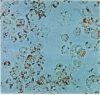
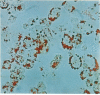
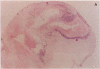
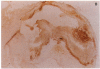
Abstract
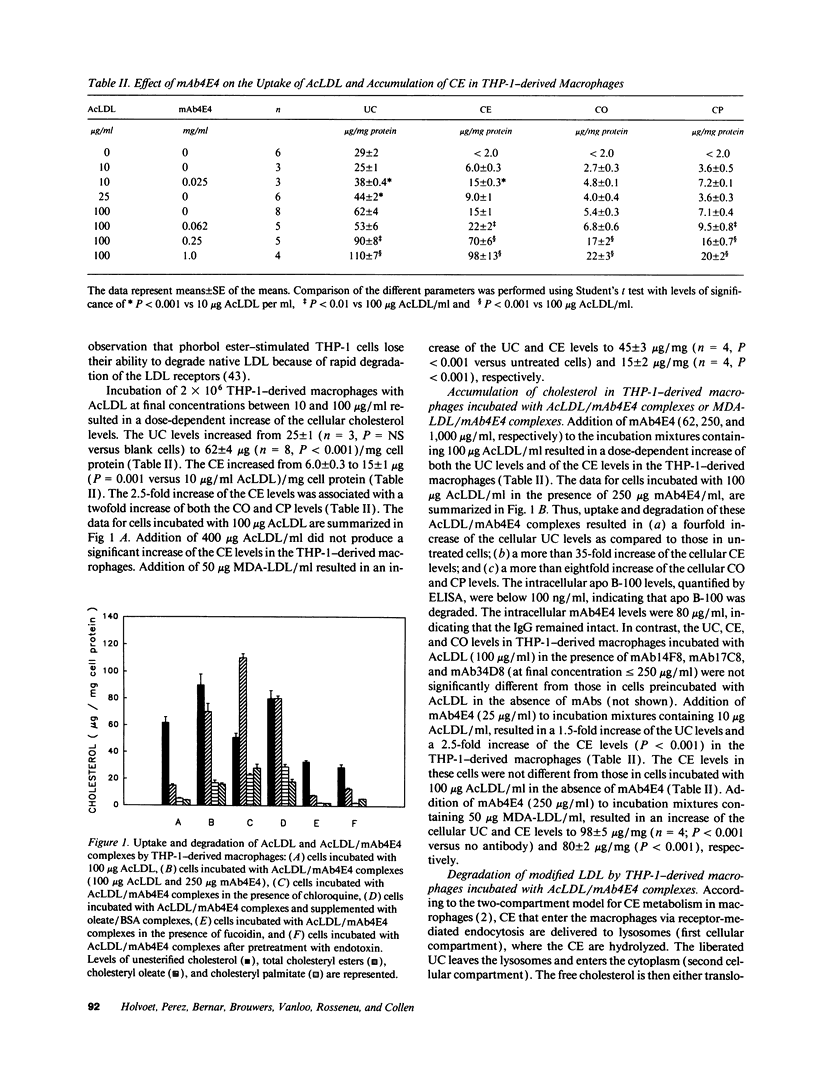
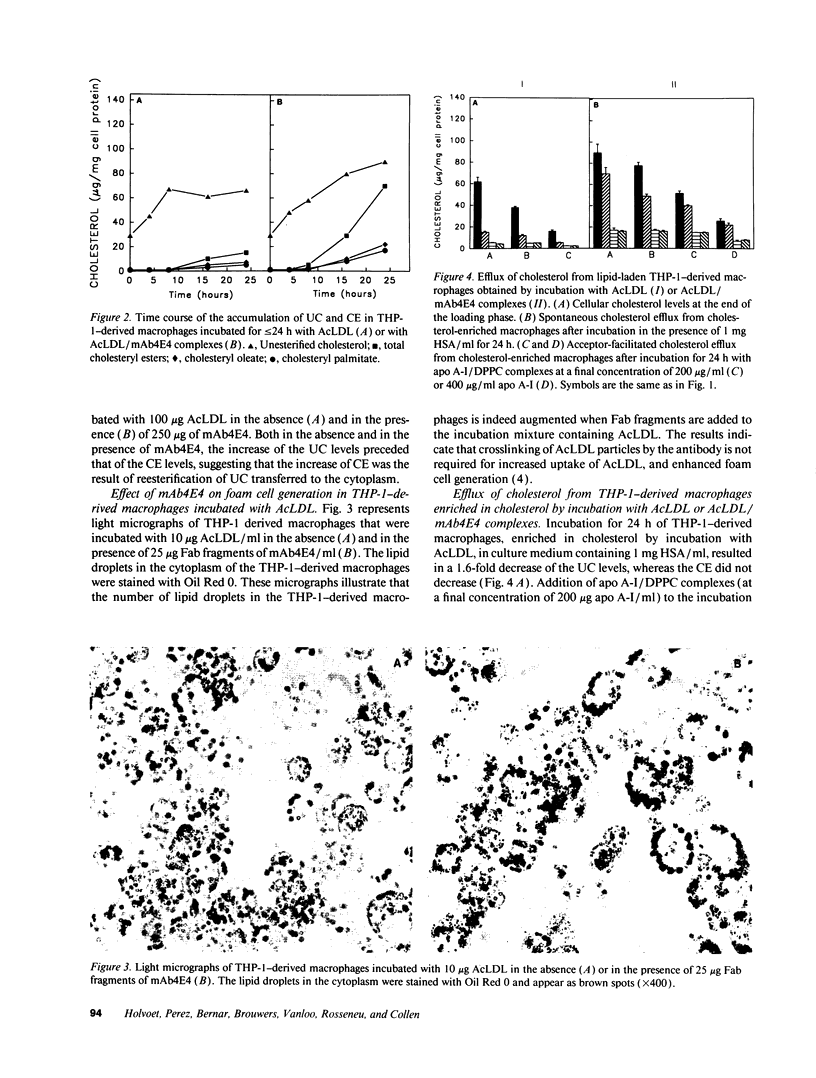
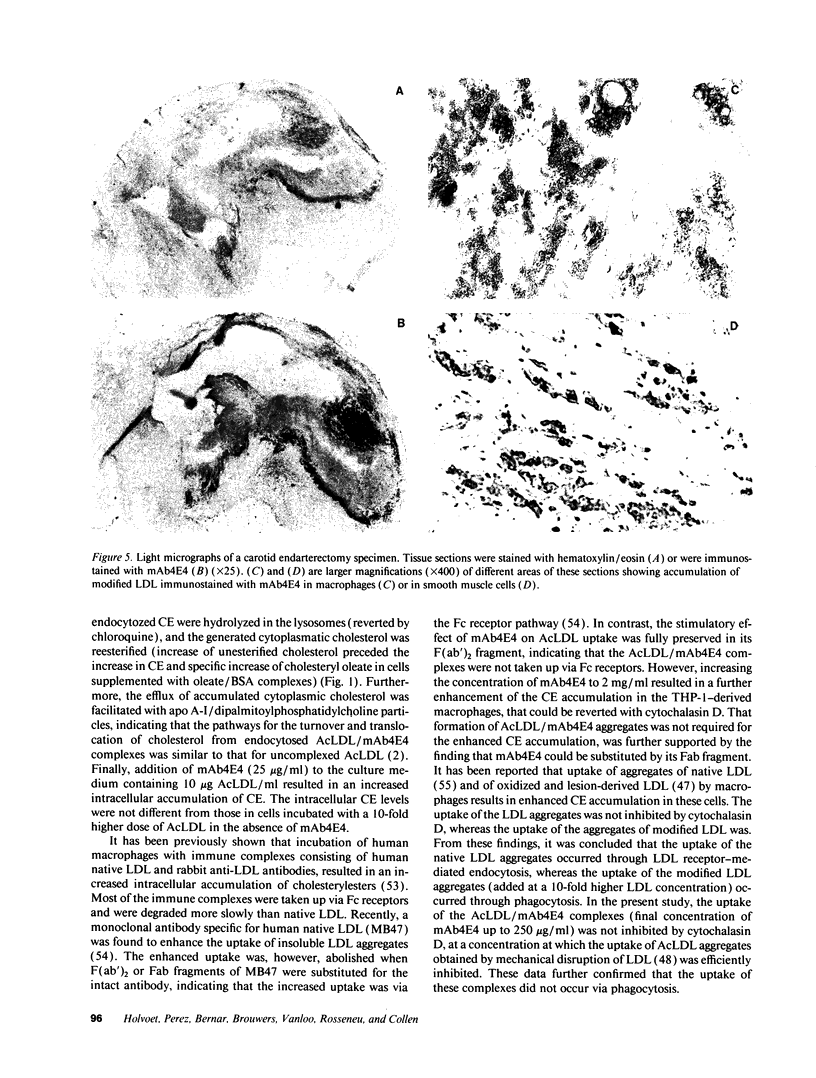
mAb4E4, a murine monoclonal antibody that is specific for acetylated LDL and malondialdehyde-treated LDL, binds specifically to modified LDL present in human atherosclerotic lesions. It is directed against an epitope that is poorly exposed in delipidated and solubilized apolipoprotein B-100 from modified LDL. mAb4E4, as well as its F(ab')2 and Fab fragments, enhanced the uptake of both acetylated LDL and malondialdehyde-treated LDL by THP-1-derived macrophages resulting in a sixfold increase of cytoplasmic cholesteryl ester levels. The increased uptake of modified LDL/mAb4E4 complexes did not occur via the Fc receptor and did not depend on aggregation of modified LDL particles. However, their uptake was inhibited by blocking the scavenger receptors with fucoidin or by downregulation of receptor expression with endotoxins or interferon-gamma, indicating that their uptake is mediated via these receptors. Thus, generation of autoimmune antibodies against modified LDL and subsequent endocytosis of soluble modified LDL/antibody complexes via scavenger receptors may enhance foam cell generation. This mechanism may contribute to the progression of atherosclerotic lesions.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbustini E., Grasso M., Diegoli M., Pucci A., Bramerio M., Ardissino D., Angoli L., de Servi S., Bramucci E., Mussini A. Coronary atherosclerotic plaques with and without thrombus in ischemic heart syndromes: a morphologic, immunohistochemical, and biochemical study. Am J Cardiol. 1991 Sep 3;68(7):36B–50B. doi: 10.1016/0002-9149(91)90383-v. [DOI] [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Suppression of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and inhibition of growth of human fibroblasts by 7-ketocholesterol. J Biol Chem. 1974 Nov 25;249(22):7306–7314. [PubMed] [Google Scholar]
- Cardin A. D., Witt K. R., Barnhart C. L., Jackson R. L. Sulfhydryl chemistry and solubility properties of human plasma apolipoprotein B. Biochemistry. 1982 Aug 31;21(18):4503–4511. doi: 10.1021/bi00261a048. [DOI] [PubMed] [Google Scholar]
- Clevidence B. A., Morton R. E., West G., Dusek D. M., Hoff H. F. Cholesterol esterification in macrophages. Stimulation by lipoproteins containing apo B isolated from human aortas. Arteriosclerosis. 1984 May-Jun;4(3):196–207. doi: 10.1161/01.atv.4.3.196. [DOI] [PubMed] [Google Scholar]
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fantappiè S., Corsini A., Sidoli A., Uboldi P., Granata A., Zanelli T., Rossi P., Marcovina S., Fumagalli R., Catapano A. L. Monoclonal antibodies to human low density lipoprotein identify distinct areas on apolipoprotein B-100 relevant to the low density lipoprotein-receptor interaction. J Lipid Res. 1992 Aug;33(8):1111–1121. [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel M. E., Gerhard W. The rapid determination of binding constants for antiviral antibodies by a radioimmunoassay. An analysis of the interaction between hybridoma proteins and influenza virus. Mol Immunol. 1979 Feb;16(2):101–106. doi: 10.1016/0161-5890(79)90051-8. [DOI] [PubMed] [Google Scholar]
- Geng Y. J., Hansson G. K. Interferon-gamma inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest. 1992 Apr;89(4):1322–1330. doi: 10.1172/JCI115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. Role of lysosomal acid lipase in the metabolism of plasma low density lipoprotein. Observations in cultured fibroblasts from a patient with cholesteryl ester storage disease. J Biol Chem. 1975 Nov 10;250(21):8487–8495. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka R., Seguchi T., Sato Y., Ono M., Kohno K., Kuwano M. Rapid turnover of low density lipoprotein receptor in human monocytic THP-1 cells. FEBS Lett. 1991 Dec 9;294(3):261–263. doi: 10.1016/0014-5793(91)81443-c. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J. Lesion-derived low density lipoprotein and oxidized low density lipoprotein share a lability for aggregation, leading to enhanced macrophage degradation. Arterioscler Thromb. 1991 Sep-Oct;11(5):1209–1222. doi: 10.1161/01.atv.11.5.1209. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J. Lesion-derived low density lipoprotein and oxidized low density lipoprotein share a lability for aggregation, leading to enhanced macrophage degradation. Arterioscler Thromb. 1991 Sep-Oct;11(5):1209–1222. doi: 10.1161/01.atv.11.5.1209. [DOI] [PubMed] [Google Scholar]
- Hogg P. J., Johnston S. C., Bowles M. R., Ponds S. M., Winzor D. J. Evaluation of equilibrium constants for antigen-antibody interactions by solid-phase immunoassay: the binding of paraquat to its elicited mouse monoclonal antibody. Mol Immunol. 1987 Jul;24(7):797–801. doi: 10.1016/0161-5890(87)90064-2. [DOI] [PubMed] [Google Scholar]
- Holvoet P., Lijnen H. R., Collen D. A monoclonal antibody specific for Lys-plasminogen. Application to the study of the activation pathways of plasminogen in vivo. J Biol Chem. 1985 Oct 5;260(22):12106–12111. [PubMed] [Google Scholar]
- Hughes D. A., Townsend P. J., Haslam P. L. Enhancement of the antigen-presenting function of monocytes by cholesterol: possible relevance to inflammatory mechanisms in extrinsic allergic alveolitis and atherosclerosis. Clin Exp Immunol. 1992 Feb;87(2):279–286. doi: 10.1111/j.1365-2249.1992.tb02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo J. C., Miller E., McLoughlin P., Steinberg D. Enhanced macrophage uptake of low density lipoprotein after self-aggregation. Arteriosclerosis. 1988 Jul-Aug;8(4):348–358. doi: 10.1161/01.atv.8.4.348. [DOI] [PubMed] [Google Scholar]
- Khoo J. C., Miller E., Pio F., Steinberg D., Witztum J. L. Monoclonal antibodies against LDL further enhance macrophage uptake of LDL aggregates. Arterioscler Thromb. 1992 Nov;12(11):1258–1266. doi: 10.1161/01.atv.12.11.1258. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liao W., Florén C. H. Endotoxins inhibit endocytotic catabolism of low-density lipoproteins in Hep G2 cells. Hepatology. 1992 Jul;16(1):224–231. doi: 10.1002/hep.1840160133. [DOI] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Lopes-Virella M. F., Griffith R. L., Shunk K. A., Virella G. T. Enhanced uptake and impaired intracellular metabolism of low density lipoprotein complexed with anti-low density lipoprotein antibodies. Arterioscler Thromb. 1991 Sep-Oct;11(5):1356–1367. doi: 10.1161/01.atv.11.5.1356. [DOI] [PubMed] [Google Scholar]
- Mahlberg F. H., Glick J. M., Lund-Katz S., Rothblat G. H. Influence of apolipoproteins AI, AII, and Cs on the metabolism of membrane and lysosomal cholesterol in macrophages. J Biol Chem. 1991 Oct 25;266(30):19930–19937. [PubMed] [Google Scholar]
- Marcel Y. L., Hogue M., Weech P. K., Milne R. W. Characterization of antigenic determinants on human solubilized apolipoprotein B. Conformational requirements for lipids. J Biol Chem. 1984 Jun 10;259(11):6952–6957. [PubMed] [Google Scholar]
- Matz C. E., Jonas A. Micellar complexes of human apolipoprotein A-I with phosphatidylcholines and cholesterol prepared from cholate-lipid dispersions. J Biol Chem. 1982 Apr 25;257(8):4535–4540. [PubMed] [Google Scholar]
- McGill H. C., Jr Fatty streaks in the coronary arteries and aorta. Lab Invest. 1968 May;18(5):560–564. [PubMed] [Google Scholar]
- Morton R. E., West G. A., Hoff H. F. A low density lipoprotein-sized particle isolated from human atherosclerotic lesions is internalized by macrophages via a non-scavenger-receptor mechanism. J Lipid Res. 1986 Nov;27(11):1124–1134. [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P., Androlewicz M. J., Brodsky F. M., Holmes N. J., Ways J. P. Monoclonal antibodies: purification, fragmentation and application to structural and functional studies of class I MHC antigens. J Immunol Methods. 1982 Sep 17;53(2):133–173. doi: 10.1016/0022-1759(82)90137-5. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Lougheed M., Kwan W. C., Dirks M. Recognition of oxidized low density lipoprotein by the scavenger receptor of macrophages results from derivatization of apolipoprotein B by products of fatty acid peroxidation. J Biol Chem. 1989 Sep 15;264(26):15216–15223. [PubMed] [Google Scholar]
- Steinbrecher U. P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987 Mar 15;262(8):3603–3608. [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Vanderyse L., Devreese A. M., Baert J., Vanloo B., Lins L., Ruysschaert J. M., Rosseneu M. Structural and functional properties of apolipoprotein B in chemically modified low density lipoproteins. Atherosclerosis. 1992 Dec;97(2-3):187–199. doi: 10.1016/0021-9150(92)90131-y. [DOI] [PubMed] [Google Scholar]
- Vanloo B., Morrison J., Fidge N., Lorent G., Lins L., Brasseur R., Ruysschaert J. M., Baert J., Rosseneu M. Characterization of the discoidal complexes formed between apoA-I-CNBr fragments and phosphatidylcholine. J Lipid Res. 1991 Aug;32(8):1253–1264. [PubMed] [Google Scholar]
- Vercaemst R., Union A., Rosseneu M., De Craene I., De Backer G., Kornitzer M. Quantitation of plasma free cholesterol and cholesteryl esters by high performance liquid chromatography. Study of a normal population. Atherosclerosis. 1989 Aug;78(2-3):245–250. doi: 10.1016/0021-9150(89)90230-x. [DOI] [PubMed] [Google Scholar]
- Via D. P., Pons L., Dennison D. K., Fanslow A. E., Bernini F. Induction of acetyl-LDL receptor activity by phorbol ester in human monocyte cell line THP-1. J Lipid Res. 1989 Oct;30(10):1515–1524. [PubMed] [Google Scholar]
- Xu X. X., Tabas I. Sphingomyelinase enhances low density lipoprotein uptake and ability to induce cholesteryl ester accumulation in macrophages. J Biol Chem. 1991 Dec 25;266(36):24849–24858. [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarron C., Ginsberg M. H., Plow E. F. A receptor-induced binding site in fibrinogen elicited by its interaction with platelet membrane glycoprotein IIb-IIIa. J Biol Chem. 1991 Aug 25;266(24):16193–16199. [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- van Lenten B. J., Fogelman A. M. Lipopolysaccharide-induced inhibition of scavenger receptor expression in human monocyte-macrophages is mediated through tumor necrosis factor-alpha. J Immunol. 1992 Jan 1;148(1):112–116. [PubMed] [Google Scholar]