Abstract
The importance of Mn2+ for pneumococcal physiology and virulence has been studied extensively. However, the specific cellular role(s) for which Mn2+ is required are yet to be fully elucidated. Here, we analyzed the effect of Mn2+ limitation on the transcriptome and proteome of Streptococcus pneumoniae D39. This was carried out by comparing a deletion mutant lacking the solute binding protein of the high-affinity Mn2+ transporter, pneumococcal surface antigen A (PsaA), with its isogenic wild-type counterpart. We provide clear evidence for the Mn2+-dependent regulation of the expression of oxidative-stress-response enzymes SpxB and Mn2+-SodA and virulence-associated genes pcpA and prtA. We also demonstrate the upregulation of at least one oxidative- and nitrosative-stress-response gene cluster, comprising adhC, nmlR, and czcD, in response to Mn2+ stress. A significant increase in 6-phosphogluconate dehydrogenase activity in the psaA mutant grown under Mn2+-replete conditions and upregulation of an oligopeptide ABC permease (AppDCBA) were also observed. Together, the results of transcriptomic and proteomic analyses provided evidence for Mn2+ having a central role in activating or stimulating enzymes involved in central carbon and general metabolism. Our results also highlight the importance of high-affinity Mn2+ transport by PsaA in pneumococcal competence, physiology, and metabolism and elucidate mechanisms underlying the response to Mn2+ stress.
Divalent cations are essential micronutrients for the growth and survival of bacteria in diverse environmental niches, including sites within higher organisms. One strategy employed by host organisms is to limit bacterial growth by restricting metal ion availability by sequestration with high-affinity metal binding proteins (7). To overcome these innate defenses, bacteria have evolved both metal ion-chelating mechanisms and high-affinity transport systems, enabling them to scavenge essential metals in vivo. Although the best-studied systems are involved in the acquisition of Fe3+, Mn2+ is increasingly being recognized as a critical micronutrient, particularly for pathogenic bacteria. Pneumococcal surface antigen A (PsaA) (47), the Mn2+-specific solute binding component of an ATP-binding cassette (ABC) cation permease encoded by the psaBCA locus, is an important virulence factor for Streptococcus pneumoniae (43). Mutation of psaA has been shown to result in massively reduced virulence in systemic, respiratory tract, and otitis media murine models of infection (4, 33, 34).
PsaA belongs to the cluster IX family of bacterial transporters of the essential metal ions Mn2+, Zn2+, and Fe2+ (9). All of the available physiological evidence indicates that it is involved in the transport of Mn2+; the optimal growth and competence for genetic transformation of a psaA mutant showed an absolute requirement for additional Mn2+ (9, 33, 34). The deletion of psaB and psaC, which encode the transmembrane and ATP-binding components of the Psa permease, also results in a requirement for Mn2+ (33, 34, 37). Despite this physiological evidence, the high-resolution structure of PsaA showed a Zn2+ ion occupying the metal binding site (29). However, Zn2+ transport seems unlikely to be the primary physiological function for PsaBCA for two reasons; first, two specific ABC cassette transporters for Zn2+ (AdcRCBA and AdcAII) are present in S. pneumoniae, and second, Zn2+ negatively regulates PsaBCA expression via PsaR (9, 32).
There is a large body of evidence that PsaA plays a role in resistance to oxidative stress (18, 19, 34, 57). The most obvious explanation for this observation is the presence of the Mn2+-dependent superoxide dismutase Mn2+ SodA, which protects the cell from superoxide, in the pneumococcus. The observation that psaA mutants exhibit a 40% reduction in Sod activity in the absence of Mn2+ is consistent with this interpretation (57, 58). Interestingly, one microarray study identified a two-component system that controls the expression of PsaA and regulates virulence and resistance to oxidative stress, albeit in a serotype-specific manner (35).
The above-described studies indicate that Mn2+ is associated with a diverse range of cellular metabolic and regulatory processes. However, the presence of high-affinity Mn2+ transporters has prevented the manipulation of intracellular Mn2+ levels in wild-type bacteria to allow elucidation of these processes. Nevertheless, the intracellular Mn2+ concentration can be manipulated in Mn2+ transport mutants (9, 34). Thus, in the present study, a combination of transcriptomic and proteomic comparisons of wild-type and psaA mutant S. pneumoniae under Mn2+-replete and Mn2+-limiting conditions has enabled the first systematic investigation of the role of Mn2+ in the regulation of metabolism and stress response physiology in S. pneumoniae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. pneumoniae strains used in this study are D39 (3) and its isogenic unmarked, in-frame psaA deletion mutant (34). Frozen stocks of the two strains were prepared by growing them at 37°C to an A600 of 0.4 in a semisynthetic casein hydrolysate medium supplemented with 0.5% yeast extract without added MnSO4 (C+Y medium) (27), as described previously (34). The cultures were concentrated 20× in C+Y medium, glycerol was added to a final concentration of 15%, and then the cultures were stored at −80°C. For in vitro growth measurements and proteomic and transcriptomic analyses, cultures from frozen stocks were added to C+Y medium or to C+Y medium with 3 μM MnSO4 (C+Y+M medium). Cultures were incubated at 37°C, and growth measured at regular intervals until an A600 of 0.5 was reached. The experiment was performed four times, and for each growth condition, cells were harvested and processed for either transcriptomic or proteomic analysis.
Microarray protocol and data analysis.
RNA was extracted from bacterial pellets with acid-phenol/chloroform/isoamyl alcohol (125:24:1 [pH 4.5]; Ambion) and checked for purity and integrity as described previously (30, 38). Microarray experiments were performed on whole-genome S. pneumoniae PCR arrays based on TIGR4 and R6 annotations (55). Array slides were obtained from the Bacterial Microarray Group at St. George's, University of London. The array design is available in BμG@Sbase (accession no. A-BUGS-14; http://bugs.sgul.ac.uk/A-BUGS-14) and, also, ArrayExpress (accession no. A-BUGS-14).
Fluorescently labeled cDNAs were hybridized to the surface of the microarray as described previously (35). The Spot plug-in (CSIRO, Australia) within the R statistical software package (http://www.R-project.org) and the Limma plug-in for R (49) were used for data processing and statistical analysis. The ratio values were normalized using the print-tip Loess normalization routine (51), and a linear model fitted to determine a final expression value for each mRNA (50). These statistics were used to rank the mRNAs from those most likely to be differentially expressed to the least likely using a false discovery rate of a P value of <0.05. Details of protocol and analysis are in the supplemental material. Fully annotated microarray data have been deposited in BμG@Sbase (accession no. E-BUGS-89; http://bugs.sgul.ac.uk/E-BUGS-89) and, also, ArrayExpress (accession no. E-BUGS-89).
2D DIGE and data analysis.
Proteins were extracted using a modification of the method described previously (12). Proteins were labeled with Cy2, Cy3, or Cy5 CyDye DIGE fluors (GE Healthcare) and subjected to two-dimensional (2D) difference gel electrophoresis (DIGE) according to the manufacturer's protocol. After electrophoresis, gels were scanned for fluorescence using an Ettan DIGE imager (GE Healthcare). Image analysis was undertaken using the Differential In-gel Analysis (DIA) module in DeCyder 2D software (version 6.5; GE Healthcare). Protein spots chosen for identification were excised robotically (Ettan SpotCutter; GE Healthcare) from two gels using pick coordinates assigned by DeCyder software. Principal components analysis (PCA) and hierarchical clustering analysis (HCA) were conducted according to the DeCyder Extended Data Analysis user manual, version 7.0. If more than one protein was identified in each spot, the relative abundance of each protein was determined from the emPAI (exponentially modified protein abundance index) score reported by MASCOT and the assumption made that the most abundant protein would account for the observed regulation (see the supplemental material). In some instances, the relative abundances of identified proteins in pair-wise comparisons were further validated by reverse transcription (RT)-PCR. Secondary identification of the protein spots was also carried out on preparative silver-stained gels. Further details of these procedures and statistical analyses are in the supplemental material.
Real-time RT-PCR.
For a subset of selected genes, differences in the levels of expression obtained by transcriptomic and proteomic analysis were validated by one-step relative quantitative real-time RT-PCR in a Roche LC480 real-time cycler, essentially as described previously (46). The specific primers used for the various RT-PCR assays are listed in Table S1 in the supplemental material and were used at a final concentration of 200 nM per reaction. As an internal control, primers specific for the 16S rRNA were employed. The amplification data were analyzed using the comparative critical threshold (2−ΔΔCT) method (31).
6-PGD enzymatic activity assay.
An enzymatic activity assay for (6-phosphogluconate dehydrogenase) 6-PGD was carried out as described previously (5) and as detailed in the supplemental material.
SDS-PAGE and Western blotting.
Bacteria were lysed in lysis buffer and subjected to SDS-PAGE as described previously (28). Separated proteins were electroblotted onto nitrocellulose (Pall Life Sciences, MI) as described previously (56). After transfer, the membrane was probed with specific polyclonal mouse anti-PsaA serum at a dilution of 1/3,000 and then reacted with blotting-grade goat anti-mouse alkaline phosphatase conjugate (Bio-Rad Laboratories, Hercules, CA).
Metal content determination.
Metal content determination was performed by inductively coupled plasma mass spectrometry (ICPMS) using wild-type and mutant cells grown to an A600 of 0.3 in either C+Y or C+Y+M medium on an Agilent 7500cx ICPMS essentially as described previously (34).
RESULTS
Transcriptome and proteome analyses of wild-type S. pneumoniae D39 and its isogenic unmarked, in-frame psaA deletion mutant.
This study used a systematic approach integrating transcriptomic (microarray) and proteomic (two-dimensional [2D] difference gel electrophoresis [DIGE]) comparisons of wild-type S. pneumoniae D39 (D) and an otherwise isogenic unmarked, in-frame psaA deletion mutant thereof (A). Each of these was grown under Mn2+-replete (+) and Mn2+-limiting (−) conditions (C+Y medium with or without 3 μM MnSO4, respectively). To confirm that the selected conditions were appropriate for the investigation of Mn2+-dependent responses, we investigated PsaA expression by Western blot analysis of whole-cell lysates using mouse polyclonal anti-PsaA serum. As expected (18), the expression of PsaA in wild-type cells grown in Mn2+-limiting conditions (D−) was markedly higher than its expression in cells grown in Mn2+-replete medium (D+), with no product observed for the mutant strain in either medium (A− or A+) (Fig. 1).
FIG. 1.

Western blot analysis of PsaA expression in S. pneumoniae D39 and its isogenic psaA deletion mutant lysates. Bacteria were grown in C+Y+M (with 3 μM added MnSO4) or C+Y (without added MnSO4) medium. D+, D39 wild type grown in C+Y+M medium; D−, D39 wild type grown in C+Y medium; A+, D39 psaA deletion mutant grown in C+Y+M medium; A−, D39 psaA deletion mutant grown in C+Y medium.
The effect of Mn2+ limitation on gene expression in wild-type cells.
The effect of Mn2+ limitation on gene expression in wild-type (D− versus D+) cells was then investigated, and based on the results of previous studies (18, 23), it was expected that genes under the control of the Mn2+-responsive transcription factor PsaR would show altered levels of expression. The results in Table 1 and, also, in Table S5 in the supplemental material show that, in the absence of Mn2+, there was a significant increase in the relative transcript levels of psaB, psaC, and psaA, consistent with derepression of the psa operon as a consequence of Mn2+ limitation. Other pneumococcal virulence-associated genes, such as pcpA (spd1965) and prtA (spd0558), also showed large increases in relative transcript levels in response to Mn2+ limitation, and it is known that these genes are also part of the PsaR regulon (16, 18, 23). The czcD gene, which encodes a zinc efflux pump, also showed increased mRNA levels in response to Mn2+ limitation (Table 1); in contrast to the other genes described above, czcD expression is activated by the Zn2+-responsive transcription factor SczA (22, 53) rather than PsaR.
TABLE 1.
Microarray analysis of S. pneumoniae D39 wild type and its isogenic psaA deletion mutant counterpart grown in C+Y medium with and without 3 μM added Mn2+
| Gene IDa |
Gene annotation | Fold changeb |
|||||
|---|---|---|---|---|---|---|---|
| Strain R6 or TIGR4 | Strain D39 | A− vs A+ | A+ vs D− | A+ vs D+ | A− vs D− | D− vs D+ | |
| 0014 | 0014 | Transcriptional regulator ComX1 | N.S. | N.S. | N.S. | −8.4 | N.S. |
| 0641 | 0558 | Serine protease, subtilase family (PrtA) | 16.1 | −13.7 | N.S. | N.S. | 21.69 |
| 0766 | 0667 | SodA; superoxide dismutase, manganese-dependent | −2.1 | N.S. | N.S. | N.S. | N.S. |
| SpR6-1191 | 1167 | AppD; ABC transporter ATP-binding protein | N.S. | 2.7 | 2.7 | N.S. | N.S. |
| SpR6-1193 | 1169 | AppB; ABC transporter membrane-spanning permease | N.S. | 3.0 | 2.7 | N.S. | N.S. |
| SpR6-1194 | 1170 | AppA; ABC transporter substrate-binding protein (oligopeptide transport) | N.S. | 2.3 | 2.4 | N.S. | N.S. |
| 1648 | 1461 | Manganese ABC transporter, ATP-binding protein (PsaB) | 6.7 | −7.6 | N.S. | N.S. | 8.51 |
| 1649 | 1462 | Manganese ABC transporter, permease protein (PsaC) | 7.6 | −7.4 | N.S. | N.S. | 12.68 |
| 1855 | 1636 | Alcohol dehydrogenase, zinc containing (AdhC) | 3.9 | N.S. | N.S. | 3.5 | N.S. |
| 1856 | 1637 | Transcriptional regulator, MerR family protein (NmlR) | 4.7 | N.S. | N.S. | 3.1 | N.S. |
| 1857 | 1638 | Cation efflux system protein (CzcD) | 7.8 | −3.4 | N.S. | N.S. | 4.60 |
| 2136 | 1965 | Choline binding protein PcpA | 27.3 | −8.7 | N.S. | 3.1 | 12.99 |
Gene sequences were obtained from the S. pneumoniae R6, D39, or TIGR4 genome as deposited in the Kyoto Encyclopedia of Genes and Genomes (KEGG) database.
A+, D39 psaA deletion mutant grown in C+Y medium with 3 μM added Mn2+; A−, D39 psaA deletion mutant grown in C+Y medium without added Mn2+; D+, D39 wild type grown in C+Y medium with 3 μM added Mn2+; D−, D39 wild type grown in C+Y medium without added Mn2+. Values are for the first indicated phenotype; N.S. indicates that the comparison did not yield a value reaching statistical significance.
The effect of Mn2+ limitation on gene expression in the psaA mutant.
The effect of Mn2+ limitation on gene expression in the psaA mutant (A− versus A+) was also investigated. The five genes that showed increased relative transcript levels in response to Mn2+ limitation in wild-type cells also showed an increase in the mutant strain. However, there were three additional changes; genes encoding the MerR family transcription factor (NmlR) and a glutathione (GSH)-dependent alcohol dehydrogenase (AdhC) also showed increased expression, while sodA (encoding Mn2+-dependent superoxide dismutase [SOD]) showed a small decrease in its relative transcript amount (Table 1; also see Tables S2A, C, and D in the supplemental material). It is notable that czcD, nmlR, and adhC form a gene cluster (spd1636 to spd1638 [spd1636-1638]) whose transcription is coupled under some conditions (22, 53).
Effect of mutation of psaA on gene expression.
Our observation that the psaA mutant grown under conditions of Mn2+ supplementation (A+) had a cellular concentration of Mn2+ comparable to that in wild-type cells grown under conditions of Mn2+ limitation (D−) enabled us to determine whether the loss of PsaA alone had an effect on gene expression. The results in Table 1 show that the relative transcript levels of genes under PsaR control were much lower in the psaA mutant than in wild-type cells even though the cellular Mn2+ concentration in both strains was similar. Similarly, the czcD gene exhibited a lower transcript level in the psaA mutant than in the wild-type strain. A gene cluster encoding an ABC cassette transport protein, predicted to encode an oligopeptide permease, was also observed to increase its relative transcript amount in the A+ cells compared to its transcript amounts in D− and D+ cells. Taken together, these data suggest that the absence of the PsaA protein might be linked to a change in gene expression that was independent of the cellular Mn2+ concentration.
Effect of Mn2+ limitation on growth.
The requirement for Mn2+ supplementation for the growth of psaA mutants was investigated. As expected (9, 19, 34), mutant bacteria grown without added Mn2+ (A−) had an extended lag phase, whereas mutant bacteria grown in the presence of 3 μM MnSO4 (A+) had an almost wild-type growth rate (see Fig. S1 in the supplemental material).
Proteome analysis.
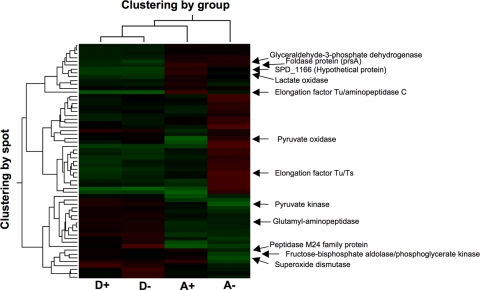
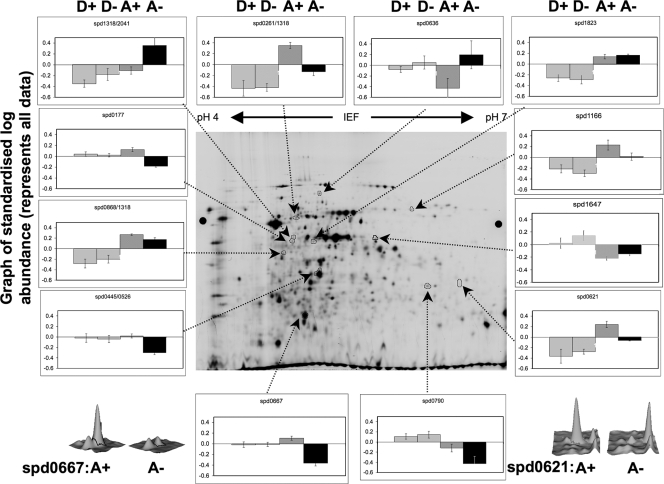
The optimized DIGE and DeCyder analyses of four replicate samples (see the supplemental material) from each of the D+, D−, A+, and A− cells resolved more than 2,000 spots, 61 of which were differentially expressed. The set of spots that showed significant regulation in at least one pair-wise comparison were subjected to PCA using the DeCyder Extended Data Analysis module (version 6.5) (13). This reduction of the dimensionality of the data showed that there was good reproducibility of the spot maps from each experimental group. The spot maps from the four conditions showed that D+ and D− samples clustered together, while A+ and A− samples clustered separately and distant from each other (Fig. 2), indicating that the Mn2+-limiting condition had little overall effect on protein expression in wild-type bacteria. The results of the hierarchical clustering analysis (11) performed subsequently correlated with the PCA results (Fig. 3), confirming the robustness of the strain- and growth medium-dependent protein expression patterns. Of this set of spots, 12 spots that satisfied the selection criteria as defined by the emPAI score reported by MASCOT (see Fig. S2 and Table S6 in the supplemental material) were chosen for identification and differential expression analysis (Table 2 and Fig. 4). Three-dimensional images of standardized log abundances of the Mn2+-dependent superoxide dismutase (SodA; spd0667) and lactate oxidase (LctO; spd0621) are illustrated as examples (Fig. 4).
FIG. 2.
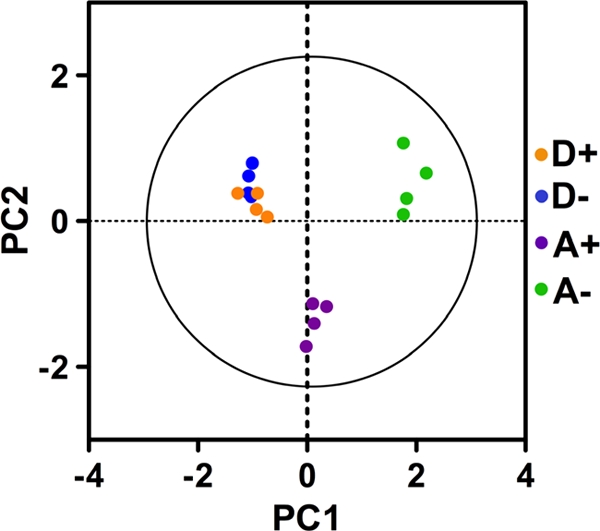
Principal component analysis of S. pneumoniae D39 and its isogenic psaA deletion mutant protein spots. Sixty-one differentially expressed protein spots were analyzed. PC1 is the single principal component that accounts for as much of the variability in the data as possible. PC2 is the succeeding uncorrelated (orthogonal) component that accounts for as much of the remaining variability as possible. D+, D39 wild type grown in C+Y+M medium; D−, D39 wild type grown in C+Y medium; A+, D39 psaA deletion mutant grown in C+Y+M medium; A−, D39 psaA deletion mutant grown in C+Y medium.
FIG. 3.
Hierarchical clustering analysis of S. pneumoniae D39 and its isogenic psaA deletion mutant protein spots. The wild type and its isogenic psaA deletion mutant were grown in C+Y+M or C+Y medium. The relative positions of 12 highly regulated protein spots from the 61 differentially expressed spots are indicated. D+, D39 wild type grown in C+Y+M medium; D−, D39 wild type grown in C+Y medium; A+, D39 psaA deletion mutant grown in C+Y+M medium; A−, D39 psaA deletion mutant grown in C+Y medium; red, upregulation; green, downregulation.
TABLE 2.
DIGE analysis of S. pneumoniae D39 wild type and its isogenic psaA deletion mutant counterpart grown in C+Y medium with and without 3 μM added Mn2+
| Locus tag (strain D39) | Protein ID (NCBI GI no.) | Relative abundance (P value)a |
||||
|---|---|---|---|---|---|---|
| A− vs A+ | A+ vs D− | A+ vs D+ | A− vs D− | D− vs D+ | ||
| spd0636 | Pyruvate oxidase (116516119) | 4.76 (0.1)b | −2.65 | −1.94 | 1.80 | 1.37 |
| spd1166 | Hypothetical protein (116515829) | −1.71 | 3.50 (0.003) | 3.35 (0.004) | 2.05 (0.014) | −1.04 |
| spd1318/spd0261/spd0343 | Elongation factor Tu (116515356)/aminopeptidase C (116516913)/6-phosphogluconate dehydrogenase | −2.95 (0.002) | 5.79 (0.0001) | 5.31 (0.001) | 1.96 | −1.09 |
| spd1318/spd2041 | Elongation factor Tu/Ts (116515356/116516076) | 3.42 (0.048) | 1.12 | 1.44 | 3.83 (0.04) | 1.29 |
| spd0177 | Peptidase M24 family protein (116516135) | −2.03 (0.0001) | 1.27 | 1.11 | −1.60 | −1.14 |
| spd0868/spd1318 | Foldase protein (PrsA) (116517083)/elongation factor Tu (116515356) | −1.23 | 2.57 (0.003) | 2.91 (0.0001) | 2.29 (0.004) | 1.03 |
| spd1823 | Glyceraldehyde-3-phosphate dehydrogenase (116516442) | 1.05 | 2.81 (0.001) | 2.22 (0.0001) | 2.70 (0.002) | −1.16 |
| spd0526/spd0445 | Fructose-biphosphate aldolase (116515860)/phosphoglycerate kinase (116516585) | −2.08 (0.001) | 1.12 | −1.15 | −1.85 | −1.29 |
| spd0621 | Lactate oxidase (116517149) | −2.06 (0.003) | 3.32 (0.001) | 3.31 (0.005) | 1.61 | −1.0 |
| spd0790 | Pyruvate kinase (116516870) | −1.80 | −1.81 (0.04) | −1.91 | −3.26 (0.010) | −1.06 |
| spd0667 | Mn2+-dependent superoxide dismutase (116515547) | −2.89 (0.0001) | 1.30 | 1.15 | −2.21 (0.002) | −1.13 |
| spd1647 | Glutamyl aminopeptidase PepA (116516201) | 1.18 | −2.39 (0.005) | −2.25 (0.015) | −2.03 (0.014) | 1.06 |
A+, D39 psaA deletion mutant grown in C+Y medium with 3 μM added Mn2+; A−, D39 psaA deletion mutant grown in C+Y medium without added Mn2+; D+, D39 wild type grown in C+Y medium with 3 μM added Mn2+; D−, D39 wild type grown in C+Y medium without added Mn2+. Scores without a P value did not reach statistical significance by t test (two-tailed).
This did not meet the t test criteria but was selected on the basis of its high Mn2+ dependence in the mutant.
FIG. 4.
DIGE analysis of protein spots from S. pneumoniae D39 wild type and its isogenic psaA deletion mutant. Graphs of standardized log abundances of 12 highly regulated spots and three-dimensional images of standardized log abundances of the Mn2+-dependent superoxide dismutase (SodA; spd0667) and lactate oxidase (LctO; spd0621) are shown. D+, D39 wild type grown in C+Y+M medium; D−, D39 wild type grown in C+Y medium; A+, D39 psaA deletion mutant grown in C+Y+M medium; A−, D39 psaA deletion mutant grown in C+Y medium.
Analysis of the changes to the proteome of strains grown under the conditions described above revealed some changes in relative protein amounts that were distinct from those observed in the microarray analysis (Table 2). In concordance with the results of PCA and HCA described above, no significant changes in protein levels were observed in the comparison of D− and D+ cells, confirming that, in wild-type bacteria, the Mn2+-limiting condition had a minimal effect on relative protein expression. Under conditions where the cellular concentration of Mn2+ was similar (A+ versus D−) a number of changes in relative protein abundance were observed. Five proteins showed a >2-fold increase in abundance in the A+ strain. These proteins included glyceraldehyde 3-phosphate dehydrogenase (GAPDH), LctO, and elongation factor Tu (EF-Tu)/PepC. Two proteins showed >2-fold lower abundance in A+ cells than in D− cells. These proteins were pyruvate oxidase (SpxB) and PepA, a glutamyl aminopeptidase. The effect of Mn2+ on the proteome of a psaA mutant was in many cases opposite to those changes observed in the A+/D− comparison. The most notable changes were an increase in the protein abundance of SpxB in the A− strain accompanied by a decrease in LctO and a decrease in SodA. Wherever possible, relative protein amounts were correlated with the corresponding relative mRNA levels.
Metal content determination.
ICPMS analysis of wild-type and mutant cells grown to an A600 of 0.3 either in C+Y or C+Y+M medium showed that A+ cells had a Mn2+ concentration similar to that in wild-type cells grown under conditions of Mn2+ limitation (Table 3). The ICPMS data also showed that the PsaA mutants had essentially half the Zn2+ concentration of wild-type cells. The Zn2+ content data are consistent with transcriptional regulation of prtA, psaBC, pcpA, and czcD by the Zn2+-responsive regulator PsaR.
TABLE 3.
Intracellular metal ion concentrations of S. pneumoniae D39 wild type and its isogenic psaA deletion mutant counterpart grown in C+Y medium with and without 3 μM added Mn2+
| Strainb | Concn (ng/g cells) ofa: |
|
|---|---|---|
| Mn2+ | Zn2+ | |
| A− | 89.81 ± 1.6 | 6874.16 ± 44.0 |
| A+ | 290.99 ± 7.1 | 6413.56 ± 116.2 |
| D− | 241.44 ± 10.3 | 12865.84 ± 1684.1 |
| D+ | 1017.69 ± 60.3 | 12702.48 ± 3697.3 |
Cells were grown to an A600 of 0.3, and metal ion values are the means ± standard errors of the means of the results for duplicate samples from two independent experiments.
A+, D39 psaA deletion mutant grown in C+Y medium with 3 μM added Mn2+; A−, D39 psaA deletion mutant grown in C+Y medium without added Mn2+; D+, D39 wild type grown in C+Y medium with 3 μM added Mn2+; D−, D39 wild type grown in C+Y medium without added Mn2+.
6-PGD enzymatic activity assay.
Secondary analysis of proteomic data on preparative silver-stained gels identified 6-phosphogluconate dehydrogenase (6-PGD) (gnd; spd0343) as a spot neighboring/overlapping EF-Tu/PepC which the original DIGE experiment was not sensitive enough to identify. Because this enzyme could potentially be activated by Mn2+ but has yet to be characterized, we investigated its activity in A+, A−, D+, and D− cells. Two independent experiments were performed; the assays showed 6-PGD activity in cell extracts of A+ and A− cells, with approximately 4.5-fold greater activity in A+ bacteria. However, no detectable activity was observed in the D+ or D− cell extracts.
DISCUSSION
The importance of Mn2+ for pneumococcal physiology and virulence has been studied extensively. However, the specific cellular role(s) for which Mn2+ is required are yet to be fully clarified. In this investigation, we have carried out an extensive analysis of the global effects of Mn2+ on the transcriptome and proteome of S. pneumoniae D39 by comparing a deletion mutant lacking the solute binding protein of the high-affinity Mn2+ transporter, PsaA, with its isogenic wild-type counterpart. Our analysis showed differences between the microarray and proteomic data, and this would not be totally unexpected. This is because while the microarray provides a comprehensive snapshot of transcripts whose levels are altered, proteomic analysis does not provide a similar overview. Some proteins may be not be identified by DIGE because of solubility issues, neighboring/overlapping spots, posttranslational modification, and pIs outside the range of the analysis. In the DIGE analysis, there were about 2,000 resolvable spots representing probably only the 1,000 most abundant proteins, taking into account protein isoforms, and we analyzed only 12 of these. Nevertheless, the protein spots identified were further validated by RT-PCR.
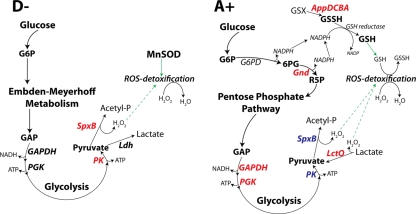
The results of the comparison between Mn2+-limited (D−) and Mn2+-replete (D+) wild-type cells highlight the central role of Mn2+ in the regulation of gene expression in the pneumococcus. Genes such as prtA, psaBCA, and pcpA are known to be regulated by the Mn2+-dependent repressor PsaR (16, 18, 23). This transcription factor is the homologue of Bacillus species MntR which is known to bind two Mn2+ ions as corepressors (15). The gene encoding the zinc efflux pump CzcD also showed increased expression under Mn2+-limited conditions in wild-type cells. The expression of czcD is known to be under the control of the transcriptional factor SczA that activates expression in response to elevated Zn2+ levels (22, 53). Thus, a decrease in intracellular Mn2+ may be associated with an increase in intracellular Zn2+. It has been reported that Zn2+ binding to PsaR may antagonize its action as a repressor (20, 40). Thus, perturbation of the Mn2+/Zn2+ ratio caused by Mn2+ limitation may cause altered gene expression in wild-type S. pneumoniae. A similar pattern of gene expression was observed in the A− versus A+ comparison. This demonstrates that PsaR is exerting its regulatory influence on gene expression in the mutant. The czcD gene was also induced but, in addition, the nmlR and adhC genes were also upregulated. czcD-nmlR-adhC form a gene cluster (spd1636-1638). Thus, it is possible that under conditions of severe Mn2+ limitation such as for the A− strain, there are changes in the transcription of this gene cluster and nmlR and adhC are expressed as part of a polycistronic transcript as a consequence of read-through from the czcD gene, or alternatively, as a distinct transcript from the nmlR promoter. In Haemophilus influenzae, adhC encodes a glutathione-dependent alcohol dehydrogenase that is required for defense against oxidative and nitrosative stress (21). This suggests that under conditions of Mn2+ limitation, a glutathione-based defense system could manifest itself in the pneumococcus. The gene expression changes may relate to the different niches in which S. pneumoniae is located within the human host; the nasopharynx is known to contain high levels of Mn2+ (36 μM quoted for saliva) (6), while in blood serum, it is about 20 nM (18, 26, 48). The relative concentrations of total glutathione in the nasopharynx and whole blood are <0.5 μM and 11 μM, respectively (41, 42). Interestingly, we have previously observed that adhC and nmlR mutants show reduced survival in blood compared to the survival of wild-type cells in a murine infection model, although the strains exhibited similar survival in the nasopharynx (22, 53). This would be consistent with a switch from a Mn2+-based to a glutathione-based stress defense system. A model for the central role of Mn2+ in this process is proposed in Fig. 5.
FIG. 5.
Diagrammatic representation of the proposed carbon metabolism in psaA mutants. The diagram shows the proposed flux of carbon in the psaA mutant strain as indicated by our biochemical, microarray, and proteomic data in D− and A+ cells. Wild-type cells utilized MnSOD to provide oxidative stress management, while the psaA mutant strains employed glutathione-based defense. Upregulated genes are indicated in red, and downregulated genes in blue. Abbreviations: G6P, glucose-6-phosphate; GAP, glyceradehyde-3-phosphate; PGK, phosphoglycerate kinase; PK, pyruvate kinase; Ldh, lactate dehydrogenase; Acetyl-P, acetyl phosphate; ROS, reactive oxygen species; G6PD, glucose-6-phosphate dehydrogenase; R5P, ribulose-5-phosphate; GSX, environmental glutathione; GSSH, oxidized glutathione; GSH, reduced glutathione.
S. pneumoniae is not thought to synthesize glutathione (14), indicating that it would need to acquire this tripeptide. This could be a function of the appDCBA operon that encodes a polyspecific oligopeptide ABC transporter. The relative increase in the mRNA abundance of the appDCBA operon coincides with a decrease in the level of the PepA protein (spd1647). In silico analysis indicated that PepA had structural motifs of a tripeptidase, an enzyme that has been shown to cleave reduced glutathione (GSH) to generate cysteine (8), which is critical for intracellular growth (1). Thus, downregulation in the psaA mutant would suggest that hydrolysis of GSH would be lower in order to preserve the GSH pool that is necessary for management of the oxidative-stress response. LctO also showed an increase in expression in the comparison of A+ and D−. This enzyme catalyzes the oxidation of lactate and generates pyruvate and H2O2 (54). Conditions where lactate would be available to the pneumococcus as a carbon source would be in blood and in the neutrophil. In contrast, in the nasopharynx, it would be expected that the organism would produce lactate as a fermentation end product. Under these conditions, pyruvate can also be oxidized by SpxB to generate acetyl phosphate and H2O2 (54). The increased expression of SpxB in the A−/A+ cells suggests that there may be some posttranslational effects of Mn2+ on SpxB.
The utilization of a glutathione-based defense system by S. pneumoniae would require a mechanism for regeneration of the intracellular glutathione pool. Glutathione reductase serves to reduce both oxidized and environmentally acquired glutathione (glutathione disulfide [GSSG]). However, a consequence of this mechanism is the rapid depletion of intracellular NADPH levels (10). Concomitant with the depletion of NADPH is the derepression of the pentose phosphate pathway (PPP) and diversion of carbon flux away from Embden-Meyerhoff metabolism. Consistent with this observation was the detection of the activity of the PPP enzyme, 6-phosphogluconate dehydrogenase (6-PGD), in both A+ and A− cells. This indicated that metabolic flux was being directed to the PPP in the psaA mutants, thus generating NADPH, consistent with the switch to a glutathione-based defense system under Mn2+ restriction.
In addition to these global changes to carbon metabolism, numerous minor alterations to cellular metabolism were also observed in response to Mn2+ concentrations. Notably, certain key enzymes involved in pyruvate metabolism, while not requiring Mn2+ for their function, exhibit their highest activity with Mn2+ as a cofactor (20). As a consequence, it would be expected that pyruvate precursor pools would be small, leading to increased flux through the glycolytic pathway and the upregulation of key enzymes. Under Mn2+-replete conditions, oxidative stress would be ameliorated as Mn2+ could act directly to detoxify superoxide and H2O2 generated during metabolism (18). Although S. pneumoniae lacks catalase, it may be less sensitive to H2O2 than many bacteria because it contains only a small number of iron-containing proteins that could drive Fenton chemistry in the presence of H2O2. Recently, it has been suggested that Mn2+ can confer protection against peroxide stress on Escherichia coli by inserting into mononuclear metalloenzymes in place of ferrous iron (2). If Mn2+ is used predominantly in equivalent enzymes in S. pneumoniae, this would explain why the effect of upregulation of SpxB can be tolerated in Mn2+-replete cells.
Colonization of the lungs is a key stage in the conversion of a local pneumococcal infection from the nasopharynx to a systemic infection. This is linked to the activation of alveolar macrophages and a subsequent heavy recruitment of neutrophils (24). A key pneumococcal virulence factor, pneumolysin, has been shown to activate the production of nitric oxide (NO) in neutrophils (17). Interestingly, NO is known to trigger the release of Zn2+ from metallothionein as a response to lung injury (52). Thus, we suggest that a coupling of the expression of a glutathione-based oxidative- and nitrosative-stress defense system and the removal of excess Zn2+ may be linked to colonization of the lower respiratory tract.
Beyond the central role of Mn2+ in metabolic and oxidative-stress processes, putative roles in the regulation and activation of other proteins were also elucidated. In the proteomic analysis, EF-Tu and EF-Ts were upregulated in A− cells. EF-Ts is essential for the elongation of the polypeptide chain and mediates the regeneration of EF-Tu·GDP into the active form, EF-Tu·GTP, which transports aminoacyl-tRNA to the ribosome in the elongation cycle of protein synthesis (25, 39). We hypothesize that upregulation of the EF-Tu/EF-Ts in cells starved of Mn2+ might be necessary for protein stability. Genes encoding the heat shock proteins GroEL/GroES (spd1711) were downregulated in A− cells, most probably due to the lack of Mn2+ to stabilize GroEL under conditions of oxidative stress (36). Moreover, a peptidase M24 family protein (spd0177), a Mn2+-dependent Xaa-Pro aminopeptidase (44), was upregulated in A+ cells, indicating the importance of Mn2+ as a cofactor in the activity of this enzyme.
Apart from the fundamental alterations to cellular metabolism and oxidative stress management, our transcriptome analyses have provided the first direct evidence for the essential role of PsaA in the induction of competence. Exogenous Mn2+ has long been implicated as a requirement for the development of competence for the genetic transformation of psaA mutants (9, 33, 34). In A− cells, downregulation of the transcriptional regulator ComX1 (spd0014), the competence-stimulating peptide (CSP; spd2065), and the competence operon (spd1857-1863) would be consistent with previous phenotypic observations (9, 33, 34).
In this study, gene expression and metabolic changes resulting from possible interaction between the genotypes of the strains (psaA mutation) and the growth conditions (with or without Mn2+) regulating the expression of particular genes/proteins were analyzed for synergy or interference. Microarray interaction analysis shows that a number of putative ABC transporters/transport systems (spd0097, spd0154, spd0616-0617, and spd1900) and a suite of genes involved in nucleotide metabolism (spd0187, spd0190, and spd1042) were downregulated in A− cells. However, a transcriptional regulator, a GntR family protein (spd1524), was highly upregulated in A− cells. Regulators belonging to this family have been shown to act as environmental sensors for controlling genes involved in responding directly or indirectly to external stimuli, such as small molecules or substrates (45). In addition, stress proteins (spd1590 and spd1793) were also upregulated in A− cells (see Table S3 in the supplemental material), probably due to oxidative stress. A further analysis to establish relationships in gene expression patterns due to psaA mutation and/or Mn2+ limitation was carried out using Venn diagrams (see Fig. S3 and Table S4 in the supplemental material). Protein interaction analysis highlighted those proteins whose regulation by the presence or absence of Mn2+ depended on the genotype. This is exemplified by the expression of EF-Tu/PepC and LctO, in which the genotypic effect is significantly suppressed under low Mn2+ conditions, and by the significant downregulation of the expression of PepM and SodA under low Mn2+ conditions in the psaA mutant (Table 2; also see Fig. S4 in the supplemental material).
Conclusions.
In this study, we have used a combination of transcriptomic and/or proteomic approaches to provide evidence for Mn2+-dependent regulation of the expression of the pneumococcal virulence-associated genes pcpA and prtA and the oxidative stress-response enzymes SpxB and Mn2+ SodA. Furthermore, the analyses provided direct evidence for high-affinity Mn2+ transport by PsaA in pneumococcal competence, physiology, and metabolism and elucidated the mechanisms underlying the response of the psaA mutant to Mn2+ stress. This serves as a paradigm for numerous mucosal pathogens which, like S. pneumoniae, are exposed to various levels of Mn2+ during disease pathogenesis, from asymptomatic nasopharyngeal colonization to the invasion of deeper host tissues.
Supplementary Material
Acknowledgments
We thank Mark Condina and Alex Colella for assistance with proteome data analysis, Megan Penno for helpful discussions, and Chris Cursaro for technical assistance. We acknowledge BμG@S (the Bacterial Microarray Group at St. George's, University of London) for supply of the microarray and advice.
This work was supported by the National Health and Medical Research Council of Australia (NHMRC) program grant 284214. We acknowledge The Wellcome Trust for funding the multicollaborative microbial pathogen microarray facility under its Functional Genomics Resources Initiative. J.C.P. is an NHMRC Australia Fellow.
The authors declare that no competing interests exist.
Footnotes
Published ahead of print on 2 July 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alkhuder, K., K. L. Meibom, I. Dubail, M. Dupuis, and A. Charbit. 2009. Glutathione provides a source of cysteine essential for intracellular multiplication of Francisella tularensis. PLoS Pathog. 5:e1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anjem, A., S. Varghese, and J. A. Imlay. 2009. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol. Microbiol. 72:844-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Inductions of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berry, A. M., and J. C. Paton. 1996. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect. Immun. 64:5255-5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, A. T., and C. L. Wittenberger. 1971. Mechanism for regulating the distribution of glucose carbon between the Embden-Meyerhof and hexose-monophosphate pathways in Streptococcus faecalis. J. Bacteriol. 106:456-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chicharro, J. L., V. Serrano, R. Urena, A. M. Gutierrez, A. Carvajal, P. Fernandez-Hernando, and A. Lucia. 1999. Trace elements and electrolytes in human resting mixed saliva after exercise. Br. J. Sports Med. 33:204-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corbin, B. D., E. H. Seeley, A. Raab, J. Feldmann, M. R. Miller, V. J. Torres, K. L. Anderson, B. M. Dattilo, P. M. Dunman, R. Gerads, R. M. Caprioli, W. Nacken, W. J. Chazin, and E. P. Skaar. 2008. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962-965. [DOI] [PubMed] [Google Scholar]
- 8.Del Corso, A., M. Cappiello, F. Buono, R. Moschini, A. Paolicchi, and U. Mura. 2006. Colorimetric coupled enzyme assay for gamma-glutamyltransferase activity using glutathione as substrate. J. Biochem. Biophys. Methods 67:123-130. [DOI] [PubMed] [Google Scholar]
- 9.Dintilhac, A., G. Alloing, C. Granadel, and J. P. Claverys. 1997. Competence and virulence of Streptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol. Microbiol. 25:727-739. [DOI] [PubMed] [Google Scholar]
- 10.Eggleston, L. V., and H. A. Krebs. 1974. Regulation of the pentose phosphate cycle. Biochem. J. 138:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. U. S. A. 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Encheva, V., S. E. Gharbia, R. Wait, S. Begum, and H. N. Shah. 2006. Comparison of extraction procedures for proteome analysis of Streptococcus pneumoniae and a basic reference map. Proteomics 6:3306-3317. [DOI] [PubMed] [Google Scholar]
- 13.Eriksson, L., E. Johansson, N. Kettaneh-Wold, and S. Wold. 2001. Multi- and megavariate data analysis: principles and applications. Umetrics Academy, Umeå, Sweden.
- 14.Fahey, R. C., W. C. Brown, W. B. Adams, and M. B. Worsham. 1978. Occurrence of glutathione in bacteria. J. Bacteriol. 133:1126-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasfeld, A., E. Guedon, J. D. Helmann, and R. G. Brennan. 2003. Structure of the manganese-bound manganese transport regulator of Bacillus subtilis. Nat. Struct. Biol. 10:652-657. [DOI] [PubMed] [Google Scholar]
- 16.Hendriksen, W. T., H. J. Bootsma, A. van Diepen, S. Estevao, O. P. Kuipers, R. de Groot, and P. W. Hermans. 2009. Strain-specific impact of PsaR of Streptococcus pneumoniae on global gene expression and virulence. Microbiology 155:1569-1579. [DOI] [PubMed] [Google Scholar]
- 17.Hirst, R. A., A. Kadioglu, C. O'Callaghan, and P. W. Andrew. 2004. The role of pneumolysin in pneumococcal pneumonia and meningitis. Clin. Exp. Immunol. 138:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston, J. W., D. E. Briles, L. E. Myers, and S. K. Hollingshead. 2006. Mn2+-dependent regulation of multiple genes in Streptococcus pneumoniae through PsaR and the resultant impact on virulence. Infect. Immun. 74:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston, J. W., L. E. Myers, M. M. Ochs, W. H. Benjamin, Jr., D. E. Briles, and S. K. Hollingshead. 2004. Lipoprotein PsaA in virulence of Streptococcus pneumoniae: surface accessibility and role in protection from superoxide. Infect. Immun. 72:5858-5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kehres, D. G., and M. E. Maguire. 2003. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol. Rev. 27:263-290. [DOI] [PubMed] [Google Scholar]
- 21.Kidd, S. P., D. Jiang, M. P. Jennings, and A. G. McEwan. 2007. Glutathione-dependent alcohol dehydrogenase AdhC is required for defense against nitrosative stress in Haemophilus influenzae. Infect. Immun. 75:4506-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloosterman, T. G., M. M. van der Kooi-Pol, J. J. Bijlsma, and O. P. Kuipers. 2007. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 65:1049-1063. [DOI] [PubMed] [Google Scholar]
- 23.Kloosterman, T. G., R. M. Witwicki, M. M. van der Kooi-Pol, J. J. Bijlsma, and O. P. Kuipers. 2008. Opposite effects of Mn2+ and Zn2+ on PsaR-mediated expression of the virulence genes pcpA, prtA, and psaBCA of Streptococcus pneumoniae. J. Bacteriol. 190:5382-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knapp, S., J. C. Leemans, S. Florquin, J. Branger, N. A. Maris, J. Pater, N. van Rooijen, and T. van der Poll. 2003. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 167:171-179. [DOI] [PubMed] [Google Scholar]
- 25.Krab, I. M., and A. Parmeggiani. 1998. EF-Tu, a GTPase odyssey. Biochim. Biophys. Acta 1443:1-22. [DOI] [PubMed] [Google Scholar]
- 26.Krachler, M., E. Rossipal, and D. Micetic-Turk. 1999. Concentrations of trace elements in sera of newborns, young infants, and adults. Biol. Trace Elem. Res. 68:121-135. [DOI] [PubMed] [Google Scholar]
- 27.Lacks, S., and R. D. Hotchkiss. 1960. A study of the genetic material determining an enzyme in Pneumococcus. Biochim. Biophys. Acta 39:508-518. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence, M. C., P. A. Pilling, V. C. Epa, A. M. Berry, A. D. Ogunniyi, and J. C. Paton. 1998. The crystal structure of pneumococcal surface antigen PsaA reveals a metal-binding site and a novel structure for a putative ABC-type binding protein. Structure 6:1553-1561. [DOI] [PubMed] [Google Scholar]
- 30.LeMessurier, K. S., A. D. Ogunniyi, and J. C. Paton. 2006. Differential expression of key pneumococcal virulence genes in vivo. Microbiology. 152:305-311. [DOI] [PubMed] [Google Scholar]
- 31.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 32.Loisel, E., L. Jacquamet, L. Serre, C. Bauvois, J. L. Ferrer, T. Vernet, A. M. Di Guilmi, and C. Durmort. 2008. AdcAII, a new pneumococcal Zn-binding protein homologous with ABC transporters: biochemical and structural analysis. J. Mol. Biol. 381:594-606. [DOI] [PubMed] [Google Scholar]
- 33.Marra, A., S. Lawson, J. S. Asundi, D. Brigham, and A. E. Hromockyj. 2002. In vivo characterization of the psa genes from Streptococcus pneumoniae in multiple models of infection. Microbiology. 148:1483-1491. [DOI] [PubMed] [Google Scholar]
- 34.McAllister, L. J., H. J. Tseng, A. D. Ogunniyi, M. P. Jennings, A. G. McEwan, and J. C. Paton. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53:889-901. [DOI] [PubMed] [Google Scholar]
- 35.McCluskey, J., J. Hinds, S. Husain, A. Witney, and T. J. Mitchell. 2004. A two-component system that controls the expression of pneumococcal surface antigen A (PsaA) and regulates virulence and resistance to oxidative stress in Streptococcus pneumoniae. Mol. Microbiol. 51:1661-1675. [DOI] [PubMed] [Google Scholar]
- 36.Melkani, G. C., R. L. Sielaff, G. Zardeneta, and J. A. Mendoza. 2008. Divalent cations stabilize GroEL under conditions of oxidative stress. Biochem. Biophys. Res. Commun. 368:625-630. [DOI] [PubMed] [Google Scholar]
- 37.Novak, R., J. S. Braun, E. Charpentier, and E. Tuomanen. 1998. Penicillin tolerance genes of Streptococcus pneumoniae: the ABC-type manganese permease complex Psa. Mol. Microbiol. 29:1285-1296. [DOI] [PubMed] [Google Scholar]
- 38.Ogunniyi, A. D., P. Giammarinaro, and J. C. Paton. 2002. The genes encoding virulence-associated proteins and the capsule of Streptococcus pneumoniae are upregulated and differentially expressed in vivo. Microbiology 148:2045-2053. [DOI] [PubMed] [Google Scholar]
- 39.Overweg, K., C. D. Pericone, G. G. Verhoef, J. N. Weiser, H. D. Meiring, A. P. De Jong, R. De Groot, and P. W. Hermans. 2000. Differential protein expression in phenotypic variants of Streptococcus pneumoniae. Infect. Immun. 68:4604-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papp-Wallace, K. M., and M. E. Maguire. 2006. Manganese transport and the role of manganese in virulence. Annu. Rev. Microbiol. 60:187-209. [DOI] [PubMed] [Google Scholar]
- 41.Pastore, A., R. Massoud, C. Motti, A. Lo Russo, G. Fucci, C. Cortese, and G. Federici. 1998. Fully automated assay for total homocysteine, cysteine, cysteinylglycine, glutathione, cysteamine, and 2-mercaptopropionylglycine in plasma and urine. Clin. Chem. 44:825-832. [PubMed] [Google Scholar]
- 42.Pastore, A., F. Piemonte, M. Locatelli, A. Lo Russo, L. M. Gaeta, G. Tozzi, and G. Federici. 2001. Determination of blood total, reduced, and oxidized glutathione in pediatric subjects. Clin. Chem. 47:1467-1469. [PubMed] [Google Scholar]
- 43.Paton, J. C. 1998. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 6:85-87. (Discussion, 6:87-88.) [DOI] [PubMed] [Google Scholar]
- 44.Rawlings, N. D., and A. J. Barrett. 1993. Evolutionary families of peptidases. Biochem. J. 290(Pt. 1):205-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rigali, S., A. Derouaux, F. Giannotta, and J. Dusart. 2002. Subdivision of the helix-turn-helix GntR family of bacterial regulators in the FadR, HutC, MocR, and YtrA subfamilies. J. Biol. Chem. 277:12507-12515. [DOI] [PubMed] [Google Scholar]
- 46.Rogers, T. J., A. W. Paton, S. R. McColl, and J. C. Paton. 2003. Enhanced CXC chemokine responses of human colonic epithelial cells to locus of enterocyte effacement-negative shiga-toxigenic Escherichia coli. Infect. Immun. 71:5623-5632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell, H., J. A. Tharpe, D. E. Wells, E. H. White, and J. E. Johnson. 1990. Monoclonal antibody recognizing a species-specific protein from Streptococcus pneumoniae. J. Clin. Microbiol. 28:2191-2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scheuhammer, A. M., and M. G. Cherian. 1985. Binding of manganese in human and rat plasma. Biochim. Biophys. Acta 840:163-169. [DOI] [PubMed] [Google Scholar]
- 49.Smyth, G. K. 2005. Limma: linear models for microarray data, p. 397-420. In V. Carey, R. Gentleman, S. Dudoit, R. Irizarry, and W. Huber (ed.), Bioinformatics and computational biology solutions using R and Bioconductor. Springer, New York, NY.
- 50.Smyth, G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article3. [DOI] [PubMed] [Google Scholar]
- 51.Smyth, G. K., and T. Speed. 2003. Normalization of cDNA microarray data. Methods 31:265-273. [DOI] [PubMed] [Google Scholar]
- 52.St. Croix, C. M., K. Leelavaninchkul, S. C. Watkins, V. E. Kagan, and B. R. Pitt. 2005. Nitric oxide and zinc homeostasis in acute lung injury. Proc. Am. Thorac. Soc. 2:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stroeher, U. H., S. P. Kidd, S. L. Stafford, M. P. Jennings, J. C. Paton, and A. G. McEwan. 2007. A pneumococcal MerR-like regulator and S-nitrosoglutathione reductase are required for systemic virulence. J. Infect. Dis. 196:1820-1826. [DOI] [PubMed] [Google Scholar]
- 54.Taniai, H., K. Iida, M. Seki, M. Saito, S. Shiota, H. Nakayama, and S. Yoshida. 2008. Concerted action of lactate oxidase and pyruvate oxidase in aerobic growth of Streptococcus pneumoniae: role of lactate as an energy source. J. Bacteriol. 190:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 56.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng, H. J., A. G. McEwan, J. C. Paton, and M. P. Jennings. 2002. Virulence of Streptococcus pneumoniae: PsaA mutants are hypersensitive to oxidative stress. Infect. Immun. 70:1635-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yesilkaya, H., A. Kadioglu, N. Gingles, J. E. Alexander, T. J. Mitchell, and P. W. Andrew. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.