Abstract
To determine the role of Ca2+ signaling in activation of the Mitogen-Activated Protein Kinase (MAPK) pathway, we subjected MC3T3-E1 pre-osteoblastic cells to inhibitors of Ca2+ signaling during application of fluid shear stress (FSS). FSS only activated ERK1/2, rapidly inducing phosphorylation within 5 minutes of the onset of shear. Phosphorylation of ERK1/2 (pERK1/2) was significantly reduced when Ca2+i was chelated with BAPTA or when Ca2+ was removed from the flow media. Inhibition of both the L-type voltage-sensitive Ca2+ channel and the mechanosensitive cation-selective channel blocked FSS-induced pERK1/2. Inhibition of phospholipase C with U73122 significantly reduced pERK1/2. This inhibition did not result from block of intracellular Ca2+ release, but a loss of PKC activation. Recent data suggests a role of ATP release and purinergic receptor activation in mechanotransduction. Apyrase-mediated hydrolysis of extracellular ATP completely blocked FSS-induced phosphorylation of ERK1/2, while addition of exogenous ATP to static cells mimicked the effects of FSS on pERK1/2. Two P2 receptors, P2Y2 and P2X7, have been associated with the anabolic responses of bone to mechanical loading. Using both iRNA techniques and primary osteoblasts isolated from P2X7 knockout mice, we found that the P2X7, but not the P2Y2, purinergic receptor was involved in ERK1/2 activation under FSS. These data suggest that FSS-induced ERK1/2 phosphorylation requires Ca2+-dependent ATP release, however both increased Ca2+i and PKC activation are needed for complete activation. Further, this ATP-dependent ERK1/2 phosphorylation is mediated through P2X7, but not P2Y2, purinergic receptors.
Keywords: Osteoblast, Purinergic, ATP, Calcium channel, Fluid shear stress
INTRODUCTION
Skeletal structure and strength is dependent on the physical strains placed upon bone. Removal of mechanical stimuli during immobilization or in microgravity results in a rapid loss of bone mass, whereas application of exogenous mechanical loading leads to increased bone formation in the modeling skeleton (for review, see [1]). Various means of mechanically stimulating bone cells in vitro have been developed to simulate forces incurred in the skeleton. While none of these loading models completely replicate the stresses endured by bone cells in vivo, application of fluid shear produces many of the cellular responses considered to be anabolic in osteoblasts including release of prostaglandins [2, 3], nitric oxide [4] and increased expression of cyclooxygenase-2 (COX-2) [5–7].
To begin to understand how osteoblasts perceive and respond to mechanical stimulation, a time course of cellular events must be considered. One of the earliest recorded responses of osteoblasts to either fluid shear or strain is a rapid increase in intracellular Ca2+ [8, 9] that is dependent on both extracellular and intracellular Ca2+ pools [10]. While we have demonstrated that changes in gene expression in response to fluid shear in osteoblasts is dependent on IP3-mediated Ca2+ release, several studies have suggested a role of Ca2+ entry through ion channels. Numerous ion channels have been characterized in osteoblasts (for review, see [11]) and two Ca2+-conducting channels have been linked to mechanotransduction in bone cells: the L-type voltage-sensitive Ca2+ channel (L-VSCC) and the mechanosensitive cation-selective channel (MSCC). Inhibition of the MSCC during mechanical stimulation has been shown to reduce the release of PGE2 in osteocytes [12], TGF-β1 in osteoblastic cells [13] and NO in ex vivo organ cultures [14]. The L-VSCC has been shown to control development and growth of bone [15] and to regulate proliferation of osteoblasts [16]. We have also shown that inhibition of this channel significantly reduces mechanically-induced bone formation in rats and mice [17].
Following the increase in Ca2+i, mechanical stimulation activates various cellular signaling pathways, including Mitogen-Activated Protein Kinase (MAPK). MAPK is a family of kinases consisting of Extracellular signal-Regulated Kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 kinase. Each of these kinases is activated by dual phosphorylation on threonine and tyrosine residues [18]. These members of the MAPK family have been implicated in the regulation of cellular growth, differentiation and apoptosis in numerous cell types [19–22] including osteoblasts [23, 24]. Several studies have shown that ERK1/2 is activated by fluid shear in osteoblasts [7, 25, 26]. Studies have also shown that Ca2+i is important to ERK1/2 activation in osteoblasts [24, 27], although it is unclear whether this Ca2+i-induced activation results from extracellular Ca2+ entry or intracellular Ca2+ release.
We have recently shown that ATP is rapidly released from MC3T3-E1 pre-osteoblastic cells within 1 minute of the onset of fluid shear [28]. This shear-induced ATP release was dependent on the Ca2+ entry through both L-VSCC and MSCC. Extracellular ATP binds to two classes of purinergic receptors: P2X receptors, which are ligand-gated ion channels, and P2Y receptors, which are G-protein coupled receptors [29]. Two isoforms of P2 receptors, P2Y2 [30] and P2X7 [31], have been associated with osteoblast activation and the anabolic response of bone to mechanical loading. However, little is known about how ATP release and P2 receptor activation regulates skeletal integrity and mechanically-induced responses in osteoblasts.
In this study, we examined the role of Ca2+i and ATP release on the activation of ERK1/2 in response to fluid shear in MC3T3-E1 pre-osteoblastic cells. We find that extracellular Ca2+ entry through both the MSCC and L-VSCC, but not Ca2+i release, was essential for the activation of ERK1/2. We further demonstrate that protein kinase C activation contributes to the Ca2+-dependent phosphorylation of ERK1/2. We also show that this activation is dependent on ATP release and that activation of the P2X7 receptor is at least partially responsible for phosphorylation of ERK1/2.
MATERIALS AND METHODS
Cell culture
The pre-osteoblastic cell line, MC3T3-E1 (passage 10–20), were cultured in α-Minimal Essential Medium (α-MEM; Sigma Chemical, St. Louis, MO, USA) containing 10% fetal bovine serum (FBS; Atlanta Biologicals, Norcross, GA), 100U/ml penicillin G (Sigma) and 100μg/ml streptomycin (Sigma). Mother cultures were maintained in a 95% air/5% CO2 humidified incubator at 37°C and subcultured every 72 hours. Primary calvarial osteoblasts from 3–5 day old neonatal WT and P2X7 null mice were harvested as previously described [32]. In brief, calvariae halves, excluding sutures, were surgically dissected and subjected to seven sequential 15-minute digestions with 1.5 U/ml collagenase P (Roche Molecular Biochemicals, Penzberg) in 0.05% trypsin/1 mM EDTA (Gibco) at room temperature, on a rocking platform. The first two digests were discarded, and the third to fifth digests of cells were pooled and centrifuged at 2000 rpm for 10 minutes. The cells were re-suspended in α-MEM, passed through 40 μm cell strainer (Falcon, Becton Dickinson, Franklin Lakes, NJ), and regularly cultured in α-MEM.
Fluid shear stress (FSS) studies
For fluid shear experiments, either MC3T3-E1 cells or primary osteoblasts were seeded onto type I collagen-coated (10μg/cm2, BD Biosciences, Bedford, MA) 75 × 38 mm2 glass slides (Fisher Scientific, Pittsburgh, PA) at a density of 2000 cells/cm2. When the glass slides reached 90% confluency (2–3 days), the cells were serum-starved for 24 hours in 0.2% FBS supplemented α-MEM prior to flow. Fluid flow was applied to the cell monolayer in a parallel plate flow chamber using a closed flow loop, as previously described [33]. This system uses a constant hydrostatic pressure head to drive media through the flow chamber, subjecting the cell monolayer to steady laminar flow and producing a well-defined FSS of 12 dynes/cm2. The entire apparatus was maintained at 37°C and the medium was aerated with 95% air/5% CO2 during experiment. In time-course studies, the cells were subjected to FSS for 0, 5, 15, 30 or 60 minutes. For the remaining experiments in this study, MC3T3-E1 cells were sheared for 30 minutes only.
Pharmacological Agents
To determine the role of Ca2+i or extracellular Ca2+ entry on pERK1/2 during shear, either 1,2-Bis(2-amino-phenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA-AM; 30μM) or Ca2+-free media (JRH Biosciences, Lenexa, KS) with an addition of 2mM EGTA was used, respectively. The role of the L-VSCC or MSCC was determined by treating the cells 30 min prior to and during shear with two L-VSCC blockers—nifedipine (5μM) or verapamil (5μM)—or with the non-specific MSCC blocker, GdCl3 (10μM). Phospholipase C (PLC) and intracellular Ca2+ release were inhibited with the selective PLC inhibitor, U73122 (10μM), or thapsigargin (1 μM), respectively. To inhibit protein kinase C (PKC), the PKC specific blocker, GF109203X (1 μM, Biomol, Plymouth Meeting, PA), was used. Each of these agents were added to the cells 30 min prior to application of FSS and maintained in the flow medium for the duration of the experiment. Exogenous ATP (10μM) was added to static, or non-sheared, cells. Extracellular ATP was hydrolyzed with apyrase (5U/ml) which was maintained in the flow medium for the duration of the experiment. All reagents were purchased from Sigma Chemical (Sigma, St. Louis, MO) unless otherwise indicated.
iRNA design and transfection
MC3T3-E1 cells were transfected with P2Y2 iRNA that was designed using software provided by Ambion (Austin, Texas) and synthesized by Dharmacon (Lafayette, CO). The sequence of the sense and antisense dsRNA of P2Y2 was 5′-AGATATAGAGAGCCACGACGCCTGTCTC-3′ (sense) and 5′-AACGTCGTGGCTCTCTATATCCCTGTCTC-3′ (anti-sense) (Gene Bank accession no. BC006613). Scrambled iRNA was obtained from Dharmacon (Lafayette, CO, USA) and used as control. To maintain identical culture conditions as used in flow experiments, cells were seeded on 75 × 38 mm2 glass slides at a density of 2–5 × 104 per slide and cultured in α-MEM supplemented with 10% FBS without antibiotics. Once the slides reached a confluency of 30–35%, the cells were rinsed with OptiMEM once, and the cell monolayer was covered with 1ml freshly prepared transfection mixture containing iRNA (50nM) and oligofectamine (0.2%, v/v) in OptiMEM for 12 hours. Afterwards, 12ml of fresh α-MEM medium with 10% FBS was added to cover the cell monolayer to rescue the cells from transfection. The cells were then lysed at a sequential time points (24, 48, 72, and 96 hrs) to determine the magnitude and duration of P2Y2 suppression. When used for flow experiments, the transfected cells were cultured for 24 hours after transfection, followed by serum starving for 24 hours prior to subjecting the cells to FSS. For FSS experiments, transfected cells were divided into 4 groups; 1) iRNA + flow, 2) iRNA static control, 3) Scrambled iRNA + flow, and 4) Scrambled iRNA static control.
Western blot analysis
The cells were washed quickly with cold PBS (1X), lysed with 2X sample buffer on ice and immediately boiled for 5 minutes. The lysis buffer contained 5mM HEPES (pH 7.9), 150mM NaCl, 26% glycerol (v/v), 1.5mM MgCl2, 0.2mM EDTA, 0.5mM dithiothreitol and 0.5mM phenylmethylsulfonyl fluoride. Before separation, the protein samples were centrifuged at 14,000g for 10 minutes at room temperature to remove any cellular debris. Twenty micrograms of whole cell lysate and a pre-stained molecular weight marker (Bio-RAD Laboratories, Hercules, CA) were boiled for 5 minutes, separated by 10% SDS-polyacrylamide gel electrophoresis and electrotransferred to a nitrocellulose membrane. Membranes were blocked in Tris-buffered saline containing 5% nonfat dry milk and 0.1% Tween-20 (TBST) and incubated with 1 μg/ml (1:1000) rabbit anti-ERK1/2, mouse anti-pERK1/2, rabbit anti-pJNK, rabbit anti-pp38 (Biosource International, Camarillo, CA) or mouse anti-vinculin antibodies overnight at 4°C. The antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) unless otherwise indicated. Following three washes in TBST, the membranes were incubated with goat anti-rabbit or goat anti-mouse IgG hydroperoxidase conjugated secondary antibodies (1:5000) for 1 hour at room temperature. Immunodetection was determined using the enhanced chemiluminescence (ECL) method. Densitometry measurement was made by using Fuji Imaging software. For quantification, densitometries of pERK1/2 gel bands were normalized to that of total ERK1/2.
Statistical analysis
In this study, each single experiment was repeated for at least 3 times on three different passages of MC3T3-E1 or three different primary cell isolations. Densitometries of gel bands were presented as mean ± SD in graphs. Differences between the means were statistically analyzed using two-way ANOVA, and the significance was considered when p values were less than 0.05.
RESULTS
Fluid shear stress induces phosphorylation of ERK1/2 in MC3T3-E1 pre-osteoblasts
Several studies have indicated that MAPK is activated in osteoblasts in response to mechanical stimulation, however which of the MAPK isoforms responds to loading is controversial [7, 24]. We examined changes in the phosphorylation of the three members of the MAPK family to determine which isoform is phosphorylated in MC3T3-E1 osteoblasts in response to FSS. MC3T3-E1 cells were subjected to 12 dynes/cm2 continuous, laminar flow for 0, 5, 15, 30, 60 minutes. Of the three MAPK isoforms, only ERK1/2 was significantly phosphorylated (pERK1/2) within 5 minutes after the onset of flow, and phosphorylation peaked at 30 min (Figure 1). Phosphorylation of p38 (pp38) was not altered by FSS and phosphorylated JNK1/2 (pJNK1/2) was not detectable. Thus, we focused the remainder of this study on phosphorylation of ERK1/2.
Figure 1. FSS-induced phosphorylation of MAPK isoforms in MC3T3-E1.
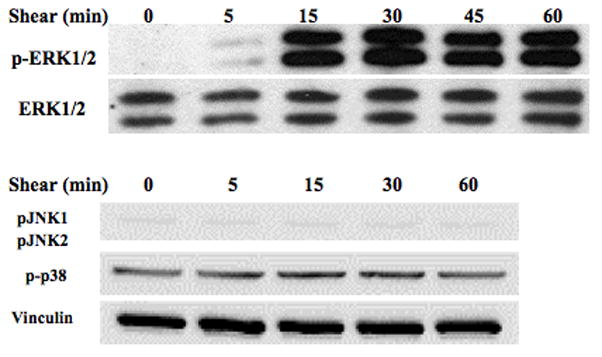
MC3T3-E1 pre-osteoblasts were subjected to 12 dynes/cm2 FSS for 0, 5, 15, 30 or 60 minutes. This representative western blot shows an increase in ERK1/2 phosphorylation within 5 min of the onset of shear, whereas JNK and p38 were not mechanoresponsive.
Ca2+ entry through MSCC and L-VSCC is essential for the FSS-induced phosphorylation of ERK1/2
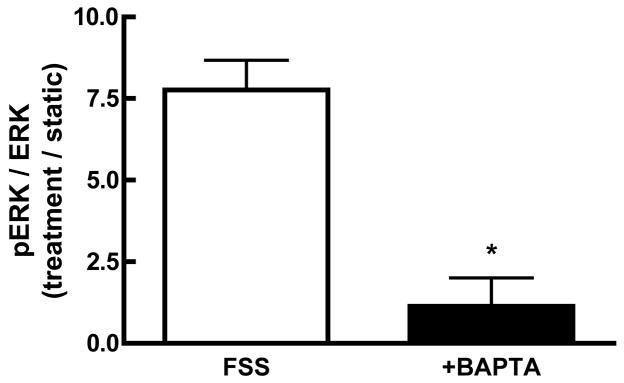
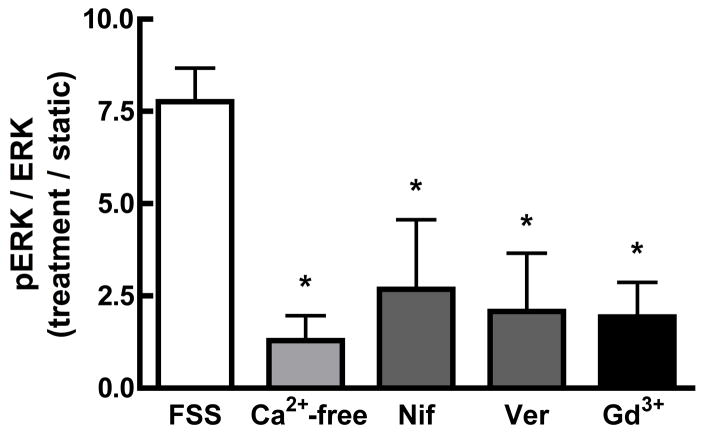
Initial studies were performed to examine the role of Ca2+i mobilization on the observed FSS-induced ERK1/2 phosphorylation. Chelation of Ca2+i with BAPTA significantly attenuated the phosphorylation of ERK1/2 in response to shear (Figure 2A), indicating that alterations in Ca2+I are required for FSS-induced ERK phosphorylation. Hung et al. demonstrated that extracellular Ca2+ entry through ion channels was essential for the shear-induced increase in Ca2+i [10]. In order to determine whether Ca2+ entry was required for ERK1/2 phosphorylation, cells were sheared in the presence of Ca2+-free media supplemented with 2mM EGTA. In contrast to cells sheared in the presence of Ca2+-containing αMEM, the use of Ca2+-free media abolished FSS-induced ERK1/2 phosphorylation (Figure 2B). To determine which ion channel was responsible for Ca2+ entry, MC3T3-E1 cells were treated with different Ca2+ channel inhibitors for 30 min prior to, and during, application of FSS. Inhibition of either the MSCC with GdCl3 (10 μM) or the L-VSCC with nifedipine (5 μM) reduced ERK1/2 phosphorylation in response to FSS by approximately 75% (Figure 2B). Similar attenuation of pERK1/2 levels in response to FSS were observed using another L-type VSCC antagonist, verapamil (5 μM). The combination of gadolinium and nifedipine did not significantly reduce FSS-induced ERK1/2 phosphorylation below that of each inhibitor alone (data not shown). These data indicate that Ca2+ entry through both the MSCC and the L-VSCC is essential for the FSS-induced ERK1/2 phosphorylation.
Figure 2. FSS-induced ERK1/2 phosphorylation requires increases in Ca2+i entry through the MSCC and L-type VSCC.
A. MC3T3-E1 osteoblasts were treated with the Ca2+i-chelating agent BAPTA (30μM) for 30 minutes prior to FSS. Addition of BAPTA significantly attenuated FSS-induced ERK1/2 phosphorylation. If Ca2+ was removed from the shear medium, ERK1/2 phosphorylation induced by shear was significantly decreased; similar to BAPTA pretreatment. B. Pretreatment of MC3T3-E1 cells with either the MSCC inhibitor, gadolinium (10μM), or the L-VSCC inhibitors, nifedipine (5μM) for 30 min significantly inhibited ERK1/2 phosphorylation by 50–75%. Values were expressed as mean ± S.D. (n=3). * p<0.05; **p<0.01) when comparing FSS groups to static controls.
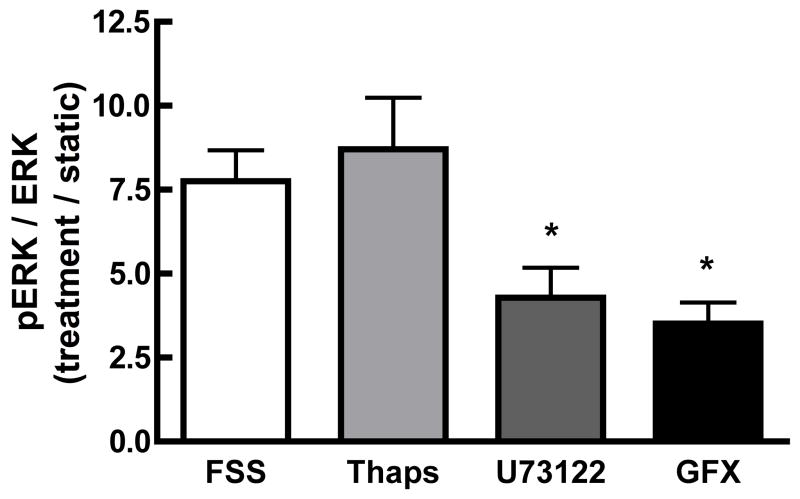
PLC/PKC signaling, but not intracellular Ca2+ release, contributes to the FSS-induced phosphorylation of ERK1/2
The Ca2+i response to shear is also dependent on intracellular Ca2+ release [10]. Since intracellular Ca2+ release is mediated by activation of the PLC-IP3 pathway in osteoblasts, we examined the effect of PLC inhibition with U73122 (10 μM) on FSS-induced pERK activation. U73122 significantly inhibited the FSS-induced ERK1/2 activation by approximately 50%. (Figure 3). Because PLC activates both IP3-medated intracellular Ca2+ release and diacylglycerol-dependent PKC activation, intracellular calcium stores were depleted with thapsigargin (1 μM), as previously used [5]. FSS-induced ERK1/2 phosphorylation was unaffected by loss of intracellular Ca2+ release (Figure 3). However, pre-treatment with the general PKC inhibitor, GF109203X (1 μM), significantly inhibited the FSS-induced pERK1/2 by 55%, mimicking the effect of U73122 (Figure 3). These data indicate that PKC activation, but not intracellular Ca2+ release, is required for maximal ERK1/2 phosphorylation in response to FSS.
Figure 3. PLC activation of PKC, but not intracellular Ca2+ release, is required for maximal shear-induced ERK1/2 phosphorylation.
MC3T3-E1 osteoblasts were treated with the PLC antagonist U73122 (10μM) or the general PKC antagonist GF109203X (1 μM) for 30 minutes prior to and during shear. To determine the role of intracellular Ca2+ release, XX μM thapsigargin was added 1 hour prior to shear. Inhibition of PLC with U73122 or PKC with GF109203X significantly attenuated pERK1/2 to approximately 50% of sheared controls, while thapsigargin had no effect on shear-induced ERK1/2 phosphorylation. Values were expressed as mean ± SD (n=5). * p<0.05; ** p<0.01 when compared to sheared controls.
Purinergic signaling mediates the effect of FSS on ERK1/2 phosphorylation
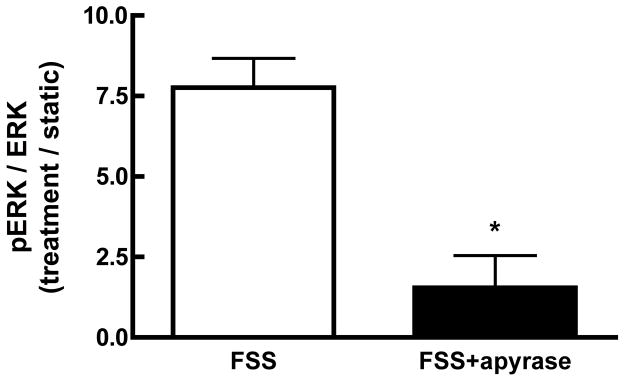
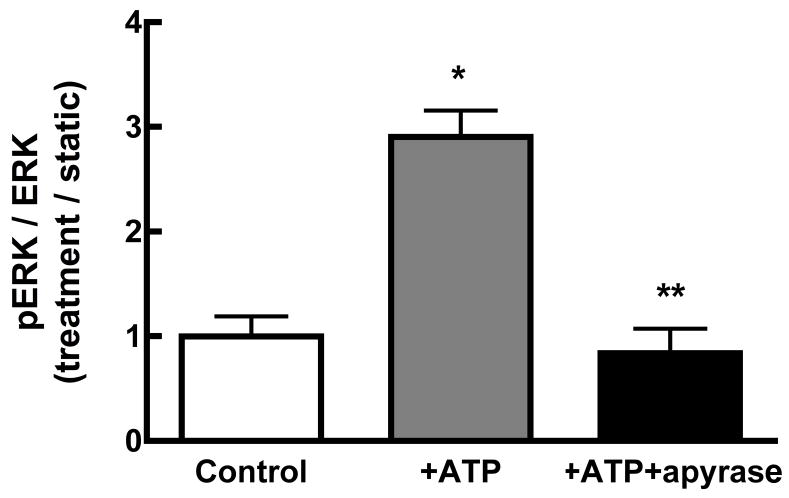
Fluid shear induces Ca2+-dependent ATP release from MC3T3-E1 osteoblasts within 1 min of the onset of shear [28]. Since we demonstrated that ERK1/2 activation is Ca2+-dependent, we sought to define the interaction of purinergic receptor activation on ERK1/2 phosphorylation in response to fluid shear. FSS-induced ERK1/2 phosphorylation was significantly attenuated when extracellular ATP was hydrolyzed with apyrase (5 U/ml) (Figure 4A). Similar results were obtained for cells treated with the non-specific purinoceptor antagonist suramin (100μM; data not shown). The addition of exogenous ATP (10 μM) to static osteoblasts mimicked the effects of FSS, inducing ERK1/2 phosphorylation within 5 min of addition (Figure 4B). Hydrolyzing exogenous ATP with apyrase (5U/ml) effectively blocked ERK1/2 phosphorylation.
Figure 4. Purinergic signaling mediates FSS-induced ERK1/2 phosphorylation.
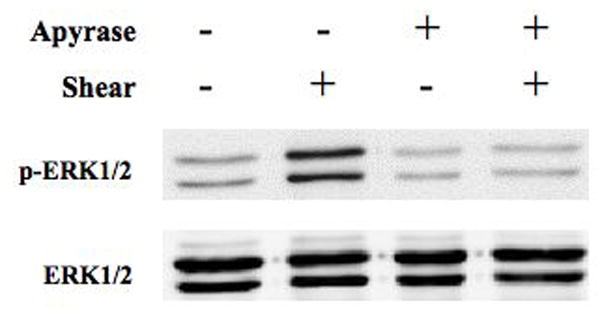
A. MC3T3-E1 cells were sheared for 30 min at 12 dynes/cm2 FSS in the presence of apyrase (5U/ml). Hydrolysis of extracellular ATP significantly inhibited the phosphorylation of ERK1/2 in response to shear. B. Static MC3T3-E1 cells were treated with exogenous ATP (10μM) or ATP + apyrase for 5 min. ATP increased phosphorylation of ERK1/2 within 5 min, but co-addition of apyrase prevented ERK1/2 phosphorylation.
FSS-induced ERK1/2 phosphorylation involves P2X7 receptor activation in MC3T3-E1 osteoblasts
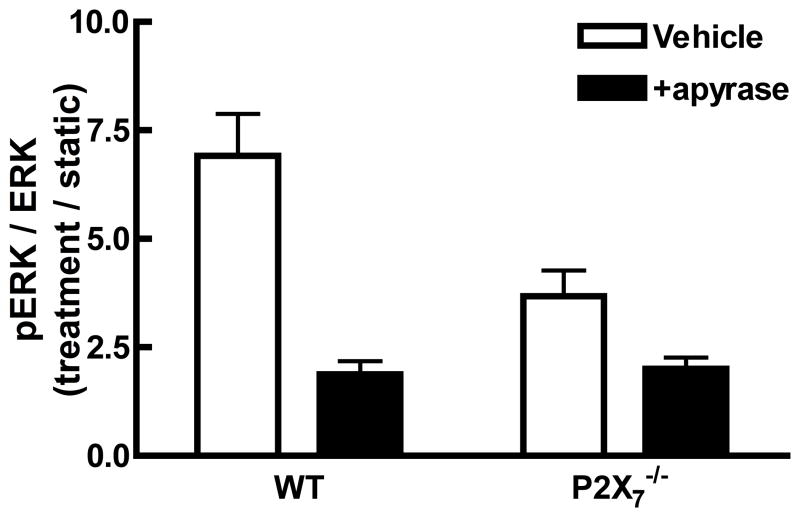
Knockout of the P2X7 receptor results in a skeletal phenotype similar to the effects of disuse [31] and significantly reduces the mechanosensitivity of the skeleton [34]. Primary osteoblasts isolated from P2X7 knockout mice demonstrated a significant reduction in ERK phosphorylation in response to shear when compared to WT primary osteoblasts (Figure 5A). Interestingly, the addition of apyrase to P2X7 KO osteoblasts further suppressed FSS-induced ERK1/2 phosphorylation in P2X7−/− osteoblasts (Figure 5A), suggesting that other P2 receptors may be involved in this response.
Figure 5. Maximal purinergic regulation of ERK1/2 phosphorylation requires the P2X7 receptor.
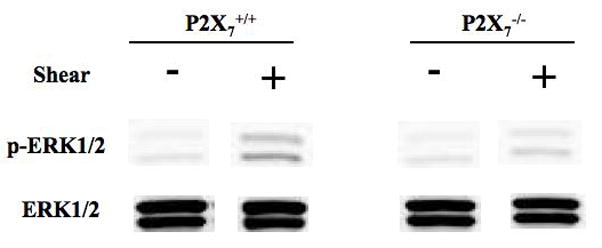

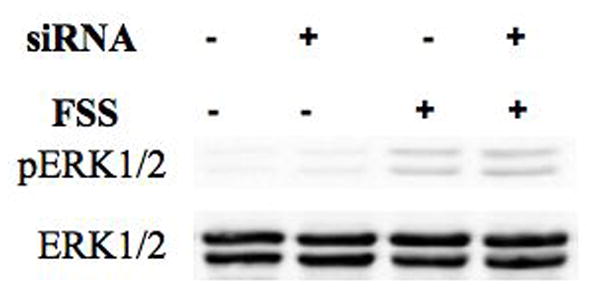
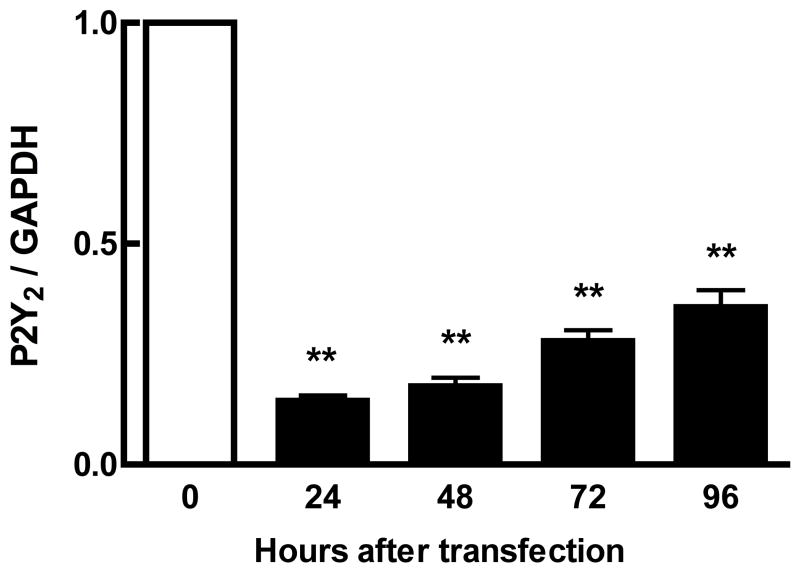
A. Primary osteoblasts, isolated from wild-type or P2X7−/− mice, were subjected to fluid shear and ERK1/2 phosphorylation was assessed as above. ERK1/2 phosphorylation was attenuated 50% in P2X7−/− osteoblasts compared to wild-type primary osteoblasts. Addition of apyrase (5U/ml) to flow media abrogated ERK1/2 phosphorylation in response to shear. B. MC3T3-E1 cells were transfected with P2Y2 iRNA (50nM) for 12 hours, followed by post-incubation for 24, 48, 72 or 96 additional hours. Western analysis indicates the magnitude and duration of P2Y2 receptor suppression. C. Fluid shear stress elicited similar increases in ERK1/2 phosphorylation in both scramble and P2Y2-iRNA-transfected cells in response to fluid shear. Values are presented as mean ± S.D. (n=3). * p<0.05, **p<0.01 compared to WT FSS; +p<0.05 compared to sheared P2X7−/− osteoblasts without apyrase.
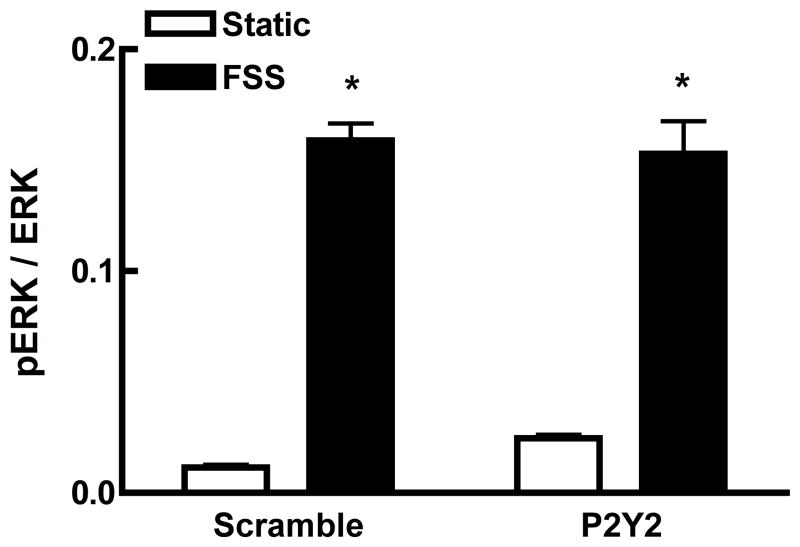
Previously, the P2Y2 receptor has been shown to be important in controlling the Ca2+i response to oscillating FSS in MC3T3-E1 cells [30]. iRNA was employed to suppress P2Y2 expression in order to examine its role in FSS-induced ERK1/2 phosphorylation. iRNA exhibited 80% suppression of the receptor protein within 24 hours of transfection, with significant suppression lasting more than 96 hours (Figure 5B). Cells were subjected to fluid shear 48 hr after transfection, a time at which P2Y2 expression was maximally suppressed. We observed no difference in shear-induced ERK1/2 phosphorylation between cells receiving either scrambled or P2Y2 iRNA (Figure 5C).
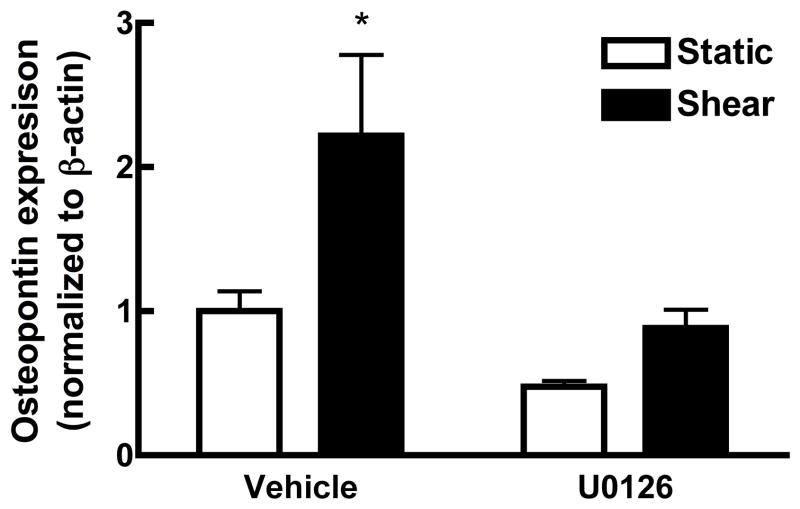
FSS-induced ERK1/2 activation is required for osteopontin expression
Previous studies have determined that activation of MAPK is essential to expression and production of osteopontin, an extracellular matrix protein associated with osteogenesis (31). Application of FSS to MC3T3-E1 osteoblasts induces a 2-fold increase in osteopontin production 6 hours after the onset of shear. To confirm that phosphorylation of ERK1/2 is required for this increase in osteopontin, MC3T3-E1 cells were treated with U0126 for 1 hr prior to application of shear (Figure 6). Inhibition of ERK1/2 by U0126 completely blocked the FSS-induced increase in osteopontin.
Figure 6. ERK1/2 phosphorylation is required for increased osteopontin (OPN) production in response to shear.
U0126 (20μM), an inhibitor of ERK, was added to MC3T3-E1 osteoblasts 1 hr prior to application of fluid shear. Western analysis indicates that inhibition of ERK phosphorylation completely abrogated the increase in OPN production in response to FSS. (n=3; * p<0.05).
DISCUSSION
Although the anabolic effects of mechanical loading on bone have been well documented, how osteogenic cells perceive a mechanical stimulus and translate it into biochemical signals still remains unclear. We have postulated that Ca2+ signals arising from mechanical activation of plasma membrane ion channels play a significant role in mechanotransduction. These studies indicate that Ca2+ entry through the MSCC and L-VSCC stimulates the phosphorylation of ERK1/2 through activation of purinergic receptors. While we have shown that knockout of the P2X7 receptor significantly reduces ERK1/2 phosphorylation in response to FSS, this response was not ablated, suggesting that other possible mechanisms or other purinergic receptors are involved in the activation of ERK1/2 in response to mechanical stimulation.
MAPK is a family of protein kinases consisting of three isoforms: ERK1/2, JNK and p38 [18]. These kinases have been shown to be important in the proliferation, growth, differentiation in many cell types [19–22] including osteoblasts [23, 24]. Activation of MAPK has been shown to be important in shear-induced increases in OPN [35] and COX-2 mRNA expression [7] and cell proliferation [26]. In the present study, we found that only ERK1/2 was activated by fluid shear, becoming phosphorylated within 5 minutes of the onset of flow with peak activation observed after 30 minutes. This observation is consistent with previous studies [24, 26], however others have shown that additional MAPK isoforms are sensitive to mechanical stimulation. You et al. [35] reported that both ERK1/2 and p38 were activated in MC3T3-E1 cells were exposed to oscillating fluid flow. These conflicting observations could result from differences in experimental procedure (i.e., serum concentrations) or design (steady versus oscillatory fluid shear). Alternately, p38 and JNK have been shown to be sensitive to oxygen pressure [20], a factor which could influence the results from cells exposed to oscillatory or steady fluid shear.
The earliest recorded response of osteoblasts to mechanical stimulation is a rapid increase in Ca2+i that is dependent on both extracellular Ca2+ entry and Ca2+ release [10]. However this increase in Ca2+i in osteoblasts in response to mechanical loading has yet to be assigned a detailed function. We have shown this increase is required for release of ATP [28] and for the translocation of NF-κB in MC3T3-E1 cells in response to shear [36]. In this study, we found that chelation of Ca2+i with BAPTA completely abolished the FSS-induced pERK1/2, indicating that Ca2+i is critical for the phosphorylation of ERK1/2.
To clarify which Ca2+ pool is important in ERK1/2 phosphorylation, we first removed Ca2+ from the shear medium. Removal of extracellular Ca2+ almost completely abrogated activation of pERK1/2 in response to shear (Figure 2B) indicating that Ca+ entry through a Ca2+-conducting channel was required for ERK1/2 phosphorylation. We have previously characterized a mechanosensitive, cation-selective channel (MSCC) and an L-type voltage-sensitive Ca2+ channel (L-VSCC) in osteoblasts [37] that, we postulate, act in concert to increase Ca2+ entry in response to mechanical stimulation. We, and others, have shown these channels to be involved in increases in production and release of paracrine/autocrine factors [4, 12, 13, 28] and changes in gene expression [36, 38] in response to mechanical stimulation. We have recently shown that inhibition of the L-VSCC significantly attenuates bone formation associated with mechanical loading in rats and mice [17]. Our results from this study demonstrate that inhibition of both the MSCC and L-VSCC significantly reduces the phosphorylation of ERK1/2 in response to fluid shear, further supporting our hypothesis that activation of these channels during mechanical stimulation are essential to mechanotransduction in osteoblasts.
The Ca2+i response to shear is also dependent on the release of Ca2+ from intracellular stores. In osteoblasts, intracellular Ca2+ release is induced by activation of PLC which hydrolyzes PIP2 to generate diacylglycerol and IP3. IP3, in turn, binds to a ligand-gated Ca2+ channel located in the membrane of the endoplasmic reticulum (ER) to enable Ca2+ release. We have previously shown that FSS can activate PLC in osteoblasts to induce translocation of NF-κB [36]. In addition, elevation of IP3 has been linked to prostaglandin and nitric oxide secretion in sheared osteoblasts [39]. Release of intracellular Ca2+ stores in response to FSS has also been shown to be involved in cytoskeletal reorganization and COX-2 gene expression in MC3T3-E1 cells [5]. In this study, inhibition of PLC with U73122 significantly reduced phosphorylation of ERK1/2 although depletion of Ca2+ stores failed to inhibit ERK1/2 activation. These data would suggest that the phosphorylation of ERK1/2 is dependent on the activation of PKC by PLC. The activation of conventional PKC isozymes, such as PKCα and PKCβ, requires not only diacylglycerol and phosphatidylserine, but also Ca2+ [40]. Activation of PKC has been shown to mediate various FSS-induced responses including gene expression and cytoskeletal organization [12, 39, 41]. Interestingly, PKC mediates increases in shear-induced prostaglandin E2 (PGE2) release in rat osteoblasts [39] but not COX-2 gene expression [7]. In this study, we found that a inhibition of PKC with GF109203X significantly inhibited the FSS-induced pERK1/2, suggesting that PKC activation also contributes to the FSS-induced phosphorylation of ERK1/2.
ATP, released into the extracellular milieu, induces a host of physiologic responses through activation of purinergic (P2) receptors. P2 receptors have been found in most tissues, including osteoblasts [42] and activation of these receptors have been shown to mediate intracellular Ca2+ signaling in osteoblasts [43]. We have recently shown that subjecting MC3T3-E1 cells to 12dynes/cm2 FSS for 15 minutes produced an increase (>5-fold) in ATP release that was predominantly released within the first minute of shear. This FSS-induced ATP release could be significantly abrogated by the L-VSCC inhibitor nifedipine. However, inhibition of intracellular Ca2+ release had no effect on ATP release [28]. Thus, a potential mechanism for the fluid shear-induced increase in pERK1/2 involves activation of P2 receptors following ATP release in response to fluid shear. Here, we find that exogenous ATP added to static MC3T3-E1 cells rapidly induced phosphorylation of ERK1/2, and that hydrolyzing ATP with apyrase significantly attenuated shear-induced ERK1/2 activation. These data indicate that release of nucleotides from osteoblasts during shear is critical for intracellular Ca2+ mobilization that in turn activates ERK1/2.
Purinergic receptors can be divided into two subgroups; metabotropic P2Y receptors that induce intracellular Ca2+ release through activation of G proteins and ionotropic P2X receptors that are ligand-gated channels [29]. Osteoblasts express a variety of P2Y and P2X receptors [42] that have been associated with increases in Ca2+i [43], propagation of Ca2+i waves [44], activation of c-fos [45] and increases in proliferation [46, 47]. Recently, two P2 receptors have been linked to mechanotransduction in bone cells. P2Y2 receptor activation has been shown to be involved in Ca2+i mobilization in response to oscillating shear [30]. Knockout of the P2X7 receptor decreases mechanosensitivity in mice in vivo and significantly reduces PGE2 release in osteoblasts [48, 49]. Previous reports indicate that P2Y2 receptors do not mediate ATP-induced bone resorption [45], and here we find that suppression of this receptor using iRNA techniques failed to reduce shear-induced ERK1/2 phosphorylation. Thus, while P2Y2 receptors may modulate the Ca2+i response to mechanical loading, this mechanism is not the sole pathway involved in mechanotransduction. Although P2Y2 suppression had no effect on FSS-induced ERK1/2 phosphorylation, ablation of the P2X7 receptor significantly attenuated pERK1/2 in sheared primary osteoblasts isolated from P2X7 knockout mice when compared to FSS-induced ERK1/2 activation in wild-type osteoblasts. This observation supports our previous data showing that P2X7 receptor activation was essential for PGE2 release and COX-2 production in MC3T3-E1 cells in response to FSS [48, 49]. However, hydrolysis of extracellular ATP by apyrase further attenuated FSS-induced ERK1/2 phosphorylation in osteoblasts isolated from P2X7 knockout mice, suggesting the involvement of another P2X or P2Y receptor in this response. Preliminary data from our laboratory (data not shown) suggests that the P2Y6 receptor may additionally mediate FSS-induced ERK1/2 phosphorylation. These data are consistent with findings by Korcok et al., who implicated both the P2X7 and P2Y6 receptors in NF-κB activation in osteoclasts [50, 51]. Such data emphasizes the need for continued study of purinergic signaling during mechanotransduction in osteoblasts.
Based on the observations outlined in this study, a putative working model of signaling cascades involved in the FSS-induced phosphorylation of ERK1/2 emerges (Figure 7). In this model, fluid shear initially triggers the MSCC which results in membrane depolarization [52]. This depolarization, in turn, activates the L-VSCC which increases intracellular calcium in a discrete domain beneath the cell membrane. This increase of intracellular Ca2+ increases membrane fusion of vesicles to the plasma membrane to release ATP. Extracellular ATP can subsequently bind to two types of purinergic receptors, P2X and P2Y, which can either gate additional entry of Ca2+ and other cations into the cell to further activate the Ca2+-dependent events or initiate signaling cascades through activation of G-proteins. We show in this study that activation of PLC and, ultimately, PKC, influences the phosphorylation of ERK1/2. However the P2Y2 receptor does not appear to be involved in this response. From our data, it is clear that our working model is incomplete. While ATP binding to the P2X7 receptor is important in shear-induced phosphorylation of ERK1/2, loss of the receptor in knockout animals does not completely block ERK1/2 phosphorylation. Further, how this ligand-gated channel activates ERK1/2 remains unknown. The data presented here indicates a direct role of calcium entry via the MSCC and L-VSCC and the resultant release of ATP in the activation of ERK1/2.
Figure 7.
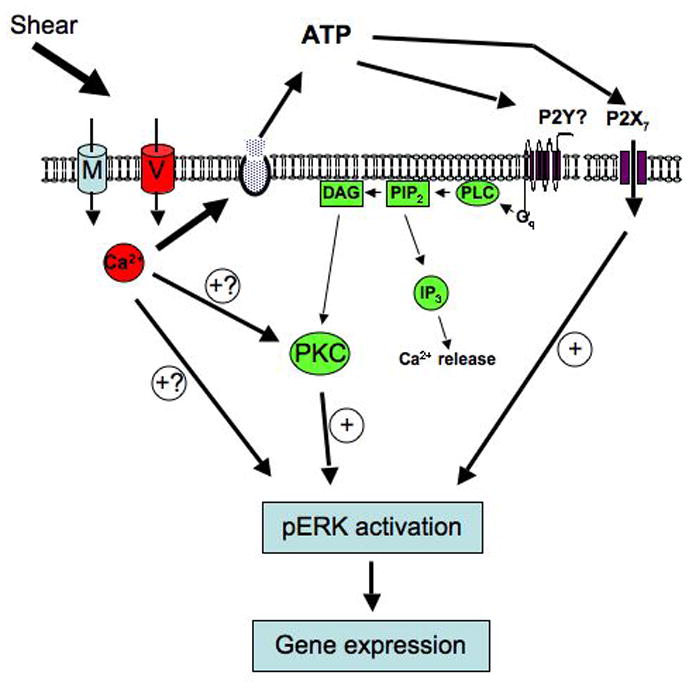
Working model of ERK1/2 activation in response to fluid shear flow in osteoblasts.
Acknowledgments
Contract grant sponsor: by NIH NIAMS grants AR043222 and AR051901 (RLD) and a NASA Pre-doctoral Fellowship NGTS-50366 (DCG).
References
- 1.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int. 1995;57:344–358. doi: 10.1007/BF00302070. [DOI] [PubMed] [Google Scholar]
- 2.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheld-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes-a cytoskeleton-dependent process. Biochem Biophys Res Comm. 1996;225:62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 3.Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol. 1991;261:C428–C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- 4.McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Min Res. 1999;14:930–936. doi: 10.1359/jbmr.1999.14.6.930. [DOI] [PubMed] [Google Scholar]
- 5.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;278:C989–C997. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 6.Pavalko FM, Chen NX, turner CH, Burr DB, Atkinson S, Hsieh Y-F, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol (Cell) 1998;275:C1591–C1601. [PubMed] [Google Scholar]
- 7.Wadhwa S, Choudhary S, Voznesensky M, Epstein M, Raisz L, Pilbeam C. Fluid flow induces COX-2 expression in MC3T3-E1 osteoblasts via a PKA signaling pathway. Biochem and Biophys Research Comm. 2002;297:46–51. doi: 10.1016/s0006-291x(02)02124-1. [DOI] [PubMed] [Google Scholar]
- 8.Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop Rel Res. 1995;313:256–269. [PubMed] [Google Scholar]
- 9.Jones DB, Nolte H, Scholubbers J-G, Turner E, Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101–110. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- 10.Hung CT, Allen FD, Pollack SR, Brighton CT. Intracellular calcium stores and extracellular calcium are required in the real-time calcium response of bone cells experiencing fluid flow. J Biomechanics. 1996;29:1411–1417. doi: 10.1016/0021-9290(96)84536-2. [DOI] [PubMed] [Google Scholar]
- 11.Duncan RL, Akanbi KA, Farach-Carson MC. Calcium signals and calcium channels in osteoblastic cells. Seminars in Nephrology. 1998;18:178–190. [PubMed] [Google Scholar]
- 12.Ajubi NE, Klein-Nulend J, Alblas MJ, Burger EH, Nijweide PJ. Signal transduction pathways involved in fluid flow-induced PGE2 production by cultured osteocytes. Am J Physiol (Endocrine) 1999;276:E171–E178. doi: 10.1152/ajpendo.1999.276.1.E171. [DOI] [PubMed] [Google Scholar]
- 13.Sakai K, Mohtai M, Iwamoto Y. Fluid shear stress increases transforming growth factor beth-1 expression in human osteoblast-like cells: modulation by cation channel blockers. Calcif Tissue Int. 1998;63:515–520. doi: 10.1007/s002239900567. [DOI] [PubMed] [Google Scholar]
- 14.Rawlinson SC, Pitsillides AA, Lanyon LE. Involvement of different ion channels in osteoblasts’ and osteocytes’ early responses to mechanical strain. Bone. 1996;19:609–614. doi: 10.1016/s8756-3282(96)00260-8. [DOI] [PubMed] [Google Scholar]
- 15.Duriez J, Flautre B, Blary MC, Hardouin P. Effects of the calcium channel blocker nifedipine on epiphyseal growth plate and bone turnover: a study in rabbit. Calcif Tissue Int. 1993;52:120–124. doi: 10.1007/BF00308320. [DOI] [PubMed] [Google Scholar]
- 16.Loza JSE, Dole C, Dziak R, Simasko S. Calcium currents in osteoblastic cells: Dependence upon cellular growth stage. Calcif Tissue Int. 1994;55:128–133. doi: 10.1007/BF00297188. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Duncan RL, Burr DB, Turner CH. L-type calcium channels mediate mechanically induced bone formation in vivo. J Bone Miner Res. 2002;17:1795–1800. doi: 10.1359/jbmr.2002.17.10.1795. [DOI] [PubMed] [Google Scholar]
- 18.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 19.Lai CF, Chaudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. Erk is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda N, Morita N, Matsuda K, Watanabe M. Proliferation and differentiation of human osteoblastic cells associated with differential activation of MAP kinases in response to epidermal growth factor, hypoxia, and mechanical stress in vitro. Biochem and Biophys Res Commun. 1998;249:350–354. doi: 10.1006/bbrc.1998.9151. [DOI] [PubMed] [Google Scholar]
- 21.Valledor AF, Comalada M, Xaus J, Celada A. The differential time-course of extracellular-regulated kinase activity correlates with the macrophage response toward proliferation or activation. J Biol Chem. 2000;275:7403–7409. doi: 10.1074/jbc.275.10.7403. [DOI] [PubMed] [Google Scholar]
- 22.Xiao G, Jiang D, Thomas P, Benson MD, Guan K, Karsenty G, Franceschi RT. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- 23.Gabarin N, Gavish H, Muhlrad A, Chen YC, Namdar-Attar M, Nissenson RA, Chorev M, Bab I. Mitogenic G(i) protein-MAP kinase signaling cascade in MC3T3-E1 osteogenic cells: activation by C-terminal pentapeptide of osteogenic growth peptide [OGP(10–14)] and attenuation of activation by cAMP. J Cell Biochem. 2001;81:594–603. doi: 10.1002/jcb.1083. [DOI] [PubMed] [Google Scholar]
- 24.Jessop HL, Rawlinson SC, Pitsillides AA, Lanyon LE. Mechanical strain and fluid movement both activate extracellular regulated kinase (ERK) in osteoblast-like cells but via different signaling pathways. Bone. 2002;31:186–194. doi: 10.1016/s8756-3282(02)00797-4. [DOI] [PubMed] [Google Scholar]
- 25.Alford AI, Jacobs CR, Donahue HJ. Oscillating fluid flow regulates gap junction communication in osteocytic MLO-Y4 cells by an ERK1/2 MAP kinase-dependent mechanism. Bone. 2003;33:64–70. doi: 10.1016/s8756-3282(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 26.Jiang GL, White CR, Stevens HY, Frangos JA. Temporal gradients in shear stimulate osteoblastic proliferation via ERK1/2 and retinoblastoma protein. Am J Physiol Endocrinol Metab. 2002;283:E383–E389. doi: 10.1152/ajpendo.00547.2001. [DOI] [PubMed] [Google Scholar]
- 27.Choudhary S, Wadhwa S, Raisz L, Alander C, Pilbeam CC. Extracellular calcium is a potent inducer of cyclo-oxygenase-2 in murine osteoblasts through an ERK signaling pathway. J Bone Miner Res. 2003;18:1813–1824. doi: 10.1359/jbmr.2003.18.10.1813. [DOI] [PubMed] [Google Scholar]
- 28.Genetos DG, Geist DJ, Liu D, Donahue HJ, Duncan RL. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. J Bone Miner Res. 2005;20:41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 30.You J, Jacobs CR, Steinberg TH, Donahue HJ. P2Y Purinoceptors Are Responsible for Oscillatory Fluid Flow-induced Intracellular Calcium Mobilization in Osteoblastic Cells. J Biol Chem. 2002;277:48724–48729. doi: 10.1074/jbc.M209245200. [DOI] [PubMed] [Google Scholar]
- 31.Ke HZ, Qi H, Weidema AF, Zhang Q, Panupinthu N, Crawford DT, Grasser WA, Paralkar VM, Li M, Audoly LP, Gabel CA, Jee WSS, Dixon SJ, Sims SM, Thompson DD. Deletion of the P2X7 Nucleotide Receptor Reveals Its Regulatory Roles in Bone Formation and Resorption. Mol Endocrinol. 2003:me.2003–0021. doi: 10.1210/me.2003-0021. [DOI] [PubMed] [Google Scholar]
- 32.Robey PG. Collagenase-treated trabecular bone fragments: a reproducible source of cells in the osteoblastic lineage. Calcif Tissue Int. 1995;56:S41–S43. [Google Scholar]
- 33.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotech Bioeng. 1988;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Liu D, Ke HZ, Duncan RL, Turner CH. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem. 2005;280:42952–9. doi: 10.1074/jbc.M506415200. [DOI] [PubMed] [Google Scholar]
- 35.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 36.Chen NX, Genetos DG, Geist D, Pavalko FM, Duncan RL. Fluid shear-induced NFκB translocation in osteoblasts is mediated by intracellular calcium release. Bone. 2003;33:399–410. doi: 10.1016/s8756-3282(03)00159-5. [DOI] [PubMed] [Google Scholar]
- 37.Duncan R, Misler S. Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106) FEBS Lett. 1989;251:17–21. doi: 10.1016/0014-5793(89)81420-6. [DOI] [PubMed] [Google Scholar]
- 38.Vadiakas GP, Banes AJ. Verapamil decreases cyclic load-induced calcium incorporation in ROS 17/2.8 osteosarcoma cell cultures. Matrix. 1992;12:439–447. doi: 10.1016/s0934-8832(11)80088-0. [DOI] [PubMed] [Google Scholar]
- 39.Reich KM, Frangos JA. Protein kinase C mediates flow-induced prostaglandin E2 production in osteoblasts. Calcif Tissue Int. 1993;52:62–66. doi: 10.1007/BF00675628. [DOI] [PubMed] [Google Scholar]
- 40.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 41.Berk BC, Corson MA, Peterson TE, Tseng H. Protein kinases as mediators of fluid shear stress stimulated signal transduction in endothelial cells: a hypothesis for calcium-dependent and calcium-independent events activated by flow. J Biomech. 1995;28:1439–1450. doi: 10.1016/0021-9290(95)00092-5. [DOI] [PubMed] [Google Scholar]
- 42.Bowler WB, Buckley KA, Gartland A, Hipskind RA, Bilbe G, Gallagher JA. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28:507–12. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- 43.Kumagi H, Sacktor B, Filburn CR. Purinergic regulation of cytosolic calcium and phosphoinsositide metabolism in rat osteoblast-like osteosarcoma cells. J Bone Miner Res. 1991;6:697–708. doi: 10.1002/jbmr.5650060707. [DOI] [PubMed] [Google Scholar]
- 44.Jorgensen NR, Henriksen Z, Sorensen OH, Eriksen EF, Civitelli R, Steinberg TH. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J Biol Chem. 2002;277:7574–80. doi: 10.1074/jbc.M104608200. [DOI] [PubMed] [Google Scholar]
- 45.Bowler WB, Dixon CJ, Halleux C, Maier R, Bilbe G, Fraser WD, Gallagher JA, Hipskind RA. Signaling in human osteoblasts by extracellular nucleotides. J Biol Chem. 1999;274:14315–14324. doi: 10.1074/jbc.274.20.14315. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura E, Uezono Y, Narusawa K, Shibuya I, Oishi Y, Tanaka M, Yanagihara N, Nakamura T, Izumi F. ATP activates DNA synthesis by acting on P2X receptors in human osteoblast-like MG-63 cells. Am J Physiol: Cell Physiol. 2000;279:C510–9. doi: 10.1152/ajpcell.2000.279.2.C510. [DOI] [PubMed] [Google Scholar]
- 47.Shimegi S. ATP and adenosine act as a mitogen for osteoblast-like cells (MC3T3-E1) Calcif Tissue Intl. 1996;58:109–13. doi: 10.1007/BF02529732. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Liu D, Duncan RL, Turner CH. Osteogenesis after mechanical loading requires the P2X7 nucleotide receptor. J Bone Miner Res. 2004;19:1032. [Google Scholar]
- 49.Liu D, Li J, Turner CH, Duncan RL. P2X7 purinergic receptor activation modulates prostaglandin synthesis and release from osteoblasts in response to fluid shear. J Bone Miner Res. 2004;19:M197. [Google Scholar]
- 50.Korcok J, Raimundo LN, Du X, Sims SM, Dixon SJ. P2Y6 nucleotide receptors activate NF-kappaB and increase survival of osteoclasts. J Biol Chem. 2005;280:16909–15. doi: 10.1074/jbc.M410764200. [DOI] [PubMed] [Google Scholar]
- 51.Korcok J, Raimundo LN, Ke HZ, Sims SM, Dixon SJ. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J Bone Miner Res. 2004;19:642–51. doi: 10.1359/JBMR.040108. [DOI] [PubMed] [Google Scholar]
- 52.Duncan RL, Hruska KA, Misler S. Parathyroid hormone activation of stretch-activated cation channels in osteosarcoma cells (UMR-106.01) FEBS Lett. 1992;307:219–23. doi: 10.1016/0014-5793(92)80771-8. [DOI] [PubMed] [Google Scholar]