plasma and RA synovial fluid, complement-mediated inflammation is evident in RA synovial tissue. On RA synovial fibroblasts and macrophages, the cell surface expression of the C5a receptor (C5aR) was elevated and positively correlated with joint swelling, ESR, and CRP levels (123). Furthermore, aside from the classic role of the C5b-C9 complex in cell lysis, it has been shown that synovial fibroblasts are activated when exposed to sublytic concentrations of C5b-C9 in vitro. Thus, enhanced activation of synovial fibroblasts provides an additional mechanism by which complement promotes inflammation in RA (124). Together, these observations support an important role for both the classical and the alternative pathways of complement activation in the pathogenesis of RA.
Therapeutic Strategies Targeting Complement in RA
Therapies that target complement activation may be effective in ameliorating disease given that it is one of the key mediators of inflammation in the effector phase of RA. In mice, it was previously shown that inhibition of C5 with a monoclonal antibody was successful at preventing the onset of CIA and was also effective at ameliorating established disease (125). This led to the development of eculizumab, which prevents the release of C5a and the formation of C5b-C9 complexes by inhibiting cleavage of C5 into C5a and C5b (126). Eculizumab has been most beneficial for patients suffering with paroxysmal nocturnal hemoglobinuria. In phase II trials, published only in abstract form, significant improvement in RA clinical score was seen after 3 months of treatment with eculizumab, and the best responders were those patients who had high baseline levels of C5b-9 complexes in the serum (127, 128). Another approach employed soluble complement receptor 1 (sCR1) derivatives that act as cofactors for complement inhibitory proteins, DAF and factor I (129). In mice with established CIA, gene therapy with sCR inhibited the progression of CIA, reduced anti-collagen antibody levels, and inhibited T-cell response to type II collagen in vitro (130). Additionally, rats with AIA were concomitantly treated with a single intra-articular dose of a membrane-targeting complement regulator derived from human CR1 (APT070), or vehicle buffer only. Animals treated with APT070 demonstrated significantly less clinical and histologic disease compared with controls after 14 days (131). APT070 is a truncated version of sCR1 with the addition of a membrane-targeting moiety that improves protein retention at the site of inflammation, and it is currently undergoing phase I/II clinical trials in RA. Other potential therapies targeting complement activation have shown positive results in animal studies, and include serine protease inhibitors, C3a and C5a receptor antagonists, and synthetic regulatory proteins involved in complement inhibition (132).
Fc RECEPTORS
Background
Fc Receptors (FcRs) are surface molecules on myeloid cells and B cells that are capable of interacting with the Fc portion of immunoglobulin molecules. FcRs help to bridge the adaptive and innate immune responses and function as mediators of effector cell activation and inhibition, antibody-dependent cellular cytotoxicity (ADCC), and release of inflammatory mediators. For human IgG, there are 3 known activating receptors: FcγRI (CD64), FcγRIIA (CD32a), and FcγRIIIA (CD16), and one inhibitory receptor: FcγRIIB (CD32b). The activation receptors are distinguished by the presence of a cytoplasmic immunoreceptor tyrosine-based activation motif (ITAM), while the inhibitory receptor possesses an immunoreceptor tyrosine-based inhibitory motif (ITIM). Initiation of the activation pathway leads to phosphorylation of ITAM sequences by Src-family kinases and recruitment and activation of spleen tyrosine kinase (Syk), ultimately resulting in activation of downstream signaling pathways such as MAP kinases that are important for cellular proliferation. On the other hand, engagement of the inhibitory pathway and phosphorylation of ITIM sequences leads to the prevention of calcium influx, and thus blocks calcium-dependent processes such as degranulation, phagocytosis, ADCC, cytokine release, and proinflammatory activation. The critical step in effector cell activation by FcRs occurs via the cross-linking of the receptors by immunoglobulin. Thus, the cross-linking of 2 ITAM-bearing FcRs leads to an activation signal whereas cross-linking of ITIM-bearing FcRs results in the arrest of effector responses. In general, both the activating and inhibitory receptors are co-expressed on myeloid cells and are engaged at the same time by circulating immune complexes or cells that have been opsonized by immunoglobulin. The threshold of effector cell activation is determined by the levels of expression of each receptor class on effector cells, as well as the ratio of an antibody’s binding affinity for an activating receptor to an inhibitory receptor (A/I ratio). Therefore, either increased expression of FcγIIB, or higher avidity of immunoglobulin subclass for FcγIIB results in a higher threshold for effector cell activation. Activating and inhibitory receptors may be up- and down-regulated by inflammatory or inhibitory cytokines. In addition, polymorphisms of FcγRs have been described which affect the binding affinities for specific IgG subclasses and result in greater or lesser activation of effector cells when stimulated. Disequilibrium between activating and inhibitory FcR pathways can result in pathologic responses, and further understanding of perturbations in the FcR system that contribute to RA will aid in the development of new therapeutic strategies that target this system (133).
Fc Receptors in Murine Models of RA
Studying mice deficient for various FcRs has led to better understanding of how these receptors contribute to experimental arthritis. A role for the FcγRIIB receptor in preventing arthritis has been demonstrated using several models of arthritis. Mice with a haplotype that confers resistance to CIA were rendered susceptible to arthritis when they were made FcγRIIB-deficient (134). Similarly, FcγRIIB-deficient mice developed accelerated arthritis when they were administered anti-GPI antibody from K/B × N mice (135). In contrast, deficiencies in activating receptors have been shown to reduce the severity of arthritis. Mice deficient in the common activating FcR γ-chain were protected from CIA (136, 137). Another study showed that, although FcγRIII was critical in early development of CIA, both FcγRIII and FcγRI were dispensible for the progression to destructive joint disease, whereas the FcR γ-chain was not, implying a likely role for FcγRIV, which is not expressed in humans, but in mice (138). Finally, studies using the K/B × N serum transfer model of arthritis showed absence of clinical arthritis in Fc γ-chain deficient mice, but erosive lesions in the bone still developed, suggesting separate mechanisms for inflammation and bony erosions (135). Together these observations support an important role for the FcgR signaling pathway in the pathogensis of experimental models of RA.
Fc Receptors in RA Patients
FcRs are found on cells from RA patients and may be associated with activation or inhibition of innate effector cells. Levels of activating receptors in plasma shed from macrophages and NK cells as well as membrane-bound activating receptors on peripheral blood monocytes were increased in RA patients compared to healthy controls (139)–(140). In addition, increases in the expression of activating FcRs on RA peripheral blood monocytes correlated with higher sedimentation rates, and DMARD-naïve patients had significantly higher levels of activating FcγRIIA compared to RA patients taking DMARDs, supporting an association between expression of activating FcRs and disease activity (140). The stimulation of activating receptors on macrophages with immune complexes results in the expression of proinflammatory molecules. In vitro stimulation of healthy peripheral blood monocyte-derived macrophages with immune complexes formed by anti-citrullinated protein antibodies from RA patient sera resulted in significantly increased TNF-α secretion via engagement of FcγRIIA. Similar findings were seen when such cells were stimulated in vitro with immune complexes derived from RA synovial fluid (141, 142). In RA synovial tissue, significantly increased levels of activating FcRs were found to correlate with the number of synovial macrophages as well as the expression of TNF-α and matrix metalloproteinases (143). Interestingly, no difference was seen between levels of inhibitory FcγRIIB expression on peripheral blood monocytes from RA patients and controls (144), while in the synovial tissue, the expression of all FcγRs, including FcγRIIB, was found to be significantly elevated in RA patients compared to biopsies from healthy volunteers (145). Thus, upregulation of activating receptors, rather than a paucity of inhibitory receptors, appears to contribute to the increased activation of monocytes and macrophages in RA. Additionally, C Reactive Protein, which is increased in RA, is capable of binding Fc receptors promoting the expression of proinflammatory cytokines, possibly contributing to the persistent expression of macrophage cytokines in RA (146, 147).
Recently it has been demonstrated that FcγRIIB was selectively up-regulated on monocyte-derived dendritic cells (DCs) from RA patients with low disease activity. In vitro incubation of FcγRIIB-bearing DCs with a TLR4 agonist and immune complexes inhibited T cell proliferation and promoted the development of regulatory T cells. Furthermore, the addition of FcγRIIB-specific blocking antibody abrogated regulatory T cell development, suggesting that FcγRIIB expression on DCs in RA patients with low activity may be important in the maintenance of tolerance (48).
Fc Receptors as Targets for Therapy in RA
Altering the balance of FcγRs in favor of inhibitory pathways is an attractive therapeutic strategy in RA. Pooled IgG from multiple donors (IVIG) is used to treat various autoimmune diseases, and suggested mechanisms of action include blockade of activating FcRs as well as upregulation of FcγRIIB on effector macrophages (148). In mice, treatment with soluble FcγRIIB significantly reduced the severity of CIA compared to controls (149). Clinical improvement in patients with RA on DMARD therapy may be associated with changes in FcγR expression or binding affinities. In one study, the levels of activating FcγRs on peripheral blood monocytes were significantly decreased after 16 weeks in RA patients receiving methotrexate (150). Additionally, recent data suggests that polymorphisms of activating FcγRs in RA patients may influence outcomes of treatment with TNF-α blocking agents (151, 152). Novel approaches are being developed that target FcRs and downstream signaling pathways associated with FcR activation. Inflammatory macrophages from RA synovial fluid treated in vitro with toxin-conjugated antibodies against FcγRI were efficiently eliminated via apoptotic cell death, resulting in a reduction of TNF-α (153, 154). Finally, a novel small molecule Syk inhibitor, R788, and its active metabolite R406, have been shown to suppress clinical arthritis, bone erosions, pannus formation, and synovitis in experimental arthritis (155). Further, in a 12-week, randomized, placebo-controlled trial with 158 active RA patients, twice-daily oral doses of R788 showed significant improvement in ACR 20, 50, and 70 scores compared to controls. Clinical efficacy was noted as early as 1 week after initiation of therapy, and correlated with serum reductions in IL-6 and MMP3 (156). Therefore, targeting the FcR signaling pathway may be an effective therapeutic strategy in RA.
SUMMARY
Innate immunity, with macrophages playing a central role, is critically important in the pathogenesis of RA (Figure 1). Experimental models document the importance in innate immune cells in the initiation of many experimental models of arthritis, promoting the development of adaptive immunity, which results in autoantibody production. In patients with RA the presence of rheumatoid factors and anti-CCP antibodies supports the importance of innate immunity in the initiation of RA. Additionally, in the joints of patients with RA there is abundant evidence for the presence of immune complexes and the activation of complement, which directly contributes to the pathogenesis of disease. Further, immune complexes bind to FcγRs, which are capable of activating macrophages and DCs. The importance of this process is supported by the preliminary observations demonstrating that suppression of the FcγR activation pathway may be effective in treating patients with RA. Finally, synovial macrophages have clearly been demonstrated to be critically important in the pathogenesis of RA and effective therapies result in a reduction of synovial macrophages, regardless of the biologic pathway targeted. The mechanisms contributing to the persistent activation of macrophages may be due to the expression of endogenous TLR ligands, such as gp96 and tenascin-C, which are upregulated in RA and are capable of activating macrophages through TLR signaling, creating a self-perpetuating, progressive chronic inflammatory process. Thus, targeting innate immunity has already proven beneficial in RA, and targeting additional pathways such as complement, the FcgR, and TLR signaling pathways holds promise for further therapeutic advances.
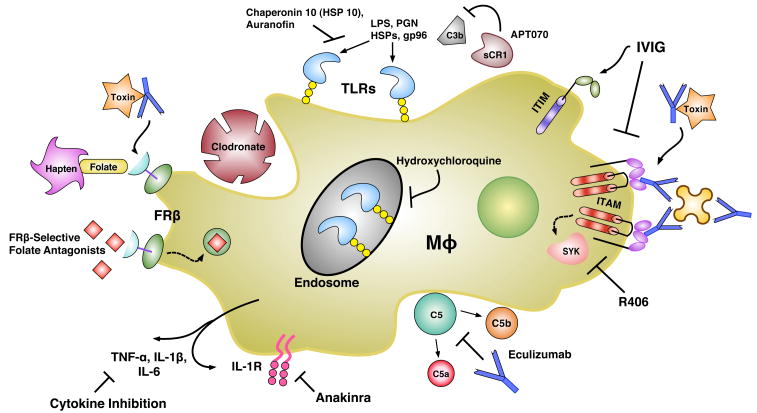
FIGURE 1.
Various current and novel innate immunity-directed therapeutics and their targets. Mφ, macrophage; IL-1R, Interleukin-1 Receptor; TNF-α, Tumor Necrosis Factor Alpha; FRβ, Folate Receptor Beta; TLR, Toll-Like Receptor; HSP, Heat-Shock Protein; LPS, Lipopolysaccharide; PGN, Peptidoglycan; sCR1, Soluble Complement Receptor 1; ITIM, Immunoreceptor Tyrosine-based Inhibitory Motif; ITAM, Immunoreceptor Tyrosine-based Activation Motif; IVIG, Intravenous Immunoglobulin; SYK, Spleen Tyrosine Kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tak PP, Bresnihan B. The pathogenesis and prevention of joint damage in rheumatoid arthritis: advances from synovial biopsy and tissue analysis. Arthritis Rheum. 2000;43(12):2619–33. doi: 10.1002/1529-0131(200012)43:12<2619::AID-ANR1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KP, Travers P, Walport M, Janeway C. Janeway’s immunobiology. 7. New York: Garland Science; 2008. [Google Scholar]
- 4.Lavagno L, Gunella G, Bardelli C, Spina S, Fresu LG, Viano I, et al. Anti-inflammatory drugs and tumor necrosis factor-alpha production from monocytes: role of transcription factor NF-kappa B and implication for rheumatoid arthritis therapy. Eur J Pharmacol. 2004;501(1–3):199–208. doi: 10.1016/j.ejphar.2004.07.101. [DOI] [PubMed] [Google Scholar]
- 5.Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39(1):115–24. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 6.Wijbrandts CA, Vergunst CE, Haringman JJ, Gerlag DM, Smeets TJ, Tak PP. Absence of changes in the number of synovial sublining macrophages after ineffective treatment for rheumatoid arthritis: Implications for use of synovial sublining macrophages as a biomarker. Arthritis Rheum. 2007;56(11):3869–71. doi: 10.1002/art.22964. [DOI] [PubMed] [Google Scholar]
- 7.Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. 2003;108(24):2957–63. doi: 10.1161/01.CIR.0000099844.31524.05. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 9.Vergunst CE, van de Sande MG, Lebre MC, Tak PP. The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. 2005;34(6):415–25. doi: 10.1080/03009740500439159. [DOI] [PubMed] [Google Scholar]
- 10.Polzer K, Baeten D, Soleiman A, Distler J, Gerlag DM, Tak PP, et al. Tumour necrosis factor blockade increases lymphangiogenesis in murine and human arthritic joints. Ann Rheum Dis. 2008;67(11):1610–6. doi: 10.1136/ard.2007.083394. [DOI] [PubMed] [Google Scholar]
- 11.Jutila MA, Banks KL. Locally dividing macrophages in normal and inflamed mammary glands. Clin Exp Immunol. 1986;66(3):615–24. [PMC free article] [PubMed] [Google Scholar]
- 12.Ceponis A, Konttinen YT, Imai S, Tamulaitiene M, Li TF, Xu JW, et al. Synovial lining, endothelial and inflammatory mononuclear cell proliferation in synovial membranes in psoriatic and reactive arthritis: a comparative quantitative morphometric study. Br J Rheumatol. 1998;37(2):170–8. doi: 10.1093/rheumatology/37.2.170. [DOI] [PubMed] [Google Scholar]
- 13.Liva SM, Kahn MA, Dopp JM, de Vellis J. Signal transduction pathways induced by GM-CSF in microglia: significance in the control of proliferation. Glia. 1999;26(4):344–52. doi: 10.1002/(sici)1098-1136(199906)26:4<344::aid-glia8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Rekhter MD, Gordon D. Active proliferation of different cell types, including lymphocytes, in human atherosclerotic plaques. Am J Pathol. 1995;147(3):668–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Liu H, Pope RM. Apoptosis in rheumatoid arthritis: friend or foe. Rheum Dis Clin North Am. 2004;30(3):603–25. x. doi: 10.1016/j.rdc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Scatizzi JC, Hutcheson J, Pope RM, Firestein GS, Koch EE, Mavers M, et al. Bim-BH3 mimetic therapy is effective at suppressing inflammatory arthritis through the activation of myeloid cell apoptosis Arthritis Rheum. 2009 doi: 10.1002/art.27198. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, et al. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86(5):398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 18.Baeten D, Boots AM, Steenbakkers PG, Elewaut D, Bos E, Verheijden GF, et al. Human cartilage gp-39+, CD16+ monocytes in peripheral blood and synovium: correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000;43(6):1233–43. doi: 10.1002/1529-0131(200006)43:6<1233::AID-ANR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168(7):3536–42. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81(3):584–92. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 21.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19(1):71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 22.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–7. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 23.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 24.Haringman JJ, Gerlag DM, Zwinderman AH, Smeets TJ, Kraan MC, Baeten D, et al. Synovial tissue macrophages: a sensitive biomarker for response to treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(6):834–8. doi: 10.1136/ard.2004.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurlings RM, Vos K, Wijbrandts CA, Zwinderman AH, Gerlag DM, Tak PP. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis. 2008;67(7):917–25. doi: 10.1136/ard.2007.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bresnihan B, Pontifex E, Thurlings RM, Vinkenoog M, El-Gabalawy H, Fearon U, et al. Synovial tissue sublining CD68 expression is a biomarker of therapeutic response in rheumatoid arthritis clinical trials: consistency across centers. J Rheumatol. 2009;36(8):1800–2. doi: 10.3899/jrheum.090348. [DOI] [PubMed] [Google Scholar]
- 27.Richards PJ, Williams BD, Williams AS. Suppression of chronic streptococcal cell wall-induced arthritis in Lewis rats by liposomal clodronate. Rheumatology (Oxford) 2001;40(9):978–87. doi: 10.1093/rheumatology/40.9.978. [DOI] [PubMed] [Google Scholar]
- 28.Solomon S, Rajasekaran N, Jeisy-Walder E, Snapper SB, Illges H. A crucial role for macrophages in the pathology of K/B × N serum-induced arthritis. Eur J Immunol. 2005;35(10):3064–73. doi: 10.1002/eji.200526167. [DOI] [PubMed] [Google Scholar]
- 29.Ceponis A, Waris E, Monkkonen J, Laasonen L, Hyttinen M, Solovieva SA, et al. Effects of low-dose, noncytotoxic, intraarticular liposomal clodronate on development of erosions and proteoglycan loss in established antigen-induced arthritis in rabbits. Arthritis Rheum. 2001;44(8):1908–16. doi: 10.1002/1529-0131(200108)44:8<1908::AID-ART329>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 30.Barrera P, Blom A, van Lent PL, van Bloois L, Beijnen JH, van Rooijen N, et al. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43(9):1951–9. doi: 10.1002/1529-0131(200009)43:9<1951::AID-ANR5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima-Matsushita N, Homma T, Yu S, Matsuda T, Sunahara N, Nakamura T, et al. Selective expression of folate receptor beta and its possible role in methotrexate transport in synovial macrophages from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42(8):1609–16. doi: 10.1002/1529-0131(199908)42:8<1609::AID-ANR7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 32.Paulos CM, Turk MJ, Breur GJ, Low PS. Folate receptor-mediated targeting of therapeutic and imaging agents to activated macrophages in rheumatoid arthritis. Adv Drug Deliv Rev. 2004;56(8):1205–17. doi: 10.1016/j.addr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Turk MJ, Breur GJ, Widmer WR, Paulos CM, Xu LC, Grote LA, et al. Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis. Arthritis Rheum. 2002;46(7):1947–55. doi: 10.1002/art.10405. [DOI] [PubMed] [Google Scholar]
- 34.Xia W, Hilgenbrink AR, Matteson EL, Lockwood MB, Cheng JX, Low PS. A functional folate receptor is induced during macrophage activation and can be used to target drugs to activated macrophages. Blood. 2009;113(2):438–46. doi: 10.1182/blood-2008-04-150789. [DOI] [PubMed] [Google Scholar]
- 35.van der Heijden JW, Oerlemans R, Dijkmans BA, Qi H, van der Laken CJ, Lems WF, et al. Folate receptor beta as a potential delivery route for novel folate antagonists to macrophages in the synovial tissue of rheumatoid arthritis patients. Arthritis Rheum. 2009;60(1):12–21. doi: 10.1002/art.24219. [DOI] [PubMed] [Google Scholar]
- 36.Nagayoshi R, Nagai T, Matsushita K, Sato K, Sunahara N, Matsuda T, et al. Effectiveness of anti-folate receptor beta antibody conjugated with truncated Pseudomonas exotoxin in the targeting of rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2005;52(9):2666–75. doi: 10.1002/art.21228. [DOI] [PubMed] [Google Scholar]
- 37.Nagai T, Tanaka M, Tsuneyoshi Y, Matsushita K, Sunahara N, Matsuda T, et al. In vitro and in vivo efficacy of a recombinant immunotoxin against folate receptor beta on the activation and proliferation of rheumatoid arthritis synovial cells. Arthritis Rheum. 2006;54(10):3126–34. doi: 10.1002/art.22082. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Murthy N. Targeted delivery of catalase and superoxide dismutase to macrophages using folate. Biochem Biophys Res Commun. 2007;360(1):275–9. doi: 10.1016/j.bbrc.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 39.Paulos CM, Varghese B, Widmer WR, Breur GJ, Vlashi E, Low PS. Folate-targeted immunotherapy effectively treats established adjuvant and collagen-induced arthritis. Arthritis Res Ther. 2006;8(3):R77. doi: 10.1186/ar1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206(3):549–59. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skoberne M, Beignon AS, Larsson M, Bhardwaj N. Apoptotic cells at the crossroads of tolerance and immunity. Curr Top Microbiol Immunol. 2005;289:259–92. doi: 10.1007/3-540-27320-4_12. [DOI] [PubMed] [Google Scholar]
- 42.Ju XS, Zenke M. Differentiation of human antigen-presenting dendritic cells from CD34+ hematopoietic stem cells in vitro. Methods Mol Biol. 2003;215:399–407. doi: 10.1385/1-59259-345-3:399. [DOI] [PubMed] [Google Scholar]
- 43.Miloud T, Hammerling GJ, Garbi N. Review of murine dendritic cells: types, location, and development. Methods Mol Biol. 2010;595:21–42. doi: 10.1007/978-1-60761-421-0_2. [DOI] [PubMed] [Google Scholar]
- 44.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, et al. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31(10):3026–37. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 45.Miossec P. Dynamic interactions between T cells and dendritic cells and their derived cytokines/chemokines in the rheumatoid synovium. Arthritis Res Ther. 2008;10 (Suppl 1):S2. doi: 10.1186/ar2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lebre MC, Jongbloed SL, Tas SW, Smeets TJM, McInnes IB, Tak PP. Rheumatoid Arthritis Synovium Contains Two Subsets of CD83-DC-LAMP-Dendritic Cells with Distinct Cytokine Profiles. Am J Pathol. 2008;172(4):940–50. doi: 10.2353/ajpath.2008.070703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takakubo Y, Takagi M, Maeda K, Tamaki Y, Sasaki A, Asano T, et al. Distribution of myeloid dendritic cells and plasmacytoid dendritic cells in the synovial tissues of rheumatoid arthritis. J Rheumatol. 2008;35(10):1919–31. [PubMed] [Google Scholar]
- 48.Wenink MH, Santegoets KC, Roelofs MF, Huijbens R, Koenen HJ, van Beek R, et al. The inhibitory Fc gamma IIb receptor dampens TLR4-mediated immune responses and is selectively up-regulated on dendritic cells from rheumatoid arthritis patients with quiescent disease. J Immunol. 2009;183(7):4509–20. doi: 10.4049/jimmunol.0900153. [DOI] [PubMed] [Google Scholar]
- 49.Baldwin HM, Ito-Ihara T, Isaacs JD, Hilkens CM. TNF{alpha} blockade impairs dendritic cell survival and function in rheumatoid arthritis. Ann Rheum Dis. 2009 doi: 10.1136/ard.2009.110502. [DOI] [PubMed] [Google Scholar]
- 50.Richez C, Schaeverbeke T, Dumoulin C, Dehais J, Moreau JF, Blanco P. Myeloid dendritic cells correlate with clinical response whereas plasmacytoid dendritic cells impact autoantibody development in rheumatoid arthritis patients treated with infliximab. Arthritis Res Ther. 2009;11(3):R100. doi: 10.1186/ar2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kavousanaki M, Makrigiannakis A, Boumpas D, Verginis P. Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy. Arthritis Rheum. 2010;62(1):53–63. doi: 10.1002/art.25037. [DOI] [PubMed] [Google Scholar]
- 52.Marti L, Golmia R, Golmia AP, Paes AT, Guilhen DD, Moreira-Filho CA, et al. Alterations in cytokine profile and dendritic cells subsets in peripheral blood of rheumatoid arthritis patients before and after biologic therapy. Ann N Y Acad Sci. 2009;1173:334–42. doi: 10.1111/j.1749-6632.2009.04740.x. [DOI] [PubMed] [Google Scholar]
- 53.Victoratos P, Kollias G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity. 2009;30(1):130–42. doi: 10.1016/j.immuni.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Jongbloed SL, Benson RA, Nickdel MB, Garside P, McInnes IB, Brewer JM. Plasmacytoid dendritic cells regulate breach of self-tolerance in autoimmune arthritis. J Immunol. 2009;182(2):963–8. doi: 10.4049/jimmunol.182.2.963. [DOI] [PubMed] [Google Scholar]
- 55.Ko HJ, Cho ML, Lee SY, Oh HJ, Heo YJ, Moon YM, et al. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34(2):111–20. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Jaen O, Rulle S, Bessis N, Zago A, Boissier MC, Falgarone G. Dendritic cells modulated by innate immunity improve collagen-induced arthritis and induce regulatory T cells in vivo. Immunology. 2009;126(1):35–44. doi: 10.1111/j.1365-2567.2008.02875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164(2):558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 58.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, et al. The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276(13):10229–33. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 59.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, et al. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195(1):99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang QQ, Sobkoviak R, Jockheck-Clark AR, Shi B, Mandelin AM, 2nd, Tak PP, et al. Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J Immunol. 2009;182(8):4965–73. doi: 10.4049/jimmunol.0801563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Janssens S, Beyaert R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem Sci. 2002;27(9):474–82. doi: 10.1016/s0968-0004(02)02145-x. [DOI] [PubMed] [Google Scholar]
- 62.Clark AR, Dean JL, Saklatvala J. Post-transcriptional regulation of gene expression by mitogen-activated protein kinase p38. FEBS Lett. 2003;546(1):37–44. doi: 10.1016/s0014-5793(03)00439-3. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301(5633):640–3. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 64.Iwahashi M, Yamamura M, Aita T, Okamoto A, Ueno A, Ogawa N, et al. Expression of Toll-like receptor 2 on CD16+ blood monocytes and synovial tissue macrophages in rheumatoid arthritis. Arthritis Rheum. 2004;50(5):1457–67. doi: 10.1002/art.20219. [DOI] [PubMed] [Google Scholar]
- 65.Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. 2004;50(12):3856–65. doi: 10.1002/art.20678. [DOI] [PubMed] [Google Scholar]
- 66.Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, et al. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162(4):1221–7. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roelofs MF, Joosten LA, Abdollahi-Roodsaz S, van Lieshout AW, Sprong T, van den Hoogen FH, et al. The expression of toll-like receptors 3 and 7 in rheumatoid arthritis synovium is increased and costimulation of toll-like receptors 3, 4, and 7/8 results in synergistic cytokine production by dendritic cells. Arthritis Rheum. 2005;52(8):2313–22. doi: 10.1002/art.21278. [DOI] [PubMed] [Google Scholar]
- 68.Ospelt C, Brentano F, Rengel Y, Stanczyk J, Kolling C, Tak PP, et al. Overexpression of toll-like receptors 3 and 4 in synovial tissue from patients with early rheumatoid arthritis: toll-like receptor expression in early and longstanding arthritis. Arthritis Rheum. 2008;58(12):3684–92. doi: 10.1002/art.24140. [DOI] [PubMed] [Google Scholar]
- 69.Kim KW, Cho ML, Lee SH, Oh HJ, Kang CM, Ju JH, et al. Human rheumatoid synovial fibroblasts promote osteoclastogenic activity by activating RANKL via TLR-2 and TLR-4 activation. Immunol Lett. 2007;110(1):54–64. doi: 10.1016/j.imlet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 70.Huang Q, Ma Y, Adebayo A, Pope RM. Increased macrophage activation mediated through toll-like receptors in rheumatoid arthritis. Arthritis Rheum. 2007;56(7):2192–201. doi: 10.1002/art.22707. [DOI] [PubMed] [Google Scholar]
- 71.Kyburz D, Rethage J, Seibl R, Lauener R, Gay RE, Carson DA, et al. Bacterial peptidoglycans but not CpG oligodeoxynucleotides activate synovial fibroblasts by toll-like receptor signaling. Arthritis Rheum. 2003;48(3):642–50. doi: 10.1002/art.10848. [DOI] [PubMed] [Google Scholar]
- 72.Jung YO, Cho ML, Kang CM, Jhun JY, Park JS, Oh HJ, et al. Toll-like receptor 2 and 4 combination engagement upregulate IL-15 synergistically in human rheumatoid synovial fibroblasts. Immunol Lett. 2007;109(1):21–7. doi: 10.1016/j.imlet.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Roelofs MF, Wenink MH, Brentano F, Abdollahi-Roodsaz S, Oppers-Walgreen B, Barrera P, et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA) Ann Rheum Dis. 2009;68(9):1486–93. doi: 10.1136/ard.2007.086421. [DOI] [PubMed] [Google Scholar]
- 74.Shahrara S, Huang Q, Mandelin AM, 2nd, Pope RM. TH-17 cells in rheumatoid arthritis. Arthritis Res Ther. 2008;10(4):R93. doi: 10.1186/ar2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat Immunol. 2009;10(4):340–7. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Abdollahi-Roodsaz S, Joosten LA, Koenders MI, Devesa I, Roelofs MF, Radstake TR, et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest. 2008;118(1):205–16. doi: 10.1172/JCI32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15(7):774–80. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 78.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TR, Matera G, Popa C, et al. Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 2007;56(9):2957–67. doi: 10.1002/art.22848. [DOI] [PubMed] [Google Scholar]
- 79.Tidswell M, Tillis W, Larosa SP, Lynn M, Wittek AE, Kao R, et al. Phase 2 trial of eritoran tetrasodium (E5564), a toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med. 2010;38(1):72–83. doi: 10.1097/CCM.0b013e3181b07b78. [DOI] [PubMed] [Google Scholar]
- 80.Vanags D, Williams B, Johnson B, Hall S, Nash P, Taylor A, et al. Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet. 2006;368(9538):855–63. doi: 10.1016/S0140-6736(06)69210-6. [DOI] [PubMed] [Google Scholar]
- 81.Tschopp J, Martinon F, Burns K. NALPs: a novel protein family involved in inflammation. Nat Rev Mol Cell Biol. 2003;4(2):95–104. doi: 10.1038/nrm1019. [DOI] [PubMed] [Google Scholar]
- 82.Joosten LA, Heinhuis B, Abdollahi-Roodsaz S, Ferwerda G, Lebourhis L, Philpott DJ, et al. Differential function of the NACHT-LRR (NLR) members Nod1 and Nod2 in arthritis. Proc Natl Acad Sci U S A. 2008;105(26):9017–22. doi: 10.1073/pnas.0710445105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ospelt C, Brentano F, Jungel A, Rengel Y, Kolling C, Michel BA, et al. Expression, regulation, and signaling of the pattern-recognition receptor nucleotide-binding oligomerization domain 2 in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2009;60(2):355–63. doi: 10.1002/art.24226. [DOI] [PubMed] [Google Scholar]
- 84.Martinon F, Burns K, Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-[beta] Molecular Cell. 2002;10(2):417–26. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 85.Stehlik C. Multiple interleukin-1beta-converting enzymes contribute to inflammatory arthritis. Arthritis Rheum. 2009;60(12):3524–30. doi: 10.1002/art.24961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201(9):1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci U S A. 2006;103(26):9982–7. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416–25. doi: 10.1056/NEJMoa0810787. [DOI] [PubMed] [Google Scholar]
- 89.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–41. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 90.Schaefer L. Extracellular matrix molecules: endogenous danger signals as new drug targets in kidney diseases. Curr Opin Pharmacol. 2009 doi: 10.1016/j.coph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 91.Rosengren S, Hoffman HM, Bugbee W, Boyle DL. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis. 2005;64(5):708–14. doi: 10.1136/ard.2004.025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolly L, Busso N, Palmer G, Talabot-Ayer D, Chobaz V, So A. Expression and function of the NALP3 inflammasome in rheumatoid synovium. Immunology. 2009 doi: 10.1111/j.1365-2567.2009.03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joosten LA, Netea MG, Fantuzzi G, Koenders MI, Helsen MM, Sparrer H, et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1beta. Arthritis Rheum. 2009;60(12):3651–62. doi: 10.1002/art.25006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guma M, Ronacher L, Liu-Bryan R, Takai S, Karin M, Corr M. Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum. 2009;60(12):3642–50. doi: 10.1002/art.24959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175(5):2851–8. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 96.Imaizumi T, Aratani S, Nakajima T, Carlson M, Matsumiya T, Tanji K, et al. Retinoic acid-inducible gene-I is induced in endothelial cells by LPS and regulates expression of COX-2. Biochem Biophys Res Commun. 2002;292(1):274–9. doi: 10.1006/bbrc.2002.6650. [DOI] [PubMed] [Google Scholar]
- 97.Imaizumi T, Yagihashi N, Hatakeyama M, Yamashita K, Ishikawa A, Taima K, et al. Expression of retinoic acid-inducible gene-I in vascular smooth muscle cells stimulated with interferon-gamma. Life Sci. 2004;75(10):1171–80. doi: 10.1016/j.lfs.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 98.Imaizumi T, Yagihashi N, Kubota K, Yoshida H, Sakaki H, Yagihashi S, et al. Expression of retinoic acid-inducible gene-I (RIG-I) in macrophages: possible involvement of RIG-I in atherosclerosis. J Atheroscler Thromb. 2007;14(2):51–5. doi: 10.5551/jat.14.51. [DOI] [PubMed] [Google Scholar]
- 99.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5(7):730–7. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki K, Imaizumi T, Tsugawa K, Ito E, Tanaka H. Expression of retinoic acid-inducible gene-I in lupus nephritis. Nephrol Dial Transplant. 2007;22(8):2407–9. doi: 10.1093/ndt/gfm175. [DOI] [PubMed] [Google Scholar]
- 101.Imaizumi T, Arikawa T, Sato T, Uesato R, Matsumiya T, Yoshida H, et al. Involvement of retinoic acid-inducible gene-I in inflammation of rheumatoid fibroblast-like synoviocytes. Clin Exp Immunol. 2008;153(2):240–4. doi: 10.1111/j.1365-2249.2008.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Imaizumi T, Matsumiya T, Yoshida H, Naraoka T, Uesato R, Ishibashi Y, et al. Tumor-necrosis factor-alpha induces retinoic acid-inducible gene-I in rheumatoid fibroblast-like synoviocytes. Immunol Lett. 2009;122(1):89–93. doi: 10.1016/j.imlet.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 103.Hietala MA, Jonsson IM, Tarkowski A, Kleinau S, Pekna M. Complement deficiency ameliorates collagen-induced arthritis in mice. J Immunol. 2002;169(1):454–9. doi: 10.4049/jimmunol.169.1.454. [DOI] [PubMed] [Google Scholar]
- 104.Banda NK, Thurman JM, Kraus D, Wood A, Carroll MC, Arend WP, et al. Alternative complement pathway activation is essential for inflammation and joint destruction in the passive transfer model of collagen-induced arthritis. J Immunol. 2006;177(3):1904–12. doi: 10.4049/jimmunol.177.3.1904. [DOI] [PubMed] [Google Scholar]
- 105.Blom AM, Nandakumar KS, Holmdahl R. C4b-binding protein (C4BP) inhibits development of experimental arthritis in mice. Ann Rheum Dis. 2009;68(1):136–42. doi: 10.1136/ard.2007.085753. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Kristan J, Hao L, Lenkoski CS, Shen Y, Matis LA. A role for complement in antibody-mediated inflammation: C5-deficient DBA/1 mice are resistant to collagen-induced arthritis. J Immunol. 2000;164(8):4340–7. doi: 10.4049/jimmunol.164.8.4340. [DOI] [PubMed] [Google Scholar]
- 107.Solomon S, Kolb C, Mohanty S, Jeisy-Walder E, Preyer R, Schollhorn V, et al. Transmission of antibody-induced arthritis is independent of complement component 4 (C4) and the complement receptors 1 and 2 (CD21/35) Eur J Immunol. 2002;32(3):644–51. doi: 10.1002/1521-4141(200203)32:3<644::AID-IMMU644>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 108.Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, et al. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–68. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 109.Aho K, Palosuo T, Raunio V, Puska P, Aromaa A, Salonen JT. When does rheumatoid disease start? Arthritis Rheum. 1985;28(5):485–9. doi: 10.1002/art.1780280503. [DOI] [PubMed] [Google Scholar]
- 110.Rantapaa-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 111.Mewar D, Wilson AG. Autoantibodies in rheumatoid arthritis: a review. Biomed Pharmacother. 2006;60(10):648–55. doi: 10.1016/j.biopha.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Moxley G, Ruddy S. Elevated C3 anaphylatoxin levels in synovial fluids from patients with rheumatoid arthritis. Arthritis Rheum. 1985;28(10):1089–95. doi: 10.1002/art.1780281003. [DOI] [PubMed] [Google Scholar]
- 113.Swaak AJ, Van Rooyen A, Planten O, Han H, Hattink O, Hack E. An analysis of the levels of complement components in the synovial fluid in rheumatic diseases. Clin Rheumatol. 1987;6(3):350–7. doi: 10.1007/BF02206833. [DOI] [PubMed] [Google Scholar]
- 114.Hogasen K, Mollnes TE, Harboe M, Gotze O, Hammer HB, Oppermann M. Terminal complement pathway activation and low lysis inhibitors in rheumatoid arthritis synovial fluid. J Rheumatol. 1995;22(1):24–8. [PubMed] [Google Scholar]
- 115.Jose PJ, Moss IK, Maini RN, Williams TJ. Measurement of the chemotactic complement fragment C5a in rheumatoid synovial fluids by radioimmunoassay: role of C5a in the acute inflammatory phase. Ann Rheum Dis. 1990;49(10):747–52. doi: 10.1136/ard.49.10.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crockard AD, Thompson JM, McBride SJ, Edgar JD, McNeill TA, Bell AL. Markers of inflammatory activation: upregulation of complement receptors CR1 and CR3 on synovial fluid neutrophils from patients with inflammatory joint disease. Clin Immunol Immunopathol. 1992;65(2):135–42. doi: 10.1016/0090-1229(92)90216-b. [DOI] [PubMed] [Google Scholar]
- 117.Corvetta A, Pomponio G, Rinaldi N, Luchetti MM, Di Loreto C, Stramazzotti D. Terminal complement complex in synovial tissue from patients affected by rheumatoid arthritis, osteoarthritis and acute joint trauma. Clin Exp Rheumatol. 1992;10(5):433–8. [PubMed] [Google Scholar]
- 118.Brodeur JP, Ruddy S, Schwartz LB, Moxley G. Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34(12):1531–7. doi: 10.1002/art.1780341209. [DOI] [PubMed] [Google Scholar]
- 119.Biro E, Nieuwland R, Tak PP, Pronk LM, Schaap MC, Sturk A, et al. Activated complement components and complement activator molecules on the surface of cell-derived microparticles in patients with rheumatoid arthritis and healthy individuals. Ann Rheum Dis. 2007;66(8):1085–92. doi: 10.1136/ard.2006.061309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sjoberg AP, Manderson GA, Morgelin M, Day AJ, Heinegard D, Blom AM. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46(5):830–9. doi: 10.1016/j.molimm.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wouters D, Voskuyl AE, Molenaar ET, Dijkmans BA, Hack CE. Evaluation of classical complement pathway activation in rheumatoid arthritis: measurement of C1q-C4 complexes as novel activation products. Arthritis Rheum. 2006;54(4):1143–50. doi: 10.1002/art.21729. [DOI] [PubMed] [Google Scholar]
- 122.Trouw LA, Haisma EM, Levarht EW, van der Woude D, Ioan-Facsinay A, Daha MR, et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum. 2009;60(7):1923–31. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 123.Yuan G, Wei J, Zhou J, Hu H, Tang Z, Zhang G. Expression of C5aR (CD88) of synoviocytes isolated from patients with rheumatoid arthritis and osteoarthritis. Chin Med J (Engl) 2003;116(9):1408–12. [PubMed] [Google Scholar]
- 124.Jahn B, Von Kempis J, Kramer KL, Filsinger S, Hansch GM. Interaction of the terminal complement components C5b-9 with synovial fibroblasts: binding to the membrane surface leads to increased levels in collagenase-specific mRNA. Immunology. 1993;78(2):329–34. [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci U S A. 1995;92(19):8955–9. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33(17–18):1389–401. doi: 10.1016/s0161-5890(96)00078-8. [DOI] [PubMed] [Google Scholar]
- 127.Tesser JKA, Fleischmann R, Mojcik C, Bombara M, Burch F. Safety and Efficacy of the Humanized Anti-C5 Antibody h5G1.1 in Patients with Rheumatoid Arthritis. Arthritis Rheum. 2001;44(9):S274. [Google Scholar]
- 128.Burch FTJ, Bell L, Kivitz A. Baseline C5b-9 Level Correlates With CRP and ACR 20 Response To The Humanized Anti-C5 Antibody h5G1.1 In Patients With Rheumatoid Arthritis. Arthritis Rheum. 2001;44(9):S214. [Google Scholar]
- 129.Krych-Goldberg M, Atkinson JP. Structure-function relationships of complement receptor type 1. Immunol Rev. 2001;180:112–22. doi: 10.1034/j.1600-065x.2001.1800110.x. [DOI] [PubMed] [Google Scholar]
- 130.Dreja H, Annenkov A, Chernajovsky Y. Soluble complement receptor 1 (CD35) delivered by retrovirally infected syngeneic cells or by naked DNA injection prevents the progression of collagen-induced arthritis. Arthritis Rheum. 2000;43(8):1698–709. doi: 10.1002/1529-0131(200008)43:8<1698::AID-ANR5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 131.Linton SM, Williams AS, Dodd I, Smith R, Williams BD, Morgan BP. Therapeutic efficacy of a novel membrane-targeted complement regulator in antigen-induced arthritis in the rat. Arthritis Rheum. 2000;43(11):2590–7. doi: 10.1002/1529-0131(200011)43:11<2590::AID-ANR29>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 132.Mizuno M. A review of current knowledge of the complement system and the therapeutic opportunities in inflammatory arthritis. Curr Med Chem. 2006;13(14):1707–17. doi: 10.2174/092986706777441959. [DOI] [PubMed] [Google Scholar]
- 133.Paul WE. Fundamental immunology. 6. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 134.Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, et al. Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med. 1999;189(1):187–94. doi: 10.1084/jem.189.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Corr M, Crain B. The role of FcgammaR signaling in the K/B × N serum transfer model of arthritis. J Immunol. 2002;169(11):6604–9. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 136.Kleinau S, Martinsson P, Heyman B. Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med. 2000;191(9):1611–6. doi: 10.1084/jem.191.9.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kagari T, Tanaka D, Doi H, Shimozato T. Essential role of Fc gamma receptors in anti-type II collagen antibody-induced arthritis. J Immunol. 2003;170(8):4318–24. doi: 10.4049/jimmunol.170.8.4318. [DOI] [PubMed] [Google Scholar]
- 138.Boross P, van Lent PL, Martin-Ramirez J, van der Kaa J, Mulder MH, Claassens JW, et al. Destructive arthritis in the absence of both FcgammaRI and FcgammaRIII. J Immunol. 2008;180(7):5083–91. doi: 10.4049/jimmunol.180.7.5083. [DOI] [PubMed] [Google Scholar]
- 139.Masuda M, Morimoto T, De Haas M, Nishimura N, Nakamoto K, Okuda K, et al. Increase of soluble FcgRIIIa derived from natural killer cells and macrophages in plasma from patients with rheumatoid arthritis. J Rheumatol. 2003;30(9):1911–7. [PubMed] [Google Scholar]
- 140.Wijngaarden S, van Roon JA, Bijlsma JW, van de Winkel JG, Lafeber FP. Fcgamma receptor expression levels on monocytes are elevated in rheumatoid arthritis patients with high erythrocyte sedimentation rate who do not use anti-rheumatic drugs. Rheumatology (Oxford) 2003;42(5):681–8. doi: 10.1093/rheumatology/keg174. [DOI] [PubMed] [Google Scholar]
- 141.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, et al. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58(3):678–88. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 142.Mathsson L, Lampa J, Mullazehi M, Ronnelid J. Immune complexes from rheumatoid arthritis synovial fluid induce FcgammaRIIa dependent and rheumatoid factor correlated production of tumour necrosis factor-alpha by peripheral blood mononuclear cells. Arthritis Res Ther. 2006;8(3):R64. doi: 10.1186/ar1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blom AB, Radstake TR, Holthuysen AE, Sloetjes AW, Pesman GJ, Sweep FG, et al. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 2003;48(4):1002–14. doi: 10.1002/art.10871. [DOI] [PubMed] [Google Scholar]
- 144.Wijngaarden S, van de Winkel JG, Jacobs KM, Bijlsma JW, Lafeber FP, van Roon JA. A shift in the balance of inhibitory and activating Fcgamma receptors on monocytes toward the inhibitory Fcgamma receptor IIb is associated with prevention of monocyte activation in rheumatoid arthritis. Arthritis Rheum. 2004;50(12):3878–87. doi: 10.1002/art.20672. [DOI] [PubMed] [Google Scholar]
- 145.Magnusson SE, Engstrom M, Jacob U, Ulfgren AK, Kleinau S. High synovial expression of the inhibitory FcgammaRIIb in rheumatoid arthritis. Arthritis Res Ther. 2007;9(3):R51. doi: 10.1186/ar2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Du Clos TW. C-reactive protein as a regulator of autoimmunity and inflammation. Arthritis Rheum. 2003;48(6):1475–7. doi: 10.1002/art.11025. [DOI] [PubMed] [Google Scholar]
- 147.Bharadwaj D, Stein MP, Volzer M, Mold C, Du Clos TW. The major receptor for C-reactive protein on leukocytes is fcgamma receptor II. J Exp Med. 1999;190(4):585–90. doi: 10.1084/jem.190.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 149.Magnusson SE, Andren M, Nilsson KE, Sondermann P, Jacob U, Kleinau S. Amelioration of collagen-induced arthritis by human recombinant soluble FcgammaRIIb. Clin Immunol. 2008;127(2):225–33. doi: 10.1016/j.clim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 150.Wijngaarden S, van Roon JA, van de Winkel JG, Bijlsma JW, Lafeber FP. Down-regulation of activating Fcgamma receptors on monocytes of patients with rheumatoid arthritis upon methotrexate treatment. Rheumatology (Oxford) 2005;44(6):729–34. doi: 10.1093/rheumatology/keh583. [DOI] [PubMed] [Google Scholar]
- 151.Tutuncu Z, Kavanaugh A, Zvaifler N, Corr M, Deutsch R, Boyle D. Fcgamma receptor type IIIA polymorphisms influence treatment outcomes in patients with inflammatory arthritis treated with tumor necrosis factor alpha-blocking agents. Arthritis Rheum. 2005;52(9):2693–6. doi: 10.1002/art.21266. [DOI] [PubMed] [Google Scholar]
- 152.Canete JD, Suarez B, Hernandez MV, Sanmarti R, Rego I, Celis R, et al. Influence of variants of Fc gamma receptors IIA and IIIA on the American College of Rheumatology and European League Against Rheumatism responses to anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68(10):1547–52. doi: 10.1136/ard.2008.096982. [DOI] [PubMed] [Google Scholar]
- 153.van Roon JA, van Vuuren AJ, Wijngaarden S, Jacobs KM, Bijlsma JW, Lafeber FP, et al. Selective elimination of synovial inflammatory macrophages in rheumatoid arthritis by an Fcgamma receptor I-directed immunotoxin. Arthritis Rheum. 2003;48(5):1229–38. doi: 10.1002/art.10940. [DOI] [PubMed] [Google Scholar]
- 154.van Roon JA, Bijlsma JW, van de Winkel JG, Lafeber FP. Depletion of synovial macrophages in rheumatoid arthritis by an anti-FcgammaRI-calicheamicin immunoconjugate. Ann Rheum Dis. 2005;64(6):865–70. doi: 10.1136/ard.2004.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pine PR, Chang B, Schoettler N, Banquerigo ML, Wang S, Lau A, et al. Inflammation and bone erosion are suppressed in models of rheumatoid arthritis following treatment with a novel Syk inhibitor. Clin Immunol. 2007;124(3):244–57. doi: 10.1016/j.clim.2007.03.543. [DOI] [PubMed] [Google Scholar]
- 156.Weinblatt ME, Kavanaugh A, Burgos-Vargas R, Dikranian AH, Medrano-Ramirez G, Morales-Torres JL, et al. Treatment of rheumatoid arthritis with a Syk kinase inhibitor: a twelve-week, randomized, placebo-controlled trial. Arthritis Rheum. 2008;58(11):3309–18. doi: 10.1002/art.23992. [DOI] [PubMed] [Google Scholar]